Abstract
In 2014, as an attempt to address the Zika health crisis by controlling the mosquito population, Brazil took the unprecedented action of applying a chemical larvicide, pyriproxyfen, to drinking water sources. The World Health Organization has established an acceptable daily intake of pyriproxyfen to be 100 micrograms per kg of body weight per day, but studies have demonstrated that at elevated doses (>5000 mg/kg), there are adverse effects in mice, rats and dogs. To better understand the potential developmental toxicity of pyriproxyfen, we utilized the embryonic zebrafish. Our results demonstrate that the concentration resulting in 50% of animals presenting adverse morphological effects (EC50), including craniofacial defects, was 5.2 μM for daily renewal exposure, and above this concentration, adverse behavioral effects were also observed in animals that followed a static exposure regimen. Thus, zebrafish data suggest that the developmental toxicity of pyriproxyfen may not be limited to insects.
Keywords: Pyriproxyfen, Zika, Development, Morphology defects, Neurotoxicology, Behavior, Zebrafish
Graphical abstract
1. Introduction
The prevalence of the Zika virus has elevated to a full-fledged health crisis in parts of South and Central America. It has rapidly spread in the Americas since first being identified in Brazil in early 2014 (Centers for Disease Control and Prevention, 2016a). Brazil is currently experiencing a significant outbreak of the Zika virus and an associated increase in babies born with microcephaly. Previous outbreaks have been recorded in Africa, the Americas, Asia and the Pacific, transmitted by the Aedes genus of mosquito. After extensive review, the US Centers for Disease Control and Prevention (CDC) concluded that Zika virus is a cause of microcephaly and other severe fetal brain defects (Centers for Disease Control and Prevention, 2016b). Prior to this report, the World Health Organization (WHO) had associated the mosquito-borne virus with microcephaly and Guillain-Barré syndrome. There currently is no treatment or vaccine available.
The mosquito population in Brazil has proliferated due to inadequate municipal sewage management, extremely dense urban development and reliance on open-reservoir, stagnant drinking water. In 2014, the insect larvicide pyriproxyfen, a growth hormone analog, was applied to drinking water supplies to prevent mosquito larvae from hatching and maturing (Government, 2014). This chemical has also been widely used on citrus crops in Israel, South Africa, Spain and Italy and is recommended by the WHO for addition to drinking water storage vessels to control malaria.
Pyriproxyfen is also a registered pesticide with the US Environmental Protection Agency (EPA) for use only on non-food crops and lawns, or to control fleas or ticks on pets. It is not recommended for use where human ingestion could occur. It underwent testing in rats and rabbits where it was determined not to produce reproductive or developmental effects at doses up to 100 mg per kg of body-weight per day (Organization, 2008; Safety, 1999), equivalent to consuming 6 grams of the compound per day in a human. The acceptable daily intake of pyriproxyfen set by the WHO is 100 micrograms per kg of body-weight per day for a lifetime. However, recent studies by WHO have found that at >5,000 mg/kg of body-weight, pyriproxyfen caused an increase in liver weight and changes in plasma lipid concentrations in rats, induced hepatic enzymes in dogs and affected cholesterol levels (2007). The WHO-recommended concentration of pyriproxyfen for treatment of drinking water containers was 0.1 mg/L. Brazil’s treatment of drinking water with pyriproxyfen following the table published in Government B. 2014, equated to 2 mg/L of pyriproxyfen (Government, 2014) and would result in a daily intake 25 – 50% of the WHO limit for an average sized adult.
Concern has been expressed that the unprecedented scale of pyriproxyfen addition to the drinking water in Brazil may itself be causing or enhancing the increased incidence of micropcephaly in Zika affected areas, though peer-reviewed evidence that directly addresses these concerns is still lacking. To explore the potential adverse effects of pyriproxyfen on vertebrate development, we used the developmental zebrafish model. Our goal was to determine if early life stage pyriproxyfen exposure would produce early developmental or behavioral changes. The high genetic and physiological orthology of this model increases the probability that observed chemical phenotypes are relevant to humans. Herein, we report that exposure to 5.2 uM pyriproxyfen, renewed daily, resulted in high incidences of mortality and morphological defects. While this was not observed with static pyriproxyfen exposures, the static regimen did uncover behavioral differences relative to the control animals.
