Abstract
The sensory organs for taste in chickens (Gallus sp.) are taste buds in the oral epithelium of the palate, base of the oral cavity, and posterior tongue. Although there is not a pan-taste cell marker that labels all chicken taste bud cells, α-Gustducin and Vimentin each label a subpopulation of taste bud cells. In the present study, we used both α-Gustducin and Vimentin to further characterize chicken taste buds at the embryonic and post-hatching stages (E17-P5). We found that both α-Gustducin and Vimentin label distinct and overlapping populations of, but not all, taste bud cells. A-Gustducin immunosignals were observed as early as E18 and were consistently distributed in early and mature taste buds in embryos and hatchlings. Vimentin immunoreactivity was initially sparse at the embryonic stages then became apparent in taste buds after hatch. In hatchlings, α-Gustducin and Vimentin immunosignals largely co-localized in taste buds. A small subset of taste bud cells were labeled by either α-Gustducin or Vimentin or were not labeled. Importantly, each of the markers was observed in all of the examined taste buds. Our data suggest that the early onset of α-Gustducin in taste buds might be important for enabling chickens to respond to taste stimuli immediately after hatch and that distinctive population of taste bud cells that are labeled by different molecular markers might represent different cell types or different phases of taste bud cells. Additionally, α-Gustducin and Vimentin can potentially be used as molecular markers of all chicken taste buds in whole mount tissue.
Keywords: α-Gustducin, Vimentin, taste bud, chicken, poultry, molecular marker, connective tissue, mesenchyme
Introduction
Like mammals, chickens respond to taste stimuli [1, 2]. Right after hatch, chickens exhibit aversive responses to bitter and sour tastes [2–4] and prefer umami and fat taste substances [5, 6]. In chickens, the sensory organs for taste are taste buds located in the palate, base of the oral cavity, and posterior region of the tongue [7, 8]. The structure and locations of the taste buds have been identified by scanning electron microscopy [7, 8] and histological analyses [9, 10]. At embryonic day 17 (E17) chicken taste bud primordia emerge as clusters of spherical cells and mature at E19 as ovoid-shaped cell clusters that penetrate the epithelium and have a taste pore [10].
While pan-taste cell markers for mammals are available, including K8 [11, 12] and KCNQ [13], similar markers for chicken taste buds are lacking. Vimentin, an intermediate filament that is expressed in the mesenchyme, connective tissue cells, and neural precursors [14, 15], labeled a population of chicken taste bud cells from embryonic to post-hatching (P) stages [16, 17]. More recently, Gustducin expression was found in mature chicken taste buds at P3, suggesting a similar signaling mechanism as that in mammals [9]. However, the following questions remain: (1) when does α-Gustducin expression begin in the developing taste buds for involvement with taste signal transduction; (2) whether α-Gustducin and Vimentin label the same or different populations of taste bud cells; and (3) whether α-Gustducin and Vimentin together label all taste bud cells. Clarification and comparison of different molecular markers in the labeling of chicken taste bud cells will provide new insight into how chicken taste buds develop and function.
In the present study, we characterized the distribution of α-Gustducin and Vimentin immunosignals in chicken taste buds in late embryos and hatchlings. We found that α-Gustducin immunosignals emerged early in premature taste buds and were consistently distributed in a large population of mature taste bud cells. Brightly labeled Vimentin+ cells were abundant in taste buds after hatch. The markers labeled distinct and overlapping taste bud cell populations. Although both markers did not label all the taste bud cells, each marker was observed in all the taste buds. These findings provide novel information regarding the development and function of chicken taste buds.
Materials and Methods
Animals and tissue collection
Animal use was approved by The University of Georgia Institutional Animal Care and Use Committee and was in compliance with the National Institutes of Health Guidelines for care and use of animals in research.
Fertilized eggs and newly hatched (P0) male chicks were obtained from the Cobb Vantress, Inc, Cleveland Hatchery, Georgia. The chicks were housed in the animal facility until P5 with brood temperature maintained at 35°C and room temperature at 30°C. The chicks were continuously monitored. Animals were maintained with food (starter feed) and water ad libitum under a 12-12 hr dark-light cycle. For tissue collected at embryonic days 17, 18 and 19 (E17, E18, and E19), the fertilized eggs were incubated in a standard egg incubator at 37.7°C and 50–60% humidity.
Tissue from the palate, base of the oral cavity and posterior tongue was collected at E17, E18, E19, P0, P1, P3 and P5. For the embryonic tissue, timed incubated eggs were cracked and embryos were collected in 0.1 M PBS solution (pH 7.3). P0-P5 chickens were euthanized by decapitation. The tissue samples were dissected and fixed in 4% paraformaldehyde (PFA) for ~4 hr at room temperature. The tissue was briefly rinsed in 0.1 M PBS followed by cryoprotection with 30% sucrose at 4°C for ~48 hr. The tissue was trimmed under a dissecting microscope to include regions that contained taste buds, and then embedded in OCT compound (Tissue Tek) at a sagittal orientation and rapidly frozen. Serial and neighboring sections were cut at 6–15 µm thickness, mounted onto gelatin-coated glass slides, and processed for different analyses as below.
