Abstract
Pathophysiological conditions such as cerebral ischemia trigger the production of new neurons from the neurogenic niche within the subgranular zone (SGZ) of the dentate gyrus. The functional significance of ischemia-induced neurogenesis is believed to be the regeneration of lost cells, thus contributing to post-ischemia recovery. However, the cell signaling mechanisms by which this process is regulated are still under investigation. Here, we investigated the role of mitogen and stress-activated protein kinases (MSK1/2) in the regulation of progenitor cell proliferation and neurogenesis after cerebral ischemia. Using the endothelin-1 model of ischemia, wild type (WT) and MSK1−/−/MSK2−/− (MSK dKO) mice were injected with BrdU and sacrificed 2 days, 4 weeks, or 6 weeks later for the analysis of progenitor cell proliferation, neurogenesis, and neuronal morphology, respectively. We report a decrease in SGZ progenitor cell proliferation in MSK dKO mice compared to WT mice. Moreover, MSK dKO mice exhibited reduced neurogenesis and a delayed maturation of ischemia-induced newborn neurons. Further, structural analysis of neuronal arborization revealed reduced branching complexity in MSK dKO compared to WT mice. Taken together, this dataset suggests that MSK1/2 plays a significant role in the regulation of ischemia-induced progenitor cell proliferation and neurogenesis. Ultimately, revealing the cell signaling mechanisms that promote neuronal recovery will lead to novel pharmacological approaches for the treatment of neurodegenerative diseases such as cerebral ischemia.
Introduction
Cerebral ischemia causes widespread neuronal degeneration, particularly in cortical and limbic regions, such as the hippocampus (Benveniste et al., 1984, Mattson et al., 1989). Given that hippocampal neuronal degeneration after ischemia leads to long-term functional and cognitive impairments (Rempel-Clower et al., 1996), there has been a longstanding interest in studying potential mechanisms of hippocampal neuronal repair. Importantly, some of the hippocampal vulnerability to cell death is mitigated by local neurogenesis: a process that is sustained throughout adulthood and is induced by ischemic injury (Jin et al., 2001, Yagita et al., 2001, Türeyen et al., 2004). Indeed, neural stem cells (NSCs) in the dentate gyrus subgranular zone (SGZ) are capable of regenerating a small, but significant, portion of the cell loss through ischemia-induced production of new neurons (Kee et al., 2001, Bendel et al., 2005). The majority of these immature neurons migrate to the granule cell layer, and integrate into the existing hippocampal network (Emsley et al., 2005). Importantly, interventions that increase post-ischemic neurogenesis promote greater functional and cognitive recovery (Chu et al., 2004, Matsumori et al., 2006b), while attenuation of neurogenesis has been shown to worsen post-ischemic recovery (Raber et al., 2004).
Given the inducible nature of SGZ neural progenitor proliferation, a good deal of effort has been focused on understanding the kinase signaling events that regulate this process. Consistent with this, an examination of the p42/44 mitogen activated protein kinase (MAPK) cascade (Wu et al., 2000b) in the SGZ progenitor cell population has revealed that this pathway is activated within minutes following ischemic and other brain insults, and that it plays a key role in regulating inducible cell proliferation (Okuyama et al., 2004, Choi et al., 2008, Tian et al., 2009, Li et al., 2010).
The MAPK cascade is formed by a three kinase signaling cassette, with the phospho-activation of extracellular signal-regulated kinases 1 and 2 (collectively referred to as ERK) (Chen et al., 1992) serving as its functional endpoint. Activation of MAPK occurs in response to extracellular stress signals produced by cerebral ischemia (Sugino et al., 2000, Lennmyr et al., 2002). Specifically, ERK phosphorylation occurs in response to growth factors, oxidative stress, glutamate receptor activation and cytokine release (Nozaki et al., 2001). These factors induce rapid activation (phosphorylation) of the MAPK/ERK pathway, measurable within minutes following cerebral ischemia (Wu et al., 2000a, Ferrer et al., 2003). Following phosphorylation, ERK translocates from the cytosol to the nucleus where it regulates transcription via the direct targeting of transcription factors, and via the actuation of effector kinase pathways. In line with this idea, our recent work has indicated that much of the proliferative capacity of MAPK signaling in the SGZ (Okuyama et al., 2004, Choi et al., 2008, Tian et al., 2009) is mediated by the ERK effector kinases mitogen and stress-activated kinases MSK1 and MSK2 (hereafter referred to as MSK1/2). MSK1/2 are a nuclear-localized family of serine/threonine kinases that tightly regulate the transactivational potential of numerous transcription factors (Deak et al., 1998, Wiggin et al., 2002, Hauge and Frödin, 2006). We recently reported that both basal and seizure-evoked progenitor cell proliferation, as well as adult neurogenesis, are disrupted in MSK1/2 null mice (Choi et al., 2012). In a separate line of work, the deletion of MSK1 was found to impair environmental enrichment-induced hippocampal neuron plasticity, as well as cognition (Karelina et al., 2012). Given the role that MSK1/2 plays in progenitor cell proliferation and neuronal plasticity, the experiments described here were designed to specifically assess the regulatory role of MSK1/2 in ischemia-induced neurogenesis. Using the endothelin-1 (ET-1) model of transient cerebral ischemia, we report a reduction in ischemia-induced progenitor cell proliferation in MSK1−/−/MSK2−/− mice (hereafter referred to as “MSK dKO”) compared to wild type mice. Moreover, our analysis of immature newborn neurons indicates that MSK dKO mice exhibit impaired neuronal development after ischemia. Finally, the dendritic branching complexity of mature neurons was reduced in MSK dKO mice compared to WT. Taken together, our dataset reveals that MSK1/2 signaling is critical for the regulation of ischemia-induced progenitor cell proliferation, neuronal differentiation, and neurite outgrowth.
