Abstract
Background
Glucagon-like peptide 1 (GLP-1) receptor agonists have been shown to decrease ethanol drinking in rodent assays. The GLP-1 system also powerfully modulates food and fluid intake, gastrointestinal functions, and metabolism. To begin to understand the neurobiological mechanisms by which GLP-1 receptor ligands may be able to control ethanol intake, it is important to ascertain whether they can modulate the direct reinforcing effects of ethanol, without the confound of effects on ingestive behaviors generally.
Methods
We trained experimentally naïve, free-fed C57BL/6J mice to self-administer ethanol intravenously. Once stable ethanol intake was acquired, we tested the effect of acute pretreatment with the GLP-1 receptor agonist Exendin-4. Effect of Exendin-4 on operant behavior reinforced by a palatable liquid food was similarly evaluated as a control.
Results
Intravenous ethanol functioned as a positive reinforcer in over half the mice tested. In mice that acquired self-administration, ethanol intake was high, indeed, reaching toxic doses. 3.2 μg/kg Exendin-4 decreased intravenous ethanol intake by at least 70%, but had no significant effect on food-maintained operant responding.
Conclusions
This experiment produced two main conclusions. First, although technically challenging and yielding only moderate throughput, the intravenous self-administration procedure in mice is feasible, and sensitive to pharmacological manipulations. Second, GLP-1 receptor agonists can powerfully attenuate voluntary ethanol intake by directly modulating the reinforcing effects of ethanol. These findings support the potential usefulness of GLP-1 receptor ligands in the treatment of alcohol use disorder.
Keywords: Alcohol, Intravenous self-administration, Exendin-4, Glucagon-like peptide 1, Mouse
INTRODUCTION
Alcohol use disorder causes great costs to individuals and to society worldwide, with an annual 3.3 million deaths attributed to alcohol use (SAMHSA, 2014; WHO, 2014). Although available therapies and counseling can be effective in some drinkers, long-term recovery remains difficult to achieve. Glucagon-like peptide 1 (GLP-1) is a peptide that has both hormone and neurotransmitter functions. GLP-1 is produced in the intestinal tract and regulates blood sugar and food intake, and GLP-1 receptor agonists are used clinically to manage type 2 diabetes. GLP-1 is also produced in the brain, and GLP-1 receptors are expressed in brain regions important in substance use disorders, such as the ventral tegmental area (VTA) and nucleus accumbens (Göke et al. 1995; Merchenthaler et al., 1999; Cork et al., 2015). GLP-1- producing neurons of the nucleus of the solitary tract project directly to the VTA and the shell and core regions of the nucleus accumbens (Rinaman, 2010; Alhadeff et al., 2012). Recent evidence indicates that one or more GLP-1 receptor variants are associated with alcohol use disorder and modulate effects of ethanol in humans (Suchankova et al., 2015).
In rodent studies, GLP-1 receptor agonists were shown to reduce ethanol drinking, although the effects were sometimes modest, or only observed in high-drinking subjects (e.g., made dependent on ethanol using vapor exposure, or spontaneously alcohol-preferring; Egecioglu et al., 2013a; Shirazi et al., 2013; Suchankova et al., 2015; Vallöf et al., 2016). The well-described role of GLP-1 systems in regulating food and fluid intake makes it difficult to differentiate how much of the observed decreases in ethanol drinking are attributable to decreased reinforcing/rewarding effects of ethanol specifically, from effects on fluid and calorie intake more generally. Indeed, the abovementioned studies often reported decreases in food or water intake in animals treated with GLP-1 receptors agonists, albeit often smaller in magnitude or more variable relative to the effects on alcohol intake. GLP-1 receptor-mediated effects on food and fluid intake are at least partly centrally mediated (Tang-Christensen et al., 1996; Turton et al., 1996; Baggio et al., 2004; Kanoski et al., 2012; Dickson et al., 2012; McKay et al., 2014), and may thus share common neurobiological mechanisms with the effects on ethanol drinking. Another potential complicating factor in using oral ethanol assays is the fact that GLP-1 receptor ligands affect gastric emptying of liquids and solids, as well as colonic motility, which could affect rates of ethanol uptake into the blood stream after oral administration (Wettergren et al., 1993; Imeryuz et al., 1997; Baggio et al., 2004; Nakade et al., 2006; Nakade et al., 2007).
