Abstract
Importance
Defining what represents a macronutritionally balanced diet remains an open question and a high priority in nutrition research. Although the amount of protein may have specific effects, from a broader dietary perspective, the choice of protein sources will inevitably influence other components of diet and may be a critical determinant for the health outcome.
Objective
To examine the associations of animal and plant protein intake with risk of mortality
Design
Prospective cohort study
Setting
Health professionals in the United States
Participants
85,013 women and 46,329 men from the Nurses’ Health Study (1980–2012) and Health Professionals Follow-up Study (1986–2012)
Exposure
Animal and plant protein intake as assessed by regularly updated validated food questionnaires
Main outcomes and measures
Hazard ratio (HR) of mortality
Results
The median intake, as assessed by percentage of energy, was 14% for animal protein (5th–95th percentile: 9–22%) and 4% for plant protein (2–6%). After adjusting for major lifestyle and dietary risk factors, animal protein intake was weakly associated with higher mortality, particularly cardiovascular mortality (HR=1.08 per 10%-energy increment, 95% confidence interval [CI], 1.01–1.16, Ptrend=0.04), whereas plant protein was associated with lower mortality (HR=0.90 per 3%-energy increment, 95% CI, 0.86–0.95, Ptrend<0.001). These associations were confined to participants with at least one of the unhealthy lifestyle factors based on smoking, heavy alcohol drinking, overweight or obesity, and physical inactivity, but not evident among those without any of these risk factors (Pinteraction<0.001). Replacing animal protein of various origins with plant protein was associated with lower mortality. In particular, the HRs (95% CI) of all-cause mortality were 0.66 (0.59–0.75) when 3% of energy from plant protein was substituted for an equivalent amount of protein from processed red meat, 0.88 (0.84–0.92) from unprocessed red meat, and 0.81 (0.75–0.88) from eggs.
Conclusions and relevance
Higher animal protein intake was positively, whereas plant protein was inversely, associated with mortality, especially among individuals with at least one lifestyle risk factors. Substitution of plant protein for animal protein, especially from processed red meat, was associated with lower mortality, suggesting the importance of protein source.
Keywords: protein, nutrient density model, longevity, survival
Introduction
Defining what represents a macronutritionally balanced diet remains an open question and a high priority in nutrition research.1,2 In short-term randomized controlled trials (RCTs), substitution of protein for carbohydrate has been shown to favor weight management, decrease blood pressure, and improve cardiometabolic biomarkers, including blood lipid and lipoprotein profiles, and glycemic regulation.3–5 These beneficial effects are partly dependent on weight loss and possibly due to the enhanced postprandial satiety and energy expenditure when exchanging protein for carbohydrate.6 Therefore, high-protein-low-carbohydrate diets have been promoted for weight loss and health improvement. Although the amount and type of protein may have specific effects,7 such as on insulin-like growth factor (IGF)-1 level,8 from a broader dietary perspective, the choice of protein sources will inevitably influence other components of diet, including macronutrients, micronutrients and phytochemicals, which can in turn influence health outcomes. Therefore, taking into account food sources is critical to better understand the health effect of protein intake and fine-tune dietary recommendations.
To date, data examining protein sources in relation to mortality are sparse. While no association was found between animal or plant protein and all-cause mortality in a cohort of postmenopausal women, substitution of plant protein for animal protein was associated with lower cardiovascular mortality.9 A positive association between animal protein and mortality was also found in the National Health and Nutrition Examination Survey (NHANES).8 Nevertheless, these data are far from conclusive due to several limitations of the studies, including the relatively small sample size, single assessment of diet at baseline, and lack of data on detailed food sources of animal and plant protein.
Therefore, utilizing data from two large U.S. cohort studies with repeated measures of diet and up to 32 years of follow-up, we prospectively examined animal versus plant protein in relation to the risk of all-cause and cause-specific mortality, and performed an isocaloric substitution analysis for a variety of food sources of protein.
Methods
Study population
The Nurses’ Health Study (NHS) included 121,700 U.S. registered female nurses who were aged 30–55 years in 1976. The Health Professionals Follow-up Study (HPFS) included 51,529 U.S. male health professionals who were aged 40–75 years in 1986. Details of the two cohorts have been described elsewhere.10,11 Briefly, follow-up questionnaires were administered at baseline enrollment and every two years thereafter to collect lifestyle and medical information. Dietary intake was assessed by the food frequency questionnaires (FFQs) every four years. The follow-up rates were 95.4% in the NHS and 95.9% in the HPFS until 2010.
Among participants who returned baseline questionnaires, we excluded those who had a history of cancer (except non-melanoma skin cancer), cardiovascular disease or diabetes at baseline, left more than 10 items blank on the baseline FFQ in the NHS and more than 70 in the HPFS, or reported implausible energy intake levels (<500 or >3500 kcal/d for women, <800 or >4200 kcal/d for men). After exclusions, 85,013 women and 46,329 men were available for the analysis. The study protocol was approved by the Institutional Review Board at the Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health.
Dietary assessment
In each FFQ, participants were asked how often, on average, they consumed each food of a standardized portion size during the previous year. The average daily nutrient intake was calculated by multiplying the consumption frequency of each food item by its nutrient content and then summing across all foods. Animal and plant protein intake was expressed as a percentage of total energy consumption. Major sources of animal protein included processed and unprocessed red meat, poultry, dairy products, fish, and egg. Major food contributors to plant protein included bread, cereals, pasta, nuts, beans, and legumes. We derived protein intake from processed red meat by summing the products between intake frequency (serving/day) and the protein content (g/serving) for various processed red meats (i.e., bacon; beef or pork hot dogs; salami, bologna or other processed meat sandwiches; other processed meats [e.g., sausage, kielbasa, etc.]). Similar calculations were done for protein intake from unprocessed red meat, poultry, fish, egg, and dairy. FFQs have demonstrated good validity in assessing protein intake and the Spearman correlation coefficient of intake assessed by the FFQs and seven-day dietary record was 0.56 for animal protein and 0.66 for plant protein,12 as detailed in the eSupplement.
Ascertainment of death
We identified deaths from state statistics records, the National Death Index, next of kin, and the postal system. Using these methods, we were able to ascertain more than 96% of the deaths in each cohort.13 Cause of death was identified from death certificates or review of medical records by physicians. For this analysis, we assessed all-cause mortality, and death from CVD (International Classification of Diseases, Eighth Revision, codes 390 to 458), cancer (codes 140 to 207), and other causes.
Statistical analysis
We calculated person-time of follow-up for each participant from the age in months at the return date of the baseline questionnaire (1980 for the NHS, 1986 for the HPFS) until the age in months at the date of death, loss to follow-up, or end of follow-up (June 1, 2012 for the NHS, January 31, 2012 for the HPFS), whichever came first. We used time-varying Cox proportional hazards regression models with age as the time scale to estimate the hazard ratio (HR) and 95% confidence interval (CI) of mortality associated with animal and plant protein intake.
