Summary
Objective
Normal cognitive function is defined by harmonious interaction among multiple neuropsychological domains. Epilepsy has a disruptive effect on cognition, but how diverse cognitive abilities differentially interact with one another compared to healthy controls (HC) is unclear. This study used graph theory to analyze the community structure of cognitive networks in adults with temporal lobe epilepsy (TLE) compared with HC.
Methods
Neuropsychological assessment was performed in 100 patients with TLE and 82 HC. For each group, an adjacency matrix was constructed representing pair-wise correlation coefficients between raw scores obtained in each possible test-combination. For each cognitive network, each node corresponded to a cognitive test; each link corresponded to the correlation coefficient between tests. Global network structure, community structure and node-wise graph theory properties were qualitatively assessed.
Results
The community structure in patients with TLE was composed of fewer, larger, more mixed modules, characterizing three main modules representing close relationships between: 1) aspects of executive function (EF), verbal and visual memory, 2) speed and fluency, and 3) speed, EF, perception, language, intelligence, and nonverbal memory. Conversely, controls exhibited a relative division between cognitive functions, segregating into more numerous, smaller modules consisting of: 1) verbal memory, 2) language, perception and intelligence, 3) speed and fluency, and 4) visual memory and EF. Overall node-wise clustering coefficient and efficiency were increased in TLE.
Significance
Adults with TLE demonstrate a less clear and poorly structured segregation between multiple cognitive domains. This panorama suggests a higher degree of interdependency across multiple cognitive domains in TLE, possibly indicating compensatory mechanisms to overcome functional impairments.
Keywords: cognitive network, temporal lobe epilepsy, graph theory
1. Introduction
Cognitive impairment in temporal lobe epilepsy (TLE) is a well-recognized complication of the disorder [1] with memory impairment one of the more prominent cognitive deficits [2], however, cognitive morbidity in TLE may extend to other cognitive domains including executive function, language, processing speed, intelligence, motor dexterity, and other abilities [3–6]. The cause(s) of these potentially widespread cognitive effects were initially uncertain, but the changing view of the neurobiology of epilepsy offers some insight. TLE has classically been viewed as a pathological process localized to a discrete seizure focus, a view that has evolved to one that focal epilepsies, including TLE, involve disruption of large-scale networks characterized by pathological hyper-excitability and other forms of seizure-related neural plasticity [7–12]. The networks implicated in TLE nearly always involve the hippocampal formation and its functional circuits, including the anterior and lateral temporal lobe, insula, thalamus, cingulate gyrus, and prefrontal cortex, providing likely substrates for memory, language, and other cognitive impairments reported in TLE. This disruption of large-scale neural networks could reasonably be expected to alter the natural relationships between the cognitive abilities that these networks moderate and result in a broad alteration of the usual relationships between the neuropsychological measures themselves.
Cognitive abilities do not exist in isolation. It is the dynamic interaction among different neuropsychological domains that permit healthy cognitive function [13], and these interactions may vary as a function of external demands and internal goals [14, 15]. Nonetheless, an altered interplay between impaired memory due to hippocampal pathology and other cognitive functions may serve to disrupt the typical overall architecture of cognitive processes in TLE. While this is a reasonable inference, there has been little empirical research to support or refute the hypothesis of an altered cognitive network using conventional neuropsychological measures.
In this study, we employed an innovative approach to investigate the impact of TLE on the global landscape of cognition, defined by the interaction of multiple cognitive domains. Knowing that cognitive domains are inter-dependent [16], it is possible to consider the associations between neuropsychological functions as a cognitive network. Thus, the architecture of the cognitive network can be assessed using formal methods to determine network conformation, i.e., graph theory. Here we assessed the influence of each cognitive domain on the conformation of neuropsychological structure and the impact that TLE has on this framework. Applying graph theory analysis to neuropsychological tests, we investigated the relationships that exist between diverse neuropsychological abilities as well as global network structures such as clustering coefficient, betweenness centrality and network efficiency in patients with TLE and healthy controls (HC), as well as in specific comparisons of HC to patients with unilateral left and right TLE.
We hypothesized that a graph theory approach could provide a novel method for investigating cognition and the organization of cognitive domains in TLE. As will be shown, by assessing the community structure of cognitive functions (i.e., cognitive modules) it was possible to observe a less clear segregation between multiple cognitive domains and more mixed modules in patients with TLE, suggesting a deficient supporting structure for neuropsychological function.
2. Methods
2.1. Subjects
Neuropsychological assessments were performed on 100 patients with TLE and 82 HC, the groups comparable in gender and years of education with the clinical and demographic characteristics presented in Table 1. As would be anticipated, Full Scale IQ was lower in the TLE group (92.7) compared to controls (106.4, p<.001) as was Performance IQ (HC= 110, TLE= 96; p<.001) and Verbal IQ (HC= 103, TLE= 92; p<.001).
Table 1.
Group characteristics.