Place conditioning experiments support the notion that GLP-1 systems modulate rewarding effects of ethanol and other drugs of abuse directly, rather than simply decreasing oral intake. GLP-1 receptor agonists decreased conditioned place preference to ethanol, sweet foods, nicotine, cocaine and amphetamine (Dickson et al., 2012; Egecioglu et al., 2013a; Egecioglu et al., 2013b, 2013c; Graham et al., 2013; Shirazi et al., 2013; Alhadeff and Grill, 2014; Richard et al., 2015; Vallöf et al., 2016), and also blunted increases in extracellular striatal dopamine induced by ethanol, nicotine, or stimulants (Egecioglu et al., 2013a; Egecioglu et al., 2013b, 2013c; Sørensen et al., 2015; Vallöf et al., 2016). However, place conditioning measures effects of experimentally administered drug, in a drug-free state, and effects do not always align with effects on voluntary drug or ethanol intake. Intravenous self-administration (IVSA) in laboratory animals is a procedure for measuring the immediate reinforcing effects of drugs, i.e., active drug taking behavior, and is frequently used as an “animal model” of human drug abuse (Panlilio and Goldberg, 2007). IVSA has generally been a better predictor of clinical efficacy for candidate medication relative to assays that rely on subjective/conditioned effects of abused substances (Haney and Spealman, 2008). Exendin-4 is a degradation-resistant, brain penetrant peptide and a long-acting GLP-1 receptor agonist (Göke et al., 1993; Thorens et al., 1993; Kastin and Akerstrom, 2003). We have recently shown that Exendin-4 can decrease IVSA of cocaine in mice (Sørensen et al., 2015). Here, we applied ethanol IVSA to measure the effects of Exendin-4 on voluntary ethanol intake without the potential confounds related to oral consumption.
MATERIALS AND METHODS
Animals and housing
Male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were acquired at 8 weeks of age and were acclimated to the housing facilities at least 7 days before experiments were initiated. Mice were group housed up to 4 per cage under a 12-hour light/dark cycle, lights on at 2300. Food and water (rodent diet 5015, PMI Feeds, Inc., St. Louis, MO) were accessible ad libitum in the home cage. For enrichment, rodent “treats”, nesting material and hiding/nesting devices were provided. Experimental sessions took place between 1300 and 1700. All procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the McLean Hospital Institutional Animal Care and Use Committee.
Apparatus
Modular mouse operant conditioning chambers were used (Med-Associates, Inc., Georgia, VT), enclosed in sound-attenuating cubicles equipped with a house light (Med Associates ENV-221M), an exhaust fan, and a syringe pump (Med Associates PHM-100, 3.3 rpm) for the delivery of intravenous solutions or liquid food reinforcers. The manipulanda were two illuminated nose-poke holes, located 1 cm above the grid floor (Med Associates ENV-313M), one on either side of a steel dish into which liquid food could be delivered. A low-torque fluid swivel (375/25, Instech Laboratories, Plymouth Meeting, PA) mounted on a balance arm was used for IV solution delivery, allowing free movement within the chamber.
Catheter implantation surgery and maintenance
An indwelling catheter was implanted into the right or left external jugular vein under oxygen/sevoflurane vapor anesthesia. The surgical procedure has been described in detail elsewhere (Thomsen and Caine, 2005). Briefly, a catheter (MIVSA, Camcaths, Cambridge, UK) was inserted 1.2 cm into the jugular vein and anchored to the vein with sutures. The catheter ran subcutaneously to the base located above the midscapular region. Mice were allowed 7 days recovery, during which 0.02 ml of 0.9% saline containing heparin (30 USP units/ml) and antibiotic (cefazolin, 67 mg/ml) was infused daily through the catheter to forestall clotting and infection. Outside self-administration sessions, the externalized end of the catheter was kept sealed. Only data from mice in which catheter patency was confirmed before and after completion of an experimental phase were included. Catheter patency was verified by loss of muscle tone and clear signs of anesthesia within 3 s after the infusion of 0.02–0.03 ml of 15 mg/ml ketamine + 0.75 mg/ml midazolam in saline.
