Abstract
Hypersecretion of corticotropin releasing factor (CRF) is linked to the pathophysiology of major depression and post-traumatic stress disorder, disorders that are more common in women than men. Notably, preclinical studies have identified sex differences in CRF receptors that can increase neuronal sensitivity to CRF in female compared to male rodents. These cellular sex differences suggest that CRF may regulate brain circuits and behavior differently in males and females. To test this idea, we first evaluated whether there were sex differences in anxiety-related behaviors induced by the central infusion of CRF. High doses of CRF increased self-grooming more in female than in male rats, and the magnitude of this effect in females was greater when they were in the proestrous phase of their estrous cycle (higher ovarian hormones) compared to the diestrous phase (lower ovarian hormones), which suggests that ovarian hormones potentiate this anxiogenic effect of CRF. Brain regions associated with CRF-evoked self-grooming were identified by correlating a marker of neuronal activation, cFOS, with time spent grooming. In the infralimbic region, which is implicated in regulating anxiety, the correlation for CRF-induced neuronal activation and grooming was positive in proestrous females, but negative for males and diestrous females, indicating that ovarian hormones altered this relationship between neuronal activation and behavior. Because CRF regulates a number of regions that work together to coordinate different aspects of responding to stress, we then examined more broadly whether CRF-activated functional connectivity networks differed between males and cycling females. Interestingly, hormonal status altered correlations for CRF-induced neuronal activation between a variety of brain regions, but the most striking differences were found when comparing proestrous females to males, particularly when comparing neuronal activation between prefrontal cortical and other forebrain regions. These results suggest that ovarian hormones alter the way brain regions work together in response to CRF, which could drive different strategies for coping with stress in males versus females. These sex differences in stress responses could also help explain female vulnerability to psychiatric disorders characterized by CRF hypersecretion.
Keywords: corticotropin releasing hormone, anxiety, stress, sex difference, estrogens, progesterone
1. Introduction
Women are roughly twice as likely as men to suffer from stress-related psychiatric disorders, such as major depression and post-traumatic stress disorder (PTSD; Kessler et al., 2012). These disorders are considered stress-related because stress is associated with their onset and severity, and stress hormone levels are altered in patients with these disorders (Breslau, 2009; Holsboer, 2001). For example, a key mediator of the stress response, corticotropin releasing factor (CRF), is hypersecreted in patients with depression and PTSD (Bremner et al., 1997; Nemeroff et al., 1984). Given that disorders characterized by CRF dysregulation occur more frequently in women than in men, sex differences in the CRF system could contribute to the sex bias in disease prevalence (Bangasser and Valentino, 2014).
In a preclinical model, we previously identified sex differences in neuronal responses to CRF. Specifically, noradrenergic neurons in the locus coeruleus (LC)-arousal system were more sensitive to CRF in female than in male rats (Bangasser et al., 2010; Bangasser et al., 2013b; Curtis et al., 2006). This physiological sex difference was linked to sex differences in CRF1 receptor coupling and signaling (Bangasser et al., 2010). Importantly, sex differences in CRF receptors are not limited to the LC. For example, CRF1 receptor binding is higher in certain regions of the cortex and amygdala in adult female compared to male rats (Weathington and Cooke, 2012; Weathington et al., 2014). Additionally, sex differences in CRF receptor co-localization with GABAergic neurons in the dorsal raphe and delta opioid receptor-containing neurons in the hippocampus have been identified (Howerton et al., 2014; Williams et al., 2011). Collectively, these studies suggest widespread sex differences in CRF receptors at the cellular level. However, systems level sex differences in CRF-mediated behaviors and activated circuitry have been largely underexplored because the majority of previous studies assessed systems level effects of CRF only in male rodents (but see, Toth et al., 2015; Toth et al., 2014).
In male rodents, central administration of CRF is known to evoke a number of stress-related behaviors, potentiating anxiety in novel environments and eliciting defensive responses (e.g., Britton et al., 1982; Korte et al., 1994; Veldhuis and De Wied, 1984). Even in a familiar environment devoid of any anxiogenic stimuli, Howard et al. (2008) found that central administration of CRF evoked head shakes, burying, and self-grooming in male rats, behaviors that are thought to be defensive or reflect an anxiety-like state (De Boer et al., 1990; Handley and Singh, 1986; Homberg et al., 2002; Spruijt et al., 1992). At the anatomical level, CRF has been shown to increase neuronal activation in cortical, limbic, and hindbrain regions in males, but again, females were not included in these studies (Arnold et al., 1992; Imaki et al., 1993).
The present study was designed to test the hypotheses that there are sex differences in CRF-evoked behavior and activated brain circuits. To this end, we first utilized the CRF-evoked behavior procedure, which was previously established in males (Howard et al, 2008) and is known to elicit both defensive and anxiety-related behavior, to determine whether CRF would increase these behaviors more in female than in male rats. We also evaluated the estrous cycle of females to assess a role for circulating ovarian hormones in regulating CRF’s behavioral effects. Putative brain regions linked to CRF-evoked behavior in males and cycling females were then assessed by correlating a marker of neuronal activation, cFOS, with behavior. Then, CRF-activated circuits were assessed more broadly by evaluating neuronal activation in a number of stress responsive brain regions and determining whether the relationships between neuronal activation in these brain regions differed by sex and cycle stage. This approach allowed us to assess the effect of sex and hormonal status on neuronal activation in stress-related functional connectivity networks.
2. Methods
2.1. Subjects, cytology, and stereotaxic surgery
Two sets of adult (>70 days old) male and female Sprague Dawley rats (Charles River Laboratories, Wilmington MA, USA) were used. The first set (male, n=11; female, n=10) was used to generate CRF dose-response curves for anxiety-related behavior. The second set (male, n=23; female, n=42) was used to test the effect of estrous cycle stage on CRF-evoked behavior and activated brain circuits. All rats were housed individually on a 12-hour reverse light/dark cycle with dark onset at 9:00am and ad libitum water and food. Females were lavaged daily to assess estrous cycle stage. All studies were conducted in accordance with the Temple University Institutional Animal Use and Care Committee and the Institutional Animal Care and Use Committee.
Rats were implanted with a cannula aimed at the lateral ventricle (−1.1 mm A/P, −1.5 mm M/L, −4.4 mm D/V) as previously described (Bangasser et al., 2013a). Then they were allowed at least 7 days to recover before testing.
2.2. CRF dose-response curve for evoked behavior
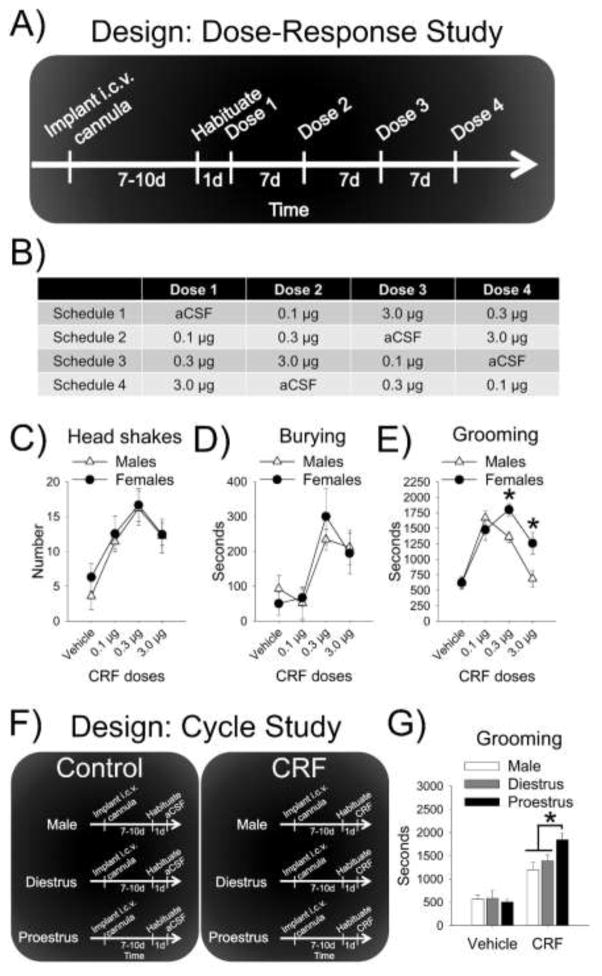
We chose to utilize the CRF-evoked behavior task developed for male rats by Howard et al. (2008) to compare the effects of CRF in males and females. This task has several advantages. First, it evokes multiple types of stress-related behaviors (e.g., defensive and anxiety-related), which can be independently assessed. Second, because it is performed in a familiar environment, baseline anxiety levels are similarly low in both males and females. Under red light, each rat in the dose-response study was habituated individually for 1 h to the experimental chamber (black plexiglass 60.96 cm × 30.48 cm × 21.59 cm, open top) that contained bedding (7.5 cm in depth) spread evenly across the floor. The following day, each rat was returned to the chamber for a 30 min acclimation session. Immediately after, the rat was infused via a microinfusion pump (Harvard Apparatus) at a rate of 1μl/min with artificial cerebrospinal fluid (aCSF) vehicle or one of three doses of ovine CRF (0.1 μg, 0.3 μg, and 3.0 μg in 3 μl of aCSF; American Peptides) as previously described (Bangasser et al., 2013a; Howard et al., 2008). Doses of CRF were chosen based on Howard et al. (2008) and were administered with a week-long washout period as described (Cole et al., 2016; Fig. 1A) and in a counterbalanced fashion using four different schedules that controlled for order and carryover effects (Fig. 1B).
