SUMMARY
The aim of the present study was to analyze the status of the American Tegumentary Leishmaniasis (ATL) in the state of Rio de Janeiro, from 2004 to 2013, through its spatiotemporal distribution. We also described variables considered relevant to the epidemiology of the disease, such as the clinical form, gender, ethnic group, age group, and progression of disease. This is a descriptive study, which used notified secondary data from the Brazilian Information System of Notifiable Diseases (SINAN), Ministry of Health, Brazil, regarding confirmed diagnoses. To help the calculation of coefficients of detection and mortality, we used population data from the Brazilian Institute of Geography and Statistics (IBGE). We analyzed 1,470 cases of ATL with the predominance of the cutaneous clinical form (1,292/87.89%). The data has also revealed seven deaths, a predominance of males (922/62.72%), and a higher incidence of ATL in the white ethnic group (731/49.72%). We observed a high incidence of ATL in the group of 20 - 39 years old (477/32.44%). We concluded that there was a decrease in the number of ATL cases in the state of Rio de Janeiro, based on a coefficient of detection of 1.44/100.000 inhabitants in 2004 decreasing to 0.20/100.000 inhabitants in 2013. The localities with the highest occurrences of ATL were the metropolitan region (843 cases) and the municipality of Rio de Janeiro (740 cases). In 2005, the highest incidence of the disease was observed (351 cases) in the study. Among the variables selected to describe the epidemiology of the disease, the following categories: cutaneous clinical form, male patients, white ethnic group, and the age group of 20 - 39 years old were more affected than the others.
KEYWORDS: American tegumentary leishmaniasis, Public health, Epidemiology, Detection rate, Mortality
INTRODUCTION
According to the World Health Organization, leishmaniasis is among the six most important parasitic infectious diseases, as it occurs in 88 countries that sum up a population of 12 million infected individuals. There are approximately 1.3 million new cases each year, and 350 million people are at risk 1 .
The American Tegumentary Leishmaniasis (ATL) is a relevant zoonosis for the Brazilian health system and is on the list of neglected diseases that receive annually, through increasing government investments, around R$75 million "reais", the Brazilian currency (four "reais" corresponding to approximately one US dollar), for studies on new treatments. This disease affects poor and developing countries; 90-95% of the cases recorded occurred in Afghanistan, Algeria, Brazil, Colombia, Bolivia, Iran, Peru, and Syria 1 , 2 .
The disease is a public health problem in Brazil, as well as in several other countries, presents cutaneous (localized, disseminated, relapsing or diffuse) and mucosal forms (late, concomitant, contiguous, primary, or undetermined). ATL mutilates and disables people, leading to profound psychosocial consequences. Mucosal lesions can be lethal due to complications resulting from secondary infections, usually in the upper respiratory tract 3 , 4 , 5 , 6 .
In Brazil, cases of leishmaniasis have been notified in all the states, with an average occurrence of 26,000 cases/year from 2000 to 2009. In 2010, there was a slight decrease, with 21,981 cases. The Northeastern region was the most representative, with 40.54% of the cases, followed by the Northern (32.34%), Midwestern (14.39%), Southeastern (11.05%), and Southern regions (1.15%) 7 , 8 .
In 1915, d'Utra & Silva 9 made the first description of the disease in the state of Rio de Janeiro. Since then, it has been reported in several municipalities such as Campos dos Goytacazes, Barra Mansa, Niterói, Rio de Janeiro, Mesquita, Mangaratiba, Maricá, Angra dos Reis, Paracambi, and Seropédica 10 , 11 , 12 , 13 , 14 , 15 .
In a country where the occurrence of ATL has been increasing year after year, the state of Rio de Janeiro, on the contrary, has drastically reduced the number of cases of the disease 8 . Therefore, the present study is the first step to inform researchers on the situation, so that they can acknowledge the effects of the control measures implemented in the state and create strategies for other localities in Brazil.
The aim of the present study was to analyze the status of ATL in the state of Rio de Janeiro, from 2004 to 2013, through its spatiotemporal distribution. We also described variables considered relevant to the epidemiology of the disease such as the clinical form, gender, ethnic group, age group, and progression of disease.
We decided to describe the epidemiological variables of ATL to contribute to the monitoring of this disease and to make the epidemiological surveillance stronger to characterize the tendencies and the magnitude of the disease.
MATERIAL AND METHODS
The state of Rio de Janeiro lies in the southeastern region of Brazil (22°54'S; 43°10'W). It has a population of 15,989,929 people, an area of 43,780,157 km2, and is the third most populous state in the country. Its climate is warm, with humid, semi-humid, and dry regions, and an average annual temperature of 24 °C. The predominant vegetation is the Atlantic forest. The state has 92 municipalities distributed into eight regions (Table 1) 16 , 17 .