Experimental Procedures
Generation of MSK1−/−/MSK2−/− mice
Double knockout (MSK dKO) and wild type (WT) mice were generated by crossing MSK1−/+/MSK2−/+ heterozygous mice (kindly provided by Dr. J. Simon Arthur; University of Dundee, Dundee, Scotland). Mice were backcrossed into a C57/bl6 line for over 10 generations. A subset of WT and MSK dKO mice were crossed with a line of transgenic mice expressing GFP under the Thy1 promoter (Thy1-GFP mice were kindly provided by Dr. Gouping Feng; Duke University). Mouse genotype was confirmed using tail DNA samples subjected to PCR amplification as previously described (Karelina et al., 2012).
Stereotaxic surgery and endothelin-1 infusion
Adult WT and MSK dKO mice (6–9 weeks old/mixed sex) were anesthetized with an intraperitoneal (i.p.) injection of ketamine (95.2 mg/kg) and xylazine (30.8 mg/kg) approximately 15 minutes before surgery. The scalp was cleaned and sterilized and ointment was applied to the eyes. Mice were then placed in the stereotaxic apparatus (Cartesian Research, Inc), a single hole was drilled into the skull (coordinates AP - 2.06 mm; ML +1.30 mm), and the tip of a 5 μL syringe was then lowered −2.00 mm to the position above the upper blade of the dentate gyrus. Mice were infused with 0.5 μL of ET-1 (1μg/μL), a potent vasoconstrictor, or the vehicle saline, at the rate of 1 μL/minute (Karelina et al., 2014). The needle was left in place for 5 minutes before retraction and suturing. All mice were returned to their home cages and monitored for the duration determined by experimental conditions.
BrdU administration
Following ischemia induction via ET-1 infusion, newly generated cells were labeled via i.p. injections of 5-bromo-2′-deoxyuridine (BrdU: 50 mg/kg in saline, Sigma Aldrich). BrdU-positive cell analysis was performed at 2 days, 4 weeks, or 6 weeks post injury. For the 2 day time point, BrdU was injected 4 and 2 hours before sacrifice (Choi et al., 2008, Karelina et al., 2014). For the 4 and 6 week time points, BrdU was injected twice daily, 6 hours apart, on days 6, 7, and 8 after injury. A parallel BrdU injection protocol was used for saline-infused control animals.
Tissue processing
Two days, 4 weeks, or 6 weeks after the induction of ischemia (or control), mice were anesthetized with an overdose of ketamine/xylazine and sacrificed via transcardial perfusion of cold saline, followed by 4% paraformaldehyde. Brains were post-fixed in 4% paraformaldehyde overnight, then cryoprotected with 30% sucrose. Coronal sections (40 μm for the 2 day and 4 week tissue, 80μm for the 6 week tissue) through the hippocampus were prepared using a freezing microtome.
Immunolabeling
All immunohistochemical labeling was performed as previously described (Karelina et al., 2014). Briefly, sections were washed with phosphate buffered saline with 0.1% Triton-X (PBST), and blocked with 10% normal goat serum before overnight incubation at 4°C with rabbit anti-phosphorylated MSK1 (pMSK1, 1:250: Ser360, Cell Signaling, catalog # 9594). Sections were processed using the ABC staining method (Vector Labs) and visualized with nickel-intensified DAB (Vector Labs).
For immunofluorescent labeling for BrdU, and doublecortin or GFP, sections were incubated overnight in rat anti-BrdU (1:200, Accurate Chemical & Scientific Corp., catalog code YSRTMCA2060GA), goat anti-doublecortin (1:1000, Santa Cruz Biotech, catalog code SC-8066) or chicken anti-green fluorescent protein (1:2500, Abcam, catalog code AB13970). Primary antibody labeling was visualized with AlexaFluor 488 and 594 antibodies (1:500; Invitrogen). DraQ5 (1:5000; Biostatus Limited) was used as a counterstain for the imaging of hippocampal cell layers. Images were captured using a Zeiss 510 Meta confocal microscope. Cresyl violet staining was performed as recently reported (Karelina et al., 2014).
For pMSK1 and Sox2 immunofluorescent labeling, we used a biotinylated tyramide labeling approach. Briefly, tissue sections were washed PBST (3X), and then treated with 0.3% hydrogen peroxide (40 min). Tissue was then washed in PBST (3X), and blocked with 10% horse serum (HS: 1 hr). Next, tissue was incubated (overnight, 4°C) with a goat antibody against Sox-2 (1:1500, Santa Cruz Biochem, catalog code SC-17320) in 5% HS. Following our noted wash paradigm, tissue was incubated (2 hr) with a donkey anti-goat Alexa 594 antibody (1:500, Invitrogen, catalog code A11058) in 5% HS. Next, tissue was incubated (1 hr) in 10% normal goat serum (NGS), and then incubated (overnight, 4°C) with the noted primary pMSK1 antibody (1:100) in 5% NGS. Tissue was then incubated (2 hr, room temperature) with a goat anti-rabbit HRP-conjugated secondary antibody (1:500, Perkin Elmer, catalog code NEF812001EA) in 5% NGS. Tissue was then incubated (1 hr) with tyramide 488 (1:1000) in PBS containing 0.0015% hydrogen peroxide, 10 mM imidazole, 0.2% BSA and 0.05% Tween 20. Both the tyramide labeling and the preparation of the tyramide substrate followed the methodological approach outlined in Vize (2009). Lastly, tissue was counterstained with Draq5 as described above and images were captured using a Zeiss 510 Meta confocal microscope.
Cell quantitation
To quantify BrdU expression, photomicrographs were captured at a 10X magnification. Cells were counted unilaterally (ipsilateral to the infusion) in every 6th dorsal hippocampal section (defined as Bregma −1.70 through Bregma −2.06) spaced 120 μm apart, summed, and multiplied by 6. The SGZ was defined as an approximately 50 μm-thick region between the hilus and GCL.