To reduce random within-person variation and to best represent long-term dietary intake, we calculated cumulative average of protein intake from our repeated FFQs.14 We stopped updating dietary information when a participant reported a diagnosis of cancer (except non-melanoma skin cancer), diabetes, stroke, coronary heart disease, or angina, because these conditions may lead to dietary change.15
A nutrient density model was used with adjustment for total energy and percentage of energy from various fats.16 Thus the coefficient for animal and plant protein reflects the substitution effect of an equal amount of energy from protein for carbohydrate. In the multivariable analysis, we adjusted for several potential dietary and lifestyle confounding factors (see footnote of Table 2). To address the possibility of residual confounding, we further adjusted for a propensity score that reflected associations of protein consumption with potential confounding covariates.17 Details about covariate assessment and propensity score analysis are provided in the eSupplement.
Table 2.
Risk of all-cause and cause-specific mortality according to percent of energy from animal and plant protein intake
| Category 1 | Category 2 | Category 3 | Category 4 | Category 5 | HR (95% CI) per certain increment | Ptrend | |
|---|---|---|---|---|---|---|---|
| Animal protein | |||||||
| Intake category | ≤ 10 % | >10, ≤ 12 % | >12, ≤ 15 % | >15, ≤ 18 % | >18 % | Per 10% increment | |
| Median intake (% energy) | 8.9 | 11 | 14 | 16 | 20 | ||
| Person-years | 317,851 | 544,922 | 1,171,916 | 893,047 | 613,056 | ||
| All-cause mortality | |||||||
| No. of deaths | 3,770 | 6,151 | 11,909 | 8,401 | 5,884 | ||
| Age-adjusted HR (95% CI)* | 1 (referent) | 0.97 (0.93–1.01) | 0.97 (0.93–1.00) | 0.98 (0.94–1.02) | 1.01 (0.96–1.06) | 1.03 (1.00–1.06) | 0.09 |
| Multivariable-adjusted HR (95% CI)† | 1 (referent) | 1.01 (0.97–1.05) | 1.03 (0.99–1.07) | 1.03 (0.98–1.07) | 1.03 (0.98–1.08) | 1.02 (0.98–1.05) | 0.33 |
| Cardiovascular mortality | |||||||
| No. of deaths | 974 | 1,527 | 2,967 | 1,987 | 1,396 | ||
| Age-adjusted HR (95% CI)* | 1 (referent) | 0.94 (0.87–1.02) | 1.01 (0.94–1.09) | 1.07 (0.99–1.17) | 1.19 (1.09–1.30) | 1.20 (1.12–1.28) | <0.001 |
| Multivariable-adjusted HR (95% CI)† | 1 (referent) | 0.98 (0.90–1.07) | 1.05 (0.97–1.14) | 1.06 (0.97–1.16) | 1.09 (0.99–1.20) | 1.08 (1.01–1.16) | 0.04 |
| Cancer mortality | |||||||
| No. of deaths | 1,322 | 2,176 | 4,325 | 3,136 | 2,200 | ||
| Age-adjusted HR (95% CI)* | 1 (referent) | 0.96 (0.89–1.02) | 0.94 (0.88–1.00) | 0.93 (0.87–1.00) | 0.94 (0.87–1.02) | 0.96 (0.91–1.01) | 0.15 |
| Multivariable-adjusted HR (95% CI)† | 1 (referent) | 1.00 (0.93–1.07) | 1.01 (0.94–1.08) | 1.01 (0.94–1.09) | 1.02 (0.94–1.11) | 1.00 (0.95–1.06) | 0.91 |
| Other mortality | |||||||
| No. of deaths | 1,474 | 2,448 | 4,617 | 3,278 | 2,288 | ||
| Age-adjusted HR (95% CI)* | 1 (referent) | 0.99 (0.92–1.05) | 0.96 (0.90–1.02) | 0.96 (0.90–1.02) | 0.97 (0.90–1.05) | 0.99 (0.95–1.04) | 0.80 |
| Multivariable-adjusted HR (95% CI)† | 1 (referent) | 1.04 (0.97–1.11) | 1.02 (0.96–1.09) | 1.01 (0.94–1.08) | 0.99 (0.92–1.07) | 0.99 (0.94–1.05) | 0.80 |
|
| |||||||
| Plant protein | |||||||
| Intake category | ≤ 3 % | >3, ≤ 4 % | >4, ≤ 5 % | >5, ≤ 6 % | >6 % | Per 3% increment | |
| Median intake (% energy) | 2.6 | 3.5 | 4.5 | 5.4 | 6.6 | ||
| Person-years | 710,592 | 1,060,873 | 929,193 | 550,015 | 290,118 | ||
| All-cause mortality | |||||||
| No. of deaths | 6,160 | 9,661 | 10,235 | 6,602 | 3,457 | ||
| Age-adjusted HR (95% CI)* | 1 (referent) | 0.92 (0.89–0.96) | 0.85 (0.82–0.89) | 0.72 (0.69–0.76) | 0.67 (0.63–0.70) | 0.73 (0.70–0.75) | <0.001 |
| Multivariable-adjusted HR (95% CI)† | 1 (referent) | 0.97 (0.94–1.01) | 0.95 (0.91–0.99) | 0.91 (0.86–0.96) | 0.89 (0.84–0.96) | 0.90 (0.86–0.95) | <0.001 |
| Cardiovascular mortality | |||||||
| No. of deaths | 1,260 | 2,126 | 2,638 | 1,811 | 1,016 | ||
| Age-adjusted HR (95% CI)* | 1 (referent) | 0.88 (0.82–0.95) | 0.78 (0.72–0.85) | 0.63 (0.57–0.69) | 0.60 (0.53–0.67) | 0.67 (0.62–0.72) | <0.001 |
| Multivariable-adjusted HR (95% CI)† | 1 (referent) | 0.93 (0.86–1.01) | 0.90 (0.82–0.99) | 0.83 (0.74–0.93) | 0.85 (0.74–0.97) | 0.88 (0.80–0.97) | 0.007 |
| Cancer mortality | |||||||
| No. of deaths | 2,372 | 3,678 | 3,664 | 2,330 | 1,115 | ||
| Age-adjusted HR (95% CI)* | 1 (referent) | 0.98 (0.92–1.03) | 0.93 (0.87–0.99) | 0.83 (0.77–0.90) | 0.72 (0.65–0.79) | 0.80 (0.75–0.85) | <0.001 |
| Multivariable-adjusted HR (95% CI)† | 1 (referent) | 1.02 (0.97–1.08) | 1.02 (0.95–1.09) | 1.01 (0.92–1.10) | 0.92 (0.82–1.03) | 0.97 (0.90–1.05) | 0.46 |
| Other mortality | |||||||
| No. of deaths | 2,528 | 3,857 | 3,933 | 2,461 | 1,326 | ||
| Age-adjusted HR (95% CI)* | 1 (referent) | 0.90 (0.85–0.95) | 0.82 (0.78–0.88) | 0.69 (0.64–0.74) | 0.66 (0.60–0.72) | 0.70 (0.66–0.74) | <0.001 |
| Multivariable-adjusted HR (95% CI)† | 1 (referent) | 0.95 (0.90–1.00) | 0.91 (0.86–0.98) | 0.86 (0.79–0.94) | 0.89 (0.80–0.99) | 0.86 (0.79–0.92) | <0.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Cox proportional hazards model with age as the time scale was stratified by sex and calendar time, and adjusted for total caloric intake, and percent of energy from saturated fat, polyunsaturated fat, monounsaturated fat, and trans fat (all continuous).
