Abstract
Learning and memory plays an important role in host preference and parasite transmission by disease vector insects. Historically there has been a dearth of standardized protocols that permit testing their learning abilities, thus limiting discussion on the potential epidemiological consequences of learning and memory to a largely speculative extent. However, with increasing evidence that individual experience and associative learning can affect processes such as oviposition site selection and host preference, it is timely to review the recently acquired knowledge, identify research gaps and discuss the implication of learning in disease vector insects in perspective with control strategies.
Keywords: Disease Vector, Conditioning, Learning, Host Selection, Individual Experience, Control Strategies
Learning in disease vectors – current knowledge gaps and potential epidemiological consequences
Learning in insect disease vectors has historically been of epidemiological interest because its potential implications for modifying the pattern of contact between vectors and hosts [1]. As reviewed by a number of authors [2-4], knowledge about the existence and extent to which these abilities modify behavioral preferences in a vector species could greatly improve our understanding of the mechanisms underlying heterogeneous patterns of biting activity and inform the design and planning of programs of vector-borne disease control. Blood-sucking insects, among them mosquitoes, kissing bugs and tsetse flies, to name only a few, are vectors of various parasites and arboviruses such as those responsible for malaria, sleeping sickness, Chagas disease, West Nile fever, Zika, and for which no vaccine exists yet. These diseases affect millions of people each year as well as livestock [5] and thus have a huge economic impact and burden on public health.
In 2002, McCall and Kelly [4] wrote the last important review on learning and memory in disease vector insects. At that time, almost fifteen years ago, only a handful of studies were explicitly suggesting that learning could be involved in processes such as host selection, choice of oviposition site, and home range (e.g. [6-9]). Therefore, the discussion on the potential epidemiological consequences of learning and memory was to a large extent speculative [3]. Since then, disease vectors remain responsible for major health concerns [5], but important discoveries have been made in the field of learning in disease vectors (Tables 1 and 2), thus making it timely to review recent studies, and to point out the paucity of data on certain aspects of this topic. Filling these knowledge gaps will be important to determine whether this information can be leveraged for disease vector control. In addition, some of the early work done on this topic revealed inconclusive results where alternative hypotheses could not always be discarded. Discussing potential reasons for these results is critical to allow progress to be made in our understanding of the biology and neuro-ethology of these disease vectors (Box 1 and 2).
Table 1.
Studies showing clear experimental demonstration for associative or non-associative learning in haematophagous insects
| Insects | Context | Type of learning | Refs |
|---|---|---|---|
| Mosquitoes | Feeding (visual and olfactory cues) | Associative learning | [13] |
| Responses to odors | Associative learning | [15-18] | |
| Kissing bugs | Aversive responses to plant odors | Non associative | [22] |
| Responses to alarm pheromone | Non associative and associative | [31] | |
| Responses to olfactory cues | Associative pavlovian learning | [25-27] | |
| Responses to thermal cues | Associative operant learning | [28] | |
| Circadian modulation of learning | Associative operant learning | [29] | |
Table 2.
Studies demonstrating experience-dependent modulation of behavior in haematophagous insects.
Box 1: Evidencing associative learning in disease vector insects.
A critical element for evidencing associative learning is the demonstration that a change in behavior in response to the conditioned stimulus (CS) is the result of the learned association between this latter and an unconditioned stimulus (US). In some of the early studies investigating learning abilities of vectors, it was impossible to determine whether individual mosquitoes exhibited site fidelity because of habituation, simply by chance, or because of characteristics of the environment itself [1, 13].
In this context, it is critical to select the CS and the US among stimuli that are detected by the insects, and to consider the innate valence of these latter. For example, not all odorants used as CS for a given conditioning protocol could evoke a learned response in mosquitoes [18], and while using the same odor as a CS, the learning performance of female Cx. quinquefasciatus differed according to the nature of the US (e.g., blood versus sugar in [15]). In triatomine bugs, the use of non-biologically relevant odors as CS in the absence of true reinforcement (e.g., using warm saline lacking phago-stimulants as US) are plausible explanations for the lack of success of the first attempt to demonstrate associative olfactory learning in these insects [23]. Finally, when adapting experimental procedures that have been developed in classical insect models (e.g., honey bees, fruit flies), experimental paradigms must be adapted to the particulars of the biology of haematophagous insects (e.g. piercing or tearing of a membrane to access blood, rhythmic host seeking and activity patterns).
If it is necessary to select the CS and US according to the biology of the investigated species, in and of itself this choice is not sufficient for clear demonstration of learning. For that, it is necessary to have a strong temporal and quantitative control of the CS and US presentations as it is the temporal relation (either positive or negative, see [35]) between presentations that will allow the formation of a learned association. Controls where the US and CS are presented either alone or together but without temporal relation (i.e. CS only, US only and most importantly unpaired CS and US controls; [35]), must be performed to bring clear evidence of the associative nature of the learning.
One must also consider that different forms of memory can underlie the performance of the insect, highlighting the necessity to test it within an appropriate time window.
Box 2: Future perspectives on the neurophysiological bases of learning.
Sensory processing of host information
Although there is increased interest and research on sensory transduction at the peripheral level, how blood host-related information is processed and stored in the brain of insect vectors remains unknown. Nonetheless, neuroanatomical studies [50-53] in concert with work from other insect models [54-55] provides impetus for elucidating the neural substrates involved in learning and memory in haematophagous insects. A major issue is evolutionary divergence, as haematophagous bugs belong to phylogenetically very distant groups, the challenge will be identifying common patterns in neural organization.