Dendritic morphometric analysis
Tissue double labeled for GFP and BrdU was used for dendritic morphology analysis. Neurons in the GCL (Bregma −1.46 mm through −2.54 mm) were selected for analysis based on the following criteria: 1) clear co-labeling of GFP/BrdU, 2) the presence of intact dendritic arbors, and 3) lack of obstruction by overlapping dendrites of neighboring cells. Once a cell was identified, a z-stack was obtained using a Zeiss 510 Meta confocal microscope and 3D reconstructions of the arborization were obtained using the Simple Neurite Tracer plugin (Longair et al., 2011) in the image processing package Fiji (Schindelin et al., 2012). Dendritic branching complexity was measured using Sholl analysis, by quantifying the number of intersections in each concentric ring, spaced 10 μm apart, and calculating the total dendritic length. Note that because only a small subpopulation of neurons express GFP (~ 10%: Feng et al., 2000), the probability of finding a neuron that co-labeled with BrdU and that met the established criteria was very low, therefore out of 10 animals per experimental condition, the morphometric analysis only includes data from 5–9 cells per condition. All analyses were performed by an experimenter blinded to genotyping and experimental conditions.
Neurosphere culture
The neurosphere culture was established using published methods (Pacey et al., 2006) with minor modifications, as previously described (Karelina et al., 2014). Briefly, the hippocampus dentate gyrus was microdissected from 5 week-old WT and MSK dKO mice, and digested with trypsin for 90 minutes at 37 °C. The tissue was then treated with a trypsin inhibitor (Roche), mechanically triturated, and plated as a single cell suspension at a density of 15 cells/μL in serum-free medium containing EGF and bFGF. Neurospheres were passaged every 7 days, and proliferation was quantified after the second passage. Neurosphere proliferation was quantified by calculating the total number of neurospheres and by profiling the neurosphere size distribution over 50 μm intervals (from 50–250 μm in diameter). All analyses were performed by an individual blinded to genotyping and experimental conditions. A total of three replicates were performed using 2–4 mice each.
Statistical Analysis
Immunohistochemistry, neurosphere proliferation, and dendritic length data comparisons were analyzed using an ANOVA, and specific group comparisons were determined via a post hoc Tukey analysis.
Results
Intrahippocampal endothelin-1 infusion and MSK1 activation
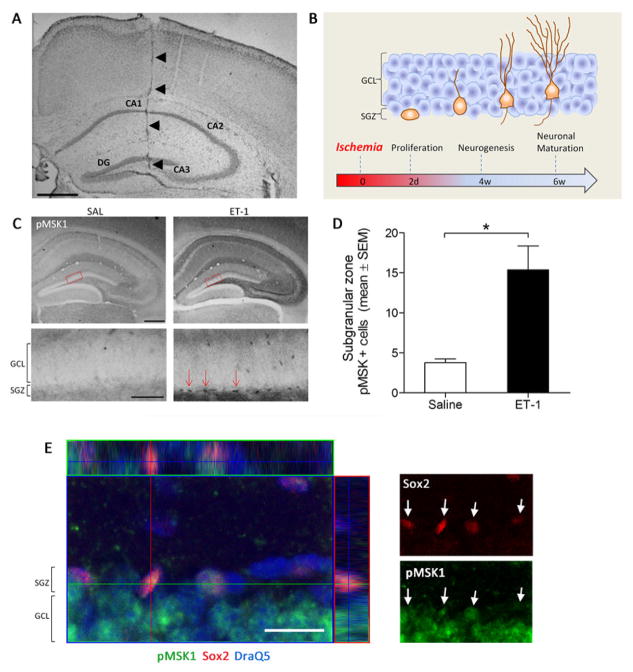
Transient hippocampal ischemia was induced via a single unilateral infusion of the potent vasoconstrictor ET-1 (Figure 1A). Our previous work validated that ET-1 leads to marked cell death within the hippocampus CA1, GCL and hilus at the 2-day post-infusion time point (Karelina et al., 2014). Depending on the experiment, post-ischemia tissue collection occurred at one of three time points: 2 days-post injury (2 DPI) for analysis of progenitor cell proliferation and MSK1 activation, 4 weeks-post injury for analysis of neurogenesis and newborn neuronal development, and 6 weeks-post injury for analysis of neuronal morphological maturation (Figure 1B). Initially, 2 DPI tissue was processed for the immunohistochemical detection of MSK1 activation via phosphorylation at Ser-360. Of note, the Ser-360 phosphorylation site on MSK1 is a direct target of ERK1/2, and is essential for its kinase activity (McCoy et al., 2005). Representative images presented in figure 1C reveal that ET-1 increased expression of phospho-MSK1 in the SGZ relative to the control, saline injection, condition. This interpretation is supported by quantitative analysis (Figure 1D), showing that ET-1 infusion induced a greater than 3-fold increase in SGZ phospho-MSK1 (pMKS1) expression. Further, fluorescent immunolabeling for pMSK1 and the progenitor cell marker Sox-2, indicated that the activated form of the kinase is expressed in progenitor cell populations of the SGZ at 2 DPI (Figure 1E). These data, coupled with prior work from our lab (Choi et al., 2012) showing MSK1 expression in SGZ progenitor cells, strongly suggest that ischemia triggers MSK1 activation in progenitor cells
Figure 1. Experimental timeline and ischemia-induced MSK1 activation.
A) Nissl staining showing needle track into the hippocampus dentate gyrus (DG). B) Animals were sacrificed 2 days, 4 weeks or 6 weeks after ischemia for analysis of progenitor cell proliferation, neurogenesis, and neuronal maturation, respectively. C) Ischemia induces phosphorylation of MSK1 in the DG subgranular zone (SGZ). Top image scale bar = 200μm, bottom image scale bar = 50μm. D) Quantification of the number of pMSK1-positive cells in the SGZ following ischemia (F1,10 = 7.199, p < 0.05). An asterisk (*) denotes a statistically significant difference between groups. E) Confocal Z-stack analysis of pMSK and Sox-2 labeling in the SGZ (2 days post ET-1 injection). Tissue was also labeled for DraQ5. An orthogonal Z-stack image is presented in the left panel. In the right panel, pMKS1 and Sox-2 labeling is presented separately; arrows denote pMSK1 and Sox-2 positive cells in the SGZ. Scale bar: 20 microns.
