Abstract
Background
There are several effective treatments for prostate cancer. To what extent a patient's functional status influences the treatment decision is unknown. We sought to examine the association of functional status with treatment among older men with prostate cancer.
Methods
Using Surveillance, Epidemiology, and End Results-Medicare Health Outcomes Survey (SEER-MHOS) data, we identified men 65 years or older diagnosed with prostate cancer between 1998 and 2009. Our primary outcome was treatment choice: conservative management, surgery, or radiation within one year of diagnosis. Our exposure was functional status assessed as 4 measures within three domains: 1) physical function (activities of daily living [ADL] and physical component summary [PCS] score), 2) cognitive function (who completed the survey [self vs. proxy]), and 3) emotional well-being (mental component summary [MCS] score). We fit a multivariable multinomial logistic regression adjusting for several patient, tumor, and regional characteristics.
Results
We identified 508 conservative management, 195 surgery, and 603 radiation patients. Compared to men with no ADL dependency, those with any ADL dependency had lower odds of receiving surgery (odds ratio [OR] 0.61; 95% confidence interval [CI] 0.38-0.99) and radiation (OR 0.58; 95% CI 0.43-0.78) relative to conservative management. ADL dependency did not differ when comparing surgery and radiation. Patients with proxy survey response were less likely to receive surgery or radiation compared with conservative management.
Conclusions
Functional status is associated with treatment choice for men with prostate cancer. Future research should examine whether this is due to physicians’ recommendations, patient preferences, or a combination.
Keywords: activities of daily living, prostate cancer, radiation, conservative management, functional status, surgery
INTRODUCTION
Several different treatment choices exist for the management of localized prostate cancer, including conservative management, surgery, and radiation. By and large, patients can experience excellent cancer control, regardless of the approach they choose.1, 2 For this reason, many factors other than cancer control weigh into the decision making process, such as a patient's overall health,3 physician preference,4 and the potential side effects associated with each approach.5
What is less clear is the degree to which functional status influences treatment choice. Traditionally, clinicians have used coarse measurements of functional status, including the Charlson Comorbidity Index6 and the Eastern Cooperative Oncology Group (ECOG) performance measure,7 to help guide decisions. While these measurements are valuable risk-assessment tools, they lack the ability to quantify subclinical physiologic deficits and cognitive disability.8 More focused measures of functional status, such as activities of daily living (ADLs), the physical component summary (PCS) score, proxy survey response, and the mental component summary (MCS) score, can predict adverse outcomes across a wide spectrum of diseases.9-12 While clinicians who manage prostate cancer generally do not assess these measures of functional status, they may indirectly account for them in their overall “gestalt” about a patient's condition,13 which ultimately influences their treatment recommendation.
For these reasons, we sought to examine the relationship between functional status and treatment choice among prostate cancer patients. Specifically, we wished to assess if a patient's physical function, cognitive function, and emotional well-being as measured by ADLs, PCS scores, proxy survey response, and MCS scores were associated with the receipt of conservative management, surgery, or radiation among older men with prostate cancer.
METHODS
Data Source and Study Population
We used Surveillance, Epidemiology, and End Results-Medicare Health Outcomes Survey (SEER-MHOS) data to identify men aged 65 years or older diagnosed with prostate cancer between 1998 and 2009. SEER-MHOS links two large population-based databases to provide detailed information about older adults with cancer.14 Specifically, SEER is a nationally representative cancer registry that comprises approximately 26% of the United States’ population15 and the MHOS is a survey that provides information about the functional status of Medicare Advantage beneficiaries.14 The MHOS survey focuses on the Medicare Advantage population, which is excluded from Medicare claims-based analyses restricted to fee-for-service beneficiaries. The MHOS includes patient-reported functional status for a subsample of beneficiaries. For participating Medicare Advantage plans, surveys are distributed every two years to a random sample of 1200 members for larger plans (i.e., > 1200 members) and distributed to all members in smaller plans (i.e., between 500-1200 members).16 The response rate is about 64% for baseline surveys and 80% for follow-up surveys.17
We identified men with localized prostate cancer as their first and only cancer who completed a survey within 1 year prior to diagnosis. We then categorized patients based on their treatment within 1 year after diagnosis.
