Abstract
Ultraviolet radiation B stimulates both the production of vitamin D3 in the skin and the activation of the skin analog of the hypothalamic-pituitary-adrenal axis (HPA) as well as the central HPA. Since the role of vitamin D3 in the regulation of the HPA is largely unknown, we investigated the impact of 1,25(OH)2D3 and its noncalcemic analogs, 20(OH)D3 and 21(OH)pD, on the expression of the local HPA in human epidermal keratinocytes. The noncalcemic analogs showed similar efficacy to 1,25(OH)2D3 in stimulating the expression of neuropeptides, CRF, urocortins and POMC, and their receptors, CRFR1, CRFR2, MC1R, MC2R, MC3R and MC4R. Interestingly, unlike other secosteroids, the activity of 21(OH)pD did not correlate with induction of differentiation, suggesting a separate but overlapping mechanism of action. Thus, biologically active forms of vitamin D can regulate different elements of the local equivalent of the HPA with implications for the systemic HPA.
Keywords: Vitamin D3, Vitamin D3 analogs, HPA axis, Corticotropin releasing factor, Keratinocytes differentiation, Calcium
1. Introduction
Skin responds to stress and possesses the cutaneous equivalent of the hypothalamic-pituitary-adrenal axis (HPA), referred to as the sHPA (reviewed in (Slominski et al., 2012; Paus et al., 2014). Its functions are coordinated by the corticotropin releasing factor (CRF) signaling system (reviewed in Slominski et al. (2013a)). An additional cutaneous regulatory system initiated by the action of ultraviolet B radiation (UVB) is the vitamin D3 signaling pathway (reviewed in Bikle (2011a,b,c), Holick (2003)). After photochemical production, vitamin D3 (which is a prohormone) (Holick et al., 1997; Holick, 1996), is activated by 25-hydroxylation catalyzed by CYP2R1 or CYP27A1 in the liver, then 1α-hydroxylation by CYP27B1 in the kidney, producing 1,25-diydroxyvitamin D3 (1,25(OH)2D3) (Holick, 2011; Bikle, 2011a,b,c, 2012; Zhu et al., 2013). Local activation is also possible since these enzymes are present in the skin (Bikle, 2011a,b,c, 2012; Zhu et al., 2013; Bikle et al., 2004a,b), as is the vitamin D receptor (VDR) (Bikle, 2012; Reichrath et al., 1996), so that the skin is not only a source of active vitamin D3 but also a target of its activity.
Recently, one of the key enzymes in steroidogenesis, CYP11A1 (also known as cytochrome P450scc), was shown to metabolize 7-dehydroholesterol and vitamin D3 providing an alternative pathway of vitamin D activation (Slominski et al., 2005c; 2015b), with products including the low calcemic but biologically active secosteroids, 21(OH)pD which has a short (2C) side chain and 20S-hydroxyvitamin D3 (20(OH)D3) (reviewed in Slominski et al. (2014, 2013c,d)).
Active forms of vitamin D3 exhibit a broad spectrum of phenotypic effects on the skin such as inhibition of cell proliferation and stimulation of cell differentiation (reviewed in Bikle (2012), Oda et al. (2007), Bikle (2004), Holick (2014)). Furthermore, there is accumulating evidence that active forms of vitamin D possess anti-cancer (Holick, 2014; Bikle, 2008; Feldman et al., 2014), antifibrotic (Slominski et al., 2011; Slominski et al., 2013b,c,d; Bonventre, 2013), antioxidative (Slominski et al., 2015a; Tongkao-On et al., 2015; Gordon-Thomson et al., 2012) and anti-inflammatory properties (Cannell et al., 2014; Barragan et al., 2015; Wei and Christakos, 2015; Bikle, 2011a,b,c).
The skin equivalent of the HPA follows the basic scheme of the central HPA, with the expression of CRF, structurally related urocortins (UCN 1–3) and their corresponding receptors: CRFR1 and CRFR2 (Slominski et al., 2001; Slominski et al., 2004; Slominski et al., 2000; Slominski et al., 1998 Slominski etal., 2006b; Zmijewski and Slominski, 2009). Stimulation of the CRFR1 receptor in skin cells results in an induction of the expression of proopiomelanocortin (POMC) with the resulting generation of POMC-derived peptides, ACTH, α-MSH and β-endorphin (Slominski et al., 2005a,b; Rousseau et al., 2007). CRF and ACTH (interacting with the MC2R receptor) stimulate the production of glucocorticoids (cortisol and corticosterone) in skin cells (Slominski et al., 2005a,b; Cirillo and Prime, 2011; Hannen et al., 2011; Slominski et al., 2006a). Activation of the glucocorticoid receptor (GR) completes the stress response via inhibition of the HPA axis by attenuating CRF and POMC peptide production (Slominski et al., 2013a; Zmijewski and Slominski, 2011; Ito et al., 2005). Furthermore, locally produced glucocorticoids play an important role in the regulation of the skin inflammatory response (Sevilla et al., 2012; Slominski et al., 2013a,d). UVB not only induces production of vitamin D3, but also stimulates cutaneous production of a variety of classical hypothalamic and pituitary peptides involved in the HPA and increases the expression of their receptors (reviewed in Chakraborty et al. 1999; Pawelek et al., 1992; Slominski et al. (2012, 2013a,d)), and stimulates local steroidogenesis (Slominski et al., 2013a,d; Skobowiat et al., 2011; Skobowiat et al., 2013a,b; Talabér et al., 2013). UVB also upregulates the central HPA axis (Skobowiat and Slominski, 2015) and the POMC signaling system in the arcuate nucleus of the hypothalamus (Skobowiat and Slominski, 2016). Therefore, it is hypothesized that the selective expression of neuropeptides and their receptors not only contributes to the process of formation of the epidermal barrier, but also participates in skin stress and immune responses.
Vitamin D3 and calcium are essential regulators of skin physiology including keratinocyte differentiation (Bikle, 2011a,b,c, 2012; Elias, 2012; Elias et al., 2013). Vitamin D and its analogs are known to modify expression of at least 3000 genes (Haussler et al., 2011), as shown by several transcriptome-wide arrays, however the effect on expression of the elements of HPA axis has not been investigated (Rid et al., 2013). Therefore, the major aim of this study was to determine whether 1,25(OH)2D3 and its low calcemic analogs, 20(OH)D3 and 21(OH)pD, as well-studied inducers of keratinocytes differentiation, also modulate the expression of the sHPA.
2. Materials and methods
2.1. Cell culture
Pooled juvenile Human Epidermal Keratinocyte Progenitors (HPEKp) were acquired from CELLnTEC (Bern, Switzerland). Cells were cultivated in Epidermal Keratinocyte Medium (CnT-07, CELL-nTEC) containing low calcium (0.07 mM), supplement mix (A, B, C), bisphenol A (BPE) and gentamycin. This medium supports the retention of proliferative progenitors and reduces differentiation according to the manufacturer. HPEKp cells were cultured at 37 °C in a humidified 5% CO2 incubator in T-75 culture flasks. Only cells from passages two to four were used for experiments. Cells were passaged at 80–90% confluency after trypsinization with TrypLE™ Express solution (Gibco, Life Technologies, USA).
2.2. Cell treatment
After 24 h of preincubation in supplemented CnT-07 medium, HPEKp cells were treated with vehicle, 0.1 µM 1,25(OH)2D3 or 2.5 mM CaCl as a source of Ca2+, alone or in combination with 1,25(OH)2D3, for 4 or 24 h. Additionally, some cells were treated with the vitamin D3 derivatives 20(OH)D3 or 21(OH)pD (0.1 µM) for 24 h. 1,25(OH)2D3 was purchased from Pharmaceutical Research Institute, Warsaw, Poland. 21(OH)pD was synthesized according to a procedure described by Zmijewski et al. (2011) by ProChimia Surfaces Sp. Zo.o. (Poland), while 20(OH)D3 was enzymatically synthesized as described previously (Slominski et al., 2005c; Tuckey et al., 2011).
2.3. Proliferation assay
The degree of proliferation of cells following treatment was measured from their protein content using the sulforhodamine B assay (SRB). Measurements were made as described previously (Wierzbicka et al., 2015), with some modifications. Cells were seeded at a density of ~12 × 103 cells per 100 µl in 96-well plates and allowed to attach for 24 h before treatment. The culture medium was replaced with fresh medium containing serial dilutions of 1,25(OH)2D3 or its analogs (0.01 nM–1 µM) in a volume of 100 µl/well. The plates were incubated at 37 °C for an additional 48 h, 100 µl of 10% TCA was added to each well and plates incubated for 1 h at 4 °C. Medium was removed and cells were washed 5 times with deionized water. Following overnight air drying, 100 µl of SRB solution [0.4% (w/v) in 1% acetic acid] was added to each well. After incubation for 15 min, plates were washed 5 times with 1% acetic acid and air-dried. The protein-bound dye was solubilized with 10 mM Tris-base solution (pH 10.5). The absorbance of the dye was recorded at 570 nm with a microplate reader. Results are expressed as percentage change in protein level ().
2.4. Real-Time PCR
Total RNA was extracted from cell cultures or skin biopsies using the Total RNA MiniPLUS kit (A&A Biotechnology, Poland) according to the manufacturer's instructions. The concentration and purity of isolated RNA were measured with an Epoch spectrophotometer (BioTek, Winooski, USA). Two micrograms of total RNA were subjected to reverse transcription using a RevertAid™ First Strand cDNA Synthesis kit (Thermo Fisher Scientific Inc., USA.). The primers used for PCR amplification are listed in Table 1. The reactions were performed in duplicate for each primer set with Real Time HS 2× PCR Master Mix SYBR® kit (A&A Biotechnology, Poland). The data were collected using the StepOnePlus™ Real-Time PCR System (Life Technologies, USA). The amount of amplified product for each gene was compared to that for the reference gene (RPL37) using a comparative ΔΔCT method and presented as a fold change ± SD. In the initial stage of the project it was found that RPL37, a gene encoding a ribosomal 60s subunit protein, is a suitable reference gene for quantitative transcript analysis for human keratinocytes out of all the potential housekeeping genes that were tested (RPL37, B2M and HPRT1).
