Abstract
The thalamus is the major source of cortical inputs shaping sensation, action and cognition. Thalamic circuits are targeted by two major inhibitory systems: the thalamic reticular nucleus (TRN) and extra-thalamic inhibitory (ETI) inputs. A unifying framework of how these systems operate is currently lacking. Here, we propose that TRN circuits are specialized to exert thalamic control at different spatiotemporal scales. Local inhibition of thalamic spike rates prevails during attentional selection whereas global inhibition more likely during sleep. In contrast, the ETI (arising from basal ganglia, zona incerta, anterior pretectum and pontine reticular formation) provides temporally-precise and focal inhibition, impacting spike timing. Together, these inhibitory systems allow graded control of thalamic output, enabling thalamocortical operations to dynamically match ongoing behavioral demands.
Keywords: thalamocortical, GABA, reticular thalamic nucleus, basal ganglia
Introduction – General questions
While several studies have elegantly delineated roles for thalamus in relaying sensory inputs to the neocortex, the extensive reciprocal connections between thalamic nuclei and all cortical regions suggest that the function of the thalamus extends well beyond sensory processing and simple relay. Thalamic nuclei are integral to processes involving motor control [1] memory [2] and arousal [3]. In one of the best studied cases, the visual thalamus, the retinal signal experiences substantial transformation on its way to the cortex that involves contrast-and context-dependent gain modulation [4] as well as temporal structuring [5]. Because these operations are prevalent across non-sensory systems as well, analogous thalamic circuits and computations are likely to subserve multiple cognitive functions [6–8].
The aforementioned thalamic operations require highly complex inhibitory control. Unlike cortex, striatum and cerebellum, the thalamus lacks a variety of interneuron types that provides spatio-temporally diverse and precise GABAergic input to its projection neurons (see Box 1). The best-studied source of thalamic inhibition derives from a thin sheet of cells, the thalamic reticular nucleus (TRN) which innervates all its individual nuclei [9]. Although some heterogeneity has been described among TRN neurons, their morphology and neurochemistry appear to be much less diverse than those of cortical interneurons [10,11]. Nevertheless, thalamic operations are under similar constraints as those of the neocortex, requiring inhibitory control across multiple spatial and temporal scales (see Box 1).
BOX 1. Scales of inhibition.
The nervous system needs to organize neuronal activity across multiple spatial and temporal scales. The spatial domain ranges from local cell populations to the entire brain, the time domain from slow oscillations (0.1 – 1Hz) to high gamma activity (up to 250Hz) [81]. In several brain regions (cortex, hippocampus, striatum, cerebellum) one solution to cope with these the wide range of scales is the emergence of distinct interneuron classes [82–85]. E.g. the size of axon arbors in cortical interneurons ranges from small, very dense (e.g neurogliaform cells) through mid-range, covering roughly a cortical column (e.g. basket cells) to long range interneuron selective cells which can simultaneously affect the activity of large cortical territories [86]. The size of axon arbor, thus, will physically determine the spatial scale of action. Along the temporal dimension, the firing rate of the interneurons as well as the exact mechanism of GABAergic action will determine the scale of action in time. The slow firing rate, sluggish kinetic of receptor activation as well as the extrasynaptic mode of action will enable neurogliaform cells to act in the time domains of slow oscillations [87]. On the other hand, high firing rate and fast GABA-A receptor mediated inhibition of basket cells allows them to control gamma oscillations [86].
The question we pose here is how can the thalamus cope with these variable spatio-temporal scales? Since a substantial proportion of cortical operations involve interactions with thalamus, thalamic inhibition needs to operate across a similar spatio-temporal range to that of the cortex. In addition, various inputs parcellate the thalamus into distinct nuclei and subnuclei which, in some situation, need to be controlled separately while in others synchronously. So what are the inhibitory mechanisms that enable different scales of control in the thalamus?
Given this challenge, the central question we pose here is how does heterogeneity of inhibition arise in the thalamus? In other words what specialized GABAergic mechanisms enable thalamic circuits to differentially process information streams in both space and time, and according to ongoing behavioral demands?
In this review, we will discuss two putative solutions to this problem:
Structural and physiological features of TRN will allow its circuits to shift the spatial and temporal scales of inhibition in various behavioral conditions.
Powerful ETI systems [12–16] will provide heterogeneous, nucleus-specific inhibitory control of thalamus involved in a well-defined set of nuclei.
We should note here that while many of the experimental data informing our view of the thalamus here are derived from studies of rodent brains, we have tried to focus on principles that are likely to be universal to mammalian thalamic function. In line with this approach, we indicate when comparative data on rodents and primates (including humans) are available.
We also note that thalamic interneurons as a third form of inhibition in the thalamus are outside the scope of the present account. These cells are found in variable numbers and distributions across distinct mammalian species [17]. Outside of their function in vision (as local spatial contrast enhancement elements [4]), there is very little information about their role in other parts of the thalamus. From our perspective their peculiar anatomical connectivity pattern (forming dendro-dendritic contacts in triadic arrangements) represent a spatially highly restricted form of inhibition acting on a single excitatory input. This may, therefore, add yet another level of complexity to thalamic inhibition.
The thalamic reticular nucleus
General overview
The thalamic reticular nucleus (TRN) is a shell of GABAergic neurons that covers the lateral and anterior aspects of the mammalian thalamus (Figure 1A). TRN-like structures exist in reptiles [18] and fish [19], suggesting an evolutionary conserved origin. Compared to the knowledge we have about the development of cortical interneuron classes, we know surprisingly little about the developmental origins of the TRN. Early reports referred to the origin of TRN as ventral (or rostral) thalamic, but more recently the term prethalamic has been introduced [20]. In adult life, the question of how exactly the TRN regulates thalamic function is an open one. Our thesis is that the TRN can operate at variable spatio-temporal scales to modulate thalamic processing according to behavioral needs. This would require specialized TRN connectivity, intrinsic properties and synaptic outputs. In the following sections, we discuss these putative mechanisms and their contribution to a spatiotemporal sliding scale of thalamic inhibition.
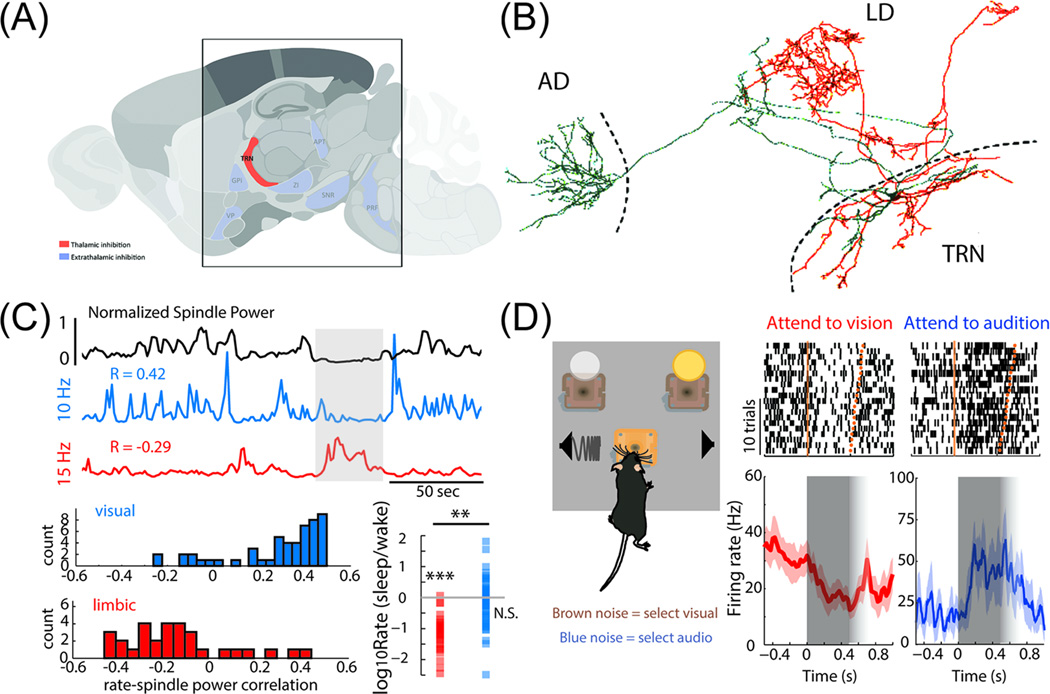
Figure 1.