MSK1/2 regulation of progenitor cell proliferation
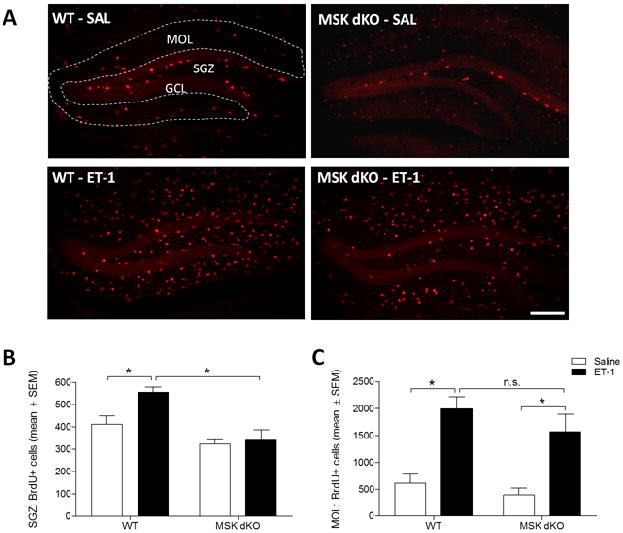
Next, we tested whether MSK1/2 plays a role in ischemia-evoked progenitor proliferation. To assess this possibility, hippocampal ischemia was induced in WT and MSK dKO mice, and BrdU was injected prior to sacrifice (2 DPI). Consistent with other reports (Takagi et al., 1999, Yagita et al., 2001, Nakatomi et al., 2002), immunohistochemical labeling for BrdU in the dentate gyrus revealed an increase in SGZ progenitor cell proliferation following ischemia in WT mice, compared to the saline controls. In striking contrast, ischemia did not increase the number of BrdU-positive cells in the SGZ of MSK dKO mice (Fig. 2A–B). It is important to note that the reduction of BrdU-positive cells in the SGZ of MSK dKO mice does not appear to be due to a global reduction of proliferation in these mice. Indeed, quantification of the total number of BrdU-positive cells in the molecular layer, which is likely a reflection of proliferating glial cells (Liu et al., 1997), revealed a comparable level of proliferation betweeen WT and MSK dKO mice after ET-1 treatment (Figure 2C). This dataset indicates that MSK1/2 plays a critical role in cell proliferation specifically among the pool of NSCs in the hippocampal SGZ.
Figure 2. Ischemia-induced progenitor cell proliferation.
Two days following ET-1 infusion, mice were injected with BrdU and sacrificed. A) Representative images of BrdU-labeled dentate gyrus. B) Quantitative analysis revealed that the number of BrdU-labeled cells in the SGZ is increased following ischemia in WT but not MSK dKO mice (F3,18 = 11.374, p < 0.05). C) The number of BrdU-positive cells in the molecular layer (MOL) is increased following ET-1 ischemia in both WT and MSK dKO mice (F3,18 = 10.189, p < 0.05). Scale bar = 200μm. Data are presented as mean ± SEM. An asterisk (*) denotes a statistically significant difference between groups.
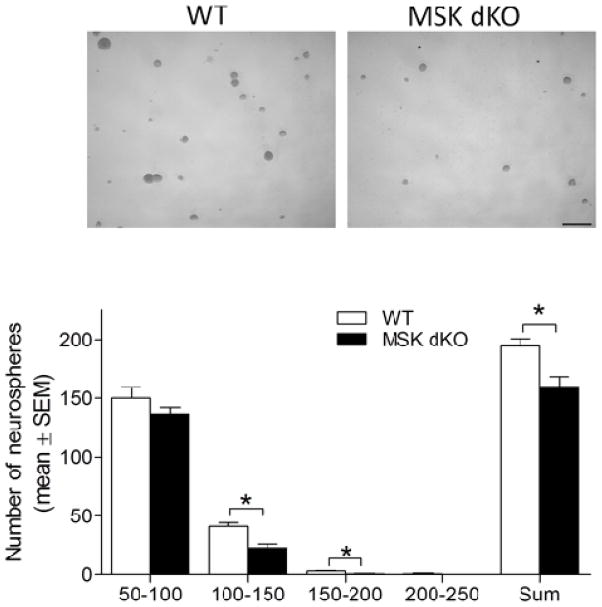
One limitation to the in vivo approach is that it is difficult to definitively conclude that the reduction in progenitor cell proliferation is specifically due to reduced MSK1/2 activation in stem/progenitor cells rather than in other CNS cell types that express MSK1/2 (Heffron and Mandell, 2005). Hence, it is conceivable that the progenitor proliferation phenotype in MSK dKO mice could result from a disruption of neuronal and/or glial cell trophic support to the NSCs of the SGZ. Thus, in order to determine whether MSK1/2 regulates progenitor cell proliferation in a cell autonomous manner, we used the neurosphere culture approach to isolate NSCs from WT and MSK dKO mice. NSCs were isolated from the dentate gyrus and grown in suspension for 7 days, and then proliferation was quantified by measuring the diameter and total number of neurospheres in each well (Figure 3). Our analysis revealed reduced NSC proliferation of MSK dKO neurospheres compared to WT. In particular, we detected fewer MSK dKO neurospheres that were 100–200 μm in diameter. SGZ-derived neurospheres typically do not reach larger sizes (i.e. >200 μm; Bonaguidi et al., 2008), and indeed were rarely detected in the present experiments. Taken together, these data indicate that MSK1/2 plays a critical role in the regulation of ischemia-induced progenitor cell proliferation. Moreover, the neurosphere assay indicates that this is a cell-autonomous effect. Of note, the reduction in the in vitro proliferation of MSK dKO progenitor cells is somewhat more subtle than was observed in the in vivo ischemia model. This raises the possibility that MSK activation in supporting cells (e.g., glia) could contribute to ischemia-evoked progenitor cell proliferation.
Figure 3. SGZ-derived neurosphere proliferation.