Table 1.
The list of PCR primers used in the study.
| Gene name | Forward primer | Reverse primer |
|---|---|---|
| RPL37 | TTCTGATGGCGGACTTTACC | CACTTGCTCTTTCTGTGGCA |
| IVL | TGGGTATTGACTGGAGGAGG | CTGCCTCAGCCTTACTGTGA |
| KRT 1 | TGACCAAGGTGGACCTTCAG | ATGATGCTGTCCAGGTCGAG |
| KRT 14 | TCTGCAGAAGGACATTGGC | GGCCTGCTGAGATCAAAGAC |
| CRF | CACCCTCAGCCCTTGGATTTC | GCCCTGGCCATTTCCAAGAC |
| UCN 1 | CAGGCGAGCGGCCGCG | CTTGCCCACCGAGTCGAAT |
| UCN 2 | GTGTCGGCCACTGCTGAGCCTGAGAGA | ATCTGATATGACCTGCATGACAGTGGCT |
| UCN 3 | TGCTGCTCCTGCTGCTGCTC | GTGTCCTGGCGTGGCTTTCCC |
| POMC | GAGGGCAAGCGCTCCTACTCC | GGGGCCCTCGTCCTTCTTCTC |
| MC1R | ACTCACCCATGTACTGCTTC | TACAGCACGGCCATGAGCAC |
| MC2R | GACTGTCCTCGTGTGGTTTTG | GGCTGCCCAGCATATCAGAT |
| NR3C1 | GAGACCAGATGTAAGCTCTCCT | GCAATCATTCCTTCCAGCAC |
| VDR | CCAGTTCGTGTGAATGATGG | GTCGTCCATGGTGAAGGA |
| PDIA3 | CTCCGACGTGCTAGAACTCA | CAGGTGTTAGTGTTGGCAGT |
| CYP24A1 | GCAGCCTAGTGCAGATTT | ATTCACCCAGAACTGTTG |
| CYP2R1 | AGAGACCCAGAAGTGTTCCAT | GTCTTTCAGCACAGATGAGGTA |
| CYP3A4 | AAGGCACCACCCACCTATGATACT | TACTTTGGGTCACGGTGAAGAGCA |
| CYP27B1 | TGTTTGCATTTGCTCAGA | CCGGGAGAGCTCATACAG |
2.5. Immunofluorescence microscopy
Prior to immunofluorescence stainings, HPEKp keratinocytes were seeded in 8-well Lab-Tek II chamber slides (Nalge Nunc Inc., USA). At selected time points after treatment, cells were fixed with 4% paraformaldehyde (PFA) and then permeabilized in 0.2% TritonX-100 solution in PBS for 10 min. Blocking was performed with 1% BSA in PBS for 1 h at room temperature (RT). Following extensive rinsing in PBS, the primary antibodies diluted in the same blocking solution were added and incubated overnight at 4 °C. Next day, slides were rinsed with PBS and incubated with the corresponding Alexa Fluor® (AF488 or AF594)-conjugated secondary antibodies for 1 h at RT. Then slides were rinsed, counterstained with DAPI (Sigma Aldrich) and closed with a cover glass. Cells treated with only the secondary antibody were used as the negative control. Images of cells were collected with a Nikon Eclipse E800, and further analyzed with the use of ImageJ® software. All primary and secondary antibodies and their dilutions for use are listed in Table 2.
Table 2.
The list of antibodies used in the study.
| Primary antibodies | ||||
|---|---|---|---|---|
| Antigen | Isotype | Dilution | Supplier | Cat. # |
| Involucrin | Mouse | 1:100 | Novocastra | NCL-INV |
| Ki67 | Mouse | 1:100 | Novocastra | NCL-Ki67-MM1 |
| CRF | Goat | 1:100 | Santa Cruz Biotechnology | sc-21675 |
| CRFR1 | Goat | 1:200 | Abcam | ab77686 |
| ACTH | 1:500 | Dr. Allen, USA | ||
| MC2R | Goat | 1:500 | Abcam | ab77347 |
| GR | Rabbit | 1:100 | Santa Cruz Biotechnology | sc-8992 |
| Secondary antibodies | |||||
|---|---|---|---|---|---|
| Antigen | Isotype | Dilution | Conjugate | Supplier | Cat. # |
| Anti-Goat IgG | Donkey | 1:500 | Alexa Fluor® 488 |
Life Technologies |
A11055 |
| Anti-Rabbit IgG |
Goat | 1:500 | Alexa Fluor® 488 |
Life Technologies |
A11008 |
| Anti-Mouse IgG |
Donkey | 1:500 | Alexa Fluor® 594 |
Life Technologies |
A21203 |
2.6. Flow cytometry
HPEKp cells were treated with 0.1 µM 1,25(OH)2D3, 20(OH)D3 or 21(OH)pD for 24 h. Simultaneously control, unstimulated cells were cultured. After treatment cells were harvested and washed twice in PBS. Cells were fixed and permeabilized with Cytofix/Cytoperm Buffer (BD Biosciences, San Jose, CA, USA) and stained with selected antibodies (Table 2) according to the manufacturer's protocol. Samples were analyzed with a BD FACS Calibur flow cytometer. FL-1 and FL-4 signals (collected from 10,000 events in side scatter/forward scatter window after debris exclusion) were recorded. Forward (relative to cell size) and side (relative to cell granularity) scatter were also recorded. Data were further analyzed with BD CellQuest Pro software (BD Biosciences, San Jose, CA, USA).
2.7. Statistical analyses
Data are presented as mean ± SD, and were analyzed with a Student's t-test (for two groups) or one-way analysis of variance with appropriate post-hoc tests (for more than two groups) using Prism 4.00 (GraphPad Software, San Diego, CA). Statistically significant differences are denoted with asterisks: *P < 0.05, **P < 0.01, ***P < 0.005.
3. Results
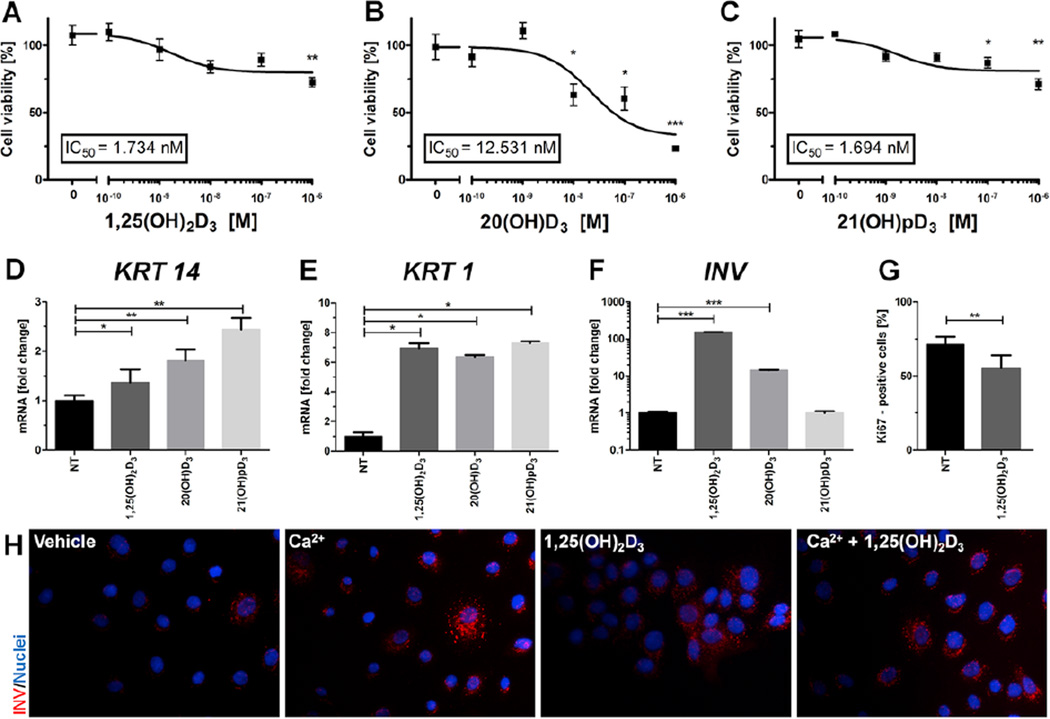
The general effects of 1,25(OH)2D3 and its analogs on HPEKp primary keratinocytes were examined (Fig. 1). All secosteroids significantly inhibited cell proliferation in a dose-dependent manner with 21(OH)pD showing a similar IC50 to that of 1,25(OH)2D3 (1.73 nM vs 1.69 nM, respectively), while 20(OH)D3 gave a higher value (12.5 nM) (Fig. 1A–C). However, 20(OH)D3 showed higher maximal inhibition (efficacy) in comparison to 1,25(OH)2D3 and 21(OH)pD, reducing the cell number by 60–70% vs 20%, for 1,25(OH)2D3 and 21(OH)pD. In addition, there was a significant difference (**P < 0.01) in the percentage of Ki67 positive cells (Fig. 1G) between control (71.3 ± 4.8%) and 1,25(OH)2D3-treated cells (55.3 ± 7.8%). We investigated the expression of keratinocyte differentiation marker genes and found that expression of the early differentiation marker genes, cytokeratin 14 (KRT14) and cytokeratin 1 (KRT1), Fig. 1D, E was elevated in HPEKp keratinocytes treated with all secosteroids tested (*P < 0.05). KRT14 expression was elevated by each secosteroid with the relative stimulation being 21(OH)pD > 20(OH)D3 > 1,25(OH)2D3 (Fig. 1D). Only 1,25(OH)2D3 and 20(OH)D3 treatment effectively increased the level of mRNA for the involucrin (INV) gene, a late differentiation marker, with the short side-chain analog, 21(OH)pD, having no effect (Fig. 1F). These results are consistent with previous data on induction of the INV gene expression in the HaCaT cell line treated with active forms of vitamin D3 (Zbytek et al., 2008). The 1,25(OH)2D3 effect was also confirmed by immunofluorescence staining for INV (Fig. 1H). 1,25(OH)2D3 proved to be as strong an inducer of INV production as Ca2+.
