Abstract
Background
Metastatic colorectal cancer (mCRC) treatment has changed substantially in the last 2 decades but the effect of age and comorbidities on chemotherapy utilization is not well studied.
Methods
mCRC patients receiving 5-FU based chemotherapy between 01/1995-12/2009 were studied using the LifeLink Health Plan Claims Database. The cohort was divided into older (>70y) and younger (≤70y) patients. Charlson Comorbidity Index (CCI) was used to assess comorbidity burden. Wilcoxon's and χ2 test were used in univariate and logistic regression in multivariate analyses (MVA).
Results
16,087 patients were identified, with 24% of the patients receiving chemotherapy aged >70y. The percentage of patients with CCI>1 receiving chemotherapy increased over time (14% in 1996 vs 40% after 2004; p<0.05). Older patients were less likely to get >2 agents compared to younger patients (15 vs. 22% and 11 vs. 16% in 2003 & 2009 respectively, p<0.001). Following FDA approval in 1998, the use of Irinotecan was lower in older compared to younger patients, a difference that resolved by 2002 (15 vs 38%, p<0.05; 62% in both groups, p=0.9, respectively). Similarly, Oxaliplatin was used more frequently in younger patients in 2003 (22% vs 15%, p<0.05), with decrease in this difference by 2009 (64% vs 60%, p=0.95). On MVA, older age [OR 0.65; p<0.001] and CCI>1 [OR 0.84; p<0.001] were associated with lower likelihood of receiving combination chemotherapy.
Conclusion
In this commercially insured population, the fraction of older patients treated for mCRC is low, and rate of chemotherapy adoption lags behind that of younger patients. However, the proportion of older patients with comorbidities receiving therapy increased over time.
Keywords: chemotherapy uptake, older adults, metastatic colorectal cancer, drug adoption
Condensed abstract
Retrospective analysis of effect of age and comorbidities on chemotherapy utilization in commercially insured metastatic colorectal cancer patients.
Introduction
The treatment of patients with metastatic colorectal cancer (mCRC) has changed substantially in the last 20 years, with new drugs and treatment regimens. Although most patients with metastatic disease remain incurable, recent studies have demonstrated a median survival of up to 30 months 1. Before the introduction of newer chemotherapeutic agents, treatment focused on optimizing the delivery of 5-Fluorouracil (FU) and enhancing its activity. New cytotoxic drugs for the treatment of mCRC began to be introduced during the late 1990s with the development of irinotecan and oxaliplatin 2, 3, both of which improved survival for patients with mCRC when added to 5-FU and leucovorin, optimally administered in an infusional schedule. These regimens (FOLFIRI and FOLFOX respectively) have been shown in clinical trials to produce similar outcomes, although they differ substantially in their toxicity profiles4. The US Food and Drug Administration (FDA) approved irinotecan and oxaliplatin for first-line treatment of mCRC in 2000 and 2004, respectively. Irinotecan was previously approved in 1998 in the second line setting. Combination therapy has since become the standard of care and recommended by the national comprehensive cancer network (NCCN)5. Since then, the FDA approval of several novel angiogenesis-targeting (Bevacizumab, Aflibercept, Regorafenib); and epidermal growth factor receptor (EGFR)–targeting agents (cetuximab, panitumumab) has improved clinical outcomes and survival.
The adoption of evidence-based new therapies among oncologists has been studied in various disease sites. A recent study by Neugut et. al. showed rapid uptake of oxaliplatin, after its approval in 2004, into adjuvant treatment regimens for node-positive early-stage colon cancer, as well as for metastatic disease6. A similar pattern was noted for the incorporation of bevacizumab into treatment of patients with mCRC6. Rapid adoption of novel therapies in oncology has been reported in other disease sites including breast cancer, lung cancer, and prostate cancer 7–11. However, the adoption of new therapeutic agents has not been well studied in older patients, and there is a paucity of literature about the role of age as a factor affecting chemotherapy use in patients with mCRC. The use of 5-FU-based combination chemotherapy (with irinotecan and oxaliplatin) has been shown to offer elderly mCRC patients a similar benefit in terms of overall and progression free survival to that seen in younger patients, with increased rates of toxicities in the older population 12–14.