Histological analysis for identification of chicken taste bud structure
Frozen, 6 µm-thick sections from E17, E18, E19 and P0 tissue samples were used for hematoxylin and eosin staining following the standard procedure. The sections were examined under a Zeiss AX10 light microscope.
Immunohistochemistry and quantification
The primary antibodies used were: α-Gustducin (1:500, serum of rabbit immunized with chicken α-Gustducin, generated by Dr. Shoji Tabata’s lab) [9]; Epcam (epithelial cell adhesion molecule markers) (1:200, MBS2027145, Mybioresource Inc, San Diego, CA); Vimentin (1:200, Abcam 28028, Vim3B4, Abcam, Cambridge, MA). Secondary antibodies were: Alexa Fluor 647 conjugated donkey anti-rabbit secondary antibody (1:500, Code: 711-605-152; Jackson Immuno Research Laboratories, West Grove, PA), Alexa Fluor 488 conjugated donkey anti-mouse (1:500, Code: 715-545-150, Jackson Immuno Research Laboratories, West Grove, PA). Frozen sections of the base of the oral cavity tissue at E17-P5 and palate at E19 and P0 were immunostained. In brief, sections were air dried for 1 hr at room temperature then rehydrated in 0.1 M PBS. Non-specific staining was blocked using 10% normal donkey serum in 0.1 M PBS containing 0.3% Triton X-100 (PBS-X) for 30 min at room temperature. Then, the sections were incubated with primary antibody in 1% normal donkey serum in PBS-X overnight at 4°C. Following 3 rinses in 0.1 M PBS (10 min) the sections were incubated with AF 488 (for Vimentin) and AF 647 (for α-Gustducin & Epcam) in 1% NDS in PBS-X for 1 hr at room temperature. The sections were then rinsed with PBS and counterstained with DAPI (200 ng/ml in PBS) for 10 min, rinsed in 0.1 M PBS, air dried and cover slipped with ProLong® Diamond antifade mounting medium (P3697, ThermoFisher Scientific). In the negative control slide, primary antibody treatment was omitted or replaced with normal serum/IgG.
Co-localization of α-Gustducin and Vimentin immunosignals was analyzed by single plane laser-scanning confocal microscopy using a Zeiss LSM 710 microscope. Representative photomicrographs were assembled and edited using Adobe Photoshop CC 2015 software. Quantitative analysis was carried out to determine the proportion of Vimentin+ and α-Gustducin+ cells in taste buds at the base of the oral cavity at P5 (n=2). Both Vimentin and α-Gustducin immunosignals, together with the structural organization of DAPI stained nuclei, were used to mark the boundaries of the taste buds on each section. The total number of DAPI stained nuclei within the boundary was quantified and described as the total number of cell profiles in a taste bud. The numbers of Vimentin+, α-Gustducin+, and Vimentin+ α-Gustducin+ cells with clear DAPI staining were counted.