Table 1. Regions and municipalities of the state of Rio de Janeiro, Brazil.
| Northwestern Region Aperibé, Bom Jesus do Itabapoana, Cambuci, Italva, Itaocara, Itaperuna, Laje do Muriaé, Miracema, Natividade, Porciúncula, Santo Antônio de Pádua, São José de Ubá, Varre-Sai. |
| Northern Region Campos dos Goytacazes, Carapebus, Cardoso Moreira, Conceição de Macabu, Macaé, Quissamã, São Fidélis, São Francisco de Itabapoana, São João da Barra. |
| Mountain Region Bom Jardim, Cantagalo, Carmo, Cordeiro, Duas Barras, Macuco, Nova Friburgo, Petrópolis, Santa Maria Madalena, São José do Vale do Rio Preto, São Sebastião do Alto, Sumidouro, Teresópolis, Trajano de Morais. |
| Mid-Southern Region Areal, Comendador Levy Gasparian, Engenheiro Paulo de Frontin, Mendes, Miguel Pereira, Paraíba do Sul, Paty do Alferes, Sapucaia, Três Rios, Vassouras. |
| Metropolitan Region Belford Roxo, Duque de Caxias, Guapimirim, Itaboraí, Japeri, Magé, Mesquita, Nilópolis, Niterói, Nova Iguaçu, Paracambi, Queimados, Rio de Janeiro, São Gonçalo, São João de Meriti, Seropédica, Tanguá. |
| Coastal Lowland Region Araruama, Armação dos Búzios, Arraial do Cabo, Cabo Frio, Cachoeiras de Macacu, Casimiro de Abreu, Iguaba Grande, Maricá, Rio Bonito, Rio das Ostras, São Pedro da Aldeia, Saquarema, Silva Jardim. |
| Middle Paraíba Region Barra do Piraí, Barra Mansa, Itatiaia, Pinheiral, Piraí, Porto Real, Quatis, Resende, Rio Claro, Rio das Flores, Valença, Volta Redonda. |
| Costa Verde Region Angra dos Reis, Itaguaí, Mangaratiba, Parati. |
Source: Fundação Ceperj16 and Instituto Brasileiro de Geografia e Estatística17 (IBGE).
This is a descriptive study, which used notified secondary data from the Brazilian Information System of Notifiable Diseases (SINAN), Ministry of Health, Brazil, regarding confirmed diagnoses, in the period from 2004 to 2013.
SINAN is a system supplied mainly by disease notifications that compose the Brazilian list of compulsorily notifiable diseases. Its main goal is to collect, transmit, and disseminate data generated on a regular basis by the Epidemiological Surveillance System at three governmental levels, through a computer network. Therefore, SINAN sets the ground for the investigation process and analysis of information on the epidemiological surveillance of compulsorily notifiable diseases8.
The system enables the use of information at multiple levels, from Municipal Health Care Units (ambulatory, medical centers, and hospitals) to the State Health Secretariat, reporting cases to the Ministry of Health. The data entry occurs through the filling of standardized forms by health professionals: the Individual Notification Form (suspicious of occurrence of a health problem of compulsory notification) and the Individual Investigation Form (specific investigation protocol for each type of disease).
The system information is available for the Department of Integrated Health System (DATASUS), with access through the software TABNET (tabnet.datasus.gov.br/tabnet/tabdescr.htm). It aims at meeting the needs of managers, scholars, and people interested on health issues, who intend to obtain and analyze rapidly and objectively the data of the entire Integrated Health System, including SINAN. TABNET allows the crossing of several variables, as it is the user who defines the content according to the user interests. This software allows selecting and organizing data according to the aim of the research. It also allows associating tables with maps, enabling a spatial visualization and assessment of the information. The data available are periodically updated 8 .
The present study used the information available at TABNET, organized per region of the state, to describe the quantitative profile and the epidemiological variables of the disease. This information comprised local notification and characteristics: clinical (clinical form), demographic data (gender, detailed age group, and ethnic group), and progression of disease (death), which we considered more relevant among the variables available.
We used census and estimated data on the population of the state of Rio de Janeiro from 2004 to 2013, available at the demographic database of the Brazilian Institute of Geography and Statistics, to help the calculation of detection and mortality rates 18 .
RESULTS
In total, there were 1,470 cases of American Tegumentary Leishmaniasis (ATL) recorded in the state of Rio de Janeiro from 2004 to 2013. On average, there were 147 cases per year, with the highest numbers found in 2005 (351) and 2006 (288). There was a reduction of 85.07% in the number of cases in the studied period (from 1.44/100,000 inhabitants in 2004 to 0.20/100,000 in 2013), with a more marked decline observed since 2005 (Fig. 1).
Fig. 1. Temporal distribution of the American Tegumentary Leishmaniasis (ATL) in the state of Rio de Janeiro, southeastern Brazil, from 2004 to 2013, highlighting the number of cases and the coefficient of detection (per 100,000 inhabitants).