2. Materials and Methods
2.1. Chemicals
Pyriproxyfen (2-[1-Methyl-2-(4-phenoxyphenoxy)ethoxy]pyridine was purchased from Sigma Aldrich (CAS 95737-68-1, Cat. No. 34174) Stock solutions (10mM) were prepared using 100% dimethyl sulfoxide (DMSO) and stored in 4C.
2.2. Zebrafish Husbandry
Wild type Tropical 5D zebrafish were housed at Oregon State University Sinnhuber Aquatic Research Laboratory in density of 1000 fish in 100 gallon tanks. The tanks were kept at standard laboratory conditions. Zebrafish were spawned, collected and staged according to Kimmel et al (1995) (Kimmel et al., 1995). To increase bioavailability, the chorion was enzymatically removed using pronase (90 μL of 25.3 U/μl; Roche, Indianapolis, In, USA) at 4 hours post fertilization (hpf) using a custom automated dechorionator (Mandrell et al., 2012).
2.3. Chemical Exposure
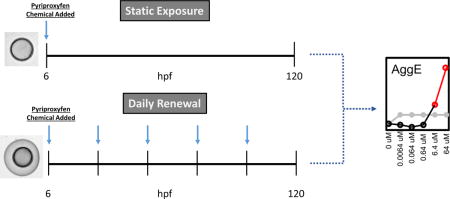
Six hours post fertilization embryos were transferred into individual wells of a 96-well plate filled with 100 μL of embryo medium (EM) (Mandrell et al., 2012). The 10 mM stock solutions were dispensed using the Hewlett-Packard D300 Digital Dispenser, an inkjet technology that can directly deliver the chemical at the desired concentration into the experimental chamber. A lower energy mixing protocol was scripted for the HP D300 and implemented to automatically mix for 1 sec between all deliveries of at least 5 nL (Truong et al., 2016), again for 15 sec after each dispense head on the cassette was exhausted (on average after every 16 wells, dependent on the protocol), then again for 15 sec after the HP D300 completed the plate (Truong et al., 2016). All wells were normalized with 0.64% DMSO. A total of 6 concentrations were tested (0, 0.0064, 0.064, 0.64, 6.4, and 64 μM) with 32 animals per concentration. The plates were sealed using parafilm to reduce evaporation and wrapped in aluminum foil to prevent photodegradation. For the static exposure, the above steps were completed by 8 hpf, and the plates placed on an orbital shaker at 235 rpm, 28°C for 16 hrs, thereafter in a 28°C incubator. The daily renewal exposures were initiated at the same time as the static exposures except the chorions were left intact. A Tecan plate washer (Tecan Hydroflex 3-in-1) was utilized to remove solution, and replace with fresh EM. Afterwards, the HP D300 was used as described above to deliver pyriproxyfen from day 2 – 4.
2.4. Developmental Toxicity Screen
At 24 and 120 hpf, a total of 22 morphological endpoints were assessed according to Truong et al (Truong et al., 2011). Briefly, at 24 hpf, mortality, developmental progression, normal spontaneous movement, and notochord were evaluated for presence of an aberrant phenotype. At 120 hpf, embryos were assessed for 17 morphological endpoints and collected in a laboratory information management system called the Zebrafish Acquisition and Analysis Program (ZAAP). Statistical significance was computed as described in Truong et al (Truong et al., 2014) and Zhang et al (Zhang et al., 2016).