Figure 1.
CRF-evoked behavior in male and female rats. (A) The schematic depicts the experimental design for the dose-response study and (B) the table displays the counterbalancing approach used for the repeated dosing. Dose-response curves were similar between males and females for (C) head shakes and (D) burying. (E) In response to higher CRF doses, females groomed more than males. (F) The schematic depicts the design for the follow-up study assessing the effect of estrous cycle on CRF-evoked behavior. (G) Although the 0.3 μg dose of CRF evoked grooming in all groups, proestrous females groomed more than diestrous females and males.
Each rat was returned to the chamber where it was individually tested. After 10 min, their behavior was recorded for 1 h. CRF-evoked behaviors were scored by an experimenter blind to the condition using the behavioral scoring software Kinoscope (Kokras et al., 2015). As in the Howard et al. (2008) study, head shakes were defined as a shaking motion originating in the head and extending through the entire body, burying—also referred to as defensive treading (Reynolds and Berridge, 2001)—was defined as repeated forward-and-backward movements of either one or both forepaws that moved bedding, and self-grooming, which we will refer to as grooming, was defined as paw strokes on the face or body, or licking of the forelimbs or body. Consistent with Howard et al. (2008) and the focus on stress-induced behaviors the other two prominent behaviors, locomotion and resting, were not scored.
2.3. Ovarian hormone effects on CRF-evoked behavior
In the first set of rats used for the dose-response curve study, the estrous cycle was tracked, but because the rat cycle is 4–5 days and testing occurred every 7 days, females were not tested in a particular cycle stage. In order to better gauge the contribution of circulating ovarian hormones, we used a second set of rats where CRF-evoked behaviors and activated brain circuits were evaluated in females in either the proestrous phase of their cycle (higher ovarian hormones) or diestrous phase (lower ovarian hormones). Specifically, proestrous females, diestrous females, and males were infused with either aCSF or the 0.3μg dose of CRF and tested on the evoked behavior procedure as detailed above (Fig. 1F). The rats were then sacrificed ~75 min after the session was complete and their tissue was processed for cFOS as detailed below.
2.4. Tissue collection and processing
Following behavioral testing, rats were deeply anesthetized and transcardially perfused. Brain tissue was sectioned (30 μm) on a cryostat as previously detailed (Bangasser et al., 2013a). Slices from the dose-response curve study were processed with cresyl violet to confirm placement. Sections from the rats in the subsequent studies were processed for immunohistochemistry as previously described (Bangasser et al., 2013b). Briefly, every 4th section throughout the brain was quenched (0.75% H2O2, 20 min), blocked (phosphate buffered saline, triton, and 0.4% bovine serum albumin, 30 min), and then incubated for 48 hours with anti-cFOS (1:1000, Santa Cruz H-125). Following rinses, sections were incubated (90 min) in donkey anti-rabbit conjugated to biotin (1:200, Jackson ImmunoResearch). Then sections were rinsed and incubated (90 min) in avidin-biotin complex reagent using a kit (ABC Vectastain, Burlingame, CA). Sections were then rinsed and processed for diaminobenzadine (DAB; Vector Laboratories, Inc. SK-4100). Finally, sections were then rinsed, dehydrated, mounted, and cover-slipped with Permount.
2.5. cFOS analysis
Regions for cFOS analysis were chosen based on their putative role in responding to stress and the presence of CRF receptors (Van Pett et al., 2000). Regions were subdivided based on differing functions as follows: infralimbic (IL) and prelimbic (PL) prefrontal cortical regions; nucleus accumbens (NAc) core and shell; bed nucleus of the stria terminalis oval subdivision (oBNST) and anterodorsal division (adBNST); basolateral (BLA) and central nuclei of the amygdala (CeA); and ventromedial dorsal raphe (vmDR) and the lateral wing of the dorsal raphe (lwDR; Crawford et al., 2010; Kim et al., 2013; Parkinson et al., 1999; Sierra-Mercado et al., 2011). The medial septum (MS) and lateral septum (LS) were analyzed separately because CRF1 receptors are denser in the MS, while CRF2 receptors are denser in the LS (Van Pett et al., 2000). The dorsomedial periaqueductal gray (dmPAG) was analyzed separately from other areas of the lateral PAG (i.e., dorsolateral PAG, lateral PAG, ventrolateral PAG) which were combined into one lPAG measure, because CRF only elicits anxiety-like behaviors on the elevated plus maze when infused into the dmPAG in male rats (Borelli and Brandão, 2008).
To quantify cFOS positive neurons, pictures of two brain sections per rat for smaller regions and four sections per rat for larger regions were taken at 10× magnification with a camera (Leica DFC450) affixed to a microscope (Leica DM5500). Rats without the predetermined minimum number of sections for a specific region were removed from the analysis for that region. Quantification of the hippocampus (HPC) was restricted to the dorsal hippocampus due to a loss of ventral hippocampus sections. Cell counting was performed using ImageJ software (NIH) by a rater blind to the experimental condition as previously detailed (Woodlee et al., 2008). Briefly, for each image, background was determined and subtracted, and thresholds were selected that best captured cFOS positive cells as detailed. Images were used to create binary “masks” to quantify labeled cells and a region of interest (ROI) was defined for each anatomical region. Cells within the ROI were automatically counted by the program.
2.6. Statistical analysis
Each dose-response curve was analyzed with a 2×4 mixed Analysis of Variance (ANOVA) with sex as the between-subjects factor and dose as the within-subjects factor. To test the effects of CRF and hormonal status, 2×3 ANOVAs (treatment × hormonal status) were used for behavior and cFOS. Significant interactions were followed by analysis of simple main effects. Differences in functional connectivity have previously been assessed by correlating cFOS activation between various brain regions, and then statistically comparing these correlations across various groups (Maras et al., 2014). We applied a similar approach here by correlating cFOS profiles with grooming behavior, as well as correlating cFOS activation between brain regions, and then comparing these correlations across hormonal condition. Brain regions included in this analysis were those that were activated by CRF in at least one hormonal condition (i.e., regions where there was a main effect of CRF or a treatment × hormonal status interaction). Pearson product moment correlations were calculated for cFOS counts between these regions, and then Fisher r-to-z transformations followed by Fisher's z-tests assessed whether these correlations differed based on hormonal status. For analyses of the between-subjects designs, values that exceeded 2 SDs above or below the group mean were considered outliers and dropped (SM, Table 1).
Table 1.
cFOS profiles following aCSF or CRF administration in male, diestrus female, and proestrus female rats. cFOS profiles ± SEM for each treatment and hormone condition are given for the brain regions analyzed.