Brain areas associated with learning and memory
There are several brain regions involved in learning and memory in insects, including the mushroom bodies, lateral horn, and central complex. The mushroom body has been shown to receive olfactory input from the antennal lobe, and is one of the centers for olfactory memories. By contrast, the lateral horn has been shown to be important for processing innate olfactory stimuli, as well as providing contextual information for learned odors, whereas the central complex is thought to be involved in the processing of visual stimuli, orientation and navigation behavior [54-55]. Each of these areas provide targets for mitigating host-seeking behaviors; for instance Vinauger et al. [18] used protein synthesis antagonists to block synaptic growth - possibly in the learning and memory centers of the Ae. aegypti brain - which eliminated appetitive learning but did not affect innate olfactory responses and other visually-guided behaviors.
State-dependent and brain-associated modulation
Diverse neuromodulators and neurohormones play critical roles in learning and memory and state-dependent behaviors. Innervation by neurons that release these substances in the sensory processing areas (e.g. antennal lobe, optic lobes) and mushroom bodies provides the relevant reinforcement system to increase the input of sensory information and mediate memory formation [565]. For example, when stimulated by shock (as US), certain dopaminergic neurons are activated leading to neurotransmitter release. When dopamine release is paired with olfactory input (as CS), mushroom body neurons increase their synaptic growth thereby providing a substrate for learning and memory [554]. Further demonstrating the importance of these neuromodulators, when their receptors are pharmacologically or genetically manipulated then insects are no longer able to learn. Beyond neuromodulators involved in learning, neurohormones play strong roles in state-dependent behaviors. For instance, the combination of blood-feeding and mating in female mosquitoes has been shown to induce release of peptides (eg, Ae. aegypti Head Peptide I (Aea-HP-I), and short neuropeptide F (sNPF)) that regulates host-attraction and inhibits peripheral olfactory neurons that respond to host odors [57-58]. Yet, the mechanisms by which these neurohormones influence the processing of host information and learning in the brain remains unclear. Nonetheless, these neuromodulators and neurohormones, and their associated receptors, provide attractive genetic targets for modifying learning and host-seeking behaviors.
Recent advances in vector learning and memory
Mosquitoes
In order to produce and grow ovocytes and lay eggs, female mosquitoes feed on blood. During the feeding process, whether they are anthropophilic or zoophilic (see Glossary), mosquitoes are exposed to host defensive behaviors (e.g. repetitive shivering) and anti-parasitic mechanisms (e.g. grooming) each time they feed [10-11]. One might thus expect mosquitoes to choose to feed on least defensive hosts and return to feed on the same host species if a past blood-feeding event was successful. Results obtained in different Culex mosquito species indicate that previous contact with a host species impacted females’ subsequent host choice, previously encountered hosts being preferred [7]. In Vantaux et al. [12], the authors investigated the impact of individual experience on the host choice of Anopheles coluzzii mosquitoes. While mosquitoes had an innate preference for rabbits when given the choice between a rabbit and a guinea-pig, a first blood-meal on a rabbit significantly decreased this preference. Also, a first blood meal on human, the host this species prefers, decreased the feeding rate on rabbit or guinea pig during the subsequent gonotrophic cycle. In other words, a successful blood meal can positively but also negatively affect subsequent host choice. Whether these changes in behaviors are mediated by state-dependent effects associated with satiation of the mosquito (see Figure 1, Key Figure), caloric differences between host blood-meals, differences in host cues (e.g., temperature, humidity, and odor), or learning induced by host defensive behaviors remains unclear. Nonetheless, in contrast to the studies with Culex sp. [7], it is worth noting that Vantaux et al. [12] used the natural host odor coupled with the live, unrestrained hosts, thereby enabling hosts to exhibit defensive behaviors.
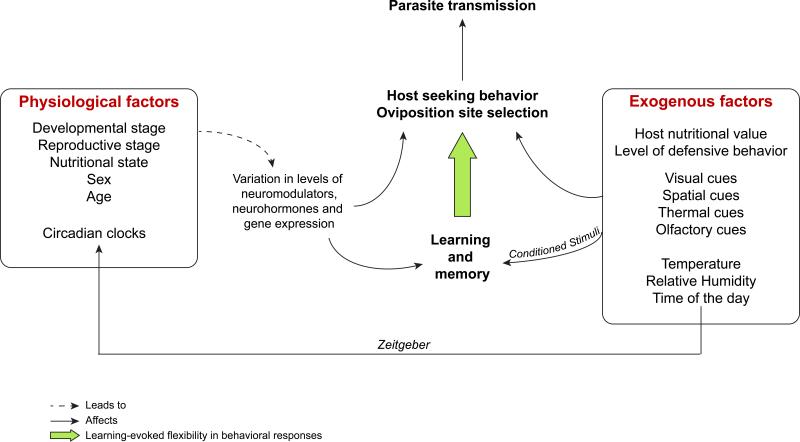
Figure 1.
Physiological and environmental factors (i.e. biotic and abiotic exogenous) factors influencing host-seeking and oviposition site selection either directly or via learning-evoked flexibility in behavioral responses.
Modulation of behavioral preferences is not only restricted to selecting potential blood hosts, but may also affect other important behaviors For example, mark and release methods were used to show that various Anopheles mosquito species returned to the places where they were first captured [9, see 4 and 13 for review]. However, in most of these studies it is impossible to determine whether these few individuals exhibit site fidelity because of habituation, simply by chance, or because of characteristics of the environment itself [1, 13] (Box1). Oviposition site fidelity has also been described in mosquitoes, as Aedes aegypti mosquitoes preferentially lay eggs in the type of water they have been reared in, including water containing a repellent, therefore affecting the effectiveness of such control strategies [14]. McCall and Eaton [8] demonstrated that Culex quinquefasciatus oviposition behavior is also influenced by prior experience.