Adult NSCs were derived from the subgranular zone of WT and MSK dKO mice. A) Representative images of neurospheres. Scale bar = 300μm. B) Quantitative analysis of the number and diameter of neurospheres per well reflects reduced proliferation of neurospheres derived from MSK dKO mice (100–150 μm diameter F1,10 = 15.948, p < 0.05; 150–200 μm diameter F1,10 = 11.575, p < 0.05; total number of neurospheres F1,10 = 10.193, p < 0.05). Data are presented as mean ± SEM. An asterisk (*) denotes a statistically significant difference between groups.
MSK1/2 regulation of ischemia-induced neurogenesis
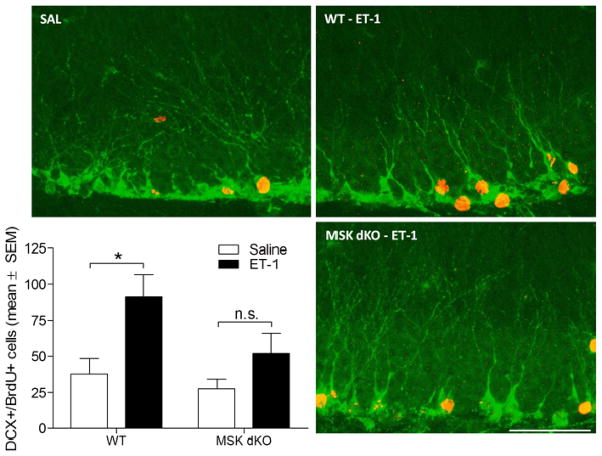
Given the role that MSK1/2 activation plays in post-ischemia hippocampal progenitor cell proliferation, we conducted additional experiments to determine whether MSK1/2 activity is also important for neuronal differentiation within a pathophysiological environment. An additional group of WT and MSK dKO mice were injected with BrdU on days 6, 7, and 8 after ET-1 or saline infusion, and sacrificed 4 weeks later. Hippocampal tissue was then immunolabeled for doublecortin (DCX: a marker of immature neurons) and BrdU. The time points for BrdU injections correspond to the greatest rate of post-ischemia neurogenesis (Takagi et al., 1999, Zhang et al., 2001). Note that because the BrdU injections occurred approximately 1 week following the infusion of ET-1/saline, the age of the newborn DCX-positive cells at the time of sacrifice is around 3 weeks. Based on the labeling pattern and timing, co-labeled (DCX/BrdU-positive) cells were indicative of immature neurons that were born after ischemia (Figure 4). Quantification of the total number of DCX/BrdU co-labeled cells indicates a significant increase in neurogenesis in WT mice following ET-1 infusion, compared to the saline controls. On the other hand, MSK dKO mice did not exhibit a significant increase in neurogenesis after ischemia. Importantly, the basal rate of neurogenesis (e.g. the saline group) was not significantly affected by the deletion of MSK1/2. Rather, it is inducible neurogenesis that appears to require MSK1/2 activation. Given the observation that MSK1/2 deletion reduces ischemia-induced proliferation (Figure 2), these data indicate that the significant reduction in neurogenesis is a consequence of an attenuated proliferative response in MSK dKO mice.
Figure 4. Ischemia-induced neurogenesis.
Post-ischemic neurogenesis was assessed 4 weeks following ET-1 or saline infusion. Newborn neurons were labeled immunohistochemically for doublecortin (green) and Brdu (red). Data are presented as the total number of BrdU-positive cells that are co-labeled with doublecortin. The number of newborn neurons is significantly increased in WT but not MSK dKO mice (F3,21 = 4.913, p<0.05). Scale bar = 50μm. Data are presented as mean ± SEM. An asterisk (*) denotes a statistically significant difference between groups, n.s. denotes that the group comparison is not significant.
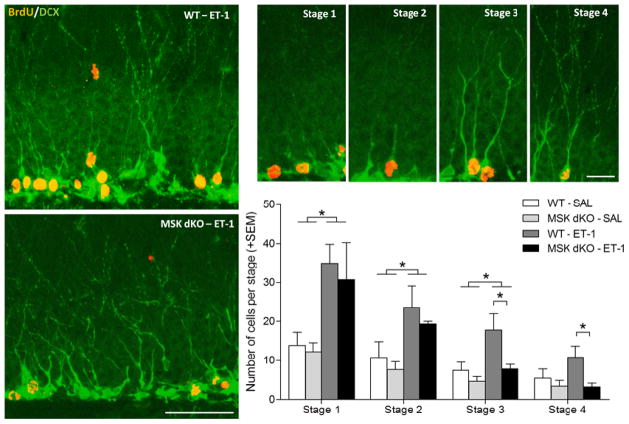
In addition to quantifying neurogenesis, we performed an extensive analysis of hippocampal neuronal development in both saline and ET-1 treated WT and MSK dKO mice. Using the same set of DCX/BrdU labeled tissues, co-labeled cells were qualitatively categorized into stages of neuronal development based on morphology (Figure 5). Stages were defined as previously reported (Choi et al., 2012). Briefly, stage 1 cells were defined as having no processes; stage 2 cells were defined as having short processes extending into the GCL; stage 3 cells were defined as those extending long processes into the molecular layer; and stage 4 cells were defined as having long processes and branches extending into the molecular layer. Our analysis revealed that following ET-1 treatment, WT and MSK dKO mice had similar numbers of stage 1 and 2 newborn neurons. On the other hand, MSK dKO mice exhibited significantly fewer late stage (stages 3 and 4) immature neurons as compared to WT mice following ischemia. Importantly, under control (saline) conditions, WT and MSK dKO mice exhibited similar numbers of newborn neurons at each stage analyzed. These data are consistent with the idea that MSK1/2 is part of a stimulus-dependent signaling cascade; as such, the effect of the MSK1/2 deletion on neuronal development is particularly evident under a pathophysiological condition. Taken together, these datasets indicate that MSK1/2 play a significant role in inducible neurogenesis and neuronal development.
Figure 5. Neuronal development following ischemia.
