SUMMARY
Toxoplasmosis is the fourth most common cause of hospitalization and the second cause of death due to food-borne infections. We conducted a cross-sectional study to determine the prevalence, disease awareness and risk factors associated with toxoplasmosis among rural communities in Northern Iran. Data were obtained from serological testing and from participant's questionnaires and were analyzed using a logistic regression. Of the 630 participants, 465 (73.8%), and 12 (1.9%) had IgG and both IgG and IgM anti-Toxoplasma gondii antibodies, respectively. In the logistic regression analysis, T. gondii seropositivity was associated with the following factors: age, occupation, consumption of undercooked meat, and of unwashed raw vegetables or fruits (p < 0.001). Our study showed a high prevalence of T. gondii infection in the general population of Northern Iran. A health program is needed to increase the public awareness of toxoplasmosis, and its associated risk factors.
KEYWORDS: Toxoplasmosis, Seroprevalence, Cross-sectional study, Rural communities, Northern Iran
INTRODUCTION
Toxoplasma gondii infection causes a zoonotic disease known as toxoplasmosis with a worldwide distribution. T. gondii infects approximately 30 to 50% of the human population in both developed and developing countries 1 . Toxoplasmosis is the fourth most common cause of hospitalization and the second cause of death due to food-borne infections in the United States 2 . The main risk factors for T. gondii infection among humans are consumption of raw or undercooked meat containing T. gondii tissue cysts, intake of sporulated oocysts from soil, water, unwashed vegetables or contaminated hands, and vertical transmission of infection through the placenta 3 .
Although primary acquired infection is mild and frequently self-limited in adults with normal immune function, exposure to T. gondii during pregnancy can lead to ocular and neurological impairment such as mental retardation, blindness, epilepsy, seizures, microcephaly and hydrocephaly 3 , 4 . Toxoplasmosis is responsible for encephalitis, brain abscesses and death when it is reactivated in immunocompromised patients 3 , 5 , 6 . Some recent studies have reported that latent toxoplasmosis could be associated with neuropsychiatric disorders such as schizophrenia, Parkinson disease, suicide, bipolar disorder, obsessive compulsive disorder and anxiety 7 , 8 . Moreover, some studies reported that latent T. gondii infection is related to male and female infertility 9 , 10 . Therefore, antibody screening in different groups of patients to identify those who are at risk of acquiring a primary Toxoplasma infection leading to an increment of epidemiological data on toxoplasmosis in the general population can be helpful to establish control measures and prevention of toxoplasmosis complications 3 , 11 .
A number of seroprevalence studies on toxoplasmosis were carried out in Iran, mostly focused on high-risk groups such as pregnant women and immunocompromised patients 12 . However, none of the studies have been conducted to evaluate the epidemiology of T. gondii infection in only rural communities. In addition, Northern Iran is a highly endemic area for toxoplasmosis 12 . Considering that there are no epidemiological data showing recent prevalence of toxoplasmosis in this area, this study was designed to investigate the prevalence of T. gondii infection and to identify the potential risk factors associated with toxoplasmosis among rural communities in Northern Iran.
MATERIAL AND METHODS
Study area and study population
This cross sectional study was performed in Northern Iran (Amol, Nor and Mahmod Abad cities) from July 2014 to March 2015. Until recently, these cities were part of a single county called Amol. This area is located in a region of mountains and forests in Northern Iran. The main economic activities in this area are agriculture and animal husbandry. According to the Statistical Centre of Iran (SCI), the number of rural inhabitants in this area is about 269,000 13 . This area (36°25'N 52°21'E) has an altitude of 76 meters above the sea level, a hot summer characterized by a Mediterranean climate, a mean annual temperature of 15.9 °C and about 829 mm of rain fall annually. We selected 13 rural villages in four different counties of the cities depending on the land area and number of inhabitants. A list of all the villages from the local governor was used to select 13 villages by means of a simple random sampling technique. The participants were selected among those who referred to health centers to undergo medical examination in the primary clinical care. Selected villages were: two from the Nor county (65 people), three from the Mahmod Abad county (128 people), four villages from the Dabodasht satae (Amol county, 219 people) and four villages from the Litkoh & Larijan community (Amol county, 208 people). Inclusion criteria to enroll rural people were: 1) belong to the Mazanderani ethnicity; 2) be a resident of the rural area in the past 10 years; 3) to be > 1 year old; 4) to consent to participate in this study. Participants were excluded from the study if they did not fulfill the inclusion criteria. Moreover, pregnant women and also individuals with a history of impaired immune response were excluded from this study.