Since Charlwood et al.'s study in Papua New Guinea [6] - possibly the first study to explicitly investigate memory in a vector - more recent studies have assessed the abilities of mosquitoes to perform associative learning (see Box 1) to visual and olfactory stimuli [13, 15-18], including some studies that failed to demonstrate learning [19]. In an example demonstrating aspects of associative learning in Cx. quinquefasciatus, Tomberlin et al. [15] suggested that both males and females could associate odor blends (synthetic vanilla and strawberry extracts) with either a blood (females only) or a 10% sugar reward (males and females) delivered on a glass pipette coated with the odor. Interestingly, when females were trained to learn the association between the blood or sugar reward (that is, as an unconditioned stimulus [US]) with the vanilla or strawberry odors (that is, the conditioned stimulus [CS]), they showed higher learning performances when sugar was used as a reward than when blood was used as a reward [15]. This was unexpected, but could be attributable to the fact that the tested odors (vanilla and strawberry) could signal a floral sugar reward, rather than a blood-host. It remains an open question whether female mosquitoes are only able learn when the appropriate US is paired with an appropriate CS – in other words, mosquitoes may only learn blood-hosts when host-specific cues are combined with the blood reward, and learn nectar sources as a combination of sugar and floral cues (e.g., flower scents, visual displays). Also, this study showed that, in contrast to females who exhibited higher responses to the trained target odor (strawberry), males trained with strawberry exhibited high response levels to both the target (strawberry) and non-target (vanilla) stimulus [15]. A confounding variable in this study is that the strawberry blend included vanilla extract, which might suggest that males either detected the vanilla contained in strawberry blend, or are capable of generalization of stimuli that share similar features. The Tomberlin group pursued working on Cx. quinquefasciatus this time using the Probing Walking Response (PWR), that they describe as a close-range search behavior that mosquitoes display when presented with a sugar solution [16]. The authors raise a series of interesting questions such as whether wild- and colony-reared mosquitoes show differences in learning, or the impact of the sex and age on learning performance. Results from their study showed that the sex of mosquitoes had the largest impact on whether the mosquitoes demonstrate a positive response to previously neutral odors. However, because both studies pre-exposed the mosquitoes to the CS and did not conduct certain controls (e.g. unpaired US and CS presentations), it remains unclear whether the behavioral responses were associative in nature.
Both males and females Cx. pipiens pipiens were also tested for their learning abilities by Jhumur et al. [20] using mixture and single compounds typically found in the floral odor of Silene otites, a plant that is pollinated by nectar-drinking mosquitoes and moths. Results suggested that both males and females increased their responses to the floral scent after being exposed to the odorant for 1 h during which they had access to a 5% sugar solution. However, training procedures used in this study did not allow explicit testing of associative learning, as here again the potential effect of the odorant presentation alone was not controlled for [20].
Employing a training procedure that provided a high degree of temporal and quantitative control of the CS and US delivery, Vinauger et al. [18] showed that Ae. aegypti mosquitoes can associate L-lactic acid, a host emitted volatile compound, with a blood-reward. Trained mosquitoes were able to retain this information for 24 h but were also able to use information learned in one experimental context in a completely different one (i.e. the testing device, a Y-maze olfactometer). Interestingly, authors tried to train mosquitoes using different volatile compounds as a CS but mosquitoes did not learn all of them as predictors of the blood reward, raising questions about the role that the valence (i.e. the intrinsic attractiveness or averseness) of the odor used as CS could play in the learning responses. Experiments using cold-shock treatments, or protein synthesis antagonists, both affected mosquitoes performances. Although this study was informative about the role of learning in odor responses in Ae. aegypti, and provided evidence for the potential role of different types of memory (e.g. long term memory and anesthesia resistant memory) in these responses, the training procedure did not allow characterization of the learning process. For instance, as training and testing occur in two different experimental contexts, it was not possible to characterize the acquisition rate of the learning.
If olfaction plays a crucial role in mosquitoes’ life-history (e.g. host-seeking, oviposition site recognition), vision is also involved and mediates mid- and short-range responses to stimuli. For example, An. gambiae females are able to associate visual patterns and olfactory cues (cheese / citronella) with either the quality of a blood-meal or the nature of the membrane used in artificial feeding experiments [13]. In these experiments, the visual CS consisted in a black and white checkerboard pattern placed around the blood-feeder as opposed to a plain white disk. Despite an innate preference for the checkered pattern, mosquitoes were able to associate the visual pattern with either a positive or a negative reinforcement (i.e. good and bad membrane qualities, or palatable and unpalatable blood) and retained this information for at least 72 h.
Similarly, the innate visual preference of Ae. aegypti was tested in an aversive training procedure. In Menda et al. [17], the innate tendency to land on a dark surface area in presence of a host related compound, 1-octen-3-ol (used as the CS), was paired with an electrical shock as US, in order to invert their innate attractiveness. Mosquitoes learned the CS-US association and, once trained, they avoided the dark area. The association was even stronger when 1-octen-3-ol was delivered during training, unraveling bimodal integration in the learning process. The association was retained for at least 60 min but not for 24 h. Interestingly, learning performances were similar for males and females despite the strong behavioral and physiological differences between the two sexes, and differences in exposure to defensive food sources: males being strictly sugar feeders while females rely on both blood (e.g. defensive hosts) and sugar. This result underlines that not only the biological meaning of a given stimulus is relevant, but also its informational value in a particular context. The learning ability is not only important for male and female adults, but may also be critical for larvae where learning of predators, or locating appropriate food sites, is essential for survival. For example, Ferrari et al. [21] demonstrated that Cx. restuans larvae exposed to a predator odor paired with an alarm cue (crushed congeners in this case) would respond with antipredator behaviors when tested to the presentation of the predator odor only, the day following training.
