Abstract
Arcuate neurons that coexpress kisspeptin (Kiss1), neurokinin B (Tac2), and dynorphin (Pdyn) mediate negative feedback of 17β-estradiol (E2) on the HPG axis. Previous studies report that fasting and caloric restriction reduce Kiss1 expression. The objective of this study was to determine the interactions of E2 with fasting, caloric restriction, and diet-induced obesity on KNDy gene and receptor expression. Ovariectomized female mice were separated into control and estradiol benzoate (E2B)-treated groups. E2B decreased Kiss1 and the tachykinin 2 receptor, Tac3r, in ARC tissue and Tac2 in Tac2 neurons. Diet-induced obesity decreased Kiss1 in oil-treated animals and the kisspeptin receptor, Kiss1r and Tac3r in the ARC of E2B-treated animals. Chronic caloric (30%) restriction reduced all three neuropeptides in oil-treated females and Kiss1r by E2B in CR animals. Taken together, our experiments suggest that steroidal environment and energy state negatively regulate KNDy gene expression in both ARC and Tac2 neurons.
Keywords: caloric restriction, diet-induced obesity, arcuate nucleus, KNDy neurons, ghrelin
1. Introduction
The growing obesity epidemic in the United States suggests a 70% increase in obesity within the past decade (Andreyeva et al., 2008). While obesity has been linked to decreased life expectancy, coronary heart disease, and type 2 diabetes mellitus (T2DM) (Fontaine et al., 2003), it also impacts reproduction. In women, obesity leads to irregular menses, infertility, and miscarriages, among other complications (Moran et al., 2011, Norman and Clark, 1998). These problems in reproductive parameters extend to individuals suffering from undernutrition. Amenorrhea is prominent in athletes and anorexia nervosa patients, largely due to their decreased body mass index (Jacobs, 1982, Sundgot-Borgen, 1994). These perturbations in energy balance can lead to problems in the reproductive hypothalamic pituitary gonadal (HPG) axis, though the mechanisms remain unclear.
Energy balance and reproduction are centrally regulated processes that are controlled, in part, by neurons in the arcuate nucleus (ARC). Regulation of reproduction in the ARC is controlled by negative feedback mechanisms of 17β-estradiol (E2) on the HPG axis mediated, in part, by neurons expressing the neuropeptide, kisspeptin. There are two main groups of kisspeptin neurons located in the rodent brain (Brock and Bakker, 2013). Kisspeptin in the anteroventral periventricular nucleus (AVPV) regulates the surge of luteinizing hormone (LH) leading to ovulation in females (Brock and Bakker, 2013). Kisspeptin is also expressed in the ARC in KNDy neurons that co-express kisspeptin (Kiss1), neurokinin B (Tac2), and dynorphin (Dyn). These neurons contribute to negative feedback of E2 on the HPG axis (Navarro and Tena-Sempere, 2011, Oakley et al., 2009). Both AVPV and ARC kisspeptin regulate the HPG axis by binding to its receptor, G protein-coupled receptor 54 (GPR54), or Kiss1r, expressed on gonadotropin releasing hormone (GnRH) neurons (Bosch et al., 2013, Oakley et al., 2009). In ARC KNDy neurons, the two neuropeptides neurokinin B and dynorphin act as positive and negative autoregulators of KNDy neuronal excitability, respectively (de Croft et al., 2013, Ruka et al., 2016, Uenoyama et al., 2014, Weems et al., 2016). Together, the three KNDy neuropeptides act as a pulse generator for GnRH release (Goodman et al., 2013).
Recent evidence suggests that KNDy neurons are also involved in the regulation of negative and positive energy balance. Ablation of KNDy neurons abrogates the post-ovariectomy weight gain associated with E2 in rats (Mittelman-Smith et al., 2012), suggesting that KNDy neurons mediate, in part, the anorectic effects of E2. In male mice, acute caloric restriction (12, 24, and 48 h fasting) decreases Kiss1 mRNA in the whole hypothalamus, although it is unclear which hypothalamic kisspeptin population is affected (Luque et al., 2007). In female rats, Kiss1 gene expression in the ARC is unchanged in a 48 h fast (Kalamatianos et al., 2008). The inconsistency between these studies is potentially due to differences in species, sexes, hypothalamic tissue, and duration of fasting. Chronic caloric restriction also suppresses ARC Kiss1 as well as Tac2 and Pdyn expression (True et al., 2011). Positive energy balance similarly disrupts ARC kisspeptin. Diet-induced obesity (DIO) reduces hypothalamic kisspeptin expression in mice, though the mechanism is unclear (Quennell et al., 2011). Furthermore, DIO suppresses E2-regulated, hypothalamic gene expression (Balasubramanian et al., 2012). Taken together, it is important to note that most of these studies fail to examine the expression of the KNDy receptors or the receptors involved in gonadal steroid negative feedback, ERα (Esr1) and PR (Pgr). Therefore, this study examines expression of the KNDy neuropeptides and receptors during positive (DIO) and acute (fasting) and chronic (caloric restriction) negative energy balance, with or without E2.
The mechanisms that link reproduction and energy balance are not well understood. One hormone of interest is the stomach-derived hormone ghrelin, which functions to increase feeding behavior and weight gain. Ghrelin is thought to function in the ARC through its cognate receptor, growth hormone secretagogue receptor (GHSR) to stimulate NPY neurons, while simultaneously suppressing POMC neurons through inhibitory γ-aminobutyric acid (GABA)-ergic inputs (Andrews, 2011). Ghrelin’s role in reproduction is the suppression of LH pulse frequency (Forbes et al., 2009). Thus, control of ghrelin/GHSR signaling may influence both reproduction and energy balance. While ghrelin signaling has been characterized in NPY neurons, there are only a few studies examining the role of ghrelin in KNDy neuronal signaling. In one study, Kiss1 mRNA expression in the ARC was unchanged with administration of ghrelin in a fed or fasted state (Forbes et al., 2009). In another, ghrelin depolarized more KNDy neurons in E2-treated females than in oil-treated females, which was due to an increase Ghsr expression in the ARC by E2 (Frazao et al., 2014, Yasrebi et al., 2016).
We hypothesize that negative and positive energy balance disrupt KNDy-associated gene expression in both ARC and Tac2 (KNDy) neurons leading to disruption of the HPG axis. To address this, we examined KNDy neuropeptide and receptor gene expression under the following paradigms: 1) oil vs. estradiol benzoate (E2B); 2) fed vs. 24 h fast; 3) DIO in ovx females; and 4) 30% caloric restriction. These experiments were conducted in wild type (WT) females to examine ARC tissue gene expression. Experiments 1–3 were repeated in Tac2-EGFP animals to examine gene expression change in Tac2 neurons. Our results indicate that both positive and negative energy balance impact KNDy neuropeptides and their receptors with and without E2 replacement.
2. Materials and Methods
2.1 Animal care and experimental design
All animal procedures were completed in compliance with institutional guidelines based on National Institutes of Health standards and were performed with Institutional Animal Care and Use Committee approval at Rutgers University. Adult mice were housed under constant photoperiod conditions (12/12 h light/dark cycle), with lights on/off at 700 h and 1900 h, and maintained at a controlled temperature (25°C). Animals were given food (LabDiet 5V75) and water ad libitum, unless otherwise noted. Animals were weaned at postnatal day 21 (PD21). Two different strains of mice, C57/BL and Swiss Webster (SW), were used for experiments and were purchased from Jackson Laboratory. SW Tac2-EGFP were used for single-cell harvesting experiments. The strain used for each experiment is noted in Table 2 and the design for each of the experiments discussed below is illustrated in Figure 1.
Table 2.
Summary of animal body and uterine weights and serum E2 levels
| Experiment | Strain | Treatment | Body weight (g) at sacrifice | Uterine weight (mg) | Uterine weight (mg)/Body weight (g) | E2 (pg/μl) |
|---|---|---|---|---|---|---|
| 1 | C57/BL | Oil fed | 23.2 ± 0.5 | 30.4 ± 1.8 | 1.3 ± 0.1 | 12.3 ± 1.5 |
| E2B fed | 24.4 ± 0.3 | 114.6 ± 8.0 c | 4.7 ± 0.4 b | 32.8 ± 6.8 a | ||
| Oil fasted | 20.4 ± 1.4 | 39.5 ± 10.9 | 2.1 ± 0.8 | 11.7 ± 1.0 | ||
| E2B fasted | 21.6 ± 0.7 | 134.9 ± 20.1 d | 6.4 ± 1.2 b | 86.5 ± 42.2 a | ||
|
| ||||||
| 2 | C57/BL | LFD oil | 28.2 ± 1.4 | 11.7 ± 1.2 | 0.4 ± 0.0 | 10.0 ± 0.4 |
| LFD E2B | 25.3 ± 0.8 | 108.5 ± 24.6 d | 4.3 ± 1.0 d | 22.6 ± 1.5 a | ||
| HFD oil | 36.6 ± 1.6 B | 14.6 ± 2.1 | 0.4 ± 0.1 | 11.1 ± 1.1 | ||
| HFD E2B | 31.7 ± 2.0 a A | 119.6 ± 11.3 d | 3.9 ± 0.5 d | 25.0 ± 5.9 a | ||
|
| ||||||
| 3 | Swiss Webster | Oil | 41.0 ± 2.2 | 55.7 ± 3.9 | 1.4 ± 0.2 | 6.6 ± 0.7 |
| E2B | 44.0 ± 2.5 | 146.2 ± 11.2 d | 3.3 ± 0.2 d | 36.4 ± 7.9 c | ||
|
| ||||||
| 4 | Swiss Webster | Oil fed | 36.8 ± 2.7 | 81.4 ± 3.9 | 2.2 ± 0.1 | 3.7 ± 0.5 |
| E2B fed | 40.7 ± 3.2 | 105.8 ± 22.7 d | 2.6 ± 0.9 c | 37.8 ± 5.4 d | ||
| Oil fasted | 38.0 ± 3.9 | 76.5 ± 5.9 | 2.0 ± 0.1 | 3.5 ± 0.3 | ||
| E2B fasted | 40.7 ± 4.8 | 119.7 ± 15.1 d | 2.9 ± 0.3 c | 24.4 ± 6.2 b A | ||
|
| ||||||
| 5 | Swiss Webster | LFD oil | 37.5 ± 1.5 | 16.8 ± 1.6 | 0.5 ± 0.0 | 2.3 ± 0.3 |
| LFD E2B | 33.3 ± 1.0 | 177.4 ± 13.6 d | 5.4 ± 0.4 d | 26.3 ± 6.0 a | ||
| HFD oil | 38.4 ± 1.7 | 23.6 ± 3.8 | 0.6 ± 0.1 | 1.8 ± 0.3 | ||
| HFD E2B | 36.4 ± 1.6 | 187.3 ± 17.2 d | 5.2 ± 0.44 d | 18.7 ± 5.6 a | ||
|
| ||||||
| 6 | Swiss Webster | Oil ad lib | 38.2 ± 1.4 | 24.1 ± 1.6 | 0.6 ± 0.0 | 3.9 ± 0.2 |
| E2B ad lib | 40.0 ± 2.1 | 239.8 ± 16.2 d | 6.0 ± 0.3 d | 224.8 ± 41.0 d | ||
| Oil CR | 33.8 ± 0.8 A | 19.2 ± 1.6 | 0.6 ± 0.0 | 4.8 ± 0.4 | ||
| E2B CR | 28.7 ± 1.3 a D | 110.2 ± 11.7 d D | 3.9 ± 0.5 d D | 37.0 ± 19.1 D | ||
Data were analyzed differently in different experiments based on experimental paradigm. See materials and methods for comprehensive statistical method outline. Lowercase letters signify differences between oil- vs. E2B-treated females, within the same treatment (different according to experiment: time of sacrifice, energy balance state, diet). Uppercase letters signify differences within steroid, across treatment. (a/A = p < 0.05; b/B = p < 0.01; c/C = p < 0.001; d/D = p < 0.0001)
Figure 1. Experimental design timelines.
