Abstract
Background
Dementia is a devastating condition typically preceded by a long prodromal phase characterized by accumulation of neuropathology and accelerated cognitive decline. A growing number of epidemiologic studies have explored the relation between air pollution exposure and dementia-related outcomes.
Methods
We undertook a systematic review, including quality assessment, to interpret the collective findings and describe methodological challenges that may limit study validity. Articles, which were identified according to a registered protocol, had to quantify the association of an air pollution exposure with cognitive function, cognitive decline, a dementia-related neuroimaging feature, or dementia.
Results
We identified 18 eligible published articles. The quality of most studies was adequate to exemplary. Almost all reported an adverse association between at least one pollutant and one dementia-related outcome. However, relatively few studies considered outcomes that provide the strongest evidence for a causal effect, such as within-person cognitive or pathologic changes. Reassuringly, differential selection would likely bias toward a protective association in most studies, making it unlikely to account for observed adverse associations. Likewise, using a formal sensitivity analysis, we found that unmeasured confounding is also unlikely to explain reported adverse associations.
Discussion
We also identified several common challenges. First, most studies of incident dementia identified cases from health system records. As dementia in the community is underdiagnosed, this could generate either non-differential or differential misclassification bias. Second, almost all studies used recent air pollution exposures as surrogate measures of long-term exposure. Although this approach may be reasonable if the measured and etiologic exposure windows are separated by a few years, its validity is unknown over longer intervals. Third, comparing the magnitude of associations may not clearly pinpoint which, if any, pollutants are the probable causal agents, because the degree of exposure misclassification differs across pollutants.
The epidemiologic evidence, alongside evidence from other lines of research, provides support for a relation of air pollution exposure to dementia. Future studies with improved design, analysis and reporting would fill key evidentiary gaps and provide a solid foundation for recommendations and possible interventions.
Keywords: air pollution, cognitive function, cognitive decline, dementia, Alzheimer disease, systematic review
1. INTRODUCTION
Dementia is a condition diagnosed when loss of cognitive function becomes severe enough to interfere with daily activities.1 It is typically preceded by a protracted period of cognitive decline,2–7 and is extremely common in older adults.8,9 The number of older adults in the US with Alzheimer’s disease dementia, the most common form of dementia, is expected to rise from approximately 4.7 million in 2010 to 13.8 million by 2050,10 with analogous increases expected worldwide.11 Dementia inflicts substantial burdens on families, friends, caretakers, and social safety nets. Those afflicted lose the ability to engage in basic self-care and social interaction. Associated healthcare use and costs exceed those associated with other common age-related conditions.12–14 No medication, including the handful approved by the US Food and Drug Administration, has yet been shown to meaningfully alter the course of Alzheimer’s dementia.14 This lack of progress in identifying effective treatments, along with recognition of dementia’s decades-long incipient phase, has led many scientists to shift their attention to prevention.15
Research on risk factors for dementia has largely emphasized the potential contribution of behaviors, medication use, and health conditions. More recently, epidemiologic studies have begun to explore the etiologic role of exposures to common environmental pollutants, notably air pollution. Unlike other putative modifiable risk factors for cognitive decline and dementia, air pollution can be modified at the population level through environmental regulation and technological innovation. Given its ubiquity, if exposure to air pollution is causally related to dementia, population-level reductions in exposure may significantly alter the population-level burden of dementia, even if the effects are modest.
Epidemiologic research has the potential to offer critical evidence on whether air pollution exposures affect dementia risk, in large part because this method is well-suited for studying long-term exposures. However, such research must contend with the inherent difficulties in studying the causes of dementia. For example, dementia is diagnosed in those whose level of cognitive function falls below a threshold, meaning that dementia’s emergence depends on achieved (i.e., “peak”) level of function as well as trajectory of decline. Thus, when incident dementia is the outcome, it is difficult to disentangle the exposure’s association with achieved function from its association with pathology. Moreover, although cognitive decline precedes dementia onset, decline does not always indicate subclinical dementia. Persons with mild cognitive impairment (MCI), a state characterized by measurable cognitive deficits that do not interfere with everyday activities,16 may remain stable, worsen, or revert to normal.17
By definition, dementia is marked by deficits that interfere with carrying out tasks of everyday life, in at least two of the following cognitive domains: memory, executive function, visuospatial ability, and language.18 The canonical cognitive function affected in Alzheimer’s disease is memory, particularly ‘impairment in learning and recall of recently learned information.’ A diagnosis of Alzheimer’s dementia requires impairment in at least one of the aforementioned cognitive domains, as well.18 Vascular dementia may initially manifest executive dysfunction, and other dementias tend to manifest early deficits in other domains.14 Nonetheless, following a proliferation in new cognitive, imaging and neuropathologic data, the previous convention of equating specific dementia types to unique cognitive fingerprints has given way to a more nuanced view, especially pertaining dementias in older adulthood,18,19 many of which may be manifestations of several neuropathologies.20,21 Dementia’s cognitive symptoms are related to the presence of one or more underlying neuropathologies (e.g., amyloid plaques and neurofibrillary tangles in Alzheimer’s disease or Lewy bodies in Lewy body dementia, neuronal loss), many of which accumulate years prior to the onset of clinical symptoms.20,22–24 Putative risk factors may influence only one or a subset of these pathologies. Ultimately, the case for air pollution’s causal effect hinges largely on consistent findings linking exposure to multiple indicators of cognitive deterioration or the accumulation of dementia-related pathology.
In addition to evaluating a variety of outcomes, the epidemiologic studies published thus far on air pollution and dementia or related outcomes vary in study design, the air pollutants considered, methods for assessing exposures and outcomes, and potential for bias. As a result, it can be difficult to interpret the collective findings. Therefore, we conducted a systematic review of epidemiologic studies reporting on the association of a measure of long-term exposure to outdoor air pollution with a cognitive or neuroimaging outcome related to cognitive decline and dementia, with the aim of summarizing and synthesizing the findings.
2. METHODS
This systematic review was developed and reported according to PRISMA reporting guidelines.25 The review methods were pre-specified and documented in protocol CRD42015016805 registered with PROSPERO (http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015016805). Briefly, we developed search strategies for PubMed and EMBASE (Appendix A) to identify relevant articles. The original search covered all articles added to either database through December 31, 2014. A limited update literature search subsequently captured articles added to either database from January 1, 2015 through August 10, 2015. Eligible articles met the following criteria: (a) reported on any epidemiologic analyses of data from a sample of adults (i.e., over age 18) in which inclusion criteria did not require presence of a specific disease (e.g., diabetes); (b) quantified long-term exposure (i.e., a 1-year or longer averaging period) to ambient outdoor ozone (O3), sulfur dioxide (SO2), nitrogen dioxide (NO2), oxides of nitrogen (NOx), particulate matter (PM), including respirable particles less than 10 μm (PM10), coarse particles between 2.5 and 10 μm in aerodynamic diameter (PM2.5–10), or fine particles less than 2.5 μm (PM2.5), and/or traffic-related air pollution, including accepted surrogates for PM or traffic-related pollution such as distance to road, soot, or black carbon; and (c) reported on the association of long-term exposure to outdoor air pollution with cognitive test scores, change in cognitive test scores, diagnosis of MCI, dementia, or dementia subtypes (e.g., Alzheimer’s disease dementia), neuroimaging features associated with dementia, or progression of neuroimaging features associated with dementia. Conference abstracts and non-peer reviewed publications were excluded. We did not impose language restrictions. Potentially overlapping publications were included if either the exposures or outcomes considered were distinct.
Two authors (MP and JW) independently reviewed titles and abstracts of all citations, determining eligibility of those selected for full-text review. MP recorded data on each eligible article (Appendix B); JW assessed study quality and risk of bias using a custom template (Appendix C). Study authors were contacted when information required for the data extraction or study quality assessment was not available from published reports. JW reviewed recorded data and MP reviewed study quality determinations. Discrepancies and disagreements were resolved via discussion.
We developed tables and narratives summarizing relevant study characteristics and quality assessments. As anticipated, heterogeneity in study design, exposures, and outcomes precluded meta-analysis. Non-comparable effect estimates also precluded statistical evaluation of the likelihood of publication bias. In addition, as registration of observational epidemiologic studies is uncommon, it was not possible to evaluate publication bias or selective reporting by comparing registered with published studies.
To better understand whether residual confounding could plausibly explain the reported associations, we conducted a formal post-hoc assessment to determine the sensitivity of study findings to omission of adjustment for an unmeasured confounder. Specifically, we quantified the characteristics of a binary variable U that would be required to produce associations observed in two studies that met our eligibility criteria and were judged to be of high quality.26,27 We implemented the method of Vanderweele and Arah28 under two simplifying assumptions, that: (1) the difference in the prevalence of U across levels of the exposure did not vary across levels of the other covariates in the model; and (2) the relationship between U and the outcome did not vary by exposure level (i.e., no interaction on the modeling scale). We specified the effect of having U=1 (versus U=0) on the outcome to be equivalent to the influence on the outcome of being 2, 5, or 10 years older in the study sample. Under these three scenarios, we then estimated the prevalence difference in U for a given exposure contrast required to produce the reported effect estimate.
3. RESULTS
We identified 212 non-duplicate records (Figure 1), one of which was known to the authors and available ahead-of-print, but had not yet been added to either PubMed or EMBASE.29 Of the 29 citations we identified for full text review,26,27,29–55 18 published articles met our eligibility criteria.26,27,29,30,33,34,36,38–41,45–47,49,50,53,54 We describe the features of these studies in Section 3.1 and discuss individual and aggregate study quality in Section 3.2.
Figure 1.
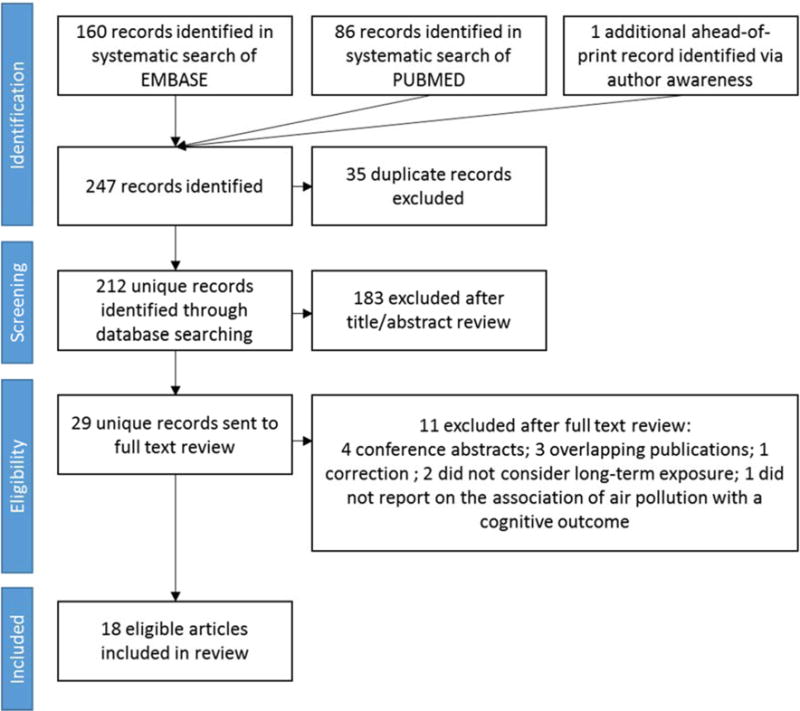
Flow chart of study selection process
3.1. Study characteristics
Table 1 summarizes information on each eligible study’s design, cohort, cohort size, exposure, and outcome measure, and findings. Individual-level study findings are described in greater detail using a narrative approach in Appendix D.
Table 1.