Characteristics of controls and temporal lobe epilepsy participants.
| Variable | Healthy Controls (n=82) | Patients (n=100) |
|---|---|---|
| Age in Years (M, SD) | 33.6 (12.5) | 37.1 (11.6) |
| Sex (F/M) | 49/33 | 33/67 |
| Years of Education (M, SD) | 14.0 (2.4) | 12.9 (2.3) |
| FSIQ (M, SD) | 106.4 (14.4) | 92.7 (16.0) |
| Age in Years at Seizure Onset (M, SD) | 14.7 (10.6) | |
| Epilepsy Duration in Months (M, SD) | 266.2 (144.3) | |
| Seizure Localization | Right Temporal: 25 (43.1%) Left Temporal: 21 (36.2%) Bilateral L = R: 6 (10.3%) Bilateral L > R: 3 (5.2%) Bilateral R > L: 1 (1.7%) Indeterminate: 2 (3.4%) |
The participants were drawn from an outpatient cohort of patients with chronic epilepsy, diagnosed according to criteria defined by the International League Against Epilepsy (ILAE) [17, 18]. All subjects were receiving pharmacological anti-epileptic treatment at the time of their neuropsychological assessment. Initial selection criteria for the participants with epilepsy included: a) chronological age between 18–65 years; b) WAIS-III IQ> 69; c) complex partial seizures of definite or probable temporal lobe origin based on consensus conference review; d) no MRI abnormalities other than medial temporal atrophy on clinical interpretation; and e) no other neurological disorder. Board-certified neurologists with special expertise in epileptology reviewed each patient's medical records. This review, blinded to all quantitative imaging and cognitive data, included seizure semiology, previous EEGs, clinical neuroimaging reports, and all available medical records. Based on this review, each patient was classified as having complex partial seizures of definite, probable, or possible temporal lobe origin. Definite TLE was defined by continuous video-EEG monitoring of spontaneous seizures demonstrating unequivocal temporal lobe onset of typical recurring spontaneous seizures as reported by patient and family members; probable TLE was determined by review of clinical semiology with features reported to identify reliably complex partial seizures of temporal lobe origin versus onset in other regions (e.g., frontal) in conjunction with interictal EEGs, neuroimaging findings, and developmental and clinical history. Only those meeting criteria for definite and probable TLE proceeded to recruitment for study participation; patients with possible TLE were excluded. Of the 100 patients with epilepsy, 58 underwent inpatient ictal EEG monitoring which revealed 21 with unilateral left temporal lobe onset and 26 with unilateral right temporal lobe onset and 12 with bilateral temporal lobe onset (Table 1). The remaining patients were not ictally monitored. The left and right TLE groups were used in secondary analyses addressing TLE laterality differences in relation to HC. Initial selection criteria for HC included: a) chronological age between 18 and 65; b) WAIS-III Full Scale IQ > 69; c) either a friend, relative, or spouse of the participant with epilepsy; d) no current substance abuse, or medical or psychiatric condition that could affect cognitive functioning; and e) no episodes of loss of consciousness greater than 5 minutes, identified developmental learning disorder, or repetition of a grade in school. This project was reviewed and approved by the University of Wisconsin Madison Institutional Review Board, and all participants were informed of the nature and purposes of this investigation, their questions were answered, and signed informed consent was obtained.
2.2. Neuropsychological Assessment
A comprehensive neuropsychological test battery from which 30 different cognitive measures were extracted was administered to all subjects. This battery assessed multiple domains including standard measures of intelligence (WAIS-III) [19], language (object naming [Boston Naming Test] [20], lexical fluency [ Controlled Oral Word Association Test] [21], several categories of semantic fluency (animals, living and nonliving objects), visuoperceptual/spatial skills (facial discrimination [Facial Recognition Test] [22], line orientation [Judgment of Line Orientation Test [22]), immediate and delayed verbal and visual memory [WMS-III [23], Verbal and Nonverbal Selective Reminding Tests [24, 25] executive function [novel problem solving (Wisconsin Card Sort Test) [26], simple and complex speeded psychomotor processing [Trail Making Test A &B] [27], response inhibition [Stroop Test] [28], speeded fine motor dexterity [Grooved Pegboard Test] [27], and word reading [WRAT III][29]. All test measures included in this investigation are listed in Table 2, a file with means and SDs for the test measures can be found in supplementary Table 1. All participants were assessed by trained research staff under the direction of clinical neuropsychologists. Administration of tests followed a fixed format.
Table 2.
Neuropsychological test battery
Neuropsychological test battery employed in this study. The ordering of tests demonstrated here is the same as from the adjacency matrix in Figure 1.