| Region | Male | Diestrus | Proestrus | Main effect hormone | Main effect CRF | Interaction | |||
|---|---|---|---|---|---|---|---|---|---|
| aCSF | CRF | aCSF | CRF | aCSF | CRF | p value | p value | p value | |
| Anterior Cingulate Cortex (ACC) | 17.7 ± 4.3 | 26.6 ± 3.7 | 19.3 ± 5.0 | 32.9 ± 4.4 | 18.8 ± 2.3 | 42.4 ± 7.6 | .259 | .001 | .344 |
| Prelimbic Cortex (PL) | 30.6 ± 4.3 | 71.9 ± 12.3 | 43.8 ± 4.9 | 73.2 ± 7.5 | 37.5 ± 6.4 | 96.6 ± 4.8 | .196 | <.001 | .262 |
| Infralimbic Cortex (IL) | 17.8 ± 3.1 | 46.4 ± 8.08 | 29.5 ± 4.1 | 42.9 ± 3.9 | 27.5 ± 5.2 | 56.9 ± 6.3 | .261 | <.001 | .372 |
| Nucleus Accumbens (NAc) Shell | 45.4 ± 9.9 | 79.9 ± 9.8 | 72.5 ± 11.6 | 73.1 ± 12.6 | 49.5 ± 10.7 | 109.3 ± 15.1 | .389 | .002 | .049 |
| Nucleus Accumbens (Nac) Core | 31.6 ± 6.6 | 68.7 ± 8.7 | 49.5 ± 7.8 | 53.2 ± 7.9 | 44.2 ± 11.9 | 92.6 ± 12.8 | .125 | .001 | .064 |
| Lateral Septum (LS) | 36.4 ± 8.5 | 102. 5 ± 16.4 | 29.2 ± 2.7 | 92.2 ± 8.3 | 33.3 ± 6.4 | 98.1 ± 9.7 | .707 | <.001 | .990 |
| Medial Septum (MS) | 14.2 ± 2.6 | 50.7 ± 7.2 | 16.3 ± 2.1 | 54.0 ± 7.0 | 20.3 ± 3.7 | 62.6 ± 5.9 | .264 | <.001 | .860 |
| anterodorsal Bed Nucleus of the Stria Terminalis (adBNST) | 3.9 ± 0.5 | 11.0 ± 1.6 | 3.8 ± 0.3 | 13.2 ± 1.7 | 4.1 ± 0.9 | 12.8 ± 1.1 | .564 | <.001 | .617 |
| oval nucleus Bed Nucleus of the Stria Terminalis (oBNST) | 3.3 ± 0.7 | 9.3 ± 1.2 | 4.0 ± 0.8 | 15.8 ± 2.7 | 1.7 ± 0.4 | 16.3 ± 2.2 | .208 | <.001 | .134 |
| Basal Nucleus of Meynert (BN) | 9.7 ± 2.2 | 28.8 ± 4.0 | 16.6 ± 3.0 | 17.5 ± 2.5 | 12.8 ± 2.0 | 36.3 ± 4.7 | .090 | <.001 | .005 |
| Paraventricul ar Nucleus (PVN) | 75.9 ± 13.4 | 100. 3 ± 14.7 | 55.8 ± 9.6 | 102. 8 ± 17.8 | 61.1 ± 8.6 | 120.1±15.4 | .709 | .001 | .489 |
| Central Amygdala (CeA) | 6.6 ± 1.5 | 17.6 ± 1.2 | 7.0 ± 0.9 | 15.0 ± 3.3 | 5.7 ± 0.9 | 22.5 ± 3.9 | .437 | <.001 | .189 |
| Basolateral Amygdala (BLA) | 8.0 ± 1.1 | 11.4 ± 1.7 | 8.9 ± 1.8 | 10.6 ± 2.1 | 9.7 ± 1.9 | 16.4 ± 2.0 | .123 | .014 | .388 |
| Hippocampus (HPC) | 14.2 ± 1.8 | 54.1 ± 7.9 | 24.1 ± 2.4 | 52.7 ± 12.2 | 19.9 ± 3.0 | 59.4 ± 4.5 | .632 | <.001 | .574 |
| dorsomedial Periaqueduct al Gray (dmPAG) | 26.0 ± 6.4 | 23.6 ± 3.2 | 42.8 ± 8.1 | 80.4 ± 10.1 | 30.1 ± 6.1 | 36.6 ± 5.7 | <.001 | .058 | .002 |
| lateral subdivisions of the Periaqueduct al Gray (lPAG) | 149.2 ± 25.3 | 136.3 ± 15.7 | 128.0 ± 20.2 | 249.7 ± 48.7 | 162.3 ± 18.9 | 251.8 ± 26.2 | .046 | .004 | .036 |
| Pedunculopo ntine Tegmental Nucleus (PPTg) | 38.2 ± 3.6 | 38.9 ± 4.9 | 41.9 ± 6.3 | 44.3 ± 4.7 | 39.7 ± 9.7 | 45.1± 5.3 | .607 | .798 | .935 |
| ventromedial Dorsal Raphe (vmDR) | 49.1 ± 15.0 | 38.9 ± 3.7 | 34.9 ± 4.7 | 96.8 ± 13.8 | 67.3 ± 13.7 | 84.0 ± 17.0 | .029 | .024 | .017 |
| lateral wing Dorsal Raphe (lwDR) | 97.8 ± 24.6 | 103.8 ± 9.2 | 104.5 ±11.8 | 150. 9 ± 22.7 | 133.3 ± 18.5 | 149.5 ± 21.0 | .261 | .074 | .509 |
| Laterodorsal Tegmental Nucleus (LDTg) | 46.7 ± 7.2 | 40.9 ± 4.9 | 35.2 ± 5.4 | 64.8 ± 7.4 | 33.5 ± 5.8 | 47.9 ± 8.3 | .362 | .021 | .031 |
| Locus Coeruleus (LC) | 66.5 ± 10.9 | 105. 7 ± 14.9 | 61.4 ± 10.8 | 235. 7 ± 27.2 | 86.1 ± 10.9 | 163.8 ± 25.4 | .004 | <.001 | .001 |
3. Results
3.1. CRF dose-response curve for evoked behavior in male and female rats
As in Howard et al. (2008), the only robust and consistent stress-related behaviors engaged in following CRF administration were head shakes, burying, and grooming. When rats were not engaging in stress-related behavior, they spent time exploring or resting as illustrated in the video. Dose-response curves were generated for CRF-evoked anxiety-related behavior. For head shakes and burying, there was only a main effect of dose [F(3, 57)=6.43, p=.001 and F(3, 57)=7.50, p<.001, respectively] (Fig. 1C and 1D). There was a sex × dose interaction for grooming [F(3, 57)=4.88, p=.004] (Fig. 1E). Post-hoc tests revealed that females groomed more than males at the 0.3μg and 3.0μg doses (p=.047 and p=.031, respectively).
3.2. Effect of estrous cycle on CRF-evoked behavior
Based on the efficacy of the 0.3μg CRF infusion, this dose was selected for the subsequent study aimed at assessing whether the magnitude of CRF-evoked behavior changed in different estrous cycle phases. Relative to vehicle infused controls, CRF increased burying and headshakes similarly for all hormonal conditions [F(1,56)=21.72, p<.001], [F(1,58)=10.12, p=.002], respectively, and there were no main effects of hormonal status or hormonal status × CRF interactions (data not shown). For grooming, there was a hormonal status × CRF interaction [F(2,58)=5.39, p=.007] (Fig. 1D). Post-hoc tests revealed that CRF increased grooming in proestrous females more than in diestrous females and males (p=.005 and p<.001, respectively). Vehicle treated rats groomed similarly, regardless of hormonal condition (ps>.05).
3.3. Sex differences in CRF-induced neuronal activation
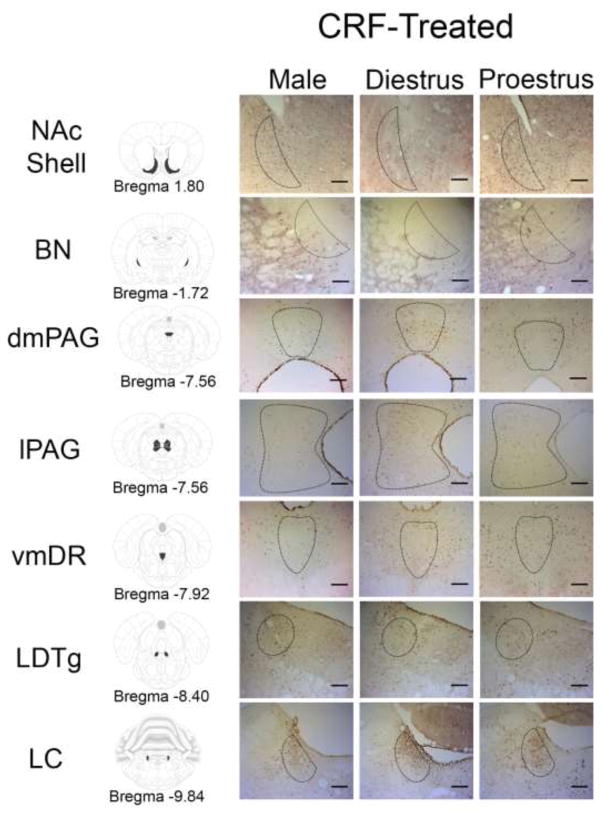
To compare brain regions activated by CRF in males, diestrus, and proestrous females, cFOS profiles were analyzed and the results are presented in Table 1. There were many brain regions where CRF increased the cFOS profiles, regardless of sex and hormonal status. However, in the lPAG and LC, sex differences were found [F(2,50)=3.586, p=.036; F(2,52)=7.539, p=.001], such that CRF-induced cFOS activation in females, regardless of hormonal condition, but not in males (ps<.05 values; Fig. 2). Additionally, hormonal status altered the pattern of CRF-induced cFOS activation for the dmPAG, LDTg, vmDR, BN, and NAc shell [F(2,44)=7.08, p=.002; F(2,54)=3.723, p=.031; F(2,51)=4.439, p=.017; F(2,58)=5.928, p=.005; F(2,54)=3.208, p=.049, respectively] (Fig. 2). Post-hoc tests revealed that, compared to their vehicle infused counterparts, CRF increased cFOS only in diestrous females in the dmPAG, LDTg, and vmDR, and increased cFOS in proestrous females and males in the BN and NAc shell (ps<.05).
Figure 2.
Photomicrographs of regions where hormonal status altered CRF-induced cFOS profiles. The left column illustrates the brain structures on the Paxinos and Watson atlas sections (Paxinos and Watson, 2007). Photomicrographs of cFOS profiles are displayed for CRF-treated males, diestrous females, and proestrous females. Black dotted lines on the photomicrographs represent the region of interest analyzed. NAc Shell, nucleus accumbens shell; BN, basal nucleus of Meynert; lPAG, lateral regions of the periaqueductal gray; vmDR, ventromedial dorsal raphe; LDTg, laterodorsal tegmental nucleus; LC, locus coeruleus. Scale bars = 200 μm.
