Abstract
BACKGROUND
Corticotropin-releasing hormone receptor 1 (CRH-R1) in the amygdala and the stria terminalis plays an important role in the activation of central stress circuits. Genetic factors may contribute to the hyperresponsiveness of these circuits in irritable bowel syndrome (IBS).
AIMS
To determine if CRH-R1 SNPs are associated with: 1) a diagnosis of IBS, 2) gastrointestinal (GI) symptoms, and 3) acoustic startle response (ASR) to threat, which is mediated by the amygdala via CRH.
METHODS
Three CRH-R1 SNPS (rs110402, rs242924, and rs7209436) were genotyped using salivary DNA from IBS and healthy control subjects (HCs). Eye blink ASR was obtained during safe (no shock), anticipation (abdominal shock may soon occur) and threat (abdominal shock likely) conditions in a subset of subjects. Associations between each SNP with IBS status, clinical traits and ASR were measured.
RESULTS
235 IBS patients (mean age 37.5 yrs, 74% F) and 264 HCs (mean age 32.1 yrs, 70% F) were studied. Of these, 57 IBS and 41 HCs underwent the ASR protocol. The presence of IBS was associated with the major allele for all three CRH-R1 SNPs (p=0.009-0.025). Within IBS, the major allele for all three SNPs (p=0.017-0.065) was associated with GI symptom anxiety scores. Within subjects with at least one copy of the major allele for the CRH-R1 SNPs, IBS had significantly lower ASR compared to HCs during threat conditions (p=0.001-0.002). Within IBS, CRH-R1 SNPs were associated with a graded increase in ASR to threat (p=0.007-0.008).
CONCLUSION
These findings support that CRH-R1 contributes to the dysregulated stress responsiveness in IBS.
Keywords: irritable bowel syndrome, corticotropin releasing hormone receptor 1, genetic polymorphism, acoustic startle response
1. INTRODUCTION
Irritable bowel syndrome (IBS) affects approximately 10.5% of the population (Wilson et al., 2004), and as currently defined, likely represents a heterogeneous group of disorders presenting with a similar pattern of symptoms, including abdominal pain, altered bowels habits and gastrointestinal (GI) symptom-specific anxiety (Su et al., 2014). Several studies suggest an interaction between genetic and environmental factors (including early adverse life events [EALs]) in the development of altered brain-gut interactions in stress-sensitive conditions including IBS (Fukudo, 2007, 2013; Sato et al., 2012). In predisposed individuals, chronic or sustained stress can result in increased responsiveness of central stress circuits and an increased vulnerability for the development of functional and affective disorders (Mayer et al., 2001).
Stress results in increased central corticotropin releasing hormone (CRH) and noradrenaline release, and activation of behavioral and autonomic nervous system responses. Converging results from preclinical and clinical studies suggest that central and peripheral effects of CRH play a role in IBS pathophysiology by modulating visceral perception, intestinal motility and permeability, immune function, and the hypothalamic-pituitary-adrenal (HPA) axis (Kiank et al., 2010). The mechanism was further elucidated in a preclinical study in rats, which showed that elevated corticosterone levels in the amygdala resulted in somatic and colonic hypersensitivity via a CRF 1 receptor-dependent mechanism in the bed nucleus of the stria terminalis (BNST) (Tran et al., 2012). In healthy individuals, peripheral administration of CRH has been shown to increase small intestine permeability via a mast cell-dependent mechanism (Vanuytsel et al., 2014), increase small bowel constriction and ascending colon volumes (Pritchard et al., 2015), decrease perception thresholds and increase perceptual ratings to rectal balloon distension (Lembo et al., 1996). All of these effects are thought to be mediated by CRH 1 receptors (CRH-R1). In IBS subjects, administration of a CRH-R1 antagonist was associated with suppression of colonic motility indices and abdominal pain ratings induced by electrical stimulation (Sagami et al., 2004), as well as with reduced cerebral blood flow in brain regions that were activated in response to an expected abdominal pain stimulus (Labus et al., 2013).
CRH-R1 receptors are widely distributed throughout the brain-gut axis, and its role in neuroendocrinology has been intensely studied and clearly defined (Fukudo, 2007; Tyrka et al., 2009). CRH-R1 also appears to play an important role in central stress circuits underlying anxiety-like sustained fear responses that are activated and modulated via the amygdala complex (Walker and Davis, 2008). Rapid, phasic responses to threat are generated from outputs of the basolateral amygdala (BLA) acting on the central nucleus of the amygdala (CeA) which in turn projects to hypothalamus and brainstem regions that regulate stress responses. In contrast, slower developing and more sustained fear responses (anxiety-like responses) involve projections of the BLA to the BNST which also projects to hypothalamic and brainstem areas similar to those innervated by the CeA. Growing animal evidence suggests that CRF plays a significant role in this latter pathway (Tran et al., 2012). CeA neurons release CRF which binds to receptors located on the terminals of glutamatergic BLA neurons which in turn increases glutamate release and BNST activation (Walker and Davis, 2008).
An important function of the extended amygdala circuit described above is to modulate the threshold for defensive reflexes such as freezing or eye blink responses. If, for example, an auditory stimulus is salient, it triggers a predictable defensive motor reflex which can be assessed as an eye blink in humans and other mammals. Circuits of the amygdala complex set the sensitivity of this system such that increased amygdala activation (e.g. during conditions of threat) causes a lower threshold for activation of the defensive reflex and this is manifested in a greater eye blink response to a sudden loud sound termed the acoustic startle response (ASR) (Gillespie et al., 2009; Naliboff et al., 2008). Potentiated ASRs during predictable or conditioned threat results from the fear circuit described above while unpredictable (or anxiety-like) threats potentiate the ASR via the BNST circuit involving CRH (Davis, 1998; Walker and Davis, 2008). CRF modulation of the BNST circuitry in the ASR was further supported in a preclinical study in stressed rats, which showed that there was a significant decrease in startle amplitude when a CRF-R1 antagonist was implanted into the anteriolateral BNST compared to the normal saline control (Tran et al., 2014). Additionally, chronic changes in the amygdala, such as CRF receptor upregulation, can also impact stress responses. For example the presence of EALs or genetic factors are associated with greater priming for stress responsiveness and enhanced ASRs to threat (Gillespie et al., 2009). Enhanced threat potentiated ASR, which may represent amygdala-dependent central pain amplification, have been demonstrated in IBS subjects (Naliboff et al., 2008), fibromyalgia (Bartley et al., 2009), anxiety (Davis, 1998), and interstitial cystitis (Twiss et al., 2009).