Ethanol self-administration
Ethanol IVSA procedures were based on methods by Grahame and Cunningham (1997). Following recovery from surgery, experimentally naïve mice were allowed to acquire nose-poking reinforced with 75 or 125 mg/kg/infusion intravenous ethanol solution under an FR 1 schedule of reinforcement, for daily 2-hour sessions, 5–6 days per week. Consistent with previous experience with other intravenous reinforcers (e.g., Caine et al., 2014), we found that varying the ethanol dose between sessions facilitated acquisition. However, once a mouse emitted at least 15 responses in the active hole in a session, the maintenance and testing dose was 75 mg/kg/infusion for all mice. Responding in the active hole (left or right) resulted in delivery of an ethanol infusion and illumination of the cue light for a 2-s timeout period during which no reinforcer could be earned. Ethanol was infused in 187.5 μl/kg over about one second, adjusted by bodyweight so that infusion duration and volume produced precisely the correct dose for each mouse (e.g., 4.7 μl in a 25g mouse). Responses in the inactive hole and during timeout periods were recorded but had no scheduled consequences. The house light was illuminated after delivery of a single noncontingent reinforcer signaling the beginning of the session, and stayed on until the session ended. Mice were considered to have acquired ethanol self-administration when they earned ≥15 infusions earned per session on the 75 mg/kg dose for three consecutive sessions, with ≥75% responses in the active hole (modified from Grahame and Cunningham, 1997). Mice were tested in two cohorts, a few weeks apart (N=7 each). Due to signs of toxicity in the first cohort, the total ethanol intake per session was limited to no more than 2.25 g/kg for subsequent studies (i.e., the session terminated if the limit was reached). In mice that did not meet criteria after at least 16 sessions, one or two sessions in which responding was reinforced with a palatable liquid food were presented to encourage responding, after which acquisition criteria were increased to a minimum of five days of stable ethanol intake.
Exendin-4 testing and food controls
Once acquisition criteria were met, mice were allowed to self-administer 75 mg/kg/infusion ethanol until intake was stable at ≤20% variation for two consecutive sessions (if this was not already met in the first three acquisition sessions), to provide a baseline level against which to gauge the effects of Exendin-4 administration. The stability criterion was met within 1–5 additional sessions. Then, on the next day, mice had access to 75 mg/kg/infusion ethanol in an identical session, but 30 min following an intraperitoneal injection of saline vehicle or Exendin-4 (3.2 μg/kg and, in a few mice, 1.8 μg/kg). Saline and Exendin-4 doses were tested in a counterbalanced order, with at least two baseline sessions between tests. To control for nonspecific decreases in rates of responding, Exendin-4 was also tested as a pretreatment to nose-poking maintained by a palatable liquid food (25 μl of vanilla flavor Ensure® nutrition drink) under the same conditions (FR 1 schedule of reinforcement, 2 hour sessions). Doses of Exendin-4 were selected based on published studies, and on pilot studies in our laboratory (Baraboi et al., 2011; Williams et al., 2011). Mice tested in the food protocol all had prior exposure to ethanol and Exendin-4, either in the ethanol IVSA experiment described herein (N=4), or in an ethanol “drinking in the dark” pilot experiment (N=4; 20% ethanol in water, no sucrose or vapor exposure).
Drugs
Non-denatured ethanol meeting USP testing specification (190 proof, Sigma-Aldrich, St. Louis, MO) was diluted to 50.7% in sterile 0.9% saline. Exendin-4 (Tocris Bioscience, Ellisville, MO) was dissolved in saline and stored in aliquots at −80C; fresh aliquots were thawed to room temperature immediately before use.