3.4. The relationship between cFOS activation and grooming behavior following CRF infusion
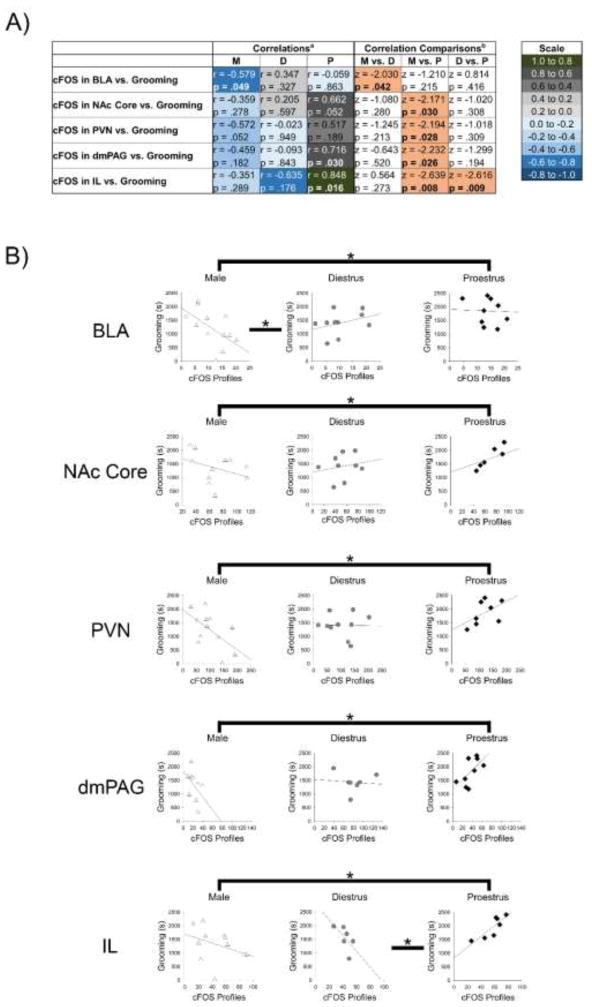
For CRF-treated rats, cFOS profiles and time spent grooming were correlated and then Fisher’s z-tests compared whether these correlations differed between hormonal conditions. The complete results are in supplementary materials (SM, Table 2). This analysis revealed differences between males and diestrous females for the BLA, as well as differences between males and proestrous females for the NAc core, PVN, and dmPAG (Fig. 3). The correlation between IL cFOS profiles and time spent grooming was significantly different in proestrous females than other groups, because these measures were positively correlated for proestrous females, but negatively correlated in males and diestrous females (Fig. 3).
Figure 3.
In CRF-treated rats, the relationship between cFOS profiles and grooming for several brain regions differed by hormonal condition. (A) Table showing acorrelations between the number of cFOS profiles for a given brain region and time spent grooming for CRF-treated rats, as well as bcorrelation comparisons that reveal the results of Fishers z-tests. Peach shading indicates significant differences between hormonal conditions. (B) Correlations between cFOS profile and grooming in CRF-treated rats for each hormonal condition are shown for the regions where the correlations differed based on hormonal status. Asterisks indicate significant differences between correlations (p<.05). BLA, basolateral amygdala; D, diestrus; dm, dorsomedial; IL, infralimbic; M, male; NAc, nucleus accumbens; P, proestrus; PAG, periaqueductal gray; PL, prelimbic; PVN, paraventricular nucleus of the hypothalamus
3.5. CRF activated networks differed depending on hormonal status
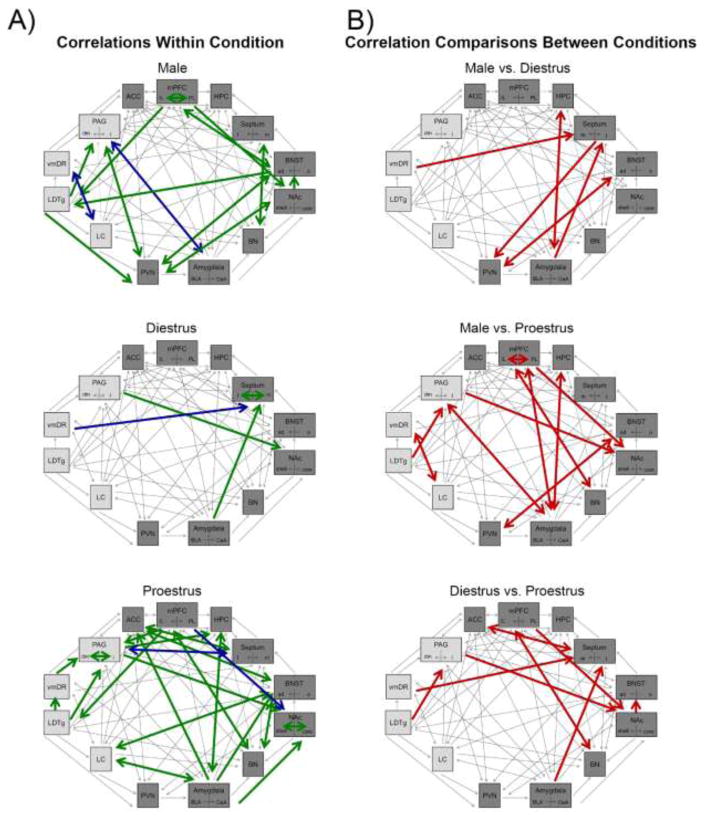
First, CRF-induced cFOS positive neurons were correlated between brain regions within each group (i.e., males, diestrous females, and proestrous females) as depicted in Figure 4a (anatomical projections are indicated with arrows) with the full analysis shown in SM, Tables 3–5. Proestrous females had a greater number of correlations for neuronal activation between brain regions than other groups. Additionally, there was a striking lack of correlations in diestrus females.
Figure 4.
Schematics depict CRF-activated networks. Brain regions included were activated by CRF, with forebrain regions shaded with the darker gray color. Arrows indicate anatomical connections. (A) Correlations between brain regions for each hormone condition are depicted with green arrows indicating significant positive correlations and blue arrows indicating significant negative correlations. (B) Schematics illustrate with red arrows the correlations that significantly differed between hormone conditions. ACC, anterior cingulate cortex; ad, anterodorsal; BLA, basolateral amygdala; BN, basal nucleus of Meynert; BNST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; DR, dorsal raphe; HPC, hippocampus; IL, infralimbic; LC, locus coeruleus; LDTg, laterodorsal tegmental nucleus; l, lateral; m, medial; mPFC, medial prefrontal cortex; NAc, nucleus accumbens; o, oval nucleus; PAG, periaqueductal gray; PL, prelimbic; PVN, paraventricular nucleus of the hypothalamus; vm, ventromedial.
Second, to assess whether patterns of CRF-induced neuronal activation differed between males and females in different estrous cycle stages, Fisher’s z-tests compared whether the correlations differed based on hormonal status. Table 2 lists the regions with different correlations between hormonal conditions, while the entire analysis is included in supplementary materials (SM, Tables 6–8). The schematics in Figure 4b show these differences. Comparisons between proestrous females and males revealed the greatest differences, but there were also many differences between diestrous and proestrous females. Fewer differences were observed between diestrous females and males.
Table 2.
Results of correlations and Fishers z-tests for areas where CRF-induced neuronal activation differed between at least one hormonal condition.