2.5. Embryo Photomotor Response behavior (EPR)
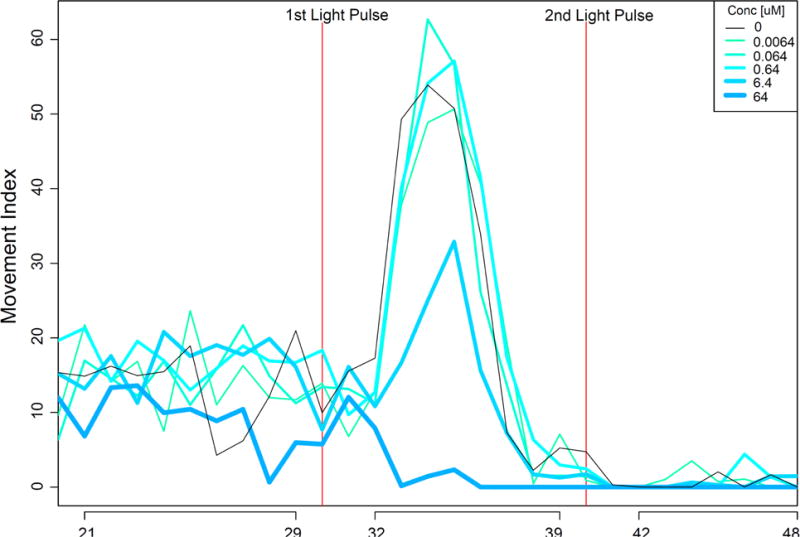
The embryonic photomotor response (EPR) was assessed at approximately 28 hpf when zebrafish typically exhibit a burst in contralateral axial bending in response to a bright flash of visible light. This activity is driven by photoreceptors in the hindbrain and the response is typically refractory to a second light flash (Kokel and Peterson, 2008; Raftery et al., 2014; Reif et al., 2016). The assay thus requires keeping the developing embryos in the dark until 28 hpf when embryos are subjected to two flashes of a bright light with a 9 sec interval between pulses. For every exposure plate, 850 frames of digital video were recorded at 17 frames s−1 from beneath a custom 96-well plate mount, and lighted from above with white LED and infrared lights. The light cycle consisted of 30 seconds of dark background (prior to the first light pulse), a short pulse of light, and 9 seconds later, a 2nd pulse of light, and then 10 more seconds of dark. Animals dead or malformed at the 24 hpf timepoint were excluded from the behavior data sets. Statistical analysis was conducted as described in Reif et al (2016) (Reif et al., 2016).
2.6. Larval Photomotor Response behavior (LPR)
At 120 hpf (5 days post fertilization) zebrafish are free swimming larvae and the photomotor response assayed total movement (swim distance) in response to multiple light -> dark transitions (Figure 3). Briefly, Zebrabox behavior chambers (ViewPoint Life Sciences, Montreal, CA) with an infrared backlit stage were used to track total movement in 96 wells during a 24-minute assay. HD video was captured at 15 frames s−1 and processed in real time by the manufacturer’s software. The assay consisted of 4 cycles of 3 minutes visible light, 3 minutes dark (IR light). The first light-dark cycle is considered acclimation, and the remaining 3 cycles were used for statistical analysis. Additional animals dead or malformed at the 120 hpf time point were excluded from the larval behavior data analysis. For each concentration, area under the curve (AUC) was calculated in R, and a Kolmogorov–Smirnov test was applied to identify statistical significance. Significance was determined as p<0.05 and a change in the AUC of > 40%).
Figure 3. Larval photomotor response to alternating light and dark cycles after static exposure.
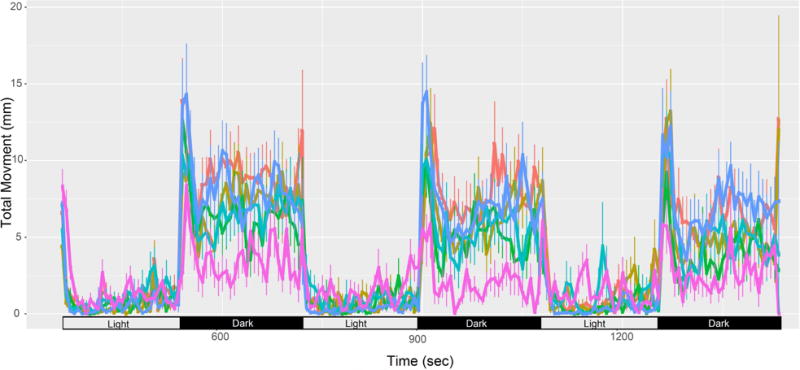
Data are reported as mean ± standard deviation at each second time bin. The number of contributing animals (originally n=32) for 0, 0.0064, 0.064, 0.64, 6.4, and 64 μM is 28, 24, 24, 28, 25, and 15, respectively.