Sample size and sampling method
To calculate the sample size we used a seroprevalence of reference of 59% 12 at a confidence level of 99% and a marginal error of 0.05. The calculation () of the sample size for the cross-sectional study was adopted 14 . The result of the calculation was 638 subjects, and 630 participants were enrolled in the study.
Ethical aspects
This study received the approval from the local health authorities and from the Shahid Beheshti University of Medical Science Ethical Committee. All the enrolled participants were informed about the study and a written informed consent was obtained. In the case of minors, written consents were signed by the parents or legal guardians.
Questionnaire survey
A previously published questionnaire was used to assess the risk factors, with some modifications, mainly regarding eating habits and the type of meat consumed, such as pork meat and salami that were removed from the questionnaire 15 . Briefly, the following variables were included to measure demographics and potential risk factors: age; sex; place of residence, educational level (illiterate, primary education, high school, college graduate education and higher); occupation; soil-related activities including gardening, washing fruits or vegetables; exposure to cats and cat feces; consumption of raw or undercooked meat; type of meat consumed (sheep, beef, goat, poultry); and sources of drinking water (well, unfiltered, spring water, filtered) as shown in Table 1.
Table 1. Seroprevalence of Toxoplasma infection among rural individuals in Northern Iran according to sociodemographic characteristics (risk factors analysis by the Pearson's chi-square test).
| Characteristic | No. participants | No. of seropositives (%) | OR (95% CI) | p value |
| Age (years) | <0.001 | |||
| ≤10 | 18 | 8 (44.4) | 1.0 | |
| 11-20 | 60 | 36 (60.0) | 1.88 (0.65-5.43) | |
| 21-30 | 141 | 101 (71.6) | 3.16 (1.16-8.57) ** | |
| 31-40 | 124 | 91 (73.4) | 3.45 (1.25- 9.48) ** | |
| 41-50 | 112 | 93 (83.0) | 6.12 (2.14-17.53) ** | |
| 51-60 | 109 | 93 (85.3) | 7.27 (2.49-21.19) ** | |
| ≥61 | 66 | 55 (83.3) | 6.25 (2.01-19.40) ** | |
| Sex | 0.512 | |||
| Male | 203 | 157 (77.3) | 1.14 (0.77 -1.69) | |
| Female | 427 | 320 (74.9) | 0.88 (0.59- 1.30) | |
| Educational level | <0.001 | |||
| Illiterate | 158 | 132 (83.5) | 1.0 | |
| Primary school | 143 | 118 (82.5) | 0.93 (0.51-1.70) | |
| High school | 234 | 166 (70.9) | 0.48 (0.29 -0.80) ** | |
| College and higher | 95 | 61 (64.2) | 0.35 (0.20-0.64) ** | |
| Occupation | 0.010 | |||
| Farmer & shepherd | 57 | 46 (80.7) | 1.0 | |
| Housewife | 312 | 251 (80.4) | 0.98 (0.48 -2.01) | |
| Student | 99 | 70 (70.7) | 0.58 (0.26 -1.27) | |
| Government employee & other | 162 | 110 (67.9) | 0.51 (0.24 -1.06) | |
| Region | 0.992 | |||
| Dabodasht | 219 | 167 (76.3) | 1.0 | |
| Litkoh & Larijan | 208 | 157 (75.5) | 0.96 (0.62 -1.49) | |
| Dashtesar | 138 | 104 (75.4) | 0.95 (0.58-1.57) | |
| Larijan | 65 | 49 (75.4) | 0.95 (0.50 -1.81) | |
| Water supply | 0.009 | |||