Heterogeneity, subnetworks and gating by TRN. A. Parasagittal section of the mouse brain, highlighting TRN in blue. B. Juxtacellular filling of two neighboring TRN neurons in the rat (red and green). Although the cell bodies are in close proximity, each neuron projects to a distinct thalamic target (AD; anteriodorsal thalamus associated with mnemonic processing and LD; laterodorsal associated with sensory processing). C. TRN neurons display distinct physiological phenotypes. In relation to spindle power, one TRN neuron (blue trace) shows positive whereas the other (red) negative firing rate correlation. Subsequent optogenetic tagging showed that these physiological phenotypes map onto anatomical projections. Consistent with this notion, several visual TRN neurons show enhanced firing rate in sleep compared to wake, while limbic neurons are exclusively suppressed during sleep (bottom right in C). D. Cross-modal divided attention in the mouse shows TRN recruitment by attentional allocation. Example raster plot and corresponding peristimulus time histograms of two visual TRN neurons when the animal is instructed to attend to vision (red) or audition (blue). Grey shading depicts TRN activity during the stimulus anticipation period following the presentation of the instruction signal. Note that visual TRN activity is reduced during visual trials but augmented during auditory trials, resulting in a corresponding decreased and increased inhibitory output to visual thalamic cells. This is consistent with a gating role of TRN during selective attention to a given modality and focal subnetwork specific TRN action Figure B is based on [24] C on [25], D on [26]
TRN connectivity that enables variable scales of action
While the sole recipients of TRN output are thalamic projection neurons (thalamocortical (TC) cells also called relay neurons), TRN neurons receive excitatory inputs from both thalamus and cortex. Based on pioneering anatomical studies, a sectorial organization of the TRN has been known for several decades [21,22]. This along with physiological mapping experiments have indicated that a TRN neuron innervating a particular thalamic nucleus receives its main inputs from topographically-aligned deep layer cortex and the corresponding thalamic nucleus [23]. The vast majority of individual TRN cells innervate a single thalamic nucleus (Figure 1B) [24]. In sensory systems, this organization would allow the TRN to provide modality-specific inhibition that is impacted by both “bottom-up” inputs coming through the thalamus and “top-down" feedback from cortex [9].
Despite this knowledge, however, the functional meaning of TRN’s topographical organization was diminished by the lack of targeted physiological measurements of TRN neurons that innervate different thalamic nuclei (Box 2). Simultaneous probing of connectivity and function was not routinely performed, and therefore it only recently became clear that the TRN is composed of functional ‘subnetworks’ based on projections to distinct thalamic nuclei [25]. Such conclusions are based on recordings from optically-tagged TRN neurons in the freely behaving mouse, which show that neurons that project to sensory thalamic nuclei are engaged in canonical sleep rhythms known as spindles, and a substantial proportion of them show elevated spiking during sleep compared to wake (Figure 1C). In contrast, TRN neurons that project to limbic thalamic nuclei (associated with hippocampal processing) do not engage in spindles and show reduced spiking in sleep [25]. These observations suggested that in sleep, inhibition is higher for sensory thalamic processing (diminishing sensory transfer) but lower for memory-associated thalamic processing (allowing the replay of memory traces) matching the brain’s needs in this state. Such observations also clearly demonstrate that thalamic inhibition is not globally controlled, but can operate on more local scales. How local and how scalable is this type of inhibition? Answering this question requires detailed information of TRN connectivity on a single cell resolution, which is currently lacking (Box 2). Available data, nonetheless, make very interesting predictions about the different functional architectures reticulo-thalamic circuits can exhibit.
BOX 2. Open vs. closed loop organization of TRN.
The TRN is composed of individual parallel networks, each innervating different thalamic target (Pinault & Deschenes, 1998, Halassa 2014). The detailed anatomical connectivity of TRN neurons is unknown and we think that this will be a critical determinant of how wide-spread thalamic inhibition will be in space and time. The most important question is to what extent TRN forms open or closed loop connections with their thalamic targets. An open loop here refers to an arrangement in which a TRN neuron projecting to a thalamic neuron would not receive input back from that neuron, whereas a closed loop indicates that the TRN neuron innervates only those TC cells that it receives input from. Closed loop organization may allow more restricted spatial scale of inhibition whereas open loop connections (depending on their degree of divergence) may involve larger part of the thalamus. In addition open loop organization would allow exerting lateral inhibition within thalamic circuits and ascending sensory signals therefore, likely to show a winner-take-all feature [88]. It is intriguing to speculate that activating subsets of TRN neurons by top-down control can result in biasing thalamic processing towards particular sensory features based on this open-loop organization. Due to technical challenges detailed investigation of the openness/closeness of the TRN-TC loops has not been performed. Closed loop organization is suggested by the TRN axon arbors restricted mainly to single nuclei [24], (or to a single subregion e.g barreloids [89], the mediolaterally strictly organized “tier” system of TRN [90–92], rebound activity even in in vitro slice preparation [75], spatially restricted oscillatory (spindle) activity in vivo under anesthesia [51]. Open loops were first indicated by a mismatch between the position of TRN axon arbors and location of their presumed TC cells [93], however, the method used here did not allow positive identification of the synaptic contacts established by the back-filled TC cells on the labeled TRN neurons. Subsequent physiological data, such as crossmodal projections [94] and interactions [95] as well as cross-nuclear inhibitory modulations [96,97] strongly supported the presence of open loops.
Open and closed loop organization is clearly the two ends of a spectrum and perhaps a more realistic scenario is a certain degree of openness in the TRN-TC circuits. The spatial scale of openness (i.e. within a subregion, within a nucleus, or open circuits among different nuclei) needs to be defined since these will results in distinct TRN gating functions ranging from lateral inhibition to selective attention. Finally, the degree of openness will likely differ in various sectors of TRN innervating different sets of thalamic nuclei. Combination of classical labeling and novel viral tracing methods will hopefully soon provide insights into these important questions which largely determine the spatial scale of TRN action.
Behaviorally-relevant variable scales of thalamic inhibition can be concluded based on recent studies of the visual TRN subnetwork (visTRN; projecting to lateral geniculate nucleus) in mice performing tasks with different requirements [26]. In a simple visual detection task, when there was no modality-specific sensory gating requirement, most visTRN neurons exhibited a stereotyped reduction in firing rate during external visual stimulus anticipation resulting in increased sensory gain of the relay cell activity (Fig 1C). Many neurons that likely project to other sensory thalamic nuclei, show this type of activity profile as well, suggesting that general enhancement of sensory processing during sensory-directed arousal may involve coordinated suppression of multiple sensory TRN subnetworks. In contrast, when behavior is controlled in a way that requires the brain to selectively gate visual or auditory information, visTRN neurons displayed richer dynamics; while these neurons showed the predicted reduction in firing rate when vision was favored, they showed elevated firing when audition was instead favored and vision suppressed (Fig 1D). Optogenetic suppression of this increased visTRN firing rate resulted in diminished performance on trials where the auditory input was the target, suggesting that distractor suppression (vision in this case) is required for optimal performance. It is likely that this type of activity is mirrored in the auditory TRN (audTRN), and as such the level of engagement of TRN subnetworks is dependent on behavioral requirements. Also, it is important to note that distractor suppression is consistent with selective attentional engagement [26], and is dependent on intact prefrontal activity in this task. The precise pathway that links prefrontal activity to TRN control, however, is unclear.
Various inputs to TRN subnetworks are likely to be important for determining their engagement and the scale of action. One clue comes from the aforementioned study that monitored sensory and limbic TRN subnetworks in sleep [25]. Because the cortex is known to oscillate between active (UP) and inactive (DOWN) states during sleep, subcortical structures will be entrained to this cortical rhythm depending on how robust their cortical inputs are. Sensory TRN neurons were much more likely to be entrained by the cortical slow oscillation than those found in limbic subnetworks [25]. This suggest that during sleep the spatial scale of TRN action can increase and can be coordinated across multiple sensory nuclei, but would still not involve the entire TRN.