Experimental numbers correspond to Table 2 experimental numbers as well as those written in the text. Abbreviations: ovx, ovariectomy; LFD, low fat diet; HFD, high fat diet; CR, calorie restricted
In Experiment #1, we used a 24 h fast prior to sacrifice to determine if acute negative energy balance regulates ARC KNDy neuropeptide and receptor gene expression. To elucidate the interactions of E2 and fasting on gene expression, adult females were bilaterally ovx under isoflurane anaesthesia using sterile no-touch technique according to the NIH Guidelines for Survival Rodent Surgery. Animals were given a dose of analgesic [4 mg/kg carprofen (Rimadyl®)] one day following surgery for pain management. Animals typically lost 1–2 grams of weight one day after surgery. Following ovx, C57/BL females were separated into 2 treatments – oil and estradiol benzoate (E2B) – and two feeding states – fed and 24 h fasted (n = 5–6, per group). An E2B injection protocol was used that has been shown to alter gene expression in the hypothalamus (Bosch et al., 2013). Animals were injected subcutaneously (s.c.) at 1000 h on post-ovx day 5 with either 0.25 μg of E2B or oil. On post-ovx day 6, a 1.5 μg dose of E2B or oil was injected at 1000 h. Females in the fed groups were allowed to feed ad libitum and females in the fasted group were food restricted 24 h prior to sacrifice at 1000 h on post-ovx day 7. Animals were sedated with ketamine (100 μl of 100 mg/ml stock, intraperitoneal [i.p.]) and rapidly decapitated. Brains were removed and rinsed in ice-cold Sorensen’s Phosphate Buffer (0.2 M sodium phosphate, dibasic and 0.2 M sodium phosphate, monobasic) for 30–60 sec. The basal hypothalamus (BH) was cut using a brain slice matrix (Ted Pella, Inc., Redding, CA, USA) into 1-mm thick coronal rostral and caudal blocks corresponding to Plates 42 to 47 and Plates 48 to 53, respectively, from The Mouse Brain in Stereotaxic Coordinates (Franklin and Paxinos, 2008). The slices were transferred to a 50/50 RNAlater®/Pyrogard water solution and fixed overnight at 4°C. The ARC was found in two slices a nd identified based on The Mouse Brain Stereotaxic Coordinates (Franklin and Paxinos, 2008). ARC nuclei were microdissected using a dissecting microscope, following our previous publications (Bosch et al., 2009, Franklin and Paxinos, 2008, Mamounis et al., 2013, Roepke et al., 2008). The microdissected sections represent the entirety of the ARC tissue. Dissected tissue was stored at −80°C until RNA extraction. Trunk blood was collected at sacrifice to measure plasma E2, LH, and FSH levels. Uteri were removed and wet weight was recorded. Uterine weights (mg) are expressed as wet weight and also as wet weight relative to body weight (g), as indicated in Table 2.
In Experiment #2, we used a diet-induced obesity (DIO) model to determine if chronic positive energy balance regulates ARC gene expression. C57/BL females were fed either a control LFD (10% fat, D12450B, Research Diets, New Brunswick, NJ) or a HFD (45% fat, D12451, Research Diets) diet. Following ovx, females were separated into four groups: 1) LFD oil, 2) LFD E2B, 3) HFD oil or 4) HFD E2B (n = 8–10). Animals were orally dosed with oil or E2B (300 μg/kg body weight) in peanut butter daily at 900 h, starting on the day of ovx. Animals were pair housed and fed either LFD or HFD ad libitum for 8 weeks post-ovx. Body weights and energy intake were measured each week. To determine feeding efficiency, we used the formula: body weight gained/kCal food consumed. Prior to sacrifice, body composition (lean mass and fat mass) was measured twice using an EchoMRI (Houston, TX, USA). Data for fat and lean mass were averaged for each animal. Animals were sacrificed at 1000 h and the ARC was microdissected. Trunk blood was collected to measure plasma E2, LH, and FSH levels. Uteri were removed to determine wet weight.
To determine if Tac2 neurons exhibit similar gene expression changes as whole ARC, we repeated our experiments in Tac2-EGFP mice, which have a Swiss Webster (SW) backbone. In Experiment #3, we wanted to confirm the effects of E2B on KNDy expression in the ARC from SW females. Following ovx, SW wild-type females were separated into a control sesame oil-treated group and an E2B-treated group and were sacrificed on post-ovx day 7 at 1100 h, n = 5–8/treatment. The E2B injection protocol for Experiment #3 is the same as Experiment #1. The ARC was microdissected for gene expression studies. Uteri were collected to determine wet weight. Trunk blood was collected to determine plasma E2 and LH concentrations. In Experiment #4, we repeated Experiment #1 in Tac2-EGFP animals (n=5–6, per treatment) and harvested single Tac2 neurons for single cell PCR co-localization in oil- and E2B-treated, fed females. Pools of 5 Tac2 neurons were also harvested from oil- and E2B-treated, fed and fasted females. Single-cell harvesting of Tac2 neurons and pools is outlined below. In Experiment #5, we repeated Experiment #2 in Tac2-EGFP animals (n=6–8, per treatment) to determine if DIO regulates gene expression in Tac2 neurons.
To determine if chronic negative energy balance regulates ARC KNDy gene expression, we conducted a caloric restriction study (Experiment #6). Wild-type SW females were separated into four groups (n = 7–8, per group): 1) oil ad libitum, 2) oil calorie restricted (CR), 3) E2B ad libitum, and 4) E2B CR. Animals were ovx prior to the experiment (Week 0) and recovered for one week. All animals were fed ad libitum for the first four weeks, while orally dosed with oil or E2B (300 μg/kg body weight) in powdered, fat-free peanut butter (peanut oil removed) daily. The caloric restriction phase followed this ad libitum feeding stage, lasting for 6 weeks. Females were dosed with oil or E2B (continuing the same treatment as Weeks 1–4) at 1700 h to minimize disruption of circadian rhythmicity. The two CR groups (oil and E2B) were fed 70% of the daily average (± 0.02 g) of each of the ad libitum groups within steroid treatment. Body weights and energy intake were measured each week. Body composition was measured using an MRI (EchoMRI) prior to and following (pre- and post-) caloric restriction. Pre-MRI was conducted on Week 4 and post-MRI was conducted on Week 9. Animals were sacrificed at 1000 h and the ARC was microdissected following the procedures described in Experiment #1.
2.2 Drugs
Estradiol benzoate (E2B) was purchased from Sigma-Aldrich and dissolved in ethanol (1mg/ml) prior to mixing in sesame oil (Sigma-Aldrich). Ketamine was purchased from Henry Schein Animal Health (Melville, NY, USA) and was used for sedation prior to sacrifice.
2.3 Blood preparation and hormone assays
Whole trunk blood was subjected to centrifugation (4°C at 1300 rpm for 30 min). The supernatant was subjected to an additional 15 min of centrifugation (4°C at 1300 rpm) and the plasma supernatant was transferred and stored in a cryotube at −80°C until E2 analysis. E2 was analyzed using the Mouse Calbiotech ELISA (ES180S-100) at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for Experiment #3 (Haisenleder et al., 2011). For the remaining experiments, E2 ELISA was run in house using the Mouse Calbiotech ELISA (ES180S-100). The Calbiotech ELISA is specific to detect 17β-estradiol and has a standard range of 3–300 pg/ml and an analytical sensitivity of 3 pg/ml. For Experiment #3, the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core measured LH. For Experiment #1, 2, and 6, LH and FSH levels were measured using a Millipore Luminex MAGPIX plate (#MPTMAG-49K). The assay has a minimum detectable concentration of 9.5 pg/mL (FSH) and 1.9 pg/mL (LH), an intrassay coefficienct of varaiance of <15% (both), and an interassay coefficient of variance of <20% (both).
2.4 Cell harvesting of dispersed Tac2 neurons
In Experiments #4–5, we collected Tac2 neurons to determine cell-type specific changes in gene expression similar to our previous publication (Yasrebi et al., 2016). Briefly, animals were sedated with ketamine (100 μl of 100 mg/ml stock, i.p.) and decapitated. Brains were transferred to a vibratome containing cold, oxygenated aCSF and sliced into 250μM thick basal hypothalamic (BH) slices. BH slices were transferred to an auxiliary chamber (~1 h) containing oxygenated aCSF. The ARC was microdissected and incubated in a protease solution (15 min at 37°C) and washed with low calcium aCSF followed by regular aCSF. The ARC was titurated using flame-polished Glass Pasteur pipettes to disperse cells, which were placed on a glass-bottomed Petri dish (60 mm) and perfused with aCSF for the duration of the experiment (2 ml/min). Tac2 cells were visualized using a Leica DM-IL fluorescent microscope, patched, and harvested by applying low negative pressure to the pipette using the Xenoworks manipulator system (Sutter Instruments, Novato, CA). Positive pressure was used to expel the contents of the pipette into a siliconized microcentrifuge tube containing: 1 μl 5x Superscript III Buffer (Life Technologies), 15 U Rnasin (Promega), 0.5 μl 100 mM DTT, and DEPC-treated water in 8 μl total volume. Tac2 neurons were harvested both individually as single cells or collected into 10–15 pools of 5 Tac2 neurons from each animal.
Harvested single cells and pools were reverse transcribed as previously described (Bosch et al., 2013, Roepke et al., 2011). In brief, tubes of harvested cells and a positive control (25 ng of total hypothalamic RNA in 1 μl) were denatured for 5 min at 65°C and cooled on ice for 5 min. Reverse transcription was conducted by adding 50 U Superscript III RT, 3 μl 5x Superscript Buffer, 5 mM MgCl2, 0.625 mM dNTPs (Clontech), 15 U Rnasin, 400 ng anchored oligo(d)T (Life Technologies), 100 ng random hexamers (Promega), 10 mM DTT in DEPC-water in a total volume of 25 μl. One single cell and one tissue RNA tube were used as negative controls, processed without RT. aCSF was collected every 2–3 pools or 10 single cells to analyze for contamination. Reverse transcription protocol is as follows: 5 min at 25°C, 60 min at 50°C, 15 min at 70°C.
For single cell colocalization experiment, Tac2 neurons were analyzed using standard PCR protocols and gel electrophoresis as previously described (Roepke et al., 2011, Xu et al., 2008). Primers for single cell PCR are those used with ARC tissue qPCR (Table 1), with the exception of Tac2: F: 5′-TCTGGAAGGATTGCTGAAAGTG-3′; R: 5′-GTAGGGAAGGGAGCCAACAG-3′. Each reaction was amplified for 50 cycles using a C1000 Thermal Cycle (Bio-Rad, Hercules, CA) at an annealing temperature of 60°C. Negative (cell and tissue samples without RT), aCSF, and positive tissue controls were analyzed with each PCR run.
Table 1.
Primer sequences for qPCR and single cell PCR
| Gene Name | Product length | % Eff | Primer sequence | Base pair # | Accession # |
|---|---|---|---|---|---|
| Kiss1 | 154 | 91 | F: TGATCTCAATGGCTTCTTGGCAGC R: CTCTCTGCATACCGCGATTCCTTT |
40–63 170–193 |
NM_178260 |
| Kiss1r | 138 | 100 | F: CCTTCACCGCACTCCTCTAC R: CATACCAGCGGTCCACACTC |
1993–2012 2111–2130 |
NM_03244 |
| Pdyn | 133 | 105 | F: AGCTTGCCTCCTCGTGATG R: GGCACTCCAGGGAGCAAAT |
335–353 441–459 |
NM_018863 |
| Pgr | 191 | 104 | F: TGAAAGAGCGTCATTCTTAC R: CAATTCGCGGATATAGCTTG |
2980–2999 3151–3170 |
NM_008829 |
| Tac2 | 220 | 103 | F: CGTGACATGCACGACTTC R: CCAACAGGAGGACCTTAC |
505–522 707–724 |
NM_001199971 |
| Tac3r | 124 | 99 | F: TACACCATCGTTGGAATTAC R: ATGTCACCACCACAATAATC |
1026–1045 1130–1149 |
NM_021382 |
| ERα | 107 | 96 | F: GCGCAAGTGTTACGAAGTG R: TTCGGCCTTCCAAGTCATC |
919–937 1007–1025 |
NM_007956 |
| β-actin | 63 | 100 | F: GCCCTGAGGCTCTTTTCCA R: TAGTTTCATGGATGCCACAGGA |
849–867 890–911 |
NM_007393 |
| Hprt | 85 | 117 | F: GCAGTACAGCCCCAAAATGG R: AACAAAGTCTGGCCTGTATCCA |
599–618 662–683 |
NM_013556 |
| Gapdh | 98 | 93 | F:TGACGTGCCGCCTGGAGAAA R:AGTGTAGCCCAAGATGCCCTTCAG |
778–797 852–875 |
NM_008084.2 |
| Kor | 237 | 110 | F: TCCTTGGAGGCACCAAAGTCAGGG R: TGGTGATGCGGCGGAGATTTCG |
799–822 1014–1035 |
NM_001204371 |
| Ghsr | 122 | 111 | F: CAGGGACCAGAACCACAAAC R: AGCCAGGCTCGAAAGACT |
1003–1022 1107–1124 |
NM_177330 |
| Ucp2 | 194 | 105 | F: CATTGGCCTCTACGACTC R: CGACAGTGCTCTGGTATC |
668–685 844–861 |
NM_011671 |
| Cpt1c | 191 | 96 | F: GGCTGGCATTGGTCAGAATC R: CGTGCAACCTCAGGAAGTC |
719–738 892–910 |
NM_153679.2 |
Forward primer (F) is listed first with the reverse primer (R) second. Kiss1, kisspeptin; Kiss1r, kisspeptin receptor; Pdyn, prodynorphin; Pgr, progesterone receptor; Tac2, tachykinin 2; Tac3r, tachykinin 3 receptor; ERα, estrogen receptor alpha; β-actin, beta-actin; Hprt, hypoxanthine-guanine phosphoribosyltransferase; Gapdh, glyceraldehyde-3-phosphate dehydrogenase; Kor, kappa opioid receptor; Ghsr, growth hormone secretagogue receptor; Ucp2, uncoupling protein 2; Cpt1c, carnitine palmitoyltransferase 1c.