Data analysis
Ethanol intake per session was analyzed by paired-sample t-tests vs. baseline and by unpaired-sample t-test (saline vs. Exendin-4). Food reinforcers earned per session were analyzed by one-way ANOVA with drug condition as a repeated measures variable (saline, Exendin-4 1.8 or 3.2 mg/kg).
RESULTS
A majority of mice acquired ethanol self-administration. In the first cohort, five of seven mice met criteria for acquisition, after 14.8±2.7 sessions (mean ± s.e.m.), at an intake level of 2.4±0.4 g/kg/session (i.e., 31.9±5.6 infusions earned per session). Four mice acquired the response without food presentation. Two mice, which had lower initial levels of nose-poking, met criteria only after nose-poking was initiated with food reinforcement (see methods for increased criteria). The remaining two mice earned over 15 ethanol infusions in individual sessions but never for three consecutive sessions. Catheters remained patent in all mice through the experiment. Due to the high ethanol intake (maximum intake ranging from 3.1 to 8.2 g/kg/session in individual mice) and high incidence of sudden deaths following high intake (6 of 7 mice), we imposed a limit of no more than 2.25 g/kg ethanol (30 reinforcers) per daily session for the second cohort. In the second cohort, 4 of 7 mice met acquisition criteria, after 17.8±5.0 sessions, at an intake level of 2.1±0.1 g/kg/session (28.1±1.3 infusions per session). One mouse acquired after prompting with food, another, after catheter failure and implantation of a second catheter. Despite the limited intake, two mice died within the first 15 sessions, of uncertain causes, and did not meet acquisition criteria.
Pretreatment with 3.2 μg/kg Exendin-4 reduced ethanol self-administration to on average 27% of baseline intake (P<0.01), while saline injection had no significant effect (95% of baseline; Fig. 1). The effect of Exendin-4 was also significant relative to saline-treated mice (P<0.05). It should be noted that because ethanol intake was cut off at 30 reinforcers under baseline conditions in some mice, the baseline intake level is underestimated, thus the actual unrestricted effect-size of Exendin-4 pretreatment may have been even larger than what is reported here. Accordingly, the rate of responding for ethanol reinforcement in responses per minute may provide a better estimate of the true effect size, and was decreased to 16% of baseline by Exendin-4 pretreatment. Cumulative responses in a representative mouse (#3518) shows that baseline intake has a similar behavioral pattern as after saline pre-treatment whereas pretreatment with 3.2 μg/kg Exendin-4 resulted in an extinction-like behavior (Fig. 2).
Figure 1. Exendin-4 reduced intravenous ethanol self-administration.
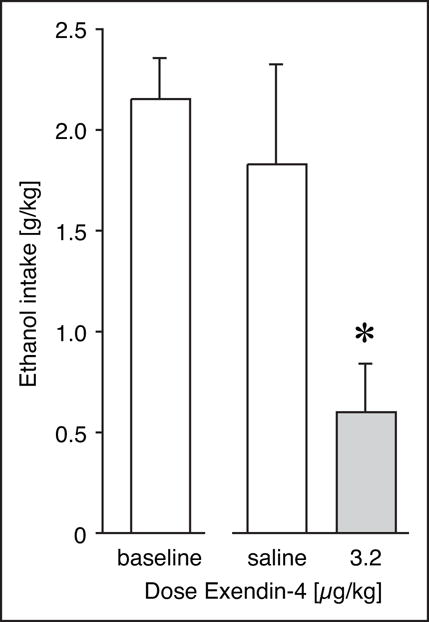
Ethanol intake per session (g/kg) at baseline, and after pretreatment with saline vehicle or 3.2 μg/kg Exendin-4. Data are group means, bars represent one standard error of the mean. Group sizes: saline, N=5; Exendin-4, N=6. * P<0.05 vs. saline.
Figure 2. Cumulative responses of intravenous ethanol self-administration.