Outcome: treatment choice
Our primary outcome was the use of conservative management, surgery, or radiation among men with prostate cancer. Patients who received both surgery and radiation were assigned based on their initial treatment.
Exposure: functional status
We focused on four measurements of functional status within three domains: 1) physical function (activities of daily living [ADL] and the physical component summary [PCS] score), 2) cognitive function (who completed the survey [self vs. proxy]), and 3) emotional well-being (the mental component summary [MCS] score). In our sample, the median number of ADL dependencies was 0 and the distribution was such that only 23%, 5%, and 12% of patients had two or more ADL dependencies for conservative management, surgery, and radiation, respectively. Accordingly, we categorized ADLs as no ADL dependency versus any ADL dependency. We examined both the physical and mental component summary scores, which were calculated from the Short Form 36 (SF-36) and the Veterans RAND 12-item health survey (VR-12) questionnaires. The SEER-MHOS database contains responses from the SF-36 from 1998 through 2005 and from the VR-12 from 2006 through the end of the study period.14 The physical and mental component scores are either calculated directly from the VR-12 or are rescored as the VR-12 equivalent of the SF-36 scores. The scores are normalized to the general population with a mean of 50 and a standard deviation of 10.18 Higher scores indicate better health.
Covariates
We assessed several patient, tumor, and regional covariates. We categorized race as white and non-white due to limited numbers of non-whites (i.e., African American, Asian, Hispanic, North American Native, and other). We examined comorbidity in several ways. First, we examined each comorbidity individually. We then examined the total number of comorbidities for each patient. Next, we aggregated similar comorbidities (e.g., angina pectoris/coronary artery disease, congestive heart failure, myocardial infarction/heart attack, and other heart conditions as “heart disease”), and lastly, we examined only those comorbidities that were included in the well-established Elixhauser method.19 Ultimately, the comorbidity findings were similar across these variations, so we used the total number of comorbidities per patient as our covariate. As described previously,20 we defined disease risk as lower risk if patients had well-differentiated tumors regardless of their age or if they had moderately differentiated tumors and were aged 70 or older at the time of diagnosis. All other patients were categorized as higher risk.
Statistical Analysis
We first compared patient, tumor, and regional characteristics among treatment types using chi-square tests. We then examined patient functional status according to treatment using chi-square tests for categorical variables and Kruskal-Wallis tests for continuous variables. Next, we fit a multivariable multinomial logistic regression model. Covariates in our model included age, race/ethnicity, marital status, comorbidity, education, household income, who completed the survey, geographic region, tumor grade, disease risk, ADLs, and MCS score. We used a multiple imputation method to account for missing education and income data. We used five imputations and then averaged the final model results over these imputations. Variances of the estimators were estimated using Rubin's formula for multiple imputations. Lastly, we estimated the adjusted probability of patients undergoing conservative management, surgery, or radiation according to whether or not they had any ADL dependency.
We performed several analyses to check the robustness of our findings. First, we examined the time from survey response to diagnosis and found that the association between ADLs and subsequent treatment was unaffected by differences in time of survey response. Next, we examined whether there was an interaction between age and ADLs, between diagnosis year and ADLs, and between race and geographic region. For ADLs, we checked for an interaction with age and diagnosis year due to the possible influence of active surveillance. Active surveillance involves younger patients and is increasing over time. Since we cannot differentiate active surveillance from watchful waiting in the dataset, we wanted to minimize the chance that this would alter our findings. The rationale for checking an interaction between race and geographic region is that the distribution of race varies by region. The interaction terms were not significant in any of these instances. We then built our model stepwise to examine the additive effects of each covariate, including who completed the survey, MCS and PCS scores, and diagnosis year. We excluded the PCS score from our final model due to the strong association between low PCS scores and having any ADL dependency. We performed several steps to check the performance of our model, including using a chi-square goodness-of-fit test based on a 10-fold cross-validation of the data and using loess estimates to confirm the linearity of the continuous variables. We then checked for multi-collinearity among the covariates by examining the variance inflation factor, which is the factor by which the variance of a regression coefficient of a covariate gets inflated in the presence of collinearity with other covariates.21 We found that all the variance inflation factors were low (i.e., < 5), suggesting that there were no concerns for multi-collinearity among the covariates in our model. Lastly, we reran our model after omitting the covariates household income and who completed the survey, which had the highest proportion of missing data. The findings were similar, so we report the subset of the sample with complete data on all the covariates.
