Abstract
Developmental exposure of rats to polychlorinated biphenyls (PCBs) causes impairments in hearing and in the functioning of peripheral and central auditory structures. Additionally, recent work from our laboratory has demonstrated an increase in audiogenic seizures. The current study aimed to further characterize the effects of PCBs on auditory brain structures by investigating whether developmental exposure altered the magnitude of activation in the auditory cortex (AC) in response to electrical stimulation of thalamocortical afferents. Long-Evans female rats were fed cookies containing either 0 or 6 mg/kg of an environmental PCB mixture daily from 4 weeks prior to breeding until postnatal day 21. Brain slices containing projections from the thalamus to the AC were collected from adult female offspring and were bathed in artificial cerebrospinal fluid (aCSF) alone, aCSF containing a gamma-aminobutyric acid (GABA) receptor antagonist (200 nM SR95531), and aCSF containing an and N-methyl-D-aspartate (NMDA) receptor antagonist (50μM AP5). During each of these drug conditions, electrical stimulations ranging from 25-600 μA were delivered to the thalamocortical afferents. Activation of the AC was measured using flavoprotein autofluorescence imaging. Although there were no differences seen between treatment groups in the aCSF condition, there were significant increases in the ratio of aCSF/SR95531 activation in slices from PCB-exposed animals compared to control animals. This effect was seen in both the upper and lower layers of the AC. No differences in activation were noted between treatment groups when slices were exposed to AP5. These data suggest that developmental PCB exposure leads to increased sensitivity to antagonism of GABAA receptors in the AC without a change in NMDA-mediated intrinsic excitability.
Keywords: polychlorinated biphenyls, endocrine disruptor, GABA
1. INTRODUCTION
Polychlorinated biphenyls (PCBs) are persistent environmental contaminants that were used as dielectric fluids in transformers and capacitors until the 1970s, when they were banned. Exposure of humans to this toxicant is thought to arise mainly from the ingestion of contaminated fish and seafood (Crinnion, 2011). However, additional sources of PCB exposure include caulking materials and fluorescent light ballasts (Anezaki and Nakano 2015; Hu and Hornbuckle et al., 2010), and recent work has also revealed that PCBs are inadvertently produced as by-products during the synthesis of paint pigments (Anezaki et al., 2015). PCBs are stable and lipophilic compounds, characteristics which lead to accumulation in adipose tissue, mobilization of PCBs into breast milk, transport across the placental barrier, and resulting exposure to both the fetus and infant (DeKoning and Karmaus, 2000; Jacobson et al., 1984). During brain development, numerous processes must occur in a time dependent and coordinated manner. As a result, this represents a particularly vulnerable period during which exposure to PCBs can induce long-lasting effects on the brain and behavior.
Impairments in auditory function are a consistent finding in human and rodent models examining effects of developmental PCBs (Jusko et al., 2014; Trnovec et al., 2010; Powers et al., 2006, 2009; Poon et al., 2011). Previously, our lab has reported that auditory brainstem response (ABR) thresholds were elevated across all frequencies tested in PCB-exposed rats, indicative of dysfunction in the cochlea or auditory nerve (Powers et al., 2006). Also, developmental PCB exposure in rats decreased amplitudes and increased thresholds for distortion product otoacoustic emissions (DPOAEs), which provide a measure of integrity of the outer hair cells of the cochlea (Powers et al., 2006; 2009; Poon et al., 2011). These studies demonstrate that peripheral structures of auditory system are vulnerable to developmental PCB exposure.
Previous work from our lab has also demonstrated that PCB exposure during early development leads to an increase in audiogenic seizure incidence, a decrease in the latency to onset of audiogenic seizures, and an increase in the severity of seizures in adult male and female rats (Poon et al., 2015). Importantly, these effects were observed in mature adult rats many months after PCB exposure ended, suggesting that PCB exposure altered developmental processes, leading to a permanent increase in susceptibility to audiogenic seizures. These findings were recently replicated in a second study (Bandara et al., 2016), in which it was also demonstrated that GAD67 (glutamic acid decarboxylase), an enzyme that converts glutamate in gamma-aminobutyric acid (GABA), was decreased in the inferior colliculus (IC) in adulthood after developmental exposure to PCBs. Given that the IC is important for the initiation and propagation of audiogenic seizures (N'Gouemo and Faingold, 1996; Ross and Coleman, 2000), this suggests that levels of inhibitory neurotransmission in the IC are permanently decreased after developmental exposure to PCBs, a potential mechanism for the increase in audiogenic seizures seen in these animals.
Although the auditory cortex (AC) has not been implicated directly in the initiation and propagation of audiogenic seizures, this structure has ascending and descending connections to other auditory structures, such as the IC, and plays a modulatory role in many aspects of the response properties of neurons from other auditory and thalamic structures, such as sensitivity to sound frequency in the IC (Bajo and King, 2013; Suga, 2008; Stebbings et al., 2014). In addition, cells within the AC from fragile X knockout mice demonstrate a hyper-responsiveness to sound, and this animal model also displays an increased incidence of audiogenic seizures (Rotschafer and Razak, 2013; 2014).