Four weeks following ET-1 or saline infusion, doublecortin and BrdU-positive co-labeled cells were assessed for stages of development. A post-hoc Tukey HSD analysis revealed a significant decrease in the number of stage 3 and 4 developing neurons following ischemia in MSK dKO mice compared to WT mice (F3,21 = 5.155, p < 0.05). Large image scale bar = 100μm, small images scale bar = 20 μm. Data are presented as mean ± SEM. An asterisk (*) denotes a statistically significant difference between groups.
Neuronal maturation following ischemia
Given that MSK1/2 appears to play an important role in neuronal development after ischemia, we conducted an additional set of experiments to determine the extent to which MSK1/2 may regulate dendritic morphology in mature neurons. The development of a transgenic mouse strain expressing GFP in a subpopulation of neurons under the control of the thy-1 promoter (Feng et al., 2000) provides an ideal model system for examining granule cell neuronal morphology. This transgenic line consistently drives robust GFP expression in the dendritic and axonal arbors of neurons and allows for accurate morphometric analysis of the structure of granule cell neurons (Danzer and McNamara, 2004). For these studies, WT and MSK dKO mice were initially crossed with Thy1-GFP transgenic mice. The GFP-expressing mice were then injected with BrdU on days 6, 7, and 8 after ET-1 or saline infusion, and sacrificed 6 weeks later. Hippocampal tissue was then labeled for GFP and BrdU expression, which allowed us to identify neurons that were born within a week of saline/ET-1 infusion (Figure 6A). This time course dates the newborn cells to around 5 weeks of age, which is consistent with the time it takes a newborn neuron to mature and integrate into the hippocampal network (Zhao et al., 2006). Of note, GFP-expressing neurons in this transgenic line do not express DCX, indicating that GFP is not expressed until neurons are mature (Walter et al., 2007).
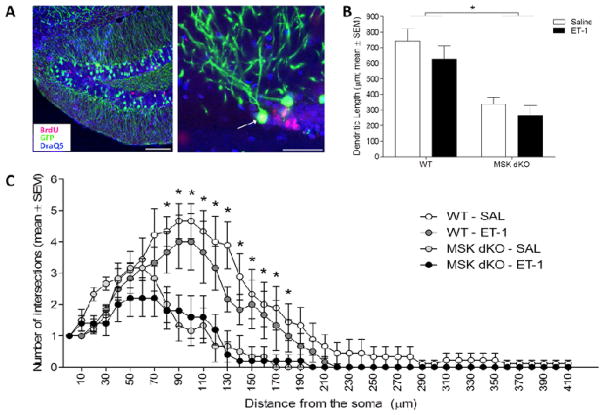
Figure 6. Maturation of ischemia-induced newborn neurons.
In order to assess morphological maturation of newborn neurons after ischemia, WT and MSK dKO mice were crossed with a transgenic line expressing green fluorescent protein (GFP) under the control of the Thy1 promoter. WT and MSK dKO Thy1-GFP expressing mice were sacrificed 6 weeks following ET-1 or saline infusion. Immunohistochemical labeling techniques were used to label GFP (green) and BrdU (red), DraQ5 was used as a nuclear counterstain (blue). A) Shown are representative images of cells that co-label for GFP and BrdU. B) The total dendritic length was significantly smaller in MSK dKO mice compared to WT (F1,24 = 29.015, p < 0.01), regardless of treatment condition. C) ANOVA tests performed at each Sholl interval revealed significant reductions in dendritic branching complexity in MSK dKO mice, regardless of treatment condition. Large image scale bar = 100μm, small images scale bar = 50 μm. Data are presented as mean ± SEM. An asterisk (*) denotes a statistically significant difference between genotypes (collapsed across treatment condition).
A previous analysis of GCL neurons from adult MSK dKO mice revealed reduced arborization in MSK dKO mice, as compared to WT mice (Karelina et al., 2012). Because MSK1/2 deletion also appears to slow the rate of development in immature neurons after ischemia (Figure 5), we hypothesized that in MSK dKO mice, mature neurons would exhibit further changes in arborization after ischemia. As previously reported, our analysis of GCL neurons revealed an overall reduction in dendritic length and branching complexity in MSK dKO mice compared to WT (Figure 6B–C). We did not observe any differences in arborization between the control and ischemia groups in either the WT or MSK dKO mice; however, the total number of neurons that could be analyzed in the MSK dKO/ischemia group is considerably lower than the other groups, suggesting that MSK1/2 may regulate the rate of neuronal maturation following ischemia. Additionally, although ischemia tends to produce ectopic migration of newborn neurons (Parent et al., 2002, Niv et al., 2012), we did not observe ectopic cells in any of the ET1-treated animals likely because this model yields a relatively mild ischemic injury in mice (Horie et al., 2008). This model of cerebral ischemia was deliberately chosen for this set of experiments because it maintains a permissive environment for neuronal development. Overall, our data confirm the previous finding that MSK1/2 is an important regulator of neuronal arborization.
Discussion
The birth and migration of new neurons in ischemic brain regions is a prominent example of an endogenous repair mechanism that persists into adulthood. While the functional relevance of hippocampal neurogenesis is becoming better understood, the cell signaling mechanisms that drive this process are still under investigation. Given that ischemia results in a rapid and sustained induction of ERK signaling (Nozaki et al., 2001), the goal of this set of experiments was to identify the role of the ERK effector kinases MSK1/2 in the regulation of ischemia-induced proliferation and neurogenesis. Our experiments reveal that MSK1 activation in the SGZ is indeed associated with ischemia-induced progenitor cell proliferation, as the deletion of MSK1/2 eliminates the increase in SGZ proliferation that is typically observed after ischemia in adult WT mice (Figure 2). Further, given that the neurosphere culture medium contains EGF and bFGF, growth factors that induce progenitor cell proliferation, our neurosphere data indicate that the role of MSK1/2 in this process is cell-autonomous (Figure 3). These data are both in-line with prior data from our lab showing that MSK1/2 couples seizure-activity to SGZ proliferation (Choi et al., 2012) and shed further light on mechanisms that drive the activity-dependent proliferative capacity of dividing cells.