| Treated | 272 | 192 (70.6) | 0.61 (0.43 -0.89) ** | |
| Untreated | 358 | 285 (79.6) | 1.63 (1.13 -2.35) ** | |
| Consumption of undercooked or raw meat | <0.001 | |||
| Yes | 386 | 318 (82.4) | 2.50 (1.72 -3.62) ** | |
| No | 244 | 159 (65.2) | 0.40 (0.28-0.58) ** | |
| Consumption of raw unwashed vegetables or fruits | <0.001 | |||
| Yes | 322 | 271 (84.2) | 2.63 (1.80 -3.85) ** | |
| No | 308 | 206 (66.9) | 0.38 (0.26 -0.56) ** | |
| Contact with cats | 0.182 | |||
| Yes | 226 | 178 (78.8) | 1.30 (0.88 -1.92) | |
| No | 404 | 299 (74.0) | 0.77 (0.52 -1.13) | |
| Contact with soil | 0.005 | |||
| Yes | 317 | 255 (80.4) | 1.69 (1.17 -2.44) ** | |
| No | 313 | 222 (70.9) | 0.59 (0.41 -0.86) ** | |
| Consumption of goat meat | 0.655 | |||
| Yes | 140 | 108 (77.1) | 1.11 (0.71 -1.73) | |
| No | 490 | 369 (75.3) | 0.90 (0.58 -1.41) | |
| Consumption of sheep meat | 0.015 | |||
| Yes | 540 | 418 (77.4) | 1.80 (1.11 -2.91) ** | |
| No | 90 | 59 (65.5) | 0.55 (0.34 -0.90) ** | |
| Consumption of cow meat | 0.663 | |||
| Yes | 429 | 327 (76.2) | 1.09 (0.74 -1.61) | |
| No | 201 | 150 (74.6) | 0.92 (0.62- 1.35) | |
| Consumption of poultry meat | <0.001 | |||
| Yes | 564 | 438 (77.7) | 2.41 (1.42 -4.09) ** | |
| No | 66 | 39 (59.1) | 0.42 (0.24 -0.71) ** |
*95% confidence interval does not include 1.
Laboratory tests
About 5 mL of whole blood samples were drawn from each participant through venipuncture. Blood samples were allowed to clot and centrifuged at 1000 g for 5 minutes. Sera were stored at 4 °C for 2-3 days until they were transported in ice to the Parasitology Laboratory of the Shahid Beheshti Univesity of Medical Science where they were kept at -20 °C until the analysis.
Serum samples were screened for anti-Toxoplasma IgG and IgM antibodies using two commercial enzyme-linked immunosorbent assays (ELISA) kits (ACON, San Diego, CA, USA) as instructed by the manufacturers. These kits have sensitivities and specificities of 99.9% and 99%, respectively. According to the manufacturer's recommendation, results were reported in International Units (IU). Sera with values of < 9.0 IU/mL were considered negative; those with values between 9.0-11.0 IU/mL were considered suspicious (gray zone), and those with values > 11.0 IU/mL were considered positive, for both T. gondii immunoglobulin G and M antibodies.
Statistical analysis
Results were analyzed using the SPSS Statistics software, version 21 (IBM, NY, USA). The mean ± standard deviation was used to describe the continuous variables such as age and frequency and percentages were used for ordinal and nominal variables. The Pearson's chi-square (c2) test was used to determine the association between seropositivity for T. gondii infection and the risk factors. A multivariate logistic analysis was performed using the Hosmer & Lemeshow selection model 16 , and odds ratios (OR) and 95% confidence intervals (CI) were calculate to associate the potential variables. A p value of < 0.05 was considered statistically significant.