Multivariable model was further adjusted for multivitamin use (yes or no), smoking status (never, past smokers, current smokers 1–14, and 15+ cigarettes/day), pack-years of smoking (in women: ≤15, 16–25, 26–45, >45; in men: <10, 11–24, 25–44, ≥45), body mass index (<23.0, 23.0–24.9, 25.0–26.9, 27.0–29.9, 30.0–34.9, ≥35 kg/m2), physical activity (quintiles), alcohol consumption (in women: 0, 0.1–5.0, 5.1–15.0, and >15.0 g/d; in men: 0, 0.1–10.0, 10.1–20.0, and >20.0 g/d), history of hypertension diagnosis (yes or no), glycemic index (in quintiles), and intake of whole grains, total fiber, fruits, and vegetables (all in quintiles). Mutual adjustment was conducted for animal protein and plant protein analysis.
We performed stratified analyses by age and lifestyle factors, and evaluated the interaction via a likelihood ratio test. To minimize the confounding effect and test for potential modification by an overall lifestyle pattern, we further performed a stratified analysis according to a priori-defined healthy lifestyle pattern, as characterized by never smoking or ever smoking with pack-years of <5, never or moderate alcohol drinking (<14 g/day in women, <28 g/day in men), BMI ≥ 18.5 and <25.0 kg/m2, and physical activity of ≥ 150-min/week at moderate level or ≥ 75 min/week at vigorous level (equivalent to ≥ 7.5 MET-hours/week) as recommended.18 Likewise, given the previous report that protein intake was associated with higher risk of diabetes-related mortality,8 we examined the protein-mortality association according to history of diabetes.
Finally, we estimated the effect of substituting 3% of energy from plant protein for an equivalent amount of animal protein from various sources, including processed and unprocessed red meat, poultry, fish, egg, and dairy by including simultaneously these protein items as continuous variables in the multivariable model. The HRs and 95% CIs for the iso-protein substitution effect were derived from the difference between the regression coefficients, variance, and covariance.19
The analyses were first conducted in each cohort separately, and because no appreciable difference was detected by cohort (eTable 1), we then conducted the pooled analysis using the sex-stratified Cox regression model in the combined dataset. More details about statistical analysis are provided in the eSupplement.
Results
In the two cohorts with 3,540,791 person-years of follow-up, we documented 36,115 deaths, of which 8,851 were due to CVD, 13,159 to cancer, and 14,105 to other causes. Participants’ median (5th–95th percentile) intake, as assessed by percent of energy, was 14% (9–22%) for animal protein and 4% (2–6%) for plant protein. Animal protein intake has decreased, whereas plant protein intake increased over time throughout follow-up (eFigure 1). Table 1 shows the basic characteristics of participants according to protein intake. Compared to participants consuming ≤ 10% of energy from animal protein, those consuming >18% were slightly heavier and less physically active, and consumed more fats (especially saturated fat) and less fiber and plant foods. In contrast, higher plant protein consumers demonstrated a clustering of positive health behaviors and had a substantially healthier diet than lower consumers.
Table 1.
Age- and sex-standardized characteristics of study participants according to percent of energy from protein intake*
| Variable | Animal protein | Plant protein | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| ≤ 10% | >12, ≤ 15% | >18% | ≤ 3% | >4, ≤ 5% | >6% | |
| Male, % | 52 | 29 | 12 | 13 | 35 | 70 |
| Age, year | 63.4 | 60.7 | 60.8 | 60.3 | 61.2 | 64.4 |
| Body mass index, kg/m2 | 25.5 | 25.7 | 26.9 | 26.3 | 25.9 | 25.3 |
| Physical activity, MET-hours/week | 21.6 | 20.4 | 20.0 | 17.2 | 20.5 | 25.2 |
| Current smoking, % | 13 | 12 | 11 | 13 | 12 | 9 |
| Pack-years of smoking† | 25.0 | 23.9 | 23.5 | 24.9 | 23.8 | 21.2 |
| Current multivitamin use, % | 50 | 52 | 53 | 47 | 51 | 58 |
| History of hypertension, % | 37 | 39 | 42 | 39 | 39 | 36 |
| Postmenopausal women, %‡ | 87 | 87 | 87 | 87 | 87 | 87 |
| Current hormone use, %§ | 14 | 18 | 18 | 17 | 17 | 18 |
| Dietary intake|| | ||||||
| Alcohol, g/d | 9.1 | 7.6 | 5.9 | 7.9 | 7.8 | 5.6 |
| Total protein, g/d | 59.2 | 76.5 | 103.6 | 79.0 | 79.2 | 78.2 |
| Animal protein, g/d | 36.0 | 58.0 | 88.1 | 68.8 | 60.0 | 48.1 |
| Plant protein, g/d | 23.2 | 18.6 | 15.6 | 10.2 | 19.1 | 30.1 |
| Carbohydrate, g/d | 226.6 | 188.0 | 155.5 | 143.6 | 189.7 | 217.9 |
| Glycemic index | 53.5 | 52.8 | 51.1 | 51.8 | 52.9 | 52.8 |
| Total fat, g/d | 60.2 | 68.9 | 72.7 | 75.9 | 67.5 | 59.3 |
| Polyunsaturated/saturated fat ratio | 0.7 | 0.6 | 0.6 | 0.5 | 0.6 | 0.8 |
| Fiber, g/d | 21.0 | 19.7 | 19.8 | 16.6 | 19.7 | 25.4 |
| Fruit, serving/week | 18.2 | 16.7 | 15.6 | 14.7 | 17.0 | 18.8 |
| Vegetable, serving/week | 22.3 | 22.3 | 23.7 | 20.3 | 22.5 | 28.3 |
| Legume, serving/week | 1.9 | 1.8 | 1.6 | 1.3 | 1.7 | 2.8 |
| Nut, serving/week | 2.2 | 1.5 | 1.1 | 1.0 | 1.4 | 3.0 |
| Bean, serving/week | 0.9 | 0.7 | 0.7 | 0.5 | 0.7 | 1.3 |
| Whole grain, g/d | 27.1 | 24.5 | 24.4 | 19.9 | 23.6 | 35.3 |
| Unprocessed red meat, serving/week | 2.9 | 4.0 | 4.0 | 4.4 | 3.9 | 2.5 |
| Processed red meat, serving/week | 1.8 | 2.1 | 1.7 | 2.1 | 2.0 | 1.1 |
| Chicken, serving/week | 1.9 | 2.5 | 3.4 | 2.4 | 2.6 | 2.5 |
| Egg, serving/week | 1.6 | 2.0 | 2.1 | 2.0 | 2.0 | 1.6 |
| Fish, serving/week | 1.4 | 1.8 | 2.6 | 1.6 | 1.9 | 2.0 |
| High-fat dairy, serving/week | 8.4 | 8.3 | 6.3 | 8.9 | 8.1 | 5.8 |
| Low-fat dairy, serving/week | 5.9 | 8.3 | 9.9 | 8.2 | 8.4 | 7.6 |
Abbreviations: MET, metabolic equivalent.