Another possible route to alter TRN engagement is the variety of inputs from subcortical structures. For example, cholinergic neurons of the basal forebrain and the brainstem project to TRN neurons [27], exerting a powerful postsynaptic response with a fast excitatory nicotinic component and a slower muscarinic inhibitory component [28]. This same logic applies to GABAergic inputs to the TRN which arise from an ill-defined zone along the basal forebrain – external globus pallidus border possibly involving some lateral hypothalamic regions as well [29–31]
In addition to extrinsic inputs, TRN neurons establish connections amongst themselves which have the potential to change the scale of thalamic inhibition. Adjacent TRN neurons can be electrically coupled by gap junctions, enabling coordinated firing [32]. Moreover gap junctions display activity-dependent plasticity which can dynamically alter their scale of action [33]. Although the available evidence suggests that gap junctions exist only among closely spaced TRN cells [34,35] the extent to which electrical coupling varies within and between subnetworks is unclear. In the somatosensory TRN most of gap junction (i.e. dye-) coupled TRN cells project to a single nucleus, probably enhancing focal subnetwork action but in certain cases dye-coupled cells can target two nuclei [36]. Thus it would be extremely important to determine whether TRN neurons that project to one thalamic target in general are more likely to be electrically coupled than ones that project to different targets. In addition to gap junctional coupling, TRN neurons may connect to one another through chemical synapses (either axo-dendritic or dendro-dendritic), establishing mutual inhibitory networks [37]. Most available evidence suggests however, that functional axo-dendritic connections are present only in young [35,38,39] but not in adult animals [24,32,40]. In adult rats no intra-TRN axon collaterals were revealed in case of over 100 TRN neurons filled in vivo [24]. In a more recent study optogenetic stimulation of TRN cells or blocking synaptic release of GABA revealed no evidence for intra-TRN inhibition in mice after P14 [40]. Lack of extensive intra-TRN connectivity certainly limits the spatial dimensions of interacting TRN subnetworks.
TRN neurons also appear to exhibit variable patterns of connections to their thalamic targets. In certain sensory systems, TRN neurons appear to receive converging inputs from multiple thalamic neurons evident by their broad receptive fields compared to thalamic projection neurons [41]. TRN neurons, in turn, project to their thalamic targets in a variety of patterns, including axonal arbors with variable sizes [24]. In general, primary sensory nuclei appear to contain more spatially compact axonal arbors compared to nuclei that receive their major excitatory input from cortical layer 5 rather than the periphery (i.e. higher order nuclei) [24]. These patterns of innervation are expected to play critical roles in determining the spatial scale of action of TRN inhibition.
Intrinsic and synaptic TRN properties that enable variable scales of action
Similar to thalamocortical neurons, TRN neurons display two types of firing mode: tonic and bursting [42] (Fig 2A). This dual firing mode may be yet another way for the TRN to switch, in this case, the temporal scale of action. Tonic spiking refers to regular tetrodotoxin (TTX)-sensitive Na+ spike trains with interval distributions that are poisson-like. A burst on the other hand, is a large amplitude TTX-insensitive Ca2+ spike that is crowned by high frequency Na+ spikes. While the Ca2+ spike is readily observable in intracellular recordings, a burst is inferred in extracellular recordings using criteria that capture their intracellular statistics (100msec silence followed by <4msec interspike intervals) [43] (Fig 2B). Thalamic Ca2+ spikes are generated by low-threshold T-type Ca2+ channels (CaV 3 family) [44], which are recruited at hyperpolarized membrane potentials that are thought to be more prevalent during periods of behavioral quiescence and sleep [45]. For the TRN, this would mean enhanced bursting during these states resulting in more widespread and longer-lasting inhibition of thalamic targets. The effect of TRN bursting is hugely aggravated by a firing pattern dependent transmitter release mechanism [46]. During tonic spikes GABA predominantly activates synaptic GABA-A receptors, however during bursts GABA spills over to extrasynaptic receptors as well resulting in large, several hundred msec long burst IPSCs [46,47] (Fig 2C–E) switching the temporal scale of inhibitory action. This spill-over is permitted by the lack of complete glia sheet around TRN terminals [15]. These burst IPSCs are especially suitable to promote de-inactivation of T-type Ca2+ channels in TC neurons, resulting in reverberating TRN-thalamic oscillations. The importance of extrasynaptic GABA-A receptors receptors in thalamus has also been demonstrated in epileptic models [48]. It is worth to mention here an even slower mode of GABA action has been proposed acting via extrasynaptically located GABA-B receptors up to tens of microns away from TRN synapses [49,50]. In our view this represents the lower end of the variable temporal scale of TRN action.
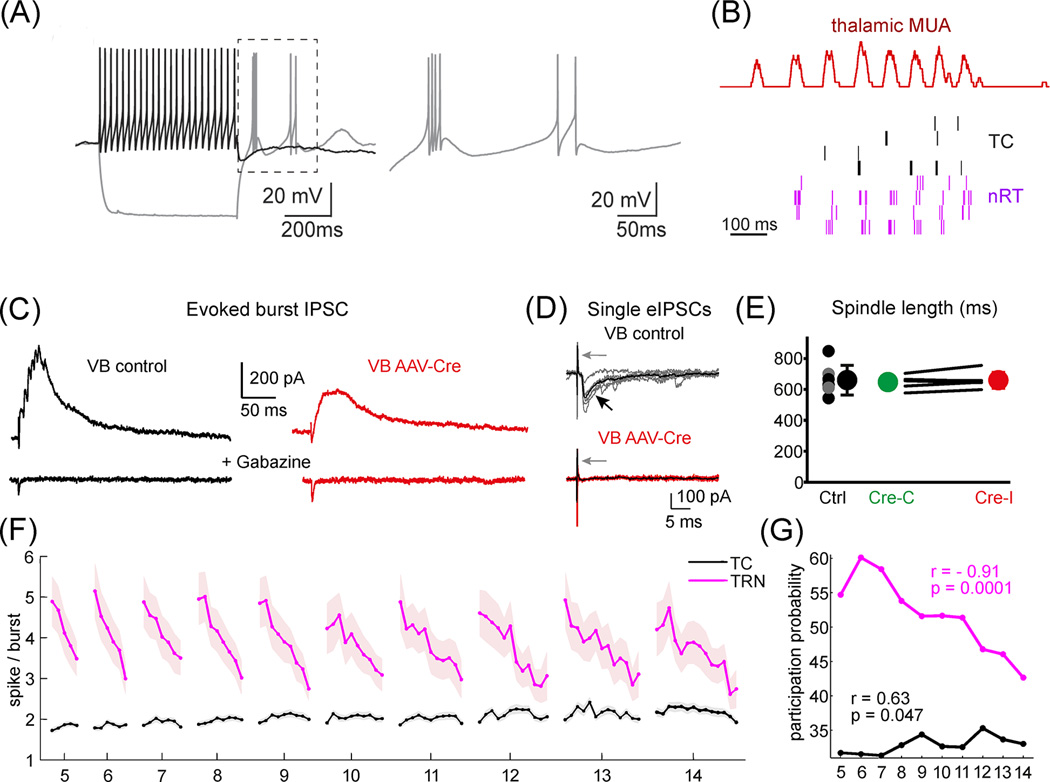
Figure 2.
Heterogeneous scales of action by TRN. A) Tonic (black) and burst (gray) response of a TRN neuron to depolarizing and hyperpolarizing current step, respectively. B) Rhythmic burst activity of interconnected TC (black) and TRN (purple) cells during sleep spindles in freely sleeping animals. C) TRN bursts generate large, slow burst IPSC in TC cells in control condition in vitro (VB control, black). Substantial amount of this burst IPSC persists after the total removal synaptic GABA-A receptors (VB AAV-Cre, red) indicating that a significant portion of the inhibitory charge is carried via extrasynaptic receptors. D) However, during single spike TRN activity only synaptic receptors are activated resulting in an order of magnitude faster response. This indicates that changes in firing pattern alter the temporal scale of TRN action D) In the absence of synaptic inhibition, phasic extrasynaptic burst IPSCs is sufficient to pace normal spindle oscillations E) Cycle-by-cycle decrease in the number of spikes/TRN bursts during sleep spindles with variable duration (5–14 cycles). Change from burst to tonic IPSCs alter the temporal scale of action, resulting in a drop in the inhibitory which leads to termination of spindles F) Participation probability of TRN cells (purple) in the first cycle of the sleep spindles display a strong correlation with spindle length. This indicates that the duration of spindles is determined at the onset by the state of the network and this state is coded best by the activity level of TRN cells. Figures B,F,G from [51], C–E from [46]
Given that recruitment of T-type channels within individual TRN neurons would depend on its intrinsic properties and inputs, it is conceivable that at any given moment TRN subnetworks can exhibit varying degrees of bursting vs tonic firing. This can precisely dictate the spatio-temporal spread of inhibition within and between thalamic nuclei across all states essentially allowing fine tuning the scale of action.