| ID | Label | Domain tested |
|---|---|---|
| 1 | PGDOM1 | Speeded dexterity—dominant hand |
| 2 | PGNDOM1 | Speeded dexterity —nondominant hand |
| 3 | WCSPR1 | Problem solving perseverative errors |
| 4 | WCSTERR1 | Problem solving errors (WCST) |
| 5 | TRLA1 | Psychomotor speed (TMT-A) |
| 6 | TRLB1 | Speeded mental flexibility (TMT-B) |
| 7 | STROOC1 | Stroop Test color naming |
| 8 | WSCAT1 | Problem solving categories (WCST) |
| 9 | VC | Verbal comprehension (WAIS-III) |
| 10 | PO | Perceptual organization (WAIS-III) |
| 11 | PS | Processing speed (WAIS-III) |
| 12 | WM | Working memory (WAIS-III) |
| 13 | AUD_I | Auditory memory-immediate (WMS-III) |
| 14 | AUD_D | Auditory memory-delayed (WMS-III) |
| 15 | VM_I | Visual memory-immediate (WMS-III) |
| 16 | VM_D | Visual memory-delayed (WMS-III) |
| 17 | AUDCRAW1 | Auditory recognition |
| 18 | BNTSPON1 | Confrontation naming (BNT) |
| 19 | FACREC1 | Face perception |
| 20 | FLUANIM1 | Fluency animals |
| 21 | FLUCFL1 | Fluency letters |
| 22 | FLULIVE1 | Fluency living |
| 23 | FLUNLIV1 | Fluency nonliving |
| 24 | VCLTR1 | Verbal recall (SRT) |
| 25 | VSUM1 | Verbal learning (SRT) |
| 26 | JOLOBENT1 | Line orientation |
| 27 | NVCLTR1 | Nonverbal recall (SRT) |
| 28 | NVSUM1 | Nonverbal learning (SRT) |
| 29 | READRAW1 | Word reading (WRAT-III) |
| 30 | STROOCW1 | Stroop Interference |
2.3. Network Analysis
In order to describe the inter-relationship between different cognitive domains, a correlation matrix was computed between the performance of different neuropsychological tests, separately for both groups, i.e., patients with TLE and HC. We evaluated the correlation coefficient between each possible pair of tests, whereby all cognitive tests were adjusted so that higher scores reflected better performance. A weighted adjacency matrix was then constructed for both groups (patients with TLE and controls) where each link represented the correlation coefficient between the tests in the corresponding row and column. We also performed separate secondary analyses comparing left TLE to healthy controls and right TLE to healthy controls. The adjacency matrices demonstrated the cross-correlations between scores for the neuropsychological tests for controls and then for patients with TLE. The adjacency matrix is a matrix with 1’s on its diagonal with truncated negative links; therefore ranging from 0 to 1. Since negative links may characterize an indirect measure of association by indicating when test performances vary in opposite directions, in order to enable the visual representation of networks, and to permit graph theory metric calculations, only positive correlation coefficients were maintained [30]. We acknowledge that a negative correlation may represent an indirect and orthogonal association between tests, nonetheless, we opted to evaluate networks composed only of links representing positive correlations since the goal of this study was to assess the synergistic association between tests and also because graph theory methods are validated for positive networks. In summary, the cognitive network was comprised of 30 nodes, whereby each node represents one cognitive test, and weighted links between the nodes represented the strength of the positive correlation of those nodes across all individuals in each group, i.e., patients with TLE or controls.
2.3.1. Visual representation of network structure
Two-dimensional graphs were reconstructed in order to define the overall structure of cognition in each group. The data were exported to the software Gephi (http://gephi.github.io/) and displayed using a Force Atlas algorithm [31] (attraction strength =10, repulsion strength =100, gravity=30). To preserve the most important relationships and to improve visualization of the network structure, graphs were reconstructed using only links above the 70% percentile of weight in each group, i.e., links below the 70% percentile were given weight=0, while the remaining links maintained their original weights.
Using Gephi the community structure of each network was calculated, and each node was coded in accordance with module participation, whereby nodes with a strong inter-relationship were arranged within a module and nodes with a relative lower relationship were arranged outside the module.
2.3.2. Local network parameters
We assessed node-wise network parameters using the Brain Connectivity Toolbox within the software MATLAB [32]. Since each group yielded one network, we compared the absolute value of node measurements between groups. Specifically, we assessed 1) clustering coefficient; 2) betweenness centrality; and 3) efficiency. These measurements provide information regarding how each node interacts and influences the remainder of the network. Specifically, clustering coefficient estimates the degree of connected nodes, i.e., cognitive tests, around the node of interest, i.e., cognitive abilities. Betweeness centrality is a metric that measures the relative importance of a node, i.e., cognitive test within the network. It estimates the fraction of the shortest paths that go through each node, i.e., cognitive test. Local efficiency is defined as the inverse of the shortest path length between linked nodes that are surrounding the node of interest.
3. Results
3.1. Visual representation of network structure
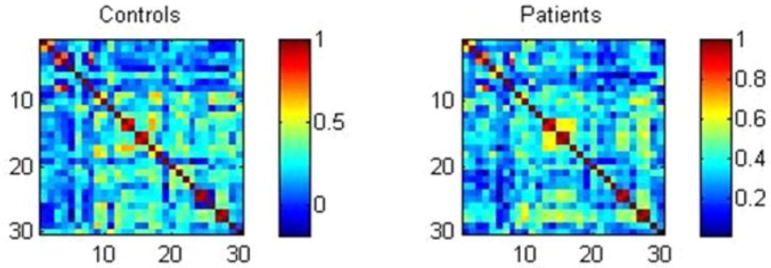
Adjacency matrices represent the correlations between administered cognitive tests. These were performed separately for the HC and TLE groups (Figure 1). The order of tests and measures in the adjacency matrices is the same order as the neuropsychological tests and measures listed in Table 2. To highlight a few findings, while Figure 1 demonstrates arguably expected high correlation between different but related measures from a particular problem solving test (WCST) (3 and 4 and 8, respectively, from Table 2) in both patients with TLE and controls, patients with TLE presented stronger cross-correlation between immediate and delayed auditory and visual memory measures (13–16 in Table 2) while controls showed higher cross-correlations between tests of verbal comprehension and confrontation naming (test 9 and 18). Thus, observed are similarities within both the TLE and control groups, disruptions of normal associations seen in controls, as well as epilepsy-specific anomalous associations.
Figure 1.
Adjacency matrices demonstrating the cross-correlations between scores in neuropsychological tests for controls and patients. Neuropsychological tests are numbered in accordance with Table 2.