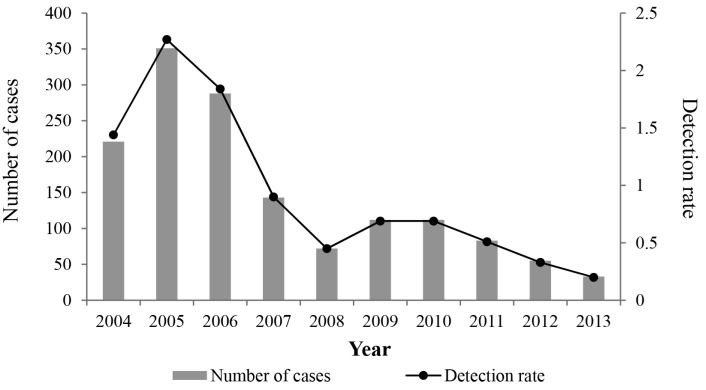
Seven cases resulted in the death of patients (0.47%; 0.04/100,000 inhabitants), considering only the years 2005, 2006, and 2008. In 2005, there was a peak, with four deaths (0.27%; 0.02/100,000 inhabitants). In 2010, 2011, and 2013 five deaths were clearly associated with other causes. As there was no notification of deaths from 2009 to 2013, we can infer a 100% reduction in mortality.
Among the clinical forms of ATL observed during the study period, the cutaneous form predominated with 1,292 cases (87.89%), followed by the mucosal form with 174 cases (11.83%), and by non-classified forms with four cases (0.27%). There was a reduction of 86.46% in the number of cases of the cutaneous form (from 1.25/100,000 inhabitants in 2004 to 0.13/100,000 inhabitants in 2013) and of 75.0% in the number of cases of the mucosal form (from 0.18/100,000 inhabitants in 2004 to 0.04/100,000 inhabitants in 2013). The number of deaths per clinical form was more strongly related to the cutaneous form, with six cases (0.40%; 0.03/100,000 inhabitants).
Males were more affected than females, with 922 (62.72%) and 548 cases (37.27%), respectively. There was a reduction of 82.09% in the number of cases in males (from 0.87/100,000 inhabitants in 2004 to 0.14/100,000 inhabitants in 2013) and 89.69% in the number of cases in females (from 0.57/100,000 inhabitants in 2004 to 0.05/100,000 inhabitants in 2013). Among males, deaths were more frequent, with five cases (0.34%; 0.03/100,000 inhabitants).
The age group of 20-39 years old was the most affected, with 477 cases (32.44%), followed by the age group of 40-59 years, with 465 cases (31.63%), and the age group of 10-19 years, with 198 cases (13.46%). The age group that underwent the steepest reduction of disease, during the study period, is not among the most affected by the disease (1-9 years old). This group showed a decrease of 93.75% (from 0.10/100,000 inhabitants in 2004 to 0.00/100,000 inhabitants in 2013), followed by the age group of 10-19 years, with a decrease of 90.0% (from 0.26/100,000 inhabitants in 2004 to 0.02/100,000 inhabitants in 2013). The highest number of deaths occurred in the age group >70 years old, with three cases reported (0.20%; 0.01/100,000 inhabitants).
The white ethnic group was the most affected during the study period, with 731 cases (49.72%), followed by those with mixed-ethnicity (mixture of black and white) 314 (21.36%), and black people with 178 (12.10%). The comparison of data between 2004 and 2013 showed a reduction of 89.39% in the white ethnic group (from 0.74/100,000 inhabitants in 2004 to 0.07/100,000 inhabitants in 2013), 71.74% in the group with mixed-ethnicity (from 0.30/100,000 inhabitants in 2004 to 0.07/100,000 inhabitants in 2013), and 94.45% in black people (from 0.11/100,000 inhabitants in 2004 to 0.00/100,000 inhabitants in 2013). The number of deaths was higher in the white ethnic group, with four cases (0.27%; 0.02/100,000 inhabitants).
To assess the distribution of ATL cases per region of the state, we present the variables analyzed in Table 2, and data on the detection rate, years with higher occurrences, the most affected municipalities, and municipalities without notification of the disease in Table 3. We also present the coefficient of detection of each municipality where the disease was notified in Table 4. The Metropolitan region showed the highest number of cases in the study period (843/57.34%), represented in this study by the municipality of Rio de Janeiro, which concentrated most notified cases (740/87.78%).