We have previously reported a regulatory role of MSK1 in stimulus-induced progenitor cell proliferation using an environmental enrichment paradigm (Karelina et al, 2012). Environmental enrichment has been shown to promote progenitor cell proliferation and neurogenesis via mechanisms involving an upregulation of growth factors including BDNF, VEGF, FGF2, and NGF (Van Praag et al., 2000, During and Cao, 2006, Rossi et al., 2006). Importantly, similar mechanisms involving enhanced growth factor production regulate progenitor cell proliferation following cerebral ischemia (Kokaia and Lindvall, 2003). Moreover, both environmental enrichment and administration of growth factors (i.e. BDNF or VEGF) increase post-ischemia progenitor cell proliferation and neurogenesis in the SGZ and SVZ (Chen et al., 2005, Matsumori et al., 2006a). Given that growth factors function in large part via activation of the MAPK/ERK signaling cascade, our data on the role of MSK1 as a mediator of both environmental enrichment- (Karelina et al, 2012) and cerebral ischemia-induced progenitor cell proliferation indicate that this process is induced by the same mechanism.
The production of new neurons after ischemia plays a role in ameliorating the physiological and behavioral consequences of stroke. Indeed, the ablation of newborn neurons after ischemia increases the infarct volume and leads to greater functional deficits (Raber et al., 2004, Jin et al., 2010). An endogenous mechanism of repair includes ischemia-induced production of various growth factors, including NGF (Lee et al., 1998), bFGF (Lin et al., 1997), BDNF (Arai et al., 1996) and VEGF (Cobbs et al., 1998), which promote neurogenesis in part through ERK signaling-dependent mechanisms (Ménard et al., 2002, Xiao et al., 2007, Shioda et al., 2009). Consistent with this, the administration of compounds that stimulate ERK activation has been shown to further enhance neurogenesis after ischemia (Yan et al., 2007, Shioda et al., 2008, Shioda et al., 2009). Specifically, downstream of ERK, CREB activation has been shown to be a critical component of both ischemia-induced proliferation and survival of newborn cells (Zhu et al., 2004). Thus, because MSK1/2 is the principal kinase that couples ERK to CREB activation, it is ideally positioned to regulate the proliferative capacity of progenitors. Indeed, our data indicate that MSK1/2 deletion resulted in a cell autonomous reduction of neural stem cell proliferation (Figure 3). Here, we posit further that MSK1/2 is a critical component downstream of ERK that couples brain ischemia to neurogenesis. In support of this idea, we report reduced neurogenesis in the dentate gyrus of MSK dKO mice after ischemia (Figure 4). Additionally, an examination of the morphological development of the surviving newborn neurons revealed fewer late stage developing neurons in MSK dKO mice after ischemia (Figure 5). This is likely a function of both a smaller pool of proliferating progenitor cells and possibly a reduced rate of newborn cell survival (Choi et al., 2012). Further, it is important to note that the numbers of newborn neurons in early stages (1 and 2) are similar in WT and MSK dKO mice, whereas the numbers of late stage neurons (3 and 4) are reduced in MSK dKO mice following ischemia (Figure 5). Thus, MSK1/2 deletion may have reduced the rate of neuronal development after ischemia. Additional experiments using long-term live cell imaging techniques will be necessary to more definitively determine a role for MSK1/2 in the rate of cell development. Taken together, our data indicate that MSK1/2 signaling is critical for the proliferation, differentiation, and development of newborn neurons after ischemia.
Within approximately 4–7 weeks after birth, newborn hippocampal neurons begin to express mature neuronal markers, migrate into the GCL and develop extensive dendritic arborizations, spines and synapses, (Kempermann et al., 2004, Emsley et al., 2005). Once the new neurons are integrated into the existing hippocampal network, they are believed to be functionally indistinguishable from the surrounding cells (Jessberger and Kempermann, 2003). Data revealing a role for MSK1/2 in the regulation of GCL neuronal morphology (Karelina et al., 2012 and Figure 5), coupled with the well-characterized role of MSK1/2 to function in an activity-dependent manner, led us to speculate that ischemia would give rise to marked abnormalities in the dendritic morphology of MSK dKO neurons born into the post-ischemic environment. However, surprisingly, MSK dKO mice did not exhibit any change in dendritic morphology following ET-1 ischemia, relative to the control, MSK dKO, mice. Likewise, ischemia did not alter dendrite morphology of WT mice. With respect to the WT mice, this lack of an effect of ET-1 is similar to the findings of Niv et al (2012), who reported that a mild ischemic event induced via photothrombosis did not produce significant changes in the arborization patterns of newborn cells. In total, our data are consistent with a role of MSK1/2 as an important regulator of ischemia-evoked progenitor proliferation and neuronal maturation.
Conclusions
This dataset sheds light on a mechanism by which MAPK activation regulates injury-induced neurogenesis. Given the functional and cognitive benefits attributed to post-ischemia neurogenesis in animal models, there is now a great push to identify treatment options that may help to facilitate neurogenesis in stroke patients. Indeed, some clinical trials have initiated treatment options directed at enhancing neuronal survival (and presumably neurogenesis) after stroke, and have thus far demonstrated limited efficacy (Kondziolka et al., 2005, Ortega and Jolkkonen, 2013). A clearer understanding of the role of cellular signaling cascades in mediating stroke recovery may ultimately help guide the development of novel therapeutic options.
Highlights.
Cerebral ischemia induces progenitor cell proliferation in the dentate gyrus SGZ.
This study examined the role of the ERK effector kinase MSK1/2 in this process.
Cerebral ischemia induced MSK activation in SGZ neural stem cells.
Genetic deletion of MSK1/2 reduced ischemia-induced progenitor cell proliferation and neurogenesis
MSK1/2 deletion reduced neurosphere proliferation.
