Abstract
Objective
Depression and bipolar disorder (negative mood disorders, NMD) are associated with dysregulated hypothalamic-pituitary-adrenal (HPA)-axis function and disrupted emotion processing. The neural networks involved in attenuation of HPA-axis reactivity overlap with the circuitry involved in perception and modulation of emotion; however, direct links between these systems are understudied. This study investigated whether cortisol activity prior to undergoing fMRI was related to neural processing of emotional information in participants with NMD.
Methods
Forty-one adults (Mage=40.33, SD=15.57) with major depression (n=29) or bipolar disorder (n=12) and 23 healthy control comparisons (Mage=36.43, SD=17.33) provided salivary cortisol samples prior to completing a facial emotion perception test during 3-Tesla fMRI.
Results
Overall, pre-scan cortisol level was positively associated with greater engagement of the dorsal anterior cingulate (dACC), inferior parietal lobule, insula, putamen, precuneus, middle and medial frontal and postcentral gyri, posterior cingulate, and inferior temporal gyrus during emotion processing of all faces. NMD status moderated this effect; in NMD participants’ pre-scan cortisol was associated with attenuated activation of the insula, postcentral gyrus, precuneus, and putamen for fearful faces and the medial frontal gyrus for angry faces relative to HCs. Cortisol-related attenuation of activation among NMD participants was also observed for facial identification in the dACC, putamen, middle temporal gyrus, precuneus, and caudate.
Conclusions
Across all participants, cortisol was associated with greater activation in several regions involved in the perception and control of emotion. However, cortisol responsivity was associated with hypoactivation of several of these regions in the NMD group, suggesting that HPA-axis activity may selectively interfere with the potentially adaptive recruitment of circuits supporting emotion perception, processing and/or regulation in mood disorders.
Keywords: depression, fMRI, cortisol, emotion processing
1. Introduction
The hypothalamic-pituitary-adrenal (HPA) axis is the primary circuit of the neuroendocrine stress system and an essential route by which the brain influences many psychological processes, including emotion processing, emotion regulation, and cognitive functioning (Tsigos and Chrousos, 2002). Major depressive disorder (MDD) and bipolar disorder (BP) are often characterized by increased activity of the HPA-axis (Kamali et al., 2012; Stokes, 1995; Watson et al., 2006), as well as deficits in emotion regulation (Joormann and Quinn, 2014) and cognitive processes involved in allocating attention and modulating affect (Murrough et al., 2011). A biobehavioral construct thought to cut across both MDD and BP is response to acute threat, which is directly relevant to the negative valence systems that are characteristic of these two disorders. Behaviorally, an acute threat response is frequently organized through emotion processing, which is supported, in part, at the neurobiological level by neuroendocrine response and the integrity of functional neural circuits. Thus, in line with the Research Domain Criteria (RDoC) initiative, it is valuable to move beyond operationalizing MDD and BP as independent constructs or homogenous disorders, and instead, leverage individual differences to better understand how neuroendocrine and neurofunctional systems contribute to the range of negative mood disorders (NMD) that are characterized by disrupted negative valence systems. By way of guiding hypotheses relevant to these constructs, the subsequent literature covered addresses 1) targets of the HPA-axis in the central nervous system (CNS), 2) functional role of brain-based endocrine targets in emotional salience, and 3) cognitive and emotional control, 4) existing neural correlates of disrupted HPA-axis functioning, and 5) how the study of neuroendocrine and functional systems can advance understanding of the pathophysiology of NMDs.
1.1 Targets of the HPA-Axis in the Central Nervous System
Acute stress (Bifulco et al., 2000; Lupien et al., 2009), such as the presentation of a fearful stimulus, can trigger temporary activation of the HPA-axis; namely, secretion of corticotropin releasing hormone from the hypothalamus, release of adrenocorticotropic hormone (ACTH) from the anterior pituitary, and increased secretion of cortisol from the adrenal cortex. Accumulation of stress exposures over time can disrupt the feedback pathways of the HPA-axis and down regulate glucocorticoid receptors (GR), resulting in hypercortisolemic disruptions that commonly characterize chronic NMDs (Parker et al., 2003). Further, some central tissues in the stress response system such as the hippocampus, express abundant mineralocorticoid receptors (MR), but are relatively deficient in enzymes that protect against cortisol binding, ultimately resulting in an aldosterone-cortisol imbalance (Funder, 2005). Importantly, elevated levels of cortisol can lead to cortical atrophy, perhaps through reduced dendritic arborization, with a preferential impact upon limbic system structures such as the hippocampus, amygdala, and medial and orbital prefrontal cortex, where densities of GR and MR are high (Bao et al., 2008; Lupien et al., 2009). Dysregulated HPA-axis function, therefore, may provide one possible mechanism for how disrupted neural structures and circuitry influence emotion processing and regulation in mood disorders.
1.2 Functional Role of CNS Endocrine Targets
1.2.1 Emotional Salience
Indeed, the networks (e.g., salience and emotion, default mode) with high GR/MR receptor densities overlap with cognitive and affective networks that process emotional experience among healthy individuals. Following fearful eliciting stimuli, increased activity in the hippocampus is understood to facilitate down-regulation of the HPA axis via inhibitory connections to the paraventricular nucleus of the hypothalamus (Jankord and Herman, 2008), resulting in an inverse association between hippocampal activation and cortisol secretion (Kern et al., 2008; Liu et al., 2012; Pruessner et al., 2008). Increased activation in both the amygdala and insula has been positively related to cortisol levels, suggesting that these regions, which underlie saliency, emotion, and attention, also participate in the recruitment and ongoing stimulation of the HPA axis (Langenecker et al., 2012). The sensitivity of these specific structures to negative emotional content is relevant to features of anxiety such as fear, threat detection, and avoidance (Etkin and Wager, 2007; Seeley et al., 2007).
Given the anatomical projections and functional connections of the insula to the amygdala (Banks et al., 2007), an extended salience and emotion network is presumed to serve broad functions in emotion perception, including mind–body integration of affective information and visceral and autonomic processing (Paulus and Stein, 2006; Zald, 2003). In addition, although neuroimaging stress literature has been historically dominated by focus on the amygdala and hippocampus as pivotal mediators of the stress response (Dedovic et al., 2009; McEwen, 2007; Phillips and LeDoux, 1992), fear evoking stimuli are also thought to alter motivational processes by increasing dopamine secretion in the brain’s “reward system”, supported primarily by striatal regions (Haber and Knutson, 2010; O’Doherty, 2004). Preclinical evidence indicates a robust role of the ventral (nucleus accumbens) and dorsal (caudate, putamen) striatum in reward and stress processing (Cabib and Puglisi-Allegra, 2012; Sesack and Grace, 2010). These findings are corroborated by human neuroimaging studies where engagement of striatal regions during reward processing (Knutson et al., 2008) and decision-making (Forbes et al., 2006) has been linked to affect regulation in response to stress (Forbes et al., 2009). Indeed, high cortisol response to psychosocial stress in healthy individuals has been shown to prevent reductions in sensitivity toward reward as evidenced by hyperactivity in the nucleus accumbens (Oei et al., 2014).
1.2.2 Cognitive and Emotional Control
The neural networks involved in cortisol modulation also share functions with the circuitry implicated in control and modulation of emotion. A frontal-subcortical problem-solving circuit operates in the “top down” regulation of cognitive and behavioral inhibition and guides the selection of actions based on reward expectations. This circuit, which is often referred to as the cognitive control network (CCN) (Bonelli and Cummings, 2007; Chudasama and Robbins, 2006; Seo et al., 2012), includes the anterior cingulate, inferior frontal gyrus, inferior parietal lobule, caudate, thalamus, globus pallidus, and putamen (Bonelli and Cummings, 2007; Chudasama and Robbins, 2006; Seo et al., 2012). Positive associations have been established between cortisol levels and activation within this network, such as in parietal, ventrolateral and dorsolateral prefrontal areas (Kern et al., 2008; Weerda et al., 2010). Left and right lateral PFC activation has been associated with increased and decreased cortisol reactivity to psychosocial stress, respectively (Kern et al., 2008; Sullivan and Gratton, 2002; Taylor et al., 2008; Wang et al., 2005), and activation of the medial PFC has been associated with decreased cortisol reactivity to stress (Kern et al., 2008). In addition, increased cortisol during emotion processing has been shown to engage the caudate while successfully directing attention away from negative content and towards positive affect (Dedovic et al., 2015). Given the functional connections between the PFC and limbic, parietal, and striatal structures that are important for the integration of emotion and cognition (Seeley et al., 2007), the CCN is likely active in the down-regulation of the stress response and might facilitate attenuation of cortisol levels (Amodio and Frith, 2006; Phelps, 2004; Urry et al., 2006; Veer et al., 2012).