2.5 RNA extraction of ARC tissue
RNA was extracted from ARC using Ambion RNAqueous® Micro Kits (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol, followed by DNase-I treatment to remove contamination by genomic DNA (Life Technologies). RNA samples were run on a NanoDrop™ ND-2000 spectrophotometer (ThermoFisher, Inc., Waltham, MA, USA) to assess quantity, followed by an Agilent 2100 Bioanalyzer analysis using the RNA 6000 Nano Kit (Agilent Technologies, Inc., Santa Clara, CA, USA) to assess quality. Samples with a RNA integrity number (RIN) > 8 were used for quantitative real-time PCR (qPCR).
2.6 Quantitative real-time PCR
For ARC, cDNA was synthesized from 200 ng of total RNA, following our previous publication (Yasrebi et al., 2016). A 1:20 dilution of the cDNA was produced using nuclease-free water (Gene Mate) for a final cDNA concentration of 0.5 ng/μl and stored at −20°C. Primers for qPCR were designed to span exon-exon junctions and were synthesized by Life Technologies using Clone Manager 5 software (Sci Ed Software, Cary, NC, USA). We used 4 μl of cDNA (equivalent to 2 ng of total RNA) amplified with SsoAdvanced™ SYBR Green (BioRad, Hercules, CA, USA) on CFX-Connect Real-time PCR Instrument (BioRad). A standard curve was generated for each primer pair using serial dilutions of BH cDNA in triplicate. Efficiencies were calculated as a percent efficiency and are approximately equal (90%–110% or one doubling per cycle). Amplification protocol for genes was as follows: initial denaturing 95°C for 3 min followed by 40 cycles of amplification at 94°C for 10 sec (denaturing), 60°C for 45 sec (annealing), and completed with a dissociation step for melting point analysis with 60 cycles of 95°C for 10 sec, 65°C to 95°C (in increments of 0.5 °C) for 5 sec and 95°C for 5 sec. The reference genes used were Gapdh, Hprt, and Actb. Positive, negative and water blank controls were included in the qPCR plate design. See Table 1 for a list of all primers used for qPCR.
Analysis of qPCR was done using the comparative Cq method using a 1:20 diluted BH cDNA (equivalent to 2 ng of RNA) sample from a male as the calibrator (Livak and Schmittgen, 2001, Pfaffl, 2001). All values were normalized and are expressed as relative mRNA expression. In all plates, we maintained a consistent threshold level, set at the lowest but steepest slope of the exponential curve. We calculated the linear quantity of target genes using the formula 2−ΔΔCq. The n-fold difference was used for statistical analysis.
2.7 Data analysis
All data are expressed as mean ± SEM. All data were analyzed using GraphPad® Prism software (GraphPad Software, La Jolla, CA, USA) except when multi-factorial and repeated measures ANOVA were conducted using Statistica (Dell Statistica, Tulsa, OK, USA; Fig. 3C, 5C, 5E). In all experiments, a p < 0.05 was considered to be significant. In Experiments #1–2 and 4–6, data were analyzed with a two-way ANOVA followed by Newman-Keuls comparison within steroid, across diet, and within diet, across steroid. In Experiment #3, data were analyzed with a t-test comparing oil and E2B samples within each gene (Fig. 4A, 4B). Data for cumulative weight gain and weekly energy intake were analyzed using a repeated measures, multi-factorial ANOVA (time x diet x steroid) over time, followed by Newman-Keuls’s multiple comparison tests.
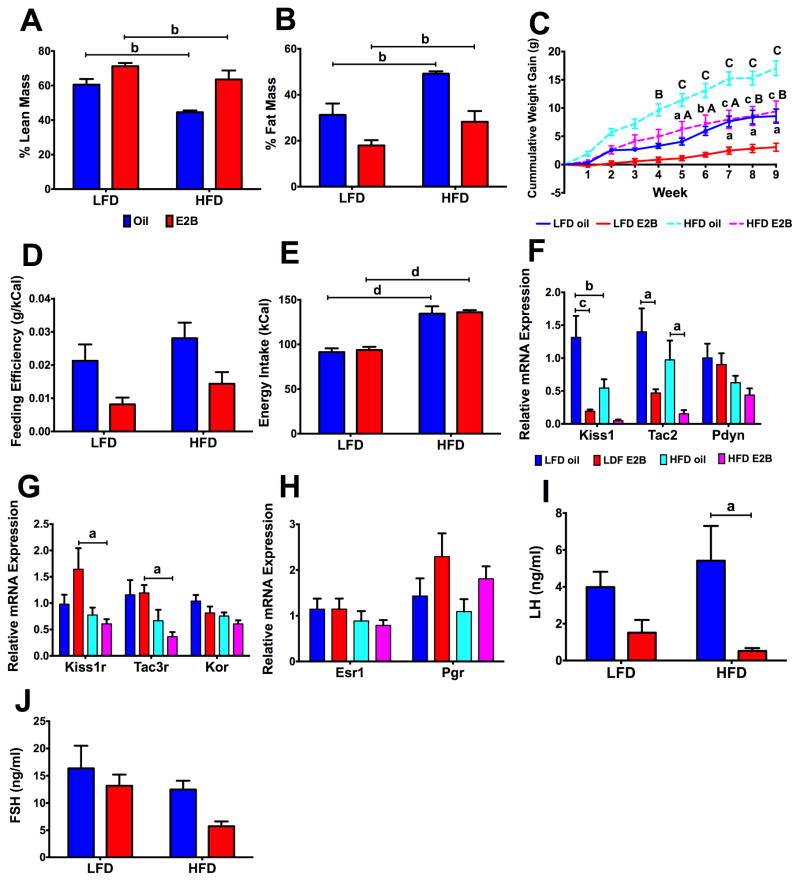
Figure 3. Diet-induced obesity disrupts KNDy-associated gene expression in the ARC (Experiment #2).
(A) Total lean mass percentage. (B) Total fat mass percentage. (C) Cumulative body weight gain. (D) Feeding efficiency. (E) Weekly energy intake (kCal). (F) KNDy genes: Kiss1, Tac2, and Pdyn; (G) KNDy-associated receptors: Kiss1r, Tac3r, and Kor; and (H) Steroid hormone receptors: Esr1 and Pgr. Gene expression data were normalized to ND-oil controls. (I) Plasma LH and (J) FSH levels. For all data, n = 8 – 10. (A–B, D–J) data were analyzed using a two-way ANOVA. (C) Data were analyzed by a repeated-measures, multifactorial ANOVA. All post hoc comparisons were Newman-Keuls multiple comparison tests. For (A–B), (D–J), a = p < 0.05; b = p < 0.01; c = p < 0.001; d = p < 0.0001). For (C), lowercase letters denote differences between steroid, within diet and uppercase letters denote differences between diet, within steroid (a/A = p < 0.05; b/B = p < 0.01; c/C= p < 0.001; d/D = p < 0.0001).
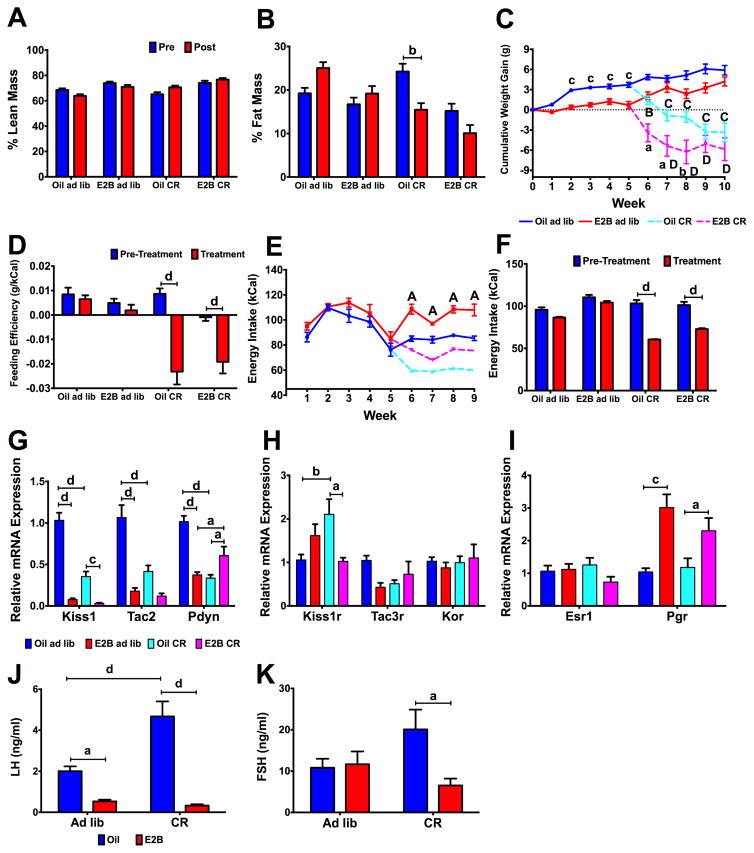
Figure 5. Caloric restriction disrupts KNDy-associated ARC gene expression (Experiment #6).
(A) Total lean mass percentage. (B) Total fat mass percentage. (C) Cumulative body weight gain (g). At week 5, animals were either fed ad libitum or put on a caloric restriction diet. (D) Weekly feeding efficiency pre- and post-diet treatment. (E) Weekly energy intake (kCal). (F) Overall average energy intake (kCal) pre- and post-diet treatment. (G) KNDy genes: Kiss1, Tac2, and Pdyn; (H) KNDy-associated receptors: Kiss1r, Tac3r, and Kor; and (I) Steroid hormone receptors: Esr1 and Pgr. Expression was normalized to oil-ad lib samples. (J) Plasma LH and (K) FSH levels. For all data, n = 7 – 8. (A–B, D, F) Data were analyzed by a repeated-measures, multifactorial ANOVA. (C, E) Data were analyzed by a repeated-measures, two-way ANOVA. (G–K) Data were analyzed using a two-way ANOVA. All post hoc comparisons were Newman-Keuls multiple comparison tests. For (A–B), (D), (F–K): a = p < 0.05; b = p < 0.01; c = p < 0.001; d = p < 0.0001. For (C) and (E), lowercase letters signify differences across steroid, within diet and uppercase letters represent differences across diet, within steroid (a/A = p < 0.05; b/B = p < 0.01; c/C = p < 0.001; d/D = p < 0.0001).