Summary of eligible studies
| PM10 | PM2.5–10 | PM2.5 | DTR or BC or traffic PM | NO2 or NOx | Ozone | API | Cognitive Test Scores | Poor Cognition (Test Scores) | Dementia (Medical Record) | Dementia (Study Assessment) | Neuroimaging | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Citation)/Cohort | N/Location | Exposures Considered | Outcomes Considered | Summary of Findings | ||||||||||
| Analyses of Cognitive Level | ||||||||||||||
| (Ailshire and Crimmins, 2014)/HRS | 13,996/US | X | X | Significant association of higher PM2.5 with worse performance on a general measure of cognition. | ||||||||||
| (Ailshire and Clarke, 2015)/ACL Survey | 780/US | X | X | Significant association of higher PM2.5 with an increasing level of cognitive impairment. | ||||||||||
| (Chen and Schwartz, 2009)/NHANES III | 1,764/US | X | X | X | Significant association of higher O3 with worse executive function and attention/short term memory test scores. Little support for associations with PM10. | |||||||||
| (Gatto et al., 2014)/WISH, BVAIT, and ELITE | 1,496/Los Angeles Basin, US | X | X | X | X | No significant adverse associations of most exposures with most cognitive outcomes. Significant association of higher PM2.5 with worse verbal learning test scores. | ||||||||
| (Power et al., 2011b)/NAS | 680/Greater Boston, US | X | X | X | Significant association of higher black carbon with greater risk of poor cognition and worse general cognitive performance. | |||||||||
| (Ranft et al., 2009)/SALIA | 399/Ruhr and adjacent area, Germany | X | X | X | Significant association of shorter distance to road with worse performance on a general assessment of cognition and a test of selective attention. No association with PM10. | |||||||||
| (Schikowski et al., 2015)/SALIA | 789/Ruhr and adjacent area, Germany | X | X | X | X | X | Association between higher PM2.5, PM10, PM2.5 absorbance, NO2, and NOx (but not traffic load) and worse performance on a test of visuo-spatial ability; association of higher NOx with worse scores on a general measure of cognition and one of three semantic memory tests. No association of any pollutant with episodic memory or executive function test scores. | |||||||
| (Tonne et al., 2014)/Whitehall II | 2,867/Greater London, UK | X | X | X | X | Higher prior exposure to PM and exhaust-related PM of all size fractions in the 5 years prior to cognitive testing appeared associated with worse reasoning test performance; PM was not associated with memory and verbal fluency scores. | ||||||||
| (Wellenius et al., 2012a)/MOBILIZE Boston | 765/Boston, US | X | X | X | Significant association of shorter distance to road with worse performance on several domain-specific cognitive tests, but not with risk of poor general cognition; significant association of higher black carbon with risk of poor general cognition and worse performance on a measure of verbal learning, but not other domain-specific cognitive tests. | |||||||||
| (Zeng et al., 2010) /CLHLS | 15,973/China | X | X | Significant association of higher API with greater risk of cognitive impairment assessed using a general test of cognition. | ||||||||||
| Analyses of Neuroimaging Level | ||||||||||||||
| (Chen et al., 2015) /WHIMS-MRI | 1,403/US | X | X | Significant association of higher PM2.5 with smaller normal-appearing white matter volumes, but not with gray matter volumes, or total ventricular, hippocampal, or basal ganglia volumes. | ||||||||||
| (Wilker et al., 2015)/FOS | 929*/New England, US | X | X | X | Significant association of higher PM2.5 with smaller total cerebral brain volume and greater risk of covert brain infarcts, but not hippocampal volume or white matter hyperintensity burden. Significant association of greater distance to road with higher white matter hyperintensity volume, but not with a dichotomous measure of severe white matter hyperintensities, hippocampal volume, or total cerebral brain volume. | |||||||||
| Analyses of Cognitive Change | ||||||||||||||
| (Tonne et al., 2014)/Whitehall II | 2,867/Greater London, UK | X | X | X | X | After excluding participants who moved, PM10 and PM2.5 in the calendar year 4 years prior to the second cognitive assessment appeared associated with faster decline in memory performance, but not reasoning or verbal fluency, over 3 to 9 years of follow-up; evidence was greatly weaker for exhaust-related PM. | ||||||||
| (Weuve et al., 2012)/NHS | 19,409/US | X | X | X | X | Higher long-term exposures to PM10, PM2.5 and PM2.5–10 were associated with faster declines on all measures of cognition, including a composite measure of general cognition. | ||||||||
| Analyses of Incident Dementia/Poor Cognition | ||||||||||||||
| (Chang et al., 2014)/NHIRD Taiwan | 29,547/Taiwan | X | X | Higher exposure to NO2 associated with greater incidence of ICD-9-CM Alzheimer’s disease or dementia codes. | ||||||||||
| (Jung et al., 2015)/NHIRD | 95,690/Taiwan | X | X | X | Higher exposure to ozone, but not PM2.5, at baseline was associated with greater risk of incident ICD-9-CM based Alzheimer’s disease dementia diagnosis over up to 10 years of follow-up; less decline in exposure over time in both ozone and PM2.5 was associated with greater risk of incident ICD-9-CM based Alzheimer’s disease dementia diagnosis. | |||||||||
| (Loop et al., 2013)/REGARDS | 20,150/US | X | X | No association of PM2.5 with incident poor cognitive performance. | ||||||||||
| (Oudin et al., 2016)/Betula | 1,806/Umeå, Sweden | X | X | Higher exposure to NOx was associated with increased risk of incident dementia diagnosed from study visit data and medical records. | ||||||||||
| Time Series Analyses of Hospital Admissions | ||||||||||||||
| (Kioumourtzoglou et al., 2016)/Medicare | 9,817,806/US | X | X | Higher than expected city-specific annual city-wide PM2.5 exposures were associated with higher than expected annual city-wide rates of ICD-9 codes for dementia and Alzheimer’s disease. | ||||||||||
Sample size varies by analysis. N=929 represents the largest reported sample size for primary analyses.
Abbreviations: ACL, Americans’ Changing Lives; BVAIT, B-Vitamin Atherosclerosis Intervention Trial; CLHLS, Chinese Longitudinal Healthy Longevity Survey; ELITE, Early Versus Late Intervention Trial; FOS, Framingham Offspring Study; HRS, Health and Retirement Study; ICD-9, International Classification of Diseases, Ninth Revision; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; MOBILIZE, Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly; NHANES III, Third National Health and Nutrition Examination Survey; NHIRD, National Health Insurance Research Database; NHS, Nurses’ Health Study; NO2, nitrogen dioxides; NOx, nitrogen oxides; PM, particulate matter; PM2.5, particulate matter with an aerodynamic diameter < 2.5 micrometers; PM10, particulate matter with an aerodynamic diameter < 10 micrometers; PM2.5–10, particulate matter with an aerodynamic diameter between 2.5 and 10 micrometers; REGARDS, Reasons for Geographic and Racial Differences in Stroke; SALIA, Study on the Influence of Air Pollution on Lung Function, Inflammation, and Aging; WHIMS-MRI, Women’s Health Initiative Memory Study Magnetic Resonance Imaging Study; WISH, Women’s Isoflavone Soy Health
3.1.1. Study design and outcome assessment
The majority of studies reported estimated associations of air pollution exposure with cognitive level (e.g., performance on cognitive tests; N=10).27,30,34,38,45–47,49,50,54 or presence and/or severity of neuroimaging markers obtained from a single brain magnetic resonance imaging (MRI) assessment (N=2).36,53 Two studies evaluated the association between air pollution and change in cognitive test scores over time,26,49 four evaluated the association between air pollution and incident cognitive impairment or dementia,29,33,39,41 and one used a time-series-like approach to link year-to-year fluctuations in air pollution with year-to-year variation in hospital admissions for dementia or Alzheimer’s disease dementia.40 Even within broad study design groupings, there was significant heterogeneity in both the approach to cognitive assessment and choice of instrument. For example, measures of “cognitive level” ranged from performance on a single cognitive test27 to performance across six cognitive domains derived from scores on a battery of 14 cognitive tests.38 Similarly, among the four studies investigating incident cognitive impairment, one defined cognitive impairment as a score of <4 on a six-item screener administered yearly,41 two used ICD-9-CM codes obtained from an administrative database,33,39 and one used dementia diagnosis according to DSM-IV criteria based on a combination of study visit assessment and medical records.29
3.1.2. Exposure assessment
Most studies considered some measure of airborne particulate matter (Table 1).26,27,30,34,36,38–41,46,47,49,53 Several studies also examined O3,34,38,39 oxides of nitrogen (NO2 or NOx),29,33,38,47 or indicators of traffic-related air pollution exposure including black carbon (BC), proximity to road, or traffic-related particulate matter.45–47,49,50,53 One study54 employed the air pollution index (API), which combines information on SO2, NO2, PM10, carbon monoxide (CO) and O3.
There was significant variation in the spatial resolution of assigned air pollution exposures. Exposure estimates were assigned based on either the participants’ community,33,40,54 county,34 postcode,39,49 census tract or census block,27,30,34 or address of residence.26,29,36,38,41,45–47,50,53 Exposure assessment methods also varied. Some studies assigned concentrations from the nearest monitor,33,46 whereas others used averages from local monitors,27,30,34,38–40 or predictions based on statistical and deterministic modeling approaches.26,29,36,41,45,47,49,50,53
3.1.3. Study findings
With one exception,41 all studies reported at least one notable association between a higher estimated exposure to air pollution and a worse cognitive or related outcome (Table 1). Even so, the specific findings pertaining to any given pollutant varied notably, as did findings within studies evaluating multiple outcomes.
3.1.3.1. Particulate matter
Most studies considering PM2.5 exposure reported an adverse association with at least one outcome of interest. Of the studies that estimated the association of PM2.5 with cognitive level or cognitive decline, two supported an adverse association with performance on a test of general ability;27,30 one supported an adverse association with verbal learning, but not general ability, executive function, logical memory, visual processing, visual episodic memory or semantic memory;38 another supported an adverse association with visuo-spatial ability, but not with episodic memory, executive function, semantic memory, or general ability;47 and yet another supported an adverse association with reasoning (but not memory or verbal fluency) in cross-sectional analyses while also reporting associations with decline in memory (but not reasoning or verbal fluency) in longitudinal analyses.49 The final study found support for an adverse association with faster decline in general ability and in performance on most individual cognitive tests.26 The first neuroimaging study reported an association of higher PM2.5 exposure with normal-appearing white matter volumes, but no association with grey matter, ventricular, hippocampal, or basal ganglia volumes.36 The second reported associations of higher PM2.5 exposure with lower total cerebral brain volume and greater risk of covert brain infarcts, but not hippocampal volume or white matter hyperintensity burden.53 Finally, neither study of PM2.5 and incident cognitive impairment found support for an adverse association,39,41 but the study using a quasi-time-series approach suggested an adverse association between higher PM2.5 levels and rates of hospitalization for dementia or Alzheimer’s disease.40
Fewer studies evaluated PM10 or PM2.5–10. Of the studies of PM10 exposure and cognitive level or decline, two found no association;34,46 one reported an adverse association with visuospatial ability but not general ability, memory, or executive function;47 and another reported an adverse cross-sectional association with reasoning, but not memory or verbal fluency, while also reporting an association with longitudinal decline in memory, but not reasoning or verbal fluency.49 The final study supported adverse associations of PM10 and PM2.5–10 exposures with faster decline on general ability and test-specific performance.26
3.1.3.2 Traffic-related air pollution
Almost all studies considering traffic-related air pollution (NO2, NOx, distance to road, BC, or traffic particulate matter) provide some support for an association with between exposure and a dementia-related outcome. Five of the six studies of traffic-related air pollution and cognitive level reported an adverse association of exposure with some measure of cognitive performance, with variation in the specific findings similar to that noted above for PM.38,45–47,49,50 Yet the one paper of the six that evaluated associations with both cognitive level and cognitive decline yielded mixed results; while the findings supported an association of traffic-sourced PM with lower cognitive level, it provided little support for an association with cognitive decline.49 Both studies of NO2 or NOX and incident cognitive impairment supported an adverse association.29,33
3.1.3.3. Ozone
Only three studies reported specifically on the association between O3 and dementia-related outcomes. The two studies of cognitive level were split, with one noting an adverse association34 and the other reporting no association.38 A third study reported greater risk of an ICD-9-CM-based dementia diagnosis with higher O3 exposure.39
3.2. Quality assessment and risk of bias
Table 2 provides the results of the study-specific bias assessment. Appendix D contains a narrative justification of noted limitations. For most reports, study quality was adequate to exemplary. Below, we discuss consequential limitations, as well as general challenges and sources of bias.
Table 2.
