Abstract
Pregnancy-associated malaria, including placental malaria, causes significant morbidity and mortality worldwide. Recently, it has been suggested that in utero exposure of the fetus to malaria antigens may negatively impact the developing immune system and result in tolerance to malaria. Here, we review our current knowledge of fetal immunity to malaria, focusing on the dynamic interactions between maternal malaria infection, placental development and the fetal immune system. A better understanding of the long-term impact of in utero malaria exposure on the development of natural immunity to malaria, immune responses to other childhood pathogens, and vaccine immunogenicity is urgently needed. This may guide the implementation of novel chemoprevention strategies during pregnancy and facilitate the push towards malaria vaccines.
Keywords: Placental Malaria, Pregnancy-associated malaria, Fetal Immune System, Fetal Tolerance
Global Impact of Pregnancy-Associated Malaria
Malaria (see Glossary) remains one of the leading causes of morbidity and mortality worldwide, resulting in an estimated 500,000 deaths each year [1,2]. Pregnant women, infants and young children are the most vulnerable populations and account for the majority of infections. More than 125 million pregnancies occur annually in regions at risk for malaria transmission and one in four pregnant women in sub-Saharan Africa have evidence of malaria infection at parturition [1,2]. Moreover, pregnant women are at a greater risk for malaria infection relative to non-pregnant adults, experiencing higher levels of parasitemia and symptomatic disease [3,4]. Pregnancy-associated malaria, including placental malaria (PM), can have profound consequences on the developing fetus, leading to intrauterine growth retardation (IUGR), low birth weight (LBW), prematurity, miscarriage, and stillbirth [1,5].
Infants and young children are highly susceptible to malaria during the first few years of life. In 2015, approximately 300,000 children under the age of five succumbed to malaria, with the highest mortality rates occurring in Africa [2]. Several epidemiological studies have found that the incidence of malaria during infancy and childhood is even higher among infants born to mothers with PM [6–8]. While this may partly due to the fact that infants of highly-exposed mothers are themselves at greater risk of environmental exposure, recent work suggests that fetuses exposed to malaria in utero may develop tolerance to malaria antigens, providing a potential immunologic mechanism for this association [9–14]. There is precedent for in utero pathogen exposure impacting infant susceptibility to infection, as offspring of filarial-infected mothers have been shown to have a 13-fold increase in filaria infection during childhood [15]. Other groups have demonstrated both antigen-specific and global alterations in immune responses from infants born to mothers with helminth infections [16,17]. Therefore, the impact of maternal infections on the development of the fetal immune system may have profound consequences for infectious disease susceptibility, vaccine immunogenicity, and predisposition to autoimmunity and allergies during childhood and beyond. Yet, we know very little about how and to what extent pathogens gain access to the fetal microenvironment and, furthermore, about precisely how the developing fetal immune system responds to early antigen exposure. Interestingly, recent studies have convincingly shown that effector-memory CD4+ T cells can be robustly primed in the fetus [18] and that naïve T cells might even exhibit novel effector functions unique to infants [19]. Here, we review our current understanding of the consequences of in utero antigen exposure on fetal immunity, with a particular focus on pregnancy-associated malaria caused by Plasmodium falciparum. We highlight key gaps in our knowledge and elaborate on what should be prioritized in order to better inform rational and effective vaccine design and chemoprevention deployment.
Current Strategies for Malaria Prevention in Pregnancy
Reducing the overwhelming burden of malaria in pregnant women and children remains a major public health goal. The use of insecticide-treated bednets (ITNs) has been shown to increase infant birth weight and reduce mortality in malaria-endemic populations [20]; however, the implementation of comprehensive and widespread coverage of endemic areas with ITNs represents a challenge. In addition to ITNs, as of October 2012, the World Health Organization (WHO) recommends that pregnant women living in areas of moderate to high malaria transmission receive intermittent preventative treatment in pregnancy (IPTp) with sulfadoxine-pyrimethamine (SP) [2]. In studies conducted in the 1990s, this regimen was shown to reduce the risk of PM and LBW [21–23]. However, anti-folate resistance is now widespread in much of East Africa [24,25] and recent studies have shown no efficacy of the use of IPTp-SP over ITNs alone [26]. In one Ugandan study, even with IPTp-SP, PM was detected in approximately 50% of women [27]. In addition, the emergence of insecticide-resistant parasite strains has now rendered ITNs ineffective in some areas [28]. Therefore, there is a pressing need for novel strategies to prevent pregnancy-associated malaria.
Two recent clinical trials have demonstrated success using prenatal chemoprevention with dihydroartemisinin-piperaquine (DP), an artemisinin combination therapy that is highly effective in treating malaria in pregnant women and children [29–31]. In a randomized controlled trial in western Kenya, Desai et al. demonstrated that IPTp-DP reduced the risk of infection at delivery by 68% and reduced the incidence of malaria during pregnancy by 84% compared to IPTp-SP [32]. In Uganda, Kakuru et al. showed that monthly administration of DP during pregnancy significantly reduced PM compared to IPTp-SP (27% vs. 50%) and led to an improvement in birth outcomes [27]. Thus, DP chemoprevention is a promising alternative strategy that may dramatically reduce the risk of malaria during pregnancy, particularly in regions with high levels of SP resistance.
Placental Malaria: Pathology and Diagnosis
Infection with P. falciparum during pregnancy commonly results in the development of PM, which is characterized by the accumulation of P. falciparum-infected erythrocytes in the placenta. Of note, Plasmodium vivax has also been shown to sequester in the placenta, but it does not appear to induce significant histopathological changes and its clinical consequences are unclear. [33,34]. During pregnancy, P. falciparum can express unique variant surface antigens that mediate binding of infected erythrocytes to chondroitin sulfate A (CSA) and other receptors on the placental synciotrophoblast layer, facilitating entry into intervillous spaces. The most ubiquitous of these antigens is Var2CSA [35], which is expressed by most CSA-binding infected erythrocytes and has unique immunological properties, (extensively reviewed elsewhere [36,37]). The development of antibodies against Var2CSA during a first or second pregnancy is believed to account for the reduction in incidence and severity of malaria with increasing parity [37–39]. Moreover, putative Var2CSA–based vaccines, which could potentially be administered to girls prior to pregnancy, are currently in development [36].
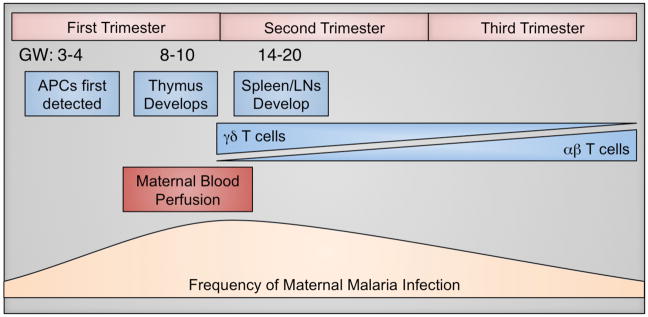
The earliest time when the placenta is susceptible to malaria infection during pregnancy is not clear. It was previously thought that maternal blood begins to flow into intervillous spaces around 10–12 weeks of gestation [40]; however, more recent studies indicate that perfusion of the placenta by maternal blood may occur as early as gestational week (GW) 6 [41]. As pregnant women may be most susceptible to malaria infection between 9–20 weeks [33], this gestational window could represent the first opportunity for P. falciparum-infected erythrocytes to sequester in the placenta. Currently, IPTp is administered from the second or third trimester onwards [2]; therefore, early pregnancy may be an especially vulnerable time for both mother and fetus (Figure 1). Then, the placenta is at risk of parasite invasion throughout the remainder of pregnancy. A recent study by Kalilani et al. has suggested that malaria infection during the second trimester might be most strongly associated with histologic evidence of PM [42]. However, many women in sub-Saharan Africa do not seek antenatal care until the second trimester or later [43], which means that there is currently limited information about malaria infections during the first half of pregnancy.