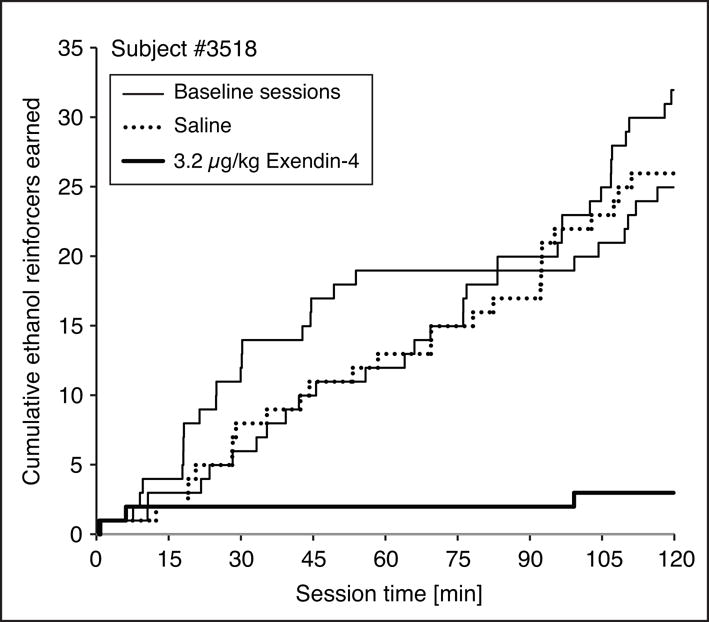
Cumulative responses in a representative mouse (#3518) describing baseline intake with similar pattern after saline pre-treatment, and an extinction-like pattern after 3.2 μg/kg Exendin-4.
Operant responding for a palatable liquid food reinforcer was measured using the same experimental design, to assess the specificity of the effect. IP injection of saline, 1.8 or 3.2 μg/kg Exendin-4 produced small decreases in rates of responding and liquid food reinforcers earned, but did not approach statistical significance (Fig. 3). All earned food reinforcers were consumed (i.e., none were left in the cup).
Figure 3. Exendin-4 had no significant effect on operant responding reinforced with a palatable liquid food.
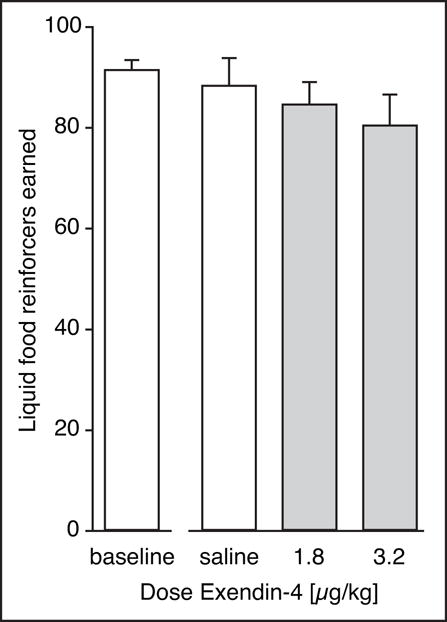
Liquid food reinforcers (25 μl of vanilla flavored Ensure protein drink) per session at baseline, and after pretreatment with saline vehicle, or 1.8 or 3.2 μg/kg Exendin-4. Group sizes: 1.8 μg/kg, N=5; all others, N=8.
DISCUSSION
Previous studies have shown that GLP-1 receptor agonists including Exendin-4 can reduce ethanol drinking, ethanol-conditioned place preference, and ethanol-induced striatal dopamine efflux in rodents (Egecioglu et al., 2013a; Shirazi et al., 2013; Suchankova et al., 2015; Vallöf et al., 2016). Preliminary reports of decreased alcohol drinking in diabetic patients treated with a GLP-1 receptor agonist liraglutide further support the potential utility of GLP-1 receptor agonists as potential treatment for alcohol use disorder (Kalra et al., 2011). However, it has not been established whether these effects of GLP-1 agonists on ethanol intake are simply an extension of their more general effects on ingestive behavior (food and fluid), conditioned effects measured in a drug-free state, or whether the direct reinforcing effects of ethanol itself are modulated. Here, we report that Exendin-4 strongly attenuated the reinforcing effects of intravenous ethanol in mice.