We performed all analyses using SAS v9.4 (Cary, NC). All tests were two-sided, and the probability of a type I error was set at 0.05. The Institutional Review Board of our institution reviewed and exempted this study from full board review. The authors have no conflicts of interest.
RESULTS
We excluded patients with missing tumor characteristics (n=420) and missing functional status information (n=163). Using these criteria, our study population consisted of 1306 patients, of whom 508 (39%) underwent conservative management, 195 (15%) underwent surgery, and 603 (46%) underwent radiation (Table 1). The three treatments differed across many patient (age, marital status, comorbidity, education, household income), tumor (disease risk), and regional (geographic region) characteristics (all p<0.05). Conversely, no differences were seen in race/ethnicity or tumor grade (both p > 0.05).
Table 1.
Characteristics of the study population
| Characteristics | Treatment | P-value* | |||
|---|---|---|---|---|---|
| All (n=1306) | Conservative management (n=508) | Surgery (n=195) | Radiation (n=603) | ||
| Age, years (%) | <0.001 | ||||
| 65-69 | 362 (28 ) | 78 (15 ) | 113 (58 ) | 171 (28 ) | |
| 70-74 | 447 (34 ) | 135 (27 ) | 68 (35 ) | 244 (40 ) | |
| 75+ | 497 (38 ) | 295 (58 ) | 14 (7 ) | 188 (31 ) | |
| Race/ethnicity (%) | 0.99 | ||||
| White | 916 (70 ) | 356 (70 ) | 136 (70 ) | 424 (70 ) | |
| Non-white | 390 (30 ) | 152 (30 ) | 59 (30 ) | 179 (30 ) | |
| Marital Status (%) | <0.001 | ||||
| Married | 871 (67 ) | 273 (54 ) | 153 (78 ) | 445 (74 ) | |
| Not Married | 435 (33 ) | 235 (46 ) | 42 (22 ) | 158 (26 ) | |
| Comorbidity (%) | 0.001 | ||||
| 0 | 199 (15 ) | 58 (11 ) | 34 (17 ) | 107 (18 ) | |
| 1 | 323 (25 ) | 134 (26 ) | 58 (30 ) | 131 (22 ) | |
| 2 | 295 (23 ) | 102 (20 ) | 46 (24 ) | 147 (24 ) | |
| 3 or more | 489 (37 ) | 214 (42 ) | 57 (29 ) | 218 (36 ) | |
| Education (%)** | <0.001 | ||||
| Less than a high school education | 321 (25 ) | 153 (31 ) | 34 (18 ) | 134 (23 ) | |
| At least a high school education | 966 (75 ) | 348 (69 ) | 158 (82 ) | 460 (77 ) | |
| Household income, $, (%)** | <0.001 | ||||
| Less than 20,000 | 333 (30 ) | 149 (34 ) | 42 (27 ) | 142 (28 ) | |
| 20,000-39,000 | 387 (35 ) | 169 (39 ) | 44 (28 ) | 174 (35 ) | |
| 40,000 or more | 376 (34 ) | 118 (27 ) | 70 (45 ) | 188 (37 ) | |
| Geographic region (%) | <0.001 | ||||
| Northeast | 250 (19 ) | 79 (16 ) | 28 (14 ) | 143 (24 ) | |
| South | 199 (15 ) | 72 (14 ) | 26 (13 ) | 101 (17 ) | |
| Central | 240 (18 ) | 108 (21 ) | 44 (23 ) | 88 (15 ) | |
| West | 617 (47 ) | 249 (49 ) | 97 (50 ) | 271 (45 ) | |
| Tumor grade (%) | 0.14 | ||||
| Well/moderately differentiated | 809 (62 ) | 312 (61 ) | 110 (56 ) | 387 (64 ) | |
| Poorly/undifferentiated | 497 (38 ) | 196 (39 ) | 85 (44 ) | 216 (36 ) | |
| Disease risk (%) | <0.001 | ||||
| Lower | 633 (48 ) | 271 (53 ) | 62 (32 ) | 300 (50 ) | |