Kissing bugs
In triatomine bugs (Rhodnius prolixus), vectors of Chagas disease, the first attempts to assess their learning abilities suggested that pre-exposure to an odor during the last larval instar could reduce its aversion in adults [22]. Subsequent studies, however, were unsuccessful in demonstrating the ability of the bugs to perform associative learning [23-24]. These negative results could have been due to the fact that the stimuli and reward did not adequately match the biology of bugs. Vinauger et al. [25-26] examined the learning abilities of kissing bugs in more detail, using the accumulated knowledge on R. prolixus biology, and demonstrated that the same single chemical compound, L-lactic acid, used as CS, could be learned as a predictor for both appetitive (blood reward) and aversive (mechanical shock) stimuli. Three appetitive training trials pairing the delivery of the odorant with a partial blood-meal, or five aversive training trials pairing the odorant with a controlled mechanical shock, were sufficient to switch the neutral responses of the bugs to L-lactic acid into a learned attraction or aversion, respectively. This work was the first demonstration that the same host volatile can be used by disease vectors to learn to recognize either a host to feed on or a potentially defensive one. In a subsequent study and using the same aversive training procedure, it was possible to show that bugs were capable of associating the complex odor bouquet of a live host (e.g. quail or rat) with a mechanical shock - when given the choice between the two host odors, the bugs showed an attraction to the host odor that had not been punished [27]. As described above, the biological meaning of a given stimulus is relevant, but the associated response can be modified according its informational value in a particular context (i.e., when it is paired with a reward or aversive stimulus). It is also worth mentioning that the experimental paradigm developed in these studies allowed a clear demonstration of the associative nature of learning in bugs, by discarding potential pre-exposure effects of the CS alone, US alone, or both CS and US presented in an unpaired way (Box 1).
Finally, a classical procedure largely employed in insects such as flies and honeybees to condition the proboscis extension response (PER) was adapted to the biology of R. prolixus, in order to characterize non-associative and associative learning, memory retention [28], and the circadian modulation of learning abilities [29]. These studies demonstrated that the PER can not only be habituated and dishabituated, but also trained in an operant manner. Conditioning the bugs to stop responding to an appetitive thermal stimulus by pairing their behavioral response (PER) with a thermal shock (negative reinforcement), made it possible to show that learned information can be retrieved up to 72 h following training, and that bugs learning performances are maximal in early scotophase (i.e. beginning of the night), corresponding to the moment of the day when kissing bugs leave their shelters and search for hosts [30]. Finally, Minoli et al. [31] provided evidence suggesting that learning may influence the way bugs respond to their own alarm pheromones.
Tsetse flies and other species of haematophagous insects
In tsetse flies, both females and males feed on vertebrate blood and are the vectors of various trypanosomes to both humans and livestock. Tsetse flies face a particular challenge for their blood meal because when their proboscis pierces the skin of the host, it evokes a quick defensive response by the host [32]. They thus need to ingurgitate as much blood as possible in the least amount of time possible to avoid host defensive behaviors. In this context being able to learn and remember which hosts are the least defensive would provide a significant advantage to these vectors.
Bouyer et al. [33-34] showed that previous feeding experience increased the probability for tsetse flies to choose the same host species for a subsequent blood-meal. However, if the interval between the two blood feeding events was extended to three days, the effect of the first blood meal could no longer be observed in host choice experiments [34]. In a previous study, it was shown that when provided the choice between two hosts species, flies with previous feeding experience returned to the host they had already fed on [33]. These two studies highlight evidence for host preferences and how it could possibly affect parasite transmission. However, experiments assessing tsetse flies cognitive abilities (sensu Rescorla [35], that is with high temporal and quantitative control over stimuli presentation and appropriate controls to determine the non-associative or associative nature of learning), have not yet been conducted in these insects, thus making it difficult to conclude whether this experience-induced host preference is due to associative learning or other physiological processes.
Using mark-recapture methods, Guerra-Silva et al. [36] showed that sand flies, the vectors of the American cutaneous leishmaniasis, were able to return to their initial location after being released in an unknown area. The use of learned spatial, visual or olfactory cues by sandflies could be just one of the possible explanations for this behavior, but further work is still needed to assess whether or not sand flies rely on learned information.
Epidemiological consequences of vector memory
Learning and vector efficiency
Beyond innate behavior, learning helps tailor responses to resources and dangers that vary from generation to generation. In this way, learning is expected to increase the fitness of insects that evolved such abilities. However, like any biological process, learning requires energy [37-39 and see Box 2] and is subjected to trade-offs with other functions [40-41]. In the end, the capacity for learning would be selected for if evolutionary benefits exceed costs [42]. Evidence that learning is ubiquitous in insects highlights its benefits and suggests that learning could influence diverse activities including avoidance of predation and host defensive behavior, choice of host for blood feeding, of sugar resource, and appropriate oviposition or resting sites. Learning may thus increase fitness of insects at any of these life activities, potentially increasing both their reproduction and longevity. As mentioned above, learning by haematophagous insects may modify responses to host defensive behaviors, host selection, and identifying appropriate oviposition sites. As such, learning could have important consequences on vector reproduction and longevity that have yet to be fully explored in models of vectorial capacity and population dynamics. For instance, in a pathogen-transmitting insect the vectorial capacity can be modeled by the equation:
where m is the vector density, a, the daily biting rate on a host, n, the extrinsic incubation period of the parasite, p, the daily survival rate of the vector ([43]). Dynamically changing the values of p (vector survival) or a (vector biting rate) to account for learning could have large effects on vectorial capacity, as well as causing a feedback on the m and p terms. However, such effects have yet to be experimentally tested in a controlled manner, and learning may have more complex consequences on disease transmission.