3. Results
3.1. Evaluating developmental toxicity under two exposure paradigms
Static, non-renewal exposure of dechorionated embryonic zebrafish to pyriproxyfen did not lead to a statistically significant increase in mortality or any adverse outcomes (Figure 1A). This is consistent with the reproductive and developmental findings reported in rats and rabbits. We also conducted studies in accordance with the Organisation for Economic Co-operation and Development (OECD) zebrafish protocol with modification(OECD): embryos with their chorions intact were placed into individual wells of a 96-well plate, and the exposure medium was renewed daily until 120hpf. At the conclusion of the daily renewal experiments, significant mortality and sublethal effects (YSE: yolk sac edema; AXIS: bent axis; SNOU: abnormal snout; JAW: jaw; PE__: pericardial edema and TR: lack of a touch response) were observed only at 6.4 and 64 μM (Figure 1B). This increase in pyriproxyfen toxicity over that seen with the static regimen may be due to the renewal offsetting losses due to metabolism. Pyriproxyfen lability may also have contributed to lower toxicity in the static exposures. We note that while its half-life is 16–21 days in aerobic lake water-sediment systems(Organization, 2008), the temperature of that study system was undoubtedly well below the constant 28°C of our system which might have hastened lability.
Figure 1. Comparison of developmental exposure to pyriproxyfen after (A) static exposure and (B) daily renewal.
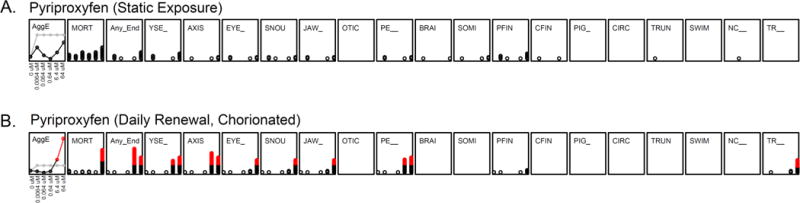
For the first panel of each chemical (Aggregated Entropy: AggE), the gray line represents the significant threshold of AggE by concentration, which is determined by summation cross all the endpoints; black line is the cumulative summation of chemical-associated AggE morphological effects by concentration, and the red colored points represents those that exceed the threshold. The other panels represent the 18 morphological endpoints assessed. The 4 endpoints measured at 24 hpf are not shown as there was no statistical significance. Each red dot represents a significant (above threshold) incidence count at a given concentration. A Fisher’s Exact test was used to identify the significance threshold.
3.2. Neurotoxicity of pyriproxyfen
To determine if pyriproxyfen exposures could impact early life stage behaviors, two neurobehavioral assays were conducted. At 24 hpf, we conduct an EPR assay. A typical EPR exhibits limited basal activity prior to the 1st light flash, a burst of axis bending immediately following illumination, and little or no movement in response to the second flash of light (Figure 2). The two highest concentrations in the daily renewal protocol produced significantly hypoactive embryos following the first light pulse with fold changes ranging from 45–90% relative to the controls (K-S test, P<0.05, delta > 40%). This behavioral phenotype would have been missed if we had only examined the EPR in statically exposed animals. In light of the high incidences of mortality and morphology defects from the daily renewal regimen, the hypoactive EPR detected earlier in the same animals suggests that the EPR is predictive of later adverse outcomes. It does not directly indicate neurotoxicity as many non-neuronal chemical effects could result in hypoactivity in this assay.
Figure 2.
The second behavioral assay was conducted at 120 hpf, to evaluate larval photomotor response (LPR) changes in swimming activity following a cyclical light – dark stimulus. The rationale for this assay is that, barring animals with morphological defects, and aberrant LPR in an otherwise normal animal is more likely to directly implicate neurotoxicity than the EPR. The typical LPR (Figure 3) exhibits modest movements in the light, and pronounced increases in motor activity in the dark phase. In the static exposure regimen, the two highest pyriproxyfen concentrations (6.4 and 64 uM) were associated with hypoactive swimming activity relative to the control fish. These concentration effects were not directly comparable to the 6.4 and 64 uM daily renewal LPR because high mortality/malformation at these daily renewal concentrations precluded a valid LPR. We did not detect an abnormal LPR below 6.4 uM, in either exposure regimen. There are no peer-reviewed reports demonstrating that developmental exposure to pyriproxyfen in rats or mice lead to behavior phenotypes. However, in honeybees it was observed that exposure to pyriproxyfen disrupted behavior and elicited malformations (Fourrier et al., 2015).