Consistent with a previous report, we found that experimentally naïve C57BL/6J mice can acquire IVSA of ethanol, demonstrating that intravenous ethanol can function as a positive reinforcer in mice (Grahame and Cunningham, 1997). However, also consistent with previous findings, the efficacy of ethanol as a reinforcer varied between cohorts, with an overall acquisition rate of 64% of the mice (see Grahame et al., 1998). In comparison, cocaine, at an optimal dose, was typically self-administered by near 100% of wild-type C57BL/6 mice under comparable experimental conditions (Thomsen et al., 2005, 2009; Thomsen and Caine, 2011; Schmidt et al., 2011; Caine et al., 2012; and ongoing projects, as yet unpublished). In mice that met acquisition criteria, total ethanol intake per session typically exceeded toxic levels for acute bolus dosing in mice (LD50 2 g/kg; JECFA, 1970). The intake levels were also consistent with previous findings (Grahame and Cunningham, 1997). Unlike oral intake that is subject to first pass metabolism, when delivered IV, the full dose of ethanol can be assumed to end up in the blood stream. In other words, ethanol intake approached or reached toxic levels, indicating that blood ethanol levels obtained using this model were high, and likely relevant to heavy (binge) drinking in humans.
Acute pretreatment with 3.2 μg/kg Exendin-4 suppressed ethanol self-administration in all mice: a 40%–50% reduction in mice that had limited access, and a 70%–100% reduction in mice that had unlimited access, relative to baseline intake. 1.8 μg/kg Exendin-4 had minimal effects in a small number of mice and was not tested further. This study is the first to report a pharmacological manipulation of ethanol IVSA in mice. It is unlikely that GLP-1 agonists decreased ethanol reward by modifying ethanol metabolism, because it did not alter blood ethanol levels following intraperitoneally administered ethanol in mice in a previous investigation (Vallöf et al., 2016). Indeed, evidence from intracranial microinfusions in rats and from mutant mice lacking GLP-1 receptors selectively in the central nervous system indicates that the effects of GLP-1 agonists on ethanol intake are centrally mediated (Shirazi et al., 2013; Sirohi et al., 2016). In comparison, 1.8 and 3.2 μg/kg Exendin-4 had no significant effect on operant responding reinforced by a palatable liquid food under the same experimental conditions. It should be noted that mice were intentionally not food- or water-deprived, so that access to the liquid food is more likely to reflect a measure of the hedonic value of the reinforcer, rather than a measure related to caloric or hydration needs.
In summary, these findings reestablish the mouse ethanol IVSA as a feasible procedure for evaluating reinforcing effects of ethanol when confounds due to general ingestive behavior are a concern. Because it remains a challenging assay with moderate throughput, its obvious benefit relative to IVSA in larger animals such as rats or non-human primates lies in experiments that require the mouse species, e.g., genetically engineered mouse strains. Our findings also show that GLP-1 agonists can strongly attenuate voluntary ethanol taking behavior even when oral intake is bypassed, by modulating the reinforcing effects of ethanol directly. The effect is not likely attributable to general hedonic, motivational, motor function impairments, because responding for a palatable liquid food was not affected under the same conditions. Future studies are needed to pinpoint the neurobiological pathways involved in this effect.
Acknowledgments
Experiments and surgical procedures were performed by MT and GS, all authors contributed to the manuscript and funding. We thank Technical Research Assistant Christopher William Adam for general lab support functions.
Sources of support
This work was supported by McLean Hospital institutional funds (MT). In addition, GS was supported by the Lundbeck Foundation and the Alfred Benzon Foundation, and MT and SBC were supported by NIH grants DA027825 and DA041173, respectively, during a portion of the work.