Interestingly, one previous study has reported changes in the AC of adult rats after developmental exposure to PCBs. Exposure to PCB 95, a specific penta-chlorinated congener, led to large changes in the functioning and organization of the AC (Kenet et al., 2007), including alterations in the tonotopic and topographic gradients and receptive field characteristics of neurons in the primary AC. Additionally, the normal plasticity that occurs in response to noise exposure in vivo was altered after developmental PCB exposure. Lastly, the best frequencies to elicit tone-evoked inhibitory postsynaptic currents (IPSCs) and excitatory postsynaptic currents (EPSCs) in the AC were correlated in control animals, while they were not correlated in PCB-exposed rats (Kenet et al., 2007). These findings suggest that the AC is susceptible to perturbations by developmental PCB exposure. However, due to recent findings demonstrating increased audiogenic seizures and changes within the AC, more work is needed to better understand the long-lasting functional changes in inhibition and excitation in the AC after developmental exposure to PCBs.
In the current study, we adapted a flavoprotein autofluorescence technique previously used in mice (Llano et al., 2012; Stebbings et al., 2016) to measure neural activation in response to electrical stimulation of thalamocortical afferents from the medial geniculate body of the thalamus (MGB) to the AC in brain slices from adult rats developmentally exposed to an environmentally relevant mixture of PCBs. In addition to measuring changes under control artificial cerebral spinal fluid (aCSF) bath conditions, GABAA antagonists and N-methyl-D-aspartate (NMDA) antagonists were added to the bath to determine whether the AC activation would differ between slices from oil and PCB-exposed rats in response to blockade of inhibitory or excitatory neurotransmitters, respectively.
2. MATERIALS AND METHODS
2.1 Animals
Female and male Long-Evans rats were purchased from Harlan (Indianapolis, IN) as adults and were individually housed in standard polycarbonate cages with woodchip bedding. All rats (including breeders and offspring) were given chow (Harlan Teklad rodent diet 8604) and water ad libitum and were kept on a 12/12-hr light cycle (lights on at 0700 hr) throughout the entire duration of the study. The rats were maintained in facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Illinois at Urbana-Champaign and were in accordance with the guidelines of the National Institutes of Health (2002) and National Research Council (2003).
2.2 PCB Exposure
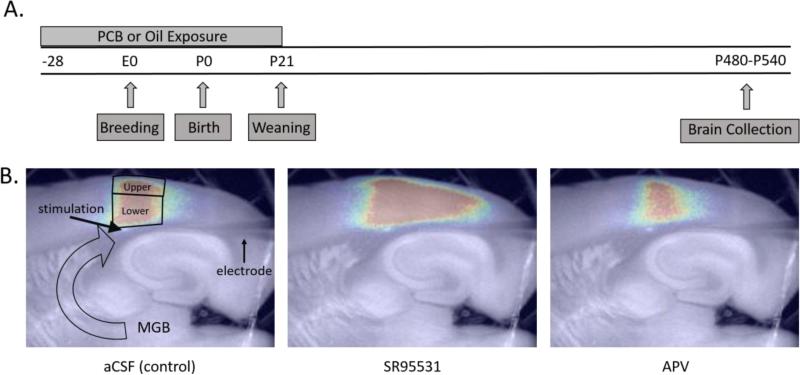
A timeline of experimental procedures is shown in Figure 1A. Beginning 4 weeks prior to breeding, female rats were orally exposed to corn oil vehicle (n = 9) or 6 mg/kg PCBs in corn oil (n = 8) daily. The treatment continued throughout gestation and lactation until the offspring were weaned on postnatal day (PND) 21. The PCB mixture (Fox River PCB mixture) was created to mimic the congener profile found in walleye from the Fox River in northeast Wisconsin. The mixture consisted of 35% Aroclor 1242 (Monsanto lot KB 05-415; St. Louis, MO), 35% Aroclor 1248 (Accustandard lot F-110; New Haven, CT), 15% Aroclor 1254 (Monsanto lot KB 05-612), and 15% Aroclor 1260 (Accustandard lot 021-020) (Kostyniak et al., 2005). The dose of 6 mg/kg PCBs was selected for this study based on previous studies demonstrating ototoxicity and increased incidence of audiogenic seizures (Powers et al., 2006, Poon et al., 2011; 2015) at this dose, without other overt signs of clinical toxicity (Kostyniak et al,. 2005). The PCB solution was diluted in corn oil (Mazola) at a concentration of 15 mg/ml. Female rats were weighed daily and a volume of 0.4 ml of solution/kg of body weight was pipetted onto ½ of a vanilla wafer cookie (Keebler Golden Vanilla Wafers). The female rats were fed oil or PCB-treated cookies daily. The experimenter was blind to the treatment groups until statistical analyses were completed.
Figure 1.
A) Timeline of experimental procedures B) Representative pictures of activation (200 μA) of the auditory cortex (AC) under aCSF (artificial cerebral spinal fluid), SR95531, and AP5 conditions. Outlined areas show the regions of interest for analyses of activation and redox ratios in response to stimulation of thalamocortical fibers. −28 = 28 days prior to breeding, E = embryonic day, P = postnatal day, MGB = medial geniculate body of the thalamus, large open arrow = thalamocortical fibers, upper = upper layers, lower = lower layers
2.3 Breeding and Weaning
Each female was paired with an unexposed male in a hanging wire breeding cage 4 weeks after the beginning of PCB exposure. Breeding continued for 8 consecutive days. Females were returned to their home cage briefly each day during breeding for consumption of the cookies.