Figure 1. Gestational Timeline of Fetal Immune Development and Maternal Factors Influencing Placental Malaria.
Fetal immune development (blue) begins as early as gestational week (GW) 3–4, with the emergence of antigen-presenting cells (APCs). Thymic development occurs between GW 8–10, followed by the appearance of secondary lymphoid structures, such as the spleen and lymph nodes (LNs). Naïve T cells can first be detected in the periphery around GW 12 and initially the T cell pool is predominated by γδ T cells. The proportion of γδ T cells declines with gestational age, concurrent with expansion of the αβ T cell population, which constitutes the majority at birth. The placenta is also growing rapidly during early gestation. A key turning point in placental growth is the perfusion of the placenta with maternal blood, which occurs between GW 6–12, and theoretically represents the first opportunity for malaria parasites to access the placenta for sequestration (maroon). Pregnant women are more susceptible than non-pregnant adults to malaria infection; however, the highest rates of infection and highest parasite densities seem to occur between 9–20 weeks (tan) in primigravidas. Collectively, these overlapping factors suggest that the first window of vulnerability for placental malaria infection and fetal immune exposure may occur late in the first trimester or early in the second trimester (~GW 10–16).
PM is characterized by pathological changes accompanying the accumulation of parasites, including the presence of hemozoin, infiltration of monocytes and macrophages, and deposition of perivillous fibrin [44]. More severe inflammation and destruction of the synciotrophoblast barrier has also been reported and complete loss of villous integrity can occur [45]. These pathological features provide information about the chronicity and severity of placental infection. For example, the presence of parasites generally designates an active acute infection of the placenta. Detection of parasites may or may not be accompanied by hemozoin and cellular immune infiltrates; however, identification of these features concurrently suggests a more inflammatory chronic infection [44]. Hemozoin, pigment deposition and fibrin deposits can also be present in the absence of parasites and are thought to indicate previous infection of the placenta [44].
The placental parasite density, degree of placental damage and inflammation can be variable. In general, it is thought that high parasite burden and severe pathology lead to placental insufficiencies that impact a variety of normal functions of the placenta, including oxygen exchange, fetal nutrient uptake and passive antibody transfer [44], directly contributing to IUGR and LBW [46–48]. The level of placental parasite burden and degree of pathology are likely to influence the amount of malaria antigen that crosses the placenta, as disruption of the synciotrophoblast layer is the most obvious entry point into the fetal circulation. True congenital infection, with propagation of P. falciparum in fetal erythrocytes, is rare. However, malaria antigens are known to cross the placental barrier and enter the fetal circulation [49,50].
Fetal and Neonatal Immune Systems
Infants exhibit higher susceptibility to many infectious diseases and there is abundant clinical evidence that the cell-mediated immune response of infants differs in fundamental ways from that of adults [51]. While the neonatal immune system has traditionally been regarded as “immature”, it is increasingly appreciated that the fetus is capable of mounting adult-level responses under certain circumstances [52,53]. Experiments in mouse infection models suggest that neonatal T cells are extremely sensitive to conditions of antigen presentation at priming. Small differences in the dose of antigen [54], type of antigen presenting cell [54,55], and the intensity of co-stimulation [54–56] dictate the efficacy of the T cell response. These properties of neonatal T cells are thought to help balance the need for tolerance to maternal antigens with the need for inflammation to mount a protective anti-pathogen response. However, the dichotomy between tolerance and inflammation creates a dilemma for the fetal immune system when pathogenic insults occur in utero. While the mechanisms that control this balance are still poorly understood, several qualitative differences in fetal cellular immunity have been described.
Antigen Presenting Cells
Antigen presenting cells (APCs), such as monocytes and dendritic cells (DCs), play an essential role in dictating the activation and differentiation of T cells. APCs can be detected as early as GW 4 in humans (Figure 1), but studies in both mice and humans suggest that fetal APCs may be suboptimal in their ability to stimulate robust adaptive immune responses. For instance, fetal APCs exhibit lower expression of major histocompatibility complex (MHC)-II and co-stimulatory molecules when compared to adult APCs, in addition to a reduced ability to produce Th1-polarizing cytokines [57,58]. Low production of IL-12 by monocytes and DCs has been demonstrated in human cord blood under various stimulation conditions [59–61]. At least one mechanism for this reduction in IL-12 synthesis seems to occur at the transcriptional level, where depressed nucleosomal remodeling at the IL-12(p35) promoter has been reported to prevent gene transcription in neonatal DCs [62]. However, some studies have shown that adult-like levels of IL-12 can be secreted by human neonatal APCs under certain activation conditions [63–65]. A recent study in mice demonstrated that neonatal DCs cross-present antigen while co-producing IL-12p40 and IL-10 [66]. While this remains to be demonstrated in humans, these findings suggest that neonatal APCs might possess the ability to stimulate adaptive immunity while simultaneously regulating inflammation.
CD4+ T cells
Naïve αβ T cells, including CD4+ and CD8+, are first detected in fetal peripheral blood around GW 12, concurrent with the development of secondary lymphoid tissues, such as the spleen and lymph nodes [67,68] (Figure 1). Fetal CD4+ T cells are largely naïve in phenotype, with very few effector or effector-memory cells present at the time of birth [67,69]. Several lines of evidence support a skewing of fetal CD4+ T cell differentiation away from inflammatory Th1 cells and towards Th2 [70] and regulatory T cells (Treg) [71,72]. Regulation of this Th2 and Treg bias occurs, at least in part, by cell-intrinsic mechanisms. For example, human neonatal CD4+ T cells are poised for Th2 functionality due to hypomethylation of the IL13 locus [73], allowing for rapid production of this Th2 cytokine. In addition, repressive chromatin marks have been identified at Th1 loci in human fetal CD4+ T cells, including greater CpG methylation at the IFNG promoter compared to adult CD4+ T cells [74] and reduced accessibility in the promoter region of TBX21, which encodes the Th1 master transcription factor Tbet [75]. Fetal CD4+ T cells are also predisposed to differentiate into FoxP3+ Tregs [71]. During normal gestation, Tregs can render the fetal immune system tolerant to non-inherited alloantigens on maternal cells crossing the placenta [71]. However, it is not known whether fetuses might respond similarly to pathogen-derived antigens encountered during fetal development. Recent evidence suggests that this propensity to differentiate into Tregs may be an intrinsic property of fetal cells, as naive CD4+ T cells from mid-gestation fetuses preferentially differentiate into Tregs upon alloantigen stimulation compared to adult naive T cells [76,77]. However, Treg differentiation also seems to be favored by the cytokine milieu present in fetal lymph nodes (LN), including the suppressive cytokine TGF-β, which promotes upregulation of FoxP3 in vitro [71]. Therefore, it is possible that fetal CD4+ T cells might be both intrinsically-biased and extrinsically-skewed towards Th2 and Treg differentiation.