Fig. 1. 1,25(OH)2D3 and it analogs inhibit cell proliferation and stimulate differentiation of primary human epidermal keratinocytes (HPEKp cell line).
Inhibition of the growth of primary human epidermal keratinocyte by 1,25(OH)2D3 (A), 20(OH)D3 (B) and 21(OH)pD (C) was measured at 48 h using the SRB protein assay. Concentration-response curves were plotted and the IC50 value determined as the concentration of the secosteroid which caused a 50% decrease in cell proliferation, calculated using Graph Pad Prism 5. Real-time quantitative PCR analyses were carried out on the expression of key differentiation markers, cytokeratin 1 (KRT1, D), cytokeratin 14 (KRT14, E) and involucrin (IVN, F), in primary human epidermal keratinocytes stimulated with 0.1 µM 1,25(OH)2D3, 20(OH)D3 or 21(OH)pD for 24 h. Immunofluorescence labeling for Ki67 in control and stimulated cells with 1,25(OH)2D3 (0.1 µM) indicated a decrease in number of cells which entered cell cycle (G). Data are presented as means ± SD. Involucrin immunofluorescent-stained primary human epidermal keratinocytes (H) displayed increases in cell differentiation between control and stimulated cells with 1,25(OH)2D3 (0.1 µM), Ca2+ (2.5 mM) or both. Magnification 400×. *P < 0.05, **P < 0.01, ***P < 0.001 compared to control.
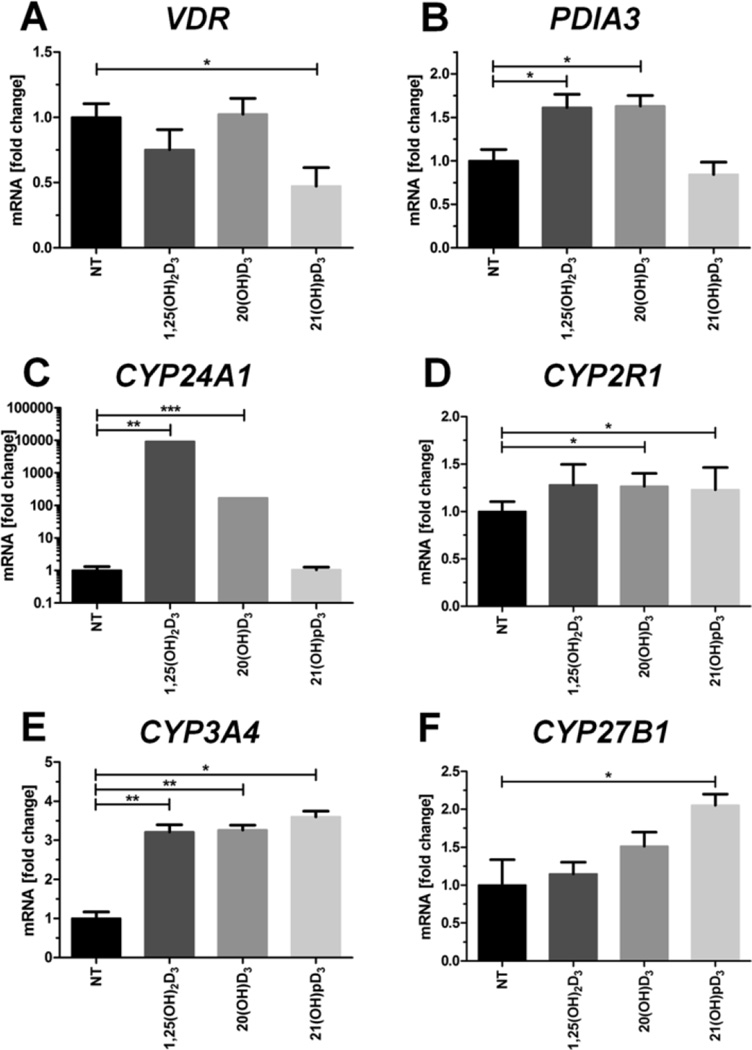
We also examined changes in the expression of genes involved in vitamin D3 signaling following treatment with the secosteroids. There was a lack of effect of 1,25(OH)2D3 and 20(OH)D3 on VDR gene expression with inhibition only occurring with 21(OH)pD (Fig. 2A). In contrast, the expression of the PDIA3 gene which encodes a plasma membrane-bound receptor for vitamin D3, was enhanced by 1,25(OH)2D3 and 20(OH)D3, but not by 21(OH)pD (Fig. 2B). Only 1,25(OH)2D3 strongly induced the expression of CYP24A1 which encodes the 24-hydroxylase that inactivates 1,25(OH)2D3, (Fig. 2C), while 20(OH)D3 caused modest stimulation, in agreement with previous investigations (reviewed in Slominski et al. (2015a,b,c)). All secosteroids tested significantly stimulated the expression of the xenobiotic metabolizing enzyme, CYP3A4, at the mRNA level (Fig. 2E), and to a lesser extent stimulated expression of the 25-hydroxylase gene, CYP2R1 (Fig. 2D). Interestingly, only 21(OH)pD significantly increased the expression of the 1α-hydroxylase gene, CYP27B1 (Fig. 2F).
Fig. 2. 1,25(OH)2D3 and it analogs modulate the expression of vitamin D-related genes in primary human epidermal keratinocytes (HPEKp cell line).
Real-time quantitative PCR analyses were carried out on the expression of key vitamin D-related genes: VDR (A), PDIA3 (B), CYP24A1 (C), CYP2R1 (D), CYP3A4 (E) and CYP27B1 (F) in primary human epidermal keratinocytes stimulated with 0.1 µM 1,25(OH)2D3, 20(OH)D3, or 21(OH)pD for 24 h. Data are presented as means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 compared to control.
3.1. Vitamin D derivatives and calcium stimulate expression of sHPA axis
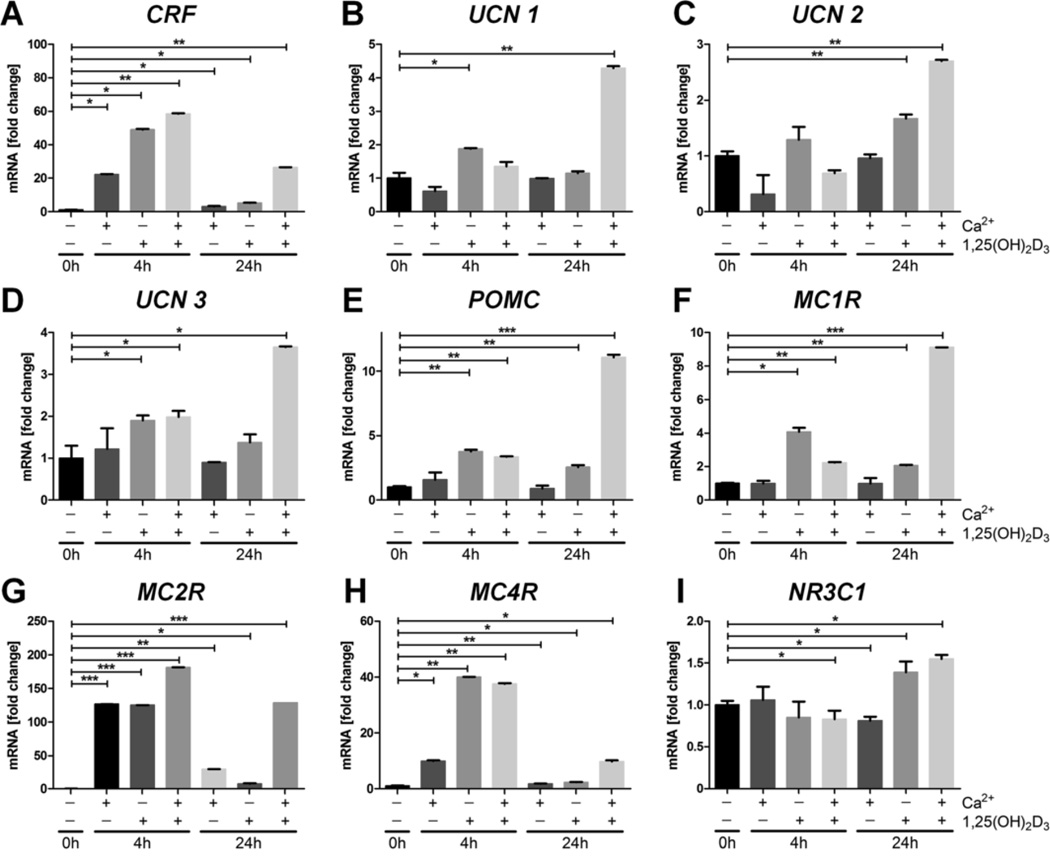
Previous studies have demonstrated that UVB can significantly stimulate the expression of HPA-related genes in epidermal keratinocytes and melanocytes (Zbytek et al., 2006a; Skobowiat et al., 2011 Slominski et al., 1996; Slominski et al., 2006c). To test whether these effects are secondary to the action of active forms of vitamin D, we investigated whether 1,25(OH)2D3 affects the expression of CRF and CRF-related genes [urocortins 1–3 (UCN1–3)] at the mRNA level (Fig. 3). HPEKp cells were treated with 0.1 µM 1,25(OH)2D3 or 2.5 mM calcium (Ca+2) separately, or simultaneously with both (Fig. 3), for 4 or 24 h. Four hours after treatment CRF mRNA was significantly increased in all groups tested (Fig. 3A). By 24 h this stimulation decreased compared to 4 h, but still was higher than the control level. The strongest effect was seen for simultaneous treatment with 1,25(OH)2D3 and Ca+2. The stimulation of the expression of UCN1–3 was less pronounced or absent, and was predominantly seen at 24 h of treatment (Fig. 3B–D). These effects were also selective with weak but significant stimulation after a 4 h of treatment with 1,25(OH)2D3 (UCN-2 and 3), and 1,25(OH)2D3 plus Ca+2 (UCN-3), with strongest stimulation at 24 h by 1,25(OH)2D3 plus Ca+2 (UCN1–3). Significant stimulation of UCN-1 and 3 expression by 1,25(OH)2D3 alone was only seen at 4 h (Fig. 3B–D).