1.3 Neural Correlates of Disrupted HPA-Axis Functioning
Although the fundamental and adaptive purpose of the HPA-axis is to mobilize resources for defense during acute stress and/or threat response, and subsequently for repair and healing (Susman, 2006), this biological response system can become dysregulated under chronic stress(McEwen, 2007). As such, these HPA-axis alterations may increase susceptibility to mental disorders such as NMDs, or exacerbate adverse effects of these disorders. Indeed, exaggerated cortisol stress response has been implicated in the pathophysiology of depression (Lopez-Duran et al., 2009) and is thought to be mediated, in part, by a) dendritic hypertrophy and enhanced activation of emotional-salience circuitry, and b) reduced dendritic arborization and activity in the hippocampus and fronto-subcortial regulatory circuits (Langenecker et al., 2012). Only a few studies have investigated links between cortisol reactivity and neural signatures supporting emotion processing in NMDs. One study among late-adolescent/young adult males undergoing fMRI during a problem-solving task found that higher cortisol output was related to hypoactivation of the inferior parietal and superior frontal cortex (Keulers et al., 2015). Elevated endocrine-stress response has been linked with enhanced amygdala reactivity to emotional faces among both adolescents (Klimes-Dougan et al., 2014) and adults (Weldon et al., 2015). In contrast, endocrine-stress response has also been related to hypo-activation of limbic regions such as the hypothalamus, subgenual ACC, amygdala and OFC (Holsen et al., 2011) in adult females.
1.4 Neuroendocrine and functional systems and the pathophysiology of NMD
fMRI is increasingly leveraged to study the neural circuits implicated in the activation and modulation of the stress response system. The psychological anticipation preceding fMRI procedures has been shown to evoke mild to moderate distress, anxiety, claustrophobia, and arousal of the sympathetic nervous system (Chapman et al., 2010; Eatough et al., 2009; Lueken et al., 2012; Wolf, 2008), thereby affecting neural functioning, network engagement and task performance, even among healthy control participants (Tessner et al., 2006; Weldon et al., 2015). If unmeasured (as is the case with the majority of fMRI experiments), basal and anticipatory cortisol levels might confound the quality of contrasts (spoiling control conditions), task performance, and functional activation patterns, introducing experimental bias and threatening clear interpretation of results. However, by measuring and modeling how HPA-dysregulation influences key outcomes (e.g., task performance, functional activation patterns) differently between clinical populations and healthy individuals, we can gain valuable insights regarding adaptive and maladaptive functioning of the HPA-axis as it relates to neuronal integrity and the pathophysiology of NMD.
1.4 Aims and Hypotheses of the Present Study
The aim of the present study was to evaluate blood-oxygen-level dependent (BOLD) activation during the anticipation of fMRI as a function of cortisol reactivity among participants with NMDs and healthy individuals. Critically, the present study extends previous work: first, by including a sample of adults with both unipolar and bipolar depression as expressing negative mood disorders, extending the domain of emotion perception in the RDoC initiative, and second, by including males and females, increasing the generalizability of findings as compared to those derived from more restricted samples (Admon et al., 2015; Holsen et al., 2013; Holsen et al., 2011; Keulers et al., 2015; Klimes-Dougan et al., 2014). We tested the following hypotheses: (1) cortisol levels immediately prior to the fMRI procedures (pre-scan cortisol) would be higher compared to estimated baseline day measurements from non-scan days; (2) pre-scan cortisol would be positively associated with activation in limbic and emotional salience regions of the brain during an emotional perception task; and (3) NMD diagnosis would moderate the relation between pre-scan cortisol and brain activation, such that increased pre-scan cortisol would be positively related to engagement of fronto-parietal and fronto-striatal regulatory regions of the brain among healthy controls, but inversely related in individuals with NMDs.
2. Method
2.1 Participants
Forty-one participants with major depression (n = 29) or bipolar disorder (n = 12) and 23 healthy control comparison participants were recruited for one of three studies; a study of emotion processing in Cushing’s disease (only control subjects included in current analysis) (Langenecker et al., 2012), an investigation of emotion processing in MDD (Briceno et al., 2013) and a study of emotion processing in BP (Ryan et al., 2015). MDD and BP participants were recruited in a depressed state or in partial-remission from a depressive episode, but currently reporting a minimum of two persistent depressive symptoms.
All studies were conducted at the University of Michigan and participants were recruited from the surrounding community. Participants were 18 to 65 years of age (M = 38.86, SD = 16.19). For all studies, participants were not taking medication for physical conditions (including hormonal contraceptives or hormone replacement therapy); current use of psychiatric medications was permitted. Participants, regardless of diagnostic status, were excluded if they used tobacco products, had a history of head injury or neurological disorder, or if their weight exceeded 220 pounds (BMI >35) for scanning purposes. Healthy control participants were also excluded if they met current or past criteria for a psychiatric disorder as assessed by the Structured Clinical Interview for DSM-IV (SCID-I; (First et al., 1995)) or if a first degree family member met current or past criteria for a psychiatric disorder based on participant report.
2.2 Procedure
After informed consent, all participants completed identical screening, diagnostic (using SCID-I and mood ratings by psychologist or clinical psychology graduate-level trainee), neuroimaging (on the same 3-Tesla Signa scanner), and salivary collection procedures. At the initial visit, participants were briefed on the scanning procedure; fMRI data were collected on a separate day following the initial screening interview. Upon arrival at the scan visit, salivary samples were collected (pre-scan). Participants then completed a practice run of the study task (e.g. Facial Emotion Perception Task; FEPT (Briceno et al., 2013; Gur et al., 1992; Langenecker et al., 2012; Weldon et al., 2015)) outside of the scanner in order to limit learning or novelty bias in the behavioral results. The study tasks were repeated in the scanner, in addition to another task not reported in the present study, for a total scan time of approximately 70 minutes, including placement. Participants were provided a brief break after completion of the scan before collecting a final saliva sample. All participants were compensated between $10 and $30 per hour for participation in basal cortisol collection, diagnostic interviewing and functional MRI.
2.3 Clinical Assessment
In addition to a diagnostic interview, all participants completed clinician-rated and self-report measures of depression and anxiety.
2.3.1 Hamilton Depression Rating Scale (HAM-D; (Hamilton, 1960)
The HAM-D is a 17-item clinical rating scale administered by an independent evaluator that includes questions about mood, feelings of guilt, suicide ideation, insomnia, agitation or retardation, anxiety, weight loss, and somatic symptoms.
2.3.2 Hamilton Anxiety Rating Scale (HAM-A; (Shear et al., 2001)
The HAM-A is a 14-item clinical rating scale administered by an independent evaluator that includes questions on worry, fear, and somatic symptoms.
2.3.3 Beck Depression Inventory (BDI; (Beck and Steer, 1984)
This depression self-report questionnaire consists of 21 items rated on a likert-scale of 0 to 3.
2.3.4 Beck Anxiety Inventory (BAI; (Beck and Steer, 1990))
This anxiety self-report questionnaire consists of 21 items rated on a likert-scale of 0 to 3.
2.4 Saliva Sample Collection and Cortisol Assay
Salivette Cortisol Tubes (Sarstedt AG & Co.) were used to collect saliva for later assessment of cortisol. Pre-scan saliva samples were collected 15–30 minutes prior to entering the scanner and after completion of the 25–35 minute FEPT to assess participant’s anticipatory stress related to the fMRI tasks/scanner environment. Scans were scheduled between 0800h and 1600h; the majority (72%) of scans were completed in the morning. All participants were awake for a minimum of one hour prior to the saliva collection to reduce the impact of the cortisol awakening response on anticipatory cortisol levels. Self-reported participant wake time was not reported, but the time of the scan was recorded and transformed into a 24-hour variable for use as a covariate in imaging regression analyses in order to control for circadian profile across the day. In addition, to validate the measurement of pre-scan cortisol, we invited the last n=13 HC participants (5 female) and n = 15 NMD participants (8 female) to provide additional saliva samples throughout a non-scan weekday. The NMD subsample was restricted to MDD participants; these data were not available for BP participants. These participants consented to provide consecutive saliva samples at 0800h, 1200h, 1600h, and 2100h on a weekday different from the fMRI scan. These measures allowed us to approximate a “normative” baseline cortisol level as compared to pre-scan cortisol assessed on the day of the fMRI scan.