A striking example of how intrinsic and synaptic TRN properties and a gradual shift from burst to tonic mode may contribute to a sliding scale of thalamic inhibition has recently been observed during sleep spindle events [51]. Spindles are 7–14Hz phasic transients that are observed in the cortical electroencephalograph (EEG) during sleep, and have been linked to sensory processing and memory consolidation [45]. Spindles are known to require interactions between TRN and connected thalamus, but the detailed synaptic interactions have only been revealed recently. Recordings from connected TRN and thalamic pairs in the somatosensory system (Fig 2B) showed that the primary determinant of spindle duration is TRN engagement on a cycle-by-cycle basis [51]. The number of spikes in each TRN burst (Fig 2F) and the percentage of TRN neurons engaged in each spindle cycle progressively decreased with spindle event progression. Thalamic projection neurons on the other hand showed the opposite modulation, indicating that the reduction of TRN engagement during a spindle event cannot be explained by reduction in their thalamic drive [51]. Instead, a strong possibility is that changes in their intrinsic properties and/or cortical inputs explain their disengagement. Progressive decrease in TRN bursting will result in a corresponding drop of TC inhibitory charge likely via the diminished extrasynaptic receptor activation [46] as indicated above. This will diminish TC rebound bursting and eventually terminate a spindle event. Importantly, the initial level of engagement of TRN neurons in a spindle event determines its length [51], indicating that whatever determines the size of TRN neuronal pool recruitment determines the temporal and perhaps spatial spread of thalamic inhibition during a spindle (Fig 2G).
Whether spindles can be coordinated across multiple sensory TRN subnetworks, and thereby multiple sensory thalamo-cortical loops, is an important question that can be answered with simultaneous optogenetic tagging of two TRN subnetworks while recording spindles across their associated thalamo-cortical circuits. This is clearly an exciting area of investigation that will not only help determine what the functional meaning of spindles is, but also how they shape thalamic inhibitory dynamics. Nonetheless, we speculate that connectivity will determine TRN engagement in thalamo-cortical network activity at the macroscale, as opposed to intrinsinc properties which will determine more microscale engagement within individual subnetworks. This, of course, may be hijacked by pathological conditions resulting in abnormally wide-spread thalamocortical network synchrony [52].
Given evidence for heterogeneity among TRN neurons with respect to their intrinsic properties [11] and axonal arbor size [53], it is likely that a wide range of spatio-temporal control of TRN inhibition can exist. Most critically, one type of inhibitory control TRN neurons exert on their thalamic targets is shunting inhibition [54]. Meaning, it reduces the overall probability of firing, and therefore, is more likely to control the overall firing rate of individual thalamic neurons rather than individual spikes times (See next section). In summary, these data together clearly demonstrated that the connectivity and intrinsic properties of TRN neurons uniquely allow them to change the scales of inhibitory action to match state-dependent requirements of the thalamocortical system.
Extrathalamic Inhibitory System
General overview
Extrathalamic GABAergic afferents arise in a set of GABAergic nuclei outside the thalamus (Fig 3A–B). Their common, defining feature is the large, multisynaptic terminals in the thalamus which are unlike any other known inhibitory terminal in the forebrain [12,15,14,16] (Fig 3C). We will consider here the following ETI nuclei: the output nuclei of basal ganglia (including substantia nigra pars compacta, SNR; intenal globus pallidus, GPi; the ventral pallidum, VP), the zona incerta (ZI), the anterior pretectal nucleus (APT) and the pontine reticular formation (PRF) (Box 3). It is important to emphasize that both the connectivity pattern of ETI inhibitory system with the thalamus and the ultrastructure of ETI terminals are evolutionary highly conserved. For example, both the basal ganglia and the PRF the ETI fibers innervate the same sets of thalamic nuclei in rodents and primates (including humans) [14,16,55] and the quantitative ultrastructural features of ETI terminals in the thalamus is indistinguishable between rats and macaques [14] (Fig 3C). ET inhibition and TRN inhibition differ conceptually regarding both connectivity (see Box 3) synaptology [15] and function.
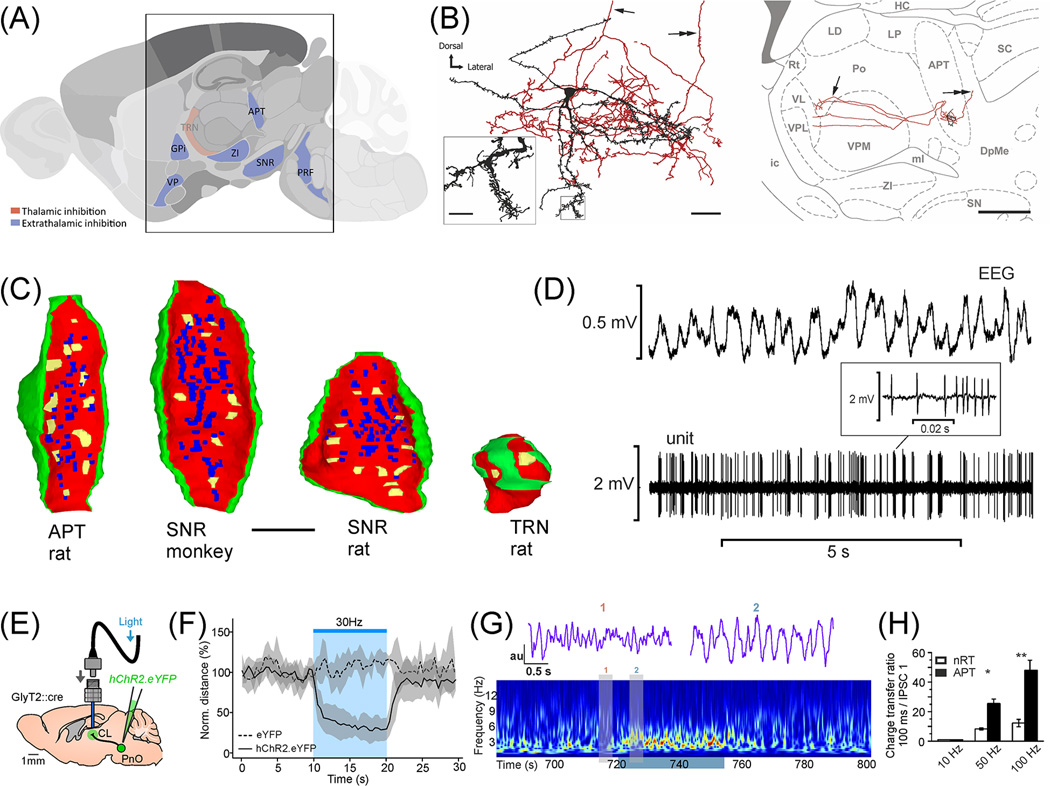
Figure 3.
Extrathalamic inhibition in the thalamus A) Parasagittal section of the brain highlighting the position of ETI nuclei (blue) around the thalamus. B) An ETI neuron in the APT. The cell have spiny dendrites, profuse local axon collaterals (left) and two ascending main axons ramifying in n.posterior of the thalamus (arrow, right). The cell also display a descending main axon (double arrow). C) Comparison of ETI and TRN terminals on the same scale. 3D reconstructions from serial electron microscopic sections. Yellow, active zones; blue, puncta adhaerentia; red, membrane of the terminal; green, glia. All active zones of ETI terminals converge on the same TC cell. Almost all TRN terminals have a single active zone per target. If they form two (arrows on the right) synapses they are separated by glia and innervate different dendrites. Note the similarity of ETI terminals among structures and taxa D) Firing activity of an APT cell in vivo with concurrent EEG recording. Note high frequency action potential clusters (inset). E–G) Activation of ETI terminals originating from PRF in the intralaminar nucleus leads to the disruption of all ongoing behavior and global alteration of the EEG activity. E) Experimental arrangement. F) Normalized travelled distance, before, during and after the stimulation. G) Wavelet spectrum of the cortical LFP. Warm color depicts higher power. Grey bars indicate the time of raw cortical LFP shown above. H) Difference in charge transfer during high frequency stimulation between ETI (APT) and TRN terminals. ETI transmission is stable at high presynaptic firing rates as well. Figures B, D from [13], C from [15,14], E–G from [16] and H from [15].