| Higher | 673 (52 ) | 237 (47 ) | 133 (68 ) | 303 (50 ) | |
| Year of diagnosis (%)*** | 0.02 | ||||
| 1998 | 42 (3 ) | 15 (3 ) | -- | 21 (3 ) | |
| 1999 | 161 (12 ) | 67 (13 ) | 28 (14 ) | 66 (11 ) | |
| 2000 | 138 (11 ) | 49 (10 ) | 18 (9 ) | 71 (12 ) | |
| 2001 | 147 (11 ) | 39 (8 ) | 31 (16 ) | 77 (13 ) | |
| 2002 | 119 (9 ) | 46 (9 ) | 14 (7 ) | 59 (10 ) | |
| 2003 | 90 (7 ) | 33 (7 ) | -- | 50 (8 ) | |
| 2004 | 79 (6 ) | 33 (7 ) | -- | 39 (6 ) | |
| 2005 | 70 (5 ) | 27 (5 ) | -- | 35 (6 ) | |
| 2006 | 58 (4 ) | 25 (5 ) | -- | 28 (5 ) | |
| 2007 | 101 (8 ) | 48 (9 ) | 20 (10 ) | 33 (5 ) | |
| 2008 | 124 (9 ) | 53 (10 ) | 18 (9 ) | 53 (9 ) | |
| 2009 | 177 (14 ) | 73 (14 ) | 33 (17 ) | 71 (12 ) | |
P-values generated from chi-square tests
Values imputed for 19 patients missing education information and 210 patients missing household income information
Exact numbers not shown in order to be compliant with SEER-Medicare guidelines, which prohibits displaying cell counts <11
Percentages might not sum to 100 because of rounding
Table 2 demonstrates the unadjusted association between treatment type and the four measurements of functional status. Patients undergoing conservative management were more likely to have ADL dependency, a lower PCS score, a proxy survey response, and a lower MCS score (all p<0.05) than patients undergoing either surgery or radiation.
Table 2.
Patient functional status according to treatment
| Characteristics | Treatment | P-value* | |||
|---|---|---|---|---|---|
| All (n=1306) | Conservative management (n=508) | Surgery (n=195) | Radiation (n=603) | ||
| Activities of daily living (%) | <0.001 | ||||
| No dependency | 934 (72 ) | 312 (61 ) | 157 (81 ) | 465 (77 ) | |
| Any dependency | 372 (28 ) | 196 (39 ) | 38 (19 ) | 138 (23 ) | |
| Physical component summary score, median (IQR) | 45 (36, 52) | 41 (32 , 51 ) | 48 (40 , 53 ) | 47 (38 , 53 ) | <0.001 |
| Who completed the survey (%) | <0.001 | ||||
| Self | 1153 (88 ) | 417 (82 ) | 190 (97 ) | 546 (91 ) | |
| Proxy | 153 (12 ) | 91 (18 ) | 5 (3 ) | 57 (9 ) | |
| Mental component summary score, median (IQR) | 56 (49, 61) | 55 (45 , 60 ) | 57 (51 , 61 ) | 57 (50 , 61 ) | 0.003 |
Abbreviations: IQR, interquartile range
P-values for continuous and categorical variables are from Kruskal-wallis test and chi-square test, respectively.
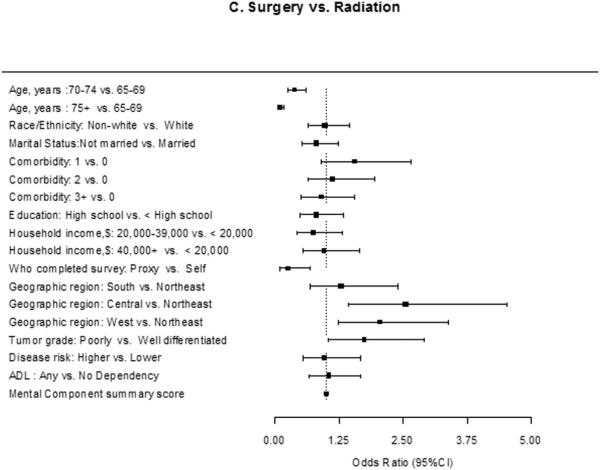
Figure 1 shows the results of our multivariable multinomial logistic regression model. Older patients (odds ratio [OR] 0.32; 95% confidence interval (CI), 0.19-0.55 [ages 70-74 vs. 65-69]; OR 0.03; 95% CI, 0.02-0.07 [ages 75+ vs. 65-69]), patients who were unmarried (OR 0.33; 95% CI, 0.21-0.51), patients who had their survey completed by a proxy (OR 0.13; 95% CI, 0.05-0.35), and patients with any ADL dependency (OR 0.61; 95% CI, 0.38-0.99) were less likely to be treated with surgery compared to conservative management.