Updated information throughout follow-up was used to calculate the mean for continuous variable and percentage for categorical variable. All variables are age- and sex-standardized except age and sex.
Among ever smokers only.
Among women only.
Among postmenopausal women only.
All dietary factors are energy-adjusted.
As shown in Table 2, higher intake of animal protein was associated with higher CVD mortality. After adjusting for major lifestyle and dietary risk factors, the HR per 10%-energy absolute increment of animal protein intake was 1.02 (95% CI, 0.98–1.05, Ptrend=0.33) for all-cause mortality and 1.08 (95% CI, 1.01–1.16, Ptrend=0.04) for CVD mortality. In contrast, higher plant protein intake was associated with lower mortality, with the multivariable HR per 3%-energy increment of 0.90 (95% CI, 0.86–0.95, Ptrend<0.001) for all-cause mortality and 0.88 (95% CI, 0.80–0.97, Ptrend=0.007) for CVD mortality. The associations did not differ by duration of follow-up (eTable 2). We did not detect any statistically significant nonlinear relationship between protein intake and mortality by spline analysis (data not shown). The results remained largely unchanged when we adjusted for a propensity score that predicted protein intake levels (eTable 3).
The increased mortality associated with higher animal protein intake was more pronounced among obese participants (Pinteraction=0.008) or heavy alcohol drinkers (Pinteraction=0.06) (eFigure 2). The association between higher plant protein intake and lower mortality was stronger among participants who were aged ≤ 65 or >80 years, currently smoked, drank alcohol of ≥ 14 g/day, and were overweight or obese, and physically inactive (all Pinteraction ≤ 0.02).
Because most of the statistically significant associations were seen among participants with unhealthy lifestyle, we further divided participants into healthy- and unhealthy-lifestyle groups according to a priori-defined criteria. Table 3 shows the basic characteristics of the two groups. Participants in the healthy-lifestyle group demonstrated slightly more homogeneous distributions in health behaviors than those in the unhealthy-lifestyle group. Of note, at similar protein amount, protein sources differed between the two groups. Compared to the healthy-lifestyle group, the unhealthy-lifestyle group with similar animal protein intake consumed more unprocessed and processed red meat, egg, and high-fat dairy, but less chicken, fish, and low-fat dairy. At similar plant protein levels, the unhealthy-lifestyle group consumed less fiber, fruit, vegetables, and whole grains than the healthy-lifestyle group.
Table 3.
Age- and sex-standardized characteristics of study participants according to percent of energy from animal and plant protein intake in the healthy- and unhealthy-lifestyle groups*
| Variable | Animal protein
|
Plant protein
|
||||||
|---|---|---|---|---|---|---|---|---|
| Healthy-lifestyle group (n=19,647)
|
Unhealthy-lifestyle group (n=106,134)
|
Healthy-lifestyle group (n=19,647)
|
Unhealthy-lifestyle group (n=106,134)
|
|||||
| ≤ 10% | >18% | ≤ 10% | >18% | ≤ 3% | >6% | ≤ 3% | >6% | |
| Male, % | 68 | 12 | 51 | 12 | 16 | 75 | 13 | 68 |
| Age, year | 64.5 | 61.8 | 63.6 | 61.1 | 61.7 | 64.3 | 60.6 | 64.7 |
| Body mass index, kg/m2 | 22.8 | 23.1 | 26.2 | 27.6 | 23.1 | 22.7 | 26.9 | 26.3 |
| Physical activity, MET-hours/week | 32.7 | 30.5 | 18.7 | 17.9 | 27.5 | 35.5 | 15.0 | 21.0 |
| Current smoking, % | 0 | 0 | 17 | 14 | 0 | 0 | 17 | 13 |
| Pack-years of smoking† | 2.9 | 2.8 | 26.8 | 25.1 | 2.8 | 2.8 | 26.2 | 23.5 |
| Current multivitamin use, % | 57 | 58 | 49 | 53 | 50 | 62 | 47 | 56 |
| History of hypertension, % | 30 | 31 | 41 | 45 | 29 | 28 | 42 | 40 |
| Postmenopausal women, %‡ | 91 | 91 | 89 | 89 | 91 | 91 | 87 | 87 |
| Current hormone use, %§ | 15 | 18 | 14 | 17 | 17 | 16 | 17 | 18 |
| Dietary intake|| | ||||||||
| Alcohol, g/d | 5.2 | 5.1 | 10.4 | 6.2 | 4.2 | 4.8 | 8.4 | 6.0 |
| Total protein, g/d | 62.2 | 107.1 | 58.7 | 103.8 | 79.3 | 79.0 | 78.5 | 77.9 |
| Animal protein, g/d | 35.6 | 89.6 | 36.2 | 88.3 | 68.9 | 47.3 | 68.3 | 48.4 |
| Plant protein, g/d | 26.6 | 17.6 | 22.5 | 15.5 | 10.3 | 31.7 | 10.2 | 29.6 |
| Carbohydrate, g/d | 245.6 | 173.8 | 222.1 | 154.4 | 149.8 | 237.4 | 140.9 | 211.2 |
| Glycemic index | 53.5 | 51.6 | 53.4 | 51.0 | 52.7 | 52.8 | 51.5 | 52.6 |
| Total fat, g/d | 59.6 | 70.4 | 60.5 | 73.0 | 77.0 | 57.8 | 75.7 | 59.9 |
| Polyunsaturated/saturated fat ratio | 0.8 | 0.6 | 0.7 | 0.6 | 0.6 | 0.8 | 0.5 | 0.7 |
| Fiber, g/d | 24.3 | 21.6 | 20.2 | 19.6 | 18.6 | 27.5 | 16.5 | 24.5 |
| Fruit, serving/week | 22.1 | 17.4 | 17.2 | 15.3 | 17.7 | 21.7 | 14.4 | 17.7 |
| Vegetable, serving/week | 25.3 | 24.7 | 21.9 | 23.8 | 22.4 | 29.6 | 20.3 | 27.9 |
| Legume, serving/week | 2.4 | 1.8 | 1.8 | 1.6 | 1.5 | 3.1 | 1.3 | 2.6 |
| Nut, serving/week | 2.7 | 1.4 | 2.1 | 1.1 | 1.3 | 3.2 | 1.0 | 2.9 |
| Bean, serving/week | 1.1 | 0.8 | 0.8 | 0.7 | 0.6 | 1.4 | 0.5 | 1.2 |
| Whole grain, g/d | 34.7 | 29.3 | 25.5 | 24.0 | 24.0 | 42.0 | 19.5 | 32.9 |
| Unprocessed red meat, serving/week | 2.3 | 3.4 | 3.0 | 4.1 | 3.9 | 2.1 | 4.4 | 2.6 |
| Processed red meat, serving/week | 1.3 | 1.2 | 2.0 | 1.7 | 1.6 | 0.8 | 2.1 | 1.3 |
| Chicken, serving/week | 2.0 | 3.8 | 1.9 | 3.4 | 2.5 | 2.5 | 2.4 | 2.5 |
| Egg, serving/week | 1.3 | 1.7 | 1.6 | 2.1 | 1.7 | 1.4 | 2.0 | 1.6 |
| Fish, serving/week | 1.5 | 2.8 | 1.4 | 2.5 | 1.7 | 2.1 | 1.6 | 2.0 |
| High-fat dairy, serving/week | 6.8 | 5.2 | 8.7 | 6.4 | 7.8 | 4.9 | 8.9 | 6.0 |
| Low-fat dairy, serving/week | 6.8 | 10.9 | 5.7 | 9.7 | 9.5 | 8.4 | 8.0 | 7.3 |
Abbreviations: MET, metabolic equivalent.