Cortisol samples were stored at −80°C until they were processed at the Clinical Ligand Assay Service Satellite Laboratory at the University of Michigan School of Public Health Department. The immunoassay was conducted using a Siemens Centaur automated analyzer via chemiluminescent technology. Inter- and intra-assay coefficients of variation were 12.4 and 3.6%, respectively (Kamali et al., 2012). Average pre-scan cortisol was .74 μg/dl (SD = .40). Post can cortisol did not change significantly from the pre-scan values and for this reason, was not included in the analysis. Natural log-transformed values were computed to allow for comparison to other studies and for use in the imaging regression analysis. The log-transformed and raw cortisol values were highly correlated (r = .92).
2.5 Facial Emotion Perception Test (FEPT)
The FEPT (Briceno et al., 2013; Gur et al., 1992; Langenecker et al., 2012; Weldon et al., 2015) was designed to assess the accuracy and speed with which participants can identify positive, negative, and neutral facial expressions. Speed and accuracy are also assessed for the identification of animals in order to control for visual processing ability and fine motor dexterity/speed, thereby isolating the face-specific performance. Participants categorize faces (MACBrain Foundation (Tottenham et al., 2009)) into one of four categories (fearful, angry, happy, or sad) and animals into one of four categories (dogs, cats, primates, or birds). Participants complete a trial of this task using the Ekman faces (Ekman et al., 1975; Langenecker et al., 2005) outside of the scanner in order to minimize the bias introduced through learning effects. To detect biases in emotional identification, neutral faces are presented in certain trials, but “neutral” is not a choice available to participants.
Each trial begins with the brief presentation of an orienting cross (500 ms), followed by presentation of the stimulus face or animal (300 ms), a visual mask (100 ms), and a response window (2,600 ms). During the response window, participants select the category of choice using a 5-button response claw. The in-scanner version of the task consisted of 56 animal and 147 face trials for a run time of 25 minutes, with 21 face blocks and 8 animal blocks of 7 consecutive stimuli separated by 2 repetition times (TRs; 3,500 ms) across 5 runs. Emotions portrayed on the faces were counterbalanced to the second order to reduce possible unanticipated effects on subsequent processing speed or accuracy; every emotion was equally likely to be followed by every other emotion. Dependent variables were the percent accuracy of categorized faces (81%) and speed of response time for each emotion (M = 1310.61 ms, SD = 167.35), consistent with performance reported in prior studies (Langenecker et al., 2005; Weldon et al., 2015).
2.6 fMRI Acquisition and Processing
Whole-brain imaging was conducted on a GE Signa 3-Tesla scanner. fMRI series consisted of 30 contiguous oblique-axial sections and acquired using a forward-reverse spiral sequence. The image matrix was 64 × 64 over a 24-cm field of view (FOV) for a 3.75 × 3.75 × 4 mm voxel. The 30-slice volume was acquired serially at 1,750 ms temporal resolution for a total of 590 time points for the FEPT. One hundred six to one hundred twenty-four high resolution Fast SPGR IR axial anatomic images [TE = 3.4 ms, TR = 10.5 ms, 27° flip angle, number of excitations = 1, slice thickness = 1–1.2 mm, FOV = 24 cm, matrix size = 256 × 256] were obtained for each participant for co-registration and normalization purposes. Processing of images was conducted using SPM8, including slice timing, realignment, motion correction, co-registration, DARTEL warping (using VBM8 toolbox), normalization to the MNI world space, and smoothing with a 5 FWHM filter. Both block and event-related analyses were completed. Contrast images were derived by subtracting the BOLD signal during the animal-processing blocks from the BOLD signal during the face processing blocks (‘faces – animals’). Images from the event-related models were created by subtracting the BOLD signal for emotional face events from neutral face events (e.g. ‘fear – neutral’). Fearful and angry faces in particular were chosen for analysis given the ability of threatening stimuli to influence HPA axis in both human and animal studies (Dedovic et al., 2009; Phillips et al., 2003; Taylor et al., 2008) and for consistency with the construct of acute threat. Sad and happy, in comparison to neutral, faces are included in online supplementary Tables 1 and 2.
2.7 Data Analytic Approach
Clinical, demographic, neuroendocrine, and behavioral measures comparing HC and NMD participants were analyzed using independent samples t-tests and chi-square tests. Change in cortisol was assessed using a mixed-effects regression model, including fixed effects for time (pre-scan, post-scan) and group (HC, NMD) and random effects (patient, patient-by-time), as well as the group by time interaction term. Pre-scan and average daily cortisol levels were compared using independent samples t-tests. The effect of group on the relationship between pre-scan cortisol and emotion recognition accuracy and response time in the scanner was tested using a moderated linear regression.
BOLD response was modeled using the SPM8 hemodynamic response function model. Whole-brain, multivariate linear regression analyses were conducted from the individual group contrasts in SPM8. All coordinates for activation are reported in MNI space. Statistical significance for second-level regression contrasts in SPM8 were set at p < .005, with a minimum cluster size of 55 2-mm cubic voxels. This whole-brain corrected alpha of 0.05 is achieved with this combined height by extent threshold strategy based upon 10,000 Monte Carlo simulations with AlphaSim inside the whole-brain search region (Ward, 2000). In addition, based upon prior work (Weldon et al., 2015) and related work on key nodes within the HPA axis, we conducted a supplemental analysis (online Supplemental Table 3) using the bilateral amygdala, subgenual ACC, hypothalamus, and hippocampus as regions of interest (ROI), with Bonferroni correction for ROIs (p < .015), as well as an extent threshold of 15 mm3 (Supplemental Table 3).
Multivariate linear regression in SPM8 was used to assess whether pre-scan cortisol levels were associated with activation in limbic, striatal, and regulatory areas of the brain across all participants. An interaction term (pre-scan cortisol × group [NMD vs. HC]) was included in the model to assess if pre-scan cortisol was differentially related to activation during facial emotion processing in NMD versus HC. All SPM8 regression analyses also included covariate terms for sex, age, percent of correctly identified faces (face accuracy), and time of scan (transformed to a 24-hour time variable). The dependent variables of interest were brain activation for ‘faces – animals’ (block design), ‘fear – neutral’ (event-related model), and ‘anger – neutral’ (event-related model). Because ‘sad – neutral’ and ‘happy – neutral’ have shown weaker links with stress reactivity (Weldon et al., 2015), these results were not the primary focus of analysis, but are included in online supplementary Tables 1 and 2 for completeness. MARSBAR was used to extract spatially averaged contrast-specific data (fear - neutral, anger -neutral, faces - animals) for each participant and merged into SPSS to conduct follow-up, exploratory analyses of clinical and demographic effects on cortisol-related BOLD activation, using pearson’s bivariate correlations. Given minimal variability on clinical measures in the HC sample, correlations between clinical measures and extracted cortisol-related BOLD activation were only computed for NMD participants.
3. Results
3.1 Participants
Demographic and clinical characteristics of the sample are reported in Table 1. NMD and HC participants were well matched on demographic variables. NMD participants reported higher scores on measures of depression and anxiety (Table 1). Mean scores on the HAM-D in NMD participants were consistent with mild depression and anxiety.
Table 1.