In this study, we explore the effect of age and comorbidities on the uptake of irinotecan and oxaliplatin in addition to 5-FU into the management of mCRC patients following the publication of studies demonstrating their efficacy and their approval by the FDA.
Methods
Data Source
This retrospective study analyzed pharmaceutical insurance claims contained in the LifeLink Health Plan Claims Database (formerly the PharMetrics Patient-Centric Database). This administrative claims database encompasses medical and pharmacy claims from various commercial health plans on 82.5 million persons, including Medicare Managed Care plans in four U.S. geographical regions (East, West, Mid-west and South). This database has been used widely in studies evaluating health care economics in oncology and other disciplines15–17. The claims database contains details such as date of service, International Classification of Diseases Ninth Revisions, Clinical Modifications (ICD-9-CM) codes, procedure codes, and national drug codes. It does not include any tumor-related features such as stage or histologic findings. De-identified data representing the national commercially insured population were obtained through a license agreement.
Time Periods of Evaluation
We reviewed claims between January, 1995 and December, 2009 in order to include the relevant events that resulted in recommendations for incorporating irinotecan and oxaliplatin into the treatment paradigm for mCRC and allow for comparision over the years. Within this time period, we divided the cohort into older (>70 years) and younger (≤70 years) age groups and analysed the results as a whole and at yearly intervals.
Patient Selection
We identified patients older than 18 years at the onset of metastatic disease, through medical claims for the diagnosis of mCRC. Patients were identified as having mCRC using the diagnosis codes of colon or rectal cancer (ICD9 codes of 153.x or 154.x). Patients with anal cancer (ICD 154.3) were excluded. We further identified patients with an ICD9 diagnosis code of metastatic disease (in organs: 197.0–197.7 and 198.0–199.0) and excluded those with only lymphnode metastases (ICD9 code 196.0, 196.1, 196.3–196.5, 196.7–196.9). Healthcare Common Procedure Coding System (HCPCS; J codes) were used for identification of drug therapy (J9190, fluorouracil therapy ; J9206, irinotecan; J9263, oxaliplatin). Recognizing that the majority of the treated patients with mCRC receive 5FU as a single agent or in combination with other chemotherapeutic agents, we only included patients who recieved 5-FU based chemotherapy for metastatic disease in our analysis. The fraction of patients receiving capecitabine was ~5% and given the lack of data on degree of compliance with the oral drug, we excluded it from the analysis. We did not include targeted antibodies (bevacizumab, cetuximab, panititumab) in the analysis as they were approved towards the later part of the study period. We also excluded adjuvant chemotherapy by including only claims submitted after the first date of metastatic diagnosis code documentation.
Comorbidity
Comorbidity burden (excluding the current mCRC diagnosis) was assessed using the Charlson Comorbidity Index calculated from claims available up to 1 year before the onset of mCRC18.
Identification of Lines of Therapy
Since this claims database did not identify specific lines of therapy, we developed an algorithm to identify treatment lines based on the drugs used in each case. This algorithm has been previously published by our group19. To identify a cohort of patients who received second-line therapy or beyond, we defined first line as any chemotherapy (fluorouracil, irinotecan or oxaliplatin) given after submission of a claim for mCRC. We subsequently defined the beginning of second-line therapy by the initiation of a new chemotherapy agent (irinotecan or oxaliplatin) after first-line therapy. We excluded any patients who only had one claim for a specific chemotherapy agent to account for coding errors.
Statistical Analysis
Categorical variables were tabulated, and means were calculated for continuous variables. The patients' characteristics were summarized and compared based on the age groups, described earlier [older (age >70 years) and younger (age ≤ 70 years)].
χ2 test (for categorical variables) and Wilcoxon's test (for continuous variables) were used to compare the groups in the univariate analysis. As for the multivariable analysis (MVA), we used multiple logistic regression models to describe the age effect on the use of one vs more agents over time, controlling for covariates such as sex, year of start of treatment, and comorbidity score. SAS 9.2 was used for all analyses. A P value of less than .05 was considered statistically significant.