Healthy lifestyle was defined as never smoking or ever smoking with pack-years of <5, never or moderate alcohol drinking (<14 g/day in women, <28 g/day in men), BMI ≥ 18.5 and <27.5 kg/m2, and physical activity of ≥150-min/week at moderate level or ≥75 min/week at vigorous level (equivalent to ≥7.5 MET-hours/week). Updated information throughout follow-up was used to calculate the mean for continuous variable and percentage for categorical variable. All variables are age- and sex-standardized except age and sex.
Among ever smokers only.
Among women only.
Among postmenopausal women only.
All dietary factors are energy-adjusted.
Table 4 shows the protein-mortality associations in the two groups. The positive association with all-cause mortality for animal protein and the inverse association for plant protein were both restricted to the unhealthy-lifestyle group (Pinteraction<0.001), although the association with animal protein did not reach statistical significance. In the unhealthy-lifestyle group, the multivariable HR per 10%-increment of animal protein was 1.03 (95% CI, 0.99–1.07, Ptrend=0.16) and the HR per 3%-increment of plant protein was 0.90 (95% CI, 0.85–0.95, Ptrend<0.001). Similar results were observed for cardiovascular mortality.
Table 4.
Risk of all-cause and cardiovascular mortality according to percent of energy from animal and plant protein intake among participants with healthy and unhealthy lifestyle*
| Category 1 | Category 2 | Category 3 | Category 4 | Category 5 | HR (95% CI) per certain increment | Ptrend | Pinteraction† | |
|---|---|---|---|---|---|---|---|---|
| Animal protein | ||||||||
| Intake category | ≤ 10 % | >10, ≤12 % | >12, ≤15 % | >15, ≤18 % | >18 % | Per 10% increment | ||
| All-cause mortality | ||||||||
| Healthy-lifestyle group | ||||||||
| No. of deaths | 626 | 978 | 1,712 | 1,012 | 587 | |||
| HR (95% CI)‡ | 1 (referent) | 0.99 (0.88–1.10) | 0.97 (0.87–1.08) | 1.01 (0.89–1.14) | 0.94 (0.81–1.09) | 0.96 (0.86–1.07) | 0.46 | <0.001 |
| Unhealthy-lifestyle group | ||||||||
| No. of deaths | 3,110 | 5,107 | 10,046 | 7,234 | 5,206 | |||
| HR (95% CI)‡ | 1 (referent) | 1.01 (0.96–1.05) | 1.01 (0.97–1.06) | 1.01 (0.96–1.06) | 1.03 (0.98–1.09) | 1.03 (0.99–1.07) | 0.16 | |
| Cardiovascular mortality | ||||||||
| Healthy-lifestyle group | ||||||||
| No. of deaths | 172 | 246 | 369 | 219 | 134 | |||
| HR (95% CI)‡ | 1 (referent) | 0.90 (0.72–1.12) | 0.81 (0.65–1.01) | 1.00 (0.77–1.29) | 0.95 (0.70–1.29) | 0.96 (0.76–1.20) | 0.72 | 0.04 |
| Unhealthy-lifestyle group | ||||||||
| No. of deaths | 793 | 1,273 | 2,569 | 1,742 | 1,249 | |||
| HR (95% CI)‡ | 1 (referent) | 0.99 (0.90–1.08) | 1.07 (0.98–1.16) | 1.05 (0.95–1.16) | 1.10 (0.98–1.23) | 1.08 (1.00–1.17) | 0.04 | |
|
| ||||||||
| Plant protein | ||||||||
| Intake category | ≤3 % | >3, ≤4 % | >4, ≤5 % | >5, ≤6 % | >6 % | Per 3% increment | ||
| All-cause mortality | ||||||||
| Healthy-lifestyle group | ||||||||
| No. of deaths | 467 | 997 | 1,312 | 1,210 | 929 | |||
| HR (95% CI)‡ | 1 (referent) | 1.07 (0.95–1.21) | 1.05 (0.91–1.21) | 0.98 (0.83–1.16) | 0.95 (0.78–1.16) | 0.95 (0.83–1.08) | 0.44 | <0.001 |
| Unhealthy-lifestyle group | ||||||||
| No. of deaths | 5,556 | 8,497 | 8,801 | 5,344 | 2,505 | |||
| HR (95% CI)‡ | 1 (referent) | 0.97 (0.94–1.01) | 0.95 (0.90–0.99) | 0.91 (0.86–0.96) | 0.89 (0.83–0.96) | 0.90 (0.85–0.95) | <0.001 | |
| Cardiovascular mortality | ||||||||
| Healthy-lifestyle group | ||||||||
| No. of deaths | 75 | 182 | 329 | 318 | 236 | |||
| HR (95% CI)‡ | 1 (referent) | 1.03 (0.76–1.39) | 0.98 (0.70–1.37) | 0.81 (0.55–1.19) | 0.69 (0.44–1.07) | 0.76 (0.57–1.01) | 0.06 | 0.01 |
| Unhealthy-lifestyle group | ||||||||
| No. of deaths | 1,159 | 1,913 | 2,293 | 1,487 | 774 | |||
| HR (95% CI)‡ | 1 (referent) | 0.93 (0.86–1.01) | 0.89 (0.81–0.98) | 0.82 (0.73–0.92) | 0.86 (0.74–1.00) | 0.88 (0.79–0.98) | 0.02 | |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Healthy lifestyle was defined as never smoking or ever smoking with pack-years of <5, never or moderate alcohol drinking (<14 g/day in women, <28 g/day in men), BMI ≥ 18.5 and <27.5 kg/m2, and physical activity of ≥ 150-min/week at moderate level or ≥ 75 min/week at vigorous level (equivalent to ≥ 7.5 MET-hours/week).
Likelihood ratio test was used to calculate the P for interaction, by comparing the model with the product term between protein intake (continuous) and healthy lifestyle (binary) to the model without this term.
Cox proportional hazards model with age as the time scale was stratified by sex and calendar time, and adjusted for total caloric intake, percent of energy from saturated fat, polyunsaturated fat, monounsaturated fat, and trans fat (continuous), multivitamin use (yes or no), smoking status (never, past smokers, current smokers 1–14, and 15+ cigarettes/day), pack-years of smoking (in women: ≤ 15, 16–25, 26–45, >45; in men: <10, 11–24, 25–44, ≥45), body mass index (<23.0, 23.0–24.9, 25.0–26.9, 27.0–29.9, 30.0–34.9, ≥35 kg/m2), physical activity (quintiles), alcohol consumption (in women: 0, 0.1–5.0, 5.1–15.0, and >15.0 g/d; in men: 0, 0.1–10.0, 10.1–20.0, and >20.0 g/d), history of hypertension diagnosis (yes or no), glycemic index (in quintiles), and intake of whole grains, total fiber, fruits, and vegetables (all in quintiles). Mutual adjustment was conducted for animal protein and plant protein analysis.
