Abstract
Behavioral evidence from phylogenetically diverse animals and humans suggests that olfaction could be much more involved in interpreting space and time than heretofore imagined by extracting temporal information inherent in the olfactory signal. If this is the case, the olfactory system must have neural mechanisms capable of encoding time at intervals relevant to the turbulent odor world in which many animals live. We review evidence that animals can use populations of rhythmically active or ‘bursting’ olfactory receptor neurons (bORNs) to extract and encode temporal information inherent in natural olfactory signals. We postulate that bORNs represent an unsuspected neural mechanism through which time can be accurately measured, and that ‘smelling time’ completes the requirements for true olfactory scene analysis.
Keywords: Olfaction, scene analysis, encoding time
Introduction: sensory discrimination of odor space and time
All sensory modalities face the common challenge of detecting and encoding the four fundamental sensory dimensions - quality, quantity, space, and time – in order to optimize the information capacity of the modality. In olfaction, the major focus of the field has been to understand how the olfactory system discriminates odor quality and quantity. Although this question remains unanswered, significant progress has been made. The extent to which the olfactory system discriminates odor space and time, however, is considerably less clear. Indeed these dimensions are frequently considered to be less salient to olfaction, leading to the common perception that animals obtain the spatio-temporal information necessary to deal with their odor worlds, i.e., scene analysis (see Glossary), through other sensory modalities. Here, we review evidence that animals can extract temporal information from natural odor cues. We then review a novel neural mechanism through which the olfactory system can encode time and propose a model for computational olfactory scene analysis. Finally, we address the question of how animals use temporal information inherent in the olfactory modality and whether such information could have diverse roles in olfaction.
Behavioral evidence that animals extract temporal information from the odor world
Behavioral evidence suggests olfaction could be much more involved in interpreting space and time than heretofore imagined, by extracting temporal information that is one of the essential ingredients needed to execute behavior in a complex spatial world. In nature, odors emitted into air or water are often advected by turbulent flows, forming a plume downstream of the source. Within the plume, odor concentration exhibits a complex, dynamic structure that evolves over time [1]. While mean odor concentration varies systematically with down-current and cross-current distance from the source, this pattern is only evident when the odor concentration is averaged over a spatial scale much larger than the physical size of an animal. At the scale of the animal, other properties of the odor field dominate, including high concentration whiffs of odor and gaps between whiffs during which concentration is low [e.g., 2, 3]. These strong fluctuations in concentration make it difficult or impossible for an animal to navigate by ascending local gradients in odor concentration alone [4]. However, spatio-temporal anisotropies in the timing of odor cues still contain information about distance and position relative to an odor source which could provide navigational cues to animals that are able to detect and neurally encode them [5].
Much of the evidence that animals detect and respond to the temporal structure of odors comes from work on insects, but the capacity to do so generalizes to a phylogenetically diverse array of animals, including humans. In wind tunnel experiments hawkmoths readily initiate navigation and feeding behavior when presented with pulses of flower odors with a limited range of frequencies that fall within the natural range of frequencies found within the plumes formed down-wind of flowers in the field [3]. The frequency range that elicits behavioral responses to flower odor is also the range in which central olfactory neurons most closely track fluctuations in odor concentration. The central olfactory neurons of the moth also closely track the complex dynamics of pheromone pulse arrival in turbulent plumes [2]. Almond moths vary their speed and the tortuosity of upwind flights depending on the timing of pheromone pulses in artificial pheromone plumes [6]. Fruit flies that exit an odor plume appear to use temporal information about the time since odor was last encountered to initiate changes in locomotion such as cross-wind casting [7]. Mosquitoes need to detect temporally separated pulses of carbon dioxide before initiating upwind flight towards prey [8]. Both lobsters and sharks respond to differences in the arrival time of prey odors at their paired olfactory organs by initiating turns toward the side that was stimulated first [9,10]. It has long been known that humans can spatially localize an odorant based on differences in concentration or time of stimulus arrival across the two nostrils [11]. Interestingly, left vs right odorant localization is not only targeted to the primary olfactory cortex but also to a portion of the superior temporal gyrus previously implicated in visual and auditory localization [12]. Taken together, such behavioral and more limited neural evidence suggest widespread use of the temporal structure of odor plumes in olfactory-mediated navigation.