Results
Baseline characteristics
Between 1995–2009, 44,376 patients with mCRC were identified in the claims database with 15,844 >70 years of age (older, 36%) and 28,532 ≤ 70 years (younger, 64%). Sixteen thousand and eighty-seven patients ( 3881 older and 12,206 younger age group) received 5FU-based chemotherapy for mCRC and comprised the study cohort. Baseline characteristics of the entire cohort are outlined in Table 1. The median age at diagnosis was 61 years (range: 18–98). The percentage of patients older than 70 years in our cohort of treated patients was 24% compared to 36% in the entire claims database (p<0.05). At each year, the majority of the patients were younger (66–76% ≤70 years old vs 23–33% >70 years old , p< 0.05). This difference did not change over the 15 years analyzed. Approximately 55% patients were males and there was no significant difference in gender distribution across both groups.
Table 1.
Patient characteristics by age groups
| Charecteristics | Total | >=70 years | < 70 years | p value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| N^ | 16087 | 100 | 3881 | 24.1 | 12206 | 75.9 | <0.05 |
| Median Age | 61 years | 76 | 57 | ||||
| Range | 18–98 | 70–98 | 18–69 | ||||
| Sex | |||||||
| Male | 8959 | 55.7 | 2192 | 56.5 | 6767 | 55.4 | >0.05 |
| Female | 7128 | 44.3 | 1689 | 43.5 | 5439 | 44.6 | >0.05 |
| CCI | |||||||
| 0–1 | 9778 | 60.8 | 1879 | 48.4 | 7899 | 64.7 | <0.05 |
| 2 or more | 6309 | 39.2 | 2002 | 51.6 | 4307 | 35.3 | <0.05 |
| Chemotherapy exposure | |||||||
| 5FU only | 3653 | 22.7 | 1167 | 30.1 | 2486 | 20.4 | <0.0001 |
| 2 agents* | 8568 | 53.3 | 1966 | 50.7 | 6602 | 54.1 | <0.05 |
| >2 agents# | 3866 | 24.0 | 748 | 19.3 | 3118 | 25.5 | <0.05 |
N^: Represents total number of patients who received 5FU based chemotherapy.
2 agents: 5Fu + Irinotecan or Oxaliplatin
>2 agents: 5FU + Irinotecan and Oxaliplatin
Chemotherapy use and comorbidity burden
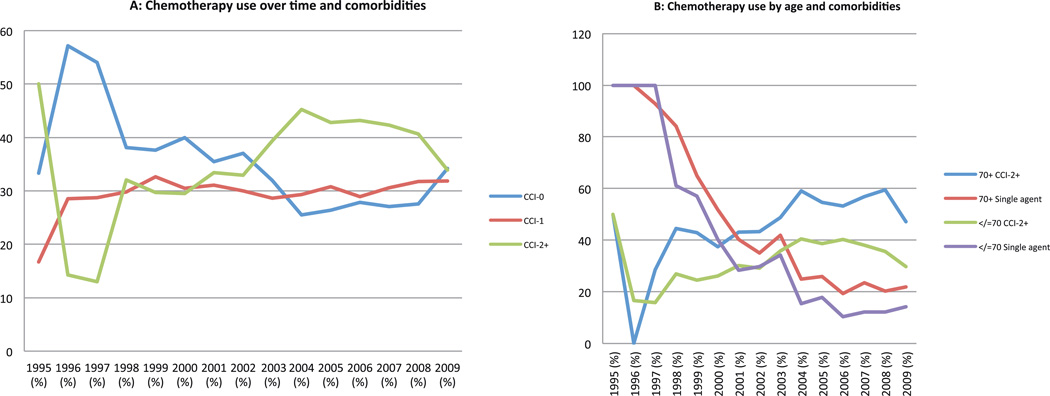
Older mCRC patients (>70 years) were more likely to have a higher CCI score than younger patients (CCI score of 2+ : 52% vs 35%, p<0.05). The proportion of patients getting chemotherapy with a higher comorbidity score increased over time from 14% in 1996 to over 40% after 2004 (p<0.05). Furthermore, chemotherapy use in older patients with CCI 2+ increased significantly over time (eg. 17%, 26%, 39% and 35% in 1996, 2000, 2005 and 2009, respectively, p<0.05) (Figure 1A,1B).