When stratified by history of diabetes, the positive association with all-cause mortality for animal protein and the inverse association for plant protein appeared to be stronger among diabetics than non-diabetics (Pinteraction=0.06 and 0.02, respectively, eTable 4)
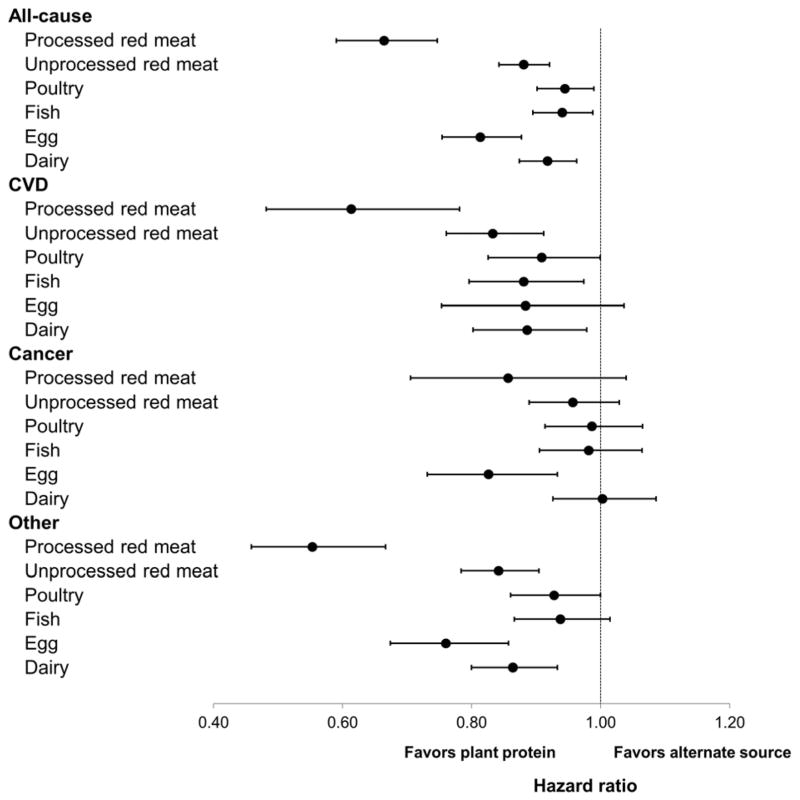
Finally, we examined the substitution association of different protein sources with mortality. The average protein intake from various foods and their correlations are shown in eTable 5, and their individual associations with mortality are summarized in eTable 6. Protein intake from processed red meat was strongly associated with mortality, whereas no association was found for protein from fish or poultry. Figure 1 presents the HRs of mortality for substitution of 3% energy from plant protein for the same amount of animal protein from different food sources.
Figure 1. Hazard ratio and 95% confidence interval for mortality associated with substitution of 3% energy from plant protein for various animal protein sources.
Protein intake from plant sources and from all the animal food items considered in the figure were included in the multivariable model that was also adjusted for all the covariates as in Table 2.
The HRs (95% CI) of all-cause mortality were 0.66 (0.59–0.75) when 3% of energy from plant protein was substituted for an equivalent amount of protein from processed red meat, 0.88 (0.84–0.92) from unprocessed red meat, 0.94 (0.90–0.99) from poultry, 0.94 (0.89–0.99) from fish, 0.81 (0.75–0.88) from eggs, and 0.92 (0.87–0.96) from dairy. The substitution associations were generally stronger for death from CVD and other causes than from cancer, except for eggs, for which substitution for 3%-energy plant protein was associated with 21% lower cancer mortality (95% CI, 7–37%).
Discussion
After adjusting for other dietary and lifestyle factors, animal protein intake was associated with higher risk of cardiovascular mortality, whereas higher plant protein intake was associated with lower all-cause mortality. However, in the stratified analysis, these associations were confined to participants with at least one lifestyle risk factor. Moreover, we observed that substitution of plant protein for animal protein from a variety of food sources, particularly processed red meat, was associated with lower risk of mortality, suggesting that protein source is important for long-term health.
Although short-term RCTs have shown a beneficial effect of high protein intake,3,4,20,21 the long-term health consequences of protein intake remain controversial.8,9,22–25 In a RCT with 2-year intervention, four calorie-restricted diets with different macronutrient compositions did not show differences in the effects on weight loss and improvement of lipid profiles and insulin levels.26 Importantly, when protein is substituted for other macronutrients, dietary source of protein appears to be a critical determinant for the outcome.
To our knowledge, only two cohort studies have examined animal and plant protein intake in relation to mortality. In the Iowa Women’s Health Study, although neither animal nor plant protein was associated with all-cause mortality, an inverse association was found between plant protein and CVD mortality, and substituting plant protein for animal protein was associated with a substantially lower CVD mortality. In a recent report from the NHANES III,8 higher protein intake was related to increased risk of all-cause mortality among participants younger than 65 years. However, when animal protein was controlled for, this association was eliminated, suggesting that animal protein was responsible for the effect of higher protein intake, if there is any, on increased mortality. While it is difficult to directly compare these studies given the variation in the study methods,27 these data together with our current findings support the importance of protein sources for the long-term health outcome and suggest that plant is a preferred protein source over animal foods.
Indeed, unlike animal protein, plant protein has not been associated with increased IGF-1 levels,28,29 and has been linked to lower blood pressure,30–32 reduced LDL,32–34 and improved insulin sensitivity.35 Substitution of plant protein for animal protein has been related to lower incidence of CVD36–39 and type 2 diabetes.40–42 Moreover, while high intake of red meat, particularly processed red meat, has been associated with increased mortality in a recent meta-analysis of 13 cohort studies,43 high consumption of nuts, a major contributor to plant protein, has been associated lower CVD and all-cause mortality.44 These results underscore the importance of protein sources for risk assessment and suggest that other components in protein-rich foods (e.g., sodium,45 nitrates and nitrites46 in processed red meat), in addition to protein per se, may have a critical health effect.