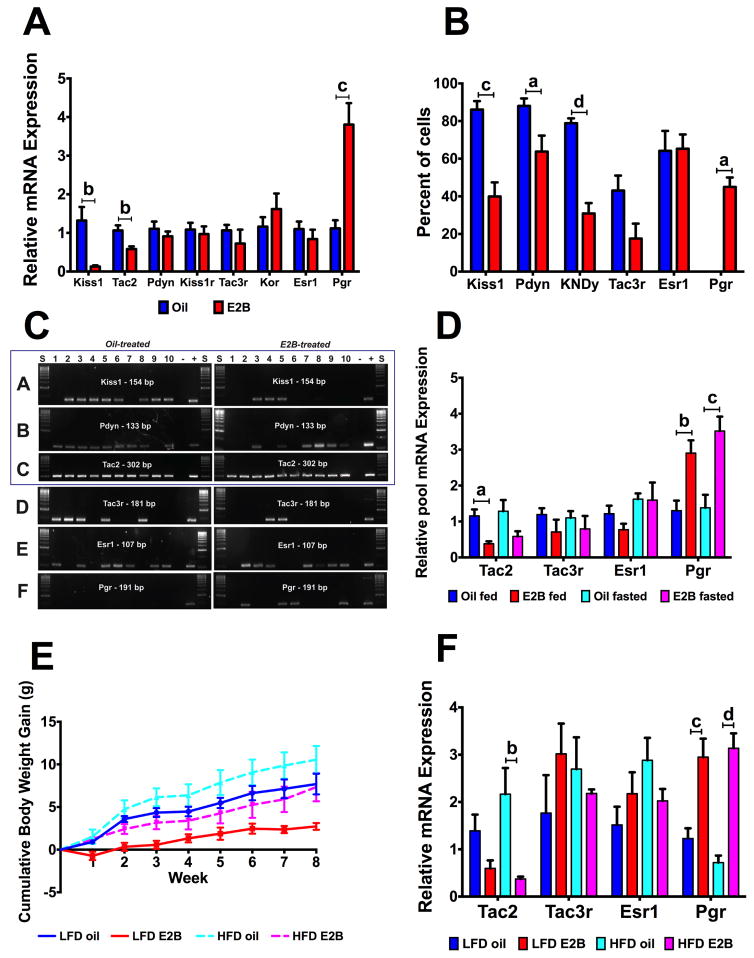
Figure 4. Differential gene expression in Tac2 neurons by E2B, fasting, and diet-induced obesity (Experiments #3–5).
Fig. 4A–C: Experiment #3; Fig. 4D: Experiment #4; Fig. 4E–F: Experiment #5.
(A) ARC KNDy neuropeptide and receptor gene expression in Swiss Webster females (n = 5 – 8). Data were normalized to oil controls, within each gene. Data were analyzed with Student’s t-test for each gene. (B) Percent of Tac2 cells coexpressing KNDy neuropeptides and receptors. Data were analyzed using a Student’s t-test, within each gene. (C) Representative gel of single-cell PCR amplification products. Panels A–C are the KNDy neuropeptides and gels represent colocalization patterns in the same 10 Tac2 neurons. Panels D–F are the receptors we analyzed for single cell PCR and represent expression patterns within gene and do not show colocalization patterns across the same 10 Tac2 neurons. (D) Gene expression in Tac2 neurons of fed-fasted females (n = 5 – 6). Data were normalized to oil fed controls, within each gene. Data were analyzed using a two-way ANOVA (fasting x steroid) followed by post-hoc Newman-Keuls analysis. (E) Cumulative body weight gain in Swiss Webster females following either ad lib LFD or HFD, orally dosed with E2B (n = 6 – 8). Data were analyzed by a repeated measures multifactorial ANOVA followed by post-hoc Newman-Keuls. (F) Gene expression in Tac2 neurons. (n = 6 – 8) Data were normalized to LFD oil controls, within each gene and analyzed using a two-way ANOVA (diet x steroid), followed by Newman-Keuls multiple comparison test. For all graphs, a = p < 0.05; b = p < 0.01; c = p < 0.001; d = p < 0.0001.
3. Results
3.1 Effects of a 24-hour fast on ARC KNDy gene expression and circulating reproductive hormones (Experiment #1)
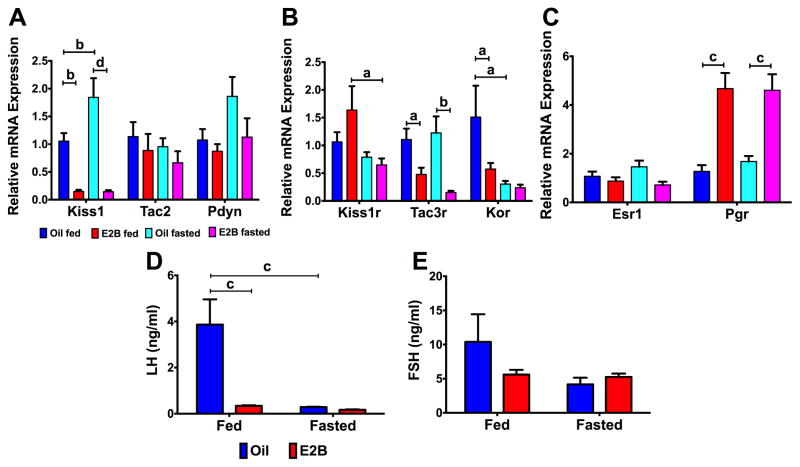
Acute caloric restriction (a 24 h fast) regulated ARC KNDy gene expression. For Kiss1 expression, there was a steroid (Fig. 2A; F(1, 20) = 48.10, p < 0.0001) and fasting*steroid (F(1, 20) = 4.442, p < 0.05) effect. E2B suppressed Kiss1 in both fed (p < 0.01) and fasted (p < 0.0001) females and fasting increased Kiss1 in oil-treated animals (p < 0.01). For the receptors, there was a steroid effect on Kiss1r expression (Fig. 2B; F(1, 20) = 6.749, p < 0.05), and a fasting effect on Kor (Fig. 2B; F(1, 20) = 7.053, p < 0.05). Both Kiss1r (p < 0.05) and Kor (p < 0.05) were reduced by fasting in E2B (Kiss1r) or oil (Kor)-treated females and Kor was decreased by E2B only in fed-animals (p < 0.05; Fig. 2B). Tac3r was reduced by E2B in fed (p < 0.05) and fasted (p < 0.01) animals (Fig. 2B; steroid: F(1, 20) = 20.22, p < 0.001). Esr1 expression did not change by steroid or fasting, but there was a steroid effect on Pgr expression (Fig. 2C; F(1, 20) = 41.69, p < 0.0001), which was augmented by E2B treatment in both fed (p < 0.001) and fasted (p < 0.001) females. For gonadotropins, there were effects of diet, (Fig. 2D; F(1, 17) = 12.96, p < 0.01), steroid (F(1, 17) = 12.26, p < 0.01), and diet*steroid (F(1, 17) = 10.70, p < 0.01) on plasma LH levels. In fed animals, LH levels were significantly decreased by E2B (p < 0.001). In addition, fasting decreased LH levels in oil-treated animals (p < 0.001). Plasma FSH levels were not changed by steroid or diet treatment (Fig. 2E).
Figure 2. Fasting (24 h) regulates KNDy-associated gene expression in the ARC (Experiment #1).
(A) KNDy genes: Kiss1, Tac2, and Pdyn; (B) KNDy-associated receptors: Kiss1r, Tac3r, and Kor; and (C) steroid hormone receptors: Esr1 and Pgr. D) Plasma LH levels and (E) Plasma FSH levels. For all, n = 5 – 6. Gene expression data were normalized to oil fed controls for each gene. All data were analyzed using a two-way ANOVA (fasting x steroid) followed by post-hoc Newman-Keuls analysis (a = p < 0.05; b = p < 0.01; c = p < 0.001; d = p < 0.0001).
There was no significant difference in body weight by diet or steroid among females at sacrifice (Table 2), although fasted females lost 2.7 ± 0.3 g in oil and 2.7 ± 0.2 g in E2B. Uterine weights were elevated by E2B in both fed (p < 0.001) and fasted animals (p < 0.0001) (Table 2; steroid: F(1, 20) = 54.58, p < 0.0001). Uterine weight relative to body weight was increased in E2B-treated compared to oil-treated animals in both fed (p < 0.01) and fasted (p < 0.01) states (Table 2; steroid: F(1, 20) = 28.63, p < 0.0001). E2 levels were elevated in E2B-treated compared to oil-treated animals, within both fed (p < 0.05) and fasted (p < 0.05) animals (Table 2; steroid: F(1, 16) = 7.922, p < 0.05).
3.2 Diet-induced obesity effects on body composition and ARC KNDy gene expression (Experiment #2)
DIO affects body composition and ARC gene expression. To measure the changes in body composition, animals were placed into an MRI at the conclusion of the study. For lean mass, there were diet (Fig. 3A; F(1, 28) = 13.34, p < 0.01) and steroid (F(1, 28) = 20.98, p < 0.0001) effects. Lean mass was higher in LFD-fed than HFD-fed animals, within both oil and E2B treatment (p < 0.01, both). For fat mass, there were diet (Fig. 3B; F(1, 28) = 15.17, p < 0.001) and steroid (F(1, 28) = 22.35, p < 0.0001) effects. Fat mass was lower in LFD-fed than HFD-fed animals within both oil and E2B treatment (p < 0.01, both). Cumulative weekly body weight gain was affected by steroid (Fig. 3C; F(1, 36) = 24.524, p < 0.0001), diet (F(1, 36) = 36.344, p < 0.0001), time (F(8, 288) = 137.196, p < 0.0001), time*steroid (F(8, 288) = 14.266, p <0.0001), and time*diet (F(8, 288,) = 15.781, p < 0.0001). Beginning on week 4, cumulative body weight gain in HFD oil was greater than in LFD oil animals. From weeks 5–9, body weight gain was higher in HFD-fed vs. LFD-fed, E2B-treated females. Beginning week 7, body weight gain in LFD-fed, E2B-treated females was less than their oil-treated counterparts. Beginning week 5, body weight gain in HFD-fed, E2B-treated females was less than their oil-treated counterparts. There was an effect of steroid (Fig. 3D; F(1, 28) = 11.45, p < 0.01) on feeding efficiency and effect of diet on energy intake (Fig. 3E; F(1, 28) = 69.51, p < 0.0001). Energy intake was higher in HFD-fed females in both steroid treatments (p < 0.0001, both).
For KNDy neuropeptide expression, there were diet (Fig. 3F; F(1, 28) = 6.648, p < 0.05) and steroid (F(1, 28) = 20.78, p < 0.0001) effects on Kiss1, which was decreased five-fold by E2B in LFD-fed animals (p < 0.001) and by two-fold in HFD-fed, oil-treated animals (p < 0.01). Tac2 decreased by E2B in both LFD (p < 0.05) and HFD (p < 0.05) fed females (Fig. 3F; steroid: F(1, 28) = 14.01; p < 0.001). For Kiss1r, there was a diet effect (Fig. 3G; F(1, 27) = 6.796, p < 0.05) with Kiss1r being suppressed two-fold by HFD in E2B-treated females (p < 0.05). Similarly, there was a diet effect (Fig. 3G; F(1, 20) = 11.58, p < 0.01) on Tac3r expression, which was decreased by HFD in E2B-treated animals (p < 0.05). There were no changes in Pdyn, Kor, Esr1, or Pgr expression.
Within HFD, plasma LH levels were suppressed by E2B (p < 0.05; Fig. 3I; steroid: F(1, 31) = 9.398, p < 0.01). There were no differences in plasma FSH levels (Fig. 3J). Uterine weight was increased by E2B treatment in both LFD- (p < 0.0001) and HFD- (p < 0.0001) fed animals (Table 2; steroid: F(1, 36) = 55.13, p < 0.0001). Uterine weight relative to body weight was increased in E2B- compared to oil-treated animals in both LFD- and HFD-fed animals, as we have previously reported (Yasrebi et al., 2016). Plasma E2 concentrations were elevated in E2B-treated animals, within both diets (Yasrebi et al., 2016).