BOX 3. Connectivity of TRN vs ETI nuclei.
Inhibitory inputs to thalamus arise from two major sources; the TRN and a set of subcortical nuclei located outside the thalamus which we jointly refer to as extrathalamic inhibitory (ETI) system due their shared morphological and physiological properties (see main text). These two systems not only differ in the organization of their thalamic outputs but display distinctively different connectivity with the rest of the brain suggesting that the regulation of their activity can be completely independent. We list here major differences:
Unlike TRN, ETI nuclei have no thalamic inputs. Therefore, they display a unidirectional, feed forward inhibitory control over thalamus which is not influenced by thalamic activity. Consequently they are not able to participate in reverberating, oscillatory activity with thalamus.
Unlike TRN, ETI neurons receives substantial and widespread inputs from subcortical glutamatergic centers. These include e.g trigeminal nuclei [71], cerebellum or the subthalamic nucleus. ZI is known to receive glutamatergic inputs from almost the entire neuroaxis [98]. Interestingly, many of these glutamatergic centers also project directly to the thalamus, where their terminals overlap with those of ETI cells [67]. As a consequence the ETI activity can temporally limit the impact of these glutamatergic afferents on the thalamus through di-synaptic, feed-forward inhibition. [67,68]. This form of inhibition is entirely missing in case of TRN.
TRN is known to receive inputs from the layer 6 of all cortical areas. In contrast ETI nuclei are innervated by layer 5 of a well-defined cortical regions (mostly frontal, motor, premotor cortical areas) or in case of basal ganglia output nuclei cortical inputs may be entirely absent. These L5 fibers are the collaterals of descending corticofugal pathways [99].
The sole output of TRN is the thalamus. In contrast, without exception all ET nuclei have extensive axon arbors in wide variety subcortical centers [98]. This creates a unique opportunity to synchronize thalamic activity with other functionally related subcortical nuclei.
In contrast to TRN, ETS cells provide extensive intranuclear axon arbors [13]. In addition, APT, ZI and PRF are mutually linked [63]. This allows various forms of interaction within ETI sectors and/or among ETI nuclei, which may involve disinhibition as well as synchronization [73].
Target regions of ETI nuclei may involve more than one thalamic nuclei but the innervation always remain selective for a well-defined thalamic territory which indicates well-focused action of ETI nuclei on the thalamus. Furthermore, any given part of an ETI nuclei will provide afferents only to a restricted part of its thalamic target nuclei [13,14]. The axons forms clusters of large terminals interspersed with bouton free regions [13].
The embryonic origins of these nuclei are quite variable and include subpallial, telencephalic (GPi, VP), rostral diencephalic (recently referred to as prethalamic) (ZI), caudal diencephalic (APT) or tegmental (SNR, PRF) structures [20]. In the adult brain ETI nuclei cover the ventral (ZI) or caudal (APT) or latera (Gpi) aspects of the thalamus, located in the basal forebrain (VP) or in the tegmentum (PRF and SNR) (Fig 3A). ETI nuclei are known to be involved in various distinct large scale neuronal circuits. We group them here based on their shared properties of the thalamic afferents. ETI nuclei are known to have significant role in cortical development [56] motor control [1] and are involved in major neurological diseases [57–61] but with the exception of basal ganglia very little data is available about their exact function.
ETI connectivity and scales of thalamic action
Unlike TRN which innervates all thalamic nuclei, ETI nuclei display selective innervation of well-defined thalamic territories. While each ETI nucleus has its own specific projection patterns, one common feature among all is the lack of innervation of primary sensory thalamus. Instead, they innervate higher order sensory nuclei (ZI, APT) [12,13], motor territories (SNR, GPi) [14], midline and intralaminar nuclei (PRF) [16] or the mediodorsal nucleus (VP) [62]. As a consequence they don’t influence the classical thalamic function, sensory relay, rather they are likely to be involved in sensory-motor integration, executive and motor control or decision making.
Since ETI nuclei, even collectively, do not innervate the entire thalamus they are not able to exert global thalamic influence. Even though some ETI nuclei are interconnected, which potentially synchronize their activity [63] their spatial scale of action will still be restricted to their target nuclei. Since they don’t receive thalamic feedback (Box 3), they may initiate but can’t maintain reverberating thalamic activity. Their influence over global brain dynamics is likely the result of inhibition of thalamic nuclei with widespread cortical projections, in contrast to the recurrent dynamics that can be established between thalamus and TRN. Such global state control has been recently demonstrated by selective optogenetic stimulation of PRF afferents to the intralaminar thalamus. Activating PRF afferents resulted in suspension of all behavior and the emergence of cortical slow oscillations with extremely short latency [16] (Fig 3).
Cortical input reaches ETI nuclei either directly [64] or, in case of BG nuclei, via the subthalamic nucleus. A given cortical region receives inputs from the thalamic nuclei where its ETI target terminates (e.g S1 innervates ZI, this in turn projects to posterior thalamic nucleus, which projects back to S1). This allows the emergence of a cortico-ET-TC loop, parallel to the cortico-TRN-TC loop. However, beside the difference in cortical origin (layer 5 vs layer 6 respectively) there is a conceptual difference between the two loops. As indicated above the TRN loop allows the emergence of local reverberating oscillations (alpha/mu/spindles) that may be dependent on the previous synaptic history of the circuit. The ETI loop on the other hand may communicate long term changes in cortical outputs as an inhibitory signal back to widespread cortical regions via the highly divergent axonal arbor of their thalamic targets.
Intrinsic and synaptic properties of ETI – precise temporal scales of action
The major features of ETI afferents in the thalamus is that a single axon terminal contacts the postsynaptic elements via several active zones [12,13,15,14,16] (Fig 3C). Almost all other inhibitory terminals, studied so far, including TRN, establish one or maximum two synapses on their target [15,65]. Indeed, ETI boutons are the largest and most complex inhibitory terminals of the brain described so far (with respect to size and number). On average one terminal establishes 7–9 synapses but this number can reach 16. All these active zones always converge on a single postsynaptic TC element [15,14,16]. The entire terminal is wrapped by glial sheets which probably restricts spillover of GABA, unlike in case of TRN where the glial cover is incomplete [15]. The terminals preferentially innervate thick proximal dendrites, electrotonically close to the soma [12,15,14,16]. As such, the activation of a single fiber is quite powerful and able to elicit rebound bursting in the innervated TC cell [13] or result in complete silencing of action potential generation. Intriguingly, despite the different embryonic origin of ETI nuclei, the ultrastructure of their thalamic terminals are similar. Moreover this structure is stable across different taxa (rodent vs. primates or even in birds) [14,16], suggesting conserved functional principles.
Unlike TRN terminals which display pronounced short term depression if activated by repetitive stimulation ETI fibers display little evidence for short term plasticity even above 50 Hz. [15,16] (Fig 3H). Modelling studies indicated that this is probably due to the overflow of GABA from one presynaptic active zone to all postsynaptic specializations belonging to one axon terminal [66]. By reaching several synapses, the release of GABA via any of the many active zones can result in similar postsynaptic response, thus the chance of release failure is minimal. This mechanism allows faithful inhibitory transmission even at high presynaptic firing rates. Indeed, the available in vivo data demonstrate high frequency firing activity of ETI cells (Fig 3D), (approaching the theoretical maximal firing rate of 1000 Hz) [13,16,64,67] where non-depressing IPSPs can maintain a constant inhibitory bombardment of the posysnaptic TC cells [67]. Precise control of thalamic outputs may be established by the reduction of firing in the sensory [67,68] or motor [69] systems studied so far or changing the exact pattern of inhibitory activity which vetoes TC firing in a well-defined time window [1].