Results
Distribution of α-Gustducin and Vimentin in early taste buds in chicken embryos
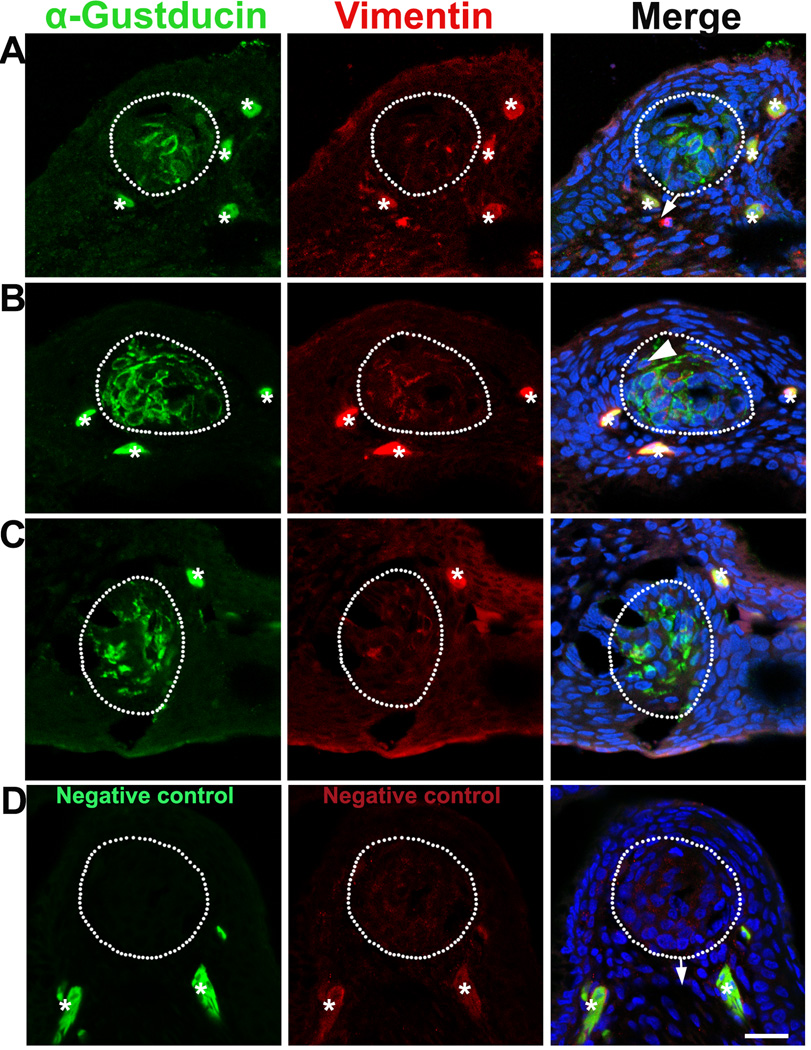
Based on a previous report stating that chicken taste buds emerge at E17 and mature by E19, we examined the immunosignals of α-Gustducin and Vimentin in E17–19 chicken embryos. At E17, taste bud structures were identified in the H & E stained sections. However, α-Gustducin and Vimentin immunoproducts were not detected in neighboring sections of those with taste bud structures (data not shown). At E18, α-Gustducin signals were observed in the specified cell clusters in the epithelium of the base of the oral cavity (Fig. 1A). Vimentin immunosignals, although sparse, were also observed in the α-Gustducin+ cell cluster region (white dotted outlines, Fig. 1A) and underlying mesenchyme (arrows, Fig. 1A). At E19, α-Gustducin+ cell clusters were apparent and larger in size (Fig. 1B, C) as compared to those observed at E18. Vimentin immunosignals were sparse in the α-Gustducin+ cell cluster region at E19, in addition to being distributed in the underlying mesenchyme. Cells double labeled with both α-Gustducin and Vimentin were observed infrequently (Fig. 1B, arrowhead).
Fig. 1.
Single plane laser-scanning confocal photomicrographs illustrate the distribution of α-Gustducin (green) and Vimentin (red) immunosignals in the oral tissue of chicken at E18 (A) and E19 (B–C) in the base of the oral cavity (A, B) and palate (C). Sections of the base of the oral cavity reacted without adding primary antibodies were used as negative controls (D, E19). Sections were counterstained with DAPI (blue) to stain cell nuclei. White dots outline the specified cell clusters where taste buds develop. Arrows point to the connective tissue. Arrowhead in B point to a cell double labeled with both α-Gustducin and Vimentin. Asterisks mark non-specifically labeled cells. Scale bars: 20 µm for all images
No signals were detected in the specified cell clusters (presumably taste buds) (white dotted outlines, Fig. 1D) in control slides without primary antibody treatment. However, solid labeling of whole cells was observed in negative control sections when omitting primary antibodies (asterisks, Fig. 1D), which suggests that the pattern of labeling outside of taste buds in the sections was non-specific (asterisks, Fig. 1A–C).