Neurally encoding olfactory time
If, as these data would suggest, animals use the timing of odor detection as navigational cues, the olfactory system must have one or more neural mechanisms to encode time at intervals relevant to the odor world in which animals live. Representation of interval timing is usually considered a higher-order brain function and various central neural mechanisms, including pacemaker-accumulators, neural oscillators, and network dynamics, have been proposed [e.g., 13, 14, 15, 16]. Central neural mechanisms have received relatively limited attention in olfaction, although network dynamics have been strongly implicated in encoding the temporal structure of odor stimuli in locusts [17], suggesting the involvement of central neural mechanisms is fertile ground for further exploration.
Peripheral mechanisms such as a system of uncoupled oscillating detectors generally have not been considered even though the primary olfactory receptor neurons (ORNs) in most animals project independently to the central nervous system and as such represent a system of uncoupled detectors. A subset of ORNs in the crustacean olfactory organ appears to have adopted just such a peripheral strategy to encode temporal intermittency [18]. These ORNs are called ‘bursting’ ORNs (bORNs) because of their periodic behavior that functionally distinguishes them from canonical, phaso-tonic ORNs (tORNs) (Fig. 1). Rhythmically active neurons are well known to be fundamental to some neuronal network functions, but typically have not been considered in the context of primary sensory signaling. Bursting is intrinsic to bORNs [18]. As shown in Fig.1, bORNs are non-linear and discharge based on the phase of bursting cycle in which the odor arrives, i.e., they are entrained by the timing of the odor stimulus. Entrainment confers on the population of bORNs the ability to encode the temporal structure of the odor stimulus, i.e., the time intervals between whiffs of odor that occur in natural odor plumes. While bORNs were characterized in a crustacean and are best understood in that animal model, ORNs with what appear to be similar properties were earlier reported from amphibians and mammals [19, 20, 21, 22], suggesting that input from rhythmically active ORNs such as bORNs could be a more general organizational feature of the olfactory periphery.
Figure 1. Activity of two different types of lobster ORNs monitored by extracellular loose patch recording.
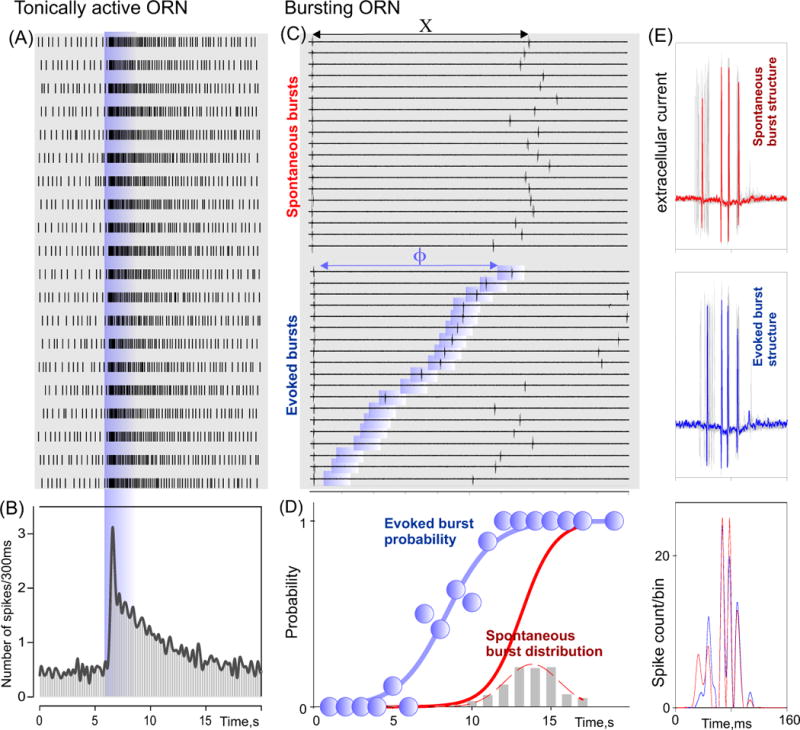
A - Raster displays of individual action potentials from a single tonically-active ORN (tORN) before and following repetitive stimulation with an odor (blue vertical bar). B - Peri-stimulus time histogram of A. C - Spontaneous (top) and odor evoked (bottom) activity of a single bursting ORN (bORN). Shaded blue areas depict odor application. Segments of the trials were aligned relative to the preceding spontaneous burst. In the bottom panel, recordings were also aligned according to time interval between last spontaneous burst and stimulus application to show the probability of generating an odor evoked burst is phase dependent. (D) Plot of the probability of eliciting a burst in response to an