Birth of offspring was considered PND 0. On PND 1, pups were examined for abnormalities, sexed, and weighed. On PND 2, large litters were culled to 10 pups with an equal number of males and females when possible. Litters with at least 7 pups had extra pups from other litters of the same treatment group cross-fostered into them so that the size of the litter ranged from 8-10 pups. Cross fostered pups were ear clipped and not used for later experiments. A total of 17 successful litters were obtained for the current study. Dosing of the dams continued until PND 21 when the offspring were weaned. Upon weaning, the female offspring were ear-punched for identification purposes and were group-housed (2-3 rats/cage) with offspring from the same treatment group. Only female offspring were available for this study since male littermates were used for other experiments (Bandara et al., 2016). 1-3 female offspring per litter were used for this experiment and to account for possible effects of litter, averages were obtained when more than one animal was tested per litter and these averages were used as the measure for analyses.
2.3 Brain Sectioning
In adulthood (approximately PND 500), female rats were injected with ketamine/xylazine (87:13) at a dose of 0.1 mg/kg of body weight. Rats were perfused with a chilled sucrose slicing solution that was carbogenated (in mM: 206 sucrose, 11 glucose, 10 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 0.5 CaCl2, 2.5 KCl, 1 kynurenic acid, pH 7.4) and the brain was removed. To obtain brain slices containing thalamocortical projections to the left AC, the brain was blocked by removing the cerebellum and anterior 3 mm of the frontal cortex. The brain was placed on its cut anterior surface and a cut was made 17° off the horizontal plane, thus removing a small dorsal flap (Cruikshank et al., 2002). Superglue was used to adhere the dorsal surface of the brain to a cutting platform and a 600 μm horizontal slice was cut on a vibratome while the brain was submerged in the slicing solution. Sections were incubated at 32°C for one hour in a carbogenated incubation solution (in mM: 10.0 glucose, 26 NaHCO3,126, NaCl, 3.0 MgCl2, 1.25 NaH2PO4, 1.0 CaCl2, 2.5 KCl, pH 7.4) before experimentation.
2.4 Stimulation and Flavoprotein Autofluorescence
Electrical stimulation was achieved with glass micropipettes broken to a diameter of 80 μm and filled with artificial cerebral spinal fluid (aCSF; in mM: 10 glucose, 26 NaHCO3, 2.5 KCl, 151 NaCl, 1.25 NaH2PO4, 2.0 MgCl2, 1.0 CaCl2). When possible, the same electrode was used across animals to reduce variability. A total of 3 electrodes were used throughout the study, and each was used equally across the 2 treatment groups.
The sections were placed into a chamber that was continuously perfused with aCSF. A coverslip was placed over the section to stabilize the imaging plane. Thalamocortical afferents corresponding to the white matter below the AC were stimulated. The electrode was placed on the surface of the subcortical white matter approximately 200 μm ventral to the AC. Trains of electrical pulses ranging from 25-600 μA were delivered using Power Lab software (Lab Chart 7) and hardware (AD Instruments; model 4/30) and a World Precision Instruments stimulus isolator (model A360). Trains were 1 s in duration and delivered at 40 pulses per second with a 2 ms pulse duration. Trains were given every 20 seconds with a total of 5 repetitions for each stimulation intensity. The 5 seconds prior to each stimulus train was used as baseline. Stimulation intensities were presented in a semi-randomized manner, but the order was consistent between the different drug conditions.
Flavoprotein autofluorescence (FAD+, flavin adenine dinucleotide) was used to measure activation of the AC. This technique utilizes the fact that mitochondrial flavoproteins endogenously fluoresce when oxidized, thus causing the tissue to fluoresce when activated, and this technique has been demonstrated to be a marker of neuronal activity (Husson and Issa, 2009; Shibuki et al., 2003). A fluorescence illuminator (Prior Lumen 200) and a U-MN49002XI E-GFP Olympus filter cube (Excitation: 470-490 nm, Dichroic 505 nm, emission 515 nm long pass) were used to illuminate with blue light to excite the tissue. Data were collected at 4 frames per second using a Retiga EXi camera, a 2.5X objective (Olympus, NA = .08), and StreamPix software (version 5.19.0.0).
2.5 Drugs
After slice activation was measured with stimulations ranging from 25-600 μA during perfusion of aCSF, identical methods were used to measure tissue activation in the presence of 200 nM SR95531 (Tocris Biosciences), a GABAA antagonist. After this was complete, activation was then measured in the presence of 50 μM AP5 (Tocris Biosciences), an NMDA antagonist. SR95531 and AP5 were dissolved in aCSF prior to the experiment with 30 minute wash-in and washout times for each drug condition. This concentration of AP5 has been previously used in examining flavoprotein autofluorescence in mouse slices (Llano et al., 2012). The concentration of SR95531 used for the experiments was based on previous experiments showing that the dissociation constant of this drug is around 170 nM for neurons (Hamann et al., 1988).