Table 2. Distribution of the American Tegumentary Leishmaniasis (ATL) according to the regions of the state of Rio de Janeiro, Brazil, from 2004 to 2013, based on the studied variables .
| Variables | Regions | |||||||
| Northwestern | Northern | Mountain | Mid-southern | Metropolitan | Coastal lowland | Middle Paraíba | Costa Verde | |
| Number of cases (%) | 67 (4.55) | 61 (4.14) | 132 (8.97) | 61 (4.14) | 843 (57.34) | 64 (4.35) | 35 (2.38) | 207 (14.08) |
| Evolution to death | 0 | 1 | 1 | 1 | 3 | 0 | 0 | 1 |
| Clinical Form Cutaneous Mucosal Unknown | 61 6 0 | 58 3 0 | 124 8 0 | 59 2 0 | 707 132 4 | 54 10 0 | 34 1 0 | 195 12 0 |
| Gender Male Female Unknown | 43 24 0 | 31 30 0 | 72 60 0 | 37 24 0 | 553 290 0 | 43 21 0 | 19 16 0 | 124 83 0 |
| Age group < 1 1-9 10-19 20-39 40-59 60-69 >70 Unknown | 1 3 4 19 30 5 5 0 | 2 4 7 18 21 5 4 0 | 0 7 19 37 41 12 16 0 | 0 2 5 17 28 4 5 0 | 4 44 117 292 245 86 55 0 | 1 6 7 18 21 7 4 0 | 0 1 7 13 10 1 3 0 | 1 20 32 63 69 12 8 2 |
| Ethnic group White Black Yellow Mixed* Indigenous Unknown | 38 6 1 16 0 6 | 14 4 1 11 0 31 | 73 26 0 17 0 16 | 28 9 0 16 1 7 | 396 100 2 198 0 147 | 30 6 0 14 0 14 | 23 5 1 4 0 2 | 129 22 1 38 1 16 |
Source: Ministério da Saúde8. The age groups arare presented in years. *Mixed ethnicity (mixture of white and black).
Table 3. Detection rate, year with the highest number of occurrences, and municipalities with the highest number of notifications or without notification of the American Tegumentary Leishmaniasis (ATL) in different regions of the state of Rio de Janeiro, Brazil, from 2004 to 2013.
| Regions | Detection rate 2004-2013/100,000 inhabitants | Year with the highest number of occurrences (Number/%) | Municipalities with the highest number of notifications 2004-2013 (Number/%) | Municipalities without notification 2004-2013 |
| Northwestern | 0.01-0.01 | 2006 (22/32.83) | Itaperuna (22/32.83) | Aperibé Miracema São José de Ubá |
| Northern | 0.00-0.01 | 2006 (18/29.50) | São Fidélis (41/67.21) | Carapebus Cardoso Moreira Quissamã São João da Barra |
| Mountain | 0.03-0.03 | 2005 (32/22.24) | Trajano de Morais (27/20.45) | Duas Barras |
| Mid-southern | 0.12-0.01 | 2004 (19/31.14) | Três Rios (15/24.59) | Paty de Alferes |
| Metropolitan | 0.78-0.09 | 2005 (222/26.33) | Rio de Janeiro (740/87.78) | Itaboraí Queimados São Gonçalo Tanguá |
| Coastal Lowland | 0.05-0.01 | 2005 (15/23.43) | Saquarema (16/25.00) | Armação dos Búzios Arraial do Cabo Cabo Frio Casimiro de Abreu Iguaba Grande Silva Jardim |
| Middle Paraíba | 0.02-0.01 | 2005 (10/28.57) | Barra Mansa (13/37.14) | Itatiaia Porto Real Quatis |
| Costa Verde | 0.40-0.00 | 2004 (62/29.95) | Angra dos Reis (104/50.24) | None |
Source: Ministry of Health (Ministério da Saúde) 8 .
Table 4. Coefficient of detection data of the American Tegumentary Leishmaniasis (ATL) per municipality where there were notifications, according to the regions of the state of Rio de Janeiro, from 2004 to 2013.
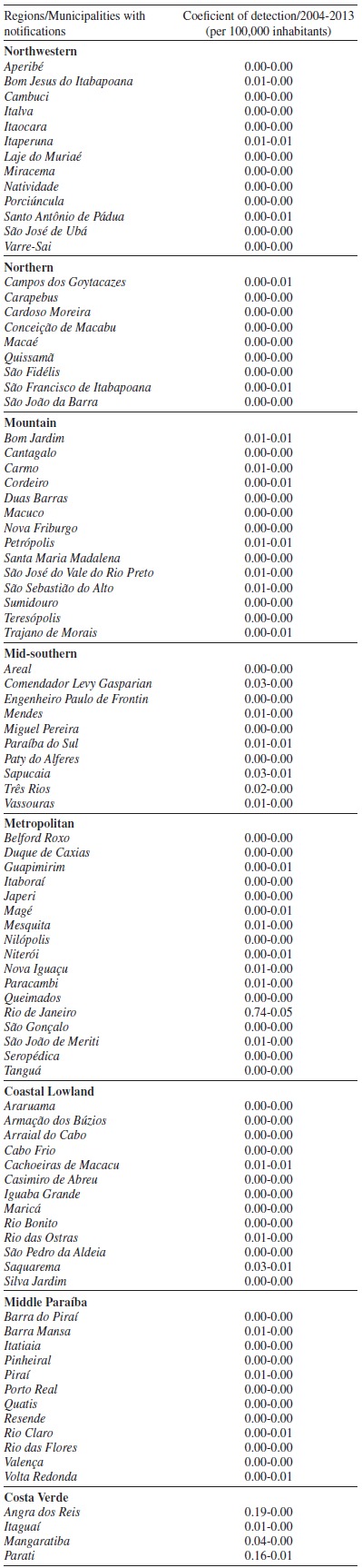
Source: Ministry of Health (Ministério da Saúde) 8 .