Demographic and Clinical Characteristics NMD and HC Participants
| NMD (n = 41) | HC (n=23) | Test Statistic | p-value | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|---|---|---|
| Variable | M (SD) | M (SD) | ||||
| Age | 40.33 (15.57) | 36.43 (17.33) | t(−.89) | .37 | −12.23 | 4.66 |
| Female % (n) | 65.9% (n=27) | 43.5% (n=10) | ||||
| Education (years) | 15.83 (2.10) | 15.65 (1.94) | t(−.33) | .74 | −1.24 | .89 |
| Verbal IQ Estimate | 111.42 (8.93) | 112.80 (6.36) | t(1.66) | .11 | −.98 | 9.92 |
| Co-morbid Anxiety, % (n) | 29.3% (n=12) | -- | ||||
| Age of MDEa Onset | 21. 09 (10.95) | -- | ||||
| Antidepressantb (AD) % (n) | 12.8% (n=5) | -- | ||||
| Current AD Plusc % (n) | 38.5% (n = 15) | -- | ||||
| HDRS* | 15.63 (5.70) | .65 (.41) | t(11.61) | <.001 | −17.56 | −12.40 |
| BDI* | 24.23 (11.18) | 1.22 (2.32) | t(−9.72) | <.001 | −27.74 | −18.27 |
| HARS* | 14.80 (6.89) | 1.05 (1.88) | t(−8.73) | <.001 | −16.91 | −10.60 |
| BAI* | 15.17 (8.76) | 1.65 (2.39) | t(−6.75) | <.001 | −17.53 | −9.52 |
| Facial Perception Accuracy | .80 (.11) | .83 (.10) | t(1.36) | .18 | −.02 | .09 |
| Pre−scan Cortisol (Ug/dl) | .76 (.40) | .69 (.41) | t(−.74) | .46 | −.29 | .13 |
| Post-scan Cortisol (Ug/dl) | .56 (.26) | .58 (.55) | t(.22) | .83 | −.18 | .23 |
| Average Daily Cortisold | .65 (.28) | .58 (.23) | t(−.62) | .55 | −.29 | .16 |
Group differences at p <.05
MDE = Major Depressive Episode
All participants taking an antidepressant were on a selective serotonin reuptake inhibitor
Refers to currently taking an antidepressant
Subsample from both groups and included for illustrative, conceptual integration purposes
Approximately half of the NMD sample was taking psychiatric medication. Pre-scan cortisol levels did not differ (p = .550) between medicated (M = .82, SD = .44) and un-medicated (M= .74, SD = .38) participants. BP and MDD participants differed in current psychiatric medication status; 83.3% (n = 10) of BP subjects were on psychiatric medications versus 37.0% (n = 10) of MDD participants (X2 = 7.13, p = .008). BP participants were also more likely to be treated with a combination of psychiatric medications (83.3%, n = 10) than MDD subjects (33.3%, n = 5), X2 = 14.95, p = .001.
3.2 fMRI Cortisol and Baseline Day Cortisol
To evaluate whether the anticipation of fMRI alters cortisol change from baseline, weekday cortisol values were used to create two interpolated trend lines from a subsample (n = 13) of healthy controls and a subsample (n = 11) of NMD participants, representing average cortisol across the day for the subsample. This allowed us to evaluate how elevations in pre-scan cortisol in NMD participants compared to elevations that have also been shown to occur in healthy participants (Weldon et al., 2015), as well as within subjects.
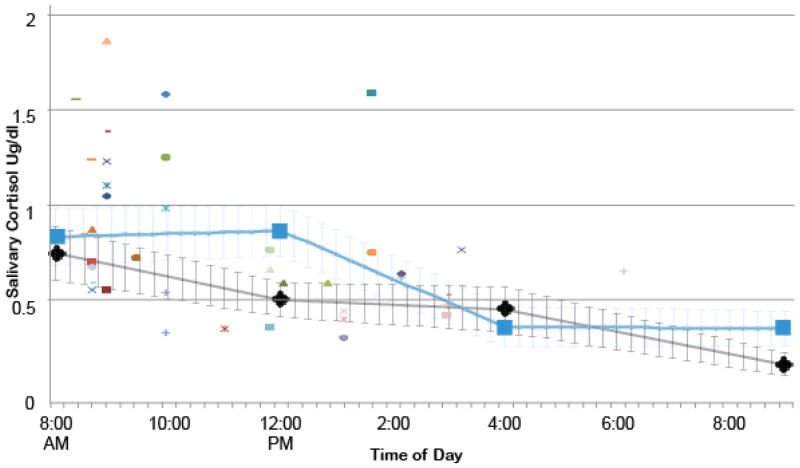
Time of day was linearly related to cortisol level. The trend line is depicted in Figure 1 and includes standard error bars with raw scores and interpolated values to evaluate fit. Pre-scan cortisol values were plotted for each NMD subject (n = 41) against the interpolated HC and NMD trend lines. There was an average of 35% increase in cortisol for the pre-scan measurement in NMD subjects compared to the baseline assessments in healthy individuals on a non-scanning day (Cohen’s d = .6; 18% and Cohen’s d = .3 in relation to NMD baseline), corresponding to a medium to large effect size. This is relative to a 16–20% increase in pre-scan cortisol in HC (Cohen’s d = .3 [small effect]) relative to the same baseline assessment trend line (Weldon et al., 2015). As we did not have baseline comparison cortisol measurements for all participants, these results are considered conceptually illustrative/qualitative; we could not run a specific test of this hypothesis with the limited pilot funding available. Cortisol levels in the whole sample did not change significantly between the pre-scan and the post-scan measurement (b = −.10, p = .66, not shown), nor did cortisol levels change differentially by the post-scan measurement in NMD versus HC subjects (b = −.10, p = .45, not shown). There were also no differences in pre-scan cortisol as a function of age (r = −.19, p = .12, 95% CI: −.41 to .06) or gender (t = .16, p = .87, 95% CI: −.19 to .22). Salivary cortisol values were not correlated with the HAM-D (r = .004, p = .98, 95% CI: −.24 to .25), HAM-A (r = .009, p = .94, 95% CI: −.24 to .25), BDI (r = .09, p = .47, 95% CI −.16 to .33), or BAI (r = .16, p = .23, 95% CI: −.09 to .39). Pre-scan cortisol was not related to emotion recognition accuracy in the scanner (b = −.04, p = .56, 95% CI −.18 to .09) or response time (b =62.68, p = .56, 95% CI =153.24 to 278.41). There was also no effect of group on the relationship between pre-scan cortisol and emotion recognition accuracy (b = .03, p = .48, 95% CI −.06 to .13) or reaction time (b = −10.19, p = .88, 95% CI −152.96 to 132.59).
Figure 1.
Pre-scan cortisol (raw values) in NMD participants plotted individually against estimated trend lines of diurnal cortisol averages on a non-scan day in 1) HC participants (grey) and 2) MDD participants (blue). Note that no BP patients had cortisol available for this trendline.
*For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article
3.3 Pre-scan Cortisol and BOLD Activation to Emotional Faces
To evaluate whether greater pre-scan cortisol was associated with increased activation in limbic regions, pre-scan cortisol levels were regressed onto activation for the faces-animals contrast, fear-neutral contrast, and anger-neutral contrast (Table 2). Activations for contrasts of sad-neutral and happy-neutral are reported in online Supplementary Table 1.
Table 2.
Foci of significant activation for pre-scan cortisol regression (positive regressor) in NMD and HC
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Contrast/lobe | BA | x | y | z | Z | k |
|
Faces-Animals
| ||||||
| Parietal | ||||||
| Precuneus | 7 | −10 | −68 | −54 | 4.16 | 82 |
| 7 | −18 | −48 | 64 | 3.74 | 100 | |
| Cingulate | 31 | −8 | −6 | 50 | 3.40 | 151 |
| Temporal Lobe | ||||||
| Inferior Temporal Gyrus | 37 | −60 | −50 | 0 | 3.52 | 91 |
|
| ||||||
| Fear-Neutral | ||||||
|
| ||||||
| Frontal | ||||||
| Middle Frontal | 6 | 32 | 0 | 58 | 3.13 | 55 |
| Dorsal Cingulate Parietal | 24 | 6 | −4 | 38 | 3.91 | 350 |
| Postcentral | 3 | 54 | −16 | 48 | 4.26 | 473 |
| Inferior Parietal | 4 | −54 | −42 | 36 | 3.98 | 210 |
| Precuneus | 7 | 30 | −50 | 62 | 3.69 | 63 |
| 7 | 4 | −56 | 54 | 3.56 | 94 | |
| 7 | 20 | −72 | 46 | 3.48 | 115 | |
| Temporal | ||||||
| Insula/Superior Temporal | 13/22 | −48 | −10 | −2 | 3.52 | 64 |
| Subcortical | ||||||
| Inferior Semi-Lunar | −16 | −60 | −36 | 3.31 | 108 | |
| Declive | −16 | −70 | −8 | 3.27 | 59 | |
| 28 | −58 | −16 | 2.98 | 74 | ||
| Culmen | −22 | −46 | −20 | 3.29 | 95 | |
| Putamen | −28 | −2 | 2 | 3.40 | 75 | |
|
| ||||||
|
Anger-Neutral
| ||||||
| Frontal | ||||||
| Medial Frontal | 9 | −6 | 60 | 14 | 3.35 | 94 |
3.3.1 Faces – animals
For the faces-animals contrast (Table 2, Figure 2 [Panels A – F]), pre-scan cortisol was positively associated with activation in the left precuneus (BA 7), left dorsal posterior cingulate (BA 31), and left inferior temporal gyrus (BA 37).