RESULTS
Distribution of participants
Among the 630 subjects of the study, 67.8% (427/630) were females and 32.2% (203/630) were males. They were 3 - 78 years old with a mean age of 36.6 years (± SD 16.3). Most of the participants (69%) had middle socioeconomic status and were aged 21-40 years (42%). The majority of women (73%) were housewives. Concerning the level of education, 158 (25%) participants were unable to read and write. Socio-demographic characteristics are presented in Table 1.
Seroprevalence of anti-T. gondii antibodies and univariate analysis of risk factors
Among the 630 participants, 477 were seropositive for anti-T. gondii antibodies (75.7%, CI 95%: 72.4-79.1). Of these, 465 cases (73.8%) tested positive for only IgG, suggesting a chronic toxoplasmosis profile. Twelve cases (1.9%) tested positive for both IgG and IgM, suggesting recent toxoplasmosis. Results of the univariate analysis showed that T. gondii seropositivity was associated with age (p < 0.001), educational level (p < 0.001), occupation (p = 0.01), water supply (p = 0.009), contact with soil (p = 0.005), consumption of undercooked meat (p < 0.001), unwashed raw vegetables or fruits (p < 0.001), sheep meat (p = 0.015) and poultry meat (p < 0.001). Complete data are presented in Table 1.
Logistic regression analysis for risk factors of seropositivity to T. gondii
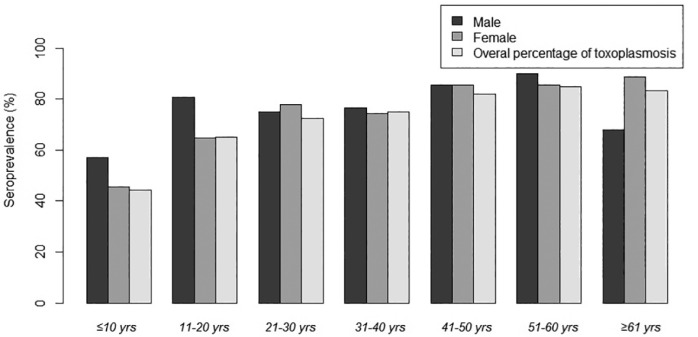
In the logistic regression analysis, four variables were identified as being associated with T. gondii infection: age, occupation, consumption of undercooked meat, and of unwashed raw vegetables or fruits (Table 2). As shown in Figure 1, an increasing seroprevalence with age was observed in this study (p = < 0.001).
Table 2. Seroprevalence of Toxoplasma infection among rural individuals in Northern Iran according to sociodemographic characteristics (estimated by a full logistic regression model).
| Characteristic | No. of participants | OR | 95% CI | p value |
| Age (years) | ||||
| ≤10 | 18 | 1.00* | 1.0 | |
| 11-20 | 60 | 3.04 | (0.90- 10.23) | 0.73 |
| 21-30 | 141 | 11.35** | (3.19- 40.34) | <0.001 |
| 31-40 | 124 | 13.31** | (3.62- 48.97) | <0.001 |
| 41-50 | 112 | 14.04** | (4.02- 49.00) | <0.001 |
| 51-60 | 109 | 17.80** | (4.89- 64.76) | <0.001 |
| ≥61 | 66 | 11.71** | (2.86- 48.00) | <0.001 |
| Educational level | ||||
| Illiterate | 158 | 1.00* | 1.0 | |
| Primary school | 143 | 1.25 | (0.57- 2.78) | 0.577 |
| High school | 234 | 0.67 | (0.30- 1.49) | 0.326 |
| College and higher | 95 | 0.44 | (0.16- 1.17) | 0,099 |
| Occupation | ||||
| Farmer & shepherd | 57 | 1.00* | 1.0 | |
| Housewife | 312 | 1.51 | (0.65- 3.49) | 0.337 |
| Student | 99 | 3.28** | (1.13- 9.53) | 0.029 |
| Government employee & other | 162 | 1.05 | (0.42- 2.62) | 0.915 |
| Water supply | 0.497 | |||
| Treated | 272 | 1.17 | (0.75- 1.81) | |
| Untreated | 358 | 1.00* | ||
| Consumption of undercooked or raw meat | 0.004 | |||
| Yes | 386 | 0.52** | (0.33- 0.81) | |
| No | 244 | 1.00* | ||
| Consumption of raw unwashed vegetables or fruits | 0.005 | |||
| Yes | 322 | 0.53** | (0.34- 0.82) | |
| No | 308 | 1.00* | ||
| Contact with soil | 0.119 | |||
| Yes | 317 | 0.72 | (0.48- 1.09) | |
| No | 313 | 1.00* | ||
| Consumption of sheep meat | 0.218 | |||
| Yes | 540 (84) | 0.71 | (0.42- 1.22) | |
| No | 90 (16) | 1.00* | ||
| Consumption of poultry meat | 0.889 | |||
| Yes | 564 (89.5) | 0.96 | (0.51- 1.80) | |
| No | 66 (10.4) | 1.00* |
* Reference. ** 95% confidence interval does not include 1.