3.2. Bioactivity comparison of other HTS assays
Pyriproxyfen was previously tested in an EPA zebrafish screen for morphological effects (daily renewal) and in the ToxCast in vitro assays. The EPA screen was similar to our daily renewal studies and the resulting EC50 was 26.13 μM(Padilla et al., 2012), whereas our estimated EC50 from the daily renewal exposures herein was 5.2 μM. The lack of significant mortality and morphology effects in the static exposures precluded estimation of an EC50. The differences between EC50s may be attributable to the chemical delivery methods and differences in the zebrafish strains used. The EPA ToxCast program (Dix et al., 2007) includes ~700 assays that covers a wide swath of biological space. A public search for the pyriproxyfen toxicity profiles across the ToxCast assays (www.actor.epa.gov/dashboard/) indicates that it was bioactive in 72 assays, 28 of which had estimated EC50 values that were predicted from the computational models with higher confidence. The biological processes queried by these assays included mitochondrial depolarization, cell cycle, regulation of transcription factors, cell death and cell proliferation. These biological processes are conserved in the embryonic zebrafish, and perturbations in all of them have been definitively linked to adverse developmental outcomes such as mortality and morphology defects.
4. Conclusion
We demonstrated the utility of the developmental zebrafish to uncover chemical bioactivity that might otherwise be missed by traditional mammalian testing of a single oral dose. Daily renewal of pyriproxyfen exposure to offset lability and metabolism losses resulted in severe mortality and teratogenicity in larval zebrafish. But static exposures resulted in low teratogenicity consistent with that seen in single dose experiments in rabbits and rats. The aberrant larval (120 hpf) photomotor behavior we detected suggested that pyriproxyfen is developmentally neurotoxic to zebrafish, at low to mid micromolar concentrations. We however did not detect abnormal LPR below 6.4 uM in either the static or the renewal exposure regimens. Obviously, the subtle differences of pyriproxyfen interactions with developmental pathways between the two exposure regimens lead to vastly different outcomes. Based on a static exposure regimen, pyriproxyfen appeared developmentally benign except for a modest potential for neuronal effects, while a daily renewal regimen under the chemical delivery, strain and test conditions that we used yielded an EC50 more than 5-fold lower than previously estimated in zebrafish. Given the higher than recommended levels of pyriproxyfen now widely used in Brazilian drinking water, daily exposure of pregnant woman and children, and its central role in attempting to ameliorate a public health crisis, much more intensive study of pyriproxyfen toxicity is warranted. One immediate goal should be assessment of chronic, low dose pyriproxyfen effects on more complex social and cognitive behaviors in zebrafish and in rodents as a more actionable measure of hazard potential. Another immediate goal should be to determine the conserved developmental pathways that pyriproxyfen targets so that we can fully understand why it and related chemistries are developmentally toxic.
Supplementary Material
Highlights.
Rapid 5-day zebrafish assay detected developmental and behavioral effects of pyriproxyfen
50% adverse effect (EC50) was 5.2μM for daily renewal exposure
Behavioral effects observed at concentrations higher than 6.4 μM
Acknowledgments
We would like to thank members of the Tanguay laboratory, SARL for assistance with fish husbandry. This work was supported by NIEHS grant P30 ES000210 and Environmental Protection Agency STAR Grant # R835168 to RLT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lisa Truong, Email: Lisa.truong@oregonstate.edu.
Greg Gonnerman, Email: Gman@oregonstate.edu.
Michael T. Simonich, Email: mtsimonich@oregonstate.edu.
Robert L. Tanguay, Email: Robert.tanguay@oregonstate.edu.