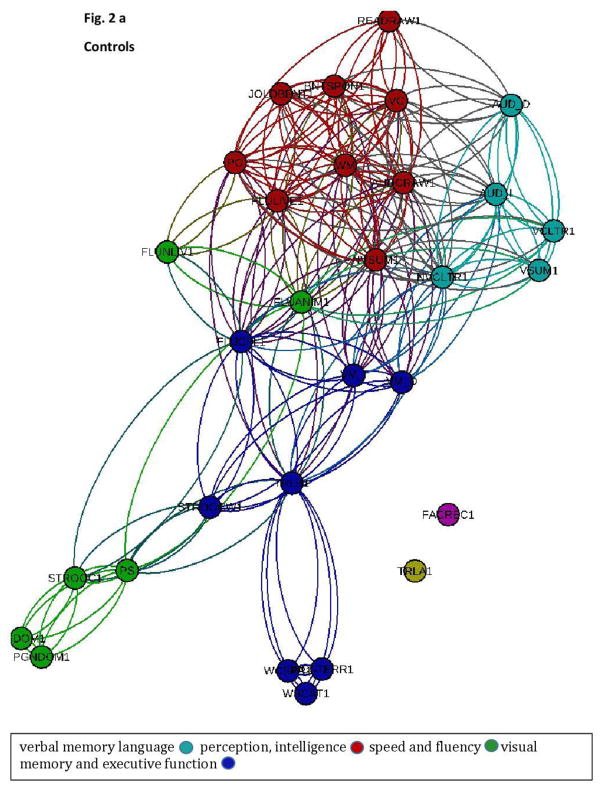
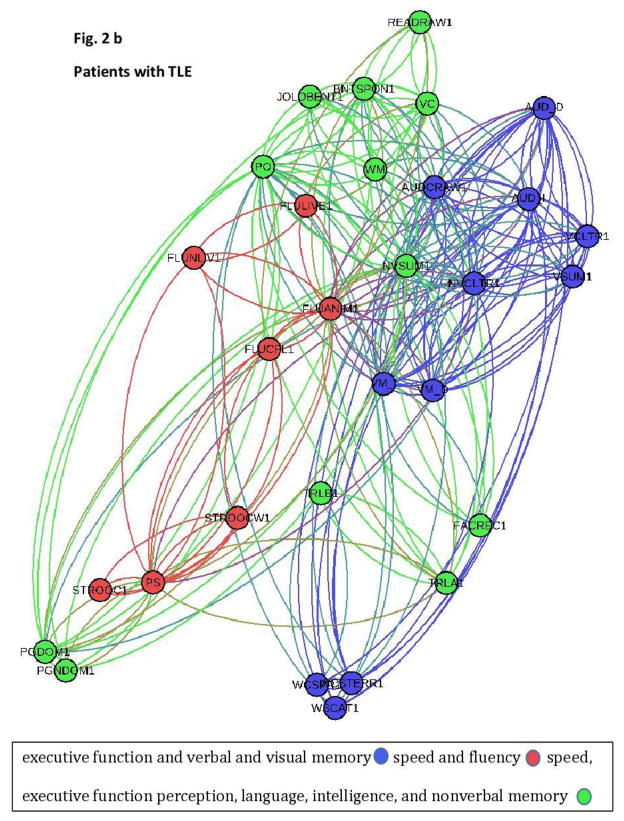
The frequency of co-associations of nodes within a module was calculated and the two-dimensional graphs illustrating the modularity, i.e., the structure of the cognitive network for each group, is demonstrated in Figure 2a (controls) and b (TLE patients).
Figure 2.
a+b This two-dimensional graph representation illustrates the cognitive networks representing the spatial relationship between cognitive tests (Figure 2 a= controls; Figure 2b= patients). The spatial distribution of nodes was calculated using a force-atlas graph algorithm. Nodes demonstrating stronger connections are located closer in space, whilst nodes with fewer connections tend to drift away. Nodes with a similar color belong to the same module, whereas each module is composed of nodes with the highest association (i.e., connectivity strength) between in-module nodes, and the lowest association with nodes outside the module.
There were notable qualitative differences in network organization between groups. While controls demonstrated a relative division between cognitive functions which segregated into more numerous and smaller modules consisting of: 1) verbal memory (light blue), 2) language, perception, and intelligence (red), 3) speed and fluency (green), and 4) visual memory and executive function (dark blue), patients with TLE showed fewer, larger and more mixed modules. Specifically, patients with TLE were characterized by three main modules representing close relationship between: 1) executive function and verbal and visual memory (dark blue), 2) speed and fluency (red), and 3) speed, executive function, perception, language, intelligence, and nonverbal memory (green).
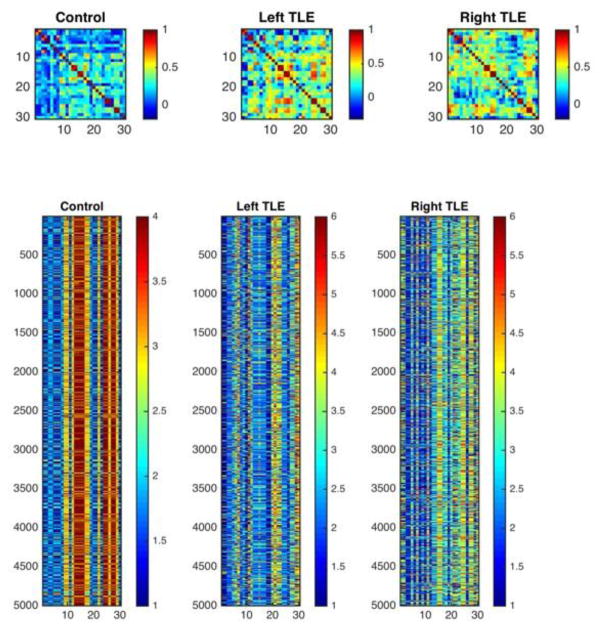
Secondary supplemental analyses were undertaken for the subset of TLE patients with ictal confirmation of left or right TLE where each group was compared to controls to inquire into possibility laterality effects. The adjacency matrices (separately for HC, left TLE and right TLE respectively) representing the correlations between tests and measures are provided in Figure 3. The order of tests in the adjacency matrices was the same order as the neuropsychological tests and measures listed in Table 2. Additional supplemental analyses controlled for IQ and education.