2.6 Flavoprotein Analysis
For each stimulation intensity, a total of 420 images were collected with the image size of 172 × 130 pixels (8 × 8 binning). The first 5 seconds (20 frames) were used as a baseline. Image analyses was performed using custom software written in MATLAB (Stebbings et al., 2016). The change in fluorescence (ΔF) with stimulation as a function of the baseline fluorescence (F) was used for measurement of activation. The upper layers (layers 1-3) and lower layers (layers 4-6) were analyzed separately (See Figure 1). Input-output curves were constructed for the AC based on the responses to electrical stimulation amplitudes ranging from 25-600 μA. The region of interest measured 1.5 mm from anterior to posterior and was centered over the area of maximum activation. (Llano et al., 2012; Stebbings et al., 2016). The changes in fluorescence over baseline fluorescence maps (Δf/f maps) were overlaid on the raw fluorescence images for identification and localization of anatomic structures.
2.7 Redox Ratios
Redox ratios were expressed as a ratio of FAD+ to nicotinamide adenine dinucleotide (NADH), a metric that has been shown to be sensitive to oxidative stress (Gerich et al., 2009; Sepehr et al., 2012, Stebbings et al., 2016). This measure was taken at the beginning of the experiment prior to any electrical stimulation. FAD+ was measured as described above and NADH imaging was measured using a Semrock Sirius A 000 cube with 350 nm-390 nm excitation and 415-465 nm emission. Redox ratios in the upper and lower layers of the cortex were measured (regions of interest outlined in Figure 1B).
2.8 Cortical Thickness
Thickness was measured in 2 areas of the AC, the area directly dorsal to the rostral edge of the hippocampus and the area that lies above the caudal edge of the CA3 region of the hippocampus. Raw images collected during flavoprotein autofluorescence were imported into Image J and the thickness in millimeters was measured.
2.9 Statistical Analyses
Repeated measures ANOVAs with PCB exposure (2 conditions: oil and PCB exposure) as the between subjects variable and stimulation amplitudes (11 levels ranging from 25-600 μA) as the repeated measure were used to analyze changes in flavoprotein autofluorescence (version 22; IBM SPSS Statistics) in either the upper (layers 1-3) or lower layers (layers 4-6) of the AC. Separate repeated measures ANOVAS were run to compare effects of PCB exposure under aCSF (control condition), the activation of SR95531/aCSF conditions, and the activation of AP5/aCSF conditions. When a significant PCB exposure or an interaction of PCB exposure × stimulation intensity were observed, student t-tests were used to determine if there were significant differences between the two treatment groups at each stimulation intensity.
Redox ratios and cortical thickness were analyzed using student t-tests with treatment as an independent factor. Redox ratios were measured separately for the upper and lower layers of the AC. SPSS (version 22; IBM SPSS Statistics) was used for all analyses.
3. RESULTS
3.1 Activation of the Auditory Cortex
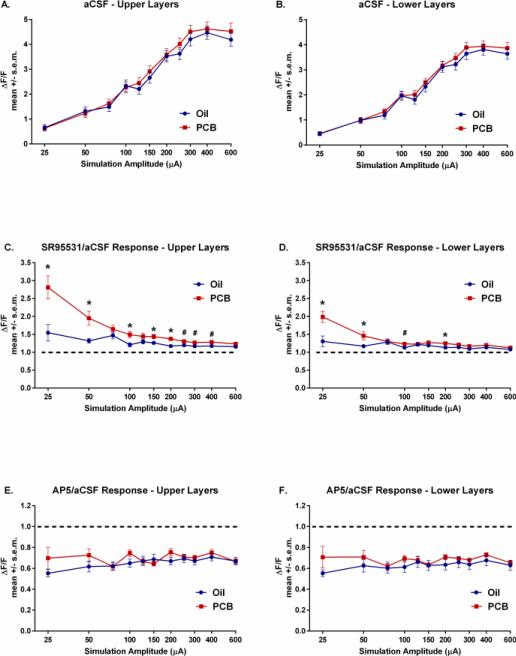
Shown in Figure 2, under control aCSF conditions, there were no significant main effects of PCB treatment and no PCB treatment x stimulation amplitude interactions in either the upper (Figure 2A) or lower layers (Figure 2B) of the AC.
Figure 2.
A) Autofluorescence (ΔF/F) in the upper layers of the AC in the aCSF condition. B) Autofluorescence of the AC in the lower layers in the aCSF condition. C) Ratio of change in autofluorescence in SR95531/aCSF conditions in the upper layers. D) Ratio of change in autofluorescence in SR95531/aCSF conditions in the lowers layers. E) Ratio of change in autofluorescence in AP5/aCSF conditions in the upper layers. F) Ratio of change in autofluorescence in AP5/aCSF conditions in the lower layers. *significant difference in activation between slices of oil and PCB-exposed animals (p<.05). dashed line = 1:1 ratio of SR95531 or AP5:aCSF condition
However, analysis of the ratio of SR-95531/aCSF activation revealed significant main effects of treatment (p<.01) and stimulation amplitude (p<.001), and a significant treatment × stimulation amplitude interaction (p<.001) in the upper layers of the AC (Figure 2C). Post-hoc comparisons showed that slices from PCB-exposed animals had increased ratios of activation at stimulation amplitudes of 25, 50, 100, 150, and 200 μA (ps<.05) and trends for increased ratios at 250, 300, and 400 μA (.05<ps<.08). No significant effects were seen at the remaining stimulation amplitudes (75, 125, 600 μA).