Figure 1.
Estimated effect (adjusted odds ratio and 95% CI) of each covariate on the use of surgery vs. conservative management (A), radiation vs. conservative management (B), and surgery vs. radiation (C): Results of a multivariable multinomial logistic regression analysis.
Older patients, patients who were not married, patients who had their survey completed by a proxy, and patients with any ADL dependencies were less likely to be treated with surgery (A) and radiation (B) compared to conservative management. In addition, patients living in the Central and West regions (compared to the Northeast) were less likely to be treated with radiation. Lastly, patients who were older or who had their surveys completed by proxies were less likely to be treated with surgery compared to radiation (C). Lastly, patients who were older or who had their surveys completed by proxies were less likely to be treated with surgery compared to radiation. Patients living in Central and West regions (compared to the Northeast) and patients with higher tumor grade were more likely to get surgery. There was no difference in the likelihood of surgery versus radiation based on ADL dependency.
Abbreviations: ADL; activities of daily living
* The effect of each covariate was adjusted for all other covariates in the model.
Similarly, older patients, patients who were unmarried, patients who had their survey completed by a proxy, and patients who had any ADL dependency were less likely to be treated with radiation compared to conservative management. In addition, patients living in central (OR 0.43; 95% CI, 0.28-0.66) or west (OR 0.54; 95% CI, 0.38-0.78) regions were less likely to be treated with radiation. Lastly, patients who were older or who had their surveys completed by proxies were less likely to be treated with surgery compared to radiation. Patients living in Central and West regions (compared to the Northeast) and patients with higher tumor grade were more likely to get surgery. There was no difference in the likelihood of surgery versus radiation based on ADL dependency (OR 1.05; 95% CI, 0.66-1.68).
Figure 2 shows the predicted probability of patients treated with conservative management, surgery, and radiation stratified by ADL dependency. Compared with patients with no ADL dependency, those with any ADL dependency were more likely to receive conservative management (53% vs. 33%), less likely to receive surgery (10% vs. 17%), and less likely to receive radiation (37% vs. 50%) (all p<0.05; chi-square tests).
Figure 2.
Estimated probability* of patients with and without ADL dependency treated with conservative management, surgery, and radiation
Compared with patients with no ADL dependency, those with any ADL dependency were more likely to receive conservative management (53% vs. 33%), less likely to receive surgery (10% vs. 17%), and less likely to receive radiation (37% vs. 50%).
Abbreviations: ADL, activities of daily living
* Adjusted for age, race, marital status, comorbidity, education, household income, who completed survey, geographic region, tumor grade, disease risk, and mental component summary score.
DISCUSSION
Functional status is associated with treatment choice for men with prostate cancer. After adjusting for a variety of patient, tumor, and regional characteristics, men undergoing treatment with radiation or surgery tended to have higher functional status than men undergoing conservative management. Supporting this view, men undergoing surgery and radiation had lower likelihoods of having an ADL deficiency than those undergoing conservative management. However, there was no association between ADLs and receipt of surgery versus radiation.
The association of ADLs with radiation and surgery may be occurring for a few reasons. Radiation oncologists and urologists may be incorporating ADL measurements (i.e., bathing, dressing, toileting, getting in and out of bed or chairs, walking, and eating) into their evaluation of whether or not a patient is a candidate for aggressive treatment. However, this is likely not the case. Although the International Society of Geriatric Oncology Prostate Cancer Working Group recommends functional status as part of the assessment of older men with prostate cancer,22, 23 specialists who treat prostate cancer receive very little geriatric training and are unlikely to perform functional status evaluations.24 A more likely scenario is that a clinician's overall “gestalt” of a patient correlates to some degree with the patient's functional status. Yet relying on cognitive processes is subject to bias and can be associated with errors in decision making.25 While cognitive processes may correctly identify patients at the extremes of functional status (e.g., none or several ADL dependencies), they may be less accurate in detecting patients with more subtle declines in functional status. A third reason why patients with ADL dependencies are less likely to receive aggressive treatment may pertain to the patients themselves. Patients with ADL dependencies may self-select conservative management over aggressive treatment due to their awareness of their physical condition and their desire for treatment associated with less morbidity.