Figure 3.
Adjacency matrices demonstrating the cross-correlations between scores in neuropsychological tests for controls and patients with left and right TLE controlling for age and education. Neuropsychological tests are numbered in accordance with Table 2.
It can be appreciated that the groups of left and right TLE continued to exhibit significant differences in cognitive correlations, as was true of the total TLE group, compared to controls. Overall, patients with left and right TLE showed higher cross-correlation between distinct cognitive tests and domains as was true of the combined group of TLE patients. Specifically, the overall pattern of less differentiation of cognitive modules in TLE compared to controls was true for both the left and right TLE groups as was the case for the overall group of TLE patients.
3.2. Local network metrics
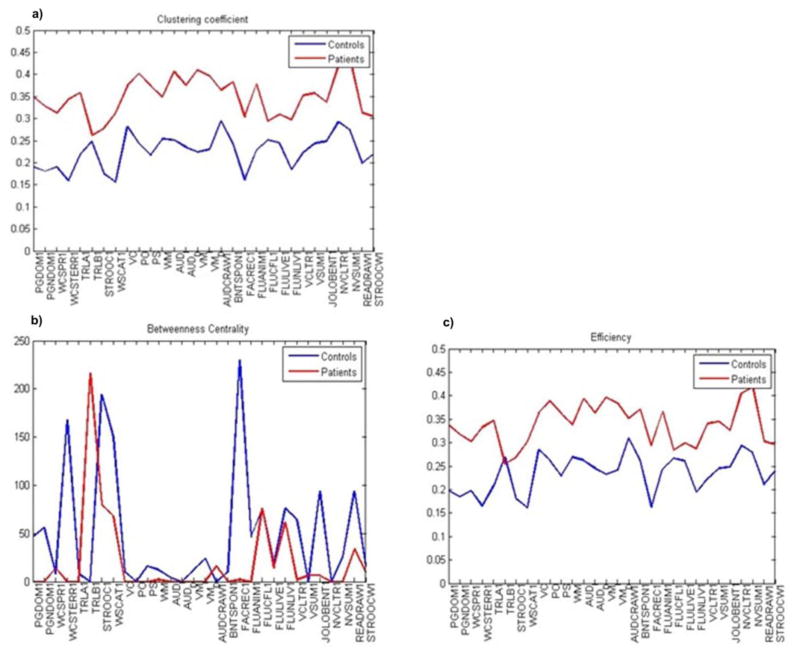
Overall, patients and controls demonstrated different network metrics as demonstrated in Figure 4a–c with overall increased node-wise clustering coefficient and efficiency in patients with TLE. Betweenness centrality showed a peak in patients with TLE in psychomotor speed and in Stroop Interference, as opposed to confrontation naming in HC, assuming suboptimal interconnections of the speed domain in patients with TLE and of executive function in HC. Increased clustering coefficient and efficiency in patients with TLE points towards a more segregated cognitive network. Also, the results suggest a higher influence of specific nodes, i.e., cognitive tests/measures, over the network, as demonstrated by higher efficiency and clustering coefficient in patients with TLE.
Figure 4.
Distribution plots demonstrating global network measures. Overall node-wise clustering coefficient and efficiency were increased in patients with TLE. Betweeness centrality showed a peak in patients with TLE in psychomotor speed and in stroop interference, as opposed to confrontation naming in HC.
High clustering coefficients indicate nodes that are part of a “clique” of densely inter-connected neighbors. Efficiency is a measure based on shortest paths between each node’s neighbors, i.e., cognitive test, which show how efficient the communication is between immediate neighbors of a node. In our study, tests of nonverbal recall demonstrated the highest clustering coefficient and efficiency in patients with TLE, which means that nonverbal tests are part of a clique of densely inter-connected nodes (i.e., cognitive tests) and have the most efficient communication with neighboring nodes. Betweeness centrality measures the importance of a node in the communication of a network. Our findings showed that in patients with TLE psychomotor speed and response inhibition measures play an important role in the communication between other nodes, i.e., cognitive tests/measures, and can be considered a hub of this network. In turn, in healthy controls confrontation naming has an important role in communicating with other nodes.
The findings in subgroups with unilateral TLE confirmed the results of higher clustering coefficient and efficiency in the combined TLE group versus HC. Betweeness centrality measures showed that in patients with left TLE speed, fluency and auditory recognition play an important role in the communication between other nodes, i.e., cognitive tests. In patients with right TLE the highest betweeness centrality was found across tests of verbal recall and response inhibition. Differences in cognitive architecture as defined by network science persist when multiple potential variables were examined either in the overall TLE versus control comparisons or similar analyses within the lateralized left TLE and right TLE versus controls (please see supplementary Figures 1–4), which shows that the reported findings are not an artifact of either IQ or education with differences persisting within both the left and right TLE groups.
4. Discussion
Cognitive function can be adversely affected in chronic TLE [2, 33]. Patterns of abnormal cognition have been characterized by analysis of individual test scores or combinations of test scores, examined both cross-sectionally and prospectively. Furthermore, specific cognitive abilities have been examined not only with paper-and-pencil tests, but with diverse imaging techniques. For example, impaired working memory performance in epilepsy has been reported in conventional neuropsychological studies [34] as well as in effective connectivity [35] and functional imaging studies [36, 37].