Despite the tendency of fetal CD4+ T cells to develop into Tregs, several recent studies have identified populations of CD4+ T cells in human cord blood with robust effector functions. In an exciting recent study, Gibbons et al. have demonstrated that naïve CD4+ T cells produce substantial amounts of IL-8, a cytokine that enhances neutrophil and γδ T cell function [19]. IL-8 secreting CD4+ T cells are rare in adults and are distinct from other inflammatory CD4+ T cells, as they do not co-produce pro-inflammatory cytokines such as IFN-γ, IL-13 or IL-17, nor do they express memory T cell markers [19]. This novel function of fetal naïve CD4+ T cells may represent a first line of defense against pathogens encountered in utero or during infancy. Zhang et al. also recently identified a population of effector-memory CD4+ T cells in human cord blood. These cells perform Th1 and Th2 functions upon activation, secreting IFN-γ, TNF-α, IL-4 and IL-13, with a mixed Th phenotype based solely on chemokine receptor expression [18]. These effector-memory cells preferentially differentiate into Th17 cells in culture, consistent with previous studies identifying CD161+ pro-Th17 cells in cord blood [78]. The origin of cord blood effector-memory CD4+ T cells and their antigenic-specificity however, remain unknown. Collectively, these new data suggest that the fetus is capable of mounting effector CD4+ T cell responses in utero.
CD8+ T cells
Like CD4+ T cells, most fetal CD8+ T cells are naïve in phenotype [67,69]. However, antigen-specific CD8+ T cell responses have been detected following in utero exposure to pathogens such as Trypanosoma cruzi [52], Human Immunodeficiency Virus (HIV) [79,80], and human Cytomegalovirus (HCMV) [53]. Fetal CD8+ T cells lack the repressive methylation marks within the IFNG locus that are characteristic of CD4+ T cells [74], suggesting that CD8+ cytotoxic T cells (CTLs) might be more capable of mounting an inflammatory response than CD4+ T cells. However, it is not clear that CTLs primed in utero are fully functional. Following congenital HCMV infection, robust CTL responses resulting in perforin-dependent cytolysis and an adult-like memory differentiation phenotype have been described [53], but more recent data indicate that HCMV-specific CD8+ T cells have a markedly reduced capacity to produce effector cytokines when compared to adult cells [81]. As CD8+ T cells are critically dependent on the presence of CD4+ T cells at priming, it’s possible that deficiencies in CD4+ T cell helper function underlie the reduced effector and memory functions of CD8+ T cells primed in utero, but as this is unclear, future studies should test this possibility.
γδ T cells
Fetal T cells bearing the γδ T cell receptor (TCR) develop prior to αβ T cells and can be detected in high proportions at GW 12 [82,83]. However, γδ T cell numbers decline with gestational age and, at birth, αβ T cells comprise the majority of the T cell compartment (Figure 1) [67,84]. Neonatal γδ T cells are capable of producing large quantities of IFN-γ compared to αβ T cells, which may be due at least in part to their less stringent activation requirements [85]. Congenital HCMV infection, for example, leads to the oligoclonal expansion of infant γδ T cells producing IFN-γ and expressing cytotoxic mediators [86]. In addition, a recent study by Dimova et al. showed that a substantial population of highly-functional γδ T cells exists during mid-gestation in humans (GW20) [83]. These γδ T cells express a phosphoantigen-reactive semi-invariant TCR and possess innate-like effector functions, including IFN-γ production and granzyme A/K expression [83]. These Vγ9Vδ2 T cells may be of importance for the fetal response to malaria, as they possess innate reactivity to Plasmodium-derived phosphoantigens, as discussed further below [87,88]. Taken together, these studies, among others, suggest that γδ T cells may represent a “first-line” of defense for the fetus.
Fetal Immune Response to Malaria Antigens
Several studies have reported P. falciparum-specific cellular immune responses in infants born to mothers with PM. Malaria-specific production of IFN-γ, TNF-α, IL-2, IL-5, IL-13 and IL-10 from whole cord blood has been described, suggesting that Th1-, Th2-and/or Treg-like responses may develop against malaria antigens in utero [13,49,89–91]. Whether these cytokines are being produced by fetal T cells or by other immune cells, however, remains elusive, as most of these analyses have been performed following stimulation of bulk cord blood mononuclear cells [13,49,89–91]. While some studies suggest that fetal CD4+ T cells are capable of producing IFN-γ in response to malaria stimulation, these responses appear to be very small in magnitude [12,89,91]. Some groups have put forth the idea that functional P. falciparum-specific T cell responses are challenging to detect because of sub-optimal priming in utero [10,12]. For example, a study in Gabon demonstrated that neonates born to women with PM presented an increased ratio of IL-10:IFN-γ that was associated with reduced expression of MHC-I and MHC-II on APCs [10,12]. These data are consistent with the argument that T cells might not be primed effectively in utero, but further studies are needed to clarify this issue.
Higher frequencies of Tregs have also been observed among infants born to women with PM [10,14], suggesting that active suppression of effector CD4+ and CD8+ T cells might also occur in utero. Depletion of cord blood CD4+ CD25+ T cells (putative Tregs) abrogates in vitro production of IL-10 and augments P. falciparum-specific IFNγ responses [10,13,14]. One interpretation of these data is that peripheral tolerance to malaria antigens occurs in utero through the expansion of Tregs. However, other groups have reported no difference in the frequency of Tregs in neonates born to mothers with or without PM [11,90], suggesting that the role of Tregs in this setting is not straightforward. Variability in the diagnosis of PM might explain some of these discrepancies, as could inconsistencies in the definition of Tregs. Indeed, studies comparing Treg frequencies in PM-exposed and unexposed neonates have used different flow cytometry gating strategies to identify Tregs, ranging from the current identification “gold standard” of CD4+CD25hiCD127lo FoxP3+ cells, to all CD4+CD25hi cells. This is problematic, as both CD25 and FoxP3 can be transiently upregulated by activated CD4+ T cells [92,93]. Therefore, the question of whether PM does indeed lead to elevated frequencies or function of cord blood Tregs remains, at present, unanswered.
Limited data suggest that PM may activate semi-innate fetal γδ T cells. Cord blood γδ T cells of PM-exposed infants are preferentially activated [94] and skewed towards a central memory phenotype [95]. Given the intrinsic reactivity of Vγ9Vδ2 T cells to malaria antigens and their direct anti-malaria effects in vitro [96], these cells may play an important role as ready-made effectors against malaria during infancy, prior to the development of an adaptive immune response.
Impact of PM on the Development of Childhood Immunity to Malaria
One fundamental question that remains unanswered is whether in utero exposure to malaria alters the long-term development of immunity to malaria. As many neonates in high prevalence settings are exposed in utero, fetal tolerance represents a potentially preventable impediment to protective anti-malarial immunity – both naturally acquired and vaccine-induced. A recent cohort study in Kenya reported that infants who were exposed to malaria in utero failed to mount malaria-specific immune responses in cord blood and exhibited lower malaria-specific Th1 responses at follow-up [9] as well as higher vulnerability to malaria during early childhood [9]. One possibility is that Tregs induced in utero may persist long-term, inhibiting priming of an effector response to malaria into childhood [97]. It has recently been shown that chronic, repeated exposure to malaria triggers several immunoregulatory mechanisms in highly exposed children, including increased generation of IL-10-producing Th1 CD4+ T cells [98], altered homeostasis of FoxP3+ Tregs [99], loss and dysfunction of malaria-reactive γδ T cells [100], as well as the generation of less functional CXCR3+ T follicular helper cells [101]. Indeed, such immunoregulatory mechanisms may play a more prominent role in childhood and might provide some protection from clinical symptoms. For example, studies of trans-migrant families have shown that while malaria-naïve adults rapidly develop protection from clinical disease relative to young children, the latter are far less susceptible to severe malaria and death during their first malaria infection [102–104]. However, the basis for suboptimal immunity to malaria in children, as well as the potential contribution of in utero antigen exposure, require further investigation.