DISCUSSION
The lower incidence of the mucosal form of ATL can be explained by its post-cutaneous origin, which compromises only 3% of the infected individuals with the cutaneous form, and can manifest concomitantly or even a few decades later 4 , 20 , 21 . Curti et al. 22 and Zea et al. 23 observed previous cutaneous lesions in patients with the mucosal form of ATL.
We did not find in the literature information on the association of the clinical form of ATL and the occurrence of deaths. We did find, though, studies that related deaths to the toxicity of the medication, quality of the medical service, and secondary infections, among other causes 4 , 24 , 25 . The higher number of deaths, in the age group above 70 years old, could be assigned to the cardiotoxic action of the first-choice drugs: pentavalent antimony compounds. Some authors reported that elderly people with comorbidities were the most susceptible to the side effects of the drug and the complications of the disease. A prolonged administration can frequently lead to the development of heart conditions in elderly people 26 , 27 , 28 .
Data from the Ministry of Health informed that males represented 74% of ATL cases in the country, in a study carried out from 1980 to 2005. Several studies confirmed that everyday tasks carried out by males close to forests or their incursion into forests to look for firewood, hunt, fish or to engage in other activities are the factors that contribute to a greater male predisposition to this zoonosis 29 , 30 , 31 , 32 , 33 .
Several authors reported the age group of 20-59 years old as the most affected by the disease and justified it claiming that this is age group of adult people that go to work 22 , 32 , 34 , 35 , 36 , 37 . The lower occurrence of ATL in population extremes, such as children and people over 60 years old, is explained by the lower transmission of infection in peridomestic or domestic environments, as the daily activities of those age groups are more concentrated in the household 38 , 39 , 40 , 41 .
We found little information in the literature to justify the higher incidence of the disease in the white ethnic group, which was observed in most investigations 25 , 42 , 43 . However, data from the Inter-Union Department of Statistics and Socio-Economical Studies, in the manuscript entitled "Racial inequality in labor market" reported the occurrence of 16.1% of infection in white and 17.5% in black people exercising agricultural and livestock raising activities, in a universe of 91,298,042 white and 10,554.336 black Brazilian residents in that year 44 , 45 . Oliveira et al. 46 reported that in a workforce of 22,218,280 white and 3,636,821 black residents in Brazil, 8.7% and 20.4%, respectively, reported working in agricultural, livestock raising, and extractive activities. IBGE data reported that among 190,755,799 million inhabitants in Brazil in 2010, out of which 91,000,000 million were classified as white and 15,000,000 million as black, 10,829,000 white and 2,160,000 black inhabitants lived in rural areas 47 . As leishmaniasis is more frequent in those who live close to forests, we believe to see in those statistics a higher occurrence of leishmaniasis according to ethnic groups.
Kawa & Sabroza 48 reported that the higher number of ATL cases in the metropolitan region could be explained by the presence of the two largest tropical forests in the world within an urban area: the Pedra Branca State Park and the Tijuca Forest. Those forests are huge parasite and vector reservoirs, where ATL found a suitable and favorable environment to develop.
The Brazilian Information System of Notifiable Diseases plays an important role as a source of health information in the country. However, the system may be not entirely accurate to determine the occurrence of ATL in the state, mainly due to sub-notification, incorrect diagnoses, and unapparent forms of the disease.
The present study listed some problems concerning the data available at TABNET (DATASUS), such as incomplete notifications, typos, and imprecise records. Despite those problems, the software is excellent, as it allows researchers to carry out investigations at reduced costs using computers, population-based studies at a national scope, with great easiness and agility to access health/epidemiological information.
We conclude that there was a decrease in the number of cases of the American tegumentary leishmaniasis in the state of Rio de Janeiro, based on a reduction of the coefficient of detection from 1.44/100,000 inhabitants in 2004 to 0.20/100,000 in 2013. Regarding the spatiotemporal distribution, the study showed that the metropolitan region (843 cases) and the municipality of Rio de Janeiro (740 cases) were the localities with the highest occurrence of ATL in the study period. In 2005, the highest incidence (351 cases) of the disease was observed. Regarding the variables chosen to describe the epidemiology of the disease in the state, we observed that the cutaneous clinical form, males, the white ethnic group, and the age group of 20-39 years old are the most susceptible to develop the disease.