Single nucleotide polymorphisms (SNPs) residing in the gene encoding for the CRH-R1 have been implicated in the pathophysiology of both IBS (Sato et al., 2012) and several psychiatric conditions including depression, (Bradley et al., 2008; Heim et al., 2009; Papiol et al., 2007; Sumner et al., 2014) which can coexist with IBS (Fond et al., 2014; Park et al., 2013). Sato and colleagues (Sato et al., 2012) found that there was a significant association between the presence of the major allele of CRH-R1 SNPs rs7209436, rs242924 and IBS and a trend with rs110402. They did not find an association of these SNPs with IBS bowel habit subtype or anxiety and depression symptoms. However, this study was conducted in a relatively small number of Japanese subjects, i.e. 103 IBS and 142 healthy controls. This group subsequently measured associations of IBS status with CRH SNPs in 111 IBS and 142 controls (Sasaki et al., 2016) and CRH receptor 2 (CRH-R2) SNPs (Komuro et al., 2016) in 142 IBS and 142 controls in a Japanese population. CRH-R2 SNP, but not CRH SNP, associations with the presence of IBS were found. Although associations of these SNPs and mood and perceived stress symptoms were assessed in these studies, they did not evaluate associations with IBS symptom severity, gastrointestinal (GI) symptom-specific anxiety, EALs or amygala function.
The major allele for the CRH-R1 SNPs was recently found to be associated with increased depressive symptoms in the presence of childhood abuse (Bradley et al., 2008; Heim et al., 2009). Additionally, another study showed that the major allele was associated with a blunted cortisol response to a mental stressor, providing evidence for the association of the major allele and blunted CRH-mediated HPA axis responses to a contextual stressor (Sumner et al., 2014).
Although studies have investigated the relationship between CRH-R1 SNPs, physiologic dysregulation, and some stress-sensitive conditions, it is not known if SNPs of the CRH-R1 are associated with changes in amygdala function, as indexed by enhanced symptom related anxiety and altered ASR in IBS subjects. In the current study conducted in well characterized, racially diverse IBS subjects and healthy controls (HCs), we aimed to determine if CRH-R1 SNPs are associated with: 1) a diagnosis of IBS with or without accounting for EALs, 2) GI symptom severity, and 3) an altered ASR. We hypothesized that: 1) there is an association between the CRH-R1 SNPs and the presence of IBS and IBS symptom severity and that these associations would be strengthened when accounting for EALs, 2) there is an association of CRH-R1 SNPs with increased ASR.
2. MATERIALS AND METHODS
2.1 Study Subjects and Recruitment
IBS subjects and HCs were primarily recruited from community advertisements although some subjects were recruited from a functional bowel disorders clinic at a university hospital setting. Subjects were between 18-55 years of age and underwent a medical history and physical examination by a gastroenterologist or nurse practitioner with expertise in IBS. IBS and bowel habit subtyping was determined by the Rome III diagnostic criteria (Drossman, 2006) in the absence of other chronic GI conditions and confirmed by a clinician with expertise in IBS. Exclusion criteria included pregnancy, substance abuse, abdominal surgery, tobacco dependence (smoked half a package of cigarettes or more daily), current psychiatric illness, and extreme strenuous exercise (exercised one hour or more per day). HCs had no history of IBS, other chronic pain disorders, or current or past psychiatric illnesses, and were not taking beta-adrenergic blockers or centrally acting drugs (antidepressants, anxiolytics, and narcotics).
All subjects were compensated for participating in the study, and written informed consent was obtained from all subjects. The study was approved by the University of California Los Angeles (UCLA) Institutional Review Board and was conducted in accordance with the institutional guidelines regulating human subjects research.
2.2 Symptom Measures
A bowel symptom questionnaire was used to assess the presence and severity of IBS symptoms (abdominal pain, bloating, usual and overall IBS symptom severity) and determine fulfillment of the Rome III diagnostic criteria (Longstreth et al., 2006). Severity of abdominal pain, IBS symptoms, and sensation of bloating during the past week were assessed on a scale from 0 (no pain) to 20 (most intense pain imaginable) (Bradford et al., 2012). Usual IBS symptom severity was ranked on a 5-point scale, which included: none, mild, moderate, severe or very severe symptoms (Bradford et al., 2012). Non-GI clinical traits were assessed using the following scales: current anxiety and depression symptoms (Hospital Anxiety and Depression Scale [HAD]) (Zigmond and Snaith, 1983), GI-symptom specific anxiety (Visceral Sensitivity Index [VSI]) (Labus et al., 2004), and somatic symptoms (Patient Health Questionnaire [PHQ-15]) (Kroenke et al., 2002). The HAD scale is a self-assessment scale that assesses current depression and anxiety symptoms (Zigmond and Snaith, 1983). The VSI is a reliable, valid measure of GI symptom-specific anxiety (Labus et al., 2004). The PHQ-12 (modified PHQ-15 without the three GI symptoms) has been shown to correlate with patient behavior and symptom severity in IBS (Kroenke et al., 2002). Early Trauma Inventory Self Report-Short Form (ETI-SR) measures the occurrence (before the age of 18) of 27 EAL items in the following domains (number of items): general trauma (11), physical (5), emotional (5), and sexual abuse (6). Each of the 27 items is scored as “Yes”=1 or “No”=0 (total score range 0-27) (Bremner et al., 2007). In addition, all the subjects had previously undergone a structured psychiatric interview (MINI) to measure past or current psychiatric illness (Sheehan et al., 1998).