Future Challenges and Perspectives
The extent to which pregnancy-associated malaria impacts fetal health and immunity is determined by multiple, overlapping factors. To begin to untangle these complex issues, several key unanswered questions should be prioritized (see Outstanding Questions and Box 1). Perhaps the biggest gaps in knowledge reside in our understanding of the fetal immune response to malaria and its consequences on the long-term development of anti-malarial immunity. Current work suggests that substantial heterogeneity exists in fetal immune responsiveness to PM and that in utero malaria exposure may result in T cell tolerance in some infants [9,13,49,89–91]. While resolving the role of Tregs remains an important issue, a major unanswered question is to what extent fetal effector T cells develop following in utero malaria exposure. The recent identification of fetal effector-memory CD4+ T cells in the cord blood of healthy infants [18] indicates that T cells can be effectively primed in the fetus in utero. Future studies should focus on further characterizing the phenotype and function of infant malaria-specific T cell responses, including CD4+, CD8+ and γδ T cell populations. The development of novel tools, such as Plasmodium-specific tetramers or pentamers for flow cytometric identification of antigen-specific T cells, could be very helpful for this endeavor. However, analysis of cord blood only provides a limited picture of fetal and infant immune responses. Indeed, recent studies have shown that cell phenotype and function are highly diverse for different anatomical locations [105]. It will be important to follow up immunological studies in cord blood with comprehensive longitudinal analyses of malaria-specific T cell responses and clinical malaria outcomes throughout infancy and childhood.
Outstanding Questions Box.
Does exposure to malaria in utero induce tolerance, mediated by Tregs or by other mechanisms? If so, does this tolerance impact the later development of immunity to malaria in childhood? And, is this tolerance malaria-specific or “global”?
Can the fetus and neonate mount protective T cell responses to malaria antigens? If so, what are the optimal conditions for priming?
What are the cellular and molecular mechanisms that limit priming of infant CD4+ effector T cell responses, and how might novel adjuvants or vaccine vectors be used to optimize the immunogenicity of vaccines for infants?
How does the timing of pregnancy-associated malaria infection impact fetal immunity?
Can maternal chemoprevention enhance infant immunity to malaria by reducing in utero exposure to malaria antigens? Could this have long-lasting effects on improving childhood immunity to pathogens and vaccines?
Box 1. The Clinician’s Corner.
Pregnancy-associated malaria causes significant obstetrical and neonatal morbidity, including prematurity and low birth weight. However, the long-term consequences of in utero malaria exposure to infant immunity remain unknown.
Several studies suggest that in utero exposure to pathogen-derived antigens, including malaria, may induce immunologic tolerance. Such tolerance may be pathogen-specific or “global”, which could impact disease susceptibility, vaccine responsiveness and/or childhood predisposition to allergic and autoimmune diseases.
Promising new interventions, including maternal chemoprophylaxis with dihydroartemisinin-piperaquine (DP) during pregnancy, have shown efficacy in preventing placental malaria and could dramatically reduce exposure to malaria in utero.
A better understanding of the fetal T cell response to malaria antigens, including how the gestational timing of exposure impacts this response, may inform the optimal use of malaria chemoprophylactic regimens and vaccination strategies.
In addition, disruption of fetal immune development by in utero malaria exposure has the potential to broadly impact immunity against other pathogens and/or vaccines. While this idea is still speculative in the setting of malaria, HIV-exposed, uninfected infants are at a high risk of developing other infections during infancy [106,107] and for example, exhibit impaired B and T cell responses against Hepatitis B, Mycobacterium tuberculosis and Tetanus Toxoid vaccines [108–110]. Indeed, some data suggests that in utero malaria exposure could impact susceptibility to childhood diseases and vaccine responsiveness [97]; however, future studies are warranted to better address this important issue.
Lastly, it is not yet known how the timing of maternal infection and PM impacts fetal outcomes and immunity (Key Figure, Figure 2). This information will be essential for targeting preventive interventions in pregnancy. The gestational timing at which the fetal immune system is first exposed to malaria antigens is likely to be critical, as the absolute numbers, proportions and functionality of APCs and T cells change throughout pregnancy [67,68,71]. Current evidence suggests that susceptibility to PM begins toward the end of the first trimester [40,41]. As we are on the brink of newer and more effective pregnancy chemoprophylaxis strategies [27,32], studies are needed to determine the optimal window for intervention. Early implementation of chemoprevention would present significant logistical hurdles, as most African women do not access prenatal care until well into the second trimester or later. Future work should focus on understanding when and how chemoprevention during pregnancy most optimally improves childhood outcomes, reduces fetal exposure to malaria antigens and promotes malaria-specific and global cellular immunity. In addition, it will be important to determine the impact of pregnancy chemoprevention on the development of Var2CSA antibodies and protection from future PM [111,112].
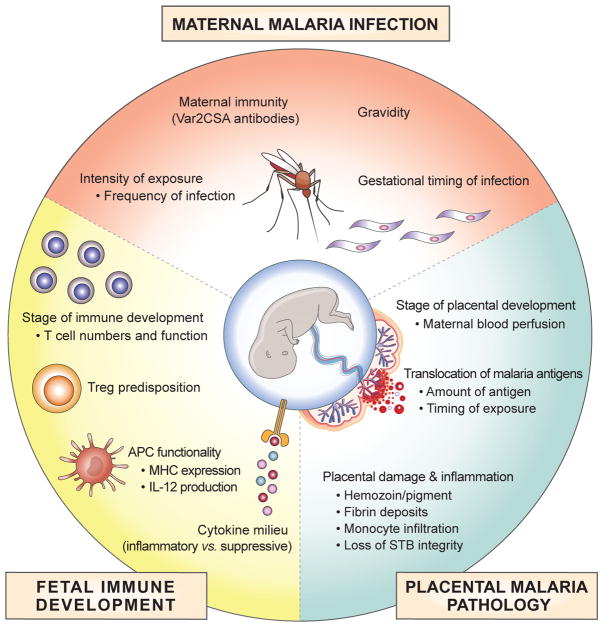
Figure 2 (Key Figure). Factors Influencing the Fetal Immune Response to Pregnancy-Associated Malaria.
Maternal, placental and fetal factors all influence how the fetal immune system will respond to in utero antigen exposure. Maternal factors play a role in when and how often parasites are present in circulation and have access to the placenta. This includes the frequency and gestational timing of infections during pregnancy. The severity of these infections and the ability of parasites to infiltrate the placenta are also impacted by maternal immunity and gravidity, particularly the generation of Var2CSA antibodies with increasing parity. Parasite sequestration in the placenta is influenced by the stage of placental development, and is dependent upon the flow of maternal blood into the placenta. The extent of placental histopathology and inflammation, including disruption of the syncytiotrophoblast (STB) layer, also play a key role in both the amount, and the timing of malaria antigen translocation into fetal circulation. Finally, the stage of fetal immune development when malaria antigen exposure occurs may greatly impact the magnitude and type of response that is mounted, as the number and function of APCs and T cells gradually change throughout gestation. In particular, predisposition toward regulatory T cell (Treg) differentiation may occur as an intrinsic property of developing fetal T cells, or could be impacted by the balance between inflammatory vs. suppressive cytokines in the microenvironment.
Concluding Remarks
Over the past decade, significant progress has been made in our understanding of fetal T cell immunity and in the development of novel approaches to prevent malaria in pregnancy (Box 1). Promising data from recent clinical trials of artemisinin-based chemoprevention in pregnancy suggest that drastic reductions in PM can be achieved. Further studies are needed to determine the impact of these interventions on fetal and infant immunity to malaria (see Outstanding Questions). Although the RTS,S vaccine was recently approved by the WHO for pilot demonstrations in Africa, it has shown only modest efficacy in infants [113]; moreover, efficacy declined over time and with increasing exposure to malaria [114]. As infants living in endemic regions are the primary target population for malaria vaccination, it will be critical to understand the basis for this suboptimal immunity and whether prior exposure to malaria, including in utero exposure, might contribute to early immune defects and reduced vaccine efficacy. Addressing the outstanding questions posed here could provide novel insights into fetal and infant immunity. Ultimately, filling in these gaps in knowledge will inform implementation of malaria chemoprevention strategies for pregnant women and children, as well as the rational design of vaccines that are maximally immunogenic in infants.