To our knowledge, it remains unknown if learning abilities in vector insects affect host choice for blood meal at species-, demographic-, or individual-level. This is unfortunate because it may have major implication in disease epidemiology. If learning increases specialization for blood meal at the host species level, this will positively or negatively affect the transmission in a quadratic relation (as the feeding rate of the vector population, “a” in the formula of vectorial capacity, is squared [43]). For instance, when pathogens, such as Plasmodium, responsible for malaria, are specific to one vertebrate host, learning would increase the transmission when it enhances the specialization for this host. However, in the same vectorial system, learning may improve the performance of mosquito populations to feed on alternative hosts when humans are not easily available (e.g., protected by bed nets), and would again affect the vectorial capacity according to a quadratic relationship, but here negatively. Moreover, learning to overcome the host defensive behavior will improve the vectors efficacy at obtaining a complete blood meal and correspondingly decrease the proportion of interrupted blood meals, which together will decrease pathogen transmission [44]. In addition, specialization for blood feeding on one species may also limit the transmission of zoonotic pathogens (e.g. yellow fever viruses) from reservoir animals to humans, and may limit the frequency of zoonose outbreaks. For instance, a recent model based on gambiense human African trypanomiasis epidemiological data showed how the sleeping disease cycle in humans depends on tsetse fly frequent host switching from wild animals to humans [45]. This suggests that, although previous individual experience influences host choice in tsetse flies [34], they remain generalist enough to bridge the transmission across host species.
Learning in blood feeding may thus have complex effects: on one hand learning could influence pathogen transmission when the vector specializes on one host species, but on the other hand learning by the vector may also cause heterogeneous biting in a host population, with some individuals being more bitten than others. Previous modeling showed such heterogeneity of biting in a host population may substantially enhance disease transmission (Box 2 in [4], Figure 4 in [46]). By contrast, if learning causes blood feeding not only on the same species or demographic groups but also on the same individual, it would then be an asset for combatting the disease as the chance for the vector to encounter a pathogen and transmit it to a new host would be decreased.
Learning and vector control strategies
Few studies have addressed the effect of previous exposures to control tools in vector insects. However, there is growing interest in determining whether repeated exposure to repellents changes vector behaviors, with the goal to better understand the repellents actual efficacy. In mosquitoes, two recent studies have investigated the effect of pre-exposure to N,N-diethyl-meta-toluamide (DEET) odor on subsequent feeding behaviors. Stanczyk et al. [47] exposed, without contact, Ae. aegypti females to DEET in presence or absence of attractive stimuli (human arm or heat source). The DEET-sensitive individuals were re- exposed 3h later and displayed a decreased sensitivity, corroborated with reduced electroantennography responses. In the same species, the study conducted by Vinauger et al. [18] suggested in addition that the reduced repellency of DEET after pre-exposure results from associative learning. In that case, the insects’ innate aversion switched to neutral behavior 24 h after training.
While both studies demonstrated that prior exposure to DEET decreased its repellency in mosquitoes under short-term conditions (~24 h), the longer-term effects (48 to 96 h), that correspond to the usual time between blood meals in mosquitoes, remains unknown. Also, the effect of DEET by contact [48] could induce aversive learning, which, to our knowledge, has yet to be documented in vector insects. Such aversive learning may counteract the decreased repellency of DEET observed after pre-exposure to volatiles. Similarly, for insecticides, Chilaka et al. [13] suggested that if a mosquito encounters a sublethal dose of insecticide during host-seeking, it would probably play a major role in avoiding the host carrying this chemical for the subsequent blood-meal. This highlights the need to better understand the behavioral and physiological effects of the chemical repellents and insecticides on the vectors. At a mechanistic level, it will be important to distinguish between chemicals and substances (repellents, insecticides) that are detected by the vector's sensory system(s), from those that act on other physiological targets (e.g., synapses), since only the first can play the role of CS.
Information on host choices and preferences are also complementary to efficiently reduce and potentially eradicate vector populations. One example where learning-evoked changes in vector host preferences could reduce disease transmission is zooprophylactic measures, which involve shifting the vector population toward other hosts (e.g., cattle). However, this type of control strategy is only applicable to vectors with a large spectrum of hosts or at least to those that are not exclusively anthropophilic. This also require a long term intervention if learning implies that an individual vector bites the same species their whole life. For instance, tsetse flies that would only bite reptiles would not be affected by the insecticide treated cattle technique and would produce an offspring able to transmit [49].
Besides host choices and preferences, increasing our knowledge on how learning may affect the choice of oviposition and resting sites is of primary importance given that it can highly impact control measures. Indeed, identifying such preferences would allow precise targeting of regional areas and application of specific treatments; for instance, reducing development of progeny (i.e., at the larval stage), or application of insecticides and repellents (i.e., for adults).
Concluding Remarks and Future Perspectives
Medical entomologists, ethologists and neurobiologists have only started to investigate the ability of insect vectors to use past experience in order to fine tune their host preference, oviposition site selection and home range. While there is now converging evidence that individual experience can affect the way vectors respond to future environmental signals, work still needs to be done on characterizing the non-associative or associative nature of learning. In addition, because associative learning can lead to long term memorization of information, determining how long these memories last, the effects on fitness, and the underlying neural mechanisms (Box 2), will be important to inform vector control strategies.
When adapting existing experimental paradigms or developing new approaches, future work on this topic needs to integrate recent advances in the description of the neurobiology of learning in insects and take into account the knowledge that is available on the physiology, behavior and activity rhythms of the studied species (Figure 1). In addition, designing experimental procedures that allow fine-scale control over the presentation of the CS and US will make it possible to perform controls where the CS and US are decoupled, thus allowing explicit testing of the associative nature of learning (See Box 1 and 2).