Shown in Figure 2D, significant differences in the ratio of activation of SR-95531/aCSF were seen in the lower layers with significant main effects of treatment (p<.05) and amplitude (p<.001), and a significant treatment × stimulation amplitude interaction (p<.001) in the lower layers of the AC. Post-hoc comparisons showed significantly increased activation in slices from PCB-exposed animals at 25, 50, and 200 μA (ps<.05) and a trend towards an increased ratio of activation with 100 μA (.10>p>.05).
Figures 2E and 2F show the ratio of activation in the AP5 condition/aCSF condition. No significant differences were found between PCB and oil-exposed rats in the ratio of AP5/aCSF activation.
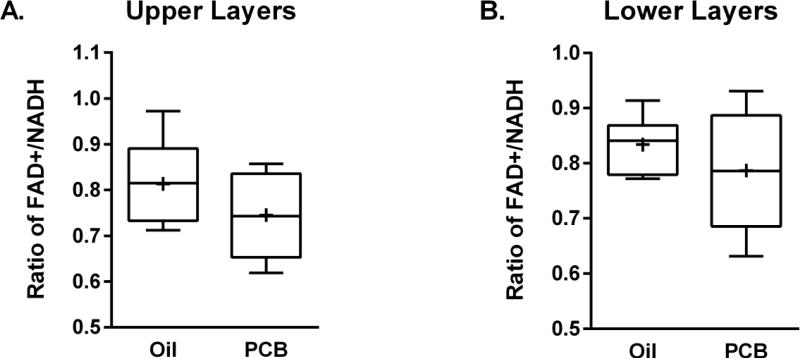
3.2 Redox Ratios
Figure 3 shows the redox ratios from both the upper (Figure 3A) and lower layers (Figure 3B) of the AC. The ratio of the FAD+/NADH in the AC did not significantly differ between rats exposed to oil vehicle and PCBs in either the upper or lower cortical layers.
Figure 3.
Box and whisker plot (Tukey) of redox ratios of upper layers (A) and lower layers (B) of the AC. + = means
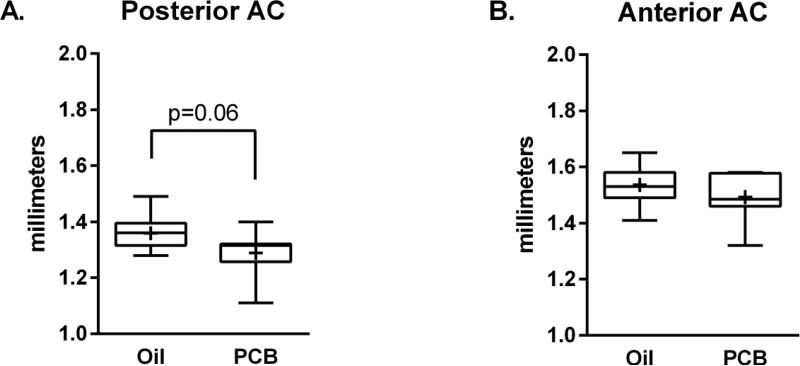
3.3 Auditory Cortex Thickness
Measurement of the thickness of the AC revealed a trend (p=.06) towards a decreased thickness of the caudal border of the AC in slices from PCB-exposed animals (Figure 4A). This trend was not seen in the anterior border (Figure 4B) of the AC.
Figure 4.
Box and whisker plot (Tukey) of cortical thickness of the anterior (A) and posterior (B) borders of the AC. + = means
4. DISCUSSION
The current study examined changes in the activation of the AC in adult female rats that were exposed to either oil vehicle or 6 mg/kg PCBs during early development. Although no differences in flavoprotein activation were seen in response to electrical stimulation of thalamocortical afferents during the aCSF bath condition or AP5/aCSF bath condition, slices from PCB-exposed rats had an increased response, most notably at the low amplitudes of stimulation, to blockade of GABAA receptors via SR-95531. These findings suggest that PCB exposure during development induces changes in the functioning of the AC. In addition, this effect occurred without alterations in ratios of FAD+/NADH, which are used as measures of oxidative stress and mitochondrial function within and outside of the central nervous system (Gerich et al., 2009; Stebbings et al., 2016; Ghanian et al, 2014). Importantly, these results illustrate that PCB exposure during the developmental period can result in long-term changes in the physiology of the AC.
The current study, together with previous studies, supports the finding that both the peripheral and central auditory systems are vulnerable to disruption by developmental PCB exposure. Developmental PCB exposure alters function of the outer hair cells in the cochlea, decreases GAD levels in the IC, and increases the incidence of audiogenic seizures in adult rats (Bandara et al., 2016; Poon et al., 2015; Powers et al., 2006; 2009). Additionally, chronic gestational exposure to an individual PCB congener (PCB95) leads to significant changes in the tonotopic organization of the AC, as well as the receptive fields, and the best frequencies to elicit excitation and inhibition in the AC (Kenet al., 2007). Importantly, this is the first study to demonstrate changes within the AC after developmental exposure to an environmentally relevant mixture of PCBs.