Interestingly, in this study, we found that the relationship of animal and plant protein with mortality varied by lifestyle factors and any statistically significant protein-mortality associations were restricted to participants with at least one of the unhealthy behaviors, including smoking, heavy alcohol drinking, overweight or obesity, and physical inactivity. Several reasons may explain these findings. First, given the remaining variation of health behaviors across protein intake categories in the unhealthy-lifestyle group, it is possible that residual confounding from lifestyle factors contributes to the observed protein-mortality associations. However, our results are robust to adjustment for a wide spectrum of potential confounders and the propensity score. Second, our results may suggest that the adverse effects of high animal protein intake and beneficial effects of plant protein may be enhanced by other unhealthy lifestyle choices and become evident among the subgroup of individuals with these behaviors who may already have had some underlying inflammatory or metabolic disorders. Finally, as shown in Table 3, with similar amount of intake, participants with and without a healthy lifestyle demonstrated distinct profiles of protein sources. Those with unhealthy lifestyles consumed more processed and unprocessed red meat, whereas the healthy-lifestyle group consumed more fish and chicken as animal protein sources, suggesting that different protein sources, at least partly, contributed to the observed variation in the protein-mortality associations according to lifestyle factors. This hypothesis is supported by our substitution analysis results. Although substituting plants for various animal foods was all associated with a lower mortality, red meat, especially processed red meat, showed a much stronger association than fish and poultry, which themselves were not associated with mortality (eTable 6). In fact, protein from certain fish, such as cod, has been suggested to improve lipid profile, glycemic control and insulin sensitivity.35,47,48
The strengths of the current study included the large sample size, repeated dietary assessments, and high follow-up rate of the two well-established cohorts up to 32 years. Moreover, we collected detailed data on a wide spectrum of lifestyle factors that allowed for rigorous confounding adjustment and subgroup analysis. In addition, to facilitate public health recommendation, we calculated protein intake according to food sources and assessed the substitution effect for protein of various origins.
A limitation of the study is the moderately higher protein consumption (median: 19% of calories) in our study population compared to the general US population (15–16%),49,50 thus limiting our ability to assess the effect of very low end of intake. Furthermore, as an observational study, residual confounding could not be excluded. However, our results were robust to the multivariable adjustment and propensity score analysis, and any confounding effect might have been minimized in our stratified analysis according to lifestyle profile.
In summary, while higher intake of animal protein was associated with higher mortality and plant protein was associated with lower mortality, these associations were confined to participants with at least one lifestyle risk factor. Substitution of plant protein for animal protein, especially from processed red meat, may confer a substantial health benefit. Therefore, public health recommendations should focus on improvement of protein sources.
Supplementary Material
Acknowledgments
M.S. is a trainee of the Harvard Transdisciplinary Research Center on Energetics and Cancer (TREC).
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study, for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Funding/Support: This work was supported by the National Institutes of Health (UM1 CA186107, P01 CA87969, and UM1 CA167552).
Role of the sponsor: The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- CVD
cardiovascular disease
- FFQ
food frequency questionnaire
- HDL
high-density lipoprotein
- HPFS
Health Professionals Follow-up Study
- HR
hazard ratio
- IGF
insulin-like growth factor
- LDL
low-density lipoprotein
- MET
metabolic equivalent
- NHANES
National Health and Nutrition Examination Survey
- NHS
Nurses’ Health Study
- RCT
randomized controlled trial
Footnotes
Conflict of Interest Disclosures: None.
Author Contributions: Drs Song and Giovannucci had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: M.S., E. G.
Acquisition of data: M.S., T.T.F., F.B.H., W.C.W., A.T.C., E. G.
Analysis and interpretation of data: M.S., F.B.H., W.C.W., V.L., A.T.C., E. G.
Drafting of the manuscript: M.S.
Critical revision of the manuscript for important intellectual content: T.T.F., F.B.H., W.C.W., V.L., A.T.C., E. G.
Statistical analysis: M.S.
Funding acquisition: F.B.H., W.C.W., A.T.C., E. G.
Administrative, technical, or material support: A.T.C., E. G.
References
- 1.Simpson SJ, Raubenheimer D. Perspective: Tricks of the trade. Nature. 2014 Apr 17;508(7496):S66. doi: 10.1038/508S66a. [DOI] [PubMed] [Google Scholar]
- 2.Solon-Biet SM, McMahon AC, Ballard JW, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014 Mar 4;19(3):418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012 Dec;96(6):1281–1298. doi: 10.3945/ajcn.112.044321. [DOI] [PubMed] [Google Scholar]
- 4.Santesso N, Akl EA, Bianchi M, et al. Effects of higher-versus lower-protein diets on health outcomes: a systematic review and meta-analysis. Eur J Clin Nutr. 2012 Jul;66(7):780–788. doi: 10.1038/ejcn.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tielemans SM, Altorf-van der Kuil W, Engberink MF, et al. Intake of total protein, plant protein and animal protein in relation to blood pressure: a meta-analysis of observational and intervention studies. J Hum Hypertens. 2013 Sep;27(9):564–571. doi: 10.1038/jhh.2013.16. [DOI] [PubMed] [Google Scholar]
- 6.Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41. doi: 10.1146/annurev-nutr-080508-141056. [DOI] [PubMed] [Google Scholar]
- 7.Rutherfurd-Markwick KJ. Food proteins as a source of bioactive peptides with diverse functions. Br J Nutr. 2012 Aug;108(Suppl 2):S149–157. doi: 10.1017/S000711451200253X. [DOI] [PubMed] [Google Scholar]
- 8.Levine ME, Suarez JA, Brandhorst S, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014 Mar 4;19(3):407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelemen LE, Kushi LH, Jacobs DR, Jr, Cerhan JR. Associations of dietary protein with disease and mortality in a prospective study of postmenopausal women. Am J Epidemiol. 2005 Feb 1;161(3):239–249. doi: 10.1093/aje/kwi038. [DOI] [PubMed] [Google Scholar]
- 10.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991 Aug 24;338(8765):464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 11.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997 Feb;6(1):49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 12.Yuan C, Spiegelman D, Rimm EB, et al. Validity of a Dietary Questionnaire Assessed by Comparison With Multiple Weighed Dietary Records or 24-Hour Recalls. Am J Epidemiol. 2016 doi: 10.1093/aje/kww104. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984 May;119(5):837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 14.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999 Mar 15;149(6):531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 15.Fung TT, van Dam RM, Hankinson SE, Stampfer M, Willett WC, Hu FB. Low-carbohydrate diets and all-cause and cause-specific mortality: two cohort studies. Ann Intern Med. 2010 Sep 7;153(5):289–298. doi: 10.1059/0003-4819-153-5-201009070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willett WC. Nutritional Epidemiology. 3. New York: Oxford University Press; 2013. Implications of Total Energy Intake for Epidemiologic Analyses. [Google Scholar]