Trends Box.
Fetal T cells are uniquely predisposed towards tolerance, including regulatory T cell differentiation and attenuated inflammatory cytokine production
Recent evidence suggests that robust effector T cell responses can be mounted during fetal life
Although the fetal microenvironment has traditionally been regarded as sterile, microbes and pathogens can disrupt the maternal-fetal interface and impact fetal development and immunity
Pregnancy-associated malaria, including placental malaria, may lead to priming of regulatory and effector T cell responses in utero
Novel interventions, including artemisinin-based chemoprevention, may enable prevention of malaria during pregnancy.
Acknowledgments
We thank members of the Feeney laboratory, especially Rachel Budker and Tara McIntyre, for careful reading of the manuscript and helpful insights. This work was supported by the National Institute of Health and National Institute of Allergy and Infectious Diseases (R01AI093615 (MEF), K24AI113002 (MEF), U19AI089674 (MEF), and P01HD059454 (MEF)).
Glossary
- Malaria
mosquito-borne disease caused by the Plasmodium sp. parasite. Malaria infection can range from being asymptomatic, to a febrile-like symptomatic disease that can even lead to death.
- Primagravidas
woman with first-time pregnancy
- Filaria infection
parasitic infection caused by one of several different types of helminthic nematodes.
- Intermittent preventative treatment in pregnancy (IPTp)
antimalarial drug therapy administered to pregnant women as part of routine antenatal care, intended to clear parasitemia and prevent placental malaria
- Sulfadoxine-pyrimethamine (SP)
combination antimalarial therapy of folic acid antagonists that inhibit the activity of dihydropteroate synthase (sulfadoxine) and dihydrofolate reductase (pyrimethamine) in the erythrocytic stage of the Plasmodium sp. life cycle.
- Dihydroartemisinin-piperaquine (DP)
combination antimalarial therapy that includes an artemisinin derivative with a short half-life that rapidly kills parasites through free radical damage to parasite membranes (dihydroartemisinin) in combination with a drug that has a longer half-life and likely works through heme detoxification within the parasite (piperaquine). The combination of these anti-malarial properties facilitates monthly dosing, rendering it an attractive preventative drug.
- Variant surface antigens
Plasmodium-derived proteins that undergo extensive antigenic variation, often driven by immune pressure. They includes the diverse P. falciparum erythrocyte membrane protein 1 (PfEMP1) family, which is expressed on the surface of infected erythrocytes and facilitates adhesion and sequestration of infected red blood cells in various anatomical compartments.
- Placental synciotrophoblast layer
specialized epithelial cell layer covering the entire surface of the placental villi, establishing the interface between mother and fetus
- Hemozoin
hemoglobin breakdown product, accumulating as a result of blood digestion by malaria parasites.
- Intervillous spaces
the spaces within the placenta where maternal blood circulates around fetal villi.
- Antigen Presenting Cells (APCs)
Immune cells that process and present antigen in the context of MHC to T cells for priming. Antigen presentation is most robust when proper co-stimulatory and inflammatory signals are present.
- Major Histocompatibility Complex (MHC)
highly polymorphic glycoproteins that present peptide antigens to T cells. Encoded by the MHC class I and MHC class II genes.
- αβ T cells
T cells bearing a TCR composed of an alpha and beta chain that recognize antigens on the cell surface in the context of major histocompability complex (MHC). Can be subdivided as CD4+ or CD8+ T cells in the periphery.
- γδ T cells
T cells with a TCR composed of a gamma and delta chain that recognize unconventional antigens and do not require antigen presentation in the context of MHC.
- Th1 CD4+ T cells
T helper cells that promote cell-mediated immunity to intracellular pathogens, characterized by high expression of the master transcription factor Tbet and by production of IFN-γ and TNF-α. Differentiation occurs preferentially in the presence of IL-12 and IFN-γ.
- Th2 CD4+ T cells
T helper cells that promote immunity to allergens and helminthes, characterized by high expression of the master transcription factor GATA-3 and production of IL-4, IL-5 and IL-13. Differentiation occurs preferentially in the presence of IL-4.
- Regulatory T cells (Tregs)
CD4+ T cells that dampen inflammatory immune responses, characterized by high expression of the master transcription factor FoxP3 and production of IL-10. Differentiation occurs preferentially in the presence of TGF-β.
- Alloantigens
Proteins that vary from individual to individual within a species, for example, human blood groups.
- Effector-memory CD4+ T cells
In this context, human memory T cells that have lost the expression of the lymph node homing factors CD62L and CCR7, allowing for homing to inflamed tissues. Defined as CD45RO+CD45RA−CD62L−CCR7−
- CD161+ pro-Th17 cells
CD4+ T cells that preferentially develop into Th17 cells, a T helper cell subset promoting immunity against bacterial and fungal infections; characterized by high expression of the master transcription factor RORγT and production of IL-17A.
- CXCR3+ T follicular helper cells
Th1-like T follicular helper cells are suboptimal in their ability to provide T cell help to B cells and to [A1] promote the development of long-lasting, high affinity antibody production.
- Natural immunity to malaria
Individuals born in highly endemic regions develop some degree of natural immunity to malaria by 2–5 years of age, which confers protection from severe and even symptomatic disease. However, adults remain susceptible to asymptomatic infections throughout life.