References
- SAMHSA, Substance Abuse and Mental Health Services Administration. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health 2014 [Google Scholar]
- WHO, World Health Organization. Global status report on alcohol and health 2014 [Google Scholar]
- JECFA, Joint FAO/WHO Expert Committee on Food Additives. Toxicological evaluation of some extraction solvents and certain other substances. World Health Organization; 1970. (Fourteenth report of the Joint FAO/WHO Expert Committee on Food Additives, FAO Nutrition Meetings Report Series). [Google Scholar]
- Alhadeff AL, Grill HJ. Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. Am J Physiol Regul Integr Comp Physiol. 2014;307:R465–470. doi: 10.1152/ajpregu.00179.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153:647–658. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio LL, Huang Q, Brown TJ, Drucker DJ. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 2004;53:2492–2500. doi: 10.2337/diabetes.53.9.2492. [DOI] [PubMed] [Google Scholar]
- Baraboi ED, St-Pierre DH, Shooner J, Timofeeva E, Richard D. Brain activation following peripheral administration of the GLP-1 receptor agonist exendin-4. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1011–1024. doi: 10.1152/ajpregu.00424.2010. [DOI] [PubMed] [Google Scholar]
- Caine SB, Collins GT, Thomsen M, Wright C, Lanier RK, Mello NK. Nicotine-like behavioral effects of the minor tobacco alkaloids nornicotine, anabasine, and anatabine in male rodents. Experimental and clinical psychopharmacology. 2014;22:9–22. doi: 10.1037/a0035749. [DOI] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Barrett AC, Collins GT, Grundt P, Newman AH, Butler P, Xu M. Cocaine self-administration in dopamine D(3) receptor knockout mice. Experimental and clinical psychopharmacology. 2012;20:352–363. doi: 10.1037/a0029135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S. Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol Metab. 2015;4:718–731. doi: 10.1016/j.molmet.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012;32:4812–4820. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS One. 2013b;8:e69010. doi: 10.1371/journal.pone.0069010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue Exendin-4 attenuates the nicotine-induced locomotor stimulation, accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice. PLoS One. 2013c;8:e77284. doi: 10.1371/journal.pone.0077284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Steensland P, Fredriksson I, Feltmann K, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue Exendin-4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology. 2013a;38:1259–1270. doi: 10.1016/j.psyneuen.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Göke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, Goke B. Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting beta-cells. The Journal of biological chemistry. 1993;268:19650–19655. [PubMed] [Google Scholar]
- Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7:2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Graham DL, Erreger K, Galli A, Stanwood GD. GLP-1 analog attenuates cocaine reward. Mol Psychiatry. 2013;18:961–962. doi: 10.1038/mp.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame NJ, Cunningham CL. Intravenous ethanol self-administration in C57BL/6J and DBA/2J mice. Alcoholism, clinical and experimental research. 1997;21:56–62. [PubMed] [Google Scholar]
- Grahame NJ, Low MJ, Cunningham CL. Intravenous self-administration of ethanol in beta-endorphin-deficient mice. Alcoholism, clinical and experimental research. 1998;22:1093–1098. [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology. 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imeryuz N, Yegen BC, Bozkurt A, Coskun T, Villanueva-Penacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol. 1997;273:G920–927. doi: 10.1152/ajpgi.1997.273.4.G920. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology. 2012;62:1916–1927. doi: 10.1016/j.neuropharm.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord. 2003;27:313–318. doi: 10.1038/sj.ijo.0802206. [DOI] [PubMed] [Google Scholar]