Fig. 3. 1,25(OH)2D3 and calcium modulate the expression of neuropeptides in primary human epidermal keratinocytes (HPEKp cell line).
Real-time quantitative PCR analyses of neuropeptide gene expression were carried out on CRF (A), UCN1 (B), UCN2 (C), UCN3 (D), POMC (E), MC1R (F), MC2R (G), MC4R (H) and NR3C1 (I) in primary human epidermal keratinocytes stimulated with 1,25(OH)2D3 (0.1 µM), Ca2+ (2.5 mM), or both, for 4 and 24 h. Data are presented as means ± SD. *P < 0.05, **P < 0.01 compared to control.
The expression of the POMC gene, encoding the second element of the sHPA, was stimulated at 4 and 24 h of treatment with 1,25(OH)2D3 alone or in combination with Ca+2, with the strongest stimulation being observed with 1,25(OH)2D3 plus Ca+2 for 24 h (Fig. 3E). 1,25(OH)2D3 and/or Ca+2 treatment of HPEKp cells also stimulated the expression of receptors for POMC-derived peptides (Fig. 3F–H). Incubation of cells with 1,25(OH)2D3 or 1,25(OH)2D3 plus Ca+2 resulted in increased expression of the MC1R gene at 4 and 24 h, with the highest stimulation seen for 1,25(OH)2D3 plus Ca+2 at 24 h, while Ca+2 alone had no effect (Fig. 3F). Stimulation of MC2R and MC4R gene expression was observed with all treatments, with the effects being greatest at 4 h (Fig. 3G and H). Finally, expression of the glucocorticoid receptor (GR) gene, NR3C1 (Fig. 3I), was only slightly elevated in cells treated with 1,25(OH)2D3 or 1,25(OH)2D3 plus Ca+2, at 24 h only.
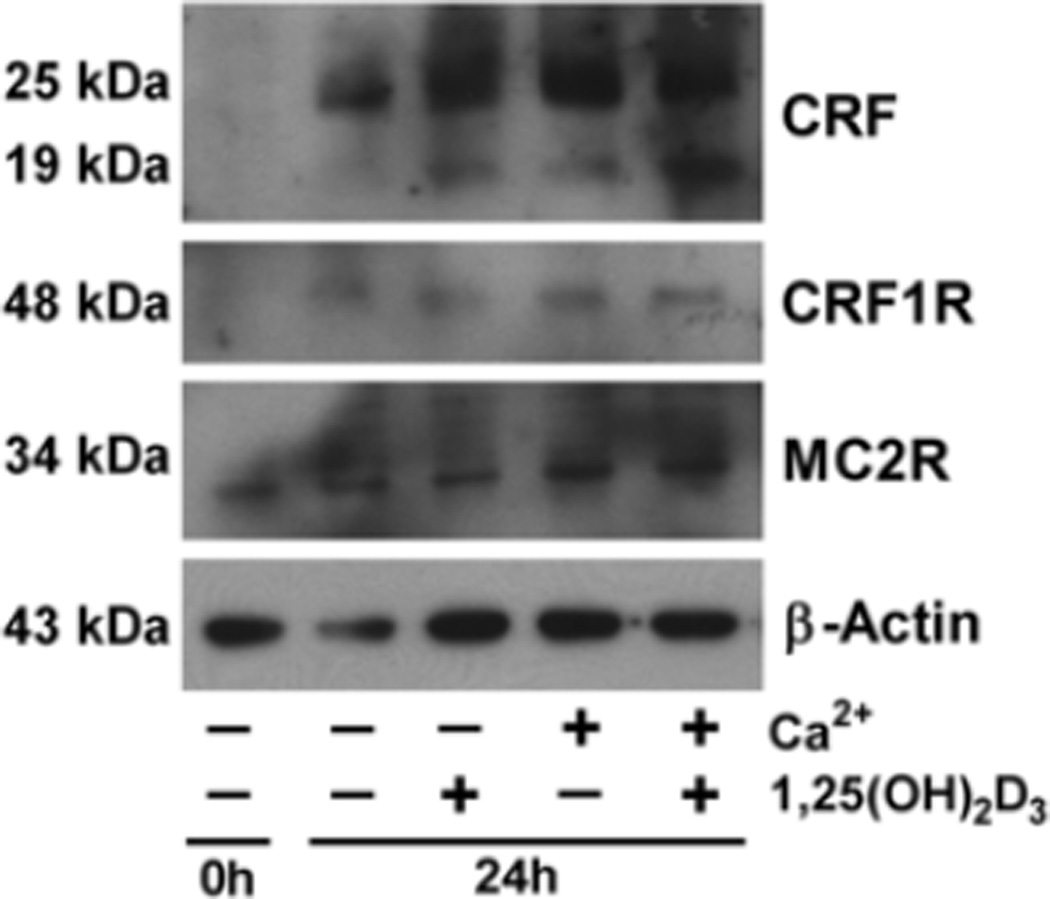
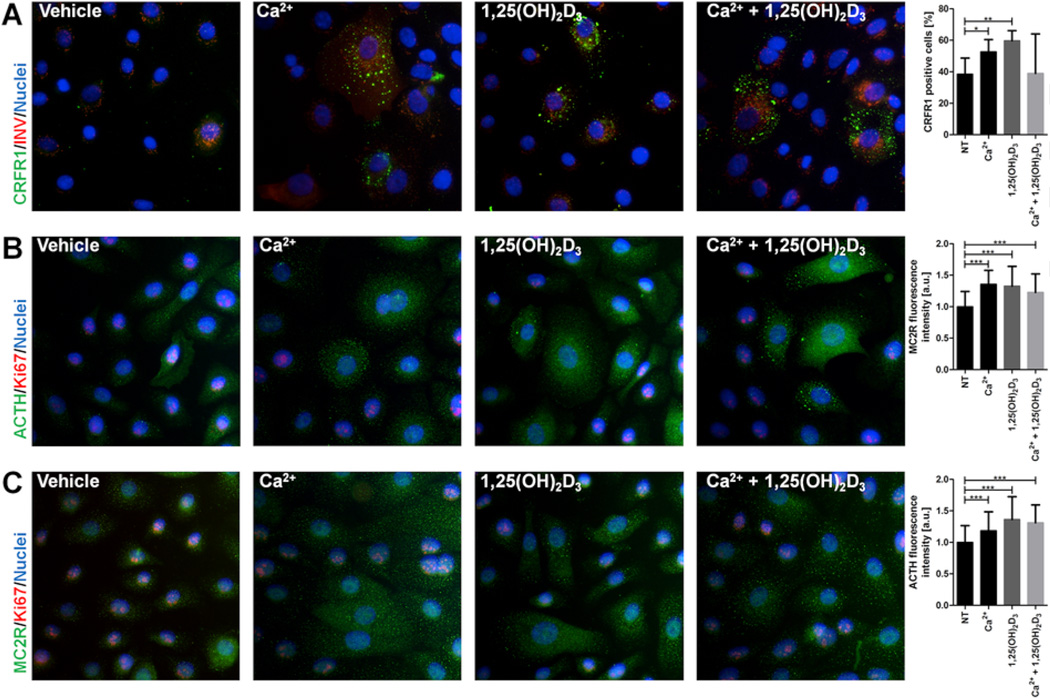
The expression of selected elements of the sHPA in human primary keratinocytes (HPEKp) at the protein level was investigated by western blotting (Fig. 4) and immunofluorescence (IF) (Fig. 5). The level of CRF peptides in HPEKp cells was increased by treatment with 1,25(OH)2D3 and Ca+2 separately or together for 24 h (Fig. 4). Some processing of the 25 kDa CRF precursor to the 19 kDa form was apparent which is consistent with previous reports in the literature (Chretien and Seidah, 1984) and with data provided by the supplier of the antiserum, with both forms displaying an increase with the treatments. The increase in CRF expression was accompanied by an increase in CRFR1 immunoreactivity as shown by western blotting (with a marked increase also occurring over 24 h without treatment) (Fig. 4) and by an elevated number of CRFR1 positive HPEKp cells (Fig. 5A). In addition, 1,25(OH)2D3, Ca+2 or 1,25(OH)2D3 plus Ca+2 stimulated the production of POMC-derived ACTH in cultured keratinocytes (Fig. 5B), consistent with stimulation of POMC gene expression. Finally, similar treatments increased the MC2R protein level as evaluated by western blotting and IF (Figs. 4 and 5C).
Fig. 4. Western Blot analyses of key proteins of the sHPA axis.
CRF, CRFR1 and MC2R protein levels were measured in primary human epidermal keratinocytes stimulated with 1,25(OH)2D3 (0.1 µM), Ca2+ (2.5 mM), or both, for 24 h. β-Actin levels were measured as a control.
Fig. 5. 1,25(OH)2D3 and calcium modulate the protein level of neuropeptides and their receptors in primary human epidermal keratinocytes (HPEKp cell line).