Assessment of study quality
| (Citation)/Cohort | Study Limitations | Justification for Noted Limitations | |||||
|---|---|---|---|---|---|---|---|
| Exposure Assessment and Variability | Outcome Assessment | Other Study Design Features | Adjustment for Confounding | Inclusion Criteria/Loss to Follow-Up | May Not Be Generalizeable | ||
| Analyses of Cognitive Level | |||||||
| (Ailshire and Crimmins, 2014)/HRS | X | No individual-level exposure assessment; restricted to regions near regulatory monitors. | |||||
| (Ailshire and Clarke, 2015) /ACL Survey | X | X | X | No individual-level exposure assessment; restricted to regions near regulatory monitors; insensitive test of cognition will likely only pick up highly impaired; crude age and education adjustment. | |||
| (Chen and Schwartz, 2009)/NHANES III | X | X | No individual-level exposure assessment; restricted to regions near regulatory monitors; adjusted for age in 10-year bands, different adjustment for socioeconomic status across exposures, specifically some models of PM10 not adjusted for both race/ethnicity and socioeconomic status. | ||||
| (Gatto et al., 2014)/WISH, BVAIT, and ELITE | X | X | X | Only modest capture of local exposure gradients; cohort was extremely healthy for age due to inclusion/exclusion criteria of original randomized controlled trials. | |||
| (Power et al., 2011b)/NAS | X | Difficult interpretation due to use of one baseline exposure and multiple cognitive follow-ups over up to 11 years after baseline. | |||||
| (Ranft et al., 2009)/SALIA | X | X | Relatively little exposure variability in recent exposure for rural participants; modest capture of local exposure gradients; crude adjustment for age and socioeconomic status. | ||||
| (Schikowski et al., 2015)/SALIA | X | Relatively little variation in PM across study participants. | |||||
| (Tonne et al., 2014)/Whitehall II | X | Relatively little variation in total PM10 and total PM2.5 across study participants; no individual-level exposure assessment. | |||||
| (Wellenius et al., 2012a)/MOBILIZE Boston | X | Lack of information on loss to follow-up despite use of repeated measures for cross-sectional analysis. | |||||
| (Zeng et al., 2010)/CLHLS | X | API is a crude measure combining multiple air pollutants with variable correlation, measured at the community level. | |||||
| Analyses of Neuroimaging Level | |||||||
| (Chen et al., 2015) /WHIMS-MRI | X | X | ~11% of the cohort were missing >40% of PM2.5 data for the exposure assessment period and point estimates are attenuated, but remain statistically significant when excluding this group; no comparison of MRI sub-cohort to full cohort. | ||||
| (Wilker et al., 2015)/FOS | X | No comparison of MRI sub-cohort to full cohort. | |||||
| Analyses of Cognitive Change | |||||||
| (Tonne et al., 2014)/Whitehall II | X | X | Relatively little variation in total PM10 and total PM2.5 across study participants; no individual-level exposure assessment; did not report whether they adjusted for time*covariate interactions in analyses of cognitive change; no individual level exposure metrics. | ||||
| (Weuve et al., 2012)/NHS | X | No discussion of correlates of attrition during follow-up. | |||||
| Analyses of Incident Cognitive Impairment | |||||||
| (Chang et al., 2014)/NHIRD Taiwan | X | X | X | X | X | X | No individual-level exposure estimates; exposure averaging period depended on date of censoring; use of ICD-9-CM codes for identification of dementia; youngest participants not at risk of dementia given <65 years of age for duration of follow-up; no adjustment for education and inappropriate adjustment for multiple potential mediating health conditions in all presented models; no information on attrition or its correlates; inclusion criteria required respiratory tract infection, which may have resulted in selection of sicker or more susceptible persons. |
| (Jung et al., 2015)/NHIRD Taiwan | X | X | X | X | X | No individual-level exposure estimates; use of ICD-9-CM codes for identification of dementia; association of change in exposure in relation to incident dementia is difficult to interpret and is susceptible to bias; no adjustment for education or socioeconomic status; no information on attrition or its correlates. | |
| (Loop et al., 2013)/REGARDS | X | X | X | No individual level exposure estimates; satellite source entails a large amount of missing data; non-standard interpretation given use of logistic regression in the presence of censoring; no information on correlates of attrition and requirement of completion of 2 cognitive assessments for inclusion in analysis. | |||
| (Oudin et al., 2016)/Betula | X | Exposures were predicted for 2009–2010, but outcome follow-up spanned 1993–2010; results using back-extrapolated exposure predictions were reported to be similar, but data not shown. | |||||
| Time Series Analyses | |||||||
| (Kioumourtzoglou et al., 2016) /Medicare | X | Use of ICD-9 codes for hospitalization for dementia or Alzheimer’s disease to define case status. | |||||
Abbreviations: ACL, Americans’ Changing Lives; BVAIT, B-Vitamin Atherosclerosis Intervention Trial; CLHLS, Chinese Longitudinal Healthy Longevity Survey; ELITE, Early Versus Late Intervention Trial; FOS, Framingham Offspring Study; HRS, Health and Retirement Study; ICD-9, International Classification of Diseases, Ninth Revision; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; MOBILIZE, Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly; MRI, magnetic resonance imaging; NHANES III, Third National Health and Nutrition Examination Survey; NHIRD, National Health Insurance Research Database; NHS, Nurses’ Health Study; PM, particulate matter; PM2.5, particulate matter with an aerodynamic diameter < 2.5 micrometers6; PM10, particulate matter with an aerodynamic diameter < 10 micrometers; REGARDS, Reasons for Geographic and Racial Differences in Stroke; SALIA, Study on the Influence of Air Pollution on Lung Function, Inflammation, and Aging; WHIMS-MRI, Women’s Health Initiative Memory Study Magnetic Resonance Imaging Study; WISH, Women’s Isoflavone Soy Health
3.2.1. Outcome assessment
Each dementia-related outcome has a distinct set of advantages and disadvantages. Cognitive test scores are informative and relatively easy to use in epidemiologic research, but are also inherently noisy, may have non-standard distributions with ceilings or floors, and are variably sensitive to differences in cognitive function across the range of normal to impaired. Tests within a battery often assess distinct yet related domains of cognition. Crucially, estimated associations with cognitive level, as assessed by cognitive test scores, are somewhat susceptible to confounding by sociocultural background. Estimated associations with cognitive change are less susceptible in this regard. This contrast may explain some of the heterogeneity of findings both within and across studies of cognitive level and cognitive decline. Despite the advantage of studying cognitive decline, only two studies have analyzed within-person cognitive change.26,49
Neuroimaging may provide insight into the underlying pathologic process, but the neuroimaging markers reported on thus far reflect only a subset of the known dementia-related pathologies. Notably, neither of the two neuroimaging studies36,53 was able to assess the association between air pollution and markers of pathologic accumulation of beta-amyloid or hyperphosphorylated tau, the pathologic hallmarks of Alzheimer’s disease. As with analyses of cognitive test scores, studies of within-person change in neuroimaging would provide stronger evidence than studies considering neuroimaging marker status at a single point in time, but no air pollution studies have yet adopted this design.
From a clinical perspective, incident dementia, including all-cause dementia and dementia subtypes (e.g., Alzheimer’s disease dementia), is arguably the most important outcome. However, repeated study-based clinical evaluation is essential for accurately capturing dementia status over time. As no surveillance system for dementia exists, and dementia is poorly documented in medical records and death certificates,56–58 reliance on these sources leads to substantial misclassification. In the Taiwan-based National Health Insurance Research Database, which was used for two of the studies of incident cognitive impairment in this review,33,39 the prevalence and incidence of dementia determined via medical claims data were one to two orders of magnitude lower than the prevalence and incidence observed in other settings with systematic evaluation59–62 suggesting potential for a striking degree of underdiagnosis in this setting. Underdiagnosis may not be independent of air pollution exposures. For example, persons with greater air pollution exposures are more likely to have cardiovascular or respiratory conditions.63,64 If having these air pollution-related conditions leads to more interaction with the medical system, persons with higher exposures who also have dementia may be more likely to obtain a clinical dementia diagnosis. In addition, misdiagnosis, even in a small percentage of non-demented persons, can further erode the validity of these records. In a US-based study,65 only 56% of participants identified as demented in Medicare data were diagnosed with dementia in study-based clinical evaluation (Figure 2). Such concerns limit our confidence in the findings from the three studies relying on ICD-9 codes to ascertain dementia.33,39,40
Figure 2.
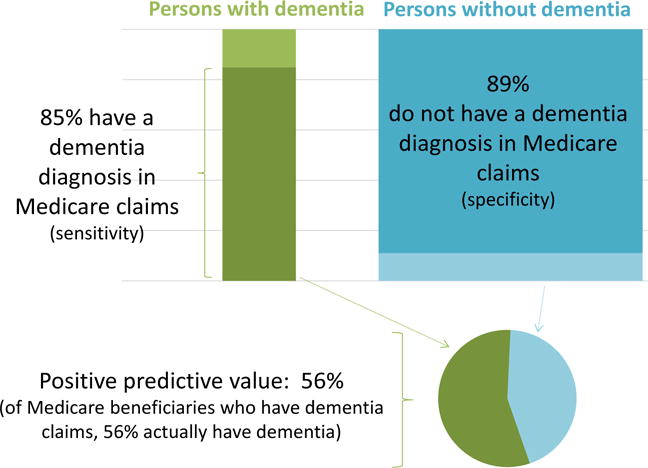
Accuracy of Medicare claims as a measure of dementia diagnosis (adapted from results reported by Taylor, Jr. DH, et al., J Alzheimer’s Dis 2009;17(4):807–816).
3.2.2. Exposure assessment
3.2.2.1. Timing
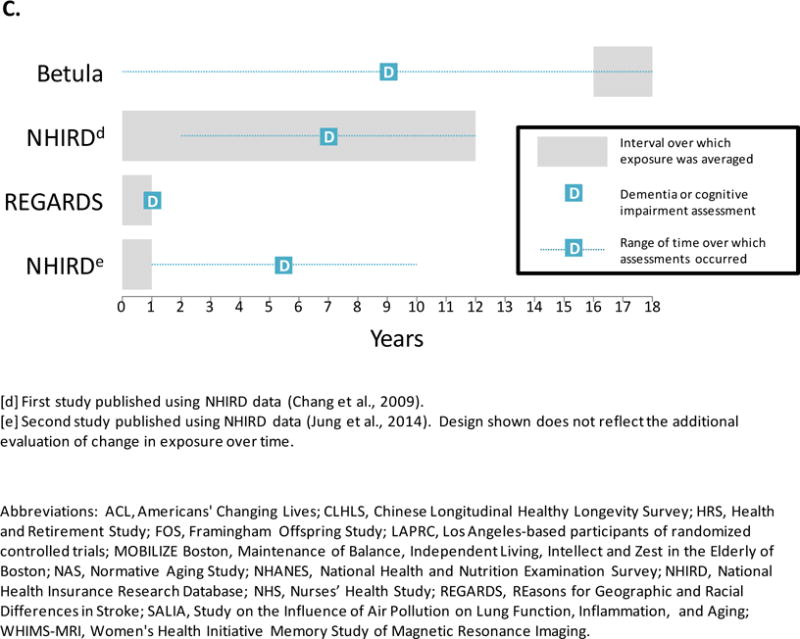
Dementia marks the end of a protracted period of pre-clinical accumulation of pathology and cognitive decline. Thus, the most relevant exposure period may be years to decades prior to dementia onset. Alternatively, the entire stretch of air pollution exposures from the distant past through the time of diagnosis may be relevant. Most of the studies in our review generated estimates of exposure averaged over one year prior to or concurrent with the year of outcome assessment (Figure 3). A few studies—including three of the four studies on incident impairment or dementia29,33,39 (Figure 3, Panel C)—used exposures estimated over intervals following the outcome assessments for some or all participants. In the most extreme case, the outcome assessment preceded the exposure window by 16 years.29 Therefore, almost all of the studies in this review implicitly assumed that current exposure levels are adequate surrogates for past exposure levels. This can be a strong assumption, especially over longer time intervals between the key exposure window and the health endpoint and in studies without consideration of residential mobility.
Figure 3. Designs of studies on air pollution exposure in relation to dementia-related outcomes.
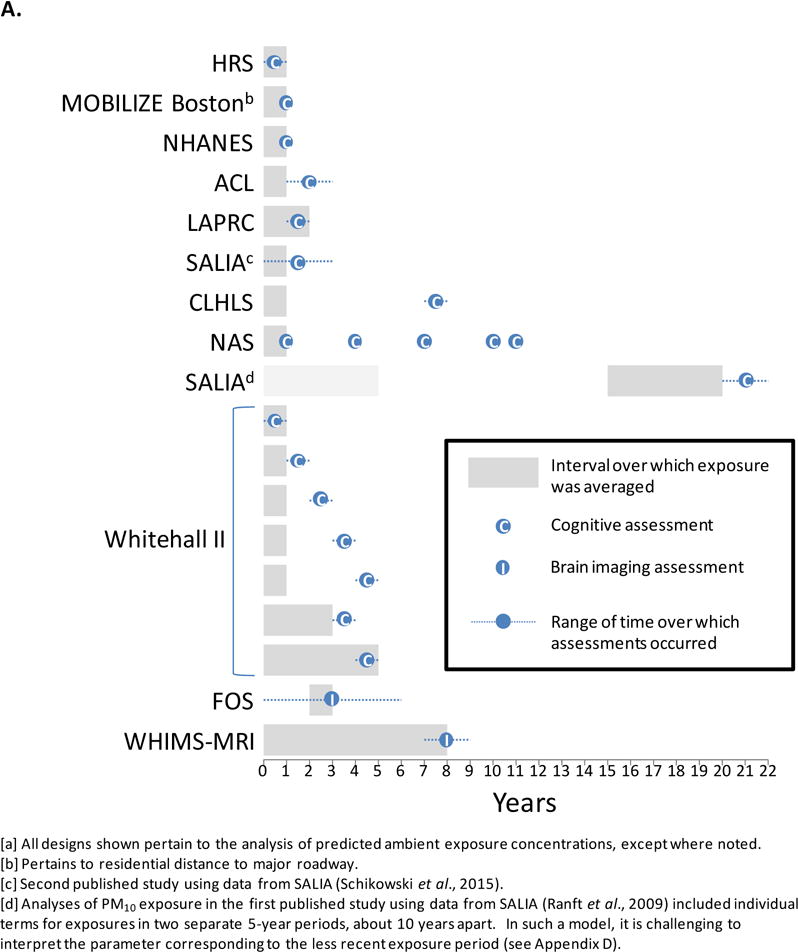
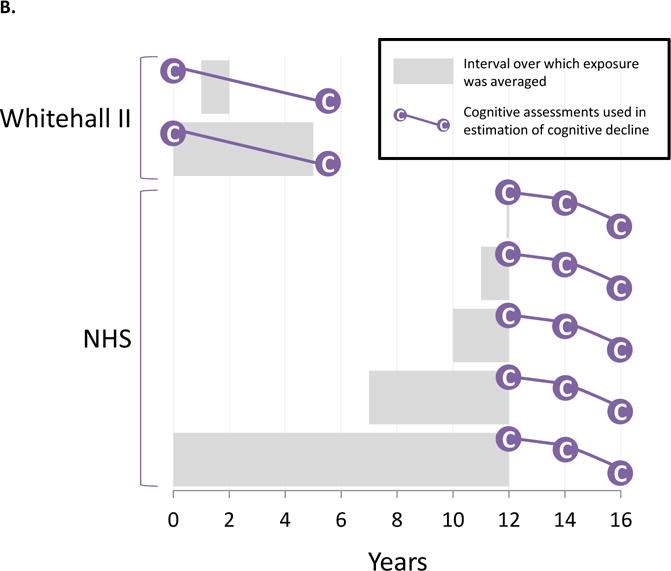
Panel A. Studies of cognitive level and magnetic resonance brain imaging.