- McKay NJ, Galante DL, Daniels D. Endogenous glucagon-like peptide-1 reduces drinking behavior and is differentially engaged by water and food intakes in rats. J Neurosci. 2014;34:16417–16423. doi: 10.1523/JNEUROSCI.3267-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Nakade Y, Tsukamoto K, Iwa M, Pappas TN, Takahashi T. Glucagon like peptide-1 accelerates colonic transit via central CRF and peripheral vagal pathways in conscious rats. Auton Neurosci. 2007;131:50–56. doi: 10.1016/j.autneu.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Nakade Y, Tsukamoto K, Pappas TN, Takahashi T. Central glucagon like peptide-1 delays solid gastric emptying via central CRF and peripheral sympathetic pathway in rats. Brain Res. 2006;1111:117–121. doi: 10.1016/j.brainres.2006.06.090. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR. Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction (Abingdon, England) 2007;102:1863–1870. doi: 10.1111/j.1360-0443.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JE, Anderberg RH, Goteson A, Gribble FM, Reimann F, Skibicka KP. Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PLoS One. 2015;10:e0119034. doi: 10.1371/journal.pone.0119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010;1350:18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LS, Thomsen M, Weikop P, Dencker D, Wess J, Woldbye DP, Wortwein G, Fink-Jensen A. Increased cocaine self-administration in M4 muscarinic acetylcholine receptor knockout mice. Psychopharmacology. 2011;216:367–378. doi: 10.1007/s00213-011-2225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi RH, Dickson SL, Skibicka KP. Gut peptide GLP-1 and its analogue, Exendin-4, decrease alcohol intake and reward. PLoS One. 2013;8:e61965. doi: 10.1371/journal.pone.0061965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi S, Schurdak JD, Seeley RJ, Benoit SC, Davis JF. Central & peripheral glucagon-like peptide-1 receptor signaling differentially regulate addictive behaviors. Physiol Behav. 2016;161:140–144. doi: 10.1016/j.physbeh.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Sørensen G, Reddy IA, Weikop P, Graham DL, Stanwood GD, Wortwein G, Galli A, Fink-Jensen A. The glucagon-like peptide 1 (GLP-1) receptor agonist exendin-4 reduces cocaine self-administration in mice. Physiol Behav. 2015;149:262–268. doi: 10.1016/j.physbeh.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchankova P, Yan J, Schwandt ML, Stangl BL, Caparelli EC, Momenan R, Jerlhag E, Engel JA, Hodgkinson CA, Egli M, Lopez MF, Becker HC, Goldman D, Heilig M, Ramchandani VA, Leggio L. The glucagon-like peptide-1 receptor as a potential treatment target in alcohol use disorder: evidence from human genetic association studies and a mouse model of alcohol dependence. Transl Psychiatry. 2015;5:e583. doi: 10.1038/tp.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, Sheikh SP. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol. 1996;271:R848–856. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Chronic intravenous drug self-administration in rats and mice. Curr Protoc Neurosci Chapter 9: Unit 9 20. 2005;9:20. doi: 10.1002/0471142301.ns0920s32. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. False positive in the intravenous drug self-administration test in C57BL/6J mice. Behavioural pharmacology. 2011;22:239–247. doi: 10.1097/FBP.0b013e328345f8f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB. Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. J Neurosci. 2009;29:1087–1092. doi: 10.1523/JNEUROSCI.4037-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Woldbye DP, Wortwein G, Fink-Jensen A, Wess J, Caine SB. Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J Neurosci. 2005;25:8141–8149. doi: 10.1523/JNEUROSCI.2077-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B, Porret A, Buhler L, Deng SP, Morel P, Widmann C. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9–39) an antagonist of the receptor. Diabetes. 1993;42:1678–1682. doi: 10.2337/diab.42.11.1678. [DOI] [PubMed] [Google Scholar]
- Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- Vallöf D, Maccioni P, Colombo G, Mandrapa M, Jornulf JW, Egecioglu E, Engel JA, Jerlhag E. The glucagon-like peptide 1 receptor agonist liraglutide attenuates the reinforcing properties of alcohol in rodents. Addict Biol. 2016;21:422–437. doi: 10.1111/adb.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ. Truncated GLP-1 (proglucagon 78–107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci. 1993;38:665–673. doi: 10.1007/BF01316798. [DOI] [PubMed] [Google Scholar]
- Williams DL, Hyvarinen N, Lilly N, Kay K, Dossat A, Parise E, Torregrossa AM. Maintenance on a high-fat diet impairs the anorexic response to glucagon-like-peptide-1 receptor activation. Physiol Behav. 2011;103:557–564. doi: 10.1016/j.physbeh.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