The forthcoming challenges for the disease vector community will be to identify whether learning influences: (1) preferences between individual hosts of the same species; (2) preferences and shifts between hosts species; and (3) how these shifts in preferences modify disease transmission and possibly explain pathogen and vector heterogeneity. Finally, such knowledge and characterization of learning abilities must not stay in the lab. Confirming the need for a precise characterization of learning abilities in vectors, acquired knowledge must be used to inform control strategies and better understand why haematophagous insects are such efficient vectors of pathogens.
Outstanding Questions Box.
What are the neurophysiological and genetic bases of learning abilities?
Are cognitive abilities modulated by physiological factors (e.g. reproductive and feeding status)?
Is it possible to impair or exploit vectors abilities to learn information about their hosts in order to reduce their ability to transmit parasites?
Can all haematophagous insect species learn?
To what extent learning impacts within species inter-individual preferences?
Can learning influence preferences and shifts between host species?
How can learning-evoked shifts and preferences impact disease transmission?
Can we quantify the relative contribution of learning to the observed heterogeneous distribution of vectors amongst host population?
Do parasites affect vectors’ ability to learn and retain information?
Trends Box.
There is growing evidence that individual experience affects processes such as host preference, choice of oviposition sites, and home range, in major disease vectors.
In triatomine bugs and mosquitoes, responses to visual and olfactory signals can be modulated by associative learning.
Experimental paradigms developed in classical models for the study of learning and memory can be adapted to haematophagous insects.
Learning abilities can contribute to increase insects’ efficiency as vectors and could, if not taken into account, reduce the effectiveness of control strategies.
Last, but not least, learning can only be assessed under rigorous conditions imposed by experimental psychology, in order to exclude more parsimonious alternative explanations for a particular behavior.
Glossary
- Anthropophilic
denotes the preference of a vector for the human host as a source of blood over other animal hosts.
- Appetitive training
refers to a training procedure where a given stimulus is paired with a food reward, and is often opposed to aversive training where the trained stimulus is paired with a punishment.
- Associative learning
the process of learning the association between two stimuli. Two forms of associative learning can be distinguished: classical conditioning, in which the animal learns the relation between a neutral stimulus and an unconditioned stimulus until eventually the neutral stimulus elicits a response on its own, and operant conditioning, in which a certain behavior is either reinforced or punished, altering the probability that the behavior will reoccur. In other words, conditioning involves the learning of relations among events, while being sensitive to relations involving the properties of the events themselves. Or, as Rescorla states, in associative learning, “the organism is [...] an information seeker using logical and perceptual relations among events, along with its own preconceptions, to form a sophisticated representation of its world.” [34].
- Conditioned Stimulus
in classical conditioning, the conditioned stimulus (CS) is a stimulus to which an organism has learned to make a response to, after becoming associated with the unconditioned stimulus (US). At the beginning of the experiment, the CS does not evoke the conditioned response by itself, i.e., it is a neutral stimulus (see below).
- Habituation
decrease or end of the response to a stimulus after repeated presentations due to central modulation. Peripheral phenomena, as sensory adaptation and motor fatigue are not formally considered as being habituation.
- Learning
there is no widely accepted definition of learning satisfying all the conditions: operational, essential, widely applicable and succinct. It is usually referred as an adaptive change in behavior resulting from individual experience. A central integration of information is mostly included, but also organisms lacking of a nervous system have been reported to learn.
- Memory retention
ability to retain information from previous experiences for a given period of time.
- Neutral stimulus
a stimulus that the animal is able to perceive, but that will trigger the response under study only once the CS-US association has been established.
- Non-associative learning
a relatively permanent change in the strength of response to a single stimulus due to repeated exposure to that stimulus. Habituation, and sensitization are examples of non-associative learning.
- Sensitization
increase in the response to a stimulus after repeated presentations.
- Unconditioned Stimulus
in classical conditioning, the unconditioned stimulus is a stimulus that unconditionally and automatically evokes an innate response.
- Zeitgeber
an environmental cue, such as a change in light or temperature, that is used by an organism to reset an internal biological clock.
- Zoophilic
denotes the preference of a vector for the other animal hosts (e.g., birds, livestock etc.) over human hosts as a source of blood.
- Zooprophylaxis
the diversion of disease-carrying insects from the reservoir to other hosts. For example, the use of cattle to divert mosquitoes from humans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Alonso WJ, Schuck-Paim C. The ‘ghosts’ that pester studies on learning in mosquitoes: guidelines to chase them off. Med. Vet. Entomol. 2006;20(2):157–165. doi: 10.1111/j.1365-2915.2006.00623.x. [DOI] [PubMed] [Google Scholar]