Panel B. Studies on longitudinal cognitive decline.
Panel C. Studies of incident cognitive impairment and dementia.
3.2.2.2. Measurement error
Many studies assigned exposures at the level of each participant’s community, county, postcode or census tract. Although this area-based approach may be adequate for certain pollutants that are dispersed relatively homogenously across space (e.g., PM2.5), it less accurately measures small-scale gradients in pollutant concentrations influenced by local sources (e.g., BC, NOx, and O3) or pollutants with shorter atmospheric residency times (e.g., PM2.5–10). Use of more sophisticated statistical and deterministic modeling approaches to predict exposures at participants’ residential addresses helps to address this issue. Such methods typically have modest to strong predictive power, so they may reduce, but not totally eliminate, exposure misclassification.66 Validation exercises can provide insight into the likely magnitude of exposure estimation error, by pollutant and method. Nonetheless, measurement error may possibly explain some heterogeneity observed in cognitive effect estimates for different pollutants and from different studies, geographic regions, and time periods.
3.2.2.3. Exposure variability
The range of exposure (actual and estimated) in a study is determined by a variety of factors, notably the spatial variability in the air pollutant of interest, the geographic region considered, and the resolution of the exposure assessment method. Greater variability in exposure increases power to detect a true health effect of air pollution. In the literature reviewed herein, exposure variability differed markedly across studies, even across studies of the same pollutant. For example, coefficients of variation (CVs) ranged from 0.0547 to 0.3026 in studies considering PM2.5. While these studies differ in other ways, those with lower CVs36,41,47,49,53 were more likely to report mixed or largely null findings than those with the largest CVs.26,27,30,38,39
3.2.2.4. Non-linear associations
Many studies of air pollution and cognition assume a linear relationship between the two, but others suggest that the steepest increase in risk accrues at lower levels of exposure.27,29,45 Although confounding or selection bias could induce such a pattern, it is also possible that the relationship is, in fact, non-linear as has been proposed for other health effects of environmental toxicant exposures.67–69
3.2.3. Confounding
3.2.3.1. Appropriate adjustment
Several studies either failed to adjust for or made very crude adjustments for sociodemographic factors,30,33,34,39,46 which are potentially moderate to strong confounders. Although cardiovascular conditions are possible intermediates of air pollution’s relation to dementia-related outcomes, several studies failed to report adjusted analyses without adjustment for these factors.33,39,46 However, in multiple studies reporting both, results were typically consistent,26,27,29,30,34,36,40,53 providing preliminary evidence against a mediating effect by cardiovascular disease. Nonetheless, a formal mediation analysis (e.g.,70–72), with careful attention to the measurement and modelling of the mediator, is warranted.
It is also under-recognized that analyses of cognitive change must include terms both for the main effect of the covariate and its cross-product with time if that covariate influences cognitive change. Only one26 of the two studies26,49 of cognitive change explicitly addressed this aspect of covariate adjustment.
Finally, it must be noted that because a dementia diagnosis depends on both one’s achieved cognitive ability and subsequent decline, analyses of incident dementia or cognitive impairment are susceptible to confounding by factors correlated with both air pollution exposures and achieved ability. For example, cognitive scores are typically much higher among those with greater educational attainment, but a similar association does not hold with change in cognition.73,74 This may explain some of the heterogeneity in the findings and again argues for the need for studies of within-person change.
3.2.3.2. Sensitivity to unmeasured or residual confounding
To test the hypothesis that an unmeasured confounder (e.g. socioeconomic disadvantage, other air pollutants) could plausibly explain the reported associations between air pollution and cognition, we conducted a post hoc sensitivity analysis to quantify the characteristics of an unmeasured confounder U that would be required to induce: (1) the difference in cognitive test performance on the Telephone Interview for Cognitive Status (TICS) from the first to third quartiles of PM2.5 exposure in the Health and Retirement Study (HRS);27 and (2) the difference in rate of cognitive change contrasting the lowest and highest quintiles of long-term average PM2.5–10 exposure in the Nurses’ Health Study (NHS).26 Assuming U had an effect on the outcome equivalent to that of being 5 years older, the difference in the prevalence of U across exposure groups would have to be 48 percentage points—equivalently, a minimum odds ratio (OR) of 13.3—to account for the results in the HRS; or 57 points—equivalently a minimum OR of 8.1—to account for the observed association in the NHS (Table 3). If U had a stronger cognitive effect, equivalent to that of being 10 years older, the difference in prevalence of U across levels of the exposure would have to be 29 percentage points (minimum OR, 3.1) or 24 points (minimum OR, 2.7), respectively, to fully account for the results in the HRS and the NHS. To account for the observed associations, these prevalence differences would have to persist after adjustment for other factors.
Table 3.
Post hoc sensitivity analyses of reported associations to bias due to an unmeasured binary confounder (U).
| Cohort (Study) | HRS (Ailshire and Crimmins, 2014) | NHS (Weuve et al., 2012) | ||||
|---|---|---|---|---|---|---|
| Cognitive outcome | total TICS score | 2-year change in global cognitive score | ||||
| Exposure contrast | 3rd versus 1st quartile of PM2.5 exposure | Highest versus lowest quintile of PM2.5–10 exposure | ||||
| Reported results | ||||||
| Effect estimate (exposure → outcome) | −0.43 units | −0.024 units | ||||
| Difference in outcome per year of agea | −0.15 units | −0.01 units | ||||
| Sensitivity analysis specifications and results | ||||||
| Hypothetical relation of U to cognitive outcome (U → outcome) | ||||||
| Difference in cognitive outcome, U=l vs U=0 | −0.3 units | −0.75 units | −1.5 units | −0.02 units | −0.05 units | −0.1 units |
| Years apart in age associated with the same difference in outcomea | 2 years | 5 years | 10 years | 2 years | 5 years | 10 years |
| Resulting relation of U to exposure (U → exposure) required to produce the reported effect estimate | ||||||
| Difference in the prevalence of Ub, high vs low exposure | >100% | 57% | 29% | >100% | 48% | 24% |
| Equivalent minimumb relative odds (OR) of high exposure, U=l vs U=0 | ∞ | 13.3 | 3.3 | ∞ | 8.1 | 2.7 |
To provide context for the magnitude of their exposure-outcome associations, studies reported the difference in cognitive outcomes corresponding to differences in participant age. For example, in Ailshire and Crimmins (2014), the 0.43-unit average decrement in cognitive score among persons in the 3rd quartile of exposure compared with the lowest quartile was equivalent to the difference in cognitive scores observed between persons who were nearly 3 years (−0.43/−0.15) apart in age. We used this age-based meter to contextualize the hypothesized U-cognitive outcome association in our sensitivity analyses.
Prevalence differences are in units of percentage points (i.e., they are absolute not relative differences). Given the difference in U prevalence shown, the minimum OR of high exposure is the smallest OR across all possible pairs of U prevalences.
An effect of U on cognitive performance equivalent to that of 5 or 10 years of age is large, but it falls within range of possible effect sizes for socioeconomic or sociodemographic factors. However, in the HRS, prevalence differences in socioeconomic and sociodemographic factors exceeding 25 percentage points from the first to third quartile of PM2.5 appear implausible given the reported differences in these factors across quartiles of exposure. For example, the difference in the percentage of non-white participants from the third to first quartile of PM2.5 exposure was only 8.5 points, and the corresponding difference in current smoking prevalence was 3.4 points.27 In the NHS, the reported distributions of an array of candidate confounders, including PM2.5 exposure, across quintiles of PM2.5–10 similarly suggest that such a vast prevalence difference is unlikely. For example, the lowest and highest quintiles of PM2.5–10 exposure differed in the prevalence of lowest quintile area-based median household income by only 3.4 points and the prevalence of lowest quintile median home value by 5.4 points.26
Given these characteristics, the most plausible class of unmeasured confounder that might account for the observed associations might be another pollutant or other environmental feature—one that is closely associated with the exposure of interest and is much more strongly associated than that exposure with the outcome. Based on our knowledge of air pollution and the built environment, we think it unlikely that such a factor exists.
3.2.4. Sample selection
Individuals who meet eligibility criteria for a study of air pollution and a dementia-related outcome, enroll in that study, continue in it, and enroll in sub-studies may differ in important ways from non-participants. These differences can bias results in two ways: the first emanates from conditioning on an intermediate variable, and the second is classical selection bias.75
3.2.4.1. Conditioning on an intermediate at enrollment
If poor health mediates the air pollution-dementia effect, excluding or under-enrolling persons with air pollution-related health problems (notably poor cardiovascular health) may amount to conditioning on an intermediate, which would be expected to bias study results towards the null.75 One example is the study that re-used data from three randomized controlled trials,38 from which enrollment criteria excluded persons with a variety of health conditions, including cardiovascular conditions or risk factors. Similarly in SALIA, compared with non-participants, participants who agreed to cognitive assessment 27 years after baseline enrollment appeared healthier than the participants of the original cohort.46 Distinct from limiting generalizability, selection based on air pollution-related mediators of dementia serve to potentially underestimate the total effect of air pollution exposure on dementia-related outcomes and likely contributed to the largely null findings from the re-analysis of the randomized controlled trials.38
3.2.4.2. Selection bias
Bias in the estimated association between air pollution exposure and dementia-related outcome can also result if enrollment or continued participation is related to both exposure and cognitive health, either directly or through common causes of participation and either the exposure or outcome.75 Air pollution exposure increases risks of both morbidity and mortality,63,64,76,77 two major forces shaping enrollment and continuation, and cognitive status is generally associated with participation in epidemiologic studies.78,79 If this combination of scenarios holds, those with the highest exposures and worst cognition are least likely to participate or continue participation. The expected resulting bias—running in the direction of benefit—cannot account for the observed adverse associations, although it may contribute to the null results observed. To partially or fully address possible selection bias, several studies reviewed herein assessed the relation of air pollution and/or cognitive outcomes to participation, and generally reported either no associations or associations with participation as described above, providing further support that this logic holds in this body of literature (e.g.,27,29,44,46,49). That said, most studies did not report sufficient information on the correlates of enrollment, especially for enrollment into sub-studies,36,53 or loss to follow-up,26,33,39,41,50 for drawing strong conclusions about the potential for selection bias in their particular settings.
We highlight two situations in which selection bias may warrant particular concern. First, studies with older baseline ages may disproportionately represent “healthy survivors,” because the probability of surviving and being free of severe disability—effects or correlates of air pollution exposure and cognitive status—diminishes with older age. As a result, associations of air pollution with cognitive outcomes may be “muted” in increasingly older cohorts.80 Second, for similar reasons, selection bias can plague brain imaging studies,81 because the technical and logistical demands of the procedures favor those with greater mobility and without contraindications for the procedures. It is not unusual for about half of all initially eligible persons to complete a MRI evaluation,81,82 and completion is often linked to cognitive status.81 For example, compared with those who did not undergo scanning for Women’s Health Initiative Memory Study Magnetic Resonance Imaging study (WHIMS-MRI), the 61% who agreed to MRI had significantly better cognitive function and less previous cognitive decline, on average, even after correcting for differences in age and education.83 Thus, in studies of air pollution exposure’s association with imaging outcomes, it is critical to either demonstrate that selection is not also linked to exposure or to take measures to correct any attendant bias.81
3.2.5. Generalizability
Even if the selection process leads to internally valid findings, those findings may not necessarily generalize to other populations. For example, the neurocognitive effects of air pollution may vary by chronic disease status; or by time and region, as a function of co-exposures such as other toxicants and diet.
3. DISCUSSION
A direct causal effect of air pollution on cognition is biologically plausible. Animal data indicate that PM may reach the brain via circulation or, bypassing the multifaceted blood-brain-barrier, via direct translocation through the olfactory bulb.84–86 In experimental, animal, and postmortem studies of animals and humans, exposures to PM and gaseous pollutants have been linked to facets of multiple pathways crucial to dementia pathogenesis.87–108 Ambient pollutants also could affect the brain indirectly. Most notably, exposure to PM2.5 and other pollutants have established cardiovascular effects,64,109–112 and because cardiovascular and cerebrovascular disease appear to promote cognitive decline and dementia,89,113,114 air pollutants could impair cognition even without reaching the brain parenchyma. Nonetheless, data supporting this pathway remains elusive.
Reductions in air pollution exposures as a consequence of environmental regulation or technological innovation have been shown to have significant impacts on the cardiovascular and respiratory health at the population level.115 For example, existing regulations under the US Clean Air Act prevent an estimated 160,000 premature deaths, 130,000 heart attacks, 1.7 million asthma attacks, and 86,000 hospital admissions each year.116 Yet associations between air pollution and poor health are still detectable at exposure levels below current regulatory standards (e.g.,117). If air pollution exposure contributes to dementia risk then further widespread reductions in air pollution exposure might also prevent or delay millions of dementia cases.