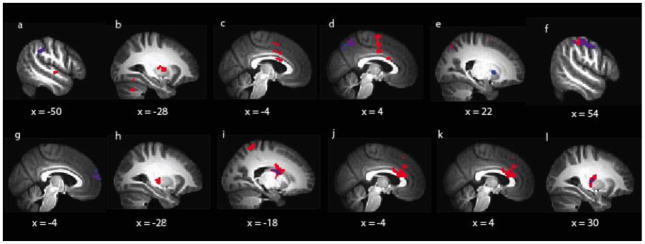
Figure 2.
Differential and overlapping patterns of activation related to cortisol in NMD versus HC subjects
*Red = Areas of significant activation related to pre-scan cortisol only (main effect of cortisol only); Blue = Areas of significant activation as a function of the interaction between cortisol and group (NMD vs. HC) only; Purple = Overlap in areas of significant activation between main effects of cortisol and cortisol x group interactions
3.3.2 Fear – neutral
For the fear-neutral contrast (Table 2, Figure 2[Panels H – L]), pre-scan cortisol was positively associated with activation in the left inferior parietal lobule (BA 4), left posterior insula (BA 13), left putamen, and left semilunar lobule, culmen, and bilateral declive of the cerebellum. Pre-scan cortisol was also positively associated with activation in the right middle frontal gyrus (BA 6), right anterior cingulate (BA 24), right postcentral gyrus (BA 3), and right precuneus (BA 7).
3.3.3 Anger – neutral
In the anger-neutral contrast (Table 2, Figure 2 [Panel G]), pre-scan cortisol was positively associated with activity of the left medial frontal gyrus (BA 9).
3.4 Pre-scan cortisol regression and BOLD activation in NMD versus HC
To evaluate whether elevations in pre-scan cortisol were differentially related to activation among NMD subjects versus healthy controls, an interaction term (pre-scan cortisol × group) was regressed onto activation for the faces-animals contrast, fear-neutral contrast, and anger-neutral contrast within the same models as reported above (Table 3). Activations for the interaction term regressed on contrasts of sad-neutral and happy-neutral are reported in online Supplementary Table 2. Results indicated that pre-scan cortisol was positively associated with activation in several regions among HCs but inversely associated with activation among NMD participants. Thus, results reported below represent significant areas of hypo-activation as a function of pre-scan cortisol in NMD relative to HC.
Table 3.
Foci of significant hypo-activation for pre-scan cortisol regression in NMD relative to HC
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Contrast/lobe | BA | x | y | z | Z | k |
| Faces-Animals | ||||||
|
| ||||||
| Frontal | ||||||
| Anterior Cingulate | 32 | −8 | 30 | 24 | 3.30 | 194 |
| 32 | 10 | 14 | 38 | 2.95 | 64 | |
| Cingulate | 24 | 10 | 4 | 28 | 3.46 | 198 |
| Parietal | ||||||
| Precuneus | 7 | −10 | −68 | 54 | 3.81 | 61 |
| Temporal | ||||||
| Middle Temporal | 21 | −60 | −48 | 0 | 3.47 | 75 |
| Subcortical | ||||||
| Putamen | 26 | −10 | 6 | 3.33 | 74 | |
| Caudate | −16 | −10 | 20 | 3.22 | 55 | |
|
| ||||||
|
Fear-Neutral
| ||||||
| Parietal | ||||||
| Postcentral | 3 | 54 | −16 | 48 | 4.55 | 239 |
| Precuneus | 7 | 4 | −54 | 56 | 3.47 | 169 |
| Temporal | ||||||
| Insula | 13 | −54 | −38 | 24 | 3.25 | 213 |
| Subcortical | ||||||
| Putamen | 24 | 8 | 2 | 3.87 | 74 | |
|
| ||||||
|
Anger-Neutral
| ||||||
| Frontal | ||||||
| Medial Frontal | 9 | −6 | 60 | 14 | 3.63 | 226 |
3.4.1 Faces – animals
For the faces – animals contrast (Table 3, Figure 2 [Panels A – F]), pre-scan cortisol was related to hypo-activity in NMD relative to HC of the bilateral dorsal anterior cingulate (BA 32; Figure 3, Panel A), right ventral anterior cingulate (BA 24), right putamen, left middle temporal gyrus (BA 21), left precuneus (BA 7), and left caudate.
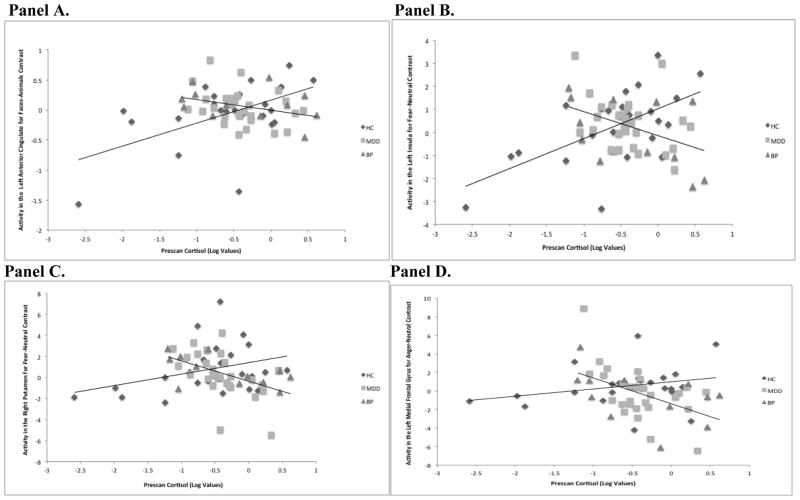
Figure 3.
Associations between cortisol and regions of activation during emotion processing in NMD versus HC subjects
Panel A: Pre-scan cortisol is associated with attenuated engagement of the left anterior cingulate in NMD but not HC subjects for Faces-Animals contrast.
Panel B: Pre-scan cortisol is associated with attenuated engagement of the left insula in NMD but not HC subjects for Fear-Neutral contrast.
Panel C: Pre-scan cortisol is associated with attenuated engagement of the right putamen in NMD but not HC subjects for Fear-Neutral contrast.
Panel D: Pre-scan cortisol is associated with attenuated engagement of the left medial frontal gyrus in NMD but not HC subjects for Anger-Neutral contrast.
3.4.2 Fear – neutral
For the fear – neutral contrast (Table 3, Figure 2 [Panels H – L]), pre-scan cortisol was related to hypo-activity in NMD relative to HC of the left posterior insula (BA 13; Figure 3, Panel B), right postcentral gyrus (BA 3), right precuneus (BA 7), and right putamen (Figure 3, Panel C).
3.4.3 Anger – neutral
For the anger – neutral contrast (Table 2, Figure 2 [Panel G]), pre-scan cortisol was related to hypo-activity in NMD relative to HC in the left medial frontal gyrus (BA 9; Figure 3, Panel D).
3.5 Clinical Correlates of Cortisol-Related BOLD Activation
Several areas of activation were significantly positively correlated with self-reported depression or anxiety in the NMD group when evaluated within the pre-scan cortisol regression models. Among NMD subjects, anxiety (BAI scores) positively correlated with both inferior semi-lunar lobule (r = .36, p = .02) and declive (r = .37, p = .02) activity for fearful faces. In addition, among NMD subjects, depression (BDI scores) positively correlated with both middle frontal gyrus (r = .43, p = .01) and precuneus (r = .33, p = .04) activity for fearful faces.
3.6 Effects of Diagnosis, Medications, Sex on Cortisol-Related BOLD Activation in NMD Subjects
While MDD and BP participants demonstrated largely similar patterns of activation, there were a few emerging differences. Several post-hoc comparisons were conducted to assess variability in the patterns of activation detected in the pre-scan cortisol regressions between BP versus MDD participants as well as the potential influence of clinical or demographic factors on these differences. In the pre-scan cortisol regression for fearful faces, the relationship between pre-scan cortisol and activation in the precuneus (p = .03) was stronger in BP compared to MDD participants and marginally stronger for the posterior insula (p = .05). Greater activation of the inferior temporal gyrus in the pre-scan cortisol regression for faces –animals occurred in participants taking psychiatric medication relative to those who were not taking medication (p = .04). There were no other effects of psychiatric medications on activation patterns. Within the NMD participants there were a few observed sex differences. In the pre-scan cortisol regression for the fearful faces – neutral faces contrast, males showed greater activity of the inferior semilunar lobule (p = .04) and less activity of the culmen (p = .04) compared to females. In the pre-scan cortisol regression for the angry faces – neutral faces contrast, males showed less activity of the medial frontal gyrus (p = .03) compared to females. Of these observed sex differences, greater activity in the inferior semi-lunar lobule among males for the fear – neutral contrast was the only effect that remained when tested across the whole sample (p = .02); the other observed sex differences were specific to the NMD group.