α -Gustducin and Vimentin largely co-localized in mature taste buds
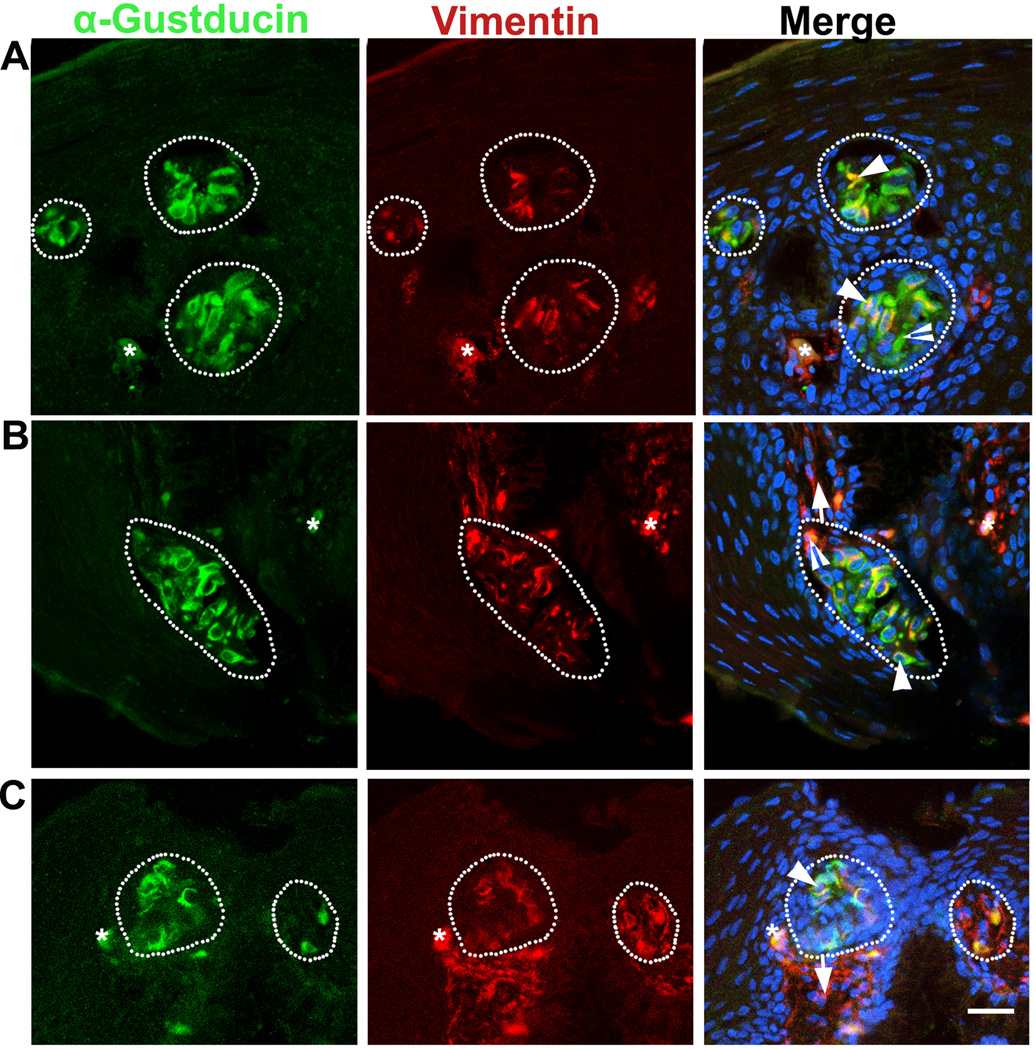
At P0, α-Gustducin immunosignals were apparent in the cluster of specified cells in the epithelium of the base of oral cavity (white dotted outlines, Fig. 2A), palate (white dotted outlines, Fig. 2B) and posterior region of the tongue (white dotted outlines, Fig. 2C). Vimentin immunosignals were extensively distributed in the underlying connective tissue. In addition, bright signals were seen in a subset of cells in all of the α-Gustducin+ cell cluster regions and presumably are taste buds. Importantly, Vimentin immunosignals were not seen in the epithelium outside of the taste buds. Within the specified cell clusters in the oral epithelium, co-localization of α-Gustducin and Vimentin immunosignals was detected in a large population of taste bud cells (arrowheads, Fig. 2A–C). Also, singly labeled α-Gustducin+ or Vimentin+ cells were seen (open arrowheads, Fig. 2A–B).
Fig. 2.
Distribution of α-Gustducin (green) and Vimentin (red) immunosignals in the oral tissue of a P0 chicken: base of the oral cavity (A), palate (B), and posterior region of the tongue (C). White dots outline the specified cell clusters, presumably taste buds. Arrows point to the connective tissue. Arrowheads in A–C point to some of the cells double labeled with α-Gustducin and Vimentin. Open arrowheads in A–B point to singly labeled α-Gustducin+ or Vimentin+ cells. Asterisks in A and C mark cells with nonspecific labeling. Scale bar: 20 µm for all images (single plane laser-scanning confocal).
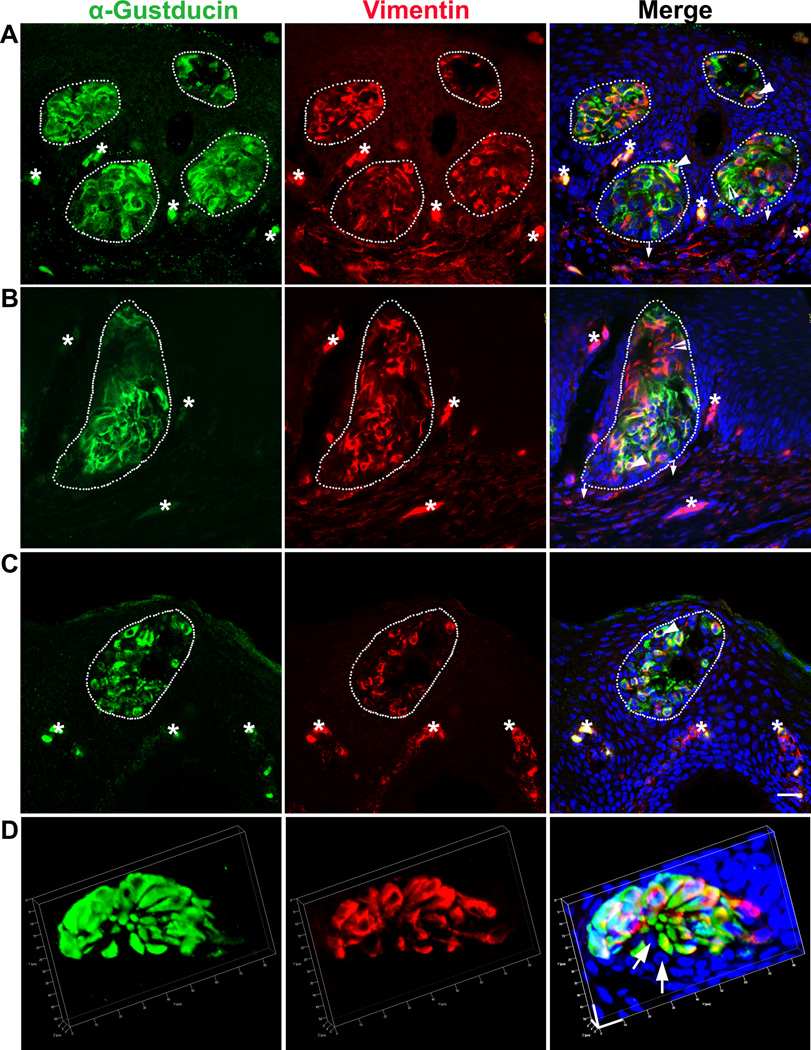
At later stages (P1–P5), intense α-Gustducin and Vimentin immunosignals were consistently distributed in all specified cell clusters in the epithelium of the base of oral cavity (Fig. 3). Vimentin+ cells were frequently observed in taste buds and the immunosignals largely co-localized with α-Gustducin (78±6% of total labeled cells at P5) (arrowheads, Fig. 3A–C) (white dotted outlines, Fig. 3A–C). A small subpopulation of labeled taste bud cells was positive for only one of the markers (9±7%Vimentin+, 13±1% α-Gustducin+ at P5) (open arrowheads, Figure 3A–B). Non-labeled cells were also found, but rarely, within taste buds (Fig. 3D). Of note, although neither α-Gustducin nor Vimentin labeled all the taste bud cells, immunosignals were detected in all examined taste buds at post-hatch stages.