The existing epidemiologic evidence on air pollution exposure’s cognitive effects in older age is highly suggestive, as almost all studies reported at least one adverse association between air pollution exposure and a dementia-related outcome. Though most studies have at least one notable limitation, a single shared limitation appears unlikely to account this pattern. In particular, we emphasize that our review suggests residual confounding is unlikely to account for the consistently positive results so far observed. Similarly, selection bias also cannot account for the observed adverse association provided the expected results of selective participation – that those least likely to participate are those with the highest exposures and worst cognition – holds in each of the individual studies. However, the evidence is too inconsistent and insufficient for concluding which pollutant is most relevant.
Most of the studies of cognition and cognitive decline employed measures of “general” or global functioning, many designed to capture the functions that decline in late-life dementia. Specific cognitive domains were evaluated by a few studies, but the representation of multiple specific domains remains too diffuse to conclude that air pollution differentially affects functioning in any particular domain and, by extension, a specific type of dementia or neuropathology. Setting aside the sparseness in the available data, there are limitations in trying to tease out domain-specific patterns this line of research. The degree to which dementias in older adults present as tidily distinct subtypes is increasingly in doubt.19 More common than the occurrence of Alzheimer’s pathology alone is the co-occurrence of pathologies from Alzheimer’s along with pathologies from other dementias,20,21 and the convention of diagnosing a dementia as Alzheimer’s by a process of exclusion is giving way to the recommendation to diagnose Alzheimer’s even if deficits indicative of others dementias accompany Alzheimer’s-typical deficits.18 Furthermore, although the Alzheimer’s dementia phenotype is defined as disorder of episodic memory, data to date suggests that rather than declining earliest and most rapidly, episodic memory may decline roughly in tandem with functioning in other domains—such as working memory, visuospatial ability, semantic memory, and perceptual speed.118
In spite of these blurred distinctions, some mechanistic insights could arise from research on air pollution’s effect on specific cognitive domains. Evidence from imaging, autopsy, chamber and animal studies might be useful for informing and interpreting this research. Epidemiologic research still serves as a complement to the serious limitations of controlled studies. Experimental studies in animals may help answer questions about potential mechanisms, but translating findings from animal studies to the human experience with dementia remains fraught with nonequivalency.119–121 Controlled studies of humans are constrained to evaluating the acute effects of short-term exposures, and a randomized controlled trial of long-term outdoor air pollution levels is simply not feasible. Thus, epidemiologic studies, such as those reviewed herein, will remain vital to answering questions about the potential effect of outdoor air pollution on cognitive decline and dementia.
Further epidemiologic investigation with improvements in design, analysis, and reporting would fill key evidentiary gaps and provide a solid foundation for future recommendations and possible interventions. Studies of within-person change provide more compelling evidence than the other study designs reviewed here, yet few have been published. Thus, research looking at the relation of a comprehensive set of air pollutants to within-person cognitive or neuropathological change would be a welcome addition. It is extremely important to note that investigating the determinants of dementia is complicated by the nature of the disease, but that many common study-level limitations can be avoided with careful analysis and adequate reporting. Thus, we advocate that all future studies adopt the MELODEM checklist80 in reporting their associations between air pollution exposures and dementia-related outcomes.
Highlights.
18 epidemiologic studies have evaluated air pollution and dementia-related outcomes.
Most reported ≥ 1 adverse association of an exposure with one of these outcomes.
Differential selection and confounding probably do not explain many of these results.
Accurate identification of dementia cases remains a major challenge.
The relevant etiologic window and most toxic agents are key gaps in the data.
Acknowledgments
The authors are grateful to Timothy English for his help in checking the accuracy of table entries and references.
Funding
Melinda C. Power was supported by the NIA (T32 AG027668). Jeff D. Yanosky was supported by NIH/NIEHS (R01ES020836-02 and R01ES019168). Jennifer Weuve was supported by the NIH/NIEHS (R21ES020404 and R21ES24700).
APPENDIX A: Database Search Terms
| DATABASE | PUBMED |
|---|---|
| STRATEGY | #1 AND #2 AND #3 AND #4 AND #5 NOT #6 |
| #1 Disease | “dementia”[mesh:noexp] OR “alzheimer Disease”[mesh] OR (“dementia”[tw] OR “alzheimer”[tw] or “alzheimers”[tw] or “alzheimer’s”[tw]) OR “Mild Cognitive Impairment”[Mesh] OR “cognitive decline” OR “neuropsycholog*” OR cognit* OR “cognitive change” OR “cognitive aging” OR “cognitive impairment” OR “neurobehavioral” |
| #2 Outcome | “risk”[mesh] OR “incidence”[mesh] OR (“risk”[tw] OR “incident”[tw] OR “incidence”[tw] OR “onset”[tw] OR “prevent”[tw] OR “prevents”[tw] OR “prevented”[tw] OR “cause”[tw] OR “causes”[tw] OR “caused”[tw] OR “effect”[TW] OR “associated”[TW] OR “association”[TW] OR “protect”[TW] OR “protects”[TW] OR “protected”[TW] OR “protective”[TW] OR “harm”[TW] OR “harms”[TW] OR “harmful”[TW] OR “develop”[TW] OR “develops”[TW] OR “developed”[TW]) |
| #3 Study Design | “intervention studies”[mesh:noexp] OR “clinical trials as topic”[mesh] OR “cohort studies”[mesh:noexp] OR “longitudinal studies”[mesh] OR “case-control studies”[mesh:noexp] OR “Health Surveys”[Mesh:noexp] OR (“longitudinal”[tw] OR “longitudinally”[tw] OR “prospective”[tw] OR “prospectively”[tw] OR “follow”[tw] OR “followed”[tw] OR “follow-up”[tw] OR “follow up”[tw] OR “cohort”[tw] OR “later”[tw] OR “case control”[tw] OR “case-control”[tw] OR “clinical trial”[tw] OR “controlled trial”[tw] OR “intervention study”[tw] or “intervention studies”[tw] or “cross-sectional”[tw] OR “regression”[tw] OR “association”[tw]) |
| #4 Exposure | “Air Pollution”[Mesh] OR “Particulate Matter”[Mesh] OR “Nitrogen Dioxide”[Mesh] OR “Ozone”[Mesh] OR “Volatile Organic Compounds”[Mesh] OR “Sulfur Dioxide”[Mesh] OR “Carbon Monoxide”[Mesh] OR “Vehicle Emissions”[Mesh] OR “distance to road”[tw] OR “PM10” [tw] OR “PM2.5” [tw] OR “traffic-related air pollution” [tw] OR “air pollution” [tw] OR “particulate matter” [tw] OR “ozone”[tw] OR “nitrogen dioxide”[tw] OR “particulates” [tw] OR “black carbon” [tw] OR “traffic pollution” [tw] OR “residential distance to nearest major”[tw] OR “traffic-related PM”[tw] |
| #5 Database Archive Date |
Initial search: Entrez date – through 2014/12/31 Update search: Entrez date – 2015/01/01 to 2015/08/10 |
| #6 (NOT) Exclude Irrelevant | “mice”[ti] OR “mouse”[ti] OR “rat”[ti] OR “rats”[ti] OR “cells”[ti] OR “plasticity”[ti] OR “synaptic”[ti] OR “signaling”[ti] OR “children”[ti] OR “children’s”[ti] OR “infant”[ti] OR “infants”[ti] OR “pediatric”[ti] OR “adolescent”[ti] OR “in vivo”[ti] OR “in vitro”[ti] OR “smoking”[ti] OR “smoker”[ti] OR “second hand smoke”[ti] OR “second-hand smoke”[ti] OR “smokers”[ti] OR “environmental tobacco”[ti] OR “cigarette”[ti] OR “tobacco”[ti] OR “secondhand”[ti] OR “childhood”[ti] OR “adolescents”[ti] OR “adolescence”[ti] OR “child”[ti] OR “preschool”[ti] OR “prenatal” |
| DATABASE | EMBASE |
|---|---|
| STRATEGY | #1 AND #2 AND #3 AND #4 AND #5 NOT #6 |
| #1 Disease | (‘dementia’/de OR ‘alzheimer disease’/de OR ‘frontotemporal dementia’/de OR ‘multiinfarct dementia’/de OR ‘presenile dementia’/de OR ‘senile dementia’/de OR dementia OR alzheimer* OR ‘mild cognitive impairment’/exp OR ‘mci’:ab,ti OR ‘cognitive decline’:ab,ti OR neuropsycholog*:ab,ti OR cognit*:ab,ti OR ‘cognitive change’:ab,ti OR ‘cognitive aging’:ab,ti OR ‘cognitive impairment’:ab,ti OR ‘neurobehavioral’:ab,ti) |
| #2 Outcome | (‘risk’ OR ‘risk factor’ OR ‘population risk’ OR ‘attributable risk’)/de OR (risk OR inciden* OR onset OR prevent* OR associat*):ti,ab |
| #3 Study Design | ‘clinical trial’/exp OR (‘intervention study’ OR ‘cohort analysis’ OR ‘longitudinal study’ OR ‘prospective study’ OR ‘evaluation and follow up’ OR ‘follow up’ OR ‘case control study’ OR ‘population based case control study’ OR ‘controlled study’ OR ‘major clinical study’)/de OR (longitudinal* OR prospective* OR follow* OR associate* OR follow-up OR ‘follow up’ OR cohort OR later OR ‘case control’ OR ‘case-control’ OR ‘clinical trial’ OR ‘controlled trial’ OR ‘intervention study’ OR ‘intervention studies’ OR ‘cross-sectional’ OR ‘regression’):ti,ab |
| #4 Exposure | ‘air pollution’/de OR ‘air pollutant’/de OR ‘particulate matter’/exp OR ‘nitrogen dioxide’/exp OR ‘ozone’/exp OR ‘volatile organic compound’/exp OR ‘sulfur dioxide’/exp OR ‘exhaust gas’/exp OR ‘distance to road’:ab,ti OR ‘pm10’:ab,ti OR ‘pm2.5’:ab,ti OR ‘traffic-related air pollution’:ab,ti OR ‘air pollution’:ab,ti OR ‘particulate matter’:ab,ti OR ‘ozone’:ab,ti OR ‘nitrogen dioxide’:ab,ti OR ‘particulates’:ab,ti OR ‘black carbon’:ab,ti OR ‘traffic pollution’:ab,ti OR ‘residential distance to nearest major’:ab,ti OR ‘traffic-related pm’:ab,ti |
| #5 Database & Archive Date |
Initial search: EMBASE ONLY, ADD DATE RESTRICTION – through 2014/12/31 Update search: EMBASE ONLY, ADD DATE RESTRICTION – 2015/01/01 to 2015/08/10 |
| #6 (NOT) Exclude Irrelevant | (‘mice’ OR ‘mouse’ OR ‘rat’ OR ‘rats’ OR ‘cells’ OR ‘plasticity’ OR ‘synaptic’ OR ‘signaling’ OR ‘children’ OR ‘infant’ OR ‘infants’ OR ‘pediatric’ OR ‘adolescent’ OR ‘in vivo’ OR ‘in vitro’ OR ‘smoking’ OR ‘smoker’ OR ‘second hand smoke’ OR ‘second-hand smoke’ OR ‘smokers’ OR ‘environmental tobacco’ OR ‘cigarette’ OR ‘tobacco’ OR ‘secondhand’ OR ‘childhood’ OR ‘adolescents’ OR ‘adolescence’ OR ‘child’ OR ‘preschool’ OR ‘prenatal’):ti |
APPENDIX B: Data Extracted On Each Eligible Article
Cohort Name
Geographic Area
Sample Size
Follow-Up Time
Exclusions
Total Number Excluded
Percent Excluded
Age of Participants at Outcome Assessment/Baseline Outcome Assessment
Race/Ethnicity
Exposures Considered
Exposure Considered, Detailed
Exposure Assessment Method, Brief
Exposure Assessment Method, Detailed
Timing/Averaging Periods Considered
Exposure Parameterization
Reported Exposure Characteristics
Calculated Exposure Characteristics
Univariate Association of Exposure With Confounders
Outcome
Outcome Assessment, Brief
Outcome Assessment, Detailed
Summary Statistics for Cognitive Outcome
Exposure Period
Cognitive Outcome Period
Regression Model
Estimate, 95% Confidence Interval, P-value
Adjustment Covariates
Sensitivity Analyses
Effect Modification
Study Design
Author Conclusions
Equivalency Reported
Summary of Study Findings
APPENDIX C: Study Quality Assessment Template
| 1 | Exposure problems, including misclassification | e.g., mistiming of exposure relative to outcome assessment, lack of adequate variability in exposure, poor exposure assessment method |
| 2 | Outcome problems, including misclassification | e.g., reliance on clinical databases, instrument grossly mismatched to participants’ abilities |
| 3 | Confounding, defined as bias due to unmeasured or poorly accounted for common causes (or correlates of such common causes) of the exposure and outcome of interest. | e.g., inadequate adjustment, overadjustment |
| 4 | Selection Bias – Cohort Formation, defined as bias which occurs when potentially eligible participants are not included in the study during enrollment in such a way that it leads to an association between the exposure and outcome that induces an association that would not have been present had those persons not been excluded | e.g., exclusion of persons with common chronic disease |
| 5 | Selection Bias – Loss to Follow-up, bias which occurs when potentially eligible participants are lost to follow-up in such a way that it leads to an association between the exposure and outcome that would not have been present had those persons not been lost | e.g. severe loss of participants (>25%) over the follow-up period in combination with lack of pertinent information on relation between exposure or outcome and loss |
| 6 | Generalizability, the expectation that the reported results would be consistent had the trial been completed in a second population of interest | e.g. highly selected population |
| 7 | Inappropriate Adjustments | e.g. main analyses adjusted for one or more possible intermediates |
| 8 | Interpretation Challenges | e.g. inappropriate statistical model, inappropriate study design |
APPENDIX D: Description of individual studies
Each of the individual studies which met eligibility criteria for inclusion in this review are described below. Additional text is also provided to give more context to the potential limitations noted in Table 2, which were identified during the individual-level bias assessment process.
