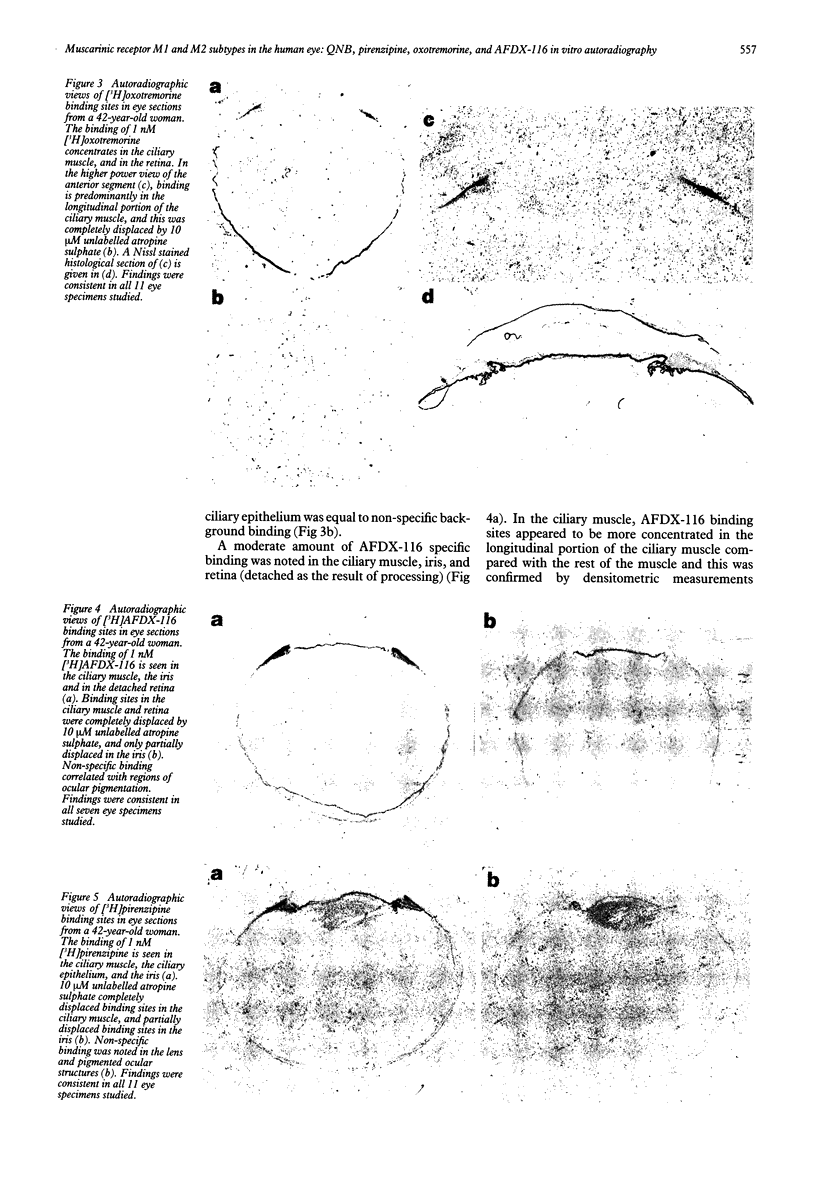
Abstract
Muscarinic cholinergic agents are used to lower intraocular pressure in the medical management of glaucoma and subtypes of muscarinic receptors have now been recognised in many tissues including the eye. To localise muscarinic receptors and their M1 and M2 subtypes in the human eye, in vitro ligand binding and autoradiographic techniques with densitometric quantitation on postmortem eye sections were used. As ligands, [3H] quinuclydinyl benzylate (QNB) (non-subtype specific muscarinic antagonist), [3H]pirenzipine (M1 antagonist), [3H]oxotremorine (M2 muscarinic agonist), [3H]AFDX-116(11[(2[diethylaminomethyl]1-piperidinyl)acetyl]5 , 11dihydro-6H-pyrido [2,3b][1,4]benzodiazepine-6-one) (M2 antagonist) were studied. Specific binding sites for QNB, pirenzipine, and AFDX-116 were localised in the entire ciliary muscle, the iris, and ciliary epithelium. [3H]oxotremorine localised only in the longitudinal portion of the ciliary muscle, and additionally, was not localised in the iris or ciliary epithelium. These results suggest that oxotremorine, by binding selectively to receptors on the longitudinal ciliary muscle and inducing its contraction, may modulate outflow facility independently from accommodation and miosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Buckley N. J., Bonner T. I., Buckley C. M., Brann M. R. Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol. 1989 Apr;35(4):469–476. [PubMed] [Google Scholar]
- Bárány E. H. The mode of action of miotics on outflow resistance. A study of pilocarpine in the vervet monkey Cercopithecus ethiops. Trans Ophthalmol Soc U K. 1966;86:539–578. [PubMed] [Google Scholar]
- Bárány E., Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. The binding properties of the muscarinic receptors of the cynomolgus monkey ciliary body and the response to the induction of agonist subsensitivity. Br J Pharmacol. 1982 Dec;77(4):731–739. doi: 10.1111/j.1476-5381.1982.tb09353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doods H. N., Mathy M. J., Davidesko D., van Charldorp K. J., de Jonge A., van Zwieten P. A. Selectivity of muscarinic antagonists in radioligand and in vivo experiments for the putative M1, M2 and M3 receptors. J Pharmacol Exp Ther. 1987 Jul;242(1):257–262. [PubMed] [Google Scholar]
- Erickson-Lamy K., Schroeder A. Dissociation between the effect of aceclidine on outflow facility and accommodation. Exp Eye Res. 1990 Feb;50(2):143–147. doi: 10.1016/0014-4835(90)90224-i. [DOI] [PubMed] [Google Scholar]
- Gabelt B. T., Kaufman P. L. Inhibition of outflow facility and accommodative and miotic responses to pilocarpine in rhesus monkeys by muscarinic receptor subtype antagonists. J Pharmacol Exp Ther. 1992 Dec;263(3):1133–1139. [PubMed] [Google Scholar]
- Gabelt B. T., Kaufman P. L., Polansky J. R. Ciliary muscle muscarinic binding sites, choline acetyltransferase, and acetylcholinesterase in aging rhesus monkeys. Invest Ophthalmol Vis Sci. 1990 Nov;31(11):2431–2436. [PubMed] [Google Scholar]