2.3 DNA collection
DNA extracted from salivary samples of IBS subjects and HCs was processed and assessed by the UCLA Biological Samples Processing Core using the Autopure LS Nucleic Acid Purification instrument (Gentra Systems, Inc., Minneapolis, MN.) The CRH-R1 SNPs were chosen based on an extensive review of the literature. The CRH-R1 polymorphisms (rs110402, rs242924, rs7209436) were genotyped using the Fluidigm Biomark system. First, 10-60 ng of DNA was pre amplified using Qiagen Multiple PCR master mix. This was then diluted and used for amplification as starting material. Next, the samples and assays were loaded onto GT 96*96 Dynamic array and processed per Fluidigm protocol. The assays used were designed using Fluidigm's proprietary technology. The genotyping calls were made using Fluidigm SNP genotyping software. The literature is inconsistent on the labeling of CRH-R1 SNP alleles. We chose alleles consistent with the reference SNP orientation on db SNP which were C and T for rs110402 and rs7209436 and A and C for rs242924 (http://www.ncbi.nlm.nih.gov/SNP/). The C allele is the major allele for all three SNPs.
2.4 Acoustic Startle Response (ASR) Protocol
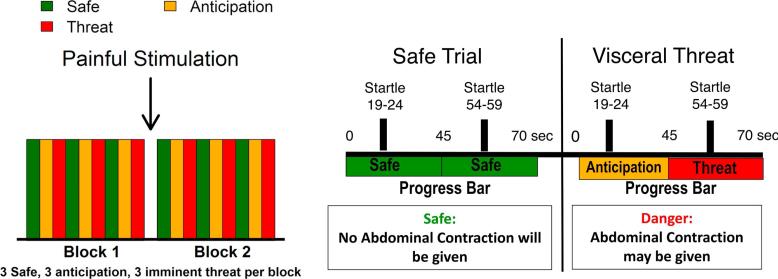
The acoustic startle protocol tested eye-blink ASRs to the relatively unpredictable threat of abdominal electrical stimulation using standard procedures for our laboratory (Figure 1) and previously described in detail (Twiss et al., 2009). Briefly, auditory startle stimuli (105 dB, 0 rise time, 50 ms white noise bursts) were presented binaurally and, immediately following, eye blink strength was measured as electromyographic activity of the orbicularis oculi muscle. A brief, intense muscle stimulation (1 second duration, 20.4 mA peak current) to the area over the lower abdomen served as an aversive stimulus. During the threat procedure, 6 safe and 6 abdominal threat periods of 70 seconds each were presented in alternating order. During Safe and Threat periods, startle stimuli were presented at random times between 19 and 24 seconds and between 54 and 59 seconds. Subjects were told that the computer monitor would inform them which type of period they were in and that no stimulations would be given during the Safe periods. However, during the danger periods they might receive a brief uncomfortable stimulation to the lower abdomen. Subjects were also informed that during all conditions they would see a progressing bar showing the time from 0 to 70 seconds, and that during the danger periods, abdominal stimulation, if it occurred, would only happen when the bar turned red (between 45 and 70 seconds). The first half of the danger period was therefore considered an anticipation condition for the analysis and the second half the threat condition. They were informed that they might receive up to 3 stimulations of increasingly stronger magnitude; however, only one abdominal stimulus was actually delivered during the study.
Figure 1.
Acoustic Startle Response (ASR) Protocol. Two blocks were analyzed. Each block consists of 3 safe (green), 3 anticipation (yellow) and 3 threat (red) conditions, which last approximately 30-45 seconds each. During Safe and Threat periods, startle stimuli were presented at random times between 19 and 24 seconds and between 54 and 59 seconds and then EMG activity measuring eye blink is obtained. A brief, intense abdominal muscle stimulation served as an aversive stimulus.
2.5 Statistical Analysis
Hardy–Weinberg equilibrium and the three different genetic models (additive, dominant, and recessive) were tested for each of the three CRH-R1 SNPs. The additive genetic model (increases in the minor alleles) was found to be the most statistically significantly associated with IBS status, and thus this model was utilized for all SNPs to focus downstream analyses. Individual logistic regressions were used to predict the odds of IBS status from each SNP while controlling for race (categorical: Caucasian, African American, Other/Mixed), sex (categorical: male or female), and HAD anxiety and depression symptom scores (continuous). These covariates were selected a priori due to known potential confounding, or association with both IBS status and the CRH-R1 SNPs. As is usually done for genetic association studies, we controlled for race and sex (Cross et al., 2010). Furthermore, there is evidence of sex differences in IBS symptoms (Houghton et al., 2016) and neurobiologic mechanisms including the HPA axis response (Videlock et al., 2016). In addition, we also controlled for anxiety and depression symptom scores because these are often higher in IBS subjects vs. HCs and CRH-R1 and their SNPs have been associated with anxiety and depression (Bradley et al., 2008; Fond et al., 2014) (Heim et al., 2009) (Papiol et al., 2007) (Park et al., 2013) (Sumner et al., 2014).
We tested for the association between pre-specified GI clinical traits (i.e. overall GI severity, usual GI severity, VSI, PHQ-12) with each SNP in linear regression models that controlled for race, sex, and HAD anxiety and depression scores within the IBS subjects as a secondary exploratory analysis. The association between CRH-R1 SNPs with ASR and interactions between CRH-R1 SNPs, IBS status, and ASR conditions on ASR were evaluated using linear mixed models to account for the multiple measurements collected for each participant. Significance was defined by p<0.05. All statistical analyses were performed using R version 3.1.1 (http://cran.r-project.org/) and all tests were two-tailed.
3. RESULTS
3.1 Baseline Clinical Characteristics
There were 499 subjects who participated in the study after 138 Asians were excluded because the major and minor alleles are reversed in this population compared to all other ethnic groups ((dbSNP)). The study group was comprised of 235 IBS subjects (mean age 37.6 yrs, 74% women) and 264 HCs (mean age 32.1 yrs, 70% women). Of these 499 subjects, a subset of 98 individuals (57 [58.2%] IBS, 41[41.8%] HCs) completed the ASR protocol. The baseline clinical characteristics of the larger group and subset who underwent ASR are summarized in Table 1. The clinical characteristics of the larger group were similar to the ASR group.
Table 1.