- 2.Hasibeder G, Dye C. Population dynamics of mosquito-borne disease: Persistence in a completely heterogeneous environment. Theor. Popul. Biol. 1988;33(1):31–53. doi: 10.1016/0040-5809(88)90003-2. [DOI] [PubMed] [Google Scholar]
- 3.Kelly DW, Thompson CE. Epidemiology and optimal foraging: modelling the ideal free distribution of insect vectors. Parasitology. 2000;120(03):319–327. doi: 10.1017/s0031182099005442. [DOI] [PubMed] [Google Scholar]
- 4.McCall PJ, Kelly DW. Learning and memory in disease vectors. Trends Parasitol. 2002;18(10):429–433. doi: 10.1016/s1471-4922(02)02370-x. [DOI] [PubMed] [Google Scholar]
- 5.World Health Statistics Annual Report. 2016 [Google Scholar]
- 6.Charlwood JD, et al. Evidence for a ‘memorized’ home range in Anopheles farauti females from Papua New Guinea. Med. Vet. Entomol. 1988;2:101–108. doi: 10.1111/j.1365-2915.1988.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 7.Mwandawiro C, et al. Heterogeneity in the host preference of Japanese encephalitis vectors in Chiang Mai, northern Thailand. Trans. Roy. Soc. Trop. Med. Hyg. 2000;94(3):238–242. doi: 10.1016/s0035-9203(00)90303-1. [DOI] [PubMed] [Google Scholar]
- 8.McCall PJ, Eaton G. Olfactory memory in the mosquito Culex quinquefasciatus. Med. Vet. Entomol. 2001;15(2):197–203. doi: 10.1046/j.0269-283x.2001.00304.x. [DOI] [PubMed] [Google Scholar]
- 9.McCall PJ, et al. Evidence of memorized site-fidelity in Anopheles arabiensis. Trans. Roy. Soc. Trop. Med. Hyg. 2001;95:587–590. doi: 10.1016/s0035-9203(01)90087-2. [DOI] [PubMed] [Google Scholar]
- 10.Day JF, Edman JD. Mosquito engorgement on normally defensive hosts depends on host activity pattern. J. med. entomol. 1984;21(6):732–740. doi: 10.1093/jmedent/21.6.732. [DOI] [PubMed] [Google Scholar]
- 11.Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annu. Rev. Entomol. 2013;58:433–53. doi: 10.1146/annurev-ento-120811-153618. [DOI] [PubMed] [Google Scholar]
- 12.Vantaux A, et al. Individual experience affects host choice in malaria vector mosquitoes. Parasite Vector. 2014;7(1):1–7. doi: 10.1186/1756-3305-7-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chilaka N, et al. Visual and olfactory associative learning in the malaria vector Anopheles gambiae sensu stricto. Malar. J. 2012;11:27. doi: 10.1186/1475-2875-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaur JS, et al. Learning and memory in the mosquito Aedes aegypti shown by conditioning against oviposition deterrence. Med. Vet. Entomol. 2003;17(4):457–460. doi: 10.1111/j.1365-2915.2003.00455.x. [DOI] [PubMed] [Google Scholar]
- 15.Tomberlin JK, et al. Associative learning of odor with food-or blood-meal by Culex quinquefasciatus Say (Diptera: Culicidae). Naturwissenschaften. 2006;93(11):551–556. doi: 10.1007/s00114-006-0143-9. [DOI] [PubMed] [Google Scholar]
- 16.Sanford MR, Tomberlin JK. Conditioning individual mosquitoes to an odor: sex, source, and time. PloS one. 2011;6(8):e24218. doi: 10.1371/journal.pone.0024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menda G, et al. Associative learning in the dengue vector mosquito, Aedes aegypti: avoidance of a previously attractive odor or surface color that is paired with an aversive stimulus. J. Exp. Biol. 2013;216(2):218–223. doi: 10.1242/jeb.074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinauger C, et al. Olfactory learning and memory in the disease vector mosquito Aedes aegypti. J. Exp. Biol. 2014;217(13):2321–2330. doi: 10.1242/jeb.101279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alonso WJ, et al. Are vectors able to learn about their hosts? A case study with Aedes aegypti mosquitoes. Mem. I. Oswaldo Cruz. 2003;98(5):665–672. doi: 10.1590/s0074-02762003000500014. [DOI] [PubMed] [Google Scholar]
- 20.Jhumur US, et al. Naive and conditioned responses of Culex pipiens pipiens biotype molestus (Diptera: Culicidae) to flower odors. J. med. entomol. 2006;43(6):1164–1170. [PubMed] [Google Scholar]
- 21.Ferrari MCO, et al. Threat-sensitive learning by the larval mosquito Culex restuans. Behav. Ecol. Sociobiol. 2008;62:1079–1083. [Google Scholar]
- 22.Abramson CI, et al. Fifth instar experience reduces aversiveness of the plant extract ruda (Ruta graveolens) in the adult triatomine Rhodnius prolixus Stal 1859. J Vector Ecol. 2006;31(1):196–197. doi: 10.3376/1081-1710(2006)31[196:fierao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Abramson CI, et al. Psychology of learning: a new approach to study behavior of Rhodnius prolixus Stal under laboratory conditions. Psychol. Rep. 2005;97:721–731. doi: 10.2466/pr0.97.3.721-731. [DOI] [PubMed] [Google Scholar]
- 24.Aldana E, et al. Learning and orientation to odor in the bug Rhodnius prolixus Stal 1859 under laboratory conditions. Parasitol. Res. 2008;103:587–594. doi: 10.1007/s00436-008-1014-4. [DOI] [PubMed] [Google Scholar]
- 25.Vinauger C, et al. Learning the way to blood: first evidence of dual olfactory conditioning in a blood-sucking insect, Rhodnius prolixus. I. Appetitive learning. J. Exp. Biol. 2011a;214:3032–3038. doi: 10.1242/jeb.056697. [DOI] [PubMed] [Google Scholar]
- 26.Vinauger C, et al. Learning the way to blood: first evidence of dual olfactory conditioning in a blood-sucking insect, Rhodnius prolixus. II. Aversive learning. J. Exp. Biol. 2011b;214:3039–3045. doi: 10.1242/jeb.057075. [DOI] [PubMed] [Google Scholar]
- 27.Vinauger C, et al. Learned host preference in a Chagas disease vector, Rhodnius prolixus. Acta Trop. 2012;122:24–28. doi: 10.1016/j.actatropica.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Vinauger C, et al. Learning and memory in Rhodnius prolixus: habituation and aversive operant conditioning of the proboscis extension response. J. Exp. Biol. 2013;216:892–900. doi: 10.1242/jeb.079491. [DOI] [PubMed] [Google Scholar]