4. Discussion
The present study evaluated whether anticipatory cortisol levels were associated with differential brain activation during an emotional face paradigm in individuals with negative mood disorders (NMD) versus healthy comparisons. Notably, individual differences in pre-scan cortisol were related to the activation of regions underlying both emotional salience and cognitive control during emotion processing. Importantly, we also observed an impact of pre-scan cortisol on neural networks for NMD participants, such that NMD participants were characterized by attenuated activation in regions of the putative frontal-subcortical regulatory circuit, as well as in some key emotional salience regions (see supplement) during emotion processing.
Consistent with our hypothesis that pre-scan cortisol would be positively associated with activation in limbic and emotional salience regions, we showed that anticipatory cortisol, in part, accounted for variation in the degree of activation in the insula, dACC, inferior parietal lobule, putamen, precuneus, middle and medial frontal and postcentral gyri, posterior cingulate, and inferior temporal gyrus. Indeed, these are areas of the brain that have been implicated in emotional salience (e.g. insula (Klumpp et al., 2012; Klumpp et al., 2013)), regulatory functions (e.g. dACC, inferior parietal lobule, putamen (Bonelli and Cummings, 2007; Chudasama and Robbins, 2006; Konishi et al., 1999)) and also include regions that are understood to support the perception of emotion and facial recognition (Haxby et al., 2000). These results were expected, given that previous neuroendocrine work has demonstrated that the “coming online” of stress response circuitry is governed by the apparent stimulatory action of HPA-axis hormones in healthy individuals (Weldon et al., 2015). The current results validate, in both healthy and individuals with mood disorders, that the mobilization of stress response systems contributes to the functional integrity of neural circuits underlying not only the detection and perceptual integration of salient emotional stimuli, but also the engagement of regulatory regions that function to modulate an emotional challenge.
Furthermore, we observed an interesting dissociation between healthy individuals and those with mood disorders, providing evidence that the exaggerated HPA-axis reactivity often observed in NMD may in turn, interfere with the engagement of neural networks supporting cognitive and emotional regulation in response to acute threat. Specifically, we found that in NMD subjects, anticipatory cortisol predicted attenuated activation in several regions of the fronto-subcortical circuit, such as the putamen during fear processing, the medial frontal gyrus during anger processing, and the dACC, putamen, and caudate during facial recognition. These findings are consistent with our hypothesis that fronto-parietal and striatal regions would be insufficiently mobilized as a function of exaggerated cortisol reactivity in NMD subjects. Observed hypoactivation of the insula during fear processing in NMD subjects is also broadly consistent with this conceptualization, as this key node in the salience and emotion network plays a central role in relaying emotional information to other brain structures that are involved in regulation and decision-making (Suzuki, 2012), especially the ACC and prefrontal cortices. In addition, our supplemental region of interest analyses indicate that cortisol might contribute to an overall “blunted” regulatory and emotional profile in NMD, as cortisol-related attenuation was observed in the amygdala, subgenual cingulate, and hypothalamus as well. Results from our brain-behavior analyses further indicate that increases in negative mood symptoms (e.g. depression) are associated with activity of frontal and cerebellar regions, which could represent either inefficient processing of regulatory networks or increased attention (Bonelli and Cummings, 2007; Chudasama and Robbins, 2006) to emotional information. Therefore, we suggest that hypo-activation of the fronto-subcortical circuit and key nodes of the emotional salience network in negative mood disorders reflects a core dysregulation in the HPA-axis feedback loop that requires study in further detail and including experimental manipulations of cortisol levels and depressive range (Sudheimer et al., 2013).
It was interesting that links between pre-scan cortisol and brain activation varied for the affective processing of specific emotions in NMD subjects. Activation in the medial frontal gyrus was unique to the anger – neutral contrast, which may reflect a fundamental inefficiency given the role of this region in deliberate suppression of response to a negative emotional signal (Hansel and von Kanel, 2008). The more diffuse findings for the fear contrast across emotional salience regions is consistent with the broader literature linking fear with HPA axis function (McEwen, 2007; Merz et al., 2010), as well as repeated findings in depression implicating corticolimbic circuit function as a key modulator of the more reactive and autonomic process of sensitivity to threat (Collins and Schiller, 2013). Indeed, threat sensitivity has been identified as a construct that cuts across negative mood disorders (Dillon et al., 2014), such as depression and bipolar disorder (Langenecker et al., 2014).
Our pattern of findings was also related to distinct clinical correlates among NMD participants; during fear processing, self-report anxiety was associated with cortisol-related cerebellum response, whereas self-report depression was related to activation of the middle frontal gyrus and precuneus. The cerebellum is a region that may be particularly susceptible to glucocorticoid exposure relative to other areas of the cerebrum, with reduced volumes and cognitive and motor deficits reported following glucocorticoid administration (Noguchi, 2014). More recently, the cerebellum has also been suggested to play a role in operant learning, which may serve to maintain the core anxiety feature of avoidance (Caulfield and Servatius, 2013). The cerebellum is also dense with cannabinoid receptors, which have been linked with anxiety (Wyrofsky et al., 2015). Regarding the functional correlates of depressive symptoms, the precuneus has been repeatedly implicated in depression in terms of allocating internal attention and maintaining rumination (Langenecker et al., 2014). Depression has also been characterized by disruptions to the functional connections between this region and the default mode network, specifically in those with active depression and not in those with depression in remission (Peters et al., 2016). Furthermore, increased connections between the PCC and the middle frontal gyrus, which may reflect a comprised ability of cognitive control regions to regulate the recruitment of areas understood to sub-serve common cognitive biases in depression. Taken together, these results indicate that the combination of neuroendocrine markers and functional assessment of fear circuits provides a neurophysiological criterion to help explain heterogeneity of symptoms that characterize depression across clinically distinct syndromes.
Pre-scan cortisol levels were non-significantly higher in NMD subjects relative to baseline averages derived from a subsample of healthy participants on a non-scan day and relative to elevations in cortisol observed in healthy individuals on a scan day, suggesting that the pathophysiology of mood disorders may be characterized by exaggerated HPA-axis sensitivity to anticipatory stress, at least at a conceptual level. It also emphasizes the fact that cortisol variability and measurement have signal to noise concerns that weaken relationships with key variables – cortisol release is affected by and interacts with a number of factors (e.g., sleep, time of day, food intake, to name a few). Nonetheless, these findings are consistent with previous reports in mood disorders of elevated levels of cortisol in plasma, cerebrospinal fluid, urine, and saliva samples (Belvederi Murri et al., 2016; Vreeburg et al., 2009), which may be exacerbated by the challenge of the fMRI environment. Although cortisol has long been understood as a core dysfunction in the pathophysiology of stress response systems in depression (Bao et al., 2008; Swaab et al., 2005), these results validate that anticipation of the fMRI environment may act as a mild acute stressor across healthy and mood-disordered individuals. Importantly, fMRI-related alterations in cortisol have also been observed in healthy individuals (Weldon et al., 2015), highlighting the critical methodological implication to consider how the anticipatory stress response impacts functional activation and task performance in the neuroimaging environment. Given that reactivity of the HPA-axis to the fMRI environment appears exaggerated in mood disorders (~36%) relative to healthy individuals (~20%), accounting for these individual differences in elevation may help reduce heterogeneity and lead to more robust results in imaging studies. We note that this was a subsample comparison of elevations compared to an aggregate group non-scan day baseline, and that there are no significant differences in pre-scan cortisol between NMD and HC groups, possibly due to small and unequal sample size. Future studies can employ a stronger comparative within subject design in this regard.