odor (blue symbols and sigmoid fit) in C as a function of the time since the last burst and odorant pulse (probability estimated over 1s intervals). Superimposed PDF and respective cumulative distribution for the spontaneous bursts (grey bars and red curves, bin width 1 sec). (E) Comparison of the structure of spontaneous (top panel) and evoked (middle panel) bursts for the same ORN as in C. The recordings are aligned relative to the first spike of the pair of spikes yielding the greatest instantaneous frequency. Superposition of 20 bursts (grey lines) is shown in each case. Red (top panel) and blue (middle panel) traces show individual spontaneous and evoked bursts, respectively. Bottom panel shows the respective spike histograms (bin width, 1 ms) to illustrate the similar structure of the spontaneous (red) and odor-evoked (blue) bursts. [after 18]
An established organizational feature of olfaction is the active sampling movements, known as sniffing in vertebrates and flicking or pulsing in arthropods, that gate access of the stimulus to the olfactory receptor cells. The question arises as to how the intermittency imposed by active sampling aligns with that inherent in the odor plume, and whether the former confounds encoding the latter. The intervals encoded by bORNs can range from hundreds of milliseconds to tens of seconds [18]. Except for the shortest intervals, they fall well outside of the dynamic range of the odor intermittency imposed by the animal’s active sampling movements. Flicking in the spiny lobster, for example, occupies approximately 100 msec, with a duty cycle of 500 msec during periods of active sampling [23, 24], and would confound encoding only the shortest time intervals between whiffs of odor. Flicking appears to trade off this loss of temporal resolution to enhance detection of the onset of the whiff [23] and thus presumably enhances the accuracy of encoding time, as may occur in other animals since active sampling movements of the antenna enhance encoding of stimulus location in locusts [25].
Computational modeling of the response of a large population of bORNs confirms the prediction that through ensemble coding bORNs have the capacity to reliably encode the time interval between two successive stimuli, i.e., the signal intermittency, using a maximum likelihood strategy and underscores that they can do so over a broad range of time intervals ranging up to tens of seconds [26]. A key finding that emerged from this analysis is that the stimulus interval can be reliably encoded in the instantaneous state of the heterogeneous population of bORNs. This makes encoding interval time through bORNs an instantaneous mechanism, distinct from more commonly assumed memory-based neural mechanisms for encoding time [e.g., 27] that require repetitive sampling to ascertain the interval using a sample-and-hold strategy. The idea of using distributed, modality-specific timing mechanisms that do not involve a centralized clock is increasingly appreciated for other sensory systems [15]. Having the capacity to encode olfactory time, however, doesn’t mean that animals actually extract and use that information. This capacity should have functional significance.