- Giachetti A., Micheletti R., Montagna E. Cardioselective profile of AF-DX 116, a muscarine M2 receptor antagonist. Life Sci. 1986 May 5;38(18):1663–1672. doi: 10.1016/0024-3205(86)90410-8. [DOI] [PubMed] [Google Scholar]
- Gillard M., Waelbroeck M., Christophe J. Muscarinic receptor heterogeneity in rat central nervous system. II. Brain receptors labeled by [3H]oxotremorine-M correspond to heterogeneous M2 receptors with very high affinity for agonists. Mol Pharmacol. 1987 Jul;32(1):100–108. [PubMed] [Google Scholar]
- Giraldo E., Hammer R., Ladinsky H. Distribution of muscarinic receptor subtypes in rat brain as determined in binding studies with AF-DX 116 and pirenzepine. Life Sci. 1987 Mar 2;40(9):833–840. doi: 10.1016/0024-3205(87)90031-2. [DOI] [PubMed] [Google Scholar]
- Hammer R., Giachetti A. Muscarinic receptor subtypes: M1 and M2 biochemical and functional characterization. Life Sci. 1982 Dec 27;31(26):2991–2998. doi: 10.1016/0024-3205(82)90066-2. [DOI] [PubMed] [Google Scholar]
- Honkanen R. E., Howard E. F., Abdel-Latif A. A. M3-muscarinic receptor subtype predominates in the bovine iris sphincter smooth muscle and ciliary processes. Invest Ophthalmol Vis Sci. 1990 Mar 1;31(3):590–593. [PubMed] [Google Scholar]
- Hutchins J. B., Hollyfield J. G. Autoradiographic identification of muscarinic receptors in human iris smooth muscle. Exp Eye Res. 1984 May;38(5):515–521. doi: 10.1016/0014-4835(84)90129-5. [DOI] [PubMed] [Google Scholar]
- Kaufman P. L., Bárány E. H. Loss of acute pilocarpine effect on outflow facility following surgical disinsertion and retrodisplacement of the ciliary muscle from the scleral spur in the cynomolgus monkey. Invest Ophthalmol. 1976 Oct;15(10):793–807. [PubMed] [Google Scholar]
- Kaufman P. L., Bárány E. H. Residual pilocarpine effects on outflow facility after ciliary muscle disinsertion in the synomolgus monkey. Invest Ophthalmol. 1976 Jul;15(7):558–561. [PubMed] [Google Scholar]
- Palacios J. M., Niehoff D. L., Kuhar M. J. Receptor autoradiography with tritium-sensitive film: potential for computerized densitometry. Neurosci Lett. 1981 Sep 1;25(2):101–105. doi: 10.1016/0304-3940(81)90315-3. [DOI] [PubMed] [Google Scholar]
- Polansky J. R., Zlock D., Brasier A., Bloom E. Adrenergic and cholinergic receptors in isolated non-pigmented ciliary epithelial cells. Curr Eye Res. 1985 Apr;4(4):517–522. doi: 10.3109/02713688509025168. [DOI] [PubMed] [Google Scholar]
- Prusky G., Cyander M. The distribution of M1 and M2 muscarinic acetylcholine receptor subtypes in the developing cat visual cortex. Brain Res Dev Brain Res. 1990 Oct 1;56(1):1–12. doi: 10.1016/0165-3806(90)90157-t. [DOI] [PubMed] [Google Scholar]
- Vanderheyden P., Ebinger G., Vauquelin G. Characterization of M1- and M2-muscarinic receptors in calf retina membranes. Vision Res. 1988;28(2):247–250. doi: 10.1016/0042-6989(88)90151-4. [DOI] [PubMed] [Google Scholar]
- Zarbin M. A., Wamsley J. K., Palacios J. M., Kuhar M. J. Autoradiographic localization of high affinity GABA, benzodiazepine, dopaminergic, adrenergic and muscarinic cholinergic receptors in the rat, monkey and human retina. Brain Res. 1986 May 21;374(1):75–92. doi: 10.1016/0006-8993(86)90396-3. [DOI] [PubMed] [Google Scholar]