3.3 Estradiol benzoate effects on ARC KNDy gene expression in SW females (Experiment #3)
We next characterized E2B regulation of ARC gene expression in SW mice. Kiss1 and Tac2 were reduced by E2B (Fig. 4A: p < 0.01, both), while Pgr expression was increased by E2B (Fig. 4A: p < 0.001). There were no changes in expression of Pdyn, Kiss1r, Tac3r, Kor, and Esr1. Animals did not have any differences in body weight. Uterine weight (Table 2; steroid: F(1, 28) = 97.33, p < 0.0001) and uterine weight relative to body weight (Table 2; steroid: F(1, 28) = 157.6, p < 0.0001) were increased by E2B (p < 0.0001, both). E2 levels were elevated in E2B-treated females (p < 0.001; Table 2; steroid: F(1, 27) = 18.98, p < 0.001). There was no difference in plasma LH between oil- and E2B-treated females (oil: 1.40 ± 0.33 ng/ml vs. E2B: 2.20 ± 0.45 ng/ml).
3.4 Single cell colocalization and Tac2 neuronal gene expression changes by fasting and HFD (Experiments #4–5)
Single Tac2 neurons were harvested from oil- and E2B-treated females to determine co-expression patterns in individual neurons. E2B decreased colocalization of Tac2 with Kiss1 (Fig. 4B; p <0.001) and of Tac2 with Pdyn (Fig. 4B; p < 0.05) and subsequently decreased KNDy co-localization by ~50% (p < 0.0001). E2B had no effect on Tac3r or Esr1 but did increase Pgr in Tac2 neurons (Fig. 4B; p < 0.05). Colocalization patterns are shown in a representative gel (Fig. 4C). The KNDy neuropeptides (Kiss1, Tac2, Pdyn) are boxed in panels A–C and the representative gels illustrate gene expression colocalization patterns within the same 10 cells. Panels D–F are the receptors examined and are not representative of colocalization patterns within the same 10 cells, but show expression patterns within 10 positive Tac2 neurons. We did not detect Kor or Kiss1r in single Tac2 neurons or pools of 5 Tac2 neurons.
To determine if fasting regulates receptor gene expression in Tac2 neurons similar to ARC, Experiment #1 was repeated and pools of 5 Tac2 neurons were harvested to use for qPCR on the following genes: Tac2, Tac3r, Esr1, and Pgr. Tac2 was decreased by E2B in fed-animals (p < 0.05), with a similar trend in fasted animals (Fig. 4D; steroid: F(1, 20) = 14.09, p < 0.01). Progesterone receptor, Pgr was increased by E2B three-fold in both fed (p < 0.01) and fasted (p < 0.001) animals (Fig. 4D; steroid: F(1, 60) = 25.83, p < 0.0001). There were no changes in Tac3r or Esr1. Uterine weights were increased by E2B (Table 2; F(1, 19) = 73.93, p < 0.0001) in fed (p < 0.0001) and fasted (p < 0.0001) females. E2B increased uterine weight relative to body weight in fed (p < 0.001) and fasted (p < 0.001) females (Table 2; steroid: F(1, 19) = 43.30, p < 0.0001).
To determine effect of DIO on Tac2 neurons, Experiment #2 was repeated in Tac2- EGFP animals and pools of 5 neurons were collected. There was no difference in cumulative body weight gain across treatments, but there was a general trend of E2B-treated animals gaining less than their oil-treated controls, within LFD and HFD (Fig. 4E). Tac2 (p < 0.01) was decreased ~75% by E2B in HFD fed animals (Fig. 4F; steroid: F(1, 20) = 14.77, p < 0.01). Pgr was augmented by E2B in both diet treatments (Fig. 4F; LFD: p < 0.001; HFD: p < 0.0001; steroid: F(1, 61) = 46.41, p < 0.001). There was no difference in body weight at sacrifice, unlike in C57/BL animals from Experiment #2 (Table 2). This is potentially due to differences in the response to DIO (HFD) between C57/BL and SW with C57/BL being more susceptible to DIO (Winzell and Ahren, 2004). E2 levels were elevated in E2B-treated females (p < 0.05; Table 2; steroid: F(1, 24) = 10.85, p < 0.01). Finally, there were steroid effects on both uterine weight (Table 2; F(1, 27) = 217.5, p < 0.0001) and uterine weight relative to body weight (Table 2; F(1, 27) = 257.0, p < 0.0001), with E2 increasing both, in LFD and HFD females (p < 0.0001; all).
3.5 Caloric restriction effects on body composition and ARC KNDy gene expression (Experiment #6)
To determine the effects of chronic caloric restriction on body composition and KNDy- associated gene expression, ovx females were orally dosed with oil or E2B and separated into ad libitum and 30% CR groups. Using an MRI, there were no changes in lean mass between pre- and post-treatment, within steroid or diet. In addition, there were steroid (Fig. 5B; F(1, 28) = 18.64, p < 0.001), diet (F(1, 28) = 8.25, p < 0.01), and time*diet (F(1, 28) = 38.89, p < 0.0001) effects on percent fat mass, which decreased in oil CR animals (p < 0.01). Cumulative body weight gain was measured; animals were fed ad lib for the first 4 weeks before they were maintained on ad lib or changed to CR. For the initial 5 weeks, there was a steroid (Fig. 5C; F(1, 20) = 31.09, p < 0.0001), time (F(4, 120) = 37.818, p < 0.0001) and steroid*time (F(4, 120) = 6.457, p < 0.0001) effect on cumulative body weight gain. During these initial weeks, oil-treated females gained more weight than E2B-treated females from week 2–5. Following diet change, there was a steroid (Fig. 5C; F(1, 27) = 14.650, p < 0.001), diet (F(1, 27) = 77.898, p < 0.0001), time (F(5, 135) = 12.599, p < 0.0001), and time*diet (F(5, 135) = 45.023, p < 00001) effect on cumulative body weight gain. Both CR groups (oil- and E2B-treated) lost body weight compared to ad lib animals in weeks 7–10. From week 6–8, there was a decrease in cumulative body weight gain in E2B CR animals compared to oil CR animals.
Feeding efficiency (g gained/kCal consumed) was also affected by CR with diet (Fig. 5D; F(1, 58) = 27.79, p < 0.0001), time (F(1, 58) = 40.07, p < 0.0001), and time*diet (F(1, 58) = 22.83, p < 0.0001) effects. Feeding efficiency decreased in CR groups within both oil- (p < 0.0001) and E2B-treated (p < 0.001) females. For energy intake, there were steroid (Fig. 5E; F(1, 10) = 72.302, p < 0.0001), diet (F(1, 10) = 251.131, p < 0.0001), time (F(4, 40) = 8.905, p < 0.0001), and time*steroid (F(4, 40) = 9.492, p < 0.0001) effects within weeks 6–9. Energy intake decreased during weeks 6–9 by CR in E2B-treated females. Average energy intake also was effected by steroid (Fig. 5E; F(1, 57) = 30.73, p < 0.0001), diet (F(1, 57) = 61.72, p < 0.0001), steroid*diet (F(1, 57) = 8.61, p < 0.01), time (F(1, 57) = 146.6, p < 0.0001), and time*diet (F(1, 37) = 59.32, p < 0.0001). Average energy intake in CR females was lower in post-CR compared to pre-CR in both oil- (p < 0.0001) and E2B- (p < 0.0001) treated animals (Fig. 5F; steroid: F(3, 132) = 46.11, p < 0.0001; time: F(1, 132) = 174.7, p < 0.0001; steroid*time: F(3, 132) = 27.77, p < 0.0001).
All relative mRNA expression were normalized to oil ad lib animals within each gene. For Kiss1, there was an effect of diet (Fig. 5G; F(1, 28) = 42.26, p < 0.0001), steroid (F(1, 28) = 132.1, p < 0.0001), and diet*steroid (F(1, 28) = 32.39, p < 0.0001) and Kiss1 was decreased by E2B (ad lib: p < 0.0001, CR: p < 0.001) and by CR, in oil-treated animals (p < 0.0001). For Tac2 expression, there was an effect of diet (Fig. 5G; F(1, 28) = 16.60, p < 0.001), steroid (F(1, 28) = 46.31, p < 0.0001), and diet*steroid (F(1, 28) = 11.55, p < 0.01). Tac2 expression was suppressed by E2B in ad lib animals (p < 0.0001) and by CR in oil-treated females (p < 0.0001). For Pdyn, there was a diet (Fig. 5G; F(1, 28) = 10.40, p < 0.01), steroid (F(1, 28) = 7.118, p < 0.05), and diet*steroid (F(1, 28) = 42.12, p < 0.0001) effect. Pdyn was suppressed by E2B in ad lib animals (p < 0.0001) and by CR in oil-treated females (p < 0.0001) and increased by both E2B in CR animals (p < 0.05) and by CR in E2B-treated animals (p < 0.05).
For the KNDy receptors, there was a diet*steroid effect on Kiss1r expression (Fig. 5H; F(1, 28) = 12.58, p < 0.01), which was augmented by CR in oil-treated animals (p < 0.01) and decreased by E2B in CR animals (p < 0.05). There were no other changes observed in KNDy-associated receptors. Pgr was increased by E2B (Fig. 5I; ad lib: p < 0.001, CR: p < 0.05; steroid: F(1, 28) = 23.61, p < 0.0001), with no change observed for Esr1. Because we did not observe any effects of energy states on the receptors, we did not repeat the chronic caloric restriction experiment in pooled Tac2 neurons.
For the gonadotropins, there were diet (Fig 5J; F(1, 28) = 10.10, < 0.01), steroid (F(1, 28) = 56.44, p < 0.0001), and diet*steroid (F(1, 28) = 13.78, p < 0.001) effects on plasma LH levels. LH levels were increased by CR in oil-treated animals (p < 0.0001) and by E2B in ad lib (p < 0.05) and CR (p < 0.0001) animals. Within CR animals, FSH levels were decreased by E2B (p < 0.05) compared to oil (Fig. 5K; diet*steroid: F(1, 28) = 5.218, p < 0.05). For uterine weights, there were diet (Table 2; F(1, 28) = 44.69, p < 0.0001), steroid (F(1, 28) = 232.4, p < 0.001), and diet*steroid (F(1, 28) = 38.40, p < 0.0001) effects. Uterine weight were decreased by CR in E2B-treated females (p < 0.0001) and increased by E2B in both ad lib and CR females (p < 0.0001, both). There were diet (Table 2; F(1, 28) = 15.92, p < 0.001), steroid (F(1, 28) = 256.9, p < 0.0001), and diet*steroid (F(1, 28) = 14.03) effects on uterine weights/body weight (mg/g). E2B increased uterine weights/body weight in both ad lib (p < 0.0001) and CR (p < 0.0001) groups and decreased by CR within E2B treated animals (p < 00001). E2 levels were affected by diet (Table 2; F(1,24) = 12.54, p < 0.01), steroid (F(1, 24) = 23.01, p < 0.0001), and diet*steroid (F(1, 24) = 12.79, p < 0.01). E2 were elevated by E2B in ad lib-fed animals only (p < 0.0001) and were reduced in E2B CR animals compared to E2B ad lib animals (p < 0.0001).