It is currently unknown whether the changes seen here are a consequence of the direct actions of PCB on the AC or whether changes in hearing and/or cellular changes in more caudal auditory centers (for example, the cochlea and IC) are responsible for the changes reported here. It is possible that PCBs are acting indirectly, given that peripheral hearing loss during a critical period of development is associated with changes in inhibitory postsynaptic currents, electrophysiological measures, and long-term plasticity in the AC of adult animals (Mowery et al., 2015; Kotak et al., 2007). However, in previous work, peripheral hearing loss is associated with a decrease in synaptic inhibition (Takesian et al., 2009), while the current study suggests that there may be an upregulation of GABAergic transmission in the AC. This discrepancy between the current results and results summarized in Takesian et al., 2009 may suggest that effect of developmental PCB exposure causes separate, direct effects on the AC. An alternative explanation is that the mechanisms for disrupting peripheral hearing structures after developmental PCB exposure differ from those seen when peripheral hearing structures are physically damaged, such as methods described in Takesian et al. (2009) for inducing conductive hearing loss.
The mechanisms responsible for the increased activation in the PCB slices after blockade of GABAA receptors are unknown and could arise from either postsynaptic or presynaptic mechanisms. Since differences were only seen with GABA blockade and not in the control aCSF condition, the results suggest an upregulation of excitation in the thalamocortical system possibly by altering functioning of glutamate receptors. Since no differences were seen with the addition of the NMDA antagonist, it is possible that other glutamate receptors, such as α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, are upregulated. Upregulation of excitation is also supported by the finding that significant increases in activation with SR95531 in slices from PCB-exposed rats were only seen at some of the lower electrical stimulation amplitudes. This may occur because at higher stimulation amplitudes, saturation of GABAA receptors and maximal activation is likely. Another possibility is that high stimulation amplitudes may activate different or additional neurochemical systems that are able to compensate for the changes seen at the lower amplitudes. Alternatively, the increased sensitivity of PCB-treated rats to SR95531 may be due to the ability of SR95531 to be an allosteric modulator of GABAA receptors and to act as an inverse agonist at GABAA receptors, although these actions of SR95531 have not been demonstrated in neuronal tissue (Ueno et al., 1997; Lindquist et al., 2005).
Additional mechanisms involved in activational changes after GABAA antagonism may include changes in the subunit composition or number of GABA receptors or changes in GABA levels. We suspect that GABA levels are not altered in the AC, given that no changes in GAD protein levels, which are indicative of levels of GABA, were seen in another cortical area, the somatosensory cortex, after developmental exposure to the same mixture of PCBs (Bandara et al., 2016). Also, no changes in the levels of the GABAAα1 receptor were seen in the IC despite changes in GAD levels in this area (Bandara et al., 2016). However, whether there are changes in GABA receptors in the AC has not been tested directly. Further studies examining functioning and number of AMPA glutamate receptors and changes to the functioning of the GABA receptors are warranted to determine the cellular mechanisms that are contributing to the changes in tissue activation seen here.
One of the most widely studied neuroendocrinological changes associated with PCB exposure is alterations to the thyroid system. Maternal exposure to the same mixture and dose of PCBs used in this study dramatically reduces circulating levels of thyroxine in the offspring (Poon et al., 2015). Although it is unknown if decreased levels of thyroxine contribute to later changes in flavoprotein activation in the AC, it is known that thyroid hormones are integral to the normal development and later functioning of the auditory system (Crofton and Zoeller, 2005; Melse-Boonstra and Mackenzie 2013, Ng et al., 2013) and are important for the normal development of white matter and establishment of layers within the cortex (Gilbert et al., 2014; Berbel et al., 2001). It would be interesting for future studies to determine the role, if any, of thyroid hormone in mediating effects on AC function by examining whether thyroid hormone replacement during development ameliorates the changes seen in the current study.
The current study used only female, and not male, rats to study differences in the activation of the AC after developmental exposure to PCBs. We suspect that males would show similar effects (increase in activation in response to SR95531), given that males also show hearing loss and increases in audiogenic seizures (Bandara et al., 2016; Powers et al., 2006; 2009). However, we would suspect that the effects in males would be attenuated compared to females, given that the magnitude of increases in audiogenic seizures is larger in females.
In conclusion, PCB exposure during early development leads to long-term changes in GABAergic inhibitory signaling in the AC. Further work is warranted to further elucidate the mechanisms by which these changes occur and whether these changes result from a direct effect of PCBs in the AC or are an indirect effect in response to disrupted function of other auditory structures, such as the cochlea or IC.
Highlights.