Fig. 3.
Overlapping but distinct distribution of α-Gustducin (green) and Vimentin (red) immunosignals in taste buds in the base of the oral cavity at P1 (A), P3 (B), and P5 (C, D). White dots outline the specified taste bud cell clusters. Arrows in A–C point to the connective tissue. Arrows in D point to the unlabeled cells. Arrowheads point to cells double labeled with both α-Gustducin and Vimentin. Open arrowheads in A–B point to singly labeled α-Gustducin+ or Vimentin+ cells. Asterisks mark non-specifically labeled cells. Scale bars: 20 µm for all images (A–C), X=Y=10 µm, Z= 8 µm (D) (single plane laser-scanning confocal in A–C, 3D confocal in D).
Vimentin+ taste bud cells acquire an epithelial cell phenotype
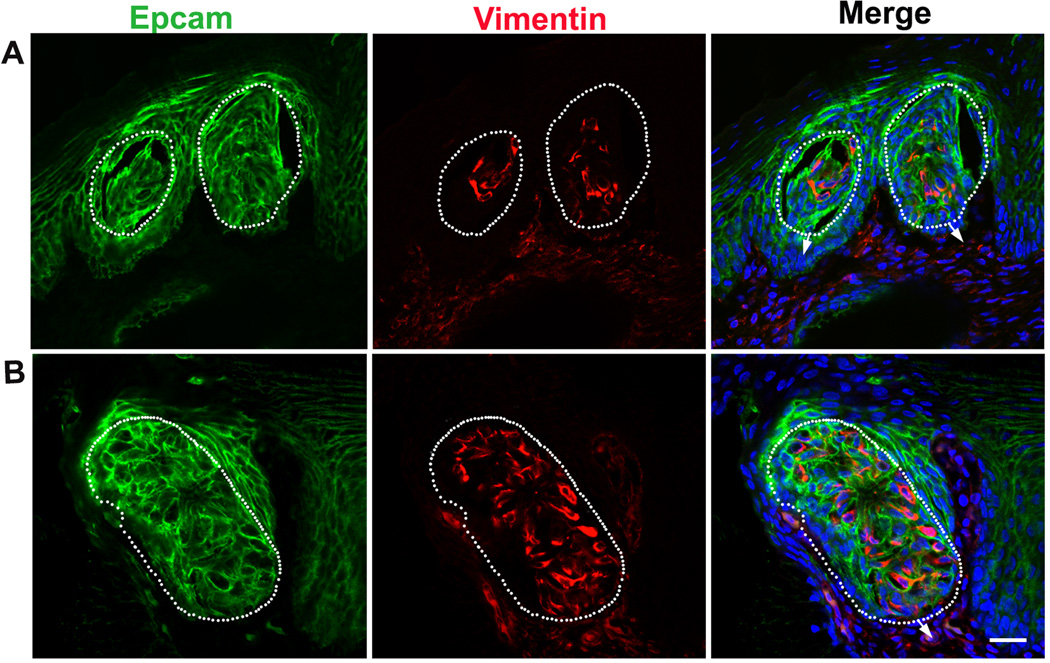
Double labeling of Vimentin and the epithelial cell adhesion molecule (Epcam) in the base of the oral cavity at P0 and P3 was performed to examine whether Vimentin+ cells in taste buds are also epithelial-like (Fig. 4). Epcam immunosignals were distributed in the epithelium (Fig. 4A–B), including in taste bud-like structures in which Vimentin immunosignals were present (Fig. 4A–B). Although Vimentin and Epcam immunosignals were in different cellular compartments, cytoplasm and membrane, respectively, co-localization of these immunosignals in taste bud cells was obvious. No Vimentin+ cells were found in the epithelium outside of the taste buds.
Fig. 4.
Distribution of Epcam (green) and Vimentin (red) immunosignals in taste buds in the base of the oral cavity of P0 (A) and P3 (B) chickens. Vimentin+ cells in taste buds were also Epcam+. White dots outline the specified taste bud cell clusters. Arrows point to the connective tissue. Vimentin and Epcam immunosignals were localized in different cellular compartments, i.e., cytoplasm and membrane, respectively, but co-localization of the signals in taste bud cells was obvious. Scale bar: 20 µm for all images (single plane laser-scanning confocal).