As noted, when patients with TLE are compared to HC across traditional measures of intelligence, language, perception, memory, executive function, and/or psychomotor speed, the resultant patterns typically indicate poorer performance to varying degrees in the TLE group [33], with the network analyses providing new and arguably more informative findings regarding disruptions in the cognitive networks of patients with TLE.
In our study, TLE versus HC performance differences were significant for all neuropsychological tests but WSCAT1 (problem solving; p=.624). Profiles such as these help to define and characterize the presence, degree and severity of cognitive morbidity associated with an epilepsy syndrome, such as TLE. Several unanswered question remain however, such as how diverse cognitive abilities and domains correlate and interact with one another to maintain cognition in epilepsy patients compared to controls; if the interrelationships are different, and if so, in what ways. Also unclear, and of considerable interest for future studies, is how these cognitive interrelationships and networks may change over time with aging, or in association with other clinical features of the disorder (e.g., increasing duration of epilepsy, seizure frequency/severaity, medication changes) — identical issues that have been raised in association with standard analyses of neuropsychological data.
Using graph theory as a novel approach we assessed the relationships that exist between diverse neuropsychological measures in the entire cognitive network organization, and identified important distinctions between participants with TLE and HC. Compared to controls, adults with TLE demonstrated a cognitive network landscape whereby segregation between multiple cognitive domains was less clear and poorly structured. Global network measures supported these findings by revealing a network in patients with TLE that was organized into a less well segregated architecture with higher clustering coefficient and average efficiency. Although specific cognitive domains were in the same module, the cognitive domains of memory and executive function were more separated in TLE than in HC. This finding is in line with studies reporting memory, cognitive/psychomotor speed and executive function to be more vulnerable to adverse changes than others [38].
The current approach provides evidence that both the interdependency of memory and executive function as well as their inter-relationship with multiple different modules may serve as a possible pathway of reorganization for cognitive deficits that are well-known in TLE. These patterns of reorganization might be related to functional compensation [39, 40] suggesting a higher degree of interdependency across multiple cognitive domains in patients with TLE. One explanation is this represents a compensatory mechanism to overcome functional impairments as networks reorganize in an attempt to maintain neurocognitive functioning.
Several lines of imaging evidence support the presence of cognitive reorganization and compensation in epilepsy. Milian and colleagues reported that by using the right hippocampus and left extratemporal mesial temporal lobe regions, patients with left mesial TLE showed a preoperative reorganization of verbal memory [41]. Benjamin et al. demonstrated that patients with mesial TLE used medial temporal lobe structures contralateral to the seizure focus differentially and extra-medial temporal lobe regions to a greater degree [42]. Functional reorganization of verbal memory processing in patients with left medial TLE has been found due to a failure of the left mesial temporal lobe system [43] and partial reorganization of the language network in pediatric epilepsy patients [44]. Although these studies demonstrated that cognitive reorganization is possible in patients with epilepsy, the results should be considered cautiously. While there is a broad consensus regarding which brain regions are involved with distinct cognitive tests, it may be possible that individuals with the same test scores (or even test scores that yield similar correlation/association patterns among the measures) may still be implementing the tasks with very different networks.
It is also possible that the interrelationship between different cognitive domains could be taken advantage of in cognitive retraining or rehabilitation attempts as has been demonstrated in training studies. For instance, working memory capacity could be expanded through training [45] which might subsequently contribute to improvements in a variety of linked cognitive skills [46]. Fluid intelligence and cognitive control have been found to improve through a combination of multiple working memory training procedures. In addition to evidence that training in a specific cognitive domain can improve cognitive skills in multiple other domains, epilepsy can activate processes of reorganization with some effectiveness.
5. Limitations and Future Directions
Neuropsychological tests, no matter how well analyzed, represent statically obtained measures of cognition, and cannot capture the dynamic interrelationships that occur in day-to-day life which vary as a function of external demands, internal goals, and the degree of relevance of a specific cognitive ability to the behavior under question [13, 14, 47]. That said, understanding the nature and pattern of relationships that exist between diverse neuropsychological measures, and how these may differ in epilepsy compared to controls, provides a novel insight into their static relationship which infers different patterns in daily life, which no one has successfully demonstrated yet.
TLE is a heterogeneous condition and going forward it will prove useful to examine patients by a number of potentially relevant factors, for example, the underlying neuropathological substrate (MTLE, MRI negative TLE, lesional syndromes of TLE) [33, 48]. It may be that patients with underlying mesial temporal sclerosis may present with networks that differ from, for example, so called MRI negative epilepsy. How common comorbidities, such as depression or anxiety, may or may not affect cognitive networks would be of interest as it would be in functional imaging studies. The impact of these and a myriad of other considerations related to the cause, course and consequences of TLE must be kept in mind.
Most importantly, the current approach introduces a new avenue to characterize and understand the impact of TLE on cognition, as assessed by traditional neuropsychological measures, by characterizing the cognitive network organization in its entirety using graph theory. Examining cognitive network differences in patients with TLE suggests that underlying networks of cognitive domains, rather than isolated domains, play a crucial role in patients with TLE. More specifically, this study provides both a method to study rich and complex cognitive data as well as a new perspective on those mechanisms by which epilepsy disrupts neuropsychological functioning. Importantly, how diverse clinical seizure features (e.g., laterality of TLE, seizure severity, specific AED dose and/or therapy across subjects, lifetime number of generalized tonic-clonic seizures) impact cognitive network organization remains a clinically important issue.
Supplementary Material
Highlights.
Cognitive domains can be impaired in TLE.
The interaction between domains compared to controls is unclear.
Graph theory has been assessed to evaluate the architecture of cognitive networks.