Fig. 1. Age-associated prevalence of Toxoplasma gondii infections among rural populations in north of Iran.
DISCUSSION
In this cross-sectional study, we found a seroprevalence of 75.7% to Toxoplasma gondii in rural communities of Northern Iran that is significantly higher than the mean (39%) seroprevalence of T. gondii infection previously reported in the general population in Iran12. In addition, the seroprevalence in our study is higher than the 23.8% of seroprevalence to T. gondii infection reported in a similar Mexican population and also the 6.2% of seroprevalence found in Kyrgyzstan, a central Asia country 17 , 18 . Our prevalence rate is also much higher than those reported in the general population from Qatar (29.8%), Pakistan (29.48%) and Turkey (31.9%) in neighboring Iran 19 - 21 . One possible factor that may explain the high seroprevalence of toxoplasmosis in the studied rural population is the geographical climate. Climate in Northern Iran has high humidity due to the proximity to the Caspian Sea which provides optimal conditions for the sporulation and survival of Toxoplasma oocysts in the environment 12 . The influence of climate conditions on the oocyst survival and T. gondii transmission has been reported by previous studies in the world and Iran 3 , 12 . Another contributing factor could be due the prosperity of agriculture and animal husbandry in Northern Iran. Toxoplasmosis is one of the most prevalent zoonotic parasitic diseases, therefore more intense contact with animals or livestock increases the risk of infection 22 . Another possibility of transmission of toxoplasmosis to humans would be through contaminated environment, contaminated unwashed vegetables and consumption of undercooked meat.
In the present study several variables were found to be associated with T. gondii infection by univariate analysis (Table 1). However, in the multivariate logistic regression analysis, only four variables were identified as potential risk factors for acquisition of infection: age, occupation, consumption of undercooked meat and of unwashed raw vegetables or fruits (Table 2). These results are in agreement with previous studies from other countries 3 , 11 , 12 . The present study found that housewives and farmers are two groups with high T. gondii seroposivity. This finding is in agreement with previous studies performed in Iran and other areas in the world such as the United States and Saudi Arabia 12 , 15 , 23 . These high-risk groups are more exposed to potential parasite sources: pet caregivers, people who work with livestock, chop meat and clean vegetables without wearing gloves and have contact with contaminated soil during gardening12. Agreeing with these facts, our results demonstrated that seroposivity was significantly higher in individuals who have contact with soil in comparison with those who did not. We found that the frequency of seropositive cases increased with age (Fig. 1). Further analysis revealed that older age groups (≥ 50 years) were three times more likely to have T. gondii seroposive results than the younger age groups (≤ 24 years). This finding is consistent with the majority of studies in the world 12 , 17 , 22 , 24 . The explanation would be an enhanced exposure to toxoplasmosis by means of consumption of raw or undercooked infected products. Another possible is that older people may have had greater exposure when they were younger and this could inflate the increase in age as a risk. It is important to remember that anti-T. gondii antibodies persist for a long time, and the increment of the seroprevalence with age is associated with life-time exposure. Moreover, our results revealed that increasing the level of education is related with reduced rate of T. gondii seroposivity. Lower levels of education are associated with a lack of awareness of the disease, lower socioeconomic status, lower hygiene status and professional activity with greater exposure to contaminated soil 12 , 15 . The results of the present study and those of others previously published on toxoplasmosis have indicated that contaminated water could be a source of T. gondii infections in outbreaks 22 , 25 , 26 . Consistent with these studies, our finding showed that using untreated water (well, spring and unfiltered water) is an important risk factor to acquisition of infection. Northern Iran has a rainy climate and abundant springs, therefore the majority of villages use these springs as a source of drinking water.