3.6 Expression of genes involved in the ghrelin signaling cascade in Tac2 neurons
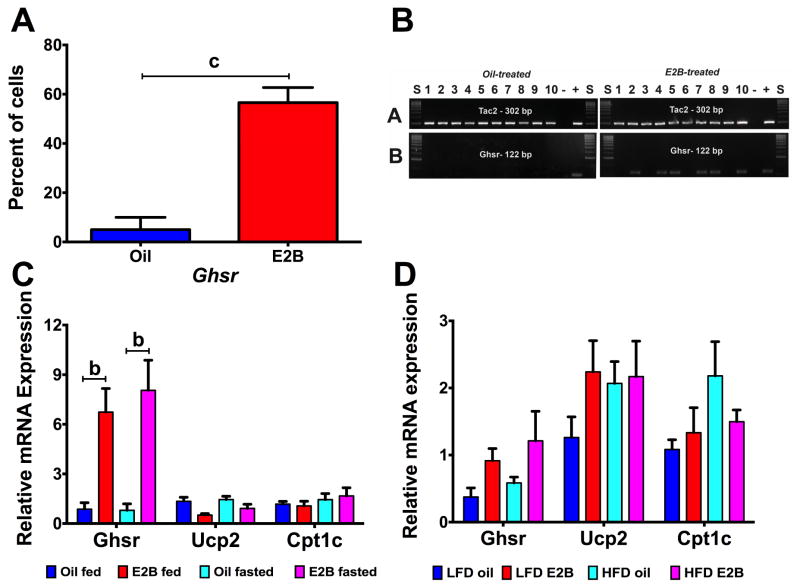
To determine if ghrelin signaling is involved in Tac2 neurons, we selected key genes involved in the ghrelin signaling cascade to conduct single cell PCR and qPCR analysis. Activation of GHSR initiates the CaMKK-AMPK-UCP2 pathway, which upregulates carnitine palmitoyl transferase 1 (CPT1) to increase fatty acid oxidation. Subsequently, uncoupling protein 2 (UCP2), which is involved in decreasing reactive oxygen species (ROS) that are produced during oxidation, is activated (Andrews, 2011). Individual Tac2 neurons were harvested and single cell PCR was conducted to determine expression of the ghrelin’s receptor, growth hormone secretagogue receptor (Ghsr). In Fig. 6A, there was a nearly ~55% increase in colocalization of Tac2 with Ghsr by E2B with products confirmed through gel electrophoresis (Fig. 6B). To determine if ghrelin signaling genes are changed in fed-fasted and DIO animals, we conducted qPCR on Tac2 pooled cells collected in Experiments #4 and #5. In the fed-fasted experiment, there were no changes in gene expression of Ghsr, Ucp2, or Cpt1c by fasting (Fig. 6C). In Tac2 pools, Ghsr expression was augmented by 6- to 8-fold in E2B-treated females in both fed (p < 0.01) and fasted (p < 0.01) groups (steroid: F(1, 20) = 5.948, p < 0.05). In diet-induced obesity animals, there were no change in any ghrelin signaling gene by steroid or diet (Fig. 6D).
Figure 6. Ghrelin signaling in Tac2 neurons is regulated by steroid but not fasting or diet-induced obesity.
(A) Percent of Tac2 cells coexpressing Ghsr. Data were analyzed using a Student’s t-test (c = p < 0.001). (B) Representative gel of single-cell PCR amplification products in oil- and E2B-treated Tac2 neurons. (C) Gene expression in Tac2 neurons of fed-fasted females. Data were normalized to oil fed controls, within each gene. Data were analyzed using a two-way ANOVA (fasting x steroid) followed by post-hoc Newman-Keuls analysis (b = p < 0.01). (D) Gene expression in Tac2 neurons. Data in C & D were normalized to LFD oil controls except for D: Ghsr which was normalized to LFD-E2B and analyzed within each gene using a two-way ANOVA (diet x steroid), followed by Newman-Keuls multiple comparison test.
4. Discussion
Disturbances in energy balance (positive and negative) are linked to reproductive problems, though the mechanisms are unclear. For example, hypogonadism secondary to obesity is common, with bariatric surgery correcting a significant percentage of infertility (Calderon et al., 2015, Milone et al., 2015). Similarly, patients with eating disorders have increased spontaneous abortions and miscarriages (Linna et al., 2013). In the present study using a mouse model, we show that there are disruptions in KNDy neuropeptide gene expression, both in the ARC and in Tac2 neuronal pools, and in KNDy receptor gene expression in the ARC by negative (anorexia) and positive (obesity) energy balance. The steroid receptors, ERα and PR, were impervious to any change in energy balance. A summary of the differential regulation of KNDy-associated neuropeptides and receptors is presented in Table 3.
Table 3.
Summary table of KNDy-associated gene expression changes in ARC and Tac2 pools.
| Gene Name | Fed vs. Fasted* Exp. #1 (ARC) & 4 (pools) | LFD vs. HFD* Exp. #2 (ARC) & 5 (pools) | SW Oil vs. E2B+ Exp. #3 | Ad lib vs. CR Exp. #6 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fed vs. Fasted | Oil vs. E2B | LFD vs. HFD | Oil vs. E2B | Ad lib vs. CR | Oil vs. E2B | ||||||||
| Oil | E2B | Fed | Fasted | Oil | E2B | LFD | HFD | Oil | E2B | Ad lib | CR | ||
| Kiss1 |
 ARC ARC |
 ARC ARC |
 ARC ARC |
 ARC ARC |
 ARC ARC |
 ARC ARC |
 ARC ARC |
 ARC ARC |
 ARC ARC |
||||
| Tac2* |
 pools pools |
□ |
 ARC ARC |
 ARC ARC pools pools |
 ARC ARC |
 ARC ARC |
 ARC ARC |
||||||
| Pdyn |
 ARC ARC |
 ARC ARC |
 ARC ARC |
 ARC ARC |
|||||||||
| Kiss1r |
 ARC ARC |
 ARC ARC |
 ARC ARC |
 ARC ARC |
|||||||||
| Tac3r* |
 ARC ARC |
 ARC ARC |
 ARC ARC |
||||||||||
| Kor |
 ARC ARC |
 ARC ARC |
|||||||||||
| Esr1* | |||||||||||||
| Pgr* |
 ARC ARC pools pools |
 ARC ARC pools pools |
 pools pools |
 pools pools |
 ARC ARC |
 ARC ARC |
 ARC ARC |
||||||
| Fig. | ARC: Fig. 2; pools: Fig. 4D | ARC: Fig. 3; pools: Fig. 4F | Fig. 4A | Fig. 5 | |||||||||
Experiments and genes with a * indicate qPCR analysis of ARC and Tac2 pools. Experiments without * were only analyzed in ARC tissue. For all experiments except oil vs E2B (+), data were analyzed with using a two-way ANOVA followed by post-hoc Newman-Keuls. For oil vs. E2B experiment, data were analyzed with a Student’s t-test Corresponding graphs of each gene expression change is noted in the “Fig.” row. Arrows indicate the direction of gene expression (
 = upregulated and
= upregulated and
 = downregulated compared to control (oil, fed, LFD, ad lib). For example, in experiment #1 (fed vs. fasted) Kiss1 in the ARC is increased by fasting in oil-treated animals (Fig. 2A) and decreased by E2B in both fed ans fasted animals (Fig. 2A).
= downregulated compared to control (oil, fed, LFD, ad lib). For example, in experiment #1 (fed vs. fasted) Kiss1 in the ARC is increased by fasting in oil-treated animals (Fig. 2A) and decreased by E2B in both fed ans fasted animals (Fig. 2A).
Disruption of energy balance may challenge E2 and P4 actions in the hypothalamus and disrupt reproduction. Previously, kisspeptin has been identified as a key regulator in reproduction and energy balance (Goodman et al., 2013, Mittelman-Smith et al., 2012, Uenoyama et al., 2014), but these studies focused primarily on AVPV kisspeptin. Of those studies that examined KNDy neurons, the effects of these physiological states on KNDy receptors and the steroid receptors, ERα and PR, which mediate negative feedback on the HPG axis (Eghlidi et al., 2010, Lehman et al., 2010), were not examined. NKB (Tac2) acts as a positive autoregulator to kisspeptin production through the NKB receptor (Tac3r) (Lehman et al., 2010) and dynorphin (DYN/Pdyn) is thought to act as a negative autoregulator, via an unidentified interneuronal network, through the κ-opioid receptor (KOR/Kor) (de Croft et al., 2013, Lehman et al., 2010, Ruka et al., 2016, Weems et al., 2016) Therefore, ERα, PR, and the KNDy-associated receptors, Kiss1r, Tac3r, and Kor, are essential players in the pulse generator and are potential targets for negative and positive energy balance.
We also demonstrate that coexpression of the KNDy neuropeptides decreases with E2B administration. Similar coexpression patterns exist in the male mice, as testosterone has been shown to decrease coexpression of KNDy neuropeptides (Navarro et al., 2011, Ruka et al., 2013). These studies report between 80–90% colocalization of both Kiss1 with Pdyn or Tac2 (gonadoectomized males) and of Tac2 with Kiss1 or Pdyn (intact males) (Navarro et al., 2011, Ruka et al., 2013). The similar colocalization percentages between our study in female Tac2-EGFP mice and in previous studies using both Tac2-EGFP and Kiss1-creGFP male mice suggests that the main driver underlying KNDy coexpression is gonadal steroids suppressing Kiss1 expression.
4.1 The effects of energy deficiency in the ARC
Previous studies suggest that energy deficiency leads to dysfunction of kisspeptin gene expression (Castellano et al., 2005, Kalamatianos et al., 2008, Luque et al., 2007, Matsuzaki et al., 2011, Polkowska et al., 2015, Roa et al., 2009, True et al., 2011, Wahab et al., 2008). In the present study, a 24 h fast increased ARC Kiss1 and decreased Kor in oil-treated females and decreased Kiss1r in E2B-treated females, but had no effect on Tac3r or steroid receptor gene expression in pools of Tac2 neurons.. Chronic caloric restriction decreased expression of all KNDy neuropeptides and increased Kiss1r in the ARC of oil-treated females. Clearly, Kiss1 expression is suppressed by negative energy balance states. However, many of the previous studies do not consider the regulatory role of E2 in modulating kisspeptin regulation, which could explain differences between our results for fasting and previous experiments.
The differences in KNDy neuropeptide and receptor gene expression between a 24 h fast and 30% CR suggest that the endocrine and neurological mechanisms controlling gene expression differ between acute and chronic negative energy balance and that the duration of caloric restriction and severity are important. Two factors may influence the apparent duration-dependent differences: age and change in body weight. Previous studies report that in the pubertal female rat ARC, kisspeptin-IR neurons decrease by a 48 h fast, but not in adult females. In the same study, change in body weight (a body weight reduction of 24% pubertal vs. 12% adult) was proposed to be important (Castellano et al., 2010). In our study, females lost <10% of body weight after a 24 h fast and lost ~20% of body weight in the CR experiment (after Week 5). In addition, the decreases in body weight are due to changes in lean vs. fat mass. In our study, CR reduced overall fat mass and did not change lean mass in oil-treated females. These changes in body weight composition may dictate kisspeptin gene expression independent of leptin (True et al., 2011). Differences in energy deficiency (undernutrition vs. elevated energy expenditure) are also important to consider; however, in our present study, we did not examine energy expenditure in the 24 h fast or the 30% CR. Collectively, considering the importance of KNDy neurons on GnRH pulsatility (Goodman et al., 2013), these data expand on our understanding of the impact of anorexia, cachexia, and other states of negative energy homeostasis on the neuroendocrine control of reproduction.
Another interesting finding in our study is the differential regulation of Kiss1 by fasting (increased) and CR (decreased) in oil-treated females, which corresponds to differential regulation of plasma LH levels by fasting (decreased) and CR (increased). These differences between the acute and chronic energy deficiency extend to the other KNDy neuropeptides, Tac2 and Pdyn, which were decreased by CR and not altered after 24 h fasting, and to Kiss1r expression (reduced by a 24 h fast and augmented by 30% CR). While the main function of Kiss1r is the activation of GnRH neurons to regulate the HPG axis, recent studies have characterized Kiss1r expression in other ARC neurons (Fu and van den Pol, 2010, Higo et al., 2016). One function of non-GnRH Kiss1r is the control of feeding behavior through the direct excitation of ARC POMC neurons by kisspeptin (Fu and van den Pol, 2010). Perhaps, a suppression of Kiss1r in POMC neurons would reduce the Kiss1-induced activation of POMC and thus increase feeding in fasting animals.
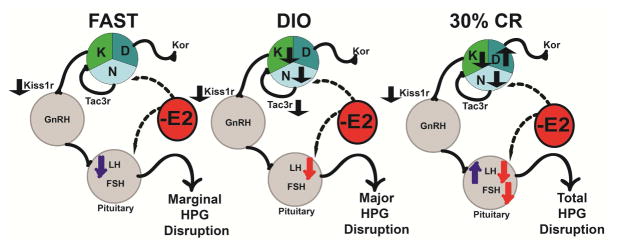
Figure 7 illustates the effects of dietary conditions on KNDy gene expression in the presence of E2 and the downstream effects on gonadotropins. With E2, which is similar to the intact state in cycling females, acute fasting does not impact KNDy-associated gene expression with the exception of Kiss1r. During chronic caloric restriction with E2, expression of all of the KNDy genes and Kiss1r are altered leading to a suppression of both LH and FSH. Suppression of Kiss1 and Tac2 (trending suppression of Tac2) and augmentation of Dyn expression potentially represses the HPG axis by blunting the pulse generator and reducing the regulation of GnRH neuronal excitability and pulsatility. Our data suggest that chronic CR leads to a total disruption of the HPG axis, which would slow folliculogenesis and block ovulation (similar to amenorrhea).