The above mentioned cellular features of ETI systems suggest that their predominant action is a strong and focal thalamic inhibition. It also indicates the primary importance of disinhibition i.e. mechanisms that results in the cessation of ETI firing consequently a temporal removal of a strong inhibition from TC cells. Indeed, disinhibition has been suggested as a primary mode of action in case of BG via an elaborate system of direct and indirect pathways both in case of dorsal and ventral striatum [1,70]. In addition the significance of ETI disinhibition in the thalamus was best studied in the somatosensory system [68,71]. Trigeminal inputs exert a strong feed-forward inhibition on posterior thalamic nucleus (Po) via collaterals to ZI. (see Box 3) [71]. The disynaptic inhibition via ZI is so fast and powerful that it overtakes monosynaptic trigeminal excitation of Po and able to completely block sensory transmission in this nucleus. Chemical lesion of ZI reveal a full-blown sensory response in Po to whisker stimulation [71]. So what mechanisms may “lesion” ZI influence on Po in real situations? Up till now three non-exclusive disinhibitory mechanisms have been suggested; i; presynaptic muscarinic receptors on ZI terminals inhibit GABA release during focused attention [72] ii, intra-incertal inhibition driven by descending cortical inputs temporally suspend inhibitory ZI output in the somatosensory ZI sector [73] iii; ZI inhibition is overcome when powerful ascending and descending inputs converge within a narrow time window on Po cells [74]. Note that in this case ETI inhibitory influence is tightly regulated both in spatial and temporal domains. Spatially by the limited extent of the thalamic axon arbor, temporally by the precise disinhibitory mechanisms.
By emphasizing the strength of ETI inhibition, we do not suggest that TRN inputs to be universally weak or modulatory. In fact, paired recordings show that some TRN neurons are capable of exerting strong and reliable inhibition of their thalamic targets [75–77] especially in case of TRN bursts [46] (see above). Moreover, it has been shown that the axon of a single TRN neuron also establishes multisynaptic contacts with the TC cells. The main difference, however, is that in case of TRN, the postsynaptic cell is contacted by single synapses of small terminals, not by many synapses of few large terminals, like ETI contacts [15]. Thus, the major difference between TRN and ETI unitary IPSCs on TC cells is not so much in their amplitude but rather in their short term dynamics [15]. Still only ETI, not TRN, inputs can provide an extremely fast inhibition that could eliminate individual spikes from a spike train without impacting the overall spike rate of a target TC neuron [1]. In contrast to TRN, ETI nuclei are unlikely to exhibit major shifts in spatial or temporal scale of action. The paucity of T-type Ca2+ channels in ETI neurons and the complete glial wrap around the terminals indicate that low threshold burst mediated phasic activation of extrasynaptic receptors are unlikely to occur.
Finally, it must be emphasized that ET inhibitory control is likely perturbed in certain disease states, for example in Parkinson’s disease, epilepsy or chronic pain [57,58,60,78–80] where aberrant synchrony among ETI nuclei and changes in their output properties may contribute to spatially extensive aberrant oscillatory patterns.
Conclusions
In summary, all available data suggest that the two inhibitory systems of the thalamus have complementary roles. ETI signals are able to exert strong, rapid and focal effects on their thalamic targets which will impact timing of individual spikes and therefore the content of the signals these thalamic cells transmit. Such precise control of content is in contrast to TRN inhibitory control, which appears to be largely involve gain control (signal amplification) and rhythm genesis. By exerting a relatively weaker and slower type of inhibitory control overall their thalamic targets, TRN subnetworks can modulate the rate of spike trains while minimally impacting their overall temporal structure. Such features allow the TRN to control the magnitude of cortical input while preserving its content.
It must be emphasized, however, the understanding of thalamic (both TRN and ET) inhibition is very limited in terms of both structure and function. Concerted efforts using the most up-to-data techniques are required to approach the level of understanding we achieved in case of cortical, striatal of cerebellar inhibition.
Outstanding questions box.
To what extent cortical and thalamic inputs of a given TRN neuron correspond to its output (i.e. to what extent are TRN loops closed vs open)?
To what extent openness/closeness vary across TRN subnetworks?
Do TRN neurons that project to different thalamic nuclei exhibit systematic differences in their inputs, synaptic or intrinsic properties?
Can TRN neurons projecting to different thalamic nuclei interact via gap-junctions?
How TRN neurons shape the activity of non-sensory thalamus?
What is the entire extent of the ETI system?
How do ETI inputs shape the output of thalamic cells?
What is the information content and behavioral correlates of ETI spiking activity? How it is shaped by cortical and subcortical inputs?
How do different ETI systems interact with one another?
How do TRN and ET inhibition interact on thalamic cells?
How does impaired ETI function contribute to aberrant thalamocortical activity in neurological diseases?
Trends.
Thalamic inhibition is a critical element of normal thalamocortical interactions and evidence for its perturbation is found in many diseases.
Compared to their cortical, striatal and cerebellar counterparts, little is known about the circuit and computational principles of thalamic inhibition.
Thalamic inhibition encompasses highly diverse circuits acting in various spatial and temporal domains.
The thalamus receives inhibitory afferents from the thalamic reticular nucleus (TRN) as well as from extrathalamic sources. These two types of inhibition display major differences in connectivity, synaptic organization and physiology suggesting distinct functions.
TRN inhibition is a scalable system that controls spike rates across behavioral states and cognitive needs whereas extrathalamic inhibition impacts spike timing in well-defined thalamic regions and limited behavioral contexts.
Acknowledgments
This work was supported by the National Research Development and Innovation Office (NKFI 109754) the Hungarian Brain Research Program (grant no. KTIA_13_NAP-A-I/1) and the Wellcome Trust (WT094513) to L.A., and grants from the US National Institute of Health (NIMH, NINDS), Brain and Behavior Foundation, Feldstein Foundation, Human Frontiers Science Program, Klingenstein Foundation, Sloan Foundation, and Simons Foundations to MMH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldberg JH, et al. Basal ganglia output to the thalamus: still a paradox. Trends Neurosci. 2013;36:695–705. doi: 10.1016/j.tins.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ketz NA, et al. Thalamic pathways underlying prefrontal cortex-medial temporal lobe oscillatory interactions. Trends Neurosci. 2015;38:3–12. doi: 10.1016/j.tins.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Schiff ND, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch JA, et al. How inhibitory circuits in the thalamus serve vision. Annu. Rev. Neurosci. 2015;38:309–329. doi: 10.1146/annurev-neuro-071013-014229. [DOI] [PubMed] [Google Scholar]
- 5.Lorincz ML, et al. Temporal framing of thalamic relay-mode firing by phasic inhibition during the alpha rhythm. Neuron. 2009;63:683–696. doi: 10.1016/j.neuron.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito HT, et al. A prefrontal–thalamo–hippocampal circuit for goal-directed spatial navigation. Nature. 2015;522:50–55. doi: 10.1038/nature14396. [DOI] [PubMed] [Google Scholar]
- 7.Xu W, Südhof TC. A neural circuit for memory specificity and generalization. Science. 2013;339:1290–1295. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward LM. The thalamic dynamic core theory of conscious experience. Conscious. Cogn. 2011;20:464–486. doi: 10.1016/j.concog.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Res Brain Res Rev. 2004;46:1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Steriade M, et al. Thalamus. Vol. 1. Elsevier; 1997. Thalamic cell types and intrinsic synaptic organization; pp. 175–269. [Google Scholar]
- 11.Lee S-H, et al. Heterogeneity of firing properties among rat thalamic reticular nucleus neurons. J. Physiol. 2007;582:195–208. doi: 10.1113/jphysiol.2007.134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barthó P, et al. Selective GABAergic innervation of thalamic nuclei from zona incerta. Eur. J. Neurosci. 2002;16:999–1014. doi: 10.1046/j.1460-9568.2002.02157.x. [DOI] [PubMed] [Google Scholar]