In our study we observed a significant association of seropositivity with the consumption of undercooked meat and unwashed raw vegetables. The latter are one of the main sources of parasitic infections in Northern Iran and around the world 27 , 28 . Several studies claimed that consumption of undercooked meat is an important factor for parasite transmission 12 , 15 , 17 , 24 , 29 . However, some other studies reported that there was no association between T. gondii infection and consumption of meat 17 , 30 . Cow, sheep, goat and chicken meat are used in almost all of the Iranian typical dishes. The consumption of poultry (turkey, chicken, geese, duck) and sheep meat was related to T. gondii seroposivity by the univariate analysis in our study. Meat is one of the major components of the Persian cuisine and is consumed in different forms. One of the most common methods of cooking meat is in the form of different Khoreshts (stews) such as Ghormeh sabzi and Gheimeh. In these forms, meat is well cooked and the risk of transmitting T. gondii is very unlikely. However, special raw meat dishes such as Kebab, Shishlik and Joje Kebab are also very popular in Iran and are consumed lightly cooked. To prepare kebab, the preferred meat is lamb, but other meats such as beef, goat and poultry are also used. Kebab and other crude meat are known festive recipes, and are served in most restaurants. These undercooked meat dishes served in most guests, and every Iranian eats it almost once a week. Previous studies revealed that seroprevalence of T. gondii infection among cow, goat and sheep in Northern Iran was 9%, 14.2-30% and 20-90%, respectively 31 , 32 .
Contact with cats was not associated with T. gondii seropositivity in the present study. Similar results were observed by Alvarado-Esquivel et al. in a study conducted in a rural Mexican population, and also by Walle et al. in a study in Ethiopia 17 , 33 . A possible explanation for this finding could be attributed to religious beliefs of the inhabitants in the studied areas, as they do not have a close contact with cats.
Our study had some limitations. It would have been ideal to include more rural communities and more participants but this was not feasible due to the time to finish the study and also financial constraints. Another limitation of our study is the risk factor analysis based on IgG antibody titers, considering that seropositivity for T. gondii persists for many years and positive individuals were likely to have been infected a long time ago, and their living conditions and behaviors may have changed over time. Therefore a direct temporal relationship between behaviors and T. gondii infection could not be established.
In conclusion, our results demonstrate a high seroprevalence of T. gondii infection in rural populations of Northern Iran. Age, consumption of undercooked meat and raw vegetables and occupation are important risk factors to acquire T. gondii infection in rural areas. A health program should be established to increase to increase people's knowledge regarding toxoplasmosis, the identified risk factors, and the negative impact on the public health that the lack of governmental measures would cause.
ACKNOWLEDGMENTS
We would like to thank the administrators, authorities and personnel of the Amol Health Care Network and especially Dr. Mojtaba Kheyri and Dr. Dehghan for their kind cooperation during the sample collection. Most importantly, the authors would like to thank all the participants for giving their permission to collect samples and for their participation in this study. We are thankful to the Protozoology Unit of the Shahid Beheshti University of Medical Sciences for provision of materials.