Involucrin (red) and CRFR1 (green) immunofluorescent-stained primary human epidermal keratinocytes demonstrated increases in CRFR1 (A) in cells stimulated with 1,25(OH)2D3 (0.1 µM), Ca2+ (2.5 mM), or both, compared to the control. Ki67 (red) and ACTH (green) immunofluorescent stained primary human epidermal keratinocytes (B) demonstrated increases in ACTH in cells stimulated with 1,25(OH)2D3 (0.1 µM), Ca2+ (2.5 mM), or both, compared to control. Ki67 (red) and MC2R (green) immunofluorescent-stained primary human epidermal keratinocytes (C) demonstrated increases in MC1R in cells stimulated with 1,25(OH)2D3 (0.1 µM), Ca2+ (2.5 mM), or both, compared to control. Fluorescence intensity, was evaluated with the image analysis software, ImageJ (graphs on the right). Magnification 400×. Data are presented as means ± SD.*P < 0.05, **P < 0.01, ***P < 0.001 compared to control. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. The effect of low-calcemic D3 analogs on the expression of HPA axis elements
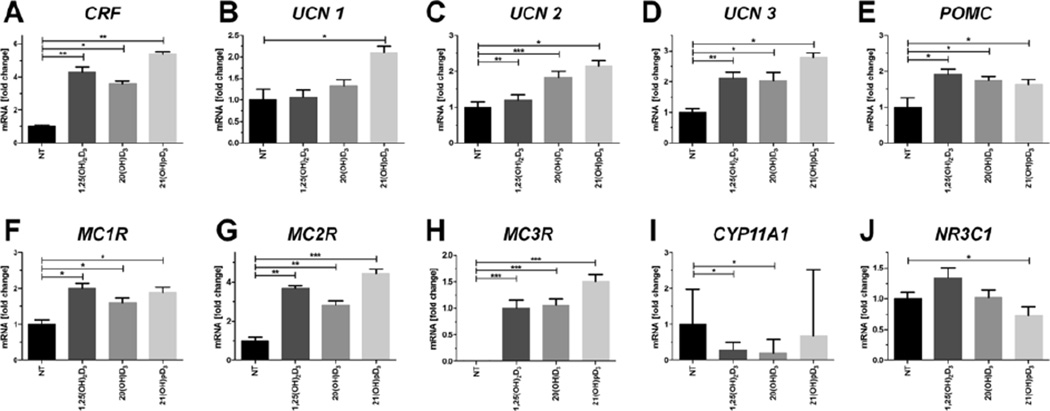
20(OH)D3 and 21(OH)pD (0.1 µM) were tested for their ability to stimulate the expression of sHPA genes in comparison to 1,25(OH)2D3. Both of these secosteroids stimulated the expression CRF similarly to 1,25(OH)2D3 (Fig. 6A). Interestingly, the highest level of stimulation of the expression of CRF and urocortins was observed for 21(OH)pD (Fig. 6B–D). In addition, 1,25(OH)2D3 and 20(OH)D3 did not affect the expression of the UCN1 gene (Fig. 6B) and only moderately increased the expression of UCN2 and 3 (Fig. 6C–D). The expression of the POMC gene was stimulated comparably by 1,25(OH)2D3, 20(OH)D3 or 21(OH)pD (Fig. 6E). Furthermore, stimulation of the expression MC1R and MC2R genes followed the same trend, with comparable effects for all the secosteroids tested (Fig. 6F–G). MC3R mRNA was undetectable in primary keratinocytes from the control culture, but its expression was effectively induced by all the secosteroids tested, with the highest stimulation obtained with 21(OH)pD (compared to 1,25(OH)2D3 treatment). Finally, 20(OH)D3 or 1,25(OH)2D3 treatment, unlike 21(OH)pD, attenuated the expression of CYP11A1 (Fig. 6I), while only 21(OH)pD moderately decreased the expression of NR3C1 (Fig. 6J).
Fig. 6. 1,25(OH)2D3 and it analogs modulate the expression of sHPA axis elements in primary human epidermal keratinocytes (HPEKp cell line).
Real-time quantitative PCR analyses of the expression of key genes of the HPA axis were carried out for CRF (A), UCN1 (B), UCN2 (C), UCN3 (D), CRFR1 (E), POMC (F), MC1R (G), MC2R (H), MC3R (I) and NR3C1 (J) in primary human epidermal keratinocytes stimulated with 0.1 µM 1,25(OH)2D3, 20(OH)D3 or 21(OH)pD for 24 h. Data are presented as means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 compared to control.
3.3. The degree of keratinocyte differentiation affects the expression of ACTH and MC2R in cells treated with vitamin D3 derivatives
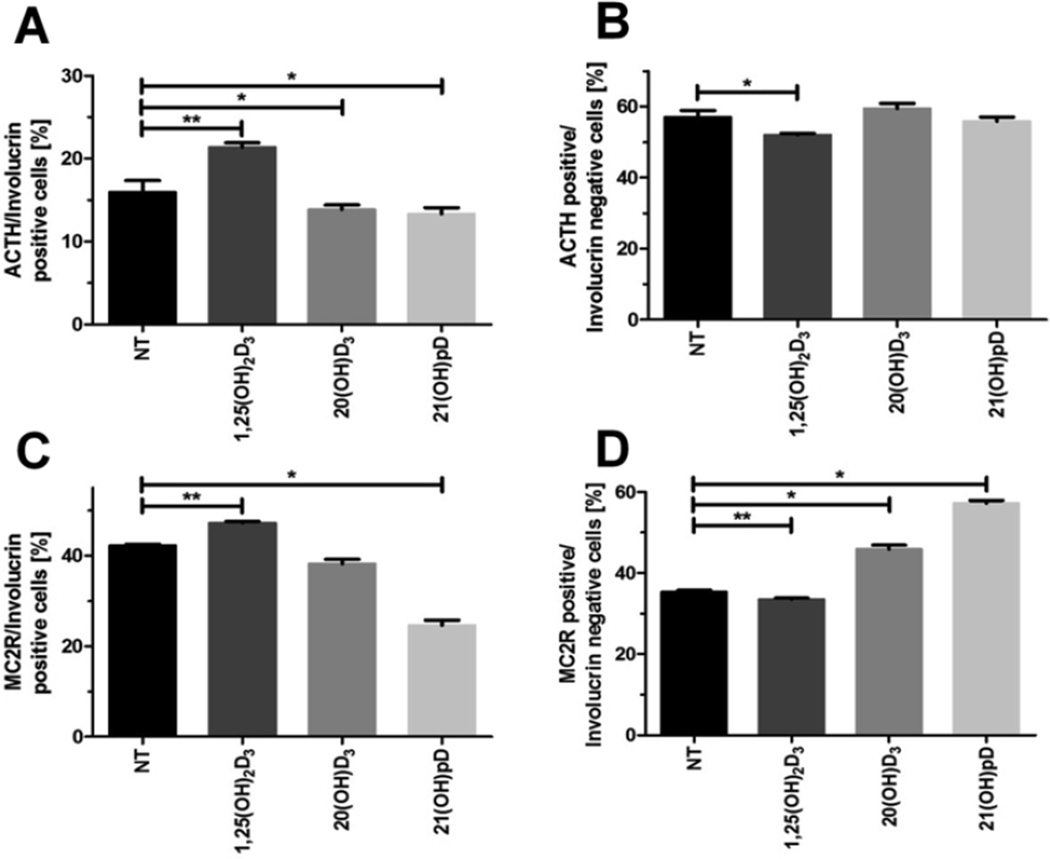
Since the differentiation of keratinocytes is accompanied by an alteration in the expression of several genes (Eckert and Rorke, 1989; Eckhart et al., 2013; Elias, 2012), we tested whether the degree of differentiation of keratinocytes, monitored by the level of expression of INV, affects the stimulation of ACTH and MC2R expression by vitamin D compounds. Using flow cytometry we tested two populations of keratinocytes, INV positive (INV+, differentiated) and INV negative (INV−, undifferentiated) cells (Fig. 7). 1,25(OH)2D3 treatment increased the number of positive cells for ACTH (Fig. 7A) and its receptor (MC2R), in INV+ keratinocytes (Fig. 7C), while their relative numbers decreased for INV− cells (Fig. 7B, D). The treatment of HPEKp with 20(OH)D3 or 21(OH)pD resulted in a decrease in the % of cells co-expressing ACTH in INV+ keratinocytes (Fig. 7A). Also, 21(OH)pD, but not 20(OH)D3, significantly decreased the % of cells co-expressing MC2R and INV+ (Fig. 7C), while the % of cells positive for MC2R in INV− cells was increased by 21(OH)pD and 20(OH)D3, but slightly decreased by 1,25(OH)2D3 (Fig. 7D).
Fig. 7. 1,25(OH)2D3 and it analogs selectively modulate the expression of ACTH and MC2R in differentiated and undifferentiated primary human epidermal keratinocytes (HPEKp cell line).
Flow cytometry analyses were performed on ACTH and MC2R in involucrin positive and negative primary human epidermal keratinocytes stimulated with 0.1 µM 1,25(OH)2D3, 20(OH)D3 or 21(OH)pD for 24 h. Data are presented as means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 compared to control.
4. Discussion
Recently, we have reported increased expression of cutaneous elements of the HPA coincides with the keratinocyte differentiation program, with both processes being stimulated by calcium addition (Wierzbicka et al., 2016). Since active forms of vitamin D3 protect the epidermis against UVB, a major environmental stressor (Slominski et al., 2015a,c; Gordon-Thomson et al., 2012; De Haes et al., 2005), clarification of the relationship between the cutaneous HPA axis and vitamin D3-activated pathways is mandatory.