Studies of Cognitive Level
In a nationally representative US sample of older adults (ages 50 to 102), the Health and Retirement Survey (HRS), census tract-level PM2.5 exposure in the year 2004 was associated with worse performance on the Telephone Interview for Cognitive Status (administered at the 2004 study visit) after adjustment for area-level and individual-level sociodemographic and socioeconomic characteristics.27 The association was non-linear, as the strongest association was reported with the third quartile of exposure for all measures of cognition, and was materially unchanged after additional adjustment for smoking and several health conditions which may mediate the air pollution-cognition association. Reassuringly, results remained consistent in sensitivity analyses designed to evaluate the potential for selection bias induced by study inclusion criteria, despite demonstrated associations between higher exposure to PM2.5 with missing cognitive data as well as worse cognitive function with missing PM2.5 data. The exposure assessment was restricted to regions with nearby regulatory monitoring and potentially limited by minimal capture of local exposure gradients by using inverse distance-weighted (IDW) interpolation of nearest regulatory monitors within 60 km of each participant’s census tract.
In a second US-based sample of older adults (ages >55), the 2001/2001 Americans’ Changing Lives (ACL) Survey, higher census tract-level PM2.5 exposure in the year 2000 was significantly associated with the number of errors on a brief screening test of cognition used to identify persons with cognitive impairment, the Short Portable Mental Status Questionnaire.30 Potential limitations of the study include: the limited spatial resolution of the exposure assessment, which followed the approach used for the HRS study described above; the relatively crude cognitive outcome (while it is a valid and reliable instrument for identifying cognitive impairment, it is unlikely to identify those with subtle deficits); and crude adjustment for age and education.
One investigation considering a subset of the 1989–1991 Third National Health and Nutrition Examination Survey (NHANES III) adult participants ages 20–59, evaluated the impact of 1 year average residential census-tract level PM10 and residential county-level ozone exposure on performance on multiple cognitive tests.34 In models adjusted for age, sex, and race/ethnicity or age, sex, and individual-level socioeconomic status, there was little evidence to support an adverse association between PM10 and performance on any cognitive test. However, higher ozone exposures were associated with worse performance on the symbol-digit substitution and serial-digit learning tests in multiple models adjusting for individual-level sociodemographic and socioeconomic characteristics. Ozone results remained robust to additional adjustment for common medical conditions and indoor air pollution. The exposure assessment was restricted to regions with nearby regulatory monitoring and potentially limited by minimal capture of local exposure gradients by using inverse distance-weighted (IDW) interpolation of nearest regulatory monitors at the county level. One minor limitation of this study pertains to attempts to control confounding; models included crude adjustment for age, different sets of confounding adjustment were presented for each exposure, and estimates were not available for all outcomes under all levels of confounding adjustment, possibly suggesting difficulties with model fit. It is also worth noting that the young age of the cohort raises questions about whether any observed associations can be attributable to underlying dementia pathogenesis, although it is now recognized that this process begins many years prior to clinically-relevant cognitive symptoms.23,24
In combined data from the baseline study visit for three randomized controlled trials of participants in the Los Angeles basin, CA, US (mean age: 61 years), higher 2-year average residential address daily PM2.5 exposure was associated with worse verbal learning test performance, but was not associated with overall or other domain-specific cognitive performance.38 While there was a marginally significant association between the highest tertile of 2-year average residential address 8-hour maximum ozone and worse executive function performance, the data also report moderate exposure to ozone is associated with better logical memory performance. There was no association between 2-year average residential address daily NO2 and overall or domain-specific cognition aside from a marginally significant association between with the highest tertile of NO2 exposure and worse logical memory. Relative lack of consistency between the NO2 and PM2.5 results is surprising given their high correlation (r=0.8) and may argue towards either truly differential effects or chance findings. The relative lack of positive associations may also be attributable to the one major limitation of the study: bias or lack of generalizability due to exclusion of “unhealthy” individuals. Approximately 14% of otherwise eligible participants were excluded due to prevalent chronic disease, and randomized controlled trial study populations are themselves often healthier (and more affluent) than the corresponding subset of the general population with potential indications for the proposed treatment due to eligibility criteria and the recruiting process.122 As such, re-use of these RCT study samples effectively conditions on mediators of the air-pollution cognition association, muting the association,75 or, even if internal validity is retained, may result in lack of generalizability to other, more susceptible populations. A minor limitation, which may have also contributed to the null findings, was the minimal capture of local exposure gradients by using inverse distance-weighted (IDW) of nearest regulatory monitors within 100 km along with supplemental measures. However, exposures were assessed at the address level.
In the Normative Aging Study (NAS), a cohort of older white men (ages 51–97), higher residential address level estimated black carbon concentrations in the year prior to baseline cognitive testing were associated with worse overall cognitive performance and relatively low MMSE scores over repeated cognitive testing in multivariable-adjusted models.45 Additional adjustment for long-term lead exposure, a historical traffic-related exposure, attenuated associations with overall cognitive performance but not associations with low MMSE scores. One minor limitation of this study relates to the use of repeated measures of cognition, but only a single measure of exposure at baseline. The resulting interpretation of these estimates is therefore a bit difficult as it estimates the impact of baseline exposure on cognitive scores obtained anywhere from 0 to 11 years after the baseline exposure. Another potential related concern given the use of variable follow-up data relates to the potential for selection bias due to loss-to-follow-up. However, reassuringly, those with fewer cognitive testing occasions typically had lower cognitive scores and higher BC, suggesting that removing any resulting selection bias would only strengthen estimates.
One study of women in the Study on the Influence of Air Pollution on Lung Function, Inflammation and Aging (SALIA) cohort considered the impact of area-level PM10 assessed at midlife and late life and residential distance to a busy road (>10k vehicles per day) on cognitive performance at ages 68–79.46 Closer residential distance to a busy road was associated with worse performance on the CERAD cognitive battery in the full sample and on the Stroop test in the younger participants. There were no associations between PM10 and cognitive test performance. One minor limitation of this study is use of relatively crude adjustment for age and education. Additionally, the study authors include adjustment for multiple chronic health conditions which may act as intermediates and report models including multiple correlated exposures within the same model, including distance to road as well as midlife and late life PM10; however, these potentially extraneous adjustments appear to have little impact on study findings. One additional potential limitation of note is the relatively little exposure variation in late life PM10; 37% of participants (all rural participants) were estimated to have the same level of late life PM10 exposures. This may have stemmed from the small study area combined with the minimal apture of local gradients by use of nearest monitor within 8 km of residential address.
A second report from the SALIA cohort, using an expanded sample of women and alternate exposure assessment methods, suggests an alternate pattern of association.47 In this report, current traffic load on nearby major roadways was not associated with performance on the CERAD cognitive battery, MMSE, or any of the considered CERAD subtests. PM10, along with PM2.5, PM2.5 absorbance, NO2, and NOX appeared to be associated with performance on a figure copying task, although there was no evidence of an association with figure recall, which also assesses visuo-spatial ability. Of all pollutants considered, only NOx was also associated with worse performance on the Boston Naming Test (but was not associated with either of two other measures of semantic memory) and overall performance on the CERAD battery. There was no suggestion of an association between any pollutant and the MMSE or tests of episodic memory or executive function. Effect modification by age was not considered in this report, despite the previous report46 in this cohort suggesting stronger results in younger women. As with the previous report, one potential limitation of note is the relatively little exposure variation in PM exposures across cohort participants. The modelling of MMSE as a continuous variable may also lead to attenuated estimates given the known issue of unequal interval scaling (i.e. ceiling effects).123
In analyses considering 2007/2009 Whitehall II participants (mean age 66), higher exposure to PM10, PM2.5 and exhaust-related PM10 or PM2.5 over various lags and averaging periods spanning 0 to 5 years before cognitive assessment appeared associated with worse performance on measures of reasoning, but not tests of verbal fluency or memory.49 Notably, the exposure variation in non-exhaust related PM is relatively low compared to that in other studies, which may contribute to the null findings with PM, but not those with exhaust-related PM. The small variation may have arisen from limited capture of local gradients due to aggregation of fine-scale predictions (20 m by 20 m) to the postcode level.
In community-dwelling seniors (mean age 78) from the Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly Boston cohort (MOBILIZE Boston), cross-sectional analyses using repeated measures of air-pollution related exposures and cognition suggested that shorter distance to road was associated with worse performance on several individual measures of cognitive function, but not with risk of poor general cognition operationalized as an MMSE <26.50 Unexpectedly, modelled black carbon exposure, which is often used to assess traffic-related air pollution exposures, appeared associated with risk of poor general cognition, but was largely unassociated with the other individual measures of cognitive function that were more domain-specific. While the repeated measures design allows full use of the available data, no information on loss to follow-up was provided, and so the potential for selection bias remains unknown. However, such loss to follow-up is likely only to result in conservative estimates, as data from other settings suggests that those most likely to be lost are those with poor cognition and higher exposures.
In data from the 2002 Chinese Longitudinal Healthy Longevity Survey of Chinese elders (ages 65+), experiencing a 1-point higher level of exposure on the 7-point ordinal categorization of the air-pollution (API) index from 1995 was associated with slightly increased odds of cognitive impairment in 2002, assessed using a Chinese version of the MMSE.54 The primary limitation of this study relates to the use of the API. Although it does reflect the general degree of overall air pollution, its component pollutants are variably correlated, and so multiple mixtures of air pollutants may result in the same API score. Furthermore, the use of a 7-point ordinal characterization makes strong assumptions about the shape of the dose response and results in loss of potentially valuable information within each category.
Studies of neuroimaging marker status
In data from the WHIMS-MRI study, higher 7-year prior cumulative average PM2.5 exposures were associated with smaller total and regional volumes of normal appearing white matter in both minimally and fully adjusted models, but were not associated with gray matter volumes, ventricular volumes, hippocampal volumes, or volumes of the basal ganglia in models adjusted only for intracranial volume.36 The magnitude of the association between PM2.5 and normal-appearing white matter volumes were significantly reduced when excluding the approximately 11% of participants who had <60% data on PM2.5 exposures during the assessment period, although these reduced estimates remained statistically significance. Limited reporting was insufficient to fully understand how or why exposure data was unavailable, the correlates of missingness, or how much data on exposure was missing among those with at least 60% coverage. Similarly, the authors provide no information on the selection process from the larger WHIMS study into the WHI-MRI study or the relation of participation to exposure.
In the Framingham Offspring Study (FOS), higher PM2.5, but not closer residential proximity to a major road, was associated with greater risk of having a covert brain infarct as well as smaller total cerebral brain volume.53 Neither exposure was adversely associated with hippocampal volume or white matter hyperintensity volume; however, paradoxically, greater distance from a major road was associated with greater white matter hyperintensity volume (but not greater risk of severe white matter hyperintensity burden). As in the WHIMS-MRI study,36 the authors provide no information on the selection process from the larger FOS study into the WHI-MRI study or the relation of participation to exposure.
Studies of cognitive change
Contrary to the cross-sectional findings in Whitehall II participants described above, analyses in the same paper considering change in cognitive between the 2002/2004 and 2007/2009 study visits found average total PM2.5 and total PM10 exposure in the calendar year 4 years prior to the year of the 2007/2009 cognitive assessment appeared associated with decline in memory, but not reasoning or verbal fluency, in persons who had not moved during follow-up.49 Associations with exhaust-related PM exposure were directionally consistent but weaker and not statistically significant. There remains potential for significant residual confounding, given lack of statements attesting the models were adjusted for covariate*time interactions aside from the baseline age*time interaction. As with the cross-sectional study, there is also relatively little variation in total PM. The small variation may have arisen from limited capture of local gradients due to aggregation of fine-scale predictions (20 m by 20 m) to the postcode level.
In 19,409 participants of the Nurses’ Health Study (NHS), higher long-term exposure to multiple size fractions of PM (PM10, PM2.5, and PM2.5–10) appeared associated with faster cognitive decline on a composite measure of general cognition, as well as with faster decline in performance on individual cognitive tests.26 Lack of information on those lost to follow-up precludes understanding of potential selection bias due to attrition; however, any bias is likely to result in conservative estimates under the assumption that those lost to follow-up are likely to have the worst cognition and worst air pollution exposures.
Studies of incident dementia or poor cognition
Higher NO2 exposures were associated with greater incidence of ICD-9-CM defined dementia using data from the National Health Insurance Research Database of Taiwan (NHIRD Taiwan).33 However, this study suffers from multiple notable limitations. First, use of health system databases for identification of dementia is generally a poor dementia assessment method, leading to numerous false positives and false negatives, and this misclassification is potentially related to air pollution exposure levels given known associations between air pollution and chronic diseases that lead to clinical encounters. Second, as subset of cohort participants are simply too young to be at risk for dementia (under age 65 for the duration of follow-up). Third, exposure was assigned as the average exposure from 1989 through dementia diagnosis, censoring, or the end of the study. As such, the exposure averaging period is dependent on whether the participant had dementia and future exposures are used to predict prior risk, both of which may lead to non-causal interpretations or misleading results given time trends in exposure. Moreover, capture of local gradients was limited by use of regulatory monitors (74 for the country of 14,000 km2) within district of participant’s clinic. Fourth, there is no adjustment for education as well as inappropriate adjustment for multiple potentially mediating health conditions in all presented models. Finally, there is no information on attrition or its correlates, and inclusion criteria required reporting of a respiratory tract infection, which may be related to air pollution susceptibility and limit generalizability of study findings.