Discussion
Chicken taste buds, located in the oral epithelium in the palate, base of the oral cavity, and posterior region of the tongue [7, 8], emerge at E17 [10]. Taste buds are identified in the epithelium as clusters adjacent to the salivary gland opening [7, 8]. In the present study, we detected α-Gustducin immunoreactivity in a significant population of taste bud cells as early as E18 in immature taste buds and in mature taste buds through the embryonic and post-hatching stages. These results suggest an important role for α-Gustducin in mediating taste signals. Vimentin, a marker that labels the underlying connective tissue of the oral cavity [16, 17], was detected in a large population of taste bud cells and Vimentin+ cells acquired an epithelial phenotype, suggesting a mesenchymal-epithelial transition. Taste bud cell populations labeled by α-Gustducin and/or Vimentin or neither may represent different origins, types, and functions of taste bud cells.
Gustducin expression in premature taste buds suggests taste function before hatch
Gustducin is a G-protein that plays a key role in signaling taste transduction [25, 26]. In mammals, it is expressed in type II taste bud cells that differentiate postnatally and comprise 20–30% of the taste receptor cells for sweet, bitter and umami tastes [26, 28]. In rats, taste bud cells that express α-Gustducin have a long life span compared to other cell types [27]. α -Gustducin is explicitly expressed in mature chicken taste buds at P3 [9] and is highly expressed in an isolated chicken taste bud-like subset of cells [30]. In the present study, we detected α-Gustducin immunosignals in immature taste buds as early as E18. As previously mentioned, chickens respond to bitter stimuli right after hatch [2]. α-Gustducin expression may be a sign of the early onset of a differentiated cell type that enables chickens to respond to gustatory stimuli before hatch [29]. Additionally, α-Gustducin+ cells, although not quantified at all stages, comprise a significant population (conservatively greater than 50%) of taste bud cells indicating the importance of GPCR signaling mechanisms for taste transduction in chickens.
Vimentin+ cells in taste buds suggest a contribution of connective tissue to taste buds
Our recent studies using transgenic mouse lines have demonstrated a novel progenitor source of taste bud cells, underlying connective tissue [18], in addition to the conventional concept that taste bud cells are derived from the surrounding epithelium [31–33]. In the both the present study and previous reports [16, 17], identification of Vimentin+ and Vimentin+Epcam+ cells in chicken taste buds [16, 17] supports the idea that underlying connective tissue cells may migrate into the epithelium and undergo a mesenchymal-epithelial transition and contribute to chicken taste buds. In humans, Vimentin is also expressed in the taste bud primordia and at later stages is restricted to the marginal cells of the bud [34]. Here, we propose that, in contrast to the hypothesis of Witt et al. [16, 17, 34] that “the mechanisms of taste bud differentiation from source tissues may differ among vertebrates of different taxa”, there may be a common principle among vertebrate species, at least in human, mouse and chicken, that connective tissue cells contribute to the formation and renewal of taste buds.
Differences do exist among these species. The relative proportions of taste bud cells that retain Vimentin expression appear to differ. The turn-over cycle and life span of taste bud cells are different in the different species - 10–12 days in mice [35] vs. 3–4 days in chickens [22, 36] - which might be the cause of different proportions of Vimentin+ taste bud cells. It is reasonable to speculate that chicken connective tissue cells retain Vimentin expression while they acquire an epithelial-like taste cell phenotype during rapid migration and differentiation to taste buds. Further studies on how interactions between the underlying connective tissue and the surrounding epithelium are regulated will be important for better understanding how taste buds are formed and renewed.
Distinct and overlapping distribution of α-Gustducin and Vimentin immunosignals indicate different features of taste bud cells
It has been reported that α-Gustducin and Vimentin each label a large subpopulation of taste bud cells in chickens [9, 16, 17]. However, this is the first study to use both markers to examine the distribution of labeled taste bud cells in early immature and mature taste buds. A large population of, but not all, the taste bud cells were labeled with both markers. The distinct and overlapping distribution of α-Gustducin and Vimentin in taste bud cells provides novel information regarding taste bud formation and function. First, both α-Gustducin and Vimentin immunosignals were distributed in all the examined taste buds (although they did not label all the taste bud cells), which indicate that they can be potential molecular markers for taste bud visualization and quantification if a protocol is available for use in whole mount tissue. Second, these two markers labeled distinct yet overlapping populations of taste bud cells. The α-Gustducin+, Vimentin+, α-Gustducin+ Vimentin+, and α-Gustducin−Vimentin− taste bud cells may represent different taste cell types or taste cells at different phases of development. Four different cell types (dark, light, intermediate and basal cells) have been identified in chickens [21, 22]. So far there are no molecular markers available that can be used to identify the specific cell types. As mentioned previously, α-Gustducin is expressed in type II taste bud cells of mammals [37] and are important for the transduction of sweet, umami and bitter tastes [38, 39]. Further studies are needed to identify whether the large population of chicken taste bud cells labeled by α-Gustducin are type II cells.