While patients with ADL dependencies were less likely to receive aggressive treatment, there was no difference in receipt of surgery or radiation. This may be attributed to the overall health of both the surgery and radiation patients in this study. The majority of patients treated aggressively had no ADL dependencies and almost half of them had minimal comorbidities (≤1).
Patients who had their survey completed by a proxy, reflecting incapacity due to physical or cognitive limitations, were less likely to undergo any aggressive treatment (i.e., surgery or radiation). Patients with surveys completed by proxies are generally older and sicker.11, 26 In addition, they have worse physical function and emotional well-being.26, 27 Taken together, patients responding to surveys by proxy likely represent a group with more severe functional status deficits, and thus, it makes sense that this patient population is less likely to receive either surgery or radiation over conservative management. Taken one step further, patients with surveys completed by proxies were less likely to receive surgery compared to radiation, highlighting that this “measurement” may correlate with being a poor surgical candidate.
These findings should give clinicians pause when evaluating patients for different prostate cancer treatments. It is well known that overtreatment of patients with low-risk prostate cancer occurs.28 In response to this, in part, the rate of active surveillance has been increasing.29 Currently, active surveillance protocols do not incorporate any specific measurements of functional status into their algorithms. Yet it may be appropriate for specific measurements of functional status to be considered in the decision-making process. As an example, a clinical pathway could be implemented that recommends a geriatric evaluation for lower risk patients with any ADL dependencies who are considering aggressive treatment.
Our findings should be interpreted in the context of several limitations. First, enrollees in Medicare Advantage plans may represent healthier individuals,30 and thus, our findings may not be generalizable to the non-Medicare Advantage population. This may explain, for example, why the average MCS scores in this study are higher than the national average of 50. However, the superior health of Medicare Advantage beneficiaries may be overestimated.31 Either way, the Medicare Advantage population has been steadily growing (as high as 25% of the Medicare population during the study period),32 and few opportunities exist to examine the health of this population. Second, for patients undergoing conservative management, we were unable to distinguish those undergoing active surveillance from those undergoing watchful waiting, which represent two different populations. Since patients undergoing active surveillance are generally younger and since rates of active surveillance are increasing,29 we stratified our analyses by age and year. In both cases, the findings for the stratified groups were similar, so we only present the overall results. Further, some patients undergoing conservative management may receive primary androgen deprivation therapy, but androgen deprivation therapy cannot be identified in SEER. Although the use of this modality for localized disease is decreasing,33 there are still men who receive this treatment. Third, aggregating patients with varying degrees of ADL deficiencies into an “any ADL dependency” group creates a heterogeneous population. It is possible that an association between ADLs and treatment choice is masked for patients with more severe ADL dependency since they are grouped with patients who have less severe ADL dependency. However, patients with localized prostate cancer generally comprise a high-functioning population, and the sparse numbers of patients with significant deficiencies in ADLs prevented us from looking at ADLs with more granularity. Fourth, there are only 195 surgery patients in our cohort of whom 38 had any ADL dependencies and 5 had surveys completed by proxy. Nonetheless, we have used two large population-based databases, imputed missing values for education and income, and identified prostate cancer patients across 12 years to help account for this.
Despite these limitations, this study illustrates some important findings. First, for patients undergoing radiation and surgery, functional status is associated with treatment choice, even after adjusting for tumor grade and disease risk. Future research should examine whether this is due to physicians’ recommendations, patient preferences, or a combination. Second, for patients in whom functional status may be a concern, treating physicians can utilize geriatricians to improve the risk assessment of patients prior to treatment and to even reverse functional impairments in some instances.34 Lastly, the use of conservative management over radiation for patients with functional status deficits may lessen as new radiotherapies for prostate cancer, such as stereotactic body radiotherapy, are adopted that reduce the burden of treatment by decreasing the number of treatment sessions.
Acknowledgments
Funding:
Bruce Jacobs is supported in part by the National Institutes of Health Institutional KL2 award (KL2TR000146-08), the GEMSSTAR award (R03AG048091), the Jahnigen career development award, and the Tippins Foundation Scholar Award.