Cognitive network organization in TLE is less well segregated and structured.
Acknowledgments
The work was supported by grants from the NIH National Institute of Neurological Disorders and Stroke (K23 NS060993, J.J.L.; 2RO1-37738, B.P.H.). The project was also supported by the Clinical and Translational Science Award program, through the NIH National Center for Advancing Translational Sciences (grant number UL1TR000427), and the University of Wisconsin. We thank Paul Rutecki MD, Raj Sheth MD, and Fred Edelman, MD for study participation and subject recruitment as well as colleagues Dr. Brian Bell, Jana Jones, Daren Jackson, and Michael Seidenberg.
Footnotes
Disclosure: None of the authors has any conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lin JJ, Mula M, Hermann BP. Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. Lancet. 2012;380:1180–92. doi: 10.1016/S0140-6736(12)61455-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helmstaedter C, Witt JA. Clinical neuropsychology in epilepsy: theoretical and practical issues. Handb Clin Neurol. 2012;107:437–59. doi: 10.1016/B978-0-444-52898-8.00036-7. [DOI] [PubMed] [Google Scholar]
- 3.Martin RC, Sawrie SM, Gilliam FG, Palmer CA, Faught E, Morawetz RB, et al. Wisconsin Card Sorting performance in patients with temporal lobe epilepsy: clinical and neuroanatomical correlates. Epilepsia. 2000;41:1626–32. doi: 10.1111/j.1499-1654.2000.001626.x. [DOI] [PubMed] [Google Scholar]
- 4.Hamberger MJ. Object naming in epilepsy and epilepsy surgery. Epilepsy & behavior : E&B. 2015 doi: 10.1016/j.yebeh.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Sung C, Jones JE, Jackson DC, Chan YC, Chan F, Seidenberg M, et al. Age-accelerated psychomotor slowing in temporal lobe epilepsy. Epilepsy research. 2013;103:231–6. doi: 10.1016/j.eplepsyres.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermann BP, Seidenberg M, Schoenfeld J, Davies K. Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Archives of neurology. 1997;54:369–76. doi: 10.1001/archneur.1997.00550160019010. [DOI] [PubMed] [Google Scholar]
- 7.Maccotta L, He BJ, Snyder AZ, Eisenman LN, Benzinger TL, Ances BM, et al. Impaired and facilitated functional networks in temporal lobe epilepsy. Neuroimage Clin. 2013;2:862–72. doi: 10.1016/j.nicl.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson MP. Large scale brain models of epilepsy: dynamics meets connectomics. J Neurol Neurosurg Psychiatry. 2012;83:1238–48. doi: 10.1136/jnnp-2011-301944. [DOI] [PubMed] [Google Scholar]
- 9.Stefan H, Lopes da Silva FH. Epileptic neuronal networks: methods of identification and clinical relevance. Front Neurol. 2013;4:8. doi: 10.3389/fneur.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terry JR, Benjamin O, Richardson MP. Seizure generation: the role of nodes and networks. Epilepsia. 2012;53:e166–9. doi: 10.1111/j.1528-1167.2012.03560.x. [DOI] [PubMed] [Google Scholar]
- 11.Gleichgerrcht E, Kocher M, Bonilha L. Connectomics and graph theory analyses: Novel insights into network abnormalities in epilepsy. Epilepsia. 2015 doi: 10.1111/epi.13133. [DOI] [PubMed] [Google Scholar]
- 12.Bernhardt BC, Bonilha L, Gross DW. Network analysis for a network disorder: The emerging role of graph theory in the study of epilepsy. Epilepsy Behav. 2015;50:162–70. doi: 10.1016/j.yebeh.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Boyer KM, JJallo OGI. Pediatric Epilepsy Surgery: Preoperative Assessment and Surgical Treatment Cataltepe. Thieme Medical Publishers; N.Y: 2010. Preoperative Neuropsychological and Cognitve Assessment; pp. 104–10. [Google Scholar]
- 14.Kellermann TS, Caspers S, Fox PT, Zilles K, Roski C, Laird AR, et al. Task- and resting-state functional connectivity of brain regions related to affection and susceptible to concurrent cognitive demand. Neuroimage. 2013;72:69–82. doi: 10.1016/j.neuroimage.2013.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci U S A. 2013;110:16616–21. doi: 10.1073/pnas.1315235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deary IJ. Individual differences in cognition: British contributions over a century. Br J Psychol. 2001;92:217–37. [PubMed] [Google Scholar]
- 17.Blume WT, Luders HO, Mizrahi E, Tassinari C, van Emde Boas W, Engel J., Jr Glossary of descriptive terminology for ictal semiology: report of the ILAE task force on classification and terminology. Epilepsia. 2001;42:1212–8. doi: 10.1046/j.1528-1157.2001.22001.x. [DOI] [PubMed] [Google Scholar]
- 18.Engel J., Jr Report of the ILAE classification core group. Epilepsia. 2006;47:1558–68. doi: 10.1111/j.1528-1167.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio TX: The Psychological Corporation; 1997. [Google Scholar]
- 20.Goodglass HKE. Boston Diagnostic Aphasia Examination. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 21.Benton A, Hamsher K, Sivan A. Multilingual Aphasia Examination. 3. Iowa City: University of Iowa; 1994. [Google Scholar]
- 22.Benton A, Sivan A, Hamsher K, Varney NR, Spreen O. Contributions to Neuropsychological Assessment: A Clinical Manual. 2. New York: Oxford University Press; 1994. [Google Scholar]
- 23.Wechsler D. Wechsler Memory Scale. 3. San Antonio TX: The Psychological Corporation; 1997. [Google Scholar]