- 13.Bokor H, et al. Selective GABAergic control of higher-order thalamic relays. Neuron. 2005;45:929–940. doi: 10.1016/j.neuron.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 14.Bodor AL, et al. Structural correlates of efficient GABAergic transmission in the basal ganglia-thalamus pathway. J. Neurosci. 2008;28:3090–3102. doi: 10.1523/JNEUROSCI.5266-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wanaverbecq N, et al. Contrasting the functional properties of GABAergic axon terminals with single and multiple synapses in the thalamus. J. Neurosci. 2008;28:11848–11861. doi: 10.1523/JNEUROSCI.3183-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giber K, et al. A subcortical inhibitory signal for behavioral arrest in the thalamus. Nat. Neurosci. 2015;18:562–568. doi: 10.1038/nn.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherman SM. Interneurons and triadic circuitry of the thalamus. Trends Neurosci. 2004;27:670–675. doi: 10.1016/j.tins.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Kenigfest N, et al. The turtle thalamic anterior entopeduncular nucleus shares connectional and neurochemical characteristics with the mammalian thalamic reticular nucleus. J. Chem. Neuroanat. 2005;30:129–143. doi: 10.1016/j.jchemneu.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Mueller T. What is the Thalamus in Zebrafish? Front. Neurosci. 2012;6:64. doi: 10.3389/fnins.2012.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puelles L, Rubenstein JL. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–476. doi: 10.1016/S0166-2236(03)00234-0. [DOI] [PubMed] [Google Scholar]
- 21.Montero VM, et al. Retinotopic organization within the thalamic reticular nucleus demonstrated by a double label autoradiographic technique. Brain Res. 1977;138:407–421. doi: 10.1016/0006-8993(77)90681-3. [DOI] [PubMed] [Google Scholar]
- 22.Jones EG. Some aspects of the organization of the thalamic reticular complex. J Comp Neurol. 1975;162:285–308. doi: 10.1002/cne.901620302. [DOI] [PubMed] [Google Scholar]
- 23.Jones EG. The Thalamus. Plenum Press; 1985. [Google Scholar]
- 24.Pinault D, Deschenes M. Projection and innervation patterns of individual thalamic reticular axons in the thalamus of the adult rat: a three-dimensional, graphic, and morphometric analysis. J Comp Neurol. 1998;391:180–203. doi: 10.1002/(sici)1096-9861(19980209)391:2<180::aid-cne3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 25.Halassa MM, et al. State-Dependent Architecture of Thalamic Reticular Subnetworks. Cell. 2014;158:808–821. doi: 10.1016/j.cell.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wimmer RD, et al. Thalamic control of sensory selection in divided attention. Nature. 2015;526:705–709. doi: 10.1038/nature15398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallanger AE, et al. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J. Comp. Neurol. 1987;262:105–124. doi: 10.1002/cne.902620109. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y-G, et al. Biphasic cholinergic synaptic transmission controls action potential activity in thalamic reticular nucleus neurons. J. Neurosci. 2013;33:2048–2059. doi: 10.1523/JNEUROSCI.3177-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asanuma C, Porter LL. Light and electron microscopic evidence for a GABAergic projection from the caudal basal forebrain to the thalamic reticular nucleus in rats. J Comp Neurol. 1990;302:159–172. doi: 10.1002/cne.903020112. [DOI] [PubMed] [Google Scholar]
- 30.Bickford ME, et al. GABAergic projection from the basal forebrain to the visual sector of the thalamic reticular nucleus in the cat. J. Comp. Neurol. 1994;348:481–510. doi: 10.1002/cne.903480402. [DOI] [PubMed] [Google Scholar]
- 31.Herrera CG, et al. Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat. Neurosci. 2015 doi: 10.1038/nn.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landisman CE, et al. Electrical synapses in the thalamic reticular nucleus. J Neurosci. 2002;22:1002–1009. doi: 10.1523/JNEUROSCI.22-03-01002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landisman CE. Long-Term Modulation of Electrical Synapses in the Mammalian Thalamus. Science (80-.) 2005;310:1809–1813. doi: 10.1126/science.1114655. [DOI] [PubMed] [Google Scholar]
- 34.Long MA, et al. Small clusters of electrically coupled neurons generate synchronous rhythms in the thalamic reticular nucleus. J Neurosci. 2004;24:341–349. doi: 10.1523/JNEUROSCI.3358-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deleuze C, Huguenard JR. Distinct electrical and chemical connectivity maps in the thalamic reticular nucleus: potential roles in synchronization and sensation. J. Neurosci. 2006;26:8633–8645. doi: 10.1523/JNEUROSCI.2333-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S-C, et al. Two functionally distinct networks of gap junction-coupled inhibitory neurons in the thalamic reticular nucleus. J. Neurosci. 2014;34:13170–13182. doi: 10.1523/JNEUROSCI.0562-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinault D, et al. Dendrodendritic and axoaxonic synapses in the thalamic reticular nucleus of the adult rat. J Neurosci. 1997;17:3215–3233. doi: 10.1523/JNEUROSCI.17-09-03215.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lam Y-W, et al. Mapping of the functional interconnections between thalamic reticular neurons using photostimulation. J. Neurophysiol. 2006;96:2593–2600. doi: 10.1152/jn.00555.2006. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Vives MV, et al. Inhibitory interactions between perigeniculate GABAergic neurons. J Neurosci. 1997;17:8894–8908. doi: 10.1523/JNEUROSCI.17-22-08894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou G, et al. Lack of Intrinsic GABAergic Connections in the Thalamic Reticular Nucleus of the Mouse. J. Neurosci. 2016;36:7246–7252. doi: 10.1523/JNEUROSCI.0607-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simm GM, et al. Discharge properties of single units in auditory part of reticular nucleus of thalamus in cat. J. Neurophysiol. 1990;63:1010–1021. doi: 10.1152/jn.1990.63.5.1010. [DOI] [PubMed] [Google Scholar]
- 42.Steriade M, et al. Reticularis thalami neurons revisited: activity changes during shifts in states of vigilance. J Neurosci. 1986;6:68–81. doi: 10.1523/JNEUROSCI.06-01-00068.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sherman SM, Guillery RW. Exploring the Thalamus. Academic Press; 2001. [Google Scholar]
- 44.Jahnsen H, Llinas R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Astori S, et al. Manipulating sleep spindles--expanding views on sleep, memory, and disease. Trends Neurosci. 2013;36:738–748. doi: 10.1016/j.tins.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Rovó Z, et al. Phasic, Nonsynaptic GABA-A Receptor-Mediated Inhibition Entrains Thalamocortical Oscillations. J. Neurosci. 2014;34:7137–7147. doi: 10.1523/JNEUROSCI.4386-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herd MB, et al. Extrasynaptic GABA(A) receptors couple presynaptic activity to postsynaptic inhibition in the somatosensory thalamus. J. Neurosci. 2013;33:14850–14868. doi: 10.1523/JNEUROSCI.1174-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cope DW, et al. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat. Med. 2009;15:1392–1398. doi: 10.1038/nm.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beenhakker MP, Huguenard JR. Astrocytes as gatekeepers of GABAB receptor function. J. Neurosci. 2010;30:15262–15276. doi: 10.1523/JNEUROSCI.3243-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kulik A, et al. Distinct localization of GABA(B) receptors relative to synaptic sites in the rat cerebellum and ventrobasal thalamus. Eur J neurosci. 2002;15:291–307. doi: 10.1046/j.0953-816x.2001.01855.x. [DOI] [PubMed] [Google Scholar]
- 51.Barthó P, et al. Ongoing Network State Controls the Length of Sleep Spindles via Inhibitory Activity. Neuron. 2014;82:1367–1379. doi: 10.1016/j.neuron.2014.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beenhakker MP, et al. Neurons that fire together also conspire together: is normal sleep circuitry hijacked to generate epilepsy? Neuron. 2009;62:612–632. doi: 10.1016/j.neuron.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pinault D, et al. Intracellular recordings in thalamic neurones during spontaneous spike and wave discharges in rats with absence epilepsy. J Physiol. 1998;509:449–456. doi: 10.1111/j.1469-7793.1998.449bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olsen SR, et al. Gain control by layer six in cortical circuits of vision. Nature. 2012;483:47–52. doi: 10.1038/nature10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ilinsky IA, et al. Mode of termination of pallidal afferents to the thalamus: a light and electron microscopic study with anterograde tracers and immunocytochemistry in Macaca mulatta. J Comp Neurol. 1997;386:601–612. doi: 10.1002/(sici)1096-9861(19971006)386:4<601::aid-cne6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 56.Chen J, Kriegstein AR. A GABAergic projection from the zona incerta to cortex promotes cortical neuron development. Science. 2015;350:554–558. doi: 10.1126/science.aac6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masri R, et al. Zona incerta: a role in central pain. J. Neurophysiol. 2009;102:181–191. doi: 10.1152/jn.00152.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plaha P, et al. Bilateral caudal zona incerta nucleus stimulation for essential tremor: outcome and quality of life. J. Neurol. Neurosurg. Psychiatry. 2011;82:899–904. doi: 10.1136/jnnp.2010.222992. [DOI] [PubMed] [Google Scholar]