Amber Barnato is supported in part by the University of Pittsburgh Clinical and Translational Science Institute – CORE C: Research Education, Training, and Career Development (UL1-RR024153)
Footnotes
Financial disclosures (these are financial disclosures, but not conflicts of interest: there are no conflicts of interest for this manuscript):
Bruce Jacobs is a consultant for ViaOncology
Amber Barnato is a former board member of the Society of Medical Decision Making
Author contributions:
Bruce Jacobs: conception and design, data acquisition, data analysis and interpretation, drafting of manuscript, critical revision of the manuscript, and statistical analyses
Samia Lopa: conception and design, data acquisition, data analysis and interpretation, critical revision of the manuscript, and statistical analyses
Jonathan Yabes: conception and design, data acquisition, data analysis and interpretation, critical revision of the manuscript, and statistical analyses
Joel Nelson: conception and design, data analysis and interpretation, critical revision of the manuscript, supervision
Amber Barnato: conception and design, data analysis and interpretation, drafting of manuscript, critical revision of the manuscript, supervision
Howard Degenholtz: conception and design, data analysis and interpretation, drafting of manuscript, critical revision of the manuscript, supervision
REFERENCES
- 1.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293:2095–2101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 2.D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 3.Mohler J, Bahnson RR, Boston B, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8:162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 4.Showalter TN, Mishra MV, Bridges JF. Factors that influence patient preferences for prostate cancer management options: A systematic review. Patient Prefer Adherence. 2015;9:899–911. doi: 10.2147/PPA.S83333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu JC, Wang Q, Pashos CL, Lipsitz SR, Keating NL. Utilization and outcomes of minimally invasive radical prostatectomy. J Clin Oncol. 2008;26:2278–2284. doi: 10.1200/JCO.2007.13.4528. [DOI] [PubMed] [Google Scholar]
- 6.Jespersen CG, Norgaard M, Jacobsen JB, Borre M. Patient comorbidity is associated with conservative treatment of localized prostate cancer. Scand J Urol. 2015:1–5. doi: 10.3109/21681805.2015.1026936. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Agarwal N, Beard C, et al. Kidney cancer. J Natl Compr Canc Netw. 2011;9:960–977. doi: 10.6004/jnccn.2011.0082. [DOI] [PubMed] [Google Scholar]
- 8.McKibben MJ, Smith AB. Evaluation and Management of the Geriatric Urologic Oncology Patient. Curr Geriatr Rep. 2015;4:7–15. doi: 10.1007/s13670-014-0106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derby R, Lettice JJ, Kula TA, Lee SH, Seo KS, Kim BJ. Single-level lumbar fusion in chronic discogenic low-back pain: psychological and emotional status as a predictor of outcome measured using the 36-item Short Form. J Neurosurg Spine. 2005;3:255–261. doi: 10.3171/spi.2005.3.4.0255. [DOI] [PubMed] [Google Scholar]
- 10.Maekawa Y, Sugimoto K, Yamasaki M, et al. Comprehensive Geriatric Assessment is a useful predictive tool for postoperative delirium after gastrointestinal surgery in old-old adults. Geriatr Gerontol Int. 2015 doi: 10.1111/ggi.12587. [DOI] [PubMed] [Google Scholar]
- 11.Bjertnaes O. Patient-reported experiences with hospitals: comparison of proxy and patient scores using propensity-score matching. Int J Qual Health Care. 2014;26:34–40. doi: 10.1093/intqhc/mzt088. [DOI] [PubMed] [Google Scholar]
- 12.Ul-Haq Z, Mackay DF, Pell JP. Association between physical and mental health-related quality of life and adverse outcomes; a retrospective cohort study of 5,272 Scottish adults. BMC Public Health. 2014;14:1197. doi: 10.1186/1471-2458-14-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trowbridge RL, Rutkowski NK, Shojania KG. Does this patient have acute cholecystitis? JAMA. 2003;289:80–86. doi: 10.1001/jama.289.1.80. [DOI] [PubMed] [Google Scholar]
- 14. [June 25, 2015];Brief Description of the SEER-MHOS Database. Available from URL: http://healthcaredelivery.cancer.gov/seer-mhos/overview/
- 15.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK, editors. SEER Cancer Statistics Review, 1975-2007. National Cancer Institute; 2010. [Google Scholar]
- 16.HEDIS [June 25, 2015];Specifications for the Medicare Health Outcomes Survey. 2015 Available from URL: http://hosonline.org/surveys/hos/download/HOS_HEDIS_Volume6_2015.pdf.