We observed that 1,25(OH)2D3 and its non-calcemic analogs (20(OH)D3 and 21(OH)pD) displayed antiproliferative properties, while only 1,25(OH)2D3 and 20(OH)D3 stimulated differentiation of keratinocytes using INV as a marker. Previous studies on immortalized human epidermal keratinocytes have shown that 20(OH)D3 possesses antiproliferative activity similar to that of 1,25(OH)2D3, with both compounds effectively stimulating INV expression (Zbytek et al., 2008). Interestingly, 21(OH)pD inhibited cell proliferation as effectively 1,25(OH)2D3 and 20(OH)D3, but caused weaker induction of keratinocytes differentiation, with only a moderate effect on the expression of KRT14 (an early differentiation marker) and no effect on INV expression (late differentiation marker). Treatment of primary keratinocytes with 21(OH)pD, which only has a 2C side chain, did not stimulate the expression of CYP24A1, a major target of the activated VDR, suggesting an alternative pathway may be activated by this secosteroid. This is consistent with previous studies showing that treatment of melanoma cells with 21(OH)pD does not stimulate the translocation of VDR to the nucleus (Zmijewski et al., 2011) nor cause stimulation of CYP24A1 expression (Wasiewicz et al., 2015), despite inhibiting melanoma cell proliferation (Zmijewski et al., 2011; Wasiewicz et al., 2015). The lack of stimulation of VDR translocation and expression of CYP24A1 and INV genes by 21(OH)pD can be explained by the recent in silico prediction that 21(OH)pD interacts poorly with the VDR, having the lowest docking score of the vitamin D analogs examined (Kim et al., 2012). Thus, the observed inhibition of cell proliferation by 21(OH)pD may be secondary to the interaction of this secosteroid, with an alternative receptor to the VDR.
1,25(OH)2D3 caused a massive increase in the expression of CYP24A1, with CYP24A1 catalyzing the inactivation of both 25(OH)D3 and 1,25(OH)2D3 (Holick, 2003). As before (Zbytek et al., 2008), 20(OH)D3 caused very low stimulation of CYP24A1 expression, with the gene product catalyzing the further activation of 20(OH)D3 instead of its inactivation, at least in melanoma cells (Tieu et al., 2012). 1,25(OH)2D3, 20(OH)D3 and 21(OH)pD all caused a 3- to 3.5-fold increase in CYP3A4 expression in keratinocytes at the mRNA level. CYP3A4, as well as acting on a wide range of xenobiotics, provides an alternate route to CYP24A1 for the metabolism of active forms of vitamin D3, including 20(OH)D3 (Cheng et al., 2016). 20(OH)D3 and 21(OH)pD, but not 1,25(OH)2D3, caused a small but significant stimulation of the expression of CYP2R1 which encodes a vitamin D 25-hydroxylase. Only 21(OH)pD significantly stimulated expression of CYP27B1 but it is unlikely that the encoded 1α-hydroxylase can act on vitamin D3 analogs like 21(OH)pD, which possess a short side chain (Chen et al., 2014).
The current study shows that treatment of primary keratinocytes with 1,25(OH)2D3 and calcium induces the expression, at least at the mRNA level, of all the elements of the HPA axis including CRF, UNC1–3, POMC, MC1R and MC2R and NR3C1. Furthermore, stimulation is generally greater than that by treatment with 1,25(OH)2D3 alone, particularly at 24 h. We have previously shown that the process of keratinocytes differentiation, which is strongly induced by the cooperation of vitamin D3 with Ca2+ (Bikle et al., 2004a,b), plays a crucial role in the stimulation of elements of the cutaneous HPA (Wierzbicka et al., 2016). However, the effect of incubation of calcium with primary keratinocytes for 4 or 24 h on the elements of the HPA was always weaker that caused by 1,25(OH)2D3. This suggests that active forms of vitamin D3 may act on the epidermal elements of the HPA through overlapping and distinct pathways to that induced by calcium.
21(OH)pD was the only secosteroid tested that was able to stimulate expression of urocortin 1 (UCN 1), and was the strongest inducer for CRF, UCN2 and UCN3 gene expression. It is well established that CRF and UCN1 enhance expression of POMC trough CRFR1 receptor-mediated activation of adenylate cyclase (Slominski et al., 2013a). It seems however, that the observed higher level of induction of CRF and UCN1 by 21(OH)pD in comparison to other secosteroids, has no impact on POMC expression, since they all showed similar effects. 21(OH)pD was marginally better at stimulating the expression of receptors for melanocortins (except MC1R) than the other secosteroids. To our knowledge, this is the first study showing the stimulation of MC3R and MC4R expression by 1,25(OH)2D3 or its non-calcemic analogs, 20(OH)D3 and 21(OH)pD. Recently, MC3R was proposed as an alternative target for corticotropins involved in immunomodulation (Zmijewski and Slominski, 2013). Although γ-MSH is the preferred ligand for MC3R, ACTH can bind to MC3R with comparable affinity (Bohm and Grassel, 2012). Moreover, MC3R may be involved in the activation of steroidogenesis (Harmer and Bicknell, 2005), but what is most interesting is the selective upregulation of IL-1β, IL-6, and NOS2 in Mc3−/− mice compared to wild type mice (Patel et al., 2010). Based on this, it was postulated that induction of MC3R contributes to the melanocortin-induced skin stress response.
The current study shows that ACTH and MC2R are preferentially found at the border of the stratum spinosum and stratum granulosum in full thickness skin biopsies. These results are supported by the observed induction of ACTH and MC2R expression in partially differentiated HPEKp keratinocytes (Wierzbicka et al., 2016). Using flow cytometry analysis and INV as a marker of moderate differentiation, we observed that 1,25(OH)2D3 enhanced ACTH and MC2R production only in INV+ cells, while 20(OH)D3 (moderately) and 21(OH)pD (more strongly) stimulated MC2R production in undifferentiated keratinocytes (INV−). Unlike 1,25(OH)2D3, treatment of primary keratinocytes with 21(OH)pD resulted in a significant decrease in MC2R immunoreactivity in differentiated (INV+) cells but an increase in MC2R immunoreactivity in undifferentiated (INV−) keratinocytes. Furthermore, in contrast to 1,25(OH)2D3 there was not a significant effect of 21(OH)pD on ACTH immunoreactivity. This suggests that 21(OH)pD action on ACTH signaling can be separated from the induction of the keratinocyte differentiation program.
Finally, our data raise the intriguing question as to whether the stimulation of the cutaneous elements of the HPA by UVB is mediated, at least in part, by locally synthesized active forms of vitamin D3. Different elements of the HPA including POMC peptides, CRF and related peptides and glucocorticoids are involved in the regulation of the epidermal skin barrier and its immune activities, as described above. Therefore, our findings may not only explain a role of vitamin D in the regulation of epidermal neuroendocrine activities, but also may represent a dawn for studies on the mechanism of regulation of systemic HPA axis and/or of POMC and CRF activities in the brain (Skobowiat and Slominski, 2016; Slominski, 2015; Slominski et al., 2015a,b,c).
In summary, we have shown for the first time that active forms of vitamin D stimulate the expression of elements of the skin analog of the HPA axis. This process occurs rapidly, is enhanced by the induction of the keratinocyte differentiation program and may follow overlapping but different mechanisms defined by the length of side chain and location of hydroxyl groups on the vitamin D scaffold.
Acknowledgments
The project was supported by Grant from the Polish Ministry of Science and Higher Education, contract grant number: N402 662840 (to M.A.Z.), and in part by NIH grants R21AR066505, 1R01AR056666 and 2R01AR052190 to AS, and the University of Western Australia to RCT.
Abbreviations
- INV
Involucrin
- HPA
hypothalamic-pituitary-adrenal axis
- sHPA
skin analog of HPA
- HPEKp
human primary epidermal keratinocytes (pulled donors)
- UVA/B
ultraviolet radiation A and B KRT1, KRT14, KRT15 keratin 1, 14, 15
- CRF
corticotropin releasing factor
- UCN1–3
urocortins 1–3
- POMC
proopiomelanocortin
- ACTH
adrenocorticotrophin
- CRFR1
corticotropin-releasing hormone receptor 1
- CRFR2
corticotropin-releasing hormone receptor 2
- MC1-5R
melanocortin receptors 1–5
- GR
glucocorticoid receptor
- 1α,25(OH)2D3
1α,25-dihydroxyvitamin D3 (calcitriol)
- 20(OH)D3
20S-hydroxyvitamin D3
- 21(OH)pD
21-hydroxypregnacalciferel
- 25(OH)D3
calcifediol (25-hydroxyvitamin D3)
- 7-DHC
7-dehydrocholesterol (provitamin D3, cholesta-5,7-dien-3β-ol)
- VDR
vitamin D receptor
- PDIA3
Protein disulfide isomerase associated 3
Footnotes
Uncited references
Chakraborty et al., 1999; Ebeling, 2014; Elias, 2012; Holick, 1996; Holick et al., 1977; Loite et al., 2013; Mason et al., 2013; Paus, 2011; Pawelek et al., 1992; Pisarchik and Slominski, 2002; Quevedo et al., 2001; Quirk et al., 2016; Searing and Leung, 2010; Slominski et al., 1996; Tang et al., 2013; Vasiadi et al., 2012; Watt, 1983; Zbytek and Slominski, 2005; Zbytek and Slominski, 2007; Zbytek et al., 2002; Zbytek et al., 2004; Zbytek et al., 2005; Zmijewski and Slominski, 2009; Zouboulis, 2009.