- RTS,S vaccine
malaria vaccine candidate that targets the P. falciparum pre-erythryocytic circumsporozoite protein and is combined with viral envelope protein from hepatitis B virus (HBsAg), as well as a chemical adjuvant (AS01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Desai M, et al. Epidemiology and burden of malaria in pregnancy. The Lancet Infectious Diseases. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. World malaria report 2015. 2015. [Google Scholar]
- 3.Brabin BJ. An analysis of malaria in pregnancy in Africa. Bull World Health Organ. 1983;61:1005–1016. [PMC free article] [PubMed] [Google Scholar]
- 4.Lindsay S, et al. Effect of pregnancy on exposure to malaria mosquitoes. The Lancet. 2000;355:1972. doi: 10.1016/S0140-6736(00)02334-5. [DOI] [PubMed] [Google Scholar]
- 5.Bardaji A, et al. Impact of Malaria at the End of Pregnancy on Infant Mortality and Morbidity. Journal of Infectious Diseases. 2011;203:691–699. doi: 10.1093/infdis/jiq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwarz NG, et al. Placental Malaria Increases Malaria Risk in the First 30 Months of Life. CLIN INFECT DIS. 2008;47:1017–1025. doi: 10.1086/591968. [DOI] [PubMed] [Google Scholar]
- 7.Mutabingwa TK, et al. Maternal Malaria and Gravidity Interact to Modify Infant Susceptibility to Malaria. PLoS Med. 2005;2:e407–9. doi: 10.1371/journal.pmed.0020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Hesran JY, et al. Maternal placental infection with Plasmodium falciparum and malaria morbidity during the first 2 years of life. American Journal of Epidemiology. 1997;146:826–831. doi: 10.1093/oxfordjournals.aje.a009200. [DOI] [PubMed] [Google Scholar]
- 9.Malhotra I, et al. Can Prenatal Malaria Exposure Produce an Immune Tolerant Phenotype?: A Prospective Birth Cohort Study in Kenya. PLoS Med. 2009;6:e1000116–13. doi: 10.1371/journal.pmed.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brustoski K, et al. Reduced cord blood immune effector-cell responsiveness mediated by CD4+ cells induced in utero as a consequence of placental Plasmodium falciparum infection. Journal of Infectious Diseases. 2006;193:146–154. doi: 10.1086/498578. [DOI] [PubMed] [Google Scholar]
- 11.Flanagan KL, et al. The effect of placental malaria infection on cord blood and maternal immunoregulatory responses at birth. Eur J Immunol. 2010;40:1062–1072. doi: 10.1002/eji.200939638. [DOI] [PubMed] [Google Scholar]
- 12.Brustoski K, et al. IFN-g and IL-10 Mediate Parasite-Specific Immune Responses of Cord Blood Cells Induced by Pregnancy-Associated Plasmodium falciparum Malaria. The Journal of Immunology. 2005;174:1738–1745. doi: 10.4049/jimmunol.174.3.1738. [DOI] [PubMed] [Google Scholar]
- 13.Bisseye C, et al. Plasmodium falciparuminfection of the placenta impacts on the T helper type 1 (Th1)/Th2 balance of neonatal T cells through CD4 +CD25 +forkhead box P3 +regulatory T cells and interleukin-10. Clinical & Experimental Immunology. 2009;158:287–293. doi: 10.1111/j.1365-2249.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackroth MS, et al. Human cord blood CD4+CD25hi regulatory T cells suppress prenatally acquired T cell responses to Plasmodium falciparum antigens. J Immunol. 2011;186:2780–2791. doi: 10.4049/jimmunol.1001188. [DOI] [PubMed] [Google Scholar]
- 15.Malhotra I, et al. Influence of maternal filariasis on childhood infection and immunity to Wuchereria bancrofti in Kenya. Infection and Immunity. 2003;71:5231–5237. doi: 10.1128/IAI.71.9.5231-5237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malhotra I, et al. Prenatal T cell immunity to Wuchereria bancrofti and its effect on filarial immunity and infection susceptibility during childhood. Journal of Infectious Diseases. 2006;193:1005–1013. doi: 10.1086/500472. [DOI] [PubMed] [Google Scholar]
- 17.Guadalupe I, et al. Evidence for in utero sensitization to Ascaris lumbricoides in newborns of mothers with ascariasis. Journal of Infectious Diseases. 2009;199:1846–1850. doi: 10.1086/599214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, et al. CD4 T cells with effector memory phenotype and function develop in the sterile environment of the fetus. Sci Transl Med. 2014;6:238ra72–238ra72. doi: 10.1126/scitranslmed.3008748. [DOI] [PubMed] [Google Scholar]
- 19.Gibbons D, et al. Interleukin-8 (CXCL8) production is a signatory T cell effector function of human newborn infants. Nature Medicine. 2014;20:1206–1210. doi: 10.1038/nm.3670. [DOI] [PubMed] [Google Scholar]
- 20.Gamble C, et al. Insecticide-treated nets for the prevention of malaria in pregnancy: a systematic review of randomised controlled trials. PLoS Med. 2007;4:e107. doi: 10.1371/journal.pmed.0040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garner P, Brabin B. A review of randomized controlled trials of routine antimalarial drug prophylaxis during pregnancy in endemic malarious areas. Bull World Health Organ. 1994;72:89–99. [PMC free article] [PubMed] [Google Scholar]
- 22.Schultz LJ, et al. The efficacy of antimalarial regimens containing sulfadoxine-pyrimethamine and/or chloroquine in preventing peripheral and placental Plasmodium falciparum infection among pregnant women in Malawi. Am J Trop Med Hyg. 1994;51:515–522. doi: 10.4269/ajtmh.1994.51.515. [DOI] [PubMed] [Google Scholar]
- 23.Verhoeff FH, et al. An evaluation of the effects of intermittent sulfadoxine-pyrimethamine treatment in pregnancy on parasite clearance and risk of low birthweight in rural Malawi. Ann Trop Med Parasitol. 1998;92:141–150. doi: 10.1080/00034989859979. [DOI] [PubMed] [Google Scholar]
- 24.ter Kuile FO, et al. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA. 2007;297:2603–2616. doi: 10.1001/jama.297.23.2603. [DOI] [PubMed] [Google Scholar]
- 25.Gesase S, et al. High resistance of Plasmodium falciparum to sulphadoxine/pyrimethamine in northern Tanzania and the emergence of dhps resistance mutation at Codon 581. PLoS ONE. 2009;4:e4569. doi: 10.1371/journal.pone.0004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ndyomugyenyi R, et al. Efficacy of malaria prevention during pregnancy in an area of low and unstable transmission: an individually-randomised placebo-controlled trial using intermittent preventive treatment and insecticide-treated nets in the Kabale Highlands, southwestern Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2011;105:607–616. doi: 10.1016/j.trstmh.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kakuru A, et al. Dihydroartemisinin–Piperaquine for the Prevention of Malaria in Pregnancy. N Engl J Med. 2016;374:928–939. doi: 10.1056/NEJMoa1509150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trape JF, et al. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. The Lancet Infectious Diseases. 2011;11:925–932. doi: 10.1016/S1473-3099(11)70194-3. [DOI] [PubMed] [Google Scholar]
- 29.McGready R, et al. Parasitological efficacy of antimalarials in the treatment and prevention of falciparum malaria in pregnancy 1998 to 2009: a systematic review. BJOG. 2011;118:123–135. doi: 10.1111/j.1471-0528.2010.02810.x. [DOI] [PubMed] [Google Scholar]
- 30.Cisse B, et al. Randomized trial of piperaquine with sulfadoxine-pyrimethamine or dihydroartemisinin for malaria intermittent preventive treatment in children. PLoS ONE. 2009;4:e7164. doi: 10.1371/journal.pone.0007164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nankabirwa J, et al. Efficacy, safety, and tolerability of three regimens for prevention of malaria: a randomized, placebo-controlled trial in Ugandan schoolchildren. PLoS ONE. 2010;5:e13438. doi: 10.1371/journal.pone.0013438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desai M, et al. Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin-piperaquine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for the control of malaria during pregnancy in western Kenya: an open-label, three-group, randomised controlled superiority trial. Lancet. 2015;386:2507–2519. doi: 10.1016/S0140-6736(15)00310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogerson SJ, et al. Malaria in pregnancy: linking immunity and pathogenesis to prevention. Am J Trop Med Hyg. 2007;77:14–22. [PubMed] [Google Scholar]