| Correlationsa | Correlation Comparisonsb | ||||||
|---|---|---|---|---|---|---|---|
| M | D | P | M vs. D | M vs. P | D vs. P | Scale | |
| BLA vs. LS | r = − 0.311 | r = 0.635 | r = 0.022 | z = − 1.986 | z = − 0.636 | z = 1.26 | 1 .0 to 0 .8 |
| p = .352 | p = .066 | p = .955 | p = .047 | 0.524 | 0.207 | 0.8 to 0.6 | |
| LS vs. PVN | r = 0.302 | r = − 0.654 | r = − 0.040 | z = 2.024 | z = 0.519 | z = − 1.049 | 0.6 to 0.4 |
| p = .367 | p = .056 | p = .940 | p = .043 | p = .604 | p = .294 | 0.4 to 0. 2 | |
| dmVR vs. MS | r = − 0.055 | r = − 0.907 | r = 0.239 | z = 2.367 | z = − 0.434 | z = − 2.297 | 0.2 to 0.0 |
| p = .880 | p = .005 | p = .648 | p = .018 | p = .665 | p = .022 | 0.00 to − 0.2 | |
| CeA vs. LS | r = 0.203 | r = 0.949 | r = − 0.288 | z = − 2.598 | z = 0.930 | z = 3.300 | −0.2 to − 0.4 |
| p = .549 | p < .001 | p = .452 | p = .009 | p = .352 | p = .001 | −0.4 to − 0.6 | |
| HPC vs. CeA | r = − 0.694 | r = 0.685 | r = 0.760 | z = − 2.027 | z = − 2.762 | z = − 0.181 | −0.6 to − 0.8 |
| p = .056 | p = .201 | p = .047 | p = .043 | p = .006 | p = .856 | −0.8 to − 1.0 | |
| adBNST PVN vs. | r = 0.812 | r = − 0.713 | r = − 0.765 | z = 2.865 | z =2.804 | z = 0.152 | |
| p = .026 | p = .072 | p = .076 | p = .004 | p = .005 | p = .879 | ||
| PL vs. IL | r = 0.943 | r = .724 | r = 0.179 | z = 1.252 | z = 2.340 | z = 0.902 | |
| p < .001 | p = .103 | p = .735 | p = .211 | p = .019 | p = .367 | ||
| PL vs. BLA | r = 0.538 | r = 0.156 | r = − 0.769 | z = 0.643 | z = 2.347 | z = 1.440 | |
| p = .109 | p = .767 | p = .074 | p = .520 | p = .019 | p = .150 | ||
| dmPAG vs. CeA | r = − 0.703 | r = − 0.526 | r = 0.425 | z = − 0.446 | z = − 2.297 | z = − 1.609 | |
| p = .035 | p = .225 | p = .255 | p = .655 | p = .022 | p = .108 | ||
| lPAG vs. NAc Shell | r = − 0.231 | r = 0.171 | r = 0.805 | z = − 0.578 | z = − 2.088 | z = − 1.230 | |
| p = .550 | p = .746 | p = .029 | p = .564 | p = .037 | p = .219 | ||
| vmDR vs. LC | r = − 0.692 | r = 0.276 | r = 0.732 | z = − 1.645 | z = − 2.848 | z = − 0.851 | |
| p = .027 | p = .597 | p = .061 | p = .100 | p = .004 | p = .395 | ||
| PL vs. NAc Shell | r = 0.836 | r = 0.710 | r = − 0.899 | z = 0.179 | z = 3.276 | ;z = 2.578 | |
| p = .005 | p = .114 | p = .038 | p = .858 | p = .001 | p = .010 | ||
| IL vs. BN | r = − 0.234 | r = − 0.581 | r = 0.859 | z = 0.603 | z = − 2.367 | z = − 2.558 | |
| p = .545 | p = .226 | p = .013 | p = .547 | p = .018 | p = .011 | ||
| LDTg vs. lPAG | r = 0.019 | r = − 0.314 | r = 0.860 | z = − 0.732 | z = − 2.100 | z = − 2.290 | |
| p = .964 | p = .544 | p = .003 | p = .464 | p = .035 | p = .022 | ||
| ACC vs. MS | r = 0.632 | r = − 0.028 | r = 0.923 | z=1.222 | z=− 1.184 | z = − 2.242 | |
| p = .093 | p =.947 | p=.009 | p=.222 | p=.236 | p = .025 | ||
| NAc Shell adBNST | r = vs. 0.782 | r = − 0.225 | r = 0.534 | z= 1.809 | z =− 0.266 | z =− 2.075 | |
| p =.038 | p = .628 | p = .217 | p = .070 | p=.790 | p = .038 | ||
| lPAG Core vs. NAc | r = 0.190 | r = − 0.789 | r = 0.776 | z= 1.384 | z = −0.907 | z = −2.754 | |
| p = .624 | p = .062 | p = .040 | p = .166 | p = .364 | p = .006 | ||
Correlations between the number of cFOS profiles.
Results of Fishers z-tests. Pink shading indicates significant differences between hormonal conditions.
M, male; D, diestrus; P, proestrus
4. Discussion
The present study evaluated how central administration of CRF affected behavior and activated networks differently in male and cycling female rats. Although CRF increased grooming, headshakes, and burying in both sexes, females groomed more than males in response to high CRF doses. CRF-evoked grooming was most pronounced in females in the proestrus cycle stage, indicating that ovarian hormones contribute to the sex difference. Anatomically, CRF activated the same regions regardless of sex and hormonal status in many cases. When compared at the network level, however, correlations of neuronal activation between these regions often differed based on hormonal status. These effects of CRF on behavior and circuitry are likely mediated by CRF1 receptors, because the ovine CRF used here preferentially binds to the CRF1 receptor (Grammatopoulos and Chrousos, 2002). Additionally, blocking CRF1 receptors, but not CRF2 receptors, is known to prevent CRF-evoked behavior in male rats (Howard et al., 2008). It will be important in future studies to replicate with a selective CRF1 agonist or block with selective CRF1 receptor antagonist CRF-evoked behavior in females to confirm that, similar to males, the CRF1 receptor subtype is critical for these behaviors in females.
4.1. Sex differences in CRF-evoked grooming
Consistent with previous findings, the only three reliably evoked stress-related behaviors were headshakes, burying, and grooming (Howard et al, 2008). Of these, grooming was increased in females compared to males at the medium and high CRF doses. One interpretation of these data could be that the dose-response curve for grooming is shifted in males, such that the inflection point occurs at a lower dose than in females. However, previous evidence suggests that, at the electrophysiological and signaling level, females are more sensitive to CRF than males (Bangasser et al., 2010; Curtis et al., 2006). Thus, a more likely explanation is that, in the present study, higher doses of CRF engaged a compensatory mechanism in males, but not in females, that reduced their stress-related behavior. One such mechanism could be CRF1 receptor internalization, which we previously found occurred in male CRF overexpressing mice, but not in female CRF overexpressing mice (Bangasser et al., 2013b). If CRF1 receptor internalization was similarly induced by the higher CRF doses used here only in males, then this would explain their lower grooming response to high levels of CRF as internalized receptors can no longer be activated.
Interestingly, the amount of CRF-evoked grooming in females differed across the estrous cycle. Specifically, CRF-treated females in the proestrous cycle phase groomed more than CRF-treated females in the diestrous cycle phase and males. There were no difference in CRF-evoked grooming between diestrous females and males. These findings indicate that the observed sex difference in CRF’s effect on grooming is attributable to adult circulating ovarian hormones. Because estradiol replacement in ovariectomized female rats reliably reduces anxiety-related behavior (Kalandakanond-Thongsong et al., 2012), it may seem surprising that females in the proestrous phase engaged in the most grooming. However, in the vehicle-treated rats, grooming was similar in males, diestrous females, and proestrous females, which is consistent with previous reports of similar grooming levels between males and females when tested in a familiar environment (Gray and Lalljee, 1974; Moore, 1986). Thus, taken together, the data suggest that ovarian hormones do not regulate grooming behavior itself, but rather that high levels of ovarian hormones potentiate the effect of CRF on grooming.
Unlike with grooming, no sex or hormonal differences were observed in CRF-evoked head shakes or burying. This may be, in part, because these behaviors are thought to reflect different aspects of responding to stress. Head shakes are a tic-like behavior that occurs under high stress conditions, although whether they have a function in alleviating stress remains unclear (Handley and Singh, 1986; Howard et al., 2008; Takao et al., 1995). The purpose of burying in the wild is to defend territory or fend off predators (Calhoun, 1963; Owings and Coss, 1977). Similarly, in the laboratory, burying of an electrified shock probe is considered a defensive response (De Boer et al., 1990; Howard et al., 2008). Grooming is thought to reflect the need for reducing arousal and anxiety, and may be a form of self-soothing (Homberg et al., 2002; Spruijt et al., 1992). Additionally, excessive grooming is thought to model aspects of obsessive-compulsive disorder (OCD, Welch et al., 2007), as well as other psychiatric conditions with pathological grooming, such as trichotillomania and body dysmorphic disorder (Feusner et al., 2009). Interestingly, trichotillomania and body dysmorphic disorder are more common in women than in men (Gupta et al., 2015; Phillips et al., 2013), while OCD has a sex difference in presentation with women displaying more cleaning rituals (Mathis et al., 2011). Taken together, the behavioral results of the present study suggest that CRF evokes similar levels of defensive behavior in males and females, but more arousal, self-soothing, and compulsive behaviors in proestrous females, which may have some relevance to certain psychiatric disorders in humans.
The behavior results of the present study have implications more broadly for the development of rodent tasks that assess aspects of stress-related psychiatric disorders. Many popular rodent tasks are limited by the fact that they fail to capture the sex differences in anxiety-like and depression-like behavior that are predicted by clinical studies (Kokras and Dalla, 2014; Shansky, 2015). This is likely because these tasks were validated only in male rodents and fail to take into account sex differences in size, activity, and other characteristics (Bangasser, 2015; Kokras and Dalla, 2014; Shansky, 2015). The CRF-evoked grooming behavior assessed here may therefore fill an unmet need for behavioral tasks that are sensitive to sex differences in anxiety-related behaviors.
4.2. The neuronal activation associated with grooming differs based on hormonal status
A precise brain circuit that mediates grooming has not been elucidated, but several forebrain areas have been implicated (Homberg et al., 2002; Lammers et al., 1987). Our goal here was not to delineate a grooming circuit, but rather to gauge which brain regions could mediate sex differences in CRF-evoked grooming by correlating neuronal activation and time spent grooming in CRF-treated rats. For the NAc core, PVN, and dmPAG, correlations differed between proestrous females and males, such that they were in opposite directions, with diestrous females falling somewhere in between. This could reflect the fact that ovarian hormone levels of diestrous females are in between those of proestrous females and males (Hawkins et al., 1975).