- 29.Vinauger C, Lazzari CR. Circadian modulation of learning ability in a disease vector insect, Rhodnius prolixus. J. Exp. Biol. 2015;218:3110–3117. doi: 10.1242/jeb.119057. [DOI] [PubMed] [Google Scholar]
- 30.Lazzari CR. Orientation towards hosts in haematophagous insects: an integrative perspective. Adv. Insect Physiol. 2009;37:1–58. [Google Scholar]
- 31.Minoli S, et al. The main component of an alarm pheromone of kissing bugs plays multiple roles in the cognitive modulation of the escape response. Front. Behav. Neurosci. 2013;7(77) doi: 10.3389/fnbeh.2013.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torr SJ, Mangwiro TNC. Interactions between cattle and biting flies: effects on the feeding rate of tsetse. Med Vet Entomol. 2000;14:400–409. doi: 10.1046/j.1365-2915.2000.00257.x. [DOI] [PubMed] [Google Scholar]
- 33.Bouyer J, et al. Learning affects host preference in tsetse flies. Revue d'elevage et de medecine veterinaire des pays tropicaux. 2005;58(1/2):27. [Google Scholar]
- 34.Bouyer J, et al. Learning influences host choice in tsetse. Biol. lett. 2007;3(2):113–117. doi: 10.1098/rsbl.2006.0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rescorla RA. Pavlovian conditioning: It's not what you think it is. Am. Psychol. 1988;43(3):151. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- 36.Guerra-Silva NMM, et al. Dispersal and memory of sand flies in an endemic area of cutaneous leishmaniasis, Southern Brazil. J. med. entomol. 2013;50(5):986–993. doi: 10.1603/me12065. [DOI] [PubMed] [Google Scholar]
- 37.Laughlin SB, et al. The metabolic cost of neural information. Nat.Neurosci. 1998;1:36–41. doi: 10.1038/236. [DOI] [PubMed] [Google Scholar]
- 38.Dukas R. Costs of memory: ideas and predictions. J. Theor. Biol. 1999;197(1):41–50. doi: 10.1006/jtbi.1998.0856. [DOI] [PubMed] [Google Scholar]
- 39.Jaumann S, et al. Energetic cost of learning and memory can cause cognitive impairment in honeybees. Biol. lett. 2013;9(4):20130149. doi: 10.1098/rsbl.2013.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snell-Rood EC, et al. Reproductive tradeoffs of learning in a butterfly. Behav. Ecol. 2011;22(2):291–302. [Google Scholar]
- 41.Mery F, Kawecki TJ. A cost of long-term memory in Drosophila. Science. 2005;308(5725):1148–1148. doi: 10.1126/science.1111331. [DOI] [PubMed] [Google Scholar]
- 42.Dukas R. Life history of learning: performance curves of honeybees in settings that minimize the role of learning. Anim. Behav. 2008;75(3):1125–1130. [Google Scholar]
- 43.Macdonald G. The epidemiology and control of malaria. 1957:212. [Google Scholar]
- 44.Churcher TS, et al. Human-to-mosquito transmission efficiency increases as malaria is controlled. Nat. Commun. 2015;19(6):6054. doi: 10.1038/ncomms7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Funk S, et al. Identifying transmission at the human-animal interface: the role of animal reservoirs in maintaining gambiense Human African Trypanosomiasis. PLoS Comput. Biol. 2013;9:e1002855. doi: 10.1371/journal.pcbi.1002855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scott TW, Takken W. Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends Parasitol. 2012;28(3):114–21. doi: 10.1016/j.pt.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Stanczyk NM, et al. Aedes aegypti mosquitoes exhibit decreased repellency by DEET following previous exposure. PloS one. 2013;8(2):e54438. doi: 10.1371/journal.pone.0054438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deletre E, et al. Repellent, irritant and toxic effects of 20 plant extracts on adults of the malaria vector Anopheles gambiae mosquito. PLoS One. 2013;8(12):e82103. doi: 10.1371/journal.pone.0082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [0]49.Vreysen MJB, et al. Tsetse flies: their biology and control using area-wide integrated pest management approaches. J. Invertebr. Pathol. 2013;112:S15–S25. doi: 10.1016/j.jip.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 50.Anton S. Central olfactory pathways in mosquitoes and other insects. In Ciba Found. Symp. 1996;200:184–192. doi: 10.1002/9780470514948.ch14. [DOI] [PubMed] [Google Scholar]
- 51.Anton S, Rospars JP. Quantitative analysis of olfactory receptor neuron projections in the antennal lobe of the malaria mosquito, Anopheles gambiae. J. Comp. Neurol. 2004;475(3):315–326. doi: 10.1002/cne.20174. [DOI] [PubMed] [Google Scholar]
- 52.Ignell R, et al. Neuronal architecture of the mosquito deutocerebrum. J. Comp. Neurol. 2005;493(2):207–240. doi: 10.1002/cne.20800. [DOI] [PubMed] [Google Scholar]
- 53.Barrozo RB, et al. Antennal pathways in the central nervous system of a blood-sucking bug, Rhodnius prolixus. Arthropod Struc. Dev. 2009;38(2):101–110. doi: 10.1016/j.asd.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 54.Haberkern H, Jayaraman V. Studying small brains to understand the building blocks of cognition. Curr. Opin. Neurobiol. 2016;37:59–65. doi: 10.1016/j.conb.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 55.Davis RL. Traces of Drosophila memory. Neuron. 2011;70(1):8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim YC, et al. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J. Neurosci. 2007;27(29):7640–7647. doi: 10.1523/JNEUROSCI.1167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klowden MJ. Endogenous factors regulating mosquito host-seeking behavior. Olfaction in Mosquito-Host Interactions. 1996;124:212. doi: 10.1002/9780470514948.ch16. [DOI] [PubMed] [Google Scholar]
- 58.Siju KP, et al. Neuropeptides in the antennal lobe of the yellow fever mosquito, Aedes aegypti. J. Comp. Neurol. 2014;522(3):592–608. doi: 10.1002/cne.23434. [DOI] [PMC free article] [PubMed] [Google Scholar]