Clinical characteristics of subjects
| Genetic Dataset | ASR Dataset | |||
|---|---|---|---|---|
| HC (n=264) | IBS (n=235) | HC (n=41) | IBS (n=57) | |
| Variable | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Age | 32.06 (11.18) | 37.55 (12.39) | 35.17 (10.54) | 39.32 (11.25) |
| BMI | 25.46 (4.84) | 25.54 (5.05) | 26.97 (5.38) | 25.31 (4.84) |
| Female (%) | 185 (70%) | 173 (74%) | 31 (76%) | 46 (81%) |
| Race | ||||
| Caucasian | 154 (58%) | 166 (71%) | 23 (58%) | 46 (84%) |
| African American | 55 (21%) | 36 (15%) | 9 (22%) | 4 (7%) |
| Other/Mixed | 55 (21%) | 36 (14%) | 8 (20%) | 5 (9%) |
| Early Adverse Life Events (ETI) | ||||
| Total Score | 4.09 (4.07) | 6.56 (5.42) | 4.20 (3.80) | 7.16 (5.17) |
| General Trauma | 1.75 (1.79) | 2.56 (2.21) | 2 (1.91) | 2.79 (2.35) |
| Physical Abuse | 1.16 (1.44) | 1.63 (1.58) | 1.12 (1.19) | 1.68 (1.58) |
| Emotional Abuse | 0.77 (1.4) | 1.66 (1.78) | 0.68 (1.27) | 1.91 (1.77) |
| Sexual Abuse | 0.42 (1.03) | 0.81 (1.61) | 0.39 (0.95) | 0.77 (1.6) |
| HAD Anxiety | 3.27 (2.75) | 7.41 (4.28) | 3.17 (3.03) | 7.67 (4.15) |
| HAD Depression | 1.23 (1.75) | 3.96 (3.53) | 0.85 (1.31) | 4.25 (3.29) |
| PHQ Score - 12 Score | 1.93 (1.85) | 6.03 (4.13) | 1.69 (1.78) | 7.59 (3.75) |
| VSI Score | 2.33 (4.74) | 34.79 (16.57) | 2.39 (4.21) | 29.46 (14.91) |
| GI Symptoms | ||||
| Overall Severity | 10.3 (4.38) | 9.27 (4.47) | ||
| Abdominal Pain | 9.5 (5.02) | 8.04 (5.26) | ||
| Usual Severity | 3.17 (0.76) | 2.91 (0.75) | ||
3.2 Association of CRH-R1 SNPs and IBS Diagnosis
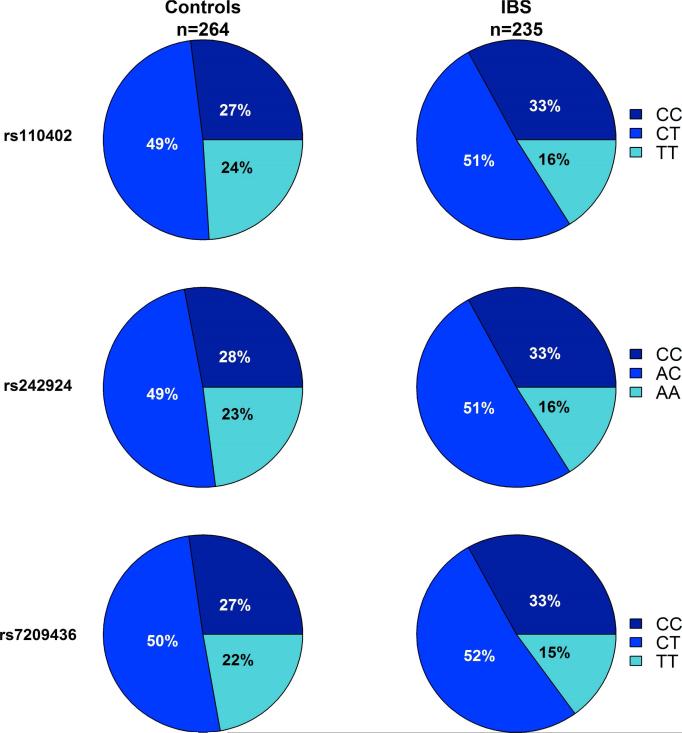
As shown in Figure 2, an increase in the number of major alleles for CRH-R1 SNPs rs110402, rs242924, and rs7209436 were significantly associated with a higher incidence of IBS (p=0.015, p=0.025, p=0.009, respectively). There were no significant interactions between CRH-R1 SNPs and ETI-SR total or subscale scores in the association with IBS (all p's>0.05).
Figure 2.
Increases in the number of major alleles (C) for all three CRH-R1 SNPs were significantly associated with a higher incidence of IBS (rs110402: p=0.015, rs242924: p=0.025, and rs7209436: p=0.009).
3.3 Association of CRH-R1 SNPs and Symptoms in IBS subjects
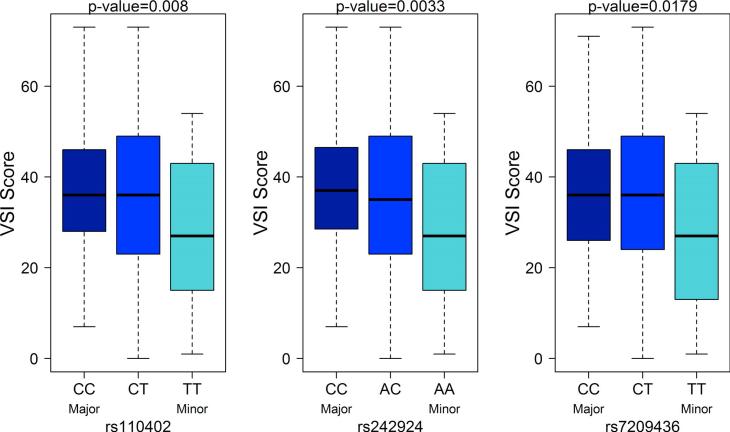
The association between GI-symptom specific anxiety (VSI score (Labus et al., 2004)) and symptom severity (BSQ, PHQ-12 (Rey et al., 2014)) with the CRH-R1 SNPs was evaluated while controlling for race, sex, HAD anxiety and HAD depression. Within the IBS subjects, an increase in the number of major alleles for CRH-R1 SNPs was associated with increased VSI scores (Figure 3; rs110402: p=0.008, rs242924: p=0.003, and rs7209436: p=0.018). There were no significant associations between the CRH-R1 SNPs and overall and usual GI symptom severity, abdominal pain severity, and PHQ-12 scores. There were also no significant interactions between CRH-R1 SNPs and ETI-SR total or subscale scores in the association with overall and usual IBS symptom severity, abdominal pain severity, PHQ score, and VSI score (all p's>0.05) except for the interaction between rs110402 with ETI-SR general trauma score with usual IBS symptom severity (p=0.02). IBS subjects with both copies of the major allele had no association between ETI-SR general trauma scores with usual severity, but IBS subjects with one or two minor allele copies had a negative association between ETI-SR general trauma score with usual IBS symptom severity. That is, the higher the ETI-SR score, the lower the usual IBS severity score in IBS subjects with at least one copy of the minor allele.