- 34.Mayor A, et al. Placental infection with Plasmodium vivax: a histopathological and molecular study. J Infect Dis. 2012;206:1904–1910. doi: 10.1093/infdis/jis614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 36.Fried M, Duffy PE. Designing a VAR2CSA-based vaccine to prevent placental malaria. Vaccine. 2015;33:7483–7488. doi: 10.1016/j.vaccine.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ataíde R, et al. Malaria, primigravidae, and antibodies: knowledge gained and future perspectives. Trends Parasitol. 2014;30:85–94. doi: 10.1016/j.pt.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Salanti A, et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. The Journal of Experimental Medicine. 2004;200:1197–1203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fried M, et al. Maternal antibodies block malaria. Nature. 1998;395:851–852. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 40.Gude NM, et al. Growth and function of the normal human placenta. Thrombosis Research. 2004;114:397–407. doi: 10.1016/j.thromres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 41.Mercé LT, et al. Intervillous and uteroplacental circulation in normal early pregnancy and early pregnancy loss assessed by 3-dimensional power Doppler angiography. YMOB. 2009;200:315.e1–315.e8. doi: 10.1016/j.ajog.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 42.Kalilani-Phiri L, et al. Timing of Malaria Infection during Pregnancy Has Characteristic Maternal, Infant and Placental Outcomes. PLoS ONE. 2013;8:e74643–8. doi: 10.1371/journal.pone.0074643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abou-Zahr CL, Wardlaw TM. World Health Organization Library Catologuing-in-Publication Data. 2003. Antenatal Care in Developing Countries: Promises, achievements and missed opportunities. [Google Scholar]
- 44.Rogerson SJ, et al. Malaria in pregnancy: pathogenesis and immunity. The Lancet Infectious Diseases. 2007;7:105–117. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- 45.Crocker IP, et al. Syncytiotrophoblast Degradation and the Pathophysiology of the Malaria-infected Placenta. Placenta. 2004;25:273–282. doi: 10.1016/j.placenta.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Rogerson SJ, et al. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg. 2003;68:115–119. [PubMed] [Google Scholar]
- 47.Leopardi O, et al. Malaric placentas. A quantitative study and clinico-pathological correlations. Pathol Res Pract. 1996;192:892–8. doi: 10.1016/S0344-0338(96)80068-9. discussion 899–900. [DOI] [PubMed] [Google Scholar]
- 48.Ordi J, et al. Massive chronic intervillositis of the placenta associated with malaria infection. Am J Surg Pathol. 1998;22:1006–1011. doi: 10.1097/00000478-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Malhotra I, et al. Umbilical cord-blood infections with Plasmodium falciparum malaria are acquired antenatally in Kenya. Journal of Infectious Diseases. 2006;194:176–183. doi: 10.1086/505150. [DOI] [PubMed] [Google Scholar]
- 50.May K, et al. Antibody-dependent transplacental transfer of malaria blood-stage antigen using a human ex vivo placental perfusion model. PLoS ONE. 2009;4:e7986. doi: 10.1371/journal.pone.0007986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu L, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. The Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 52.Hermann E, et al. Human fetuses are able to mount an adultlike CD8 T-cell response. Blood. 2002;100:2153–2158. [PubMed] [Google Scholar]
- 53.Marchant A, et al. Mature CD8+ T lymphocyte response to viral infection during fetal life. J Clin Invest. 2003;111:1747–1755. doi: 10.1172/JCI17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarzotti M, et al. Induction of protective CTL responses in newborn mice by a murine retrovirus. Science. 1996;271:1726–1728. doi: 10.1126/science.271.5256.1726. [DOI] [PubMed] [Google Scholar]
- 55.Ridge JP, et al. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 56.Forsthuber T, et al. Induction of TH1 and TH2 immunity in neonatal mice. Science. 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 57.De Wit D, et al. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood. 2004;103:1030–1032. doi: 10.1182/blood-2003-04-1216. [DOI] [PubMed] [Google Scholar]
- 58.Renneson J, et al. IL-12 and type I IFN response of neonatal myeloid DC to human CMV infection. Eur J Immunol. 2009;39:2789–2799. doi: 10.1002/eji.200939414. [DOI] [PubMed] [Google Scholar]
- 59.Goriely S, et al. Deficient IL-12(p35) Gene Expression by Dendritic Cells Derived from Neonatal Monocytes. The Journal of Immunology. 2001;166:2141–2146. doi: 10.4049/jimmunol.166.3.2141. [DOI] [PubMed] [Google Scholar]
- 60.Upham JW, et al. Development of Interleukin-12-Producing Capacity throughout Childhood. Infection and Immunity. 2002;70:6583–6588. doi: 10.1128/IAI.70.12.6583-6588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aksoy E, et al. Interferon regulatory factor 3-dependent responses to lipopolysaccharide are selectively blunted in cord blood cells. Blood. 2007;109:2887–2893. doi: 10.1182/blood-2006-06-027862. [DOI] [PubMed] [Google Scholar]
- 62.Goriely S, et al. A Defect in Nucleosome Remodeling Prevents IL-12(p35)Gene Transcription in Neonatal Dendritic Cells. The Journal of Experimental Medicine. 2004;199:1011–1016. doi: 10.1084/jem.20031272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karlsson H, et al. Innate Immune Responses of Human Neonatal Cells to Bacteria from the Normal Gastrointestinal Flora. Infection and Immunity. 2002;70:6688–6696. doi: 10.1128/IAI.70.12.6688-6696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dadaglio G, et al. Efficient In Vivo Priming of Specific Cytotoxic T Cell Responses by Neonatal Dendritic Cells. The Journal of Immunology. 2002;168:2219–2224. doi: 10.4049/jimmunol.168.5.2219. [DOI] [PubMed] [Google Scholar]
- 65.Salio M. Efficient priming of antigen-specific cytotoxic T lymphocytes by human cord blood dendritic cells. International Immunology. 2003;15:1265–1273. doi: 10.1093/intimm/dxg123. [DOI] [PubMed] [Google Scholar]
- 66.Torres D, et al. IL-12p40/IL-10 Producing preCD8α/Clec9A+ Dendritic Cells Are Induced in Neonates upon Listeria monocytogenes Infection. PLoS Pathog. 2016;12:e1005561–21. doi: 10.1371/journal.ppat.1005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palmer AC. Nutritionally Mediated Programming of the Developing Immune System. Advances in Nutrition: An International Review Journal. 2011;2:377–395. doi: 10.3945/an.111.000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ygberg S, Nilsson A. The developing immune system - from foetus to toddler. Acta Paediatrica. 2011;101:120–127. doi: 10.1111/j.1651-2227.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 69.Byrne JA, et al. A novel subpopulation of primed T cells in the human fetus. The Journal of Immunology. 1994;152:3098–3106. [PubMed] [Google Scholar]
- 70.Prescott SL, et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. The Journal of Immunology. 1998;160:4730–4737. [PubMed] [Google Scholar]
- 71.Mold JE, et al. Maternal Alloantigens Promote the Development of Tolerogenic Fetal Regulatory T Cells in Utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burt TD. Fetal Regulatory T Cells and Peripheral Immune Tolerance In Utero: Implications for Development and Disease. Am J Reprod Immunol. 2013;69:346–358. doi: 10.1111/aji.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Webster RB, et al. The Human IL-13 Locus in Neonatal CD4+ T Cells Is Refractory to the Acquisition of a Repressive Chromatin Architecture. Journal of Biological Chemistry. 2006;282:700–709. doi: 10.1074/jbc.M609501200. [DOI] [PubMed] [Google Scholar]
- 74.White GP, et al. Differential Patterns of Methylation of the IFN- Promoter at CpG and Non-CpG Sites Underlie Differences in IFN- Gene Expression Between Human Neonatal and Adult CD45RO- T Cells. The Journal of Immunology. 2002;168:2820–2827. doi: 10.4049/jimmunol.168.6.2820. [DOI] [PubMed] [Google Scholar]
- 75.Osamu Kaminuma DVMP, et al. T-box 21 transcription factor is responsible for distorted TH2 differentiation in human peripheral CD4+ T cells. Journal of Allergy and Clinical Immunology. 2009;123:813–823. e3. doi: 10.1016/j.jaci.2009.01.055. [DOI] [PubMed] [Google Scholar]