Several limitations to the current study must be considered in the context of these results. It would have been preferential to collect ‘baseline’ cortisol data from the entire sample, which would have allowed a more robust understanding of how individual differences in diurnal cortisol may have influenced results, and to conduct a more formal, well powered test of enhanced anticipatory stress in the participants with NMD. Second, there was some variation in scan times, which could increase variability in the results. We did include scanner start time as a covariate in the imaging analyses, and many participants were scanned early in the day, a period when cortisol is typically descending from peak awakening response (Stalder et al., 2016). It will be important for future studies to collect data on the menstrual cycle phase of female participants and subject wake time on the day of scanning, to account for variation in cortisol measurements. Afternoon scanning may allow for a better estimate of anticipatory stress responses at a time when circulating cortisol levels are typically much lower. Furthermore, we did not evaluate subjective levels of stress prior to the fMRI. Although associations between subjective stress and cortisol response are not typically found (Campbell and Ehlert, 2012), given that cortisol-related functional activation was associated with depression severity, even negative results would yield important insights towards the specificity of cortisol’s effects on the clinical features of depression. A more general measure of recent anxiety, the Beck Anxiety Inventory, was not associated with pre-scan cortisol levels. Finally, clinical features of our MDD and BP samples, including age, length of illness, diagnosis, and medication status could influence relationships or interactions between cortisol measurements and activation. In particular, taking an SSRI has been associated with higher evening cortisol and decreased cortisol suppression after dexamethasone ingestion (Manthey et al., 2011), which could have introduced bias into the results. There is great heterogeneity both within and across these disorders; we have attempted to address the potential impact of sample clinical characteristics through post-hoc analyses, but future studies should be stratified by these factors to better understand their effects.
In summary, results of our study revealed significant associations between HPA-axis dysfunction in disorders characterized by negative valence and alterations in activity of brain regions supporting emotional and regulatory functions. Altered neuroendocrine dynamics reduced the functional integrity of frontal and subcortical areas understood to sub-serve cognitive and emotional regulatory functions. Moreover, our approach has critical implications for the design of imaging studies in mood disorders. The present findings underscore the importance of attending to the effects of neuroendocrine stress systems on targets in the central nervous system through the parallel measurement of peripheral HPA-axis hormones with neuroimaging data. As a result, we would argue that the findings reported here provide important insights toward understanding the pathophysiology of negative mood disturbances through the cumulative assessment of neuroendocrine, functional, and clinical characteristics.
Supplementary Material
Highlights.
Cortisol predicted activation in several regions involved in emotion perception and control.
Cortisol predicted hypoactivation of several of these regions in negative mood disorders.
Cortisol may interfere with fronto-subcortical regulatory function in negative mood disorders.
Acknowledgments
We thank the University of Michigan fMRI Laboratory for assistance in collecting data, and some pilot (control) fMRI scans from the University of Michigan Functional MRI lab (SAL, SLW).
Role of the Funding Source
Support for this work was provided by the MH 091811 (SAL), MH 101487 (SAL) and a National Alliance for Research in Schizophrenia and Depression Award (SAL).
Footnotes
Contributors
ATP conducted all analyses, wrote the first draft of the manuscript and incorporated revisions. AVM, MH, and ALW assisted with analytic plan, analyses, figures, and reviewed and edited the manuscript. PJP and MTK assisted with data collection and provided feedback on the manuscript. EMB, KAR, AV, JKZ, MM, and SLW assisted with overall study design and provided input on the analyses and final draft of the manuscript. SAL oversaw all aspects of manuscript preparation, including study design, analytic plan, and analyses.
Conflict of Interest
M. G. M. is on the Speakers Bureau for Merck Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Admon R, Holsen LM, Aizley H, Remington A, Whitfield-Gabrieli S, Goldstein JM, Pizzagalli DA. Striatal Hypersensitivity During Stress in Remitted Individuals with Recurrent Depression. Biol Psychiatry. 2015;78:67–76. doi: 10.1016/j.biopsych.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao AM, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev. 2008;57:531–553. doi: 10.1016/j.brainresrev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol. 1984;40:1365–1367. doi: 10.1002/1097-4679(198411)40:6<1365::aid-jclp2270400615>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. BAI, Beck anxiety inventory. Psychological Corporation; 1990. [Google Scholar]
- Belvederi Murri M, Prestia D, Mondelli V, Pariante C, Patti S, Olivieri B, Arzani C, Masotti M, Respino M, Antonioli M, Vassallo L, Serafini G, Perna G, Pompili M, Amore M. The HPA axis in bipolar disorder: Systematic review and meta-analysis. Psychoneuroendocrinology. 2016;63:327–342. doi: 10.1016/j.psyneuen.2015.10.014. [DOI] [PubMed] [Google Scholar]
- Bifulco A, Bernazzani O, Moran P, Ball C. Lifetime stressors and recurrent depression: preliminary findings of the Adult Life Phase Interview (ALPHI) Social psychiatry and psychiatric epidemiology. 2000;35:264–275. doi: 10.1007/s001270050238. [DOI] [PubMed] [Google Scholar]
- Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9:141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briceno EM, Weisenbach SL, Rapport LJ, Hazlett KE, Bieliauskas LA, Haase BD, Ransom MT, Brinkman ML, Pecina M, Schteingart DE, Starkman MN, Giordani B, Welsh RC, Noll DC, Zubieta JK, Langenecker SA. Shifted inferior frontal laterality in women with major depressive disorder is related to emotion-processing deficits. Psychol Med. 2013;43:1433–1445. doi: 10.1017/S0033291712002176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S. The mesoaccumbens dopamine in coping with stress. Neurosci Biobehav Rev. 2012;36:79–89. doi: 10.1016/j.neubiorev.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Campbell J, Ehlert U. Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology. 2012;37:1111–1134. doi: 10.1016/j.psyneuen.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Caulfield MD, Servatius RJ. Focusing on the Possible Role of the Cerebellum in Anxiety Disorders. In: Durbano F, editor. New Insights into Anxiety Disorders. InTech; 2013. [Google Scholar]
- Chapman HA, Bernier D, Rusak B. MRI-related anxiety levels change within and between repeated scanning sessions. Psychiatry Res. 2010;182:160–164. doi: 10.1016/j.pscychresns.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Collins KA, Schiller D. What can fear and reward learning teach us about depression? Curr Top Behav Neurosci. 2013;14:223–242. doi: 10.1007/7854_2012_236. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47:864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Giebl S, Duchesne A, Lue SD, Andrews J, Efanov S, Engert V, Beaudry T, Baldwin MW, Pruessner JC. Psychological, endocrine, and neural correlates of attentional bias in subclinical depression. Anxiety Stress Coping. 2015:1–18. doi: 10.1080/10615806.2015.1101457. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Rosso IM, Pechtel P, Killgore WD, Rauch SL, Pizzagalli DA. Peril and pleasure: an rdoc-inspired examination of threat responses and reward processing in anxiety and depression. Depress Anxiety. 2014;31:233–249. doi: 10.1002/da.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatough EM, Shirtcliff EA, Hanson JL, Pollak SD. Hormonal reactivity to MRI scanning in adolescents. Psychoneuroendocrinology. 2009;34:1242–1246. doi: 10.1016/j.psyneuen.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen WV, Press CP. Pictures of facial affect. consulting psychologists press; 1975. [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M. Structured clinical interview for DSM-IV axis I disorder. New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Forbes EE, Christopher May J, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, Birmaher B, Axelson DA, Dahl RE. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry. 2006;47:1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder JW. Mineralocorticoid receptors: distribution and activation. Heart Fail Rev. 2005;10:15–22. doi: 10.1007/s10741-005-2344-2. [DOI] [PubMed] [Google Scholar]
- Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Res. 1992;42:241–251. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel A, von Kanel R. The ventro-medial prefrontal cortex: a major link between the autonomic nervous system, regulation of emotion, and stress reactivity? Biopsychosoc Med. 2008;2:21. doi: 10.1186/1751-0759-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Lancaster K, Klibanski A, Whitfield-Gabrieli S, Cherkerzian S, Buka S, Goldstein JM. HPA-axis hormone modulation of stress response circuitry activity in women with remitted major depression. Neuroscience. 2013;250:733–742. doi: 10.1016/j.neuroscience.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Spaeth SB, Lee JH, Ogden LA, Klibanski A, Whitfield-Gabrieli S, Goldstein JM. Stress response circuitry hypoactivation related to hormonal dysfunction in women with major depression. J Affect Disord. 2011;131:379–387. doi: 10.1016/j.jad.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Quinn ME. Cognitive processes and emotion regulation in depression. Depress Anxiety. 2014;31:308–315. doi: 10.1002/da.22264. [DOI] [PubMed] [Google Scholar]