Figure 1.
Chemotherapy use and comorbidity burden (assessed by Charlson Index)
A. By time period: Use of chemotherapy in metastatic colorectal cancer patients with CCI>1 increased over time (14% in 1996 vs 40% after 2004; p<0.05)
B. By age groups: Use of chemotherapy in metastatic colorectal cancer patients with CCI>1 increased in both older and younger patients over time
Chemotherapy usage pattern in older versus younger patients
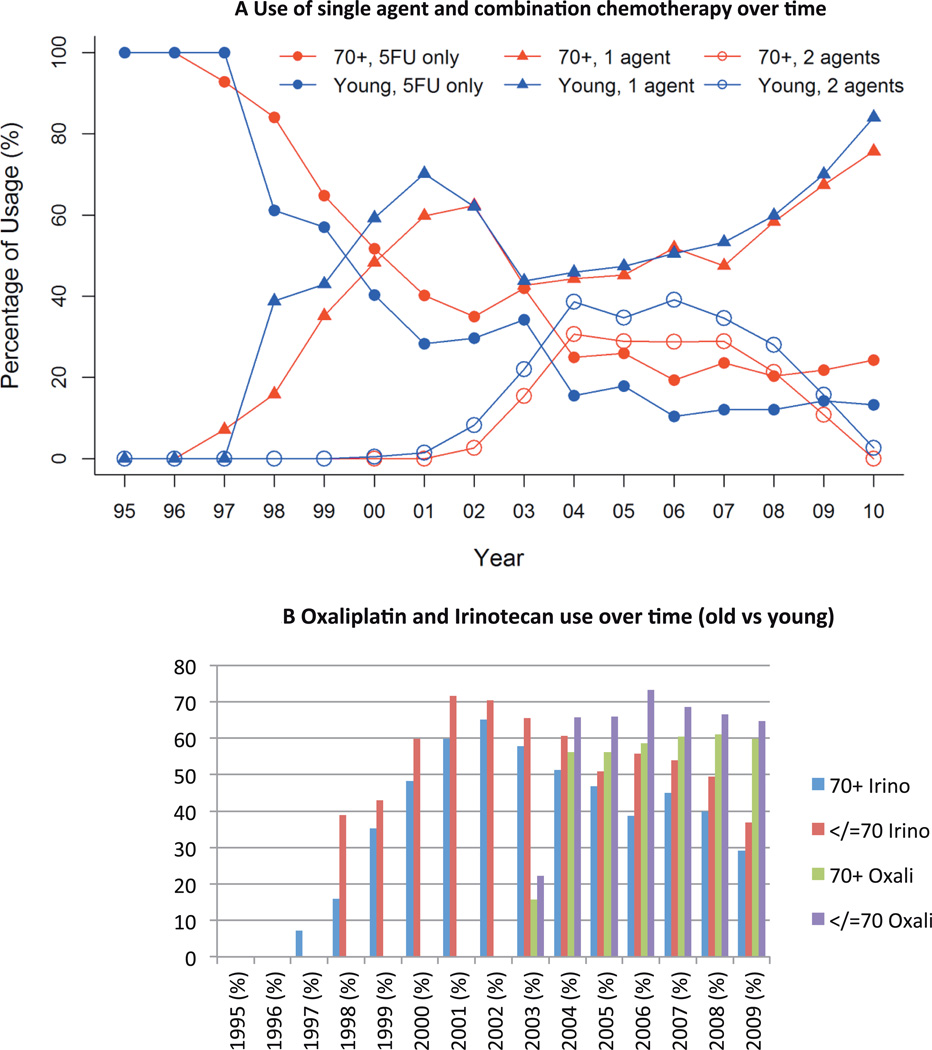
The use of single agent and combination therapy in the two groups is described in Table 1. Use of single agent 5-FU in the full cohort decreased from 100% in 1995 to 43% in 2000 and to only 14.5% in 2009. Simultaneously, the use of all three agents (5-FU, oxaliplatin, irinotecan) over the disease course in a single patient, increased over time to a high of 36% in 2006 before taking a downturn (14% in 2009). Comparing the results in older vs younger patients, the use of single agent chemotherapy declined over time in both groups (Figure 2A). However, at any given time point older patients were less likely to receive >2 agents when compared to younger patients(15 vs 22%, 29 vs 39% and 11 vs 16% in 2003, 2006 & 2009 respectively, p<0.001) (Figure 2A).