- 76.Mold JE, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McCune JM. Distinct functional programs in fetal T and myeloid lineages. 2014 doi: 10.3389/fimmu.2014.00314/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Black A, et al. Developmental regulation of Th17-cell capacity in human neonates. Eur J Immunol. 2011;42:311–319. doi: 10.1002/eji.201141847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luzuriaga K, et al. HIV-1-specific cytotoxic T lymphocyte responses in the first year of life. The Journal of Immunology. 1995;154:433–443. [PubMed] [Google Scholar]
- 80.Sanchez-Merino V, et al. HIV-1-Specific CD8+ T Cell Responses and Viral Evolution in Women and Infants. The Journal of Immunology. 2005;175:6976–6986. doi: 10.4049/jimmunol.175.10.6976. [DOI] [PubMed] [Google Scholar]
- 81.Huygens A, et al. Functional Exhaustion Limits CD4+ and CD8+ T-Cell Responses to Congenital Cytomegalovirus Infection. Journal of Infectious Diseases. 2015;212:484–494. doi: 10.1093/infdis/jiv071. [DOI] [PubMed] [Google Scholar]
- 82.Pardoll DM, et al. Differential expression of two distinct T-cell receptors during thymocyte development. Nature. 1987;326:79–81. doi: 10.1038/326079a0. [DOI] [PubMed] [Google Scholar]
- 83.Dimova T, et al. Effector Vγ9Vδ2 T cells dominate the human fetal γδ T-cell repertoire. Proc Natl Acad Sci USA. 2015;112:E556–E565. doi: 10.1073/pnas.1412058112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Rosa SC, et al. Ontogeny of T Cells in Humans. The Journal of Immunology. 2004;172:1637–1645. doi: 10.4049/jimmunol.172.3.1637. [DOI] [PubMed] [Google Scholar]
- 85.Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 86.Vermijlen D, et al. Human cytomegalovirus elicits fetal gammadelta T cell responses in utero. The Journal of Experimental Medicine. 2010;207:807–821. doi: 10.1084/jem.20090348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Langhorne J. gammadelta T cells in malaria infections. Parasitol Today (Regul Ed) 1996;12:200–203. doi: 10.1016/0169-4758(96)10009-0. [DOI] [PubMed] [Google Scholar]
- 88.Guenot M, et al. Phosphoantigen Burst upon Plasmodium falciparum Schizont Rupture Can Distantly Activate Vγ9Vδ2 T Cells. Infect Immun. 2015;83:3816–3824. doi: 10.1128/IAI.00446-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malhotra I, et al. Distinct Th1- and Th2-Type Prenatal Cytokine Responses to Plasmodium falciparum Erythrocyte Invasion Ligands. Infection and Immunity. 2005;73:3462–3470. doi: 10.1128/IAI.73.6.3462-3470.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soulard V, et al. Placental Malaria-Associated Suppression of Parasite-Specific Immune Response in Neonates Has No Major Impact on Systemic CD4 T Cell Homeostasis. Infection and Immunity. 2011;79:2801–2809. doi: 10.1128/IAI.00203-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Flanagan KL, et al. The effect of placental malaria infection on cord blood and maternal immunoregulatory responses at birth. Eur J Immunol. 2009;40:1062–1072. doi: 10.1002/eji.200939638. [DOI] [PubMed] [Google Scholar]
- 92.Wang J, et al. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 93.Scholzen A, et al. Plasmodium falciparum–Mediated Induction of Human CD25hiFoxp3hi CD4 T Cells Is Independent of Direct TCR Stimulation and Requires IL-2, IL-10 and TGFβ. PLoS Pathog. 2009;5:e1000543–20. doi: 10.1371/journal.ppat.1000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Engelmann I, et al. Activation status of cord blood gamma delta T cells reflects in utero exposure to Plasmodium falciparum antigen. Journal of Infectious Diseases. 2005;191:1612–1622. doi: 10.1086/429336. [DOI] [PubMed] [Google Scholar]
- 95.Cairo C, et al. Cord Blood V 2V 2 T Cells Provide a Molecular Marker for the Influence of Pregnancy-Associated Malaria on Neonatal Immunity. Journal of Infectious Diseases. 2014;209:1653–1662. doi: 10.1093/infdis/jit802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Costa G, et al. Control of Plasmodium falciparum erythrocytic cycle: γδ T cells target the red blood cell-invasive merozoites. Blood. 2011;118:6952–6962. doi: 10.1182/blood-2011-08-376111. [DOI] [PubMed] [Google Scholar]
- 97.Labeaud AD, et al. Do antenatal parasite infections devalue childhood vaccination? PLoS Negl Trop Dis. 2009;3:e442. doi: 10.1371/journal.pntd.0000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jagannathan P, et al. IFNγ/IL-10 co-producing cells dominate the CD4 response to malaria in highly exposed children. PLoS Pathog. 2014;10:e1003864. doi: 10.1371/journal.ppat.1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boyle MJ, et al. Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to Malaria. PLoS Pathog. 2015;11:e1005041–21. doi: 10.1371/journal.ppat.1005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jagannathan P, et al. Loss and dysfunction of Vδ2* γδ T cells are associated with clinical tolerance to malaria. Sci Transl Med. 2014;6:251ra117. doi: 10.1126/scitranslmed.3009793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Obeng-Adjei N, et al. Circulating Th1-Cell-type Tfh Cells that Exhibit Impaired B Cell Help Are Preferentially Activated during Acute Malaria in Children. Cell Reports. 2015;13:425–439. doi: 10.1016/j.celrep.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baird JK, et al. Age-specific prevalence of Plasmodium falciparum among six populations with limited histories of exposure to endemic malaria. Am J Trop Med Hyg. 1993;49:707–719. doi: 10.4269/ajtmh.1993.49.707. [DOI] [PubMed] [Google Scholar]
- 103.Baird JK, et al. Age-dependent susceptibility to severe disease with primary exposure to Plasmodium falciparum. Journal of Infectious Diseases. 1998;178:592–595. doi: 10.1086/517482. [DOI] [PubMed] [Google Scholar]
- 104.Doolan DL, et al. Acquired Immunity to Malaria. Clinical Microbiology Reviews. 2009;22:13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thome JJC, et al. Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nature Medicine. 2016;22:72–77. doi: 10.1038/nm.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.von Mollendorf C, et al. Increased risk for and mortality from invasive pneumococcal disease in HIV-exposed but uninfected infants aged <1 year in South Africa, 2009–2013. CLIN INFECT DIS. 2015;60:1346–1356. doi: 10.1093/cid/civ059. [DOI] [PubMed] [Google Scholar]
- 107.Adler C, et al. Severe Infections in HIV-Exposed Uninfected Infants Born in a European Country. PLoS ONE. 2015;10:e0135375. doi: 10.1371/journal.pone.0135375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singh DK, et al. Immunogenicity of Hepatitis B Vaccine in HIV Exposed Uninfected Infants. Indian J Pediatr. 2016;83:172–174. doi: 10.1007/s12098-015-1905-1. [DOI] [PubMed] [Google Scholar]
- 109.Jones CE, et al. The impact of HIV exposure and maternal Mycobacterium tuberculosis infection on infant immune responses to bacille Calmette-Guérin vaccination. AIDS. 2015;29:155–165. doi: 10.1097/QAD.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garcia-Knight MA, et al. Altered Memory T-Cell Responses to Bacillus Calmette-Guerin and Tetanus Toxoid Vaccination and Altered Cytokine Responses to Polyclonal Stimulation in HIV-Exposed Uninfected Kenyan Infants. PLoS ONE. 2015;10:e0143043. doi: 10.1371/journal.pone.0143043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Babakhanyan A, et al. Influence of Intermittent Preventive Treatment on Antibodies to VAR2CSA in Pregnant Cameroonian Women. Am J Trop Med Hyg. 2016;94:640–649. doi: 10.4269/ajtmh.15-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Staalsoe T, et al. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet. 2004;363:283–289. doi: 10.1016/S0140-6736(03)15386-X. [DOI] [PubMed] [Google Scholar]
- 113.White MT, et al. Immunogenicity of the RTS,S/AS01 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial. The Lancet Infectious Diseases. 2015;15:1450–1458. doi: 10.1016/S1473-3099(15)00239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Olotu A, et al. Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children. N Engl J Med. 2016;374:2519–2529. doi: 10.1056/NEJMoa1515257. [DOI] [PMC free article] [PubMed] [Google Scholar]