Taken together, our data suggest that the early onset of α-Gustducin in taste buds is important in enabling chickens to respond to taste stimuli immediately after hatch. Different taste bud cell populations labeled by different molecular markers might represent different cells types, phases, or lineages of taste bud cells.
Supplementary Material
Acknowledgments
We give thanks to the staff at Cobb Vantress, Inc, Cleaveland Hatchery, GA for providing the chickens and eggs. This study was supported by the National Institutes of Health (grant number R01 DC012308 to HXL) and University of Georgia Start-up fund to HXL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roura E, Baldwin MW, Klasing K. The avian taste system: Potential implications in poultry nutrition. Anim. Feed Sci. Tech. 2013;180:1–9. [Google Scholar]
- 2.Ganchrow JR, Steiner JE, Bartana A. Behavioral reactions to gustatory stimuli in young chicks (Gallus gallus domesticus) Dev. Psychobiol. 1990;23:103–117. doi: 10.1002/dev.420230202. [DOI] [PubMed] [Google Scholar]
- 3.Gentle M. Taste preference in the chicken (Gallus domesticus L.) Br. Poul. Sci. 1972;13:141–155. doi: 10.1080/00071667208415928. [DOI] [PubMed] [Google Scholar]
- 4.Hirose N, Kawabata Y, Kawabata F, Nishimura S, Tabata S. Bitter taste receptor T2R1 activities were compatible with behavioral sensitivity to bitterness in chickens. Biochem. Biophys. Res. Commun. 2015;460:464–468. doi: 10.1016/j.bbrc.2015.03.056. [DOI] [PubMed] [Google Scholar]
- 5.Sawamura R, Kawabata Y, Kawabata F, Nishimura S, Tabata S. The role of G-protein-coupled receptor 120 in fatty acids sensing in chicken oral tissues. Biochem. Biophys. Res. Commun. 2015;458:387–391. doi: 10.1016/j.bbrc.2015.01.125. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida Y, Kawabata Y, Kawabata F, Nishimura S, Tabata S. Expressions of multiple umami taste receptors in oral and gastrointestinal tissues, and umami taste synergism in chickens. Biochem. Biophys. Res. Commun. 2015;466:346–349. doi: 10.1016/j.bbrc.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 7.Ganchrow D, Ganchrow JR. Number and distribution of taste buds in the oral cavity of hatchling chicks. Physiol. Behav. 1985;34:889–894. doi: 10.1016/0031-9384(85)90009-5. [DOI] [PubMed] [Google Scholar]
- 8.Kudo K-i, Nishimura S, Tabata S. Distribution of taste buds in layer-type chickens: Scanning electron microscopic observations. Anim. Sci. J. 2008;79:680–685. [Google Scholar]
- 9.Kudo K-i, Wakamatsu Ki, Nishimura S, Tabata S. Gustducin is expressed in the taste buds of the chicken. Anim. Sci. J. 2010;81:666–672. doi: 10.1111/j.1740-0929.2010.00796.x. [DOI] [PubMed] [Google Scholar]
- 10.Ganchrow JR, Ganchrow D. Taste bud development in chickens (Gallus gallus domesticus) Anat. Rec. 1987;218:88–93. doi: 10.1002/ar.1092180113. [DOI] [PubMed] [Google Scholar]
- 11.Knapp L, Lawton A, Oakley B, Wong L, Zhang C. Keratins as markers of differentiated taste cells of the rat. Differentiation. 1995;58:341–349. doi: 10.1046/j.1432-0436.1995.5850341.x. [DOI] [PubMed] [Google Scholar]
- 12.Mbiene JP, Roberts JD. Distribution of keratin 8-containing cell clusters in mouse embryonic tongue: Evidence for a prepattern for taste bud development. J. Comp. Neurol. 2003;457:111–122. doi: 10.1002/cne.10551. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Iguchi N, Rong Q, Zhou M, Ogunkorode M, Inoue M, Pribitkin EA, Bachmanov AA, Margolskee RF, Pfeifer K. Expression of the voltage-gated potassium channel KCNQ1 in mammalian taste bud cells and the effect of its null-mutation on taste preferences. J. Comp. Neurol. 2009;512:384–398. doi: 10.1002/cne.21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franke WW, Schmid E, Osborn M, Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc. Natl. Acad. Sci. 1978;75:5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cochard P, Paulin D. Initial expression of neurofilaments and vimentin in the central and peripheral nervous system of the mouse embryo in vivo. J. Neurosci. 1984;4:2080–2094. doi: 10.1523/JNEUROSCI.04-08-02080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witt M, Ganchrow J, Ganchrow D. Distribution of vimentin in the developing chick taste bud during the perihatching period. Mol. Cell. Biol. 1999;45:303–316. [PubMed] [Google Scholar]