A role for olfactory time in navigation
The intermittency inherent in odor plumes is a severe constraint to odor source localization dependent on concentration [e.g., 28]. Computational modeling of the response of bORNs in simulated turbulent odor plumes suggests a way around this constraint. As mentioned, information about position in a turbulent odor plume relative to the source theoretically is embedded in the timing between odor pulses [4, 5, 29]. Much of this information can be captured by the recurrence time [30, 31]. Park et al. [32] demonstrated that the timing between odor pulses captured by bORNs is precisely a low-dimensional stochastic estimate of recurrence time. Among other things, this finding implies that bORN-mediated input is highly sensitive to changes in the local structure of turbulent odor plumes and can potentially be used to determine position in the plume relative to the odor source.
Park et al. [32] further used computational modelling to show that an ‘animat’ using populations of bORNs to capture and encode temporal information instantaneously in real time and challenged to repeatedly use this information to navigate the odor plume can find the source far more efficiently than it could by using measurements of concentration (Fig. 2). They showed that ‘animats’ with paired sensors can find the source far more efficiently by instantaneously detecting the time since the last odor encounter and steering towards the shorter time interval, notwithstanding the extreme irregularity in the timing of encounters in the simulated ‘natural’ turbulent plume. By not requiring repetitive sampling in one spot, bORN-based encoding of temporal information confers navigational speed and efficiency [32]. The sequence of intervals between odor encounters therefore appears to be useful for solving the online navigational problems animals face in nature. Recurrence times are ideally suited to this task because, unlike other metrics that are typically applied to time series analysis, recurrence times are highly sensitive to changes in the rate of arrival of the odor pulses [30], as would occur when an animal actively moves through the odor plume as it searches. Thus, bORNs provide a neural mechanism through which timing can be accurately measured online and encoded in a simple model, strongly implying (perhaps counterintuitively) that the most important spatio-temporal information captured by the olfactory system for navigation may come in the form of measurements of time rather than concentration.
Figure 2. Computer simulations of search strategies in a simulated odor plume using time or concentration with paired sensors.
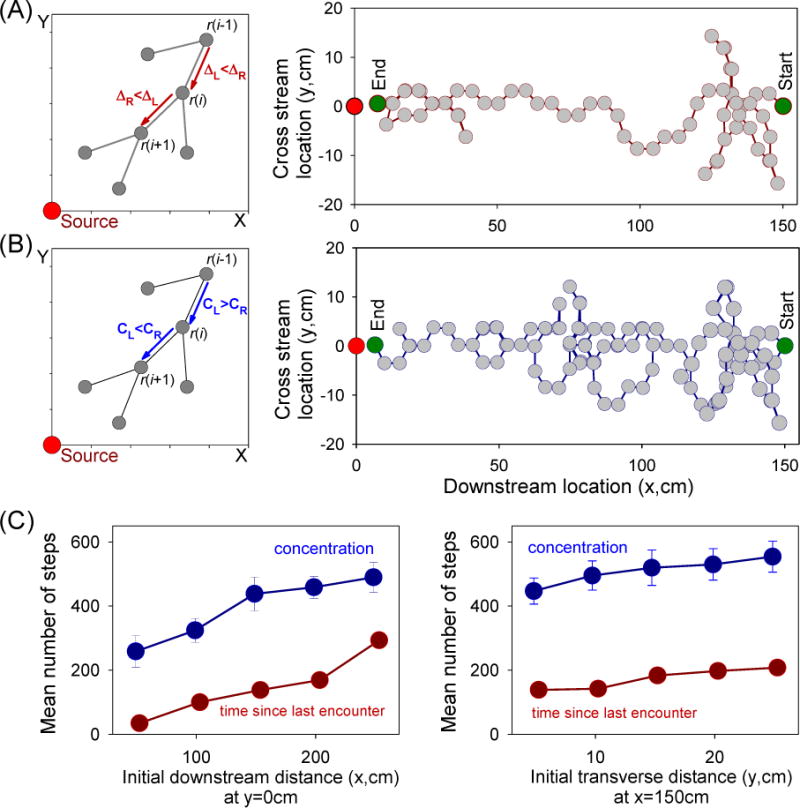
A - Search strategy based on the ability of bursting ORNs (bORNs) to code time since last odor stimulus encounter. The searcher or ‘animat’ compares the time since the last odor arrival detected by the left and right sensors and steers in the direction of the shorter interval. B – Search strategy based on the ability of tonically active ORNs (tORNs) to code stimulus intensity. The searcher in this case compares the concentration detected by the left and right sensors and steers in the direction of the higher concentration. The left panel in each case illustrates the respective search strategy. The right panel in each case depicts the respective search trajectory from the downstream starting point (x = 150 cm, y = 0 cm) to the end (x = 0 cm, y = 0 cm) with decisions made at every dot. Searchers that exit the plume backtrack to their previous position. (C) Plots of the mean number of steps required to reach the source (left panel) or the midline (right panel) of the plume using concentration (blue) or time since last encounter (red). Note in both instances that using time is significantly faster than using concentration. Plotted are the mean and SE. [after 32]