In a second study using data from the NHIRD Taiwan,33 higher exposure to ozone, but not PM2.5, in the year 2000 was associated with higher risk of Alzheimer’s disease, defined as the presence of two ICD-9-CM codes for Alzheimer’s Disease (AD), over up to 10 years of follow-up. In associations considering change in exposure levels over time and incident AD, less decline in exposure to both ozone and PM2.5 were associated with greater risk of incident AD; however this association is difficult to interpret, and may be biased, given the date of censoring determines the time period over which change in exposure levels are calculated. One significant limitation of the study includes use of ICD-9-CM codes to diagnose AD, which may lead to differential misclassification of dementia with respect to health status and other exposure-related characteristics and an arguably incomplete capture of incident AD given the small proportion of persons with such a diagnosis. Additional limitations include failure to adjust for education or other measures of lifetime socioeconomic status and lack of information on the degree of censoring and its correlates. Finally, exposure assessments were performed at the postal code level, and capture of local exposure gradients was constrained by use of IDW interpolation of three regulatory monitors within 25 km.
In the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, higher baseline PM2.5 exposures were not associated with greater risk of incident cognitive impairment.41 However, the interpretation of this finding may be largely attributable to use of logistic regression in the presence of censoring – essentially the comparison becomes one of whether the participant became demented versus the combined alternative of dead prior to dementia or alive and dementia-free. The authors also provide no information on the amount or correlates of censoring and require completion of at least two cognitive assessments for inclusion in analysis, which may result in an informatively selected sample. The exposure assessment may have also possible contributed to the null findings. By generating exposure predictions at the 10 km level, the approach may have limited capture of local exposure gradients. In addition, there is a large amount of missingness in satellite data used to derive exposure estimates.
In the Betula study, higher exposure to NOx was significantly associated with greater risk of incident dementia, diagnosed based on a combination of study visit data and medical records.29 Similar results were observed in analyses considering AD and vascular dementia subtypes. One potential limitation of this study is the timing of exposure assessment; long-term NOx exposures were assessed using the annual average from 2009–2010, the last year of dementia follow-up, which spanned from 1993 to 2010. While analyses based on back-extrapolated exposure data were reportedly similar, this data was not shown, and so strong conclusions from this study require assumptions that the rank-order of exposure from the exposure estimates available approximates the rank-order of the true etiologic time period of interest.
Other study designs
One study using a quasi-time series approach nested within Medicare fee-for-service hospital claims data reported that higher than expected year-to-year city-specific annual city-wide PM2.5 exposures are associated with higher than expected annual city-wide rates of hospital admission for dementia and Alzheimer’s disease.40 While an intriguing approach, this study does not provide strong evidence for a causal effect of PM2.5 on dementia. While requiring dementia or Alzheimer’s disease to be listed as the primary or secondary diagnosis is likely to increase specificity of using ICD-9 codes to identify persons with dementia, it remains possible that the study findings reflect impact of PM2.5 on other co-morbid conditions that lead to a hospitalization, rather than an effect of PM2.5 on dementia, especially given that persons with dementia are hospitalized more often for all types of causes.124
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare they have no actual or potential competing financial interests.
Contributor Information
Melinda C. Power, Email: power@gwu.edu.
Sara D. Adar, Email: sadar@umich.edu.
Jeff D. Yanosky, Email: jyanosky@phs.psu.edu.
Jennifer Weuve, Email: jweuve@bu.edu.
References
- 1.American Psychological Association. Neurocognitive Disorders. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 2013 [Google Scholar]
- 2.Linn RT, Wolf PA, Bachman DL, et al. The ‘preclinical phase’ of probable Alzheimer’s disease. A 13-year prospective study of the Framingham cohort. Arch Neurol. 1995;52(5):485–490. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- 3.Small BJ, Fratiglioni L, Viitanen M, Winblad B, Backman L. The course of cognitive impairment in preclinical Alzheimer disease: three- and 6-year follow-up of a population-based sample. Arch Neurol. 2000;57(6):839–844. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- 4.Kawas CH, Corrada MM, Brookmeyer R, et al. Visual memory predicts Alzheimer’s disease more than a decade before diagnosis. Neurology. 2003;60(7):1089–1093. doi: 10.1212/01.wnl.0000055813.36504.bf. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59(2):198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 6.Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. J Int Neuropsychol Soc. 2008;14(2):266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Arch Neurol. 2009;66(10):1254–1259. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol. 2007;6(12):1106–1114. doi: 10.1016/S1474-4422(07)70291-0. [DOI] [PubMed] [Google Scholar]
- 9.Weuve J, Hebert LE, Scherr PA, Evans DA. Deaths in the United States among persons with Alzheimer’s disease (2010–2050) Alzheimers Dement. 2014;10(2):e40–46. doi: 10.1016/j.jalz.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75 e62. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Alzheimer’s Association. 2014 Alzheimer’s disease facts and figures. Alzheimers Dement. 2014;10(2):e47–e92. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11(3):332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Friedrich MJ. Researchers Test Strategies to Prevent Alzheimer Disease [news] JAMA. 2014;311(16):1596–1598. doi: 10.1001/jama.2014.3891. [DOI] [PubMed] [Google Scholar]
- 16.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward A, Tardiff S, Dye C, Arrighi HM. Rate of Conversion from Prodromal Alzheimer’s Disease to Alzheimer’s Dementia: A Systematic Review of the Literature. Dementia and Geriatric Cognitive Disorders Extra. 2013;3(1):320–332. doi: 10.1159/000354370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson RS, Weir DR, Leurgans SE, et al. Sources of variability in estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimers Dement. 2011;7(1):74–79. doi: 10.1016/j.jalz.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 21.Petrovitch H, Ross GW, Steinhorn SC, et al. AD lesions and infarcts in demented and non-demented Japanese- American men. Ann Neurol. 2005;57(1):98–103. doi: 10.1002/ana.20318. [DOI] [PubMed] [Google Scholar]
- 22.Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology. 2015;85(6):535–542. doi: 10.1212/WNL.0000000000001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta- Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med. 2012;172(3):219–227. doi: 10.1001/archinternmed.2011.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ailshire JA, Crimmins EM. Fine Particulate Matter Air Pollution and Cognitive Function Among Older US Adults. American Journal of Epidemiology. 2014 doi: 10.1093/aje/kwu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanderweele TJ, Arah OA. Bias formulas for sensitivity analysis of unmeasured confounding for general outcomes, treatments, and confounders. Epidemiology (Cambridge, Mass.) 2011;22(1):42–52. doi: 10.1097/EDE.0b013e3181f74493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oudin A, Forsberg B, Nordin Adolfsson A, et al. Traffic-Related Air Pollution and Dementia Incidence in Northern Sweden: A Longitudinal Study. Environmental health perspectives. 2015 doi: 10.1289/ehp.1408322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ailshire JA, Clarke P. Fine Particulate Matter Air Pollution and Cognitive Function Among U.S. Older Adults. The journals of gerontology Series B, Psychological sciences and social sciences. 2014 doi: 10.1093/geronb/gbu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Hamdan MZ, Crosson WL, Economou SA, et al. Environmental Public Health Applications Using Remotely Sensed Data. Geocarto international. 2014;29(1):85–98. doi: 10.1080/10106049.2012.715209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bullinger M. Association between air pollution and well-being. Sozial- und Praventivmedizin. 1989;34(5):231–238. doi: 10.1007/BF02083446. [DOI] [PubMed] [Google Scholar]
- 33.Chang KH, Chang MY, Muo CH, Wu TN, Chen CY, Kao CH. Increased risk of dementia in patients exposed to nitrogen dioxide and carbon monoxide: a population-based retrospective cohort study. PloS one. 2014;9(8):e103078. doi: 10.1371/journal.pone.0103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen JC, Schwartz J. Neurobehavioral effects of ambient air pollution on cognitive performance in US adults. Neurotoxicology. 2009;30(2):231–239. doi: 10.1016/j.neuro.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Chen JC, Wang X, Espeland MA, Chui H. Particulate air pollutants and white matter brain aging. Alzheimer’s and Dementia. 2014;10:P266. [Google Scholar]
- 36.Chen JC, Wang X, Wellenius GA, et al. Ambient air pollution and neurotoxicity on brain structure: Evidence from women’s health initiative memory study. Annals of Neurology. 2015 doi: 10.1002/ana.24460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen YC, Wu YC, Lin YC, et al. The spatial relationship between air pollutants and the risk of dementia. Alzheimer’s and Dementia. 2014;10:P748. [Google Scholar]
- 38.Gatto NM, Henderson VW, Hodis HN, et al. Components of air pollution and cognitive function in middle-aged and older adults in Los Angeles. Neurotoxicology. 2014;40:1–7. [Google Scholar]
- 39.Jung CR, Lin YT, Hwang BF. Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: A population-based cohort study in Taiwan. Journal of Alzheimer’s Disease. 2015;44(2):573–584. doi: 10.3233/JAD-140855. [DOI] [PubMed] [Google Scholar]
- 40.Kioumourtzoglou MA, Schwartz JD, Weisskopf MG, et al. Long-term PM Exposure and Neurological Hospital Admissions in the Northeastern United States. Environmental health perspectives. 2015 doi: 10.1289/ehp.1408973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loop MS, Kent ST, Al-Hamdan MZ, et al. Fine particulate matter and incident cognitive impairment in the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort. PloS one. 2013;8(9):e75001. doi: 10.1371/journal.pone.0075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loop MS, Kent ST, Al-Hamdan MZ, et al. Correction: Fine particulate matter and incident cognitive impairment in the reasons for geographic and racial differences in stroke (REGARDS) cohort(PLoS ONE (2013) 8:9 (e75001) 10.1371/journal.pone.0075001) PloS one. 2015;10(4) doi: 10.1371/journal.pone.0075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pedata P, Grella R, Lamberti M, Bergamasco N. Using the Mini-Mental State Examination (MMSE) for preliminary assessment of cognitive impairment in subjects exposed to air pollution with particulate matter. Giornale italiano di medicina del lavoro ed ergonomia. 2014;36(2):124–128. [PubMed] [Google Scholar]
- 44.Power MC, Weisskopf MG, Alexeeff SE, Coull BA, Spiro IA, Schwartz J. Exposure to black carbon and cognitive function in a cohort of older men. Epidemiology (Cambridge, Mass.) 2011;22:S203. [Google Scholar]
- 45.Power MC, Weisskopf MG, Alexeeff SE, Coull BA, Spiro A, Iii, Schwartz J. Traffic-Related Air Pollution and Cognitive Function in a Cohort of Older Men. Environmental health perspectives. 2011;119(5):682–687. doi: 10.1289/ehp.1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranft U, Schikowski T, Sugiri D, Krutmann J, Kramer U. Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. Environ Res. 2009;109(8):1004–1011. doi: 10.1016/j.envres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Schikowski T, Vossoughi M, Vierkötter A, et al. Association of air pollution with cognitive functions and its modification by APOE gene variants in elderly women. Environmental Research. 2015;142:10–16. doi: 10.1016/j.envres.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 48.Sun R, Gu D. Air pollution, economic development of communities, and health status among the elderly in urban China. Am J Epidemiol. 2008;168(11):1311–1318. doi: 10.1093/aje/kwn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tonne C, Elbaz A, Beevers S, Singh-Manoux A. Traffic-related Air Pollution in Relation to Cognitive Function in Older Adults. Epidemiology (Cambridge, Mass.) 2014;25(5):674–681. doi: 10.1097/EDE.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wellenius GA, Boyle LD, Coull BA, et al. Residential proximity to nearest major roadway and cognitive function in community-dwelling seniors: results from the MOBILIZE Boston Study. Journal of the American Geriatrics Society. 2012;60(11):2075–2080. doi: 10.1111/j.1532-5415.2012.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wen M, Gu D. Air pollution shortens life expectancy and health expectancy for older adults: the case of China. The journals of gerontology. Series A, Biological sciences and medical sciences. 2012;67(11):1219–1229. doi: 10.1093/gerona/gls094. [DOI] [PubMed] [Google Scholar]
- 52.Weng SW, Lin YT, Hwang BF. Air pollution and alzheimer’s disease: A population-based prospective cohort study in Taiwan. Epidemiology (Cambridge, Mass.) 2012;23(5):S143. [Google Scholar]
- 53.Wilker EH, Preis SR, Beiser AS, et al. Long-Term Exposure to Fine Particulate Matter, Residential Proximity to Major Roads and Measures of Brain Structure. Stroke. 2015;46(5):1161–1166. doi: 10.1161/STROKEAHA.114.008348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeng Y, Gu D, Purser J, Hoenig H, Christakis N. Associations of Environmental Factors With Elderly Health and Mortality in China. American Journal of Public Health. 2010;100(2):298–305. doi: 10.2105/AJPH.2008.154971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung CR, Lin YT, Hwang BF. Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: A population-based cohort study in Taiwan. Journal of Alzheimer’s Disease. 2015 doi: 10.3233/JAD-140855. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 56.Newcomer R, Clay T, Luxenberg JS, Miller RH. Misclassification and selection bias when identifying Alzheimer’s disease solely from Medicare claims records. Journal of the American Geriatrics Society. 1999;47(2):215–219. doi: 10.1111/j.1532-5415.1999.tb04580.x. [DOI] [PubMed] [Google Scholar]