Epidemiological studies such as ours are important, as they help the health professionals and researchers to improve their studies, making them question the real situation of the disease, and contributing to the planning of control strategies and the establishment of public health governmental policies.
ACKNOWLEDGMENTS
The authors are grateful to the Coordination for the Improvement of Higher Education Personnel (CAPES) for the fellowship awarded to Gilmar Ferreira Vita.
REFERENCES
- 1.World Health Organization Leishmaniasis: fact sheet. [2016]. 2016. Available from : http://www.who.int/mediacentre/factsheets/fs375/en/
- 2.Pontes F. Doenças negligenciadas ainda matam 1 milhão por ano no mundo. Inov Pauta. 2009;6:69–73. [Google Scholar]
- 3.Gontijo B, Carvalho ML. Leishmaniose tegumentar americana. Rev Soc Bras Med Trop. 2003;36:71–80. doi: 10.1590/s0037-86822003000100011. [DOI] [PubMed] [Google Scholar]
- 4.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica . Atlas de leishmaniose tegumentar americana: diagnóstico clínico e diferencial. Brasília: Ministério da Saúde; 2006. [Google Scholar]
- 5.Palheta FX, Neto, Rodrigues AC, Silva LL, Palheta AC, Rodrigues LG, Silva FA. Otorhinolaryngologic manifestations relating american tegumentary leishmaniasis: literature review. Int Arch Otorhinolaryngol. 2008;12:531–537. [Google Scholar]
- 6.Sampaio RN, Sampaio JH, Marsden PD. Pentavalent antimonial treatment in mucosal leishmaniasis. Lancet. 1985;1:1097–1097. doi: 10.1016/s0140-6736(85)92393-1. [DOI] [PubMed] [Google Scholar]
- 7.Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde. Departamento de Atenção Básica . Vigilância em saúde: zoonoses. Brasília: Ministério da Saúde; 2009. [Google Scholar]
- 8.Ministério da Saúde Sistema de Informação de Agravos de Notificação. [2016]. 2016. Available from: http://sinan.saude.gov.br/sinan/login/login.jsf.
- 9.d'Utra e Silva O. Sobre a leishmaniose tegumentar e seu tratamento. Mem Inst Oswaldo Cruz. 1915;7:213–248. [Google Scholar]
- 10.Cardoso PG, Souza MB, Sanavria A, Meira AM, Meródio JC. Flebótomos de áreas com ocorrências de casos humanos de leishmaniose tegumentar americana no Município de Seropédica, Estado do Rio de Janeiro. Rev Soc Bras Med Trop. 2009;42:146–150. doi: 10.1590/s0037-86822009000200010. [DOI] [PubMed] [Google Scholar]
- 11.Madeira MF, Serra CM, Uchôa CM, Duarte R, Cruz DA, Perdomo CC. Leishmaniose canina: avaliação sorológica de 310 cães na região de Itaipu, Rio de Janeiro. Cad Saude Publica. 2000;16:568–568. doi: 10.1590/s0102-311x2000000200028. [DOI] [PubMed] [Google Scholar]
- 12.Mattos DG, Jr, Pinheiro JM, Menezes RC, Costa DA. Aspectos clínicos e de laboratório de cães soropositivos para leishmaniose. Arq Bras Med Vet Zootec. 2004;56:119–122. [Google Scholar]
- 13.Pereira MA, Távora MP, Vita GF, Silva VL. Diagnóstico sorológico pelo método de imunofluorescência indireta (IFI) para detecção de anticorpos anti-leishmania sp., em cães errantes do município de Campos dos Goytacazes, estado do Rio de Janeiro, no período de 2000 a 2001, após surgimento de caso humano autóctone. Ars Vet. 2008;24:177–180. [Google Scholar]
- 14.Santos GP, Sanavria A, Marzochi MC, Santos EG, Silva VL, Pacheco RS, et al. Prevalência da infecção canina em áreas endêmicas de leishmaniose tegumentar americana, do município de Paracambi, Estado do Rio de Janeiro, no período entre 1992 e 1993. Rev Soc Bras Med Trop. 2005;38:161–166. doi: 10.1590/s0037-86822005000200007. [DOI] [PubMed] [Google Scholar]
- 15.Souza MB, Carvalho RW, Machado RN, Wermelinger ED. Flebotomíneos de áreas com notificações de casos autóctones de leishmaniose visceral canina e leishmaniose tegumentar americana em Angra dos Reis, Rio de Janeiro, Brasil. Rev Bras Entomol. 2009;53:147–150. [Google Scholar]
- 16.Fundação Centro Estadual de Estatísticas, Pesquisas e Formação de Servidores Públicos do Rio de Janeiro Estado do Rio de Janeiro : divisão político-administrativa. [2016]. 2016. Available from http://www.fesp.rj.gov.br/ceep/info_territorios/divis_politico_administrativo.html.