Figure 3.
In the IBS subjects only, increases in the number of major alleles for CRH-R1 SNPs are associated with increased VSI scores (rs110402: p=0.008, rs242924: p=0.003, and rs7209436: p=0.018).
3.4 Association of CRH-R1 SNPs with ASR
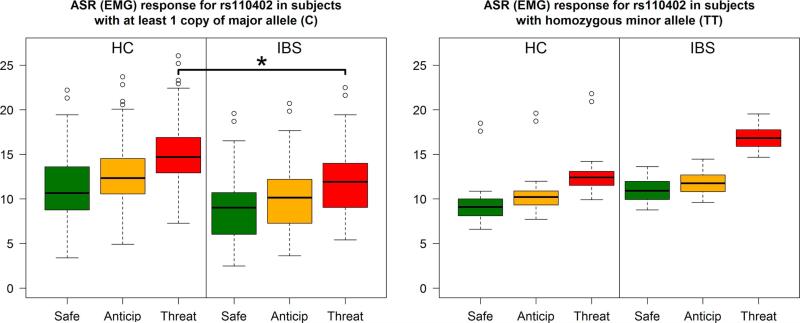
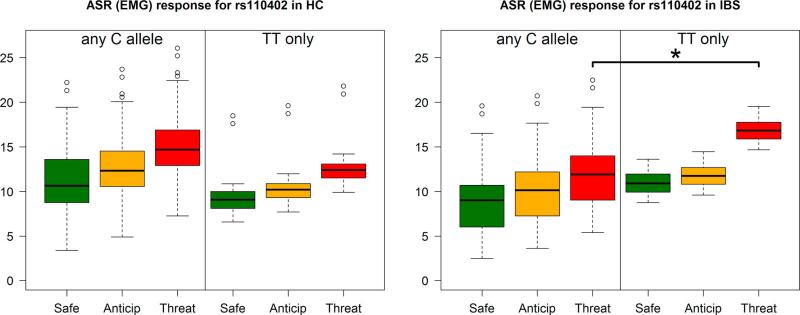
There was a significant group × condition × CRH-R1 SNP interaction (p<0.001 for both rs110402 and rs242924 and p=0.009 for rs720943). Individual contrasts showed that IBS subjects with at least one copy of the major allele for the CRH-R1 SNPs had significantly lower ASR compared to HCs during the threat condition (Figure 4, p=0.002 for both rs110402 or rs242924 and p=0.001 for rs720943). In contrast, IBS subjects with homozygous minor alleles for CRH-R1 SNPs had a numerically higher ASR to threat compared to HCs but this was not statistically significant (p=0.289). Unfortunately, there were only 20 individuals (10 IBS, 10 HCs) that were homozygous for the minor allele, which limited the power of this comparison. In addition, within the IBS group only, subjects with at least one copy of the major allele for the CRH-R1 SNPs had significantly lower ASR compared to subjects with no copies within the threat condition (Figure 5, p=0.008 for both rs110402 and rs242924 and p=0.007 for rs720943). While the same data is shown in Figures 4 and 5, Figure 4 highlights the significant differences in ASR between IBS subjects and HCs with at least one copy of the major allele (C), while Figure 5 more clearly demonstrates the diference in ASR within IBS subjects with distinct genotypes.
Figure 4.
In subjects with at least one copy of the major allele (C) for rs110402, the IBS group had a significantly lower ASR, as measured by EMG, compared to HCs during the threat condition (p=0.002, left panel). Similar significant findings were seen with rs242924 and rs7209436. In contrast, IBS subjects with homozygous minor alleles (TT) for CRH SNPs had a numerically higher ASR to threat compared to HCs but this was not statistically significant (right panel).
Figure 5.
In IBS subjects only, subjects with at least one copy of the major allele for rs110402 had significantly lower ASR, as measured by EMG, compared to subjects with no copies within the threat condition (p=0.008, right panel). Similar significant findings were seen with rs242924 and rs7209436. However, there were no significant differences in the HCs (left panel).
4. DISCUSSION
We aimed to test the hypothesis that gene polymorphisms of the CRH-R1 influence IBS status, GI symptomology and the amygdala-dependent central stress response in a relatively large, well phenotyped cohort of 499 IBS and HC subjects. The main findings of the study showed that CRH-R1 SNPs, specifically the presence of the major allele, were associated with IBS, increased GI-symptom specific anxiety, and altered amygdala function as evidenced by a blunted ASR. Despite the fact that altered CRH-R1 signaling has been associated with stress-sensitive conditions such as depression and more recently IBS, to our knowledge, this is the first demonstration of the interaction of these genetic factors influencing GI symptom-specific anxiety related to altered central stress processing in IBS.