Dams were exposed to 0 or 6 mg/kg/day PCBs during gestation and lactation
Electrical stimulation of auditory cortex slices from adult female offspring
Flavoprotein autofluorescence measured activation of auditory cortex
PCB-treated females had greater activation when GABA receptors were antagonized
These data suggest that PCBs modulate synaptic transmission in the auditory cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anezaki K, Kannan N, Nakano T. Polychlorinated biphenyl contamination of paints containing polycyclic- and Naphthol AS-type pigments. Environmental Science and Pollution Research International. 2015;22:14478–14488. doi: 10.1007/s11356-014-2985-6. [DOI] [PubMed] [Google Scholar]
- Anezaki K, Nakano T. Unintentional PCB in chlorophenylsilanes as a source of contamination in environmental samples. Journal of Hazardous Materials. 2015;287:111–117. doi: 10.1016/j.jhazmat.2015.01.026. [DOI] [PubMed] [Google Scholar]
- Bajo VM, King AJ. Cortical modulation of auditory processing in the midbrain. Frontiers in Neural Circuits. 2013;6:114, 1–12. doi: 10.3389/fncir.2012.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandara SB, Eubig PA, Sadowski RN, Schantz SL. Developmental PCB exposure increases audiogenic seizures and decreases glutamic acid decarboxylase in the inferior colliculus. Toxicological Sciences. 2016;149:335–345. doi: 10.1093/toxsci/kfv237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbel P, Auso E, Garcia-Velasco JV, Molina ML, Camacho M. Role of thyroid hormones in the maturation and organisation of rat barrel cortex. Neuroscience. 2001;107:383–394. doi: 10.1016/s0306-4522(01)00368-2. [DOI] [PubMed] [Google Scholar]
- Crinnion WJ. Polychlorinated biphenyls: persistent pollutants with immunological, neurological, and endocrinological consequences. Alternative Medicine Review: A Journal of Clinical Therapeutic. 2011;16:5–13. [PubMed] [Google Scholar]
- Crofton KM, Zoeller RT. Mode of action: neurotoxicity induced by thyroid hormone disruption during development--hearing loss resulting from exposure to PHAHs. Critical Reviews in Toxicology. 2005;35:757–769. doi: 10.1080/10408440591007304. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Rose HJ, Metherate R. Auditory thalamocortical synaptic transmission in vitro. Journal of Neurophysiology. 2002;87:361–384. doi: 10.1152/jn.00549.2001. [DOI] [PubMed] [Google Scholar]
- DeKoning EP, Karmaus W. PCB exposure in utero and via breast milk. A review. Journal of Exposure Analysis and Environmental Epidemiology. 2000;10:285–293. doi: 10.1038/sj.jea.7500090. [DOI] [PubMed] [Google Scholar]
- Gerich FJ, Funke F, Hildebrandt B, Fasshauer M, Muller M. H(2)O(2)-mediated modulation of cytosolic signaling and organelle function in rat hippocampus. European Journal of Physiology. 2009;458:937–952. doi: 10.1007/s00424-009-0672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanian Z, Maleki S, Park S, Sorenson CM, Sheibani N, Ranji M. Organ specific optical imaging of mitochondrial redox state in a rodent model of hereditary hemorrhagic telangiectasia-1. Journal of Biophotonics. 2014;7:799–809. doi: 10.1002/jbio.201300033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert ME, Ramos RL, McCloskey DP, Goodman JH. Subcortical band heterotopia in rat offspring following maternal hypothyroxinaemia: structural and functional characteristics. Journal of Neuroendocrinology. 2014;26:528–541. doi: 10.1111/jne.12169. [DOI] [PubMed] [Google Scholar]
- Hamann M, Desarmenien M, Desaulles E, Bader MF, Feltz P. Quantitative evaluation of the properties of a pyridazinyl GABA derivative (SR 95531) as a GABAA competitive antagonist. An electrophysiological approach. Brain Research. 1988;442:287–296. doi: 10.1016/0006-8993(88)91514-4. [DOI] [PubMed] [Google Scholar]
- Hu D, Hornbuckle KC. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environmental Science & Technology. 2010;44:2822–2827. doi: 10.1021/es902413k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson TR, Issa NP. Functional Imaging with Mitochondrial Flavoprotein Autofluorescence: Theory, Practice, and Applications. In: Frostig RD, editor. Vivo Optical Imaging of Brain Function. Taylor & Francis Group, LLC.; Boca Raton FL: 2009. [PubMed] [Google Scholar]
- Jacobson JL, Fein GG, Jacobson SW, Schwartz PM, Dowler JK. The transfer of polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs) across the human placenta and into maternal milk. American Journal of Public Health. 1984;74:378–379. doi: 10.2105/ajph.74.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusko TA, Sisto R, Iosif AM, Moleti A, Wimmerova S, Lancz K, Tihanyi J, Sovcikova E, Drobna B, Palkovicova L, Jureckova D, Thevenet-Morrison K, Verner MA, Sonneborn D, Hertz-Picciotto I, Trnovec T. Prenatal and postnatal serum PCB concentrations and cochlear function in children at 45 months of age. Environmental Health Perspectives. 2014;122:1246–1252. doi: 10.1289/ehp.1307473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenet T, Froemke RC, Schreiner CE, Pessah IN, Merzenich MM. Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and plasticity in rat primary auditory cortex. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7646–7651. doi: 10.1073/pnas.0701944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyniak PJ, Hansen LG, Widholm JJ, Fitzpatrick RD, Olson JR, Helferich JL, Kim KH, Sable HJ, Seegal RF, Pessah IN, Schantz SL. Formulation and characterization of an experimental PCB mixture designed to mimic human exposure from contaminated fish. Toxicological Sciences. 2005;88:400–411. doi: 10.1093/toxsci/kfi338. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Breithaupt AD, Sanes DH. Developmental hearing loss eliminates long-term potentiation in the auditory cortex. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3550–3555. doi: 10.1073/pnas.0607177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist CE, Laver DR, Birnir B. The mechanism of SR95531 inhibition at GABA receptors examined in human alpha1beta1 and alpha1beta1gamma2S receptors. Journal of Neurochemistry. 2005;94:491–501. doi: 10.1111/j.1471-4159.2005.03240.x. [DOI] [PubMed] [Google Scholar]