Figure 2.
Chemotherapy use by age groups
A. Use of single agent 5FU declined and combination therapy increased over time in both older and younger patients
B. Use of individual chemotherapy agents over time in older and younger patients
Adoption of irinotecan and oxaliplatin into the treatment paradigm for mCRC increased following FDA approval of the drugs (1998 for irinotecan and 2002 for oxaliplatin), (Figure 2B). Utilization of irinotecan increased to 32% in 1998 and then to 68% by 2001, with a drop to 47% in 2004 following the introduction of oxaliplatin. Oxaliplatin utilization increased from 6% in 2002 to 34% in 2004 and 63% in 2006 but did not increase further after that. The use of irinotecan was lower in older patients as compared to younger patients following the FDA approval (15% vs 38% in 1998, p<0.05), but this difference resolved by 2002 (62% both groups, p=0.9). Similarly, oxaliplatin was more frequently used in younger patients as compared to older patients after initial approval (22% vs 15% in 2003, p<0.05), with no statistical difference noted in 2009 (64% vs 60%, p=0.95) (Figure 2B).
Multivariate analysis
On multivariate analysis, the odds of receiving combination chemotherapy was higher with each passing year (OR 1.2; CI 1.18–1.26; p<0.001). The likelihood of receiving combination chemotherapy was lower in older patients (OR 0.65; CI 0.59–0.71, p<0.0010) and those with CCI 2+ (OR 0.84; CI 0.77–0.91; p<0.001).
Discussion
With the average age of the population steadily rising and the increased incidence of cancer in elderly patients, the field of geriatric oncology is rapidly evolving. Colorectal cancer (CRC) is the third most commonly diagnosed cancer in adults with a median age at diagnosis of 68 years and 35% of patients diagnosed after age 75 20, 21. The treatment of mCRC has improved dramatically over the last 2 decades with a corresponding improvement in the overall survival1. Older patients are underrepresentated in clinical trials. In this specific patient population, therapy is challenging and must be tailored to incorporate several factors: the patient’s overall functional status, life expectancy, risk of morbidity from their cancer and it’s treatment, competing co-morbidities, and the patient’s desire for treatment.
In our cohort of commercially insured patients with over 36% of the population > 70 years, we found that a lower fraction of older patients (24%) are treated for mCRC and adoption of new agents into their treatment appears to lag behind that of younger patients. However, our data suggest that oncologists have become more comfortable treating older patients with comorbidities over time. Prior studies have demonstrated rapid uptake of new therapies by oncologists6, 8 especially after presentation of clinical trial results and publication of new guidelines. Our group previously reported a reduction of anti-EGFR agent use for mCRC after presentation of data demonstarting lack of efficacy in KRAS mutant mCRC19. However, studies evaluating the effect of patient’s age and comorbidities on the uptake of newer regimens are few.
The low percentage of older patients in this database (36% patients over the age of 70) is not surprising as most older patients are insured by Medicare rather than commercial insurance. But, our cohort is not very different from the proportion of older CRC patients in the SEER database (35% ≥75 years of age)21. However, within the patients receiving chemotherapy (included in our analysis), the percentage of older patients was significantly lower than in the entire claims database (24% vs 36%, p<0.05), indirectly suggesting lower utilitization of chemotherapy in older patients. Prior work has shown that older adults with CRC are less likely to be referred to a medical oncologist, and those patients who are referred to oncology are less likely to receive chemotherapy 22. In addition, their treatment is more likely to be discontinued early 23, 24. This points out the continuing disparity that exists with the care of older versus younger mCRC patients. Additional studies are needed inorder to understand the barriers to quality care for older adults.