4.1 Effect of CRH-R1 SNPs on IBS Diagnosis and Symptoms
We found a statistically significant relationship between the major allele of all three CRH-R1 SNPs and the presence of IBS. This observation is consistent with the previously published finding by Sato et al., which showed that the major allele in the Japanese population for the same CRH-R1 SNPs (rs7209436, rs242924), was significantly more common in IBS subjects than HCs (Sato et al., 2012). Interestingly, in a follow-up study, they did not find an association of CRH and CRH-binding protein SNPs with IBS (Sasaki et al., 2016). Although a number of rodent studies demonstrate the role of CRH-R1 in modulating GI function (Larauche et al., 2012), there is very limited data in humans that describes the CRH-R1 activity in the GI tract suggesting that the receptor functions to enhance colonic motility and increase visceral perception (Fukudo, 2007). A small study by Fukudo et al. showed that exogenous administration of CRH induced exaggerated colonic motility indices compared to HCs (Fukudo et al., 1998). Furthermore, the administration of a CRH receptor antagonist inhibited this exaggerated response in IBS subjects, and also decreased self-reported pain scores during electrical rectal stimulation in IBS subjects (Fukudo et al., 1998). Similarly, we found that the major alleles for the CRH-R1 SNPs were associated with GI symptom anxiety as measured by VSI, which has been shown to positively correlate with IBS severity in both U.S (Labus et al., 2007) and Japanese (Saigo et al., 2014) cohorts of IBS subjects, further suggesting that CRH receptor activation results in increased visceral perception. The presence of general trauma events early in life impacted the association of one of the CRH SNPs and usual IBS symptom severity, but only in IBS subjects with at least one copy of the minor allele of CRH-R1 SNP, rs110402. This finding, however, should be interpreted with caution because multiple comparisons were evaluated and the finding was with only one of the CRH-R1 SNP (as opposed to our other results). In addition, IBS subjects with the minor allele had a higher ASR during threat and we would have expected that it would be associated with greater, and not lower, IBS symptom severity.
Although the CRH/CRH-R1 signaling system plays an important role in brain gut interactions, it has been studied more extensively in psychiatric conditions. Several studies have shown that gene polymorphisms in the CRH-R1 appear to predispose individuals to anxiety and depression (Bradley et al., 2008; Heim et al., 2009). Bradley et al showed that individuals who were homozygous for the major allele of the CRH-R1 SNPs rs110402 and rs7209436 and who were exposed to moderate to severe childhood abuse, reported more depression symptoms compared to those with at least one copy of the minor allele (Bradley et al., 2008). While HAD scores were higher in IBS, only a small subgroup had abnormal HAD scores (>11) and the IBS subjects were excluded for current psychiatric illness.
4.2 Effect of CRH-R1 SNPs on ASR
We found that within subjects that have at least one copy of the major allele for the CRH-R1 SNPs, IBS subjects had significantly lower ASR compared to HCs during the threat condition. In contrast, IBS subjects that were homozygous for the minor alleles for CRH-R1 SNPs had numerically higher ASR to threat compared to HCs. This data suggests that individuals with the major allele CRH-R1 SNPs’ genotype may have CRH-R1 downregulation, resulting in a blunted CRH-mediated ASR response to stress while individuals who are homozygous for the minor allele may have an enhanced neurobiologic response to stress. Our threat task involves a relatively unpredictable threat based on the inconsistent delivery of the noxious stimulus and the long ‘threat’ periods. Therefore we hypothesize that it primarily activates the sustained or anxiety-like amygdala/BNST circuit mediated by CRH. Our findings are similar to those obtained in adolescents from a high violence, low socioeconomic status community which showed that major allele carriers for rs110402 had a blunted cortisol response during a mental stressor (Sumner et al., 2014). We have previously shown an enhanced startle response to abdominal pain threat in subjects with IBS, and it is possible that this sample was disproportionately composed of homozygous minor individuals (Naliboff et al., 2008). Furthermore, genotype-determined disease state phenotypes may explain the mixed data on the efficacy of the CRH-R1 antagonist, where some studies have shown that CRH-R1 antagonist attenuates visceral hypersensitivity and inflammation in rats (Saito-Nakaya et al., 2008) and other studies have shown no effect on the gut function (Sweetser et al., 2009).
The finding that IBS subjects with at least one copy of the major allele for CRH-R1 SNPs have significantly lower ASR compared to IBS subjects with two copies of the minor allele further supports our theory that within IBS there are two distinct genotype-determined disease state phenotypes. In the IBS group, whether the stress response is exaggerated or blunted, there is impairment of the dynamic amygdala-dependent emotional circuit, which results in improper interpretation of threat responses (Gillespie et al., 2009). This CRH-mediated impaired central interpretation model is further supported by the study by Labus et al., which characterized the effects of the CRH-R1 antagonist on brain and skin conductance responses during acquisition and extinction of conditioned fear to the threat of abdominal pain (Labus et al., 2007). They showed that with increased doses of the CRH-R1 antagonist, there was normalization of the brain activity in the IBS group during the extinction phase (Labus et al., 2013). Additionally, the CRH-R1 antagonist normalized altered skin conductance response in fear acquisition and extinction learning in IBS (Labus et al., 2013). This study suggests that the greater involvement in IBS subjects during the extinction period might be secondary to impairments in emotional learning due alterations in CRH-R1 activity (Labus et al., 2013). Thus, the CRH-R1 genotype may encode for alterations in CRH-R1 activity in IBS as evidenced by a blunted or exaggerated ASR, which results in two separate endophenotypes associated with stress responsiveness to an abdominal threat.
4.3 Limitations
The study has several limitations. The subject selection was limited to a relatively younger age range (18-55 years) and non-Asians, because the major and minor alleles are reversed in the Asian population compared to all other ethnic groups. Thus, our findings may not be applicable to other populations. ASR was performed in a smaller subgroup of subjects and therefore, the findings need to be replicated in a larger study. We only evaluated amygdala function using ASR and did not include other measures such as mechanistic studies in animal models or manipulation of response with a CRH-R1 antagonist. In addition, HPA axis response was not measured.
4.4 Conclusions and possible clinical implications
In summary, we provide evidence that CRH-R1 SNPs are associated with IBS and GI symptom-specific anxiety, and may also play an important role in the activation and modification of central stress circuits. Additionally, we provide evidence that CRH-R1 genotype may represent a vulnerability factor for IBS and could predispose IBS patients to separate neuroendocrine signaling abnormalities. If replicated in other samples, IBS subjects with one or more copies of the major allele could be studied separately from the homozygous minor IBS subjects to further characterize the unique central stress response and the response to a CRF-R1 antagonist. Based on the different ASRs to abdominal threat, it is conceivable that a CRH-1 antagonist could be efficacious in homozygous minor IBS subjects but not in those with one or more copy of the major allele of the CRH-R1 SNPs. Further studies in populations of individuals with psychiatric and chronic pain conditions will be needed to determine if this potential endophenotype is disease specific or represents a general vulnerability factor, which predicts development of psychiatric or chronic pain conditions.
Highlights.