Other possible roles for time in olfaction
Given the capacity of the olfactory system to extract and encode time, could time play additional roles in olfaction? We posit that one such role could be predictive gain control. A fundamental confound in all sensory biology is gain control. If the sensory system maintains maximum sensitivity the incidence of false positives increases to the point where the noise can mask the signal. On the other hand, as the set point of the system moves towards minimal sensitivity to filter out noise, the probability of missing salient signals increases. Various neural mechanisms have been proposed for gain control in sensory systems, which may well be a distributed process. We speculate that bORN input could potentially contribute to resolving this confound by modulating tORN-mediated input such that the sensitivity is turned up momentarily and concurrently with the decision that quantifies the last time the bORNs were reset, while the odorant presumably is still in the neighborhood. This process could be implemented by functional feedback between the two populations of ORNs. The central projection of bORNs is unknown, but maximum likelihood decoding of the time since the last odor encounter could occur within a single projection layer, with lateral inhibition implementing a winner-take-all strategy [26]. The requisite neural anatomy is inherent in the first olfactory relay, called the antennal lobe (AL) or the olfactory lobe (OL) in arthropods and the olfactory bulb in vertebrates, which is the target of the canonical olfactory projection (tORNs). tORNs specifically synapse in the glomerular neuropil of the OL [33], so the OL glomeruli would be a logical site of interaction assuming that, as a population, bORNs project to most or all glomeruli. Interestingly, glomerular presynaptic inhibition mediates gain control in the AL of Drosophila [34]. Experimental verification of this speculative hypothesis is required, but one can at least envision a scenario in which bORN input could serve an additional function by feeding back on the canonical input to enhance sensory accuracy. We hope positing this idea will foster further exploration into the functional utility of encoding olfactory time.
Conclusions and prospects
Not all animals are necessarily capable of using bORNs to encode the time intervals inherent in natural odor stimuli. Ensemble coding by bORNs appears to be specifically adapted to longer stimulus intervals [26], which may be more salient to the dynamics of the turbulent odor worlds of some animals than others. Animals could use a different coding strategy than the bORN ensemble strategy to encode short time scales that match or approach the time scale of typical neuronal dynamics. In moths, for example, PNs temporally integrate weak odor signals to increase the sensitivity of the system over a time scale that corresponds well to the most rapid (<80 msec) temporal dynamics of odor signals in the natural environment [35]. While insects also deal with slower odor signals, how they do so is unclear. bORNs have not been reported in insects even though insects are a major group of animal models used in olfactory research. On the other hand, bORNs were not recognized by us in lobsters, another arthropod animal model used extensively in olfactory research, for some years. Even if bORNs are not present in all olfactory systems, understanding bORN-based encoding of time could provide clues about the structure and function of other potential oscillatory neural mechanisms for encoding time in animals that lack them.