REFERENCES
- 1.Flegr J, Prandota J, Sovičková M, Israili ZH. Toxoplasmosis: a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PloS One. 2014;9:70. doi: 10.1371/journal.pone.0090203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States - major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25:264–296. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. 2009;39:1385–1394. doi: 10.1016/j.ijpara.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Walker M, Zunt JR. Parasitic central nervous system infections in immunocompromised hosts. Clin Infect Dis. 2005;40:1005–1015. doi: 10.1086/428621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rostami A, Keshavarz H, Shojaee S, Mohebali M, Meamar AR. Frequency of Toxoplasma gondii in HIV positive patients from West of Iran by ELISA and PCR. Iran J Parasitol. 2014;9:474–481. [PMC free article] [PubMed] [Google Scholar]
- 7.McConkey GA, Martin HL, Bristow GC, Webster JP. Toxoplasma gondii infection and behaviour-location, location, location? J Exp Biol. 2013;216:113–119. doi: 10.1242/jeb.074153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutterland AL, Fond G, Kuin A, Koeter MW, Lutter R, van Gool T, et al. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: systematic review and meta-analysis. Acta Psychiatr Scand. 2015;132:161–179. doi: 10.1111/acps.12423. [DOI] [PubMed] [Google Scholar]
- 9.Nourollahpour Shiadeh M, Niyyati M, Fallahi S, Rostami A. Human parasitic protozoan infection to infertility: a systematic review. Parasitol Res. 2016;115:469–477. doi: 10.1007/s00436-015-4827-y. [DOI] [PubMed] [Google Scholar]
- 10.Dalimi A, Abdoli A. Toxoplasma gondii and male reproduction impairment: a new aspect of toxoplasmosis research. Jundishapur J Microbiol. 2013;6:70 [Google Scholar]
- 11.Montoya J, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 12.Daryani A, Sarvi S, Aarabi M, Mizani A, Ahmadpour E, Shokri A, et al. Seroprevalence of Toxoplasma gondii in the Iranian general population: a systematic review and meta-analysis. Acta Trop. 2014;137:185–194. doi: 10.1016/j.actatropica.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Statistical Centre of Iran . Summary and statistical report of the 2012 population and housing census. Tehran: population census commission Mazandaran province: population census commission Amol County. 2012. [Google Scholar]
- 14.Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013;35:121–126. doi: 10.4103/0253-7176.116232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones JL, Dargelas V, Roberts J, Press C, Remington JS, Montoya JG. Risk factors for Toxoplasma gondii infection in the United States. Clin Infect Dis. 2009;49:878–884. doi: 10.1086/605433. [DOI] [PubMed] [Google Scholar]
- 16.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd . New York: Wiley; 2000. [Google Scholar]
- 17.Alvarado-Esquivel C, Cruz-Magallanes HM, Esquivel-Cruz R, Estrada-Martínez S, Rivas-González M, Liesenfeld O, et al. Seroepidemiology of Toxoplasma gondii infection in human adults from three rural communities in Durango State, Mexico. J Parasitol. 2008;94:811–816. doi: 10.1645/GE-1524.1. [DOI] [PubMed] [Google Scholar]
- 18.Minbaeva G, Schweiger A, Bodosheva A, Kuttubaev O, Hehl AB, Tanner I, et al. Toxoplasma gondii infection in Kyrgyzstan: seroprevalence, risk factor analysis, and estimate of congenital and AIDS-related toxoplasmosis. PLoS Negl Trop Dis. 2013;7:70. doi: 10.1371/journal.pntd.0002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu-Madi MA, Al-Molawi N, Behnke JM. Seroprevalence and epidemiological correlates of Toxoplasma gondii infections among patients referred for hospital-based serological testing in Doha, Qatar. Parasit Vectors. 2008;1:39–39. doi: 10.1186/1756-3305-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tasawar Z, Aziz F, Lashari MH, Shafi S, Ahmad M, Lal V, et al. Seroprevalence of Human toxoplasmosis in southern Punjab, Pakistan. Pak J Life Soc Sci. 2012;10:48–52. [Google Scholar]