Figure 7. Chronic caloric overnutrition (DIO) and undernutrition (30% CR) augments the negative feedback effects of E2 leading to disruption of the HPG axis.
K = Kisspeptin; N = Neurokinin B (Tac2); D = Dynorphin. Black arrows indicate the direction of gene expression (
 = upregulated and
= upregulated and
 = downregulated) in E2B-treated females. Blue arrows indicate the direction of effects on gonadotropins by diet condition in oil-treated and red arrows indicate the direction of effects by E2B within that diet condition.
= downregulated) in E2B-treated females. Blue arrows indicate the direction of effects on gonadotropins by diet condition in oil-treated and red arrows indicate the direction of effects by E2B within that diet condition.
4.2 The effects of diet-induced obesity in the ARC
The obesity epidemic has fueled the need to study reproductive problems in obese populations. Factors such as inflammation, leptin, and E2 may contribute to the dysregulation of the hypothalamic KISS1 system in obese human males (George et al., 2010). This hypothesis has been supported by rodent studies, which suggest that positive energy balance decreases hypothalamic Kiss1 mRNA (Castellano et al., 2006, Iwasa et al., 2015). Kiss1 is also expressed in peripheral tissues (Dudek et al., 2016) and its expression is regulated by positive energy balance in the ovary (Zhou et al., 2014) and the testes (Dudek et al., 2016). In our study, DIO decreased Kiss1 in oil-treated females and Kiss1r in E2B-treated females in the ARC, which did not correlate with changes in either LH nor FSH plasma levels. Therefore, steroid treatement is important to consider when evaluating steroid hormone feedback under the influence of diet or energy state. Interestingly, in pools of Tac2 neurons, there were no changes in Tac3r or steroid receptor gene expression induced by DIO.
We hypothesize that chronic DIO leads to a major disruption in the HPG axis (Figure 7). Collectively, the decrease in Kiss1, Tac2, Kiss1r, and Tac3r expression in E2B-treated females by DIO reduces positive autoregulatory function of Tac2 and the activity of the pulse generator. Subsequently, negative feedback of E2 on the KNDy system is augmented, decreasing LH output. Suppression of LH will inhibit the late stages of folliculogenesis and ovulation, compromising reproduction in females. Nonetheless, it is important to note that our studies are conducted on ovx females supplemented with E2B. Future studies will be expanded by examining changes in energy balance in intact, cycling females.
It is unclear what central or peripheral mechanisms regulate this interaction between positive energy balance and KNDy neurons. One potential peripheral signal is leptin, which is produced by adipocyties. Leptin receptor, LepR, is expressed in >40% of ARC Kiss1 neurons (Hill et al., 2008), and the decrease in ARC KiSS-1 in male ob/ob mice is restored by leptin (Smith et al., 2006). In both oil- and E2B-treated animals, DIO increased fat mass; thus, the increase in leptin production by excess adipose tissue may be important in the disruption of KNDy neuronal functions including neuropeptide and receptor expression, unlike the Kiss1 disruption associated with caloric restriction. In our analyses, we did not consider differences across the interaction of steroid and diet (that is, differences between LFD oil vs. HFD E2B and LFD E2B vs. HFD oil). Interestingly, body composition between these groups are similar, while the effects on gene expression are distinct, suggesting that body composition may be important only to a degree in regulation of gene expression.
4.3 Estradiol benzoate increases Ghsr in Tac2 neurons
To compare receptor expression between the ARC and KNDy neurons, we harvested Tac2 neurons and analyzed using qPCR. Due to the availability of transgenic animals, mice used in the ARC studies (C57/BL) and the single cell studies (SW) are of a different strain, which is important factor to consider in interpreting our data. As expected (Bosch et al., 2012, Frazao et al., 2014, Gottsch et al., 2009, Zuloaga et al., 2012), E2 induced expression of ARC Pgr expression, which is recapitulated in Tac2 neurons. Therefore, the increase in ARC Pgr expression by E2B is due, in part, to the striking increase in KNDy Pgr expression. These data also suggest that E2’s priming of KNDy neurons for the negative feedback of P4 during the transition of pro/estrus is not disrupted by changes in energy balance (Eghlidi and Urbanski, 2015).
Another receptor that was similarly regulated by E2B in the ARC and Tac2 neurons was Ghsr, E2B increased the percentage of Ghsr-expressing Tac2 neurons and increased Ghsr expression in Tac2 pools by 6-fold, regardless of fed state. We have previously shown that in both fed and fasted females, E2B increases Ghsr expression in the ARC, but not in NPY neurons (Yasrebi et al., 2016). Therefore, the E2B-induced Ghsr expression in the ARC is due, in large part, to Ghsr expression in Tac2 neurons. Unlike in the ARC or in NPY neurons (Yasrebi et al., 2016), there were no changes in genes of the ghrelin signaling cascade by fasting or by DIO in Tac2 neurons. We hypothesize that E2 augments ghrelin sensitivity in KNDy neurons in its role as an anorectic steroid in females. We formulate this hypothesis because ablation of KNDy neurons suppressed post-ovariectomy body weight gain and its attenuation by E2 replacement (Mittelman-Smith et al., 2012) and because KNDy neurons simultaneously excite POMC neurons and suppress NPY/AgRP neurons through both kisspeptin (Fu and van den Pol, 2010) and the pluripotent actions of glutamate (Nestor et al., 2016). By increasing Ghsr expression specifically in KNDy neurons and thus their sensitivity to either circulating or local ghrelin, E2 indirectly augments the activity of these neurons, which eventually leads to a suppression of food intake in females and counteracts the effects of ghrelin on feeding.
Therefore, we hypothesize that the brain-gut peptide, ghrelin, may mediate, in part, the communication between energy homeostasis (feeding) and KNDy-associated reproduction. Furthermore, ghrelin also controls reproduction by suppressing LH pulse frequency (Forbes et al., 2009). There are few studies examining ghrelin signaling in KNDy neurons. In one recent study, ghrelin depolarized ARC kisspetin (KNDy) neurons in an E2-dependent manner with these neurons being more sensitive to ghrelin from ovx+E2 females (Frazao et al., 2014). Nonetheless, the interaction of ghrelin, E2, and KNDy neurons is largely unexplored.
5. Conclusion
Collectively, our data suggest that negative (24 h fast and 30% CR) and positive (DIO) states of energy balance differentially impact the expression of ARC KNDy neuropeptides and their receptors to alter the activity of the HPG axis. While the link between energy balance and reproduction is not clear, we demonstrate that E2 can both augment and oppose the effects of positive or negative energy states on KNDy neuropeptides and receptors, potentially leading to a disruption to the HPG axis. We see a progression in severity of HPG disruption from acute fasting to chronic DIO and CR. These disruptions in HPG disruption are elucidated through changes in LH and FSH levels, which may produce downstream problems with reproductive functions. Furthermore, the E2-induced increase in Ghsr expression in Tac2 neurons suggests that steroid and peripheral peptides interact in the ARC to control both energy balance and reproduction. Future experiments will use electrophysiology to examine the E2B-regulated increase in Ghsr expression in Tac2 neurons. In addition, it is important to note that peripheral tissues express Kiss1 and Kiss1r suggesting that the interaction of central and periphal signals is crucial to understanding this complicated system (Dudek et al., 2016, Song et al., 2014).
Infertility due to poor or excess nutrition may continue to worsen with the rise of the obesity epidemic. According to a study published by the Centers for Disease Control (CDC) in 2013, 6% of women and 12% of men are infertile (Chandra et al., 2013). Hypothalamic amenorrhea is common in both underweight and overweight females. While new technologies are making progress in addressing the number of infertile individuals, it is imperative to consider additional mechanisms of action that impact reproduction. Multiple factors lead to reproductive problems including genetics, which are equally important in the regulation of energy homeostasis. For many individuals experiencing infertility problems, energy balance dysfunction may be a underlying factor that is unexplored and unconsidered when evaluating potential solutions. Therefore, understanding the effects and causal mechanisms are critical to the development of reproductive therapy.
Highlights.
Steroidal environment (E2) regulates KNDy-associated gene expression in the arcuate nucleus and in single Tac2 neurons
KNDy-associated gene expression in the arcuate nucleus and in Tac2 neurons are dysregulated in negative and positive energy balance states
Gene expression of the KNDy-associated receptors are regulated by changes in energy balance and E2 in arcuate tissue, but not in Tac2 neurons
Gene expression of ghrelin’s receptor, Ghsr, is augmented by E2B in Tac2 neurons
Acknowledgments
The authors wish to thank Drs. Judith Storch and Sara Campbell for the use of the EchoMRI Body Composition Analyzer and the Millipore Multiplex Luminex System, respectively. This work was supported by the National Institudes of Health [R00DK083457, R00DK083457-S1, and P30ES005022]; and from the U.S. Department of Agriculture, National Institute of Food and Agriculture [NJ06107].