We clearly have much more to learn about how animals use bORN-mediated time, but the prospect is exciting (see Outstanding Questions). While the question ultimately needs to be addressed behaviorally, doing so is not trivial until we know more about the unique molecular characteristics of bORNs that would allow selectively knocking out or reducing bORN-mediated input and studying its effect on the resulting phenotype. Animals generally bring to bear all available sensory information to mediate behavior, especially under constrained conditions, and this certainly applies to navigating odor space [e.g., 7, 36]. Search strategies can also change with experience [28]. Thus, we assume that bORN-based encoding of time ultimately contributes to a broad, multi-modal strategy for navigating odor space.
In summary, we postulate that the olfactory system is likely to be much more involved in interpreting time than heretofore imagined, and that ‘smelling time’ provides the basis for what can be considered true olfactory scene analysis based on information about all four fundamental sensory dimensions that is inherent in the olfactory modality.
Outstanding Questions Box.
Is oscillatory-based neural coding of time between odor detections a general feature of olfactory organization, or specific to species and/or the dynamics of an animal’s particular odor world?
Does the ability to encode the temporal structure of odor encounters have functional implications beyond navigation, for example in predictive gain control?
If and how does the amplitude (concentration) of the odor signal influence oscillatory-based neural coding of olfactory time?
Is bORN-mediated input decoded at the first olfactory relay through inter-glomerular inhibition? If not, what is the central target of this neural projection?
Is coding of stimulus intermittency by a heterogeneous population of uncoupled oscillatory neurons unique to olfaction or do similar mechanisms occur in other sensory modalities?
How does oscillatory-based neural coding of olfactory time interact with input from other sensory modalities to mediate broad-scale navigation in odor plumes?
Could the mechanism for time encoding discussed here – encoding time intervals in the instantaneous state of populations of oscillatory neurons – have more general applicability to understanding the nervous system?
Trends Box.
Recent behavioral studies suggest that olfaction could be much more involved in interpreting space and time than heretofore imagined.
This has led researchers to search for neural mechanisms that might encode time at intervals relevant to the turbulent odor world in which many animals live.
Recent physiological and computational studies suggest a functional subclass of oscillatory primary olfactory receptor neurons has the capacity to faithfully encode the intermittency inherent in odor signals.
The ability to encode the spatio-temporal structure inherent in the odor signal, together with the well-established ability of the olfactory system to discriminate odor quality and quantity, provides the basis for true olfactory scene analysis.
Acknowledgments
Data reviewed in Figures 1 and 2 was originally obtained in the laboratories of BWA and JCP with support from the National Institute on Deafness and Other Communication Disorders through award DC011859 to BWA. AMH was supported by a fellowship from the James S. McDonnell Foundation.
Glossary Box
- ‘Animat’
A synthetic or man-made ‘creature’ that senses and moves in a world by mathematical models, perhaps most commonly embodied in robots with sensors.
- Ensemble coding
In ensemble coding the identity of the stimulus is an emergent feature of the collective response of a population of neurons and not inherent in the response of any one neuron. This contrasts with ‘labeled line’ coding in which the identity of the stimulus is inherent in the response of which neuron or subset of neurons in the population is activated, as seen for example in coding particular taste qualities.
- Entrainment
The rhythmicity of neural oscillators can usually be reset by an external cue relevant to the operational context, e.g., a flash of light for visual oscillators. Entrainment occurs within a limited window of time relative to when the oscillatory element would have discharged in its regular rhythmic pattern that is referred to as the entrainment window.
- Gain control
Another term for signal amplification. It is necessary to carefully set the sensitivity of a sensory system so as to not respond to spurious signals but at the same time not miss salient ones.
- Maximum likelihood
A statistical procedure to find the value of a parameter that maximizes the probability of occurrence of a function, such as an empirical distribution, that depends on the parameter.