In addition to age, co-morbidities are a major determinant of chemotherapy tolerance, and affect treatment recommendations in clinical practice. Our work suggests that oncologists are treating more older patients with comorbidities as depicted by the increase in percentage of patients with higher co-morbidity score that received therapy over time. A systematic review had previously shown that chemotherapy use among cancer patients with comorbidities and adjuvant therapy use among CRC patients with co-morbidity is lower compared to their counterparts25. This is despite the fact that the risk of all-cause mortality in patients with co-morbidities, as assessed by CCI, was significantly lower in patients who received guideline based therapy26. With increasing awareness, widespread use of treatment guidelines, and better supportive care, oncologists are treating more patients with co-morbidities and this will help improve cancer outcomes in the long run.
We observed a temporal decline in the use of single agent 5FU and a simultaneous increase in the use of multi agent chemotherapy in both older and younger patients, in addition to a quick adoption of irinotecan and oxaliplatin into the treatment paradigm of patients with mCRC near the time of their FDA approval. When analyzing this uptake in older patients we noted older patients were less likely to get new agents initially. However, this difference resolved a few years after the approval of the drugs. This suggests a slower uptake of novel agents among older patients. Our data demonstrates that once oncologists become comfortable with a new agent, they will apply it to the management of older patients as well. This trend is supported by studies reporting similar benefit in terms of overall survival, response rate, and progression free survival from 5-FU-based combination chemotherapy (with oxaliplatin and/or irinotecan) in older and younger mCRC 12, 14, 27. The incremental benefit comes at the expense of an increased rate of toxicities. It is likely that with more experience and longer follow up with the new agents, oncologists become better at handling the side effects, and are ready to use them among older or less fit patients. These factor likely account for the delayed adoption of new agents into the treatment of older patients. However other factors such as patient/physician preference, which are not captured in the database may play a role as well.
Observational studies such as ours carry some limitations. This data set lacked clinical details (ie, disease presentation, stage, pathology, etc), which may have led to misclassification of the patients included in the analysis. We identified our cohort by using ICD9 codes for disease and metastatic site and HCPCS for therapy, with the goal to optimize subject identification. We excluded adjuvant therapy from this analysis by reviewing claims submitted only after the first date of metastatic diagnosis code documentation. However, inaccurate coding may account for inclusion of patients who do not fit these criteria. The median length of claims in our data set was 15 months, suggesting that we did not have a significant misidentification bias (consistent with median survival for mCRC during the time). As mentioned earlier, we also excluded any patients who only had one claim for a specific chemotherapy agent to account for coding errors. Our results also suggest a decline in the use of multiagent chemotherapy over time towards the end of the study period (Figure 2), which is contrary to current practice patterns. This downward deflection in the curve may be explained by the fact that many patients who started first line therapy during this time continued on it without progression at the time of data cutoff, thus falsely lowering the percentage of patients receiving all three agents.
Another limitation of our study is that the patient population included in this administrative claims database consisted of only commercially insured patients (with some enrolled in Medicare managed care plans). As such it may not be generalizable, as older patients with commerical insurance may be healthier than patients who have Medicare as their primary insurance. Also, additional unmeasureable differences in socioeconomic status may exist between our cohort and the general mCRC population. Other factors that contribute to the oncologist's therapeutic decisions, including oncologist's previous experience, perception of benefit, and interaction with other physicians or industry may have affected our results, but could not be examined in this analysis. The applicability of the data in current time may also be affected but the time period during which the study was conducted. Furthermore, we cannot comment on the appropriate use of these medications from these data.
Older patients may forgo cancer directed therapy in the setting of a terminal disease to preserve quality of life and may have different treatement preferences when compared to their younger counterparts. The lack of data on the patient’s treatment preferences/choices limits the interpretation of these data. Similarly, although we examined the affect of comorbidities on therapy in this analysis, evaluation of the patients’ performance status and other geriatric factors that strongly influence therapy would have improved our understanding of the patterns of care. Unfortunately, this data was not available in this database.