References
- Barragan M, Good M, Kolls JK. Regulation of dendritic cell function by vitamin D. Nutrients. 2015;7:8127–8151. doi: 10.3390/nu7095383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD. Vitamin D regulated keratinocyte differentiation. J. Cell. Biochem. 2004;92:436–444. doi: 10.1002/jcb.20095. [DOI] [PubMed] [Google Scholar]
- Bikle DD. Vitamin D receptor, UVR, and skin cancer: a potential protective mechanism. J. Invest. Dermatol. U. S. 2008:2357–2361. doi: 10.1038/jid.2008.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD. Vitamin D metabolism and function in the skin. Mol. Cell. Endocrinol. 2011a;347:80–89. doi: 10.1016/j.mce.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD. Vitamin D: an ancient hormone. Exp. Dermatol. 2011b;20:7–13. doi: 10.1111/j.1600-0625.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- Bikle DD. Vitamin D regulation of immune function. Vitam. Horm. 2011c;86:1–21. doi: 10.1016/B978-0-12-386960-9.00001-0. [DOI] [PubMed] [Google Scholar]
- Bikle DD. Vitamin D and the skin: physiology and pathophysiology. Rev. Endocr. Metab. Disord. 2012;13:3–19. doi: 10.1007/s11154-011-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Choi EH, Dardenne O, Xie Z, Arnaud RS, Feingold K, Elias PM. 25 Hydroxyvitamin D 1 alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J. Invest. Dermatol. 2004a;122:984–992. doi: 10.1111/j.0022-202X.2004.22424.x. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Oda Y, Xie Z. Calcium and 1,25(OH)2D: interacting drivers of epidermal differentiation. J. Steroid Biochem. Mol. Biol. 2004b;89–90:355–360. doi: 10.1016/j.jsbmb.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Bohm M, Grassel S. Role of proopiomelanocortin-derived peptides and their receptors in the osteoarticular system: from basic to translational research. Endocr. Rev. 2012;33:623–651. doi: 10.1210/er.2011-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre JV. Antifibrotic vitamin D analogs. J. Clin. Invest. 2013;123:4570–4573. doi: 10.1172/JCI72748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell JJ, Grant WB, Holick MF. Vitamin D and inflammation. Dermatoendocrinol. 2014;6:e983401. doi: 10.4161/19381980.2014.983401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty AK, Funasaka Y, Slominski A, Bolognia J, Sodi S, Ichihashi M, Pawelek JM. UV light and MSH receptors. Ann. N. Y. Acad. Sci. 1999;885:100–116. doi: 10.1111/j.1749-6632.1999.tb08668.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang J, Kim TK, Tieu EW, Tang EK, Lin Z, Kovacic D, Miller DD, Postlethwaite A, Tuckey RC, Slominski AT, Li W. Novel vitamin D analogs as potential therapeutics: metabolism, toxicity profiling, and antiproliferative activity. Anticancer Res. 2014;34:2153–2163. [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Slominski AT, Tuckey RC. Hydroxylation of 20-hydroxyvitamin D3 by human CYP3A4. J. Steroid Biochem. Mol. Biol. 2016;159:131–141. doi: 10.1016/j.jsbmb.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chretien M, Seidah NG. Precursor polyproteins in endocrine and neuroendocrine systems. Int. J. Pept. Protein Res. 1984;23:335–341. doi: 10.1111/j.1399-3011.1984.tb02728.x. [DOI] [PubMed] [Google Scholar]
- Cirillo N, Prime SS. Keratinocytes synthesize and activate cortisol. J. Cell. Biochem. 2011;112:1499–1505. doi: 10.1002/jcb.23081. [DOI] [PubMed] [Google Scholar]
- De Haes P, Garmyn M, Verstuyf A, De Clercq P, Vandewalle M, Degreef H, Vantieghem K, Bouillon R, Segaert S. 1,25-Dihydroxyvitamin D3 and analogues protect primary human keratinocytes against UVB-induced DNA damage. J. Photochem Photobiol. B. 2005;78:141–148. doi: 10.1016/j.jphotobiol.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Ebeling PR. Vitamin D and bone health: epidemiologic studies. Bonekey Rep. 2014;3:511. doi: 10.1038/bonekey.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert RL, Rorke EA. Molecular biology of keratinocyte differentiation. Environ. Health Perspect. 1989;80:109–116. doi: 10.1289/ehp.8980109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart L, Lippens S, Tschachler E, Declercq W. Cell death by cornification. Biochim. Biophys. Acta. 2013;1833:3471–3480. doi: 10.1016/j.bbamcr.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Elias PM. Structure and function of the stratum corneum extracellular matrix. J. Invest. Dermatol. 2012;132:2131–2133. doi: 10.1038/jid.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Eichenfield LF, Fowler JF, Horowitz P, McLeod RP. Update on the structure and function of the skin barrier: atopic dermatitis as an exemplar of clinical implications. Semin. Cutan. Med. Surg. 2013;32:S21–S24. doi: 10.12788/j.sder.0022. [DOI] [PubMed] [Google Scholar]
- Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- Gordon-Thomson C, Gupta R, Tongkao-on W, Ryan A, Halliday GM, Mason RS. 1α,25 dihydroxyvitamin D3 enhances cellular defences against UV-induced oxidative and other forms of DNA damage in skin. Photochem Photobiol. Sci. 2012;11:1837–1847. doi: 10.1039/c2pp25202c. [DOI] [PubMed] [Google Scholar]
- Hannen RF, Michael AE, Jaulim A, Bhogal R, Burrin JM, Philpott MP. Steroid synthesis by primary human keratinocytes; implications for skin disease. Biochem. Biophys. Res. Commun. 2011;404:62–67. doi: 10.1016/j.bbrc.2010.11.059. [DOI] [PubMed] [Google Scholar]
- Harmer SC, Bicknell AB. Role of gamma-MSH peptides in the regulation of adrenal steroidogenesis. Peptides. 2005;26:1944–1951. doi: 10.1016/j.peptides.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2 vitamin D3: genomic and non-genomic mechanisms. Best. Pract. Res. Clin. Endocrinol. Metab. 2011;25:543–559. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D and bone health. J. Nutr. 1996;126:1159s–1164s. doi: 10.1093/jn/126.suppl_4.1159S. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D: a millenium perspective. J. Cell. Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr. Drug Targets. 2011;12:4–18. doi: 10.2174/138945011793591635. [DOI] [PubMed] [Google Scholar]
- Holick MF. Sunlight, ultraviolet radiation, vitamin D and skin cancer: how much sunlight do we need? Adv. Exp. Med. Biol. 2014;810:1–16. [PubMed] [Google Scholar]
- Holick MF, Frommer JE, McNeill SC, Richtand NM, Henley JW, Potts JT., Jr Photometabolism of 7-dehydrocholesterol to previtamin D3 in skin. Biochem. Biophys. Res. Commun. 1977;76:107–114. doi: 10.1016/0006-291x(77)91674-6. [DOI] [PubMed] [Google Scholar]
- Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19:1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- Kim TK, Wang J, Janjetovic Z, Chen J, Tuckey RC, Nguyen MN, Tang EK, Miller D, Li W, Slominski AT. Correlation between secosteroid-induced vitamin D receptor activity in melanoma cells and computer-modeled receptor binding strength. Mol. Cell. Endocrinol. 2012;361:143–152. doi: 10.1016/j.mce.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Ishikawa MH, Hawker NP, Yun QC, Bikle DD. Differential role of two VDR coactivators, DRIP205 and SRC-3, in keratinocyte proliferation and differentiation. J. Steroid Biochem. Mol. Biol. 2007;103:776–780. doi: 10.1016/j.jsbmb.2006.12.069. [DOI] [PubMed] [Google Scholar]
- Patel HB, Bombardieri M, Sampaio AL, D'Acquisto F, Gray M, Grieco P, Getting SJ, Pitzalis C, Perretti M. Anti-inflammatory and antiosteoclastogenesis properties of endogenous melanocortin receptor type 3 in experimental arthritis. FASEB J. 2010;24:4835–4843. doi: 10.1096/fj.10-167759. [DOI] [PubMed] [Google Scholar]
- Paus R, Langan EA, Vidali S, Ramot Y, Andersen B. Neuroendocrinology of the hair follicle: principles and clinical perspectives. Trends Mol. Med. 2014;20:559–570. doi: 10.1016/j.molmed.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Pawelek JM, Chakraborty AK, Osber MP, Orlow SJ, Min KK, Rosenzweig KE, Bolognia JL. Molecular cascades in UV-induced melanogenesis: a central role for melanotropins? Pigment. Cell. Res. 1992;5:348–356. doi: 10.1111/j.1600-0749.1992.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Reichrath J, Collins ED, Epple S, Kerber A, Norman AW, Bahmer FA. Immunohistochemical detection of 1,25-dihydroxyvitamin D3 receptors (VDR) in human skin. a comparison of five antibodies. Pathol. Res. Pract. 1996;192:281–289. doi: 10.1016/S0344-0338(96)80231-7. [DOI] [PubMed] [Google Scholar]
- Rid R, Wagner M, Maier CJ, Hundsberger H, Hintner H, Bauer JW, Onder K. Deciphering the calcitriol-induced transcriptomic response in keratinocytes: presentation of novel target genes. J. Mol. Endocrinol. 2013;50:131–149. doi: 10.1530/JME-11-0191. [DOI] [PubMed] [Google Scholar]
- Rousseau K, Kauser S, Pritchard LE, Warhurst A, Oliver RL, Slominski A, Wei ET, Thody AJ, Tobin DJ, White A. Proopiomelanocortin (POMC), the ACTH/melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. FASEB J. 2007;21:1844–1856. doi: 10.1096/fj.06-7398com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla LM, Latorre V, Sanchis A, Perez P. Epidermal inactivation of the glucocorticoid receptor triggers skin barrier defects and cutaneous inflammation. J. Invest. Dermatol. 2012 doi: 10.1038/jid.2012.281. [DOI] [PubMed] [Google Scholar]