- Neural oscillators
Neurons that approximately rhythmically discharge (stochastic oscillators) either individually or as an emergent property of a neural network. The rhythmicity can be inherent in the cell or network or imposed by modulation from a cell or network that itself is rhythmic.
- Recurrence time
A simple metric to quantify the time evolution of trajectories in nonlinear dynamics theory. It is an extension of the concept of periodicity in traditional signal analysis.
- Scene analysis
The process by which an animal’s sensory system organizes the sensory world into perceptually meaningful elements using information about any or all of the fundamental sensory dimensions inherent in the stimulus modality – quality, quantity, space, and time. Scene analysis is best understood in relation to visual and auditory stimuli.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shraiman BI, Siggia ED. Scalar turbulence. Nature. 2000;405:639–646. doi: 10.1038/35015000. [DOI] [PubMed] [Google Scholar]
- 2.Vickers NJ, et al. Odour-plume dynamics influence the brain’s olfactory code. Nature. 2001;410:466–470. doi: 10.1038/35068559. [DOI] [PubMed] [Google Scholar]
- 3.Riffell JA, et al. Flower discrimination by pollinators in a dynamic chemical environment. Science. 2014;344:1515–1518. doi: 10.1126/science.1251041. [DOI] [PubMed] [Google Scholar]
- 4.Vergassola M, et al. ‘Infotaxis’ as a strategy for searching without gradients. Nature. 2007;445:406–409. doi: 10.1038/nature05464. [DOI] [PubMed] [Google Scholar]
- 5.Celani A, et al. Odor landscapes in turbulent environments. Phys Rev X. 2014;4:041015. [Google Scholar]
- 6.Mafra-Neto A, Carde RT. Fine-scale structure of pheromone plumes modulates upwind orientation of flying moths. Nature. 1994;369:142–144. [Google Scholar]
- 7.van Breugel F, Dickinson MH. Plume-tracking behavior of flying drosophila emerges from a set of distinct sensory-motor reflexes. Curr Biol. 2014;24:274–286. doi: 10.1016/j.cub.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Geier M, et al. Amonia as an attractive component of host odour for the yellow fever mosquito, Aedes aegypti. Chem Senses. 1999;24:647–653. doi: 10.1093/chemse/24.6.647. [DOI] [PubMed] [Google Scholar]
- 9.Basil J, Atema J. Lobster orientation in turbulent odor plumes -Simultaneous measurement of tracking behavior and temporal odor patterns. Biol Bull. 1994;187:272–273. doi: 10.1086/BBLv187n2p272. [DOI] [PubMed] [Google Scholar]
- 10.Gardiner JM, Atema J. The function of bilateral odor arrival time differences in olfactory orientation of sharks. Curr Biol. 2010;20:1187–1191. doi: 10.1016/j.cub.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 11.Von Bekesy G. Olfactory analogue to directional hearing. J Appl Physiol. 1964;19:369–373. doi: 10.1152/jappl.1964.19.3.369. [DOI] [PubMed] [Google Scholar]
- 12.Porter J, et al. Brain mechanisms for extracting spatial information from smell. Neuron. 2005;47:581–592. doi: 10.1016/j.neuron.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 13.Miall C. The storage of time intervals using oscillating neurons. Neural Comp. 1989;1:359–371. [Google Scholar]
- 14.Buhusi CV, et al. Memory for timing visual and auditory signals in albino and pigmented rats. J Exp Psychol. 2005;31:18–30. doi: 10.1037/0097-7403.31.1.18. [DOI] [PubMed] [Google Scholar]
- 15.Bueti D. The sensory representation of time. Front Integr Neurosci. 2011;5:1–3. doi: 10.3389/fnint.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laje R, Buonomano DV. Robust timing and motor patterns by taming chaos in recurrent neural networks. Nat Neurosci. 2013;16:925–933. doi: 10.1038/nn.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown SL, et al. Encoding a temporally structured stimulus with a temporally structured neural representation. Nat Neurosci. 2005;8:1568–1576. doi: 10.1038/nn1559. [DOI] [PubMed] [Google Scholar]