The correlation between CRF-induced neuronal activation in the IL and grooming differed between proestrous females and the other groups. Specifically, unlike in males and diestrous females, where the amount of IL neuronal activation and grooming following CRF administration were negatively correlated, in proestrous females, IL neuronal activation was positively related to the amount of time spent grooming. Given the behavior data in which CRF-evoked grooming was distinguished in proestrus, the IL may be a site of ovarian hormone regulation of CRF-evoked grooming. Direct regulation of the IL by estrogens and progesterone is possible, because their receptors are present in this region (Kato et al., 1994; Shughrue et al., 1997).
Although the medial prefrontal cortex has been implicated in mediating grooming in an anxiogenic environment (Homberg et al., 2002), the exact role of the IL subregion in grooming is unknown. It is well known, however, that the IL is critical for fear extinction (i.e., learning that a cue associated with a fearful event no longer predicts that fearful event), and this region is thought to suppress the fear response initiated by the amygdala (Sierra-Mercado et al., 2011). The grooming behavior observed following CRF administration in our task more likely reflects an anxiety rather than fear to a specific threat, because rats are tested in a familiar environment and the grooming behavior is observed throughout the session (Howard et al., 2008). However, similar to the fear conditioning studies, the IL has also been implicated in inhibiting anxiety-related behavior (Jinks and McGregor, 1997). Thus, in the CRF-evoked burying procedure, it is possible that ovarian hormones potentiate the anxiogenic effects of CRF, causing the need for a greater anxiety reduction, or self-soothing, via grooming in proestrous females and this response is mediated by the IL. Future studies will be needed to clarify the role of ovarian hormone regulation of the IL in stress-related responses.
4.3. Hormonal status alters CRF-activated networks
Previous studies have used cFOS to assess CRF-induced neuronal activation in males (Arnold et al., 1992; Imaki et al., 1993), but our interest was in determining whether hormonal status altered patterns of CRF-induced neuronal activation. In the majority of brain regions analyzed, CRF increased neuronal activation similarly in all groups, regardless of hormonal status. However in the lPAG and LC, a sex difference was observed such that CRF did not alter neuronal activation in males, but increased neuronal activation in females, regardless of estrous cycle stage. To our knowledge, sex differences in the effects of CRF in the lPAG have not been explored, but this region is known to regulate active versus passive stress coping strategies (Keay and Bandler, 2001). Thus, CRF may differently impact these coping strategies in females compared to males, a possibility that could be tested in future studies. The sex difference observed in the LC is consistent with our previous studies revealing that female LC neurons are more sensitive to CRF than those of males—an effect linked to increased CRF1 receptor coupling to Gs—and these sex differences are not modulated by circulating ovarian hormones (Bangasser et al., 2010; Curtis et al., 2006).
For several brain regions, the pattern of neuronal activation was distinguished in diestrous females, such that they were the only group in which neuronal activation was either increased (e.g., dmPAG, LDTg, vmDR) or unaltered (e.g., BN, NAc shell) following CRF treatment. These results diverge from the finding that it was CRF-treated proestrous, not diestrous, females that were significantly different from the other two groups on the grooming measure. One explanation is that the amount of grooming is not primarily determined by the brain regions where diestrous females differed from other groups in their CRF-induced neuronal activation. However, another interpretation is based on De Vries’ (2004) idea that, in order to maintain similar patterns of behavior between males and females, some sex differences in the brain exist to compensate for sex differences in biology (e.g., gonadal hormones, genes on sex chromosomes; De Vries, 2004). Specifically, it is possible that the difference in CRF-evoked grooming between diestrous and proestrous females is due to the fact that infusions of CRF activate regions that promote grooming behavior similarly in both cycle phases, but in diestrus, other regions are also engaged that attenuate or inhibit this grooming response. Such a compensatory response could explain why diestrous females groom a similar amount to males following CRF infusions. Future studies assessing the specific contribution to grooming and other behaviors of regions uniquely regulated in diestrus would help elucidate this issue.
Typically, neuronal activation is assessed in individual brain regions (e.g., Arnold et al., 1992; Imaki et al., 1993). However, here we applied a technique to help identify how CRF-activated regions may work in concert, forming functional neuronal networks that interact in different ways. First, CRF-induced regional cFOS profiles were correlated for males, diestrous females, and proestrous females separately. This analysis revealed different patterns of correlated brain regions for each hormonal condition. Specifically, in males, cFOS profiles in the IL, adBNST, PVN, and LDTg, were each highly correlated with cFOS profiles in other brain regions, suggesting that these four areas may be critical nodes on the male CRF-activated brain network. In diestrous females, the LS was the region in which cFOS activation correlated with the greatest number of other regions. It is also notable that there were very few significant correlations in the diestrous females. In contrast, correlations of neuronal activation between brain regions were most common in proestrous females with cFOS profiles in the IL, NAc shell, BLA, and lPAG being the most frequently correlated with profiles in other areas.
After correlating the data we next assessed whether the correlations of neuronal activation between regions statistically differed based on hormonal status. This analysis revealed pronounced differences between males and proestrous females, especially regarding those correlations between prefrontal cortical and other forebrain regions. Compared to proestrous females, there were fewer differences between diestrous females and males. However, these groups did differ, particularly in the way neuronal activation in the septum correlated with neuronal activation in certain midbrain and hindbrain regions. A comparison of females in the two different cycle stages revealed that the pattern of CRF-induced network activation was also distinguished by hormonal status, suggesting that these circuits are dynamically regulated by ovarian hormones.
In addition to the behaviors evoked by CRF in a familiar environment that were assessed in the present study, CRF regulates performance on many other tasks, such as those that evaluate aspects of cognition (e.g., Cole et al. 2016, Snyder et al., 2012). Hormonal regulation of CRF-activated brain networks could also contribute to differences between males and cycling females in these other responses to CRF. For example, central administration of CRF impairs sustained attention, the ability to monitor a situation for rare and unpredictable events, in males and diestrous females (Cole et al., 2016). However, surprisingly, central CRF has no effect on sustained attention in females with high levels of ovarian hormones (Cole et al., 2016). The process of sustaining attention is mediated by reciprocal connections between the BN and prefrontal cortex (Sarter et al., 2001). In the present study, we found that the correlation for CRF-induced neuronal activation between these two regions differed in proestrous females compared to other groups. When taken together, the behavioral and circuitry results could indicate that high levels of ovarian hormones interact with CRF to regulate the BN–cortical connection in a way that confers resilience to CRF’s negative impact on attention.
As illustrated with the above example, this type of network analysis can help explain how CRF induces divergent behaviors under differing hormonal conditions. It is important to note that, although the relationship between neuronal activation for several brain regions is distinguished by hormonal status, not every relationship appears to change. Why this occurs remains unclear, but one possibility is that differences in the distribution of steroid receptors play a role. For example, network correlations between regions with high densities of estrogen receptors, such as the adBNST and PVN, appear to be more sensitive to differences between hormonal conditions than network correlations between regions with lower densities of estrogen receptors, such as the vmDR and LDTg (Shughrue et al., 1997). The exploratory nature of the correlation approach used here means that future, more targeted studies will be needed to first confirm the CRF-activated circuits that are sensitive to hormonal status, and then to investigate why certain connections are more affected by sex and estrous cycle phase than others. These data do indicate, however, that for studies investigating stress-related behaviors that require circuits that are sensitive to hormonal fluctuations, males and females in different estrous cycle phases should be included in the experimental design.
The functional connectivity analysis used in the current study is rarely conducted in preclinical studies (but see Maras et al., 2014), so perhaps not surprisingly this is the first study, to our knowledge, to suggest that sex and cycle stage can regulate how stress-responsive brain regions work together. In contrast to the preclinical literature, human neuroimaging studies have assessed functional connectivity between activated brain regions in stressed subjects (Henckens et al., 2010; Mather et al., 2010; Veer et al., 2011; Vogel et al., 2015). Although most of these studies have not considered sex differences, Mather et al. (2010) found that, when viewing angry faces, stressed women have greater coordination between the amygdala and fusiform face area than stressed men. Although a homologous cortical region is not present in rodents, our study identified a significant difference in the correlation for neuronal activation between the BLA and the prelimbic cortical region when comparing male to proestrous female rats. Together these cross-species findings using two different measures of activity (i.e., BOLD signal and cFOS) highlight that the amygdala and certain cortical regions may work together differently to respond to stress in males and females in different cycle stages, but, clearly, more studies looking at sex differences in stress-activated networks are needed.