- 21.Acici M, Babur C, Kilic S, Hokelek M, Kurt M. Prevalence of antibodies to Toxoplasma gondii infection in humans and domestic animals in Samsun province, Turkey. Trop Anim Health Prod. 2008;40:311–315. doi: 10.1007/s11250-007-9101-6. [DOI] [PubMed] [Google Scholar]
- 22.Bahia-Oliveira LM, Jones JL, Azevedo-Silva J, Alves CC, Oréfice F, Addiss DG. Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro state, Brazil. Emerg Infect Dis. 2003;9:55–62. doi: 10.3201/eid0901.020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Qurashi A. Seroepidemiological study of toxoplasmosis in rural areas in the eastern region of Saudi Arabia. J Egypt Soc Parasitol. 2004;34:23–34. [PubMed] [Google Scholar]
- 24.Alvarado-Esquivel C, Pacheco-Vega SJ, Hernández-Tinoco J, Sánchez-Anguiano LF, Berumen-Segovia LO, Rodríguez-Acevedo FJ, et al. Seroprevalence of Toxoplasma gondii infection and associated risk factors in Huicholes in Mexico. Parasit Vectors. 2014;7:301–301. doi: 10.1186/1756-3305-7-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benenson MW, Takafuji ET, Lemon SM, Greenup RL, Sulzer AJ. Oocyst-transmitted toxoplasmosis associated with ingestion of contaminated water. N Engl J Med. 1982;307:666–669. doi: 10.1056/NEJM198209093071107. [DOI] [PubMed] [Google Scholar]
- 26.Bowie WR, King AS, Werker DH, Isaac-Renton JL, Bell A, Eng SB, et al. Outbreak of toxoplasmosis associated with municipal drinking water. Lancet. 1997;350:173–177. doi: 10.1016/s0140-6736(96)11105-3. [DOI] [PubMed] [Google Scholar]
- 27.Rostami A, Ebrahimi M, Mehravar M, Fallah Omrani V, Fallahi S, Behniafar H. Contamination of commonly consumed raw vegetables with soil transmitted helminth eggs in Mazandaran province, northern Iran. Int J Food Microbiol. 2016;225:54–58. doi: 10.1016/j.ijfoodmicro.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Siyadatpanah A, Tabatabaei F, Emami Zeydi A, Spotin A, Fallah-Omrani V, Assadi M, et al. Parasitic contamination of raw vegetables in Amol, north of Iran. Arch Clin Infect Dis. 2013;8:70 [Google Scholar]
- 29.Studenicová C, Bencaiová G, Holková R. Seroprevalence of Toxoplasma gondii antibodies in a healthy population from Slovakia. Eur J Intern Med. 2006;17:470–473. doi: 10.1016/j.ejim.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Alvarado-Esquivel C, Alanis-Quiñones OP, Arreola-Valenzuela MA, Rodríguez-Briones A, Piedra-Nevarez LJ, Duran-Morales E, et al. Seroepidemiology of Toxoplasma gondii infection in psychiatric inpatients in a northern Mexican city. BMC Infect Dis. 2006;6:178–178. doi: 10.1186/1471-2334-6-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharif M, Sarvi S, Shokri A, Hosseini Teshnizi S, Rahimi MT, Mizani A, et al. Toxoplasma gondii infection among sheep and goats in Iran: a systematic review and meta-analysis. Parasitol Res. 2015;114:1–16. doi: 10.1007/s00436-014-4176-2. [DOI] [PubMed] [Google Scholar]
- 32.Sarvi S, Daryani A, Rahimi MT, Aarabi M, Shokri A, Ahmadpour E, et al. Cattle toxoplasmosis in Iran: a systematic review and meta-analysis. Asian Pac J Trop Med. 2015;8:120–126. doi: 10.1016/S1995-7645(14)60301-1. [DOI] [PubMed] [Google Scholar]
- 33.Walle F, Kebede N, Tsegaye A, Kassa T. Seroprevalence and risk factors for toxoplasmosis in HIV infected and non-infected individuals in Bahir Dar, Northwest Ethiopia. Parasit Vectors. 2013;6:15–15. doi: 10.1186/1756-3305-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]