- 17.Instituto Brasileiro de Geografia e Estatística . Estados@. Rio de Janeiro: 2013. http://www.ibge.gov.br/estadosat/perfil.php?sigla=rj [Google Scholar]
- 18.Instituto Brasileiro de Geografia e Estatística Estatísticas : projeção da população. [2016]. 2016. Available from: http://downloads.ibge.gov.br/downloads_estatisticas.htm?caminho=Projecao_da_Populacao.
- 19.SPSS Statistics: version 19.0. Chicago: IBM: SPSS Inc; 2010. [Google Scholar]
- 20.Lessa MM, Lessa HA, Castro TW, Oliveira A, Scherifer A, Machado P, et al. Mucosal leishmaniasis: epidemiological and clinical aspects. Braz J Otorhinolaryngol. 2007;73:843–847. doi: 10.1016/S1808-8694(15)31181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendonça SC, Souza WJ, Nunes MP, Marzochi MC, Coutinho SG. Indirect immunofluorescence test in New World leishmaniasis: serological and clinical relationship. Mem Inst Oswaldo Cruz. 1988;83:347–355. doi: 10.1590/s0074-02761988000300012. [DOI] [PubMed] [Google Scholar]
- 22.Curti MC, Silveira TG, Arraes SM, Bertolini DA, Zanzarini PD, Venazzi EA, et al. Aspectos epidemiológicos da Leishmaniose Tegumentar Americana na região Noroeste do Estado do Paraná. Rev Cienc Farm Basica Apl. 2009;30:63–68. [Google Scholar]
- 23.Zea DF, Prager M, Figueroa RA, Miranda MC. Complicación mucosa de la leishmaniasis cutánea. Biomedica. 2009;29:9–11. [PubMed] [Google Scholar]
- 24.Lima MV, Oliveira RZ, Lima AP, Cerino DA, Silveira TG. Leishmaniose cutânea com desfecho fatal durante tratamento com antimonial pentavalente. An Bras Dermatol. 2007;82:269–271. [Google Scholar]
- 25.Passos VM, Barreto SM, Romanha AJ, Krettli AU, Volpini AC, Gontijo CM, et al. Leishmaniose tegumentar na Região Metropolitana de Belo Horizonte: aspectos clínicos, laboratoriais, terapêuticos e evolutivos (1989-1995) Rev Soc Bras Med Trop. 2001;34:5–12. doi: 10.1590/s0037-86822001000100002. [DOI] [PubMed] [Google Scholar]
- 26.Nunes CS, Yoshizawa JK, Oliveira RZ, Lima AP, Oliveira LZ, Lima MV. Leishmaniose mucosa: considerações epidemiológicas e de tratamento. Rev Bras Med Fam Comunidade. 2011;6:52–56. [Google Scholar]
- 27.de Paula CD, Sampaio JH, Cardoso DR, Sampaio RN. Estudo comparativo da eficácia de isotionato de pentamidina administrada em três doses durante uma semana e de N-metil-glucamina 20mgSbV/Kg/dia durante 20 dias para o tratamento da forma cutânea da Leishmaniose tegumentar americana. Rev Soc Bras Med Trop. 2003;36:365–371. [PubMed] [Google Scholar]
- 28.Kopke LF, Café ME, Neves LB, Scherrer MA, Pinto JM, Souza MS, et al. An Bras Dermatol. 1993;68:259–261. [Google Scholar]
- 29.Antonildes NA, Jr, Silva O, Moraes JL, Nascimento FR, Pereira YN, Costa JM, et al. Foco emergente de leishmaniose tegumentar (LT) no entorno do Parque Nacional dos Lençóis Maranhenses, Nordeste, Brasil. Gaz Med Bahia. 2009;79(3):103–109. [Google Scholar]
- 30.de Castro EA, Soccol VT, Membrive N, Luz E. Estudo das características epidemiológicas e clínicas de 332 casos de leishmaniose tegumentar notificados na região norte do Estado do Paraná de 1993 a 1998. Rev Soc Bras Med Trop. 2002;35:445–452. [PubMed] [Google Scholar]
- 31.de Lima MV, Oliveira RZ, de Lima AP, Felix ML, Silveira TG, Rossi RM, et al. Atendimento de pacientes com leishmaniose tegumentar americana: avaliação nos serviços de saúde de municípios do noroeste do Estado do Paraná, Brasil. Cad Saude Publica. 2007;23:2938–2948. doi: 10.1590/s0102-311x2007001200015. [DOI] [PubMed] [Google Scholar]
- 32.Martins LM, Rebêlo JM, dos Santos MC, Costa JM, da Silva AR, Ferreira LA. Ecoepidemiologia da leishmaniose tegumentar no Município de Buriticupu, Amazônia do Maranhão, Brasil, 1996 a 1998. Cad Saude Publica. 2004;20:735–743. doi: 10.1590/s0102-311x2004000300010. [DOI] [PubMed] [Google Scholar]