4.4. Ovarian hormone regulation of the CRF system
The results from these studies indicate that ovarian hormones regulate CRF’s effects on a wide variety of interconnected brain regions. Interactions between gonadal hormones and CRF are not surprising because estrogen and progesterone receptors are found in CRF producing brain regions (Kato et al., 1994; Shughrue et al., 1997). Additionally, there are putative estrogen response elements on the CRF gene, and thus estrogens can directly regulate CRF expression (Vamvakopoulos and Chrousos, 1993). Such regulation could contribute to the high levels of CRF found in the PVN and amygdala in proestrous females (Iwasaki-Sekino et al., 2009). Ovarian hormones can similarly regulate CRF receptor expression via putative response elements on their promotor regions (Karteris et al., 2010). In addition to genomic effects, estrogen can also induce rapid cellular changes via membrane estrogen receptors that couple to intracellular signaling pathways (Srivastava et al., 2011). Therefore, another possibility is that estrogen-induced signaling potentiates CRF-induced signaling. This may occur, for example, in the IL region to increase grooming in proestrous females, because in the medial PFC (which includes the IL) estrogen receptor α, estrogen receptor β, and the highly abundant G protein-coupled estrogen receptor 1 (GPER1) are predominantly localized on the plasma membrane, a location that suggests that rapid estrogen signaling in this region is likely (Almey et al., 2014). Finally, the GPER1 can associate directly with the CRF1 receptor (Akama et al., 2013), and although their heterodimeric function remains unclear, it is tempting to speculate that this could be another mechanism by which estrogens directly alter CRF1 signaling. Collectively, these data highlight a multitude of ways that ovarian hormones can impact the CRF system, thereby regulating CRF-activated circuits and evoked behavior.
4.5. Conclusion
While sex differences in the rates of psychiatric disorders are well documented (Kessler et al., 2012), traditionally sex differences in the brain were studied in the context of reproductive behaviors. The results of the experiments conducted here add to the growing body of literature indicating that sex differences in the brain also occur within stress response systems (reviewed in, Bangasser and Valentino, 2014). Specifically, sex differences in the CRF system have now been documented to occur from the cellular to the systems level (Bangasser et al., 2010; Cole et al., 2016). If true in humans, such sex differences could contribute to the sex bias in psychiatric disorders characterized by CRF hypersecretion. Perhaps even more broadly, the effects of hormones on the brain are typically assessed in a region specific manner, yet the analyses in the current study suggest that ovarian hormones regulate CRF-activated brain networks. Thus, appreciating how ovarian hormones regulate the brain at the network level may reveal new ways in which sex differences in behavior are established.
Supplementary Material
Highlights.
Corticotropin releasing factor (CRF) evokes more grooming in female than male rats
Circulating ovarian hormones moderate the sex difference in CRF-evoked grooming
CRF-activated functional connectivity networks differ based on hormonal status
Hormonal regulation of stress circuitry may establish sex differences in anxiety
Acknowledgments
We would like to thank George McClung, Mina Youssef, Gerald Van Buskirk, Hamidou Keita, Dominique Losen, Nina Duncan, and Joy Bergmann for their assistance with the immunohistochemistry, as well as Adam Hawkins and Michelle Lerner for their assistance with the behavior. Additionally, we would like to thank Dr. Christina Dalla and Dimitris Baltas for developing Kinoscope behavior analysis program that was used to assist in behavioral scoring.
Role of funding sources
This work was supported by the National Institutes of Health grant NIMH 092438 and NSF IOS-1552416 to D.A.B. The funding source had no involvement in study design, the collection, analysis and interpretation of data, the writing of the report, or the decision to submit the article for publication.
Footnotes
Contributions
K.R.W., B.W., and D.A.B. designed the studies, oversaw data collection, and drafted sections of the manuscript. K.R.W., B.W., H.S., S.C., S.K., and N.B. collected and analyzed data. D.E.W. consulted on the statistical procedures and helped perform the circuitry analysis. All authors contributed to and have approved the final manuscript
Conflict of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akama KT, Thompson LI, Milner TA, McEwen BS. Post-synaptic density-95 (PSD-95) binding capacity of G-protein-coupled receptor 30 (GPR30), an estrogen receptor that can be identified in hippocampal dendritic spines. J Biol Chem. 2013;288:6438–6450. doi: 10.1074/jbc.M112.412478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Cannell E, Bertram K, Filardo E, Milner TA, Brake WG. Medial Prefrontal Cortical Estradiol Rapidly Alters Memory System Bias in Female Rats: Ultrastructural Analysis Reveals Membrane-Associated Estrogen Receptors as Potential Mediators. Endocrinology. 2014;155:4422–4432. doi: 10.1210/en.2014-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold FJL, de Lucas Bueno M, Shiers H, Hancock DC, Evan GI, Herbert J. Expression of c-fos in regions of the basal limbic forebrain following intra-cerebroventricular corticotropin-releasing factor in unstressed or stressed male rats. Neuroscience. 1992;51:377–390. doi: 10.1016/0306-4522(92)90322-s. [DOI] [PubMed] [Google Scholar]
- Bangasser D. To freeze or not to freeze. eLife. 2015:4. doi: 10.7554/eLife.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15:877, 896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Lee CS, Cook PA, Gee JC, Bhatnagar S, Valentino RJ. Manganese-enhanced magnetic resonance imaging (MEMRI) reveals brain circuitry involved in responding to an acute novel stress in rats with a history of repeated social stress. Physiol Behav. 2013a;122:228–236. doi: 10.1016/j.physbeh.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Reyes BA, Piel D, Garachh V, Zhang XY, Plona ZM, Van Bockstaele EJ, Beck SG, Valentino RJ. Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Mol Psychiatry. 2013b;18:166–173. doi: 10.1038/mp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borelli KG, Brandão ML. Effects of ovine CRF injections into the dorsomedial, dorsolateral and lateral columns of the periaqueductal gray: A functional role for the dorsomedial column. Hormones and behavior. 2008;53:40–50. doi: 10.1016/j.yhbeh.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse. 2009;10:198–210. doi: 10.1177/1524838009334448. [DOI] [PubMed] [Google Scholar]
- Britton DR, Koob GF, Rivier J, Vale W. Intraventricular corticotropin-releasing factor enhances behavioral effects of novelty. Life Sci. 1982;31:363–367. doi: 10.1016/0024-3205(82)90416-7. [DOI] [PubMed] [Google Scholar]
- Calhoun JB. The ecology and sociology of the Norway rat. Bethesda, Md: U.S. Dept. of Health, Education, and Welfare, Public Health Service; 1963. [Google Scholar]
- Cole RD, Kawasumi Y, Parikh V, Bangasser DA. Corticotropin releasing factor impairs sustained attention in male and female rats. Behav Brain Res. 2016;296:30–34. doi: 10.1016/j.bbr.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford LK, Craige CP, Beck SG. Increased intrinsic excitability of lateral wing serotonin neurons of the dorsal raphe: a mechanism for selective activation in stress circuits. J Neurophysiol. 2010;103:2652–2663. doi: 10.1152/jn.01132.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31:544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Slangen JL, Van der Gugten J. Plasma catecholamine and corticosterone levels during active and passive shock-prod avoidance behavior in rats: Effects of chlordiazepoxide. Physiol Behav. 1990;47:1089–1098. doi: 10.1016/0031-9384(90)90357-a. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Minireview: Sex Differences in Adult and Developing Brains: Compensation, Compensation, Compensation. Endocrinology. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Hembacher E, Phillips KA. The mouse who couldn't stop washing: pathologic grooming in animals and humans. CNS spectrums. 2009;14:503–513. doi: 10.1017/s1092852900023567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab. 2002;13:436–444. doi: 10.1016/s1043-2760(02)00670-7. [DOI] [PubMed] [Google Scholar]
- Gray JA, Lalljee B. Sex differences in emotional behaviour in the rat: Correlation between open-field defecation and active avoidance. Animal Behaviour. 1974;22(Part 4):856–861. doi: 10.1016/0003-3472(74)90008-6. [DOI] [PubMed] [Google Scholar]
- Gupta MA, Gupta AK, Knapp K. Trichotillomania: Demographic and Clinical Features From a Nationally Representative US Sample. Skinmed. 2015;13:455–460. [PubMed] [Google Scholar]
- Handley SL, Singh L. Neurotransmitters and shaking behaviour—more than a ‘gut-bath’for the brain? Trends in pharmacological sciences. 1986;7:324–328. [Google Scholar]
- Hawkins RA, Freedman B, Marshall A, Killen E. Oestradiol-17 beta and prolactin levels in rat peripheral plasma. British journal of cancer. 1975;32:179–185. doi: 10.1038/bjc.1975.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJAG, van Wingen GA, Joëls M, Fernández G. Time-Dependent Effects of Corticosteroids on Human Amygdala Processing. The Journal of Neuroscience. 2010;30:12725–12732. doi: 10.1523/JNEUROSCI.3112-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disorders. 2001;62:77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- Homberg JR, van den Akker M, Raaso HS, Wardeh G, Binnekade R, Schoffelmeer AN, de Vries TJ. Enhanced motivation to self-administer cocaine is predicted by self-grooming behaviour and relates to dopamine release in the rat medial prefrontal cortex and amygdala. Eur J Neurosci. 2002;15:1542–1550. doi: 10.1046/j.1460-9568.2002.01976.x. [DOI] [PubMed] [Google Scholar]