- 17.Witt M, Reutter K, Ganchrow D, Ganchrow JR. Fingerprinting taste buds: intermediate filaments and their implication for taste bud formation. Phil. Trans. R. Soc. B. 2000;355:1233–1237. doi: 10.1098/rstb.2000.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boggs K, Venkatesan N, Mederacke I, Komatsu Y, Stice S, Schwabe RF, Mistretta CM, Mishina Y, Liu H-X. Contribution of Underlying Connective Tissue Cells to Taste Buds in Mouse Tongue and Soft Palate. PloS One. 2016;11:e0146475. doi: 10.1371/journal.pone.0146475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H-X, Komatsu Y, Mishina Y, Mistretta CM. Neural crest contribution to lingual mesenchyme, epithelium and developing taste papillae and taste buds. Dev. Biol. 2012;368:294–303. doi: 10.1016/j.ydbio.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurosawa T, Kusuhara S, Ishida K, Niimura S. Morphological studies of taste buds in chickens. Jpn. J. Zootech. Sci. 1983;54:502–510. [Google Scholar]
- 21.Ganchrow D, Ganchrow JR, Goldstein RS. Ultrastructure of palatal taste buds in the perihatching chick. Am. J. Anat. 1991;192:69–78. doi: 10.1002/aja.1001920108. [DOI] [PubMed] [Google Scholar]
- 22.Ganchrow JR, Ganchrow D, Royer SM, Kinnamon JC. Aspects of vertebrate gustatory phylogeny: morphology and turnover of chick taste bud cells. Microsc. Res. Techniq. 1993;26:106–119. doi: 10.1002/jemt.1070260204. [DOI] [PubMed] [Google Scholar]
- 23.Ganchrow D, Ganchrow JR, Royer SM, Dovidpor S, Kinnamon JC. Identified taste bud cell proliferation in the perihatching chick. Chem. Senses. 1998;23:333–341. doi: 10.1093/chemse/23.3.333. [DOI] [PubMed] [Google Scholar]
- 24.Ganchrow D, Ganchrow JR, Gross-lsseroff R, Kinnamon JC. Taste bud cell generation in the perihatching chick. Chem. Senses. 1995;20:19–28. doi: 10.1093/chemse/20.1.19. [DOI] [PubMed] [Google Scholar]
- 25.Lagerström MC, Hellström AR, Gloriam DE, Larsson TP, Schiöth HB, Fredriksson R. The G protein–coupled receptor subset of the chicken genome. PLoS Comp. Biol. 2006;2:e54. doi: 10.1371/journal.pcbi.0020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLaughlin SK, McKinnon PJ, Margolskee R. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- 27.Cho YK, Farbman AI, Smith DV. The timing of α-gustducin expression during cell renewal in rat vallate taste buds. Chem. Senses. 1998;23:735–742. doi: 10.1093/chemse/23.6.735. [DOI] [PubMed] [Google Scholar]
- 28.Zhang G-H, Zhang H-Y, Deng S-P, Qin Y-M. Regional Differences in Taste Bud Distribution and α-Gustducin Expression Patterns in the Mouse Fungiform Papilla. Chem. Senses. 2008;33:357–362. doi: 10.1093/chemse/bjm093. [DOI] [PubMed] [Google Scholar]
- 29.Vince MA. Taste sensitivity in the embryo of the domestic fowl. Anim. Behav. 1977;25:797–805. doi: 10.1016/0003-3472(77)90033-1. [DOI] [PubMed] [Google Scholar]
- 30.Kudo Ki, Kawabata F, Nomura T, Aridome A, Nishimura S, Tabata S. Isolation of chicken taste buds for real-time Ca2+ imaging. Anim. Sci. J. 2014;85:904–909. doi: 10.1111/asj.12222. [DOI] [PubMed] [Google Scholar]
- 31.Stone LM, Finger TE, Tam P, Tan S-S. Taste receptor cells arise from local epithelium, not neurogenic ectoderm. Proc. Natl. Acad. Sci. 1995;92:1916–1920. doi: 10.1073/pnas.92.6.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okubo T, Clark C, Hogan BL. Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells. 2009;27:442–450. doi: 10.1634/stemcells.2008-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone LM, Finger TE. Mosaic analysis of the embryonic origin of taste buds. Chem. Senses. 1994;19:725–735. doi: 10.1093/chemse/19.6.725. [DOI] [PubMed] [Google Scholar]
- 34.Witt M, Kasper M. Distribution of cytokeratin filaments and vimentin in developing human taste buds. Anat. Embryol. 1999;199:291–299. doi: 10.1007/s004290050229. [DOI] [PubMed] [Google Scholar]
- 35.Farbman A. Renewal of taste bud cells in rat circumvallate papillae. Cell Prolif. 1980;13:349–357. doi: 10.1111/j.1365-2184.1980.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 36.Ganchrow D, Ganchrow JR, Romano R, Kinnamon JC. Ontogenesis and taste bud cell turnover in the chicken. I. Gemmal cell renewal in the hatchling. J. Comp. Neurol. 1994;345:105–114. doi: 10.1002/cne.903450108. [DOI] [PubMed] [Google Scholar]
- 37.Yang R, Tabata S, Crowley HH, Margolskee RF, Kinnamon JC. Ultrastructural localization of gustducin immunoreactivity in microvilli of type II taste cells in the rat. J. Comp. Neurol. 2000;425:139–151. doi: 10.1002/1096-9861(20000911)425:1<139::aid-cne12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 38.Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 39.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.