- 33.Ministério da Saúde. Secretaria de Vigilância em Saúde . Manual de vigilância da leishmaniose tegumentar americana. 2ª ed. Brasília: Ministério da Saúde; 2007. [2016]. Available from: http://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_leishmaniose_2ed.pdf. [Google Scholar]
- 34.Guerra JA, Ribeiro JA, Coelho LI, Barbosa MG, Paes MG. Epidemiologia da leishmaniose tegumentar na Comunidade São João, Manaus, Amazonas, Brasil. Cad Saude Publica. 2006;22:2319–2327. doi: 10.1590/s0102-311x2006001100006. [DOI] [PubMed] [Google Scholar]
- 35.Lima AP, Minelli L, Teodoro U, Comunello E. Tegumentary leishmaniasis distribution by satellite remote sensing imagery, in Paraná State, Brazil. An Bras Dermatol. 2002;77:681–692. [Google Scholar]
- 36.Nogueira LS, Sampaio RN. Estudo hospitalar da leishmaniose tegumentar americana (LTA): epidemiologia e tratamento. An Bras Dermatol. 2001;76:51–62. [Google Scholar]
- 37.Silva LM, Cunha PR. A urbanização da leishmaniose tegumentar americana no município de Campinas - São Paulo (SP) e região: magnitude do problema e desafios. An Bras Dermatol. 2007;82:515–519. [Google Scholar]
- 38.Naiff RD, Jr, Pinheiro FG, Naiff MF, Souza IS, Castro LM, Menezes MP, et al. Estudo de uma série de casos de leishmaniose tegumentar americana no município de Rio Preto da Eva, Amazonas, Brasil. Rev Patol Trop. 2009;38:103–114. [Google Scholar]
- 39.Luz ZM, Pimenta DN, Cabral AL, Fiúza VO, Rabello A. A urbanização das leishmanioses e a baixa resolutividade diagnóstica em municípios da Região Metropolitana de Belo Horizonte. Rev Soc Bras Med Trop. 2001;34:249–254. [PubMed] [Google Scholar]
- 40.Santiago MC, Maciel FL, Fraga CA, Pereira MG, Horta FM, Botelho AC. Leishmaniose tegumentar americana: estudo epidemiológico entre crianças e adolescentes. Rev Mult FIPMoc. 2012;15(Supl):45–50. [Google Scholar]
- 41.Monteiro WM, Neitzke HC, Lonardoni MV, Silveira TG, Ferreira ME, Teodoro U. Distribuição geográfica e características epidemiológicas da Leishmaniose tegumentar americana em áreas de colonização antiga do estado do Paraná, Sul do Brasil. Cad Saude Publica. 2008;24:1291–1303. doi: 10.1590/s0102-311x2008000600010. [DOI] [PubMed] [Google Scholar]
- 42.Garcia FC, Santos SS, Chociay MF, Medeiros AC, Roselino AM. Métodos subsidiários para o diagnóstico da Leishmaniose tegumentar americana (LTA): comparação dos resultados do seqüenciamento de DNA e da PCR-RFLP para determinação da espécie de leishmania em amostras cutâneo-mucosas. An Bras Dermatol. 2005;80(3):S339–S344. [Google Scholar]
- 43.Rodrigues AM, Hueb M, Santos TA, Fontes CJ. Fatores associados ao insucesso do tratamento da leishmaniose cutânea com antimoniato de meglumina. Rev Soc Bras Med Trop. 2006;39:139–145. doi: 10.1590/s0037-86822006000200001. [DOI] [PubMed] [Google Scholar]
- 44.Departamento Intersindical de Estatística e Estudos Socioeconômicos . A desigualdade racial no mercado de trabalho. São Paulo: Dieese; 2002. [Google Scholar]
- 45.Instituto Brasileiro de Geografia e Estatística . Censo Demográfico 2000 : características gerais da população, resultados da amostra, tabelas de resultados. [2016]. 2016. Available from: http://www.ibge.gov.br/home/estatistica/populacao/censo2000/populacao/cor_raca_Censo2000.pdf. [Google Scholar]
- 46.Oliveira LE, Porcaro RM, Araújo TC. O lugar do negro na força de trabalho. Rio de Janeiro: IBGE; 1985. [Google Scholar]
- 47.Instituto Brasileiro de Geografia e Estatística . Censo demográfico 2010: características gerais da população, religião e pessoas com deficiência. [2016]. 2016. Available fromt: http://biblioteca.ibge.gov.br/visualizacao/periodicos/94/cd_2010_religiao_deficiencia.pdf. [Google Scholar]
- 48.Kawa H, Sabroza PC. Espacialização da leishmaniose tegumentar na cidade do Rio de Janeiro. Cad Saude Publica. 2002;18:853–865. doi: 10.1590/s0102-311x2002000300034. [DOI] [PubMed] [Google Scholar]