- 18.Bobkov YV, Ache B. Intrinsically bursting olfactory receptor neurons. J Neurophysiol. 2007;97:1052–1057. doi: 10.1152/jn.01111.2006. [DOI] [PubMed] [Google Scholar]
- 19.Sicard G. Electrophysiological recordings from olfactory receptor cells in adult mice. Brain Res. 1986;397:405–408. doi: 10.1016/0006-8993(86)90648-7. [DOI] [PubMed] [Google Scholar]
- 20.Frings S, Lindemann B. Odorant response of isolated olfactory receptor cells is blocked by amiloride. J Memb Biol. 1988;105:233–243. doi: 10.1007/BF01871000. [DOI] [PubMed] [Google Scholar]
- 21.Reisert J, Matthews HR. Simultaneous recording of receptor current and intraciliary Ca2+ concentration in salamander olfactory receptor cells. J Physiol London. 2001;535:637–645. doi: 10.1111/j.1469-7793.2001.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reisert J, Matthews HR. Responses to prolonged odour stimulation in frog olfactory receptor cells. J Physiol London. 2001;534:179–191. doi: 10.1111/j.1469-7793.2001.t01-1-00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt BC, Ache BW. Olfaction: responses of a decapod crustacean are enhanced by flicking. Science. 1979;205:204–206. doi: 10.1126/science.205.4402.204. [DOI] [PubMed] [Google Scholar]
- 24.Koehl MA, et al. Lobster sniffing: antennule design and hydrodynamic filtering of information in an odor plume. Science. 2001;294:1948–1951. doi: 10.1126/science.1063724. [DOI] [PubMed] [Google Scholar]
- 25.Huston SJ, et al. Neural encoding of odors during active sampling and in turbulent plumes. Neuron. 2015;88:403–418. doi: 10.1016/j.neuron.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park IM, et al. Intermittency coding in the primary olfactory system: A neural substrate for olfactory scene analysis. J Neurosci. 2014;34:941–952. doi: 10.1523/JNEUROSCI.2204-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gamache PL, Grondin S. Sensory-specific clock components and memory mechanisms: investigation with parallel timing. Euro J Neurosci. 2010;31:1908–1914. doi: 10.1111/j.1460-9568.2010.07197.x. [DOI] [PubMed] [Google Scholar]
- 28.Gire DH, et al. Mice develop efficient strategies for foraging and navigation using complex naturel stimuli. Curr Biol. 2016;26:1–13. doi: 10.1016/j.cub.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hein AM, McKinley SA. Sensing and decision-making in random search. Vol. 109. PNAS; USA: 2012. pp. 12070–12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao JB. Recurrence time statistics for chaotic systems and their applications. Phys Rev Lett. 1999;83:3178–3181. [Google Scholar]
- 31.Ngamga E, et al. Distinguishing dynamics using recurrence-time statistics. Phys Rev. 2012;85:026217. doi: 10.1103/PhysRevE.85.026217. [DOI] [PubMed] [Google Scholar]
- 32.Park IJ, et al. Neurally encoding time for olfactory navigation. PLoS Comp Biol. 2016 doi: 10.1371/journal.pcbi.1004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wachowiak M, et al. Presynaptic inhibition of olfactory receptor neurons in crustaceans. Microsc Res Tech. 2002;58:365–375. doi: 10.1002/jemt.10144. [DOI] [PubMed] [Google Scholar]
- 34.Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452:956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabuchi M, et al. Pheromone responsiveness threshold depends on temporal integration by antennal lobe projection neurons. Vol. 110. PNAS; USA: 2013. pp. 15455–15460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Breugel F, et al. Mosquitoes use vision to associate odor plumes with thermal targets. Curr Biol. 2015;25:2123–212. doi: 10.1016/j.cub.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]