- Howard O, Carr GV, Hill TE, Valentino RJ, Lucki I. Differential blockade of CRF-evoked behaviors by depletion of norepinephrine and serotonin in rats. Psychopharmacology (Berl) 2008;199:569–582. doi: 10.1007/s00213-008-1179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howerton AR, Roland AV, Fluharty JM, Marshall A, Chen A, Daniels D, Beck SG, Bale TL. Sex differences in corticotropin-releasing factor receptor-1 action within the dorsal raphe nucleus in stress responsivity. Biol Psychiatry. 2014;75:873–883. doi: 10.1016/j.biopsych.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34:226–237. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Imaki T, Shibasaki T, Hotta M, Demura H. Intracerebroventricular administration of corticotropin-releasing factor induces c-fos mRNA expression in brain regions related to stress responses: comparison with pattern of c-fos mRNA induction after stress. Brain Res. 1993;616:114–125. doi: 10.1016/0006-8993(93)90199-w. [DOI] [PubMed] [Google Scholar]
- Jinks AL, McGregor IS. Modulation of anxiety-related behaviours following lesions of the prelimbic or infralimbic cortex in the rat. Brain Res. 1997;772:181–190. doi: 10.1016/s0006-8993(97)00810-x. [DOI] [PubMed] [Google Scholar]
- Kalandakanond-Thongsong S, Daendee S, Srikiatkhachorn A. Effect of the acute and chronic estrogen on anxiety in the elevated T-maze. Physiol Behav. 2012;105:357–363. doi: 10.1016/j.physbeh.2011.08.034. [DOI] [PubMed] [Google Scholar]
- Karteris E, Markovic D, Chen J, Hillhouse EW, Grammatopoulos DK. Identification of a novel corticotropin-releasing hormone type 1beta-like receptor variant lacking Exon 13 in human pregnant myometrium regulated by estradiol-17beta and progesterone. Endocrinology. 2010;151:4959–4968. doi: 10.1210/en.2010-0622. [DOI] [PubMed] [Google Scholar]
- Kato J, Hirata S, Nozawa A, Yamada-Mouri N. Gene Expression of Progesterone Receptor Isoforms in the Rat Brain. Horm Behav. 1994;28:454–463. doi: 10.1006/hbeh.1994.1043. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neuroscience and biobehavioral reviews. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International journal of methods in psychiatric research. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, Lo M, Pak S, Mattis J, Lim BK, Malenka RC, Warden MR, Neve R, Tye KM, Deisseroth K. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496:219–223. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokras N, Antoniou K, Mikail HG, Kafetzopoulos V, Papadopoulou-Daifoti Z, Dalla C. Forced swim test: what about females? Neuropharm. 2015;99:408–421. doi: 10.1016/j.neuropharm.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Kokras N, Dalla C. Sex differences in animal models of psychiatric disorders. Br J Pharmacol. 2014;171:4595–4619. doi: 10.1111/bph.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte SM, Korte-Bouws GA, Bohus B, Koob GF. Effect of corticotropin-releasing factor antagonist on behavioral and neuroendocrine responses during exposure to defensive burying paradigm in rats. Physiol Behav. 1994;56:115–120. doi: 10.1016/0031-9384(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Lammers JHCM, Meelis W, Kruk MR, van der Poel AM. Hypothalamic substrates for brain stimulation-induced grooming, digging and circling in the rat. Brain Research. 1987;418:1–19. doi: 10.1016/0006-8993(87)90956-5. [DOI] [PubMed] [Google Scholar]
- Maras PM, Molet J, Chen Y, Rice C, Ji SG, Solodkin A, Baram TZ. Preferential loss of dorsal-hippocampus synapses underlies memory impairments provoked by short, multimodal stress. Mol Psychiatry. 2014;19:811–822. doi: 10.1038/mp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Lighthall NR, Nga L, Gorlick MA. Sex differences in how stress affects brain activity during face viewing. Neuroreport. 2010;21:933–937. doi: 10.1097/WNR.0b013e32833ddd92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis MA, Alvarenga P, Funaro G, Torresan RC, Moraes I, Torres AR, Zilberman ML, Hounie AG. Gender differences in obsessive-compulsive disorder: a literature review. Rev Bras Psiquiatr. 2011;33:390–399. doi: 10.1590/s1516-44462011000400014. [DOI] [PubMed] [Google Scholar]
- Moore CL. A hormonal basis for sex differences in the self-grooming of rats. Horm Behav. 1986;20:155–165. doi: 10.1016/0018-506x(86)90014-0. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin–releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Owings DH, Coss RG. Snake Mobbing by California Ground Squirrels: Adaptive Variation and Ontogeny. Behaviour. 1977;62:50–69. [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in Effects of Lesions of the Nucleus Accumbens Core and Shell on Appetitive Pavlovian Approach Behavior and the Potentiation of Conditioned Reinforcement and Locomotor Activity byd-Amphetamine. The Journal of Neuroscience. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Academic Press/Elsevier; Amsterdam ; Boston: 2007. [Google Scholar]
- Phillips KA, Menard W, Quinn E, Didie ER, Stout RL. A 4-year prospective observational follow-up study of course and predictors of course in body dysmorphic disorder. Psychological Medicine. 2013;43:1109–1117. doi: 10.1017/S0033291712001730. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Fear and Feeding in the Nucleus Accumbens Shell: Rostrocaudal Segregation of GABA-Elicited Defensive Behavior Versus Eating Behavior. The Journal of Neuroscience. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM. Sex differences in PTSD resilience and susceptibility: Challenges for animal models of fear learning. Neurobiology of stress. 2015;1:60–65. doi: 10.1016/j.ynstr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable Roles of Prelimbic and Infralimbic Cortices, Ventral Hippocampus, and Basolateral Amygdala in the Expression and Extinction of Conditioned Fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder K, Wang WW, Han R, McFadden K, Valentino RJ. Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology. 2012;37:520–530. doi: 10.1038/npp.2011.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruijt BM, van Hooff JA, Gispen WH. Ethology and neurobiology of grooming behavior. Physiological reviews. 1992;72:825–852. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Waters EM, Mermelstein PG, Kramár EA, Shors TJ, Liu F. Rapid Estrogen Signaling in the Brain: Implications for the Fine-Tuning of Neuronal Circuitry. The Journal of Neuroscience. 2011;31:16056–16063. doi: 10.1523/JNEUROSCI.4097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Takao K, Nagatani T, Kitamura Y, Kawasaki K, Hayakawa H, Yamawaki S. Chronic forced swim stress of rats increases frontal cortical 5-HT2 receptors and the wet-dog shakes they mediate, but not frontal cortical beta-adrenoceptors. Eur J Pharmacol. 1995;294:721–726. doi: 10.1016/0014-2999(95)00620-6. [DOI] [PubMed] [Google Scholar]
- Toth M, Flandreau EI, Deslauriers J, Geyer MA, Mansuy IM, Merlo Pich E, Risbrough VB. Overexpression of Forebrain CRH During Early Life Increases Trauma Susceptibility in Adulthood. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M, Gresack JE, Bangasser DA, Plona Z, Valentino RJ, Flandreau EI, Mansuy IM, Merlo-Pich E, Geyer MA, Risbrough VB. Forebrain-Specific CRF Overproduction During Development is Sufficient to Induce Enduring Anxiety and Startle Abnormalities in Adult Mice. Neuropsychopharmacology. 2014;39:1409–1419. doi: 10.1038/npp.2013.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamvakopoulos NC, Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. J Clin Invest. 1993;92:1896–1902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Veer IM, Oei NY, Spinhoven P, van Buchem MA, Elzinga BM, Rombouts SA. Beyond acute social stress: increased functional connectivity between amygdala and cortical midline structures. Neuroimage. 2011;57:1534–1541. doi: 10.1016/j.neuroimage.2011.05.074. [DOI] [PubMed] [Google Scholar]
- Veldhuis HD, De Wied D. Differential behavioral actions of corticotropin-releasing factor (CRF) Pharmacol Biochem Behav. 1984;21:707–713. doi: 10.1016/s0091-3057(84)80007-6. [DOI] [PubMed] [Google Scholar]
- Vogel S, Klumpers F, Krugers HJ, Fang Z, Oplaat KT, Oitzl MS, Joels M, Fernandez G. Blocking the mineralocorticoid receptor in humans prevents the stress-induced enhancement of centromedial amygdala connectivity with the dorsal striatum. Neuropsychopharmacology. 2015;40:947–956. doi: 10.1038/npp.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathington JM, Cooke BM. Corticotropin-Releasing Factor Receptor Binding in the Amygdala Changes Across Puberty in a Sex-Specific Manner. Endocrinology. 2012;153:5701–5705. doi: 10.1210/en.2012-1815. [DOI] [PubMed] [Google Scholar]
- Weathington JM, Hamki A, Cooke BM. Sex- and region-specific pubertal maturation of the corticotropin-releasing factor receptor system in the rat. J Comp Neurol. 2014;522:1284–1298. doi: 10.1002/cne.23475. [DOI] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, Dudek SM, Weinberg RJ, Calakos N, Wetsel WC, Feng G. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Akama KT, Knudsen MG, McEwen BS, Milner TA. Ovarian hormones influence corticotropin releasing factor receptor colocalization with delta opioid receptors in CA1 pyramidal cell dendrites. Experimental neurology. 2011;230:186–196. doi: 10.1016/j.expneurol.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodlee MT, Kane JR, Chang J, Cormack LK, Schallert T. Enhanced function in the good forelimb of hemi-parkinson rats: compensatory adaptation for contralateral postural instability? Exp Neurol. 2008;211:511–517. doi: 10.1016/j.expneurol.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.