- Kamali M, Saunders EF, Prossin AR, Brucksch CB, Harrington GJ, Langenecker SA, McInnis MG. Associations between suicide attempts and elevated bedtime salivary cortisol levels in bipolar disorder. J Affect Disord. 2012;136:350–358. doi: 10.1016/j.jad.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33:517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keulers EH, Stiers P, Nicolson NA, Jolles J. The association between cortisol and the BOLD response in male adolescents undergoing fMRI. Brain Res. 2015;1598:1–11. doi: 10.1016/j.brainres.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Eberly LE, Westlund Schreiner M, Kurkiewicz P, Houri A, Schlesinger A, Thomas KM, Mueller BA, Lim KO, Cullen KR. Multilevel assessment of the neurobiological threat system in depressed adolescents: interplay between the limbic system and hypothalamic-pituitary-adrenal axis. Dev Psychopathol. 2014;26:1321–1335. doi: 10.1017/S0954579414001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Angstadt M, Phan KL. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol Psychol. 2012;89:273–276. doi: 10.1016/j.biopsycho.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Post D, Angstadt M, Fitzgerald DA, Phan KL. Anterior cingulate cortex and insula response during indirect and direct processing of emotional faces in generalized social anxiety disorder. Biol Mood Anxiety Disord. 2013;3:7. doi: 10.1186/2045-5380-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biol Psychiatry. 2008;63:686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain Res. 1999;122:981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Bieliauskas LA, Rapport LJ, Zubieta JK, Wilde EA, Berent S. Face emotion perception and executive functioning deficits in depression. J Clin Exp Neuropsychol. 2005;27:320–333. doi: 10.1080/13803390490490515720. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Jacobs RH, Passarotti AM. Current Neural and Behavioral Dimensional Constructs across Mood Disorders. Curr Behav Neurosci Rep. 2014;1:144–153. doi: 10.1007/s40473-014-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Weisenbach SL, Giordani B, Briceno EM, Guidotti Breting LM, Schallmo MP, Leon HM, Noll DC, Zubieta JK, Schteingart DE, Starkman MN. Impact of chronic hypercortisolemia on affective processing. Neuropharmacology. 2012;62:217–225. doi: 10.1016/j.neuropharm.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chaplin TM, Wang F, Sinha R, Mayes LC, Blumberg HP. Stress reactivity and corticolimbic response to emotional faces in adolescents. J Am Acad Child Adolesc Psychiatry. 2012;51:304–312. doi: 10.1016/j.jaac.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: a meta-analysis. Psychoneuroendocrinology. 2009;34:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueken U, Muehlhan M, Evens R, Wittchen HU, Kirschbaum C. Within and between session changes in subjective and neuroendocrine stress parameters during magnetic resonance imaging: A controlled scanner training study. Psychoneuroendocrinology. 2012;37:1299–1308. doi: 10.1016/j.psyneuen.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009a;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Manthey L, Leeds C, Giltay EJ, van Veen T, Vreeburg SA, Penninx BW, Zitman FG. Antidepressant use and salivary cortisol in depressive and anxiety disorders. Eur Neuropsychopharmacol. 2011;21:691–699. doi: 10.1016/j.euroneuro.2011.03.002. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT. Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology. 2010;35:33–46. doi: 10.1016/j.psyneuen.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iacoviello B, Neumeister A, Charney DS, Iosifescu DV. Cognitive dysfunction in depression: neurocircuitry and new therapeutic strategies. Neurobiol Learn Mem. 2011;96:553–563. doi: 10.1016/j.nlm.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Noguchi KK. Glucocorticoid Induced Cerebellar Toxicity in the Developing Neonate: Implications for Glucocorticoid Therapy during Bronchopulmonary Dysplasia. Cells. 2014;3:36–52. doi: 10.3390/cells3010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Oei NY, Both S, van Heemst D, van der Grond J. Acute stress-induced cortisol elevations mediate reward system activity during subconscious processing of sexual stimuli. Psychoneuroendocrinology. 2014;39:111–120. doi: 10.1016/j.psyneuen.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav. 2003;43:60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Peters AT, Burkhouse K, Feldhaus CC, Langenecker SA, Jacobs RH. Aberrant resting-state functional connectivity in limbic and cognitive control networks relates to depressive rumination and mindfulness: A pilot study among adolescents with a history of depression. J Affect Disord. 2016;200:178–181. doi: 10.1016/j.jad.2016.03.059. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry. 2008;63:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Ryan KA, Dawson EL, Kassel MT, Weldon AL, Marshall DF, Meyers KK, Gabriel LB, Vederman AC, Weisenbach SL, McInnis MG, Zubieta JK, Langenecker SA. Shared dimensions of performance and activation dysfunction in cognitive control in females with mood disorders. Brain. 2015;138:1424–1434. doi: 10.1093/brain/awv070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M, Lee E, Averbeck BB. Action selection and action value in frontal-striatal circuits. Neuron. 2012;74:947–960. doi: 10.1016/j.neuron.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear MK, Vander Bilt J, Rucci P, Endicott J, Lydiard B, Otto MW, Pollack MH, Chandler L, Williams J, Ali A, Frank DM. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A) Depress Anxiety. 2001;13:166–178. [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wust S, Dockray S, Smyth N, Evans P, Hellhammer DH, Miller R, Wetherell MA, Lupien SJ, Clow A. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology. 2016;63:414–432. doi: 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Stokes PE. The potential role of excessive cortisol induced by HPA hyperfunction in the pathogenesis of depression. Eur Neuropsychopharmacol. 1995;5(Suppl):77–82. doi: 10.1016/0924-977x(95)00039-r. [DOI] [PubMed] [Google Scholar]
- Sudheimer KD, Abelson JL, Taylor SF, Martis B, Welsh RC, Warner C, Samet M, Manduzzi A, Liberzon I. Exogenous glucocorticoids decrease subgenual cingulate activity evoked by sadness. Neuropsychopharmacology. 2013;38:826–845. doi: 10.1038/npp.2012.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27:99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: stress, early vulnerabilities and the attenuation hypothesis. Neurosci Biobehav Rev. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Suzuki A. Emotional functions of the insula. Brain Nerve. 2012;64:1103–1112. [PubMed] [Google Scholar]
- Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Burklund LJ, Eisenberger NI, Lehman BJ, Hilmert CJ, Lieberman MD. Neural bases of moderation of cortisol stress responses by psychosocial resources. J Pers Soc Psychol. 2008;95:197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- Tessner KD, Walker EF, Hochman K, Hamann S. Cortisol responses of healthy volunteers undergoing magnetic resonance imaging. Hum Brain Mapp. 2006;27:889–895. doi: 10.1002/hbm.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer IM, Oei NY, Spinhoven P, van Buchem MA, Elzinga BM, Rombouts SA. Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. Psychoneuroendocrinology. 2012;37:1039–1047. doi: 10.1016/j.psyneuen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Smit JH, Zitman FG, Penninx BW. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009;66:617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci U S A. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous Inference for FMRI Data. Biophysics Research Institute, Medical College of Wisconsin; 2000. [Google Scholar]
- Watson S, Thompson JM, Ritchie JC, Ferrier IN, Young AH. Neuropsychological impairment in bipolar disorder: the relationship with glucocorticoid receptor function. Bipolar Disord. 2006;8:85–90. doi: 10.1111/j.1399-5618.2006.00280.x. [DOI] [PubMed] [Google Scholar]
- Weerda R, Muehlhan M, Wolf OT, Thiel CM. Effects of acute psychosocial stress on working memory related brain activity in men. Hum Brain Mapp. 2010;31:1418–1429. doi: 10.1002/hbm.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon AL, Hagan M, Van Meter A, Jacobs RH, Kassel MT, Hazlett KE, Haase BD, Vederman AC, Avery E, Briceno EM, Welsh RC, Zubieta JK, Weisenbach SL, Langenecker SA. Stress Response to the Functional Magnetic Resonance Imaging Environment in Healthy Adults Relates to the Degree of Limbic Reactivity during Emotion Processing. Neuropsychobiology. 2015;71:85–96. doi: 10.1159/000369027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf OT. The influence of stress hormones on emotional memory: relevance for psychopathology. Acta Psychol (Amst) 2008;127:513–531. doi: 10.1016/j.actpsy.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Wyrofsky R, McGonigle P, Van Bockstaele EJ. Drug discovery strategies that focus on the endocannabinoid signaling system in psychiatric disease. Expert Opin Drug Discov. 2015;10:17–36. doi: 10.1517/17460441.2014.966680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.