- 24.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–25. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 25.HB Selective reminding for analysis of memory and learning. J Verbal Learning Behav. 1973;12:543–50. [Google Scholar]
- 26.Kongs S, Thompson L, Iverson G, Heaton R. Wisconsin Card Sorting Test - 64 Card Version. Lutz FL: Psychological Assessment Resources; 2000. [Google Scholar]
- 27.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tuscon AZ: Neuropsychology Press; 1993. [Google Scholar]
- 28.Trenerry M, Crosson B, DeBoe J, Leber W. Stroop Neuropsychological Screening Test manual. Odessa, FL: Psychological Assessment Resources (PAR); 1989. [Google Scholar]
- 29.Wilkinson GS. Wide Range Achievement Test Revision 3. Wilmington, DE: Jastak Association; 1993. [Google Scholar]
- 30.Kaiser M. A tutorial in connectome analysis: topological and spatial features of brain networks. NeuroImage. 2011;57:892–907. doi: 10.1016/j.neuroimage.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 31.Jacomy M, Venturini T, Heymann S, Bastian M. ForceAtlas2, a continuous graph layout algorithm for handy network visualization designed for the Gephi software. PLoS ONE. 2014;9:e98679. doi: 10.1371/journal.pone.0098679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52:1059–69. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Oyegbile TO, Dow C, Jones J, Bell B, Rutecki P, Sheth R, et al. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;62:1736–42. doi: 10.1212/01.wnl.0000125186.04867.34. [DOI] [PubMed] [Google Scholar]
- 34.Stretton J, Thompson PJ. Frontal lobe function in temporal lobe epilepsy. Epilepsy Res. 2012;98:1–13. doi: 10.1016/j.eplepsyres.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campo P, Garrido MI, Moran RJ, Garcia-Morales I, Poch C, Toledano R, et al. Network reconfiguration and working memory impairment in mesial temporal lobe epilepsy. Neuroimage. 2013;72:48–54. doi: 10.1016/j.neuroimage.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stretton J, Winston G, Sidhu M, Centeno M, Vollmar C, Bonelli S, et al. Neural correlates of working memory in Temporal Lobe Epilepsy--an fMRI study. Neuroimage. 2012;60:1696–703. doi: 10.1016/j.neuroimage.2012.01.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campo P, Maestu F, Garcia-Morales I, Gil-Nagel A, Strange B, Morales M, et al. Modulation of medial temporal lobe activity in epilepsy patients with hippocampal sclerosis during verbal working memory. Journal of the International Neuropsychological Society : JINS. 2009;15:536–46. doi: 10.1017/S135561770909078X. [DOI] [PubMed] [Google Scholar]
- 38.Hermann BP, Seidenberg M, Dow C, Jones J, Rutecki P, Bhattacharya A, et al. Cognitive prognosis in chronic temporal lobe epilepsy. Annals of neurology. 2006;60:80–7. doi: 10.1002/ana.20872. [DOI] [PubMed] [Google Scholar]
- 39.Guedj E, Bettus G, Barbeau EJ, Liegeois-Chauvel C, Confort-Gouny S, Bartolomei F, et al. Hyperactivation of parahippocampal region and fusiform gyrus associated with successful encoding in medial temporal lobe epilepsy. Epilepsia. 2011;52:1100–9. doi: 10.1111/j.1528-1167.2011.03052.x. [DOI] [PubMed] [Google Scholar]
- 40.Tracy JI, Osipowicz K, Spechler P, Sharan A, Skidmore C, Doucet G, et al. Functional connectivity evidence of cortico-cortico inhibition in temporal lobe epilepsy. Hum Brain Mapp. 2014;35:353–66. doi: 10.1002/hbm.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milian M, Zeltner L, Erb M, Klose U, Wagner K, Frings L, et al. Incipient preoperative reorganization processes of verbal memory functions in patients with left temporal lobe epilepsy. Epilepsy & behavior : E&B. 2015;42:78–85. doi: 10.1016/j.yebeh.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 42.Benjamin CF, Saling MM, Wood AG, Reutens DC. Elemental spatial and temporal association formation in left temporal lobe epilepsy. PloS one. 2014;9:e100891. doi: 10.1371/journal.pone.0100891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alessio A, Pereira FR, Sercheli MS, Rondina JM, Ozelo HB, Bilevicius E, et al. Brain plasticity for verbal and visual memories in patients with mesial temporal lobe epilepsy and hippocampal sclerosis: an fMRI study. Human brain mapping. 2013;34:186–99. doi: 10.1002/hbm.21432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.You X, Adjouadi M, Guillen MR, Ayala M, Barreto A, Rishe N, et al. Sub-patterns of language network reorganization in pediatric localization related epilepsy: a multisite study. Hum Brain Mapp. 2011;32:784–99. doi: 10.1002/hbm.21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, et al. Computerized training of working memory in children with ADHD--a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44:177–86. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Chein JM, Morrison AB. Expanding the mind's workspace: training and transfer effects with a complex working memory span task. Psychon Bull Rev. 2010;17:193–9. doi: 10.3758/PBR.17.2.193. [DOI] [PubMed] [Google Scholar]
- 47.Luria AR. The working brain: An introduction to neuropsychology. Basic Books; New York: 1973. [Google Scholar]
- 48.Hermann B, Seidenberg M, Lee EJ, Chan F, Rutecki P. Cognitive phenotypes in temporal lobe epilepsy. Journal of the International Neuropsychological Society : JINS. 2007;13:12–20. doi: 10.1017/S135561770707004X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.