- Skobowiat C, Slominski AT. UVB activates hypothalamic-pituitary-adrenal Axis in C57BL/6 mice. J. Invest. Dermatol. 2015;135:1638–1648. doi: 10.1038/jid.2014.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skobowiat C, Slominski AT. Ultraviolet B stimulates proopiomelanocortin signalling in the arcuate nucleus of the hypothalamus in mice. Exp. Dermatol. 2016;25:120–123. doi: 10.1111/exd.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. Am. J. Physiol. Endocrinol. Metab. 2011;301:E484–E493. doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skobowiat C, Sayre RM, Dowdy JC, Slominski AT. Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. Br. J. Dermatol. 2013a;168:595–601. doi: 10.1111/bjd.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skobowiat C, Nejati R, Lu L, Williams RW, Slominski AT. Genetic variation of the cutaneous HPA axis: an analysis of UVB-induced differential responses. Gene. 2013b;530:1–7. doi: 10.1016/j.gene.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT. Ultraviolet radiation (UVR) activates central neuro-endocrine-immune system. Photodermatol. Photoimmunol. Photomed. 2015;31:121–123. doi: 10.1111/phpp.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Baker J, Ermak G, Chakraborty A, Pawelek J. Ultraviolet B stimulates production of corticotropin releasing factor (CRF) by human melanocytes. FEBS Lett. 1996;399:175–176. doi: 10.1016/s0014-5793(96)01315-4. [DOI] [PubMed] [Google Scholar]
- Slominski A, Ermak G, Mazurkiewicz JE, Baker J, Wortsman J. Characterization of corticotropin-releasing hormone (CRH) in human skin. J. Clin. Endocrinol. Metab. 1998;83:1020–1024. doi: 10.1210/jcem.83.3.4650. [DOI] [PubMed] [Google Scholar]
- Slominski A, Roloff B, Curry J, Dahiya M, Szczesniewski A, Wortsman J. The skin produces urocortin. J. Clin. Endocrinol. Metab. 2000;85:815–823. doi: 10.1210/jcem.85.2.6381. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Pisarchik A, Zbytek B, Linton EA, Mazurkiewicz JE, Wei ET. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15:1678–1693. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology. 2004;145:941–950. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, Wortsman J. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am. J. Physiol. Endocrinol. Metab. 2005a;288:E701–E706. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J. Neuroimmunol. 2005b;162:97–102. doi: 10.1016/j.jneuroim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, Tuckey RC. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005c;272:4080–4090. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Szczesniewski A, Wortsman J. Cultured human dermal fibroblasts do produce cortisol. J. Invest. Dermatol. U. S. 2006a:1177–1178. doi: 10.1038/sj.jid.5700204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Pisarchik A, Slominski RM, Zmijewski MA, Wortsman J. CRH functions as a growth factor/cytokine in the skin. J. Cell. Physiol. 2006b;206:780–791. doi: 10.1002/jcp.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Zmijewski M, Slominski RM, Kauser S, Wortsman J, Tobin DJ. Corticotropin releasing hormone and the skin. Front. Biosci. 2006c;11:2230–2248. doi: 10.2741/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Li W, Bhattacharya SK, Smith RA, Johnson PL, Chen J, Nelson KE, Tuckey RC, Miller D, Jiao Y, Gu W, Postlethwaite AE. Vitamin D analogs 17,20S(OH)2pD and 17,20R(OH)2pD are noncalcemic and exhibit antifibrotic activity. J. Invest. Dermatol. 2011;131:1167–1169. doi: 10.1038/jid.2010.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012;212(v, vii):1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr. Rev. 2013a;34:827–884. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Janjetovic Z, Tuckey RC, Nguyen MN, Bhattacharya KG, Wang J, Li W, Jiao Y, Gu W, Brown M, Postlethwaite AE. 20S-Hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. J. Clin. Endocrinol. Metab. 2013b;98:E298–E303. doi: 10.1210/jc.2012-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Kim TK, Zmijewski MA, Janjetovic Z, Li W, Chen J, Kusniatsova EI, Semak I, Postlethwaite A, Miller DD, Zjawiony JK, Tuckey RC. Novel vitamin D photoproducts and their precursors in the skin. Dermatoendocrinology. 2013c;5:7–19. doi: 10.4161/derm.23938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Nikolakis G, Manna PR, Skobowiat C, Zmijewski M, Li W, Janjetovic Z, Postlethwaite A, Zouboulis CC, Tuckey RC. Steroidogenesis in the skin: implications for local immune functions. J. Steroid Biochem. Mol. Biol. 2013d;137:107–123. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, Tuckey RC. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J. Steroid Biochem. Mol. Biol. 2014;144(Pt A):28–39. doi: 10.1016/j.jsbmb.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Janjetovic Z, Kim TK, Wasilewski P, Rosas S, Hanna S, Sayre RM, Dowdy JC, Li W, Tuckey RC. Novel non-calcemic secosteroids that are produced by human epidermal keratinocytes protect against solar radiation. J. Steroid Biochem. Mol. Biol. 2015a;148:52–63. doi: 10.1016/j.jsbmb.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Li W, Kim TK, Semak I, Wang J, Zjawiony JK, Tuckey RC. Novel activities of CYP11A1 and their potential physiological significance. J. Steroid Biochem. Mol. Biol. 2015b;151:25–37. doi: 10.1016/j.jsbmb.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Manna PR, Tuckey RC. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids. 2015c;103:72–88. doi: 10.1016/j.steroids.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talabér G, Jondal M, Okret S. Extra-adrenal glucocorticoid synthesis: immune regulation and aspects on local organ homeostasis. Mol. Cell. Endocrinol. 2013;380:89–98. doi: 10.1016/j.mce.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Tang EK, Chen J, Janjetovic Z, Tieu EW, Slominski AT, Li W, Tuckey RC. Hydroxylation of CYP11A1-derived products of vitamin D3 metabolism by human and mouse CYP27B1. Drug Metab. Dispos. 2013;41:1112–1124. doi: 10.1124/dmd.113.050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieu EW, Tang EK, Chen J, Li W, Nguyen MN, Janjetovic Z, Slominski A, Tuckey RC. Rat CYP24A1 acts on 20-hydroxyvitamin D(3) producing hydroxylated products with increased biological activity. Biochem. Pharmacol. 2012;84:1696–1704. doi: 10.1016/j.bcp.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongkao-On W, Carter S, Reeve VE, Dixon KM, Gordon-Thomson C, Halliday GM, Tuckey RC, Mason RS. CYP11A1 in skin: an alternative route to photoprotection by vitamin D compounds. J. Steroid Biochem. Mol. Biol. 2015;148:72–78. doi: 10.1016/j.jsbmb.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Tuckey RC, Li W, Shehabi HZ, Janjetovic Z, Nguyen MN, Kim TK, Chen J, Howell DE, Benson HA, Sweatman T, Baldisseri DM, Slominski A. Production of 22-hydroxy metabolites of vitamin d3 by cytochrome p450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab. Dispos. 2011;39:1577–1588. doi: 10.1124/dmd.111.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasiewicz T, Szyszka P, Cichorek M, Janjetovic Z, Tuckey RC, Slominski AT, Zmijewski MA. Antitumor effects of vitamin d analogs on hamster and mouse melanoma cell lines in relation to melanin pigmentation. Int. J. Mol. Sci. 2015;16:6645–6667. doi: 10.3390/ijms16046645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM. Involucrin and other markers of keratinocyte terminal differentiation. J. Invest. Dermatol. 1983;81:100s–103s. doi: 10.1111/1523-1747.ep12540786. [DOI] [PubMed] [Google Scholar]
- Wei R, Christakos S. Mechanisms underlying the regulation of innate and adaptive immunity by vitamin D. Nutrients. 2015;7:8251–8260. doi: 10.3390/nu7105392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicka JM, Binek A, Ahrends T, Nowacka JD, Szydłowska A, Turczyk, Wąsiewicz T, Wierzbicki PM, Sądej R, Tuckey RC, Slominski AT, Chybicki J, Adrych K, Kmieć Z, Żmijewski MA. Differential antitumor effects of vitamin D analogues on colorectal carcinoma in culture. Int. J. Oncol. 2015;47:1084–1096. doi: 10.3892/ijo.2015.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicka JM, Zmijewski MA, Antoniewicz J, Sobjanek M, Slominski AT. Differentiation of keratinocytes modulates expression of epidermal elements of the cutaneous analog of hypothalamus-pituitary-adrenal axis. J. Cell. Physiol. 2016 doi: 10.1002/jcp.25400. [DOI] [PubMed] [Google Scholar]
- Zbytek B, Pfeffer LM, Slominski AT. Corticotropin-releasing hormone stimulates NF-kappaB in human epidermal keratinocytes. J. Endocrinol. 2004;181:R1–R7. doi: 10.1677/joe.0.181r001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbytek B, Wortsman J, Slominski A. Characterization of a ultraviolet B-induced corticotropin-releasing hormone-proopiomelanocortin system in human melanocytes. Mol. Endocrinol. 2006a;20:2539–2547. doi: 10.1210/me.2006-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbytek B, Pfeffer LM, Slominski AT. CRH inhibits NF-kappa B signaling in human melanocytes. Peptides. 2006b;27:3276–3283. doi: 10.1016/j.peptides.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbytek B, Janjetovic Z, Tuckey RC, Zmijewski MA, Sweatman TW, Jones E, Nguyen MN, Slominski AT. 20-hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J. Invest. Dermatol. 2008;128:2271–2280. doi: 10.1038/jid.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JG, Ochalek JT, Kaufmann M, Jones G, Deluca HF. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc. Natl. Acad. Sci. U. S. A. 2013;110:15650–15655. doi: 10.1073/pnas.1315006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmijewski MA, Slominski AT. CRF1 receptor splicing in epidermal keratinocytes: potential biological role and environmental regulations. J. Cell. Physiol. 2009;218:593–602. doi: 10.1002/jcp.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmijewski MA, Slominski AT. Neuroendocrinology of the skin: an overview and selective analysis. Dermatoendocrinology. 2011;3:3–10. doi: 10.4161/derm.3.1.14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmijewski MA, Slominski AT. Is Mc1r an important regulator of non-pigmentary responses to UV radiation? Exp. Dermatol. 2013;22:790–791. doi: 10.1111/exd.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmijewski MA, Li W, Chen J, Kim TK, Zjawiony JK, Sweatman TW, Miller DD, Slominski AT. Synthesis and photochemical transformation of 3beta,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity. Steroids. 2011;76:193–203. doi: 10.1016/j.steroids.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]