- 17.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 18.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: US Dept of Health and Human Services; 2008. [DOI] [PubMed] [Google Scholar]
- 19.Kipnis V, Freedman LS, Brown CC, Hartman A, Schatzkin A, Wacholder S. Interpretation of energy adjustment models for nutritional epidemiology. Am J Epidemiol. 1993 Jun 15;137(12):1376–1380. doi: 10.1093/oxfordjournals.aje.a116647. [DOI] [PubMed] [Google Scholar]
- 20.Layman DK, Clifton P, Gannon MC, Krauss RM, Nuttall FQ. Protein in optimal health: heart disease and type 2 diabetes. Am J Clin Nutr. 2008 May;87(5):1571S–1575S. doi: 10.1093/ajcn/87.5.1571S. [DOI] [PubMed] [Google Scholar]
- 21.Hession M, Rolland C, Kulkarni U, Wise A, Broom J. Systematic review of randomized controlled trials of low-carbohydrate vs. low-fat/low-calorie diets in the management of obesity and its comorbidities. Obes Rev. 2009 Jan;10(1):36–50. doi: 10.1111/j.1467-789X.2008.00518.x. [DOI] [PubMed] [Google Scholar]
- 22.Eisenstein J, Roberts SB, Dallal G, Saltzman E. High-protein weight-loss diets: are they safe and do they work? A review of the experimental and epidemiologic data. Nutr Rev. 2002 Jul;60(7 Pt 1):189–200. doi: 10.1301/00296640260184264. [DOI] [PubMed] [Google Scholar]
- 23.Lagiou P, Sandin S, Weiderpass E, et al. Low carbohydrate-high protein diet and mortality in a cohort of Swedish women. J Intern Med. 2007 Apr;261(4):366–374. doi: 10.1111/j.1365-2796.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson LM, Winkvist A, Eliasson M, et al. Low-carbohydrate, high-protein score and mortality in a northern Swedish population-based cohort. Eur J Clin Nutr. 2012 Jun;66(6):694–700. doi: 10.1038/ejcn.2012.9. [DOI] [PubMed] [Google Scholar]
- 25.Trichopoulou A, Psaltopoulou T, Orfanos P, Hsieh CC, Trichopoulos D. Low-carbohydrate-high-protein diet and long-term survival in a general population cohort. Eur J Clin Nutr. 2007 May;61(5):575–581. doi: 10.1038/sj.ejcn.1602557. [DOI] [PubMed] [Google Scholar]
- 26.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009 Feb 26;360(9):859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willett WC. Low-carbohydrate diets: a place in health promotion? J Intern Med. 2007 Apr;261(4):363–365. doi: 10.1111/j.1365-2796.2007.01804.x. [DOI] [PubMed] [Google Scholar]
- 28.Holmes MD, Pollak MN, Willett WC, Hankinson SE. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev. 2002 Sep;11(9):852–861. [PubMed] [Google Scholar]
- 29.Allen NE, Appleby PN, Davey GK, Kaaks R, Rinaldi S, Key TJ. The associations of diet with serum insulin-like growth factor I and its main binding proteins in 292 women meat-eaters, vegetarians, and vegans. Cancer Epidemiol Biomarkers Prev. 2002 Nov;11(11):1441–1448. [PubMed] [Google Scholar]
- 30.Elliott P, Stamler J, Dyer AR, et al. Association between protein intake and blood pressure: the INTERMAP Study. Arch Intern Med. 2006 Jan 9;166(1):79–87. doi: 10.1001/archinte.166.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He J, Gu D, Wu X, et al. Effect of soybean protein on blood pressure: a randomized, controlled trial. Ann Intern Med. 2005 Jul 5;143(1):1–9. doi: 10.7326/0003-4819-143-1-200507050-00004. [DOI] [PubMed] [Google Scholar]
- 32.Appel LJ, Sacks FM, Carey VJ, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005 Nov 16;294(19):2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 33.Lamarche B, Desroches S, Jenkins DJ, et al. Combined effects of a dietary portfolio of plant sterols, vegetable protein, viscous fibre and almonds on LDL particle size. Br J Nutr. 2004 Oct;92(4):657–663. doi: 10.1079/bjn20041241. [DOI] [PubMed] [Google Scholar]
- 34.Anderson JW, Johnstone BM, Cook-Newell ME. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med. 1995 Aug 3;333(5):276–282. doi: 10.1056/NEJM199508033330502. [DOI] [PubMed] [Google Scholar]
- 35.Tremblay F, Lavigne C, Jacques H, Marette A. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu Rev Nutr. 2007;27:293–310. doi: 10.1146/annurev.nutr.25.050304.092545. [DOI] [PubMed] [Google Scholar]
- 36.Preis SR, Stampfer MJ, Spiegelman D, Willett WC, Rimm EB. Dietary protein and risk of ischemic heart disease in middle-aged men. Am J Clin Nutr. 2010 Nov;92(5):1265–1272. doi: 10.3945/ajcn.2010.29626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010 Aug 31;122(9):876–883. doi: 10.1161/CIRCULATIONAHA.109.915165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lagiou P, Sandin S, Lof M, Trichopoulos D, Adami H-O, Weiderpass E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ. 2012 Jun 26;:344. doi: 10.1136/bmj.e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halton TL, Willett WC, Liu S, et al. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006 Nov 9;355(19):1991–2002. doi: 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- 40.Halton TL, Liu S, Manson JE, Hu FB. Low-carbohydrate-diet score and risk of type 2 diabetes in women. Am J Clin Nutr. 2008 Feb;87(2):339–346. doi: 10.1093/ajcn/87.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Koning L, Fung TT, Liao X, et al. Low-carbohydrate diet scores and risk of type 2 diabetes in men. Am J Clin Nutr. 2011 Apr;93(4):844–850. doi: 10.3945/ajcn.110.004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malik VS, Li Y, Tobias DK, Pan A, Hu FB. Dietary Protein Intake and Risk of Type 2 Diabetes in US Men and Women. Am J Epidemiol. 2016 Apr 15;183(8):715–728. doi: 10.1093/aje/kwv268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abete I, Romaguera D, Vieira AR, Lopez de Munain A, Norat T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: a meta-analysis of cohort studies. Br J Nutr. 2014 Sep 14;112(5):762–775. doi: 10.1017/S000711451400124X. [DOI] [PubMed] [Google Scholar]
- 44.Luo C, Zhang Y, Ding Y, et al. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a systematic review and meta-analysis. Am J Clin Nutr. 2014 May 21;100(1):256–269. doi: 10.3945/ajcn.113.076109. [DOI] [PubMed] [Google Scholar]
- 45.Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010 Feb 18;362(7):590–599. doi: 10.1056/NEJMoa0907355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker R. Nitrates, nitrites and N-nitrosocompounds: a review of the occurrence in food and diet and the toxicological implications. Food additives and contaminants. 1990 Nov-Dec;7(6):717–768. doi: 10.1080/02652039009373938. [DOI] [PubMed] [Google Scholar]
- 47.Lavigne C, Tremblay F, Asselin G, Jacques H, Marette A. Prevention of skeletal muscle insulin resistance by dietary cod protein in high fat-fed rats. American journal of physiology. Endocrinology and metabolism. 2001 Jul;281(1):E62–71. doi: 10.1152/ajpendo.2001.281.1.E62. [DOI] [PubMed] [Google Scholar]
- 48.Tremblay F, Lavigne C, Jacques H, Marette A. Dietary cod protein restores insulin-induced activation of phosphatidylinositol 3-kinase/Akt and GLUT4 translocation to the T-tubules in skeletal muscle of high-fat-fed obese rats. Diabetes. 2003 Jan;52(1):29–37. doi: 10.2337/diabetes.52.1.29. [DOI] [PubMed] [Google Scholar]
- 49.Yancy WS, Jr, Wang CC, Maciejewski ML. Trends in energy and macronutrient intakes by weight status over four decades. Public Health Nutr. 2014 Feb;17(2):256–265. doi: 10.1017/S1368980012005423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ford ES, Dietz WH. Trends in energy intake among adults in the United States: findings from NHANES. Am J Clin Nutr. 2013 Apr;97(4):848–853. doi: 10.3945/ajcn.112.052662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.