Abbreviations
- T2DM
type 2 diabetes, mellitus
- HPG
hypothalamic pituitary gonadal
- ARC
arcuate nucleus
- POMC
pro-opiomelanocortin
- CART
cocaine-and-amphetamine-regulated transcript
- NPY
neuropeptide Y
- AgRP
agouti-related peptide
- E2
17β-estradiol
- AVPV
anteroventral periventricular nucleus
- LH
luteinizing hormone
- Kiss1
kisspeptin
- Tac2
neurokinin B
- Pdyn
prodynorphin
- GPR54
G protein-coupled receptor 54
- DIO
diet-induced obesity
- Kiss1r
kisspeptin receptor
- GnRH
gonadotropin releasing hormone
- SCN
suprachiasmatic nucleus
- ovx
ovariectomy
- E2B
estradiol benzoate
- ND
normal diet
- HFD
high fat diet
- PD
postnatal day
- SW
Swiss Webster
- sc
subcutaneous
- ip
intraperitoneal
- BH
basal hypothalamus
- CR
caloric restriction
- RIN
RNA integrity number
- qPCR
quantitative real-time PCR
- FSH
follicle-stimulating hormone
- Gapdh
glyceraldehyde-3-phosphate dehydrogenase
- Hprt
hypoxanthine-guanine phosphoribosyltransferase
- Actb
beta-actin
- Tac3r
tachykinin 3 receptor
- Kor
kappa-opiod receptor
- ERα (Esr1)
estrogen receptor alpha
- Pgr
progesterone receptor
- P4
progesterone
- Ghsr
growth hormone secretagogue receptor
- Ucp2
uncoupling protein 2
- Cpt1c
carnitine palmitoyltransferase 1c
Footnotes
Disclosure statement: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews ZB. Central mechanisms involved in the orexigenic actions of ghrelin. Peptides. 2011;32:2248–2255. doi: 10.1016/j.peptides.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Andreyeva T, Puhl RM, Brownell KD. Changes in perceived weight discrimination among Americans, 1995–1996 through 2004–2006. Obesity. 2008;16:1129–1134. doi: 10.1038/oby.2008.35. [DOI] [PubMed] [Google Scholar]
- Balasubramanian P, Jagannathan L, Mahaley RE, Subramanian M, Gilbreath ET, Mohankumar PS, Mohankumar SM. High fat diet affects reproductive functions in female diet-induced obese and dietary resistant rats. J Neuroendocrinol. 2012;24:748–755. doi: 10.1111/j.1365-2826.2011.02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch MA, Hou J, Fang Y, Kelly MJ, Ronnekleiv OK. 17beta-estradiol regulation of the mRNA expression of T-type calcium channel subunits: role of estrogen receptor alpha and estrogen receptor beta. J Comp Neurol. 2009;512:347–358. doi: 10.1002/cne.21901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch MA, Xue C, Ronnekleiv OK. Kisspeptin expression in guinea pig hypothalamus: effects of 17beta-estradiol. J Comp Neurol. 2012;520:2143–2162. doi: 10.1002/cne.23032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch MA, Tonsfeldt KJ, Ronnekleiv OK. mRNA expression of ion channels in GnRH neurons: subtype-specific regulation by 17beta-estradiol. Mol Cell Endocrinol. 2013;367:85–97. doi: 10.1016/j.mce.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock O, Bakker J. The two kisspeptin neuronal populations are differentially organized and activated by estradiol in mice. Endocrinology. 2013;154:2739–2749. doi: 10.1210/en.2013-1120. [DOI] [PubMed] [Google Scholar]
- Calderon B, Gomez-Martin JM, Vega-Pinero B, Martin-Hidalgo A, Galindo J, Luque-Ramirez M, Escobar-Morreale HF, Botella-Carretero JI. Prevalence of male secondary hypogonadism in moderate to severe obesity and its relationship with insulin resistance and excess body weight. Andrology. 2015;4:62–67. doi: 10.1111/andr.12135. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Roa J, Vigo E, Pineda R, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes. 2006;55:2602–2610. doi: 10.2337/db05-1584. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Bentsen AH, Mikkelsen JD, Tena-Sempere M. Kisspeptins: bridging energy homeostasis and reproduction. Brain Res. 2010;1364:129–138. doi: 10.1016/j.brainres.2010.08.057. [DOI] [PubMed] [Google Scholar]
- Chandra A, Copen CE, Stephen EH. Infertility and impaired fecundity in the United States, 1982–2010: data from the National Survey of Family Growth. Natl Health Stat Report. 2013:1–18. [PubMed] [Google Scholar]
- de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154:2750–2760. doi: 10.1210/en.2013-1231. [DOI] [PubMed] [Google Scholar]
- Dudek M, Kolodziejski PA, Pruszynska-Oszmalek E, Sassek M, Ziarniak K, Nowak KW, Sliwowska JH. Effects of high-fat diet-induced obesity and diabetes on Kiss1 and GPR54 expression in the hypothalamic-pituitary-gonadal (HPG) axis and peripheral organs (fat, pancreas and liver) in male rats. Neuropeptides. 2016;56:41–49. doi: 10.1016/j.npep.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Eghlidi DH, Haley GE, Noriega NC, Kohama SG, Urbanski HF. Influence of age and 17beta-estradiol on kisspeptin, neurokinin B, and prodynorphin gene expression in the arcuate-median eminence of female rhesus macaques. Endocrinology. 2010;151:3783–3794. doi: 10.1210/en.2010-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghlidi DH, Urbanski HF. Effects of age and estradiol on gene expression in the rhesus macaque hypothalamus. Neuroendocrinology. 2015;101:236–245. doi: 10.1159/000381063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- Forbes S, Li XF, Kinsey-Jones J, O’Byrne K. Effects of ghrelin on kisspeptin mRNA expression in the hypothalamic medial preoptic area and pulsatile luteinising hormone secretion in the female rat. Neurosci Lett. 2009;460:143–147. doi: 10.1016/j.neulet.2009.05.060. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3. Academic Press; 2008. [Google Scholar]
- Frazao R, Lemko HM, da Silva RP, Ratra DV, Lee CE, Williams KW, Zigman JM, Elias CF. Estradiol modulates Kiss1 neuronal response to ghrelin. Am J Physiol Endocrinol Metab. 2014;306:E606–614. doi: 10.1152/ajpendo.00211.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu LY, van den Pol AN. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci. 2010;30:10205–10219. doi: 10.1523/JNEUROSCI.2098-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JT, Millar RP, Anderson RA. Hypothesis: kisspeptin mediates male hypogonadism in obesity and type 2 diabetes. Neuroendocrinology. 2010;91:302–307. doi: 10.1159/000299767. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, Millar RP, Cernea M, Coolen LM, Lehman MN. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154:4259–4269. doi: 10.1210/en.2013-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29:9390–9395. doi: 10.1523/JNEUROSCI.0763-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haisenleder DJ, Schoenfelder AH, Marcinko ES, Geddis LM, Marshall JC. Estimation of estradiol in mouse serum samples: evaluation of commercial estradiol immunoassays. Endocrinology. 2011;152:4443–4447. doi: 10.1210/en.2011-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo S, Honda S, Iijima N, Ozawa H. Mapping of kisspeptin receptor mRNA in the whole rat brain and its co-localisation with oxytocin in the paraventricular nucleus. J Neuroendocrinol. 2016:28. doi: 10.1111/jne.12356. [DOI] [PubMed] [Google Scholar]
- Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab. 2008;294:E827–832. doi: 10.1152/ajpendo.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa T, Matsuzaki T, Munkhzaya M, Tungalagsuvd A, Yamasaki M, Kuwahara A, Yasui T, Irahara M. The effects of prenatal undernutrition and postnatal high-fat diet on hypothalamic Kiss1 mRNA and serum leptin levels. Int J Dev Neurosci. 2015;42:76–79. doi: 10.1016/j.ijdevneu.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Jacobs HS. Amenorrhea in athletes. Br J Obstet Gynaecol. 1982;89:498–500. doi: 10.1111/j.1471-0528.1982.tb03647.x. [DOI] [PubMed] [Google Scholar]
- Kalamatianos T, Grimshaw SE, Poorun R, Hahn JD, Coen CW. Fasting reduces KiSS-1 expression in the anteroventral periventricular nucleus (AVPV): effects of fasting on the expression of KiSS-1 and neuropeptide Y in the AVPV or arcuate nucleus of female rats. J Neuroendocrinol. 2008;20:1089–1097. doi: 10.1111/j.1365-2826.2008.01757.x. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linna MS, Raevuori A, Haukka J, Suvisaari JM, Suokas JT, Gissler M. Reproductive health outcomes in eating disorders. Int J Eat Disord. 2013;46:826–833. doi: 10.1002/eat.22179. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luque RM, Kineman RD, Tena-Sempere M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: analyses using mouse models and a cell line. Endocrinology. 2007;148:4601–4611. doi: 10.1210/en.2007-0500. [DOI] [PubMed] [Google Scholar]
- Mamounis KJ, Yang JA, Yasrebi A, Roepke TA. Estrogen response element-independent signaling partially restores post-ovariectomy body weight gain but is not sufficient for 17beta-estradiol’s control of energy homeostasis. Steroids. 2013;81:88–98. doi: 10.1016/j.steroids.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki T, Iwasa T, Kinouchi R, Yoshida S, Murakami M, Gereltsetseg G, Yamamoto S, Kuwahara A, Yasui T, Irahara M. Fasting reduces the Kiss1 mRNA levels in the caudal hypothalamus of gonadally intact adult female rats. Endocr J. 2011;58:1003–1012. doi: 10.1507/endocrj.k11e-131. [DOI] [PubMed] [Google Scholar]
- Milone M, De Placido G, Musella M, Sosa Fernandez LM, Sosa Fernandez LV, Campana G, Di Minno MN, Milone F. Incidence of successful pregnancy after weight loss interventions in infertile women: a systematic review and meta-analysis of the literature. Obes Surg. 2015;26:443–451. doi: 10.1007/s11695-015-1998-7. [DOI] [PubMed] [Google Scholar]
- Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153:2800–2812. doi: 10.1210/en.2012-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LJ, Dodd J, Nisenblat V, Norman RJ. Obesity and reproductive dysfunction in women. Endocrinol Metab Clin North Am. 2011;40:895–906. doi: 10.1016/j.ecl.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Wu M, Garcia-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152:4265–4275. doi: 10.1210/en.2011-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Tena-Sempere M. Kisspeptins and the neuroendocrine control of reproduction. Front Biosci (Schol Ed) 2011;3:267–275. doi: 10.2741/s150. [DOI] [PubMed] [Google Scholar]
- Nestor CC, Qiu J, Padilla SL, Zhang C, Bosch MA, Fan W, Aicher SA, Palmiter RD, Ronnekleiv OK, Kelly MJ. Optogenetic stimulation of arcuate nucleus Kiss1 neurons reveals a steroid-dependent glutamatergic input to POMC and AgRP neurons in male mice. Mol Endocrinol. 2016;30:630–644. doi: 10.1210/me.2016-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RJ, Clark AM. Obesity and reproductive disorders: a review. Reprod Fertil Dev. 1998;10:55–63. doi: 10.1071/r98010. [DOI] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polkowska J, Cieslak M, Wankowska M, Wojcik-Gladysz A. The effect of short fasting on the hypothalamic neuronal system of kisspeptin in peripubertal female lambs. Anim Reprod Sci. 2015;159:184–190. doi: 10.1016/j.anireprosci.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Quennell JH, Howell CS, Roa J, Augustine RA, Grattan DR, Anderson GM. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology. 2011;152:1541–1550. doi: 10.1210/en.2010-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa J, Garcia-Galiano D, Varela L, Sanchez-Garrido MA, Pineda R, Castellano JM, Ruiz-Pino F, Romero M, Aguilar E, Lopez M, Gaytan F, Dieguez C, Pinilla L, Tena-Sempere M. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology. 2009;150:5016–5026. doi: 10.1210/en.2009-0096. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Ronnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology. 2008;149:6113–6124. doi: 10.1210/en.2008-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Qiu J, Smith AW, Ronnekleiv OK, Kelly MJ. Fasting and 17beta-estradiol differentially modulate the M-current in neuropeptide Y neurons. J Neurosci. 2011;31:11825–11835. doi: 10.1523/JNEUROSCI.1395-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and kappa-opioid receptors in adult male mice. Endocrinology. 2013;154:2761–2771. doi: 10.1210/en.2013-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruka KA, Burger LL, Moenter SM. Both estrogen and androgen modify the response to activation of neurokinin-3 and kappa-opioid receptors in arcuate kisspeptin neurons from male mice. Endocrinology. 2016;157:752–763. doi: 10.1210/en.2015-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- Song WJ, Mondal P, Wolfe A, Alonso LC, Stamateris R, Ong BW, Lim OC, Yang KS, Radovick S, Novaira HJ, Farber EA, Farber CR, Turner SD, Hussain MA. Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell Metab. 2014;19:667–681. doi: 10.1016/j.cmet.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundgot-Borgen J. Risk and trigger factors for the development of eating disorders in female elite athletes. Med Sci Sports Exerc. 1994;26:414–419. [PubMed] [Google Scholar]
- True C, Kirigiti MA, Kievit P, Grove KL, Smith MS. Leptin is not the critical signal for kisspeptin or luteinising hormone restoration during exit from negative energy balance. J Neuroendocrinol. 2011;23:1099–1112. doi: 10.1111/j.1365-2826.2011.02144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uenoyama Y, Tsukamura H, Maeda K. KNDy neuron as a gatekeeper of puberty onset. J Obstet Gynaecol Res. 2014;40:1518–1526. doi: 10.1111/jog.12398. [DOI] [PubMed] [Google Scholar]
- Wahab F, Aziz F, Irfan S, Zaman WU, Shahab M. Short-term fasting attenuates the response of the HPG axis to kisspeptin challenge in the adult male rhesus monkey (Macaca mulatta) Life Sci. 2008;83:633–637. doi: 10.1016/j.lfs.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Weems PW, Witty CF, Amstalden M, Coolen LM, Goodman RL, Lehman MN. Kappa-opioid receptor is colocalized in GnRH and KNDy cells in the female ovine and rat brain. Endocrinology. 2016;157:2367–2379. doi: 10.1210/en.2015-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53:S215–219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- Xu C, Roepke TA, Zhang C, Ronnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone (GnRH) activates the M-current in GnRH neurons: an autoregulatory negative feedback mechanism? Endocrinology. 2008;149:2459–2466. doi: 10.1210/en.2007-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasrebi A, Hsieh A, Mamounis KJ, Krumm EA, Yang JA, Magby J, Hu P, Roepke TA. Differential gene regulation of GHSR signaling pathway in the arcuate nucleus and NPY neurons by fasting, diet-induced obesity, and 17beta-estradiol. Mol Cell Endocrinol. 2016;422:42–56. doi: 10.1016/j.mce.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Chen H, Yang S, Li Y, Wang B, Chen Y, Wu X. High-fat diet decreases the expression of Kiss1 mRNA and kisspeptin in the ovary, and increases ovulatory dysfunction in postpubertal female rats. Reprod Biol Endocrinol. 2014:12. doi: 10.1186/1477-7827-1112-1127.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Yahn SL, Pang Y, Quihuis AM, Oyola MG, Reyna A, Thomas P, Handa RJ, Mani SK. Distribution and estrogen regulation of membrane progesterone receptor-beta in the female rat brain. Endocrinology. 2012;153:4432–4443. doi: 10.1210/en.2012-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]