- 57.Taylor DH, Jr, Ostbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. Journal of Alzheimer’s disease: JAD. 2009;17(4):807–815. doi: 10.3233/JAD-2009-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.2011 Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7(2):208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 59.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of Dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29(1–2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plassman BL, Langa KM, McCammon RJ, et al. Incidence of Dementia and Cognitive Impairment Not Dementia in the United States. Annals of neurology. 2011;70(3):418–426. doi: 10.1002/ana.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hebert LE, Beckett LA, Scherr PA, Evans DA. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer disease and associated disorders. 2001;15(4):169–173. doi: 10.1097/00002093-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Anderson JO, Thundiyil JG, Stolbach A. Clearing the air: a review of the effects of particulate matter air pollution on human health. Journal of medical toxicology: official journal of the American College of Medical Toxicology. 2012;8(2):166–175. doi: 10.1007/s13181-011-0203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brook RD, Rajagopalan S. Particulate matter air pollution and atherosclerosis. Curr Atheroscler Rep. 2010;12(5):291–300. doi: 10.1007/s11883-010-0122-7. [DOI] [PubMed] [Google Scholar]
- 65.Taylor DH, Østbye T, Langa KM, Weir D, Plassman BL. The Accuracy of Medicare Claims as an Epidemiological Tool: The Case of Dementia Revisited. Journal of Alzheimer’s disease: JAD. 2009;17(4):807–815. doi: 10.3233/JAD-2009-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kioumourtzoglou MA, Spiegelman D, Szpiro AA, et al. Exposure measurement error in PM2.5 health effects studies: a pooled analysis of eight personal exposure validation studies. Environmental health: a global access science source. 2014;13(1):2. doi: 10.1186/1476-069X-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lanphear BP, Hornung R, Khoury J, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environmental health perspectives. 2005;113(7):894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pope CA, 3rd, Burnett RT, Turner MC, et al. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure-response relationships. Environmental health perspectives. 2011;119(11):1616–1621. doi: 10.1289/ehp.1103639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith KR, Peel JL. Mind the gap. Environmental health perspectives. 2010;118(12):1643–1645. doi: 10.1289/ehp.1002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychological methods. 2013;18(2):137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bind MC, Vanderweele TJ, Coull BA, Schwartz JD. Causal mediation analysis for longitudinal data with exogenous exposure. Biostatistics (Oxford, England) 2015 doi: 10.1093/biostatistics/kxv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.VanderWeele TJ, Vansteelandt S. Mediation Analysis with Multiple Mediators. Epidemiologic methods. 2014;2(1):95–115. doi: 10.1515/em-2012-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Early DR, Widaman KF, Harvey D, et al. Demographic predictors of cognitive change in ethnically diverse older persons. Psychol Aging. 2013;28(3):633–645. doi: 10.1037/a0031645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gross AL, Mungas DM, Crane PK, et al. Effects of Education and Race on Cognitive Decline: An Integrative Study of Generalizability Versus Study-Specific Results. Psychol Aging. 2015 doi: 10.1037/pag0000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weisskopf MG, Sparrow D, Hu H, Power MC. Biased Exposure-Health Effect Estimates from Selection in Cohort Studies: Are Environmental Studies at Particular Risk? Environmental health perspectives. 2015 doi: 10.1289/ehp.1408888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pope CA, 3rd, Burnett RT, Thurston GD, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109(1):71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 77.Weuve J, Kaufman JD, Szpiro AA, et al. Exposure to traffic-related air pollution in relation to progression in disability. Environmental health perspectives. doi: 10.1289/ehp.1510089. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Euser SM, Schram MT, Hofman A, Westendorp RG, Breteler MM. Measuring cognitive function with age: the influence of selection by health and survival. Epidemiology (Cambridge, Mass.) 2008;19(3):440–447. doi: 10.1097/EDE.0b013e31816a1d31. [DOI] [PubMed] [Google Scholar]
- 79.Alonso A, Seguí-Gómez M, Irala J, Sánchez-Villegas A, Beunza J, Martínez-Gonzalez M. Predictors of follow-up and assessment of selection bias from dropouts using inverse probability weighting in a cohort of university graduates. Eur J Epidemiol. 2006;21(5):351–358. doi: 10.1007/s10654-006-9008-y. [DOI] [PubMed] [Google Scholar]
- 80.Weuve J, Proust-Lima C, Power MC, et al. Guidelines for reporting methodological challenges and evaluating potential bias in dementia research. Alzheimers Dement. 2015;11(9):1098–1109. doi: 10.1016/j.jalz.2015.06.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ganguli M, Lee CW, Hughes T, et al. Who wants a free brain scan? Assessing and correcting for recruitment biases in a population-based sMRI pilot study. Brain imaging and behavior. 2015;9(2):204–212. doi: 10.1007/s11682-014-9297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Debette S, Beiser A, DeCarli C, et al. Association of MRI Markers of Vascular Brain Injury with Incident Stroke, Mild Cognitive Impairment, Dementia and Mortality: the Framingham Offspring Study. Stroke; a journal of cerebral circulation. 2010;41(4):600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jaramillo SA, Felton D, Andrews L, et al. Enrollment in a Brain Magnetic Resonance Study: Results From the Women’s Health Initiative Memory Study Magnetic Resonance Imaging Study (WHIMS-MRI) Academic radiology. 2007;14(5):603–612. doi: 10.1016/j.acra.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oberdorster G, Utell MJ. Ultrafine particles in the urban air: to the respiratory tract–and beyond? Environmental health perspectives. 2002;110(8):A440–441. doi: 10.1289/ehp.110-1240959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peters A, Veronesi B, Calderon-Garciduenas L, et al. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part Fibre Toxicol. 2006;3:13. doi: 10.1186/1743-8977-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oberdorster G, Sharp Z, Atudorei V, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16(6–7):437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 87.Maccioni RB, Farias G, Morales I, Navarrete L. The revitalized tau hypothesis on Alzheimer’s disease. Arch Med Res. 2010;41(3):226–231. doi: 10.1016/j.arcmed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 88.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62(7):1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 89.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sagare AP, Bell RD, Zlokovic BV. Neurovascular dysfunction and faulty amyloid beta-peptide clearance in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(10) doi: 10.1101/cshperspect.a011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Revett TJ, Baker GB, Jhamandas J, Kar S. Glutamate system, amyloid ss peptides and tau protein: functional interrelationships and relevance to Alzheimer disease pathology. J Psychiatry Neurosci. 2013;38(1):6–23. doi: 10.1503/jpn.110190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cetin F. Role of Oxidative Stress in Aβ Animal Model of Alzheimer’s Disease: Vicious Circle of Apoptosis, Nitric Oxide and Age. Neurodegenerative Diseases. 2013 http://www.intechopen.com/books/neurodegenerative-diseases/role-of-oxidative-stress-in-a-animal-model-of-alzheimer-s-disease-vicious-circle-of-apoptosis-nitric. Accessed April 24, 2014.
- 93.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1(1):a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu L, Chan C. The role of inflammasome in Alzheimer’s disease. Ageing Res Rev. 2014;15C:6–15. doi: 10.1016/j.arr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012;2(1):a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zawia NH, Lahiri DK, Cardozo-Pelaez F. Epigenetics, oxidative stress, and Alzheimer disease. Free Radic Biol Med. 2009;46(9):1241–1249. doi: 10.1016/j.freeradbiomed.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque P. Neurotoxicants are in the air: convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. Biomed Res Int. 2014;2014:736385. doi: 10.1155/2014/736385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Calderon-Garciduenas L, Reed W, Maronpot RR, et al. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol. 2004;32(6):650–658. doi: 10.1080/01926230490520232. [DOI] [PubMed] [Google Scholar]
- 100.Morgan TE, Davis DA, Iwata N, et al. Glutamatergic neurons in rodent models respond to nanoscale particulate urban air pollutants in vivo and in vitro. Environmental health perspectives. 2011;119(7):1003–1009. doi: 10.1289/ehp.1002973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Calderon-Garciduenas L, Azzarelli B, Acuna H, et al. Air pollution and brain damage. Toxicol Pathol. 2002;30(3):373–389. doi: 10.1080/01926230252929954. [DOI] [PubMed] [Google Scholar]
- 102.Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. 2008;36(2):289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- 103.Kim SH, Knight EM, Saunders EL, et al. Rapid doubling of Alzheimer’s amyloid-beta40 and 42 levels in brains of mice exposed to a nickel nanoparticle model of air pollution [brief report] F1000Res. 2012;1:70. doi: 10.12688/f1000research.1-70.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Calderon-Garciduenas L, Maronpot RR, Torres-Jardon R, et al. DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol Pathol. 2003;31(5):524–538. doi: 10.1080/01926230390226645. [DOI] [PubMed] [Google Scholar]
- 105.Calderon-Garciduenas L, Mora-Tiscareno A, Ontiveros E, et al. Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cogn. 2008;68(2):117–127. doi: 10.1016/j.bandc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 106.Rivas-Arancibia S, Hernández-Zimbrón L, Rodríguez-Martínez E, Borgonio-Pérez G, Velumani V, Durán-Bedolla J. Chronic exposure to low doses of ozone produces a state of oxidative stress and blood-brain barrier damage in the hippocampus of rat. Advances in Bioscience and Biotechnology. 2013;4:24–29. [Google Scholar]
- 107.Block ML, Elder A, Auten RL, et al. The outdoor air pollution and brain health workshop. Neurotoxicology. 2012;33(5):972–984. doi: 10.1016/j.neuro.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sang N, Yun Y, Li H, Hou L, Han M, Li G. SO2 inhalation contributes to the development and progression of ischemic stroke in the brain. Toxicological sciences: an official journal of the Society of Toxicology. 2010;114(2):226–236. doi: 10.1093/toxsci/kfq010. [DOI] [PubMed] [Google Scholar]
- 109.Krishnan RM, Adar SD, Szpiro AA, et al. Vascular responses to long- and short-term exposure to fine particulate matter: MESA Air (Multi-Ethnic Study of Atherosclerosis and Air Pollution) J Am Coll Cardiol. 2012;60(21):2158–2166. doi: 10.1016/j.jacc.2012.08.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. Particulate Matter Air Pollution and Cardiovascular Disease: An Update to the Scientific Statement From the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 111.Wellenius GA, Burger MR, Coull BA, et al. Ambient air pollution and the risk of acute ischemic stroke. Arch Intern Med. 2012;172(3):229–234. doi: 10.1001/archinternmed.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dadvand P, Figueras F, Basagana X, et al. Ambient air pollution and preeclampsia: a spatiotemporal analysis. Environmental health perspectives. 2013;121(11–12):1365–1371. doi: 10.1289/ehp.1206430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.IOM (Institute of Medicine) Cognitive aging: Progress in understanding and opportunities for action. Washington, D.C.: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 114.Qiu C, Fratiglioni L. A major role for cardiovascular burden in age-related cognitive decline. Nat Rev Cardiol. 2015;12(5):267–277. doi: 10.1038/nrcardio.2014.223. [DOI] [PubMed] [Google Scholar]
- 115.Weuve J. Invited commentary: how exposure to air pollution may shape dementia risk, and what epidemiology can say about it. Am J Epidemiol. 2014;180(4):367–371. doi: 10.1093/aje/kwu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Radiation OoAa, editor. United States Environmental Protection Agency. The Benefits and Costs of the Clean Air Act from 1990 to 2020. 2011. [Google Scholar]
- 117.Lee M, Koutrakis P, Coull B, Kloog I, Schwartz J. Acute effect of fine particulate matter on mortality in three Southeastern states from 2007–2011. Journal of exposure science & environmental epidemiology. 2015 doi: 10.1038/jes.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wilson RS, Leurgans SE, Boyle PA, Bennett DA. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch Neurol. 2011;68(3):351–356. doi: 10.1001/archneurol.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Laurijssens B, Aujard F, Rahman A. Animal models of Alzheimer’s disease and drug development. Drug Discov Today Technol. 2013;10(3):e319–327. doi: 10.1016/j.ddtec.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 120.Shanks N, Greek R, Greek J. Are animal models predictive for humans? Philos Ethics Humanit Med. 2009;4:2. doi: 10.1186/1747-5341-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Woodruff-Pak DS. Animal models of Alzheimer’s disease: therapeutic implications. Journal of Alzheimer’s disease: JAD. 2008;15(4):507–521. doi: 10.3233/jad-2008-15401. [DOI] [PubMed] [Google Scholar]
- 122.Rothwell PM. External validity of randomised controlled trials: “To whom do the results of this trial apply?”. The Lancet. 2005;365(9453):82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 123.Proust-Lima C, Dartigues JF, Jacqmin-Gadda H. Misuse of the linear mixed model when evaluating risk factors of cognitive decline. Am J Epidemiol. 2011;174(9):1077–1088. doi: 10.1093/aje/kwr243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. Jama. 2012;307(2):165–172. doi: 10.1001/jama.2011.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]