In summary, within our cohort of commercially insured patients with mCRC, the adoption of new agents into the treatment paradigm of older patients appears to lag behind that of younger patients. However, the use of multiagent chemotherapy increased over time. Oncologists have become more comfortable treating older patients and those with comorbidities over the past 2 decades. Medical oncology is changing at a very rapid pace and concerted efforts are required to promote use of novel agents in healthy older patients to provide them with optimal care. Further research to explain and overcome this healthcare disparity is warranted.
Acknowledgments
Supported by a Cancer Center Support Grant 3 P30 CA006927-47S4 and a K07CA136995 from the National Cancer Institute, an Institutional Research Grant from the American Cancer Society (IRG 92-027-15) and a Cancer Center Support Grant P30 CA43703
Footnotes
Prior presentations: Poster presentation at the 2016 GI Cancers Symposium, San Francisco, CA (Jan 21–23, 2016)
Author contributions:
Namrata Vijayvergia: Conceptualization, methodology, validation formal analysis, investigation, resources, data curation, writing (initial, review and editing), visualization, supervision, project administration and funding acquisition
Tianyu Li: Methodology, validation, formal analysis, writing (review and editing), visualization, supervision and project administration
Yu-Ning Wong: Conceptualization, methodology, validation, writing (review and editing), visualization, supervision and project administration
Michael Hall: Conceptualization, methodology, validation, writing (review and editing), visualization, supervision and project administration
Steven J Cohen: Conceptualization, writing (review and editing), visualization, supervision and project administration
Efrat Dotan: Conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing (initial, review and editing), visualization, supervision, project administration and funding acquisition
Conflict of Interest disclosure
Dr Vijayvergia, Ms Li, Dr Wong and Dr Hall have no conflicts to disclose
Dr. Cohen reports other from Bayer, other from Genentech, other from Taiho, outside the submitted work
Dr Dotan received grant funding from NCI and ACS
REFERENCES
- 1.Venook AP, Niedzwiecki D, Lenz H-J, et al. CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC); ASCO Annual Meeting Proceedings; 2014. LBA3. [Google Scholar]
- 2.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 3.de Gramont Ad, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. Journal of Clinical Oncology. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 4.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 5.Benson AB, Bekaii-Saab T, Chan E, et al. Metastatic Colon Cancer, Version 3.2013 Featured Updates to the NCCN Guidelines. Journal of the National Comprehensive Cancer Network. 2013;11:141–152. doi: 10.6004/jnccn.2013.0022. [DOI] [PubMed] [Google Scholar]
- 6.Neugut AI, Becker DJ, Insel BJ, Hershman DL. Uptake of Oxaliplatin and Bevacizumab for Treatment of Node-Positive and Metastatic Colon Cancer. Journal of Oncology Practice. 2012;8:156–163. doi: 10.1200/JOP.2011.000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hershman DL, Wilde ET, Wright JD, et al. Uptake and economic impact of first-cycle colony-stimulating factor use during adjuvant treatment of breast cancer. J Clin Oncol. 2012;30:806–812. doi: 10.1200/JCO.2011.37.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith BD, Pan IW, Shih YC, et al. Adoption of intensity-modulated radiation therapy for breast cancer in the United States. J Natl Cancer Inst. 2011;103:798–809. doi: 10.1093/jnci/djr100. [DOI] [PubMed] [Google Scholar]
- 9.Shirvani SM, Pan IW, Buchholz TA, et al. Impact of evidence-based clinical guidelines on the adoption of postmastectomy radiation in older women. Cancer. 2011;117:4595–4605. doi: 10.1002/cncr.26081. [DOI] [PubMed] [Google Scholar]