- Llano DA, Turner J, Caspary DM. Diminished cortical inhibition in an aging mouse model of chronic tinnitus. Journal of Neuroscience. 2012;32:16141–16148. doi: 10.1523/JNEUROSCI.2499-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melse-Boonstra A, Mackenzie I. Iodine deficiency, thyroid function and hearing deficit: a review. Nutrition Research Reviews. 2013;26:110–117. doi: 10.1017/S0954422413000061. [DOI] [PubMed] [Google Scholar]
- Mowery TM, Kotak VC, Sanes DH. Transient Hearing Loss Within a Critical Period Causes Persistent Changes to Cellular Properties in Adult Auditory Cortex. Cerebral Cortex. 2015;25:2083–2094. doi: 10.1093/cercor/bhu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health . Public health service policy on humane care and use of laboratory animals. NIH/Office of Laboratory Animal Welfare; Rockville, MD: 2002. [Google Scholar]
- National Research Council. Institute for Laboratory Animal Research . Guidelines for the care of use of mammals in neuroscience and behavioral research. National Academy Press; Washington, DC: 2003. [Google Scholar]
- Ng L, Kelley MW, Forrest D. Making sense with thyroid hormone--the role of T(3) in auditory development. Nature Reviews: Endocrinology. 2013;9:296–307. doi: 10.1038/nrendo.2013.58. [DOI] [PubMed] [Google Scholar]
- N'Gouemo P, Faingold CL. Repetitive audiogenic seizures cause an increased acoustic response in inferior colliculus neurons and additional convulsive behaviors in the genetically-epilepsy prone rat. Brain Research. 1996;710:92–96. doi: 10.1016/0006-8993(95)01356-3. [DOI] [PubMed] [Google Scholar]
- Poon E, Bandara SB, Allen JB, Sadowski RN, Schantz SL. Developmental PCB exposure increases susceptibility to audiogenic seizures in adulthood. Neurotoxicology. 2015;46:117–124. doi: 10.1016/j.neuro.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon E, Powers BE, McAlonan RM, Ferguson DC, Schantz SL. Effects of developmental exposure to polychlorinated biphenyls and/or polybrominated diphenyl ethers on cochlear function. Toxicological Sciences. 2011;124:161–168. doi: 10.1093/toxsci/kfr214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers BE, Poon E, Sable HJ, Schantz SL. Developmental exposure to PCBs, MeHg, or both: long-term effects on auditory function. Environmental Health Perspectives. 2009;117:1101–1107. doi: 10.1289/ehp.0800428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers BE, Widholm JJ, Lasky RE, Schantz SL. Auditory deficits in rats exposed to an environmental PCB mixture during development. Toxicological Sciences. 2006;89:415–422. doi: 10.1093/toxsci/kfj051. [DOI] [PubMed] [Google Scholar]
- Ross KC, Coleman JR. Developmental and genetic audiogenic seizure models: behavior and biological substrates. 2000;24:639–653. doi: 10.1016/s0149-7634(00)00029-4. [DOI] [PubMed] [Google Scholar]
- Rotschafer S, Razak K. Altered auditory processing in a mouse model of fragile X syndrome. Brain Research. 2013;1506:12–24. doi: 10.1016/j.brainres.2013.02.038. [DOI] [PubMed] [Google Scholar]
- Rotschafer SE, Razak KA. Auditory processing in fragile x syndrome. Frontiers in Cellular Neuroscience. 2014;8:19. doi: 10.3389/fncel.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehr R, Staniszewski K, Maleki S, Jacobs ER, Audi S, Ranji M. Optical imaging of tissue mitochondrial redox state in intact rat lungs in two models of pulmonary oxidative stress. Journal of Biomedical Optics. 2012;17(4) doi: 10.1117/1.JBO.17.4.046010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuki K, Hishida R, Murakami H, Kudoh M, Kawaguchi T, Watanabe M, Watanabe S, Kouuchi T, Tanaka R. Dynamic imaging of somatosensory cortical activity in the rat visualized by flavoprotein autofluorescence. The Journal of Physiology. 2003;549:919–927. doi: 10.1113/jphysiol.2003.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbings KA, Choi HW, Ravindra A, Caspary DM, Turner JG, Llano DA. Aging-related changes in GABAergic inhibition in mouse auditory cortex, measured using in vitro flavoprotein autofluorescence imaging. The Journal of Physiology. 2016;594:207–221. doi: 10.1113/JP271221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbings KA, Lesicko AM, Llano DA. The auditory corticocollicular system: molecular and circuit-level considerations. Hear Research. 2014;314:51–59. doi: 10.1016/j.heares.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N. Role of corticofugal feedback in hearing. Journal of Comparative Physiology. 2008;194:169–183. doi: 10.1007/s00359-007-0274-2. [DOI] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Developmental hearing loss disrupts synaptic inhibition: implications for auditory processing. Future neurology. 2009;4:331–349. doi: 10.2217/FNL.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trnovec T, Sovcikova E, Pavlovcinova G, Jakubikova J, Jusko TA, Hustak M, Jureckova D, Palkovicova L, Kocan A, Drobna B, Lancz K, Wimmerova S. Serum PCB concentrations and cochlear function in 12-year-old children. Environmental Science & Technology. 2010;44:2884–2889. doi: 10.1021/es901918h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Bracamontes J, Zorumski C, Weiss DS, Steinbach JH. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. Journal of Neuroscience. 1997;17:625–634. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]