Corticotropin releasing hormone receptor 1 (CRH-R1) single gene polymorphisms (SNPs), specifically the major allele, are associated with the presence of irritable bowel syndrome (IBS)
Within IBS, the major allele for the CRH-R1 SNPs is associated with increased gastrointestinal symptom anxiety scores
In subjects with at least one copy of the major allele for the CRH-R1 SNPs, IBS had significantly lower acoustic startle response (ASR) compared to HCs during abdominal threat conditions
Within IBS, CRH-R1 SNPs were associated with a graded increase in ASR to abdominal threat
Acknowledgements
We thank the study participants and the staff of the Oppenheimer Center for Neurobiology of Stress and Resilience, startle laboratory and Genetics Core at UCLA. We specifically want to thank Cathy Liu for management of the database.
Role of funding source
This project was supported by NIH grants P50 DK64539 (EAM, LC), RO1 AR46122 (LC), RO1 DK048351 (EAM), R01 NR007768 (BN), P30 DK041301.
Abbreviations
- IBS
irritable bowel syndrome
- CRH-R1
corticotropin-releasing hormone receptor 1
- ASR
acoustic startle response
- EAL
early adverse life event
- GI
gastrointestinal
- ETI
early life trauma
- VSI
visceral sensitivity index
- HADS
hospital anxiety and depression scale
- PNS
peripheral nervous system
- CNS
central nervous system
- ENS
enteric nervous system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
LC designed and implemented the trial, monitored data collection, wrote the statistical analysis plan, analyzed data, drafted and revised the paper, and provided funding and is the guarantor of the paper. AO wrote the data analysis plan, analyzed data and drafted and revised the paper. BN designed and implemented the trial, monitored data collection, analyzed data, and revised the paper. MG analyzed the data, drafted and reviewed the paper. WS and APP wrote the data analysis plan, analyzed the data and revised the drafted paper. TJ collected the data and revised the paper. EAM initiated the collaborative project, revised the drafted paper and provided funding.
Disclosures: No potential conflicts of interest.
Conflict of interest statement
All authors have no conflicts of interest to report.
REFERENCES
- (dbSNP), D.o.S.N.P. National Center for Biotechnology Information, National Library of Medicine; Bethesda, Maryland: [Google Scholar]
- Bartley EJ, Rhudy JL, Williams AE. Experimental assessment of affective processing in fibromyalgia. J Pain. 2009;10:1151–1160. doi: 10.1016/j.jpain.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Bradford K, Shih W, Videlock EJ, Presson AP, Naliboff BD, Mayer EA, Chang L. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2012;10:385–390. e381–383. doi: 10.1016/j.cgh.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Bolus R, Mayer EA. Psychometric properties of the Early Trauma Inventory-Self Report. J Nerv Ment Dis. 2007;195:211–218. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DS, Ivacic LC, Stefanski EL, McCarty CA. Population based allele frequencies of disease associated polymorphisms in the Personalized Medicine Research Project. BMC Genet. 2010;11:51. doi: 10.1186/1471-2156-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Fond G, Loundou A, Hamdani N, Boukouaci W, Dargel A, Oliveira J, Roger M, Tamouza R, Leboyer M, Boyer L. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2014;264:651–660. doi: 10.1007/s00406-014-0502-z. [DOI] [PubMed] [Google Scholar]
- Fukudo S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J Gastroenterol. 2007;42(Suppl 17):48–51. doi: 10.1007/s00535-006-1942-7. [DOI] [PubMed] [Google Scholar]
- Fukudo S. Stress and visceral pain: focusing on irritable bowel syndrome. Pain. 2013;154(Suppl 1):S63–70. doi: 10.1016/j.pain.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. 1998;42:845–849. doi: 10.1136/gut.42.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: genetic and environmental influences on development of the stress response. Depress Anxiety. 2009;26:984–992. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB, Ressler KJ, Binder EB. Effect of Childhood Trauma on Adult Depression and Neuroendocrine Function: Sex-Specific Moderation by CRH Receptor 1 Gene. Front Behav Neurosci. 2009;3:41. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton LA, Heitkemper M, Crowell M, Emmanuel A, Halpert A, McRoberts JA, Toner B. Age, Gender and Women's Health and the Patient. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Ishitobi Y, Nakayama S, Yamaguchi K, Kanehisa M, Higuma H, Maruyama Y, Ninomiya T, Okamoto S, Tanaka Y, Tsuru J, Hanada H, Isogawa K, Akiyoshi J. Association of CRHR1 and CRHR2 with major depressive disorder and panic disorder in a Japanese population. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:429–436. doi: 10.1002/ajmg.b.32046. [DOI] [PubMed] [Google Scholar]
- Kiank C, Tache Y, Larauche M. Stress-related modulation of inflammation in experimental models of bowel disease and post-infectious irritable bowel syndrome: role of corticotropin-releasing factor receptors. Brain Behav Immun. 2010;24:41–48. doi: 10.1016/j.bbi.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Sato N, Sasaki A, Suzuki N, Kano M, Tanaka Y, Yamaguchi-Kabata Y, Kanazawa M, Warita H, Aoki M, Fukudo S. Corticotropin-Releasing Hormone Receptor 2 Gene Variants in Irritable Bowel Syndrome. PloS one. 2016;11:e0147817. doi: 10.1371/journal.pone.0147817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosomatic medicine. 2002;64:258–266. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, Mayer EA, Naliboff BD. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- Labus JS, Hubbard CS, Bueller J, Ebrat B, Tillisch K, Chen M, Stains J, Dukes GE, Kelleher DL, Naliboff BD, Fanselow M, Mayer EA. Impaired emotional learning and involvement of the corticotropin-releasing factor signaling system in patients with irritable bowel syndrome. Gastroenterology. 2013;145:1253–1261. e1251–1253. doi: 10.1053/j.gastro.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labus JS, Mayer EA, Chang L, Bolus R, Naliboff BD. The central role of gastrointestinal-specific anxiety in irritable bowel syndrome: further validation of the visceral sensitivity index. Psychosomatic medicine. 2007;69:89–98. doi: 10.1097/PSY.0b013e31802e2f24. [DOI] [PubMed] [Google Scholar]