- 10.Maione P, Perrone F, Gallo C, et al. Pretreatment Quality of Life and Functional Status Assessment Significantly Predict Survival of Elderly Patients With Advanced Non—Small-Cell Lung Cancer Receiving Chemotherapy: A Prognostic Analysis of the Multicenter Italian Lung Cancer in the Elderly Study. Journal of Clinical Oncology. 2005;23:6865–6872. doi: 10.1200/JCO.2005.02.527. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29:1517–1524. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folprecht G, Cunningham D, Ross P, et al. Efficacy of 5-fluorouracil-based chemotherapy in elderly patients with metastatic colorectal cancer: a pooled analysis of clinical trials. Annals of Oncology. 2004;15:1330–1338. doi: 10.1093/annonc/mdh344. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. Journal of Clinical Oncology. 2006;24:4085–4091. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 14.Folprecht G, Seymour MT, Saltz L, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. Journal of Clinical Oncology. 2008;26:1443–1451. doi: 10.1200/JCO.2007.14.0509. [DOI] [PubMed] [Google Scholar]
- 15.Lang K, Sussman M, Friedman M, et al. Incidence and costs of treatment-related complications among patients with advanced squamous cell carcinoma of the head and neck. Archives of Otolaryngology–Head & Neck Surgery. 2009;135:582–588. doi: 10.1001/archoto.2009.46. [DOI] [PubMed] [Google Scholar]
- 16.Crawford E, Black L, Eaddy M, Kruep E. A retrospective analysis illustrating the substantial clinical and economic burden of prostate cancer. Prostate cancer and prostatic diseases. 2010;13:162–167. doi: 10.1038/pcan.2009.63. [DOI] [PubMed] [Google Scholar]
- 17.Hatoum HT, Lin S-J, Smith MR, Guo A, Lipton A. Treatment persistence with monthly zoledronic acid is associated with lower risk and frequency of skeletal complications in patients with breast cancer and bone metastasis. Clinical breast cancer. 2011;11:177–183. doi: 10.1016/j.clbc.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Dotan E, Li T, Hall MJ, Meropol NJ, Beck JR, Wong Y-N. Oncologists' response to new data regarding the use of epidermal growth factor receptor inhibitors in colorectal cancer. Journal of Oncology Practice. 2014;10:308–314. doi: 10.1200/JOP.2014.001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 21. [accessed February 8, 2016];SEER Stat Fact Sheets: Colon and Rectum Cancer. Available from URL: http://seer.cancer.gov/statfacts/html/colorect.html.
- 22.Luo R, Giordano SH, Freeman JL, Zhang D, Goodwin JS. Referral to medical oncology: a crucial step in the treatment of older patients with stage III colon cancer. Oncologist. 2006;11:1025–1033. doi: 10.1634/theoncologist.11-9-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abrams TA, Brightly R, Mao J, et al. Patterns of adjuvant chemotherapy use in a population-based cohort of patients with resected stage II or III colon cancer. J Clin Oncol. 2011;29:3255–3262. doi: 10.1200/JCO.2011.35.0058. [DOI] [PubMed] [Google Scholar]
- 24.Ho C, Ng K, O'Reilly S, Gill S. Outcomes in elderly patients with advanced colorectal cancer treated with capecitabine: a population-based analysis. Clin Colorectal Cancer. 2005;5:279–282. doi: 10.3816/ccc.2005.n.040. [DOI] [PubMed] [Google Scholar]
- 25.Lee L, Cheung WY, Atkinson E, Krzyzanowska MK. Impact of Comorbidity on Chemotherapy Use and Outcomes in Solid Tumors: A Systematic Review. Journal of Clinical Oncology. 2010 doi: 10.1200/JCO.2010.31.3049. [DOI] [PubMed] [Google Scholar]
- 26.Cronin DP, Harlan LC, Potosky AL, Clegg LX, Stevens JL, Mooney MM. Patterns of Care for Adjuvant Therapy in a Random Population-Based Sample of Patients Diagnosed with Colorectal Cancer. Am J Gastroenterol. 2006;101:2308–2318. doi: 10.1111/j.1572-0241.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- 27.Sastre J, Marcuello E, Masutti B, et al. Irinotecan in Combination With Fluorouracil in a 48-Hour Continuous Infusion As First-Line Chemotherapy for Elderly Patients With Metastatic Colorectal Cancer: A Spanish Cooperative Group for the Treatment of Digestive Tumors Study. Journal of Clinical Oncology. 2005;23:3545–3551. doi: 10.1200/JCO.2005.03.004. [DOI] [PubMed] [Google Scholar]