References
- 1.Organization, W.H., editor. Water Quality Guidelines: Pyriproxyfen. 2007. [Google Scholar]
- 2.Centers for Disease Control and Prevention, C. All Countries and Territories with Active Zika Virus Transmission 2016a [Google Scholar]
- 3.Centers for Disease Control and Prevention, C. CDC Concludes Zika Causes Microcephaly and Other Birth Defects 2016b [Google Scholar]
- 4.Dix DJ, Houck KA, Martin MT, Richard AM, Setzer RW, Kavlock RJ. The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicol Sci. 2007;95:5–12. doi: 10.1093/toxsci/kfl103. [DOI] [PubMed] [Google Scholar]
- 5.Fourrier J, Deschamps M, Droin L, Alaux C, Fortini D, Beslay D, Le Conte Y, Devillers J, Aupinel P, Decourtye A. Larval Exposure to the Juvenile Hormone Analog Pyriproxyfen Disrupts Acceptance of and Social Behavior Performance in Adult Honeybees. PLoS ONE. 2015;10:e0132985. doi: 10.1371/journal.pone.0132985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Government, B. Orientações técnica para utilização do larvicida pyriproxyfen (0,5 G) no controle de Aedes aegypti 2014 [Google Scholar]
- 7.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 8.Kokel D, Peterson RT. Chemobehavioural phenomics and behaviour-based psychiatric drug discovery in the zebrafish. Brief Funct Genomic Proteomic. 2008;7:483–490. doi: 10.1093/bfgp/eln040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandrell D, Truong L, Jephson C, Sarker MR, Moore A, Lang C, Simonich MT, Tanguay RL. Automated Zebrafish Chorion Removal and Single Embryo Placement. Journal of Laboratory Automation. 2012;17:66–74. doi: 10.1177/2211068211432197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test. OECD Publishing; [Google Scholar]
- 11.Organization, W.H. Guidelines for Drinking-Water Quality. (3rd) 2008 [Google Scholar]
- 12.Padilla S, Corum D, Padnos B, Hunter DL, Beam A, Houck KA, Sipes N, Kleinstreuer N, Knudsen T, Dix DJ, Reif DM. Zebrafish developmental screening of the ToxCast Phase I chemical library. Reprod Toxicol. 2012;33:174–187. doi: 10.1016/j.reprotox.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Raftery TD, Isales GM, Yozzo KL, Volz DC. High-content screening assay for identification of chemicals impacting spontaneous activity in zebrafish embryos. Environ Sci Technol. 2014;48:804–810. doi: 10.1021/es404322p. [DOI] [PubMed] [Google Scholar]
- 14.Reif DM, Truong L, Mandrell D, Marvel S, Zhang G, Tanguay RL. High-throughput characterization of chemical-associated embryonic behavioral changes predicts teratogenic outcomes. Arch Toxicol. 2016;90:1459–1470. doi: 10.1007/s00204-015-1554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sciences, N.I.o.H., editor. Safety, I.P.o.C. Pesticide residues in food – Pyriproxyfen. Tokyo, Japan: 1999. [Google Scholar]
- 16.Truong L, Bugel SM, Chlebowski A, Usenko CY, Simonich MT, Simonich SL, Tanguay RL. Optimizing multi-dimensional high throughput screening using zebrafish. Reprod Toxicol. 2016;65:139–147. doi: 10.1016/j.reprotox.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Truong L, Harper SL, Tanguay RL. Evaluation of Embryotoxicity Using the Zebrafish Model. In: Gautier J-C, editor. Drug Safety Evaluation: Methods and Protocols. Humana Press; Totowa, NJ: 2011. pp. 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Truong L, Reif DM, St Mary L, Geier MC, Truong HD, Tanguay RL. Multidimensional in vivo hazard assessment using zebrafish. Toxicol Sci. 2014;137:212–233. doi: 10.1093/toxsci/kft235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang G, Marvel S, Truong L, Tanguay RL, Reif DM. Aggregate entropy scoring for quantifying activity across endpoints with irregular correlation structure. Reprod Toxicol. 2016;62:92–99. doi: 10.1016/j.reprotox.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