- Larauche M, Mulak A, Tache Y. Stress and visceral pain: from animal models to clinical therapies. Exp Neurol. 2012;233:49–67. doi: 10.1016/j.expneurol.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo T, Plourde V, Shui Z, Fullerton S, Mertz H, Tache Y, Sytnik B, Munakata J, Mayer E. Effects of the corticotropin-releasing factor (CRF) on rectal afferent nerves in humans. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 1996;8:9–18. doi: 10.1111/j.1365-2982.1996.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Naliboff BD, Chang L, Coutinho SV. V. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519–524. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Waters AM, Labus JS, Kilpatrick L, Craske MG, Chang L, Negoro H, Ibrahimovic H, Mayer EA, Ornitz E. Increased acoustic startle responses in IBS patients during abdominal and nonabdominal threat. Psychosomatic medicine. 2008;70:920–927. doi: 10.1097/PSY.0b013e318186d858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papiol S, Arias B, Gasto C, Gutierrez B, Catalan R, Fananas L. Genetic variability at HPA axis in major depression and clinical response to antidepressant treatment. J Affect Disord. 2007;104:83–90. doi: 10.1016/j.jad.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Park AJ, Collins J, Blennerhassett PA, Ghia JE, Verdu EF, Bercik P, Collins SM. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25:733–e575. doi: 10.1111/nmo.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard SE, Garsed KC, Hoad CL, Lingaya M, Banwait R, Thongborisute W, Roberts E, Costigan C, Marciani L, Gowland PA, Spiller RC. Effect of experimental stress on the small bowel and colon in healthy humans. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2015;27:542–549. doi: 10.1111/nmo.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey E, Balboa A, Mearin F. Chronic constipation, irritable bowel syndrome with constipation and constipation with pain/discomfort: similarities and differences. Am J Gastroenterol. 2014;109:876–884. doi: 10.1038/ajg.2014.18. [DOI] [PubMed] [Google Scholar]
- Sagami Y, Shimada Y, Tayama J, Nomura T, Satake M, Endo Y, Shoji T, Karahashi K, Hongo M, Fukudo S. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958–964. doi: 10.1136/gut.2003.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigo T, Tayama J, Hamaguchi T, Nakaya N, Tomiie T, Bernick PJ, Kanazawa M, Labus JS, Naliboff BD, Shirabe S, Fukudo S. Gastrointestinal specific anxiety in irritable bowel syndrome: validation of the Japanese version of the visceral sensitivity index for university students. Biopsychosoc Med. 2014;8:10. doi: 10.1186/1751-0759-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito-Nakaya K, Hasegawa R, Nagura Y, Ito H, Fukudo S. Corticotropinreleasing hormone receptor 1 antagonist blocks colonic hypersensitivity induced by a combination of inflammation and repetitive colorectal distension. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2008;20:1147–1156. doi: 10.1111/j.1365-2982.2008.01151.x. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Sato N, Suzuki N, Kano M, Tanaka Y, Kanazawa M, Aoki M, Fukudo S. Associations between Single-Nucleotide Polymorphisms in Corticotropin- Releasing Hormone-Related Genes and Irritable Bowel Syndrome. PloS one. 2016;11:e0149322. doi: 10.1371/journal.pone.0149322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Suzuki N, Sasaki A, Aizawa E, Obayashi T, Kanazawa M, Mizuno T, Kano M, Aoki M, Fukudo S. Corticotropin-releasing hormone receptor 1 gene variants in irritable bowel syndrome. PloS one. 2012;7:e42450. doi: 10.1371/journal.pone.0042450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 Suppl. 1998;20:22–33. quiz 34-57. [PubMed] [Google Scholar]
- Su AM, Shih W, Presson AP, Chang L. Characterization of symptoms in irritable bowel syndrome with mixed bowel habit pattern. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2014;26:36–45. doi: 10.1111/nmo.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, McLaughlin KA, Walsh K, Sheridan MA, Koenen KC. CRHR1 genotype and history of maltreatment predict cortisol reactivity to stress in adolescents. Psychoneuroendocrinology. 2014;43:71–80. doi: 10.1016/j.psyneuen.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetser S, Camilleri M, Linker Nord SJ, Burton DD, Castenada L, Croop R, Tong G, Dockens R, Zinsmeister AR. Do corticotropin releasing factor-1 receptors influence colonic transit and bowel function in women with irritable bowel syndrome? Am J Physiol Gastrointest Liver Physiol. 2009;296:G1299–1306. doi: 10.1152/ajpgi.00011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L, Schulkin J, Greenwood-Van Meerveld B. Importance of CRF receptor-mediated mechanisms of the bed nucleus of the stria terminalis in the processing of anxiety and pain. Neuropsychopharmacology. 2014;39:2633–2645. doi: 10.1038/npp.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L, Wiskur B, Greenwood-Van Meerveld B. The role of the anteriolateral bed nucleus of the stria terminalis in stress-induced nociception. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1301–1309. doi: 10.1152/ajpgi.00501.2011. [DOI] [PubMed] [Google Scholar]
- Twiss C, Kilpatrick L, Craske M, Buffington CA, Ornitz E, Rodriguez LV, Mayer EA, Naliboff BD. Increased startle responses in interstitial cystitis: evidence for central hyperresponsiveness to visceral related threat. The Journal of urology. 2009;181:2127–2133. doi: 10.1016/j.juro.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivity. Biol Psychiatry. 2009;66:681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanuytsel T, van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, Salim Rasoel S, Tomicronth J, Holvoet L, Farre R, Van Oudenhove L, Boeckxstaens G, Verbeke K, Tack J. Psychological stress and corticotropinreleasing hormone increase intestinal permeability in humans by a mast celldependent mechanism. Gut. 2014;63:1293–1299. doi: 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- Videlock EJ, Shih W, Adeyemo M, Mahurkar-Joshi S, Presson AP, Polytarchou C, Alberto M, Iliopoulos D, Mayer EA, Chang L. The effect of sex and irritable bowel syndrome on HPA axis response and peripheral glucocorticoid receptor expression. Psychoneuroendocrinology. 2016;69:67–76. doi: 10.1016/j.psyneuen.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Wilson S, Roberts L, Roalfe A, Bridge P, Singh S. Prevalence of irritable bowel syndrome: a community survey. Br J Gen Pract. 2004;54:495–502. [PMC free article] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]