- 17. [June 25, 2015];Response rates. Available from URL: http://appliedresearch.cancer.gov/surveys/seermhos/aboutdata/table.response.rates.html.
- 18. [June 26, 2015];SF-36 scales measures. Available from URL: http://healthcaredelivery.cancer.gov/seer mhos/aboutdata/table.scale.measures.html#howto.
- 19.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Miller DC, Gruber SB, Hollenbeck BK, Montie JE, Wei JT. Incidence of initial local therapy among men with lower-risk prostate cancer in the United States. J Natl Cancer Inst. 2006;98:1134–1141. doi: 10.1093/jnci/djj308. [DOI] [PubMed] [Google Scholar]
- 21.O'Brien RM. A Caution Regarding Rules of Thumb for Variance Inflation Factors. Quality and Quantity. 2007;41:673–690. [Google Scholar]
- 22.Droz JP, Balducci L, Bolla M, et al. Management of prostate cancer in older men: recommendations of a working group of the International Society of Geriatric Oncology. BJU Int. 2010;106:462–469. doi: 10.1111/j.1464-410X.2010.09334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Droz JP, Balducci L, Bolla M, et al. Background for the proposal of SIOG guidelines for the management of prostate cancer in senior adults. Crit Rev Oncol Hematol. 2010;73:68–91. doi: 10.1016/j.critrevonc.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Ploussard G, Albrand G, Rozet F, Lang H, Paillaud E, Mongiat-Artus P. Challenging treatment decision-making in older urologic cancer patients. World J Urol. 2013 doi: 10.1007/s00345-013-1158-4. [DOI] [PubMed] [Google Scholar]
- 25.Klein JG. Five pitfalls in decisions about diagnosis and prescribing. Bmj. 2005;330:781–783. doi: 10.1136/bmj.330.7494.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellis BH, Bannister WM, Cox JK, et al. Utilization of the propensity score method: an exploratory comparison of proxy-completed to self-completed responses in the Medicare Health Outcomes Survey. Health Qual Life Outcomes. 2003;1:47. doi: 10.1186/1477-7525-1-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yip JY, Wilber KH, Myrtle RC, Grazman DN. Comparison of older adult subject and proxy responses on the SF-36 health-related quality of life instrument. Aging Ment Health. 2001;5:136–142. doi: 10.1080/13607860120038357. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs BL, Zhang Y, Schroeck FR, et al. Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA. 2013;309:2587–2595. doi: 10.1001/jama.2013.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filson CP, Schroeck FR, Ye Z, Wei JT, Hollenbeck BK, Miller DC. Variation in Use of Active Surveillance among Men Undergoing Expectant Treatment for Early Stage Prostate Cancer. J Urol. 2014 doi: 10.1016/j.juro.2014.01.105. [DOI] [PubMed] [Google Scholar]
- 30.Morgan RO, Virnig BA, DeVito CA, Persily NA. The Medicare-HMO revolving door--the healthy go in and the sick go out. N Engl J Med. 1997;337:169–175. doi: 10.1056/NEJM199707173370306. [DOI] [PubMed] [Google Scholar]
- 31.Jensen GA, Morrisey MA. Are healthier older adults choosing managed care? Gerontologist. 2004;44:85–94. doi: 10.1093/geront/44.1.85. [DOI] [PubMed] [Google Scholar]
- 32. [June 25, 2015];Medicare Advantage Enrollees as a Percent of Total Medicare Population. Available from URL: http://kff.org/medicare/state-indicator/enrollees-as-a-of-total-medicare-population/#table.
- 33.Shahinian VB, Kuo YF, Gilbert SM. Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med. 2010;363:1822–1832. doi: 10.1056/NEJMsa0910784. [DOI] [PubMed] [Google Scholar]
- 34.Droz JP, Aapro M, Balducci L, et al. Management of prostate cancer in older patients: updated recommendations of a working group of the International Society of Geriatric Oncology. Lancet Oncol. 2014;15:e404–414. doi: 10.1016/S1470-2045(14)70018-X. [DOI] [PubMed] [Google Scholar]