- 59.Plaha P, et al. Stimulation of the caudal zona incerta is superior to stimulation of the subthalamic nucleus in improving contralateral parkinsonism. Brain. 2006;129:1732–1747. doi: 10.1093/brain/awl127. [DOI] [PubMed] [Google Scholar]
- 60.Helmich RC, et al. Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain. 2012;135:3206–3226. doi: 10.1093/brain/aws023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shaw F-Z, et al. The zona incerta modulates spontaneous spike-wave discharges in the rat. J. Neurophysiol. 2013;109:2505–2516. doi: 10.1152/jn.00750.2011. [DOI] [PubMed] [Google Scholar]
- 62.Kuroda M, Price JL. Synaptic organization of projections from basal forebrain structures to the mediodorsal thalamic nucleus of the rat. J. Comp. Neurol. 1991;303:513–533. doi: 10.1002/cne.903030402. [DOI] [PubMed] [Google Scholar]
- 63.Giber K, et al. Heterogeneous output pathways link the anterior pretectal nucleus with the zona incerta and the thalamus in rat. J. Comp. Neurol. 2008;506:122–140. doi: 10.1002/cne.21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barthó P, et al. Cortical control of zona incerta. J. Neurosci. 2007;27:1670–1681. doi: 10.1523/JNEUROSCI.3768-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Biró AA, et al. Release probability-dependent scaling of the postsynaptic responses at single hippocampal GABAergic synapses. J. Neurosci. 2006;26:12487–12496. doi: 10.1523/JNEUROSCI.3106-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Telgkamp P, et al. Maintenance of high-frequency transmission at purkinje to cerebellar nuclear synapses by spillover from boutons with multiple release sites. Neuron. 2004;41:113–126. doi: 10.1016/s0896-6273(03)00802-x. [DOI] [PubMed] [Google Scholar]
- 67.Lavallee P, et al. Feedforward inhibitory control of sensory information in higher-order thalamic nuclei. J Neurosci. 2005;25:7489–7498. doi: 10.1523/JNEUROSCI.2301-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trageser JC, Keller A. Reducing the uncertainty: gating of peripheral inputs by zona incerta. J Neurosci. 2004;24:8911–8915. doi: 10.1523/JNEUROSCI.3218-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma TP. Saccade-related omnivectoral pause neurons in the primate zona incerta. Neuroreport. 1996;7:2713–2716. doi: 10.1097/00001756-199611040-00061. [DOI] [PubMed] [Google Scholar]
- 70.Voorn P, et al. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 71.Lavallée P, et al. Feedforward inhibitory control of sensory information in higher-order thalamic nuclei. J. Neurosci. 2005;25:7489–7498. doi: 10.1523/JNEUROSCI.2301-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Masri R, et al. Cholinergic regulation of the posterior medial thalamic nucleus. J. Neurophysiol. 2006;96:2265–2273. doi: 10.1152/jn.00476.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Urbain N, Deschênes M. Motor cortex gates vibrissal responses in a thalamocortical projection pathway. Neuron. 2007;56:714–725. doi: 10.1016/j.neuron.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 74.Groh A, et al. Convergence of Cortical and Sensory Driver Inputs on Single Thalamocortical Cells. Cereb. Cortex. 2014;24:3167–3179. doi: 10.1093/cercor/bht173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim U, et al. Functional dynamics of GABAergic inhibition in the thalamus. Science (80-.) 1997;278:130–134. doi: 10.1126/science.278.5335.130. [DOI] [PubMed] [Google Scholar]
- 76.Cox CL, et al. Nucleus reticularis neurons mediate diverse inhibitory effects in thalamus. Proc Natl Acad Sci U S A. 1997;94:8854–8859. doi: 10.1073/pnas.94.16.8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim U, McCormick DA. The functional influence of burst and tonic firing mode on synaptic interactions in the thalamus. J Neurosci. 1998;18:9500–9516. doi: 10.1523/JNEUROSCI.18-22-09500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murray PD, et al. Abnormal anterior pretectal nucleus activity contributes to central pain syndrome. J. Neurophysiol. 2010;103:3044–3053. doi: 10.1152/jn.01070.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elazar Z, Paz M. Similarities between the akinesia induced by carbachol microinjections into the pontine reticular formation and neuroleptic catalepsy. Life Sci. 1992;51:1373–1380. doi: 10.1016/0024-3205(92)90637-5. [DOI] [PubMed] [Google Scholar]
- 80.Paz JT, et al. Activity of ventral medial thalamic neurons during absence seizures and modulation of cortical paroxysms by the nigrothalamic pathway. J. Neurosci. 2007;27:929–941. doi: 10.1523/JNEUROSCI.4677-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Buzsaki G. Rhythms of the Brain. Oxford Uni.; 2006. [Google Scholar]
- 82.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:345–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 83.Gittis AH, Kreitzer AC. Striatal microcircuitry and movement disorders. Trends Neurosci. 2012;35:557–564. doi: 10.1016/j.tins.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DeFelipe J, et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat. Rev. Neurosci. 2013;14:202–216. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jörntell H, et al. Cerebellar molecular layer interneurons - computational properties and roles in learning. Trends Neurosci. 2010;33:524–532. doi: 10.1016/j.tins.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 86.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Overstreet-Wadiche L, McBain CJ. Neurogliaform cells in cortical circuits. Nat. Rev. Neurosci. 2015;16:458–468. doi: 10.1038/nrn3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Binas J, et al. Learning and stabilization of winner-take-all dynamics through interacting excitatory and inhibitory plasticity. Front. Comput. Neurosci. 2014;8:68. doi: 10.3389/fncom.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Desilets-Roy B, et al. Substrate for cross-talk inhibition between thalamic barreloids. J Neurosci. 2002;22:RC218. doi: 10.1523/JNEUROSCI.22-09-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lam Y-W, Sherman SM. Functional topographic organization of the motor reticulothalamic pathway. J. Neurophysiol. 2015;113:3090–3097. doi: 10.1152/jn.00847.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lam Y-W, Sherman SM. Functional organization of the thalamic input to the thalamic reticular nucleus. J. Neurosci. 2011;31:6791–6799. doi: 10.1523/JNEUROSCI.3073-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pinault D, et al. The axonal arborization of single thalamic reticular neurons in the somatosensory thalamus of the rat. Eur J neurosci. 1995;7:31–40. doi: 10.1111/j.1460-9568.1995.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 93.Pinault D, Deschenes M. Anatomical evidence for a mechanism of lateral inhibition in the rat thalamus. Eur J neurosci. 1998;10:3462–3469. doi: 10.1046/j.1460-9568.1998.00362.x. [DOI] [PubMed] [Google Scholar]
- 94.Kimura A, et al. Axonal projections of single auditory neurons in the thalamic reticular nucleus: implications for tonotopy-related gating function and cross-modal modulation. Eur. J. Neurosci. 2007;26:3524–3535. doi: 10.1111/j.1460-9568.2007.05925.x. [DOI] [PubMed] [Google Scholar]
- 95.Kimura A. Diverse subthreshold cross-modal sensory interactions in the thalamic reticular nucleus: implications for new pathways of cross-modal attentional gating function. Eur. J. Neurosci. 2014;39:1405–1418. doi: 10.1111/ejn.12545. [DOI] [PubMed] [Google Scholar]
- 96.Crabtree JW, et al. A new intrathalamic pathway linking modality-related nuclei in the dorsal thalamus. Nat. Neurosci. 1998;1:389–394. doi: 10.1038/1603. [DOI] [PubMed] [Google Scholar]
- 97.Crabtree JW, Isaac JT. New intrathalamic pathways allowing modality-related and cross-modality switching in the dorsal thalamus. J Neurosci. 2002;22:8754–8761. doi: 10.1523/JNEUROSCI.22-19-08754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mitrofanis J. Some certainty for the “zone of uncertainty”? Exploring the function of the zona incerta. Neuroscience. 2005;130:1–15. doi: 10.1016/j.neuroscience.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 99.Guillery RW, Sherman SM. Branched thalamic afferents: what are the messages that they relay to the cortex? Brain Res. Rev. 2011;66:205–219. doi: 10.1016/j.brainresrev.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]