Acknowledgments
This work was supported by The American Heart Association Postdoctoral Fellowship number 11POST7410015: and the following National Institutes of Health Grants: NS066345, NS06740 and NS045758. The authors have declared that no competing interests exist.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arai S, Kinouchi H, Akabane A, Owada Y, Kamii H, Kawase M, Yoshimoto T. Induction of brain-derived neurotrophic factor (BDNF) and the receptor trk B mRNA following middle cerebral artery occlusion in rat. Neurosci Lett. 1996;211:57–60. doi: 10.1016/0304-3940(96)12720-8. [DOI] [PubMed] [Google Scholar]
- Bendel O, Bueters T, von Euler M, Ögren SO, Sandin J, von Euler G. Reappearance of hippocampal CA1 neurons after ischemia is associated with recovery of learning and memory. J Cerebr Blood F Met. 2005;25:1586–1595. doi: 10.1038/sj.jcbfm.9600153. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984;43:1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- Bonaguidi MA, Peng C-Y, McGuire T, Falciglia G, Gobeske KT, Czeisler C, Kessler JA. Noggin expands neural stem cells in the adult hippocampus. J Neurosci. 2008;28:9194–9204. doi: 10.1523/JNEUROSCI.3314-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. Journal of Cerebral Blood Flow & Metabolism. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Sarnecki C, Blenis J. Nuclear localization and regulation of erk-and rsk-encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Cho HY, Hoyt KR, Naegele JR, Obrietan K. IGF-1 receptor-mediated ERK/MAPK signaling couples status epilepticus to progenitor cell proliferation in the subgranular layer of the dentate gyrus. Glia. 2008;56:791–800. doi: 10.1002/glia.20653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Karelina K, Alzate-Correa D, Hoyt KR, Impey S, Arthur JS, Obrietan K. Mitogen-and stress-activated kinases regulate progenitor cell proliferation and neuron development in the adult dentate gyrus. J Neurochem. 2012;123:676–688. doi: 10.1111/jnc.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Kim M, Park K-I, Jeong S-W, Park H-K, Jung K-H, Lee S-T, Kang L, Lee K, Park D-K. Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res. 2004;1016:145–153. doi: 10.1016/j.brainres.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Cobbs CS, Chen J, Greenberg DA, Graham SH. Vascular endothelial growth factor expression in transient focal cerebral ischemia in the rat. Neurosci Lett. 1998;249:79–82. doi: 10.1016/s0304-3940(98)00377-2. [DOI] [PubMed] [Google Scholar]
- Danzer SC, McNamara JO. Localization of brain-derived neurotrophic factor to distinct terminals of mossy fiber axons implies regulation of both excitation and feedforward inhibition of CA3 pyramidal cells. J Neurosci. 2004;24:11346–11355. doi: 10.1523/JNEUROSCI.3846-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak M, Clifton AD, Lucocq JM, Alessi DR. Mitogen-and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426–4441. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During MJ, Cao L. VEGF, a mediator of the effect of experience on hippocampal neurogenesis. Current Alzheimer Research. 2006;3:29–33. doi: 10.2174/156720506775697133. [DOI] [PubMed] [Google Scholar]
- Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Friguls B, Dalfo E, Planas A. Early modifications in the expression of mitogen-activated protein kinase (MAPK/ERK), stress-activated kinases SAPK/JNK and p38, and their phosphorylated substrates following focal cerebral ischemia. Acta neuropathologica. 2003;105:425–437. doi: 10.1007/s00401-002-0661-2. [DOI] [PubMed] [Google Scholar]
- Hauge C, Frödin M. RSK and MSK in MAP kinase signalling. J Cell Sci. 2006;119:3021–3023. doi: 10.1242/jcs.02950. [DOI] [PubMed] [Google Scholar]
- Heffron D, Mandell JW. Differential localization of MAPK-activated protein kinases RSK1 and MSK1 in mouse brain. Mol Brain Res. 2005;136:134–141. doi: 10.1016/j.molbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Horie N, Maag A-L, Hamilton SA, Shichinohe H, Bliss TM, Steinberg GK. Mouse model of focal cerebral ischemia using endothelin-1. J Neurosci Meth. 2008;173:286–290. doi: 10.1016/j.jneumeth.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl A Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Greenberg DA. Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc Natl A Sci USA. 2010;107:7993–7998. doi: 10.1073/pnas.1000154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelina K, Alzate-Correa D, Obrietan K. Ribosomal S6 kinase regulates ischemia-induced progenitor cell proliferation in the adult mouse hippocampus. Exp Neurol. 2014;253:72–81. doi: 10.1016/j.expneurol.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelina K, Hansen KF, Choi Y-S, DeVries AC, Arthur JSC, Obrietan K. MSK1 regulates environmental enrichment-induced hippocampal plasticity and cognitive enhancement. Learn Memory. 2012;19:550–560. doi: 10.1101/lm.025775.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N, Preston E, Wojtowicz J. Enhanced neurogenesis after transient global ischemia in the dentate gyrus of the rat. Exp Brain Res. 2001;136:313–320. doi: 10.1007/s002210000591. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Lindvall O. Neurogenesis after ischaemic brain insults. Current opinion in neurobiology. 2003;13:127–132. doi: 10.1016/s0959-4388(03)00017-5. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Steinberg GK, Wechsler L, Meltzer CC, Elder E, Gebel J, DeCesare S, Jovin T, Zafonte R, Lebowitz J. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. Journal of neurosurgery. 2005;103:38–45. doi: 10.3171/jns.2005.103.1.0038. [DOI] [PubMed] [Google Scholar]
- Lee T-H, Kato H, Chen S-T, Kogure K, Itoyama Y. Expression of nerve growth factor and trkA after transient focal cerebral ischemia in rats. Stroke. 1998;29:1687–1697. doi: 10.1161/01.str.29.8.1687. [DOI] [PubMed] [Google Scholar]
- Lennmyr F, Karlsson S, Gerwins P, Ata K, Terent A. Activation of mitogen-activated protein kinases in experimental cerebral ischemia. Acta neurologica scandinavica. 2002;106:333–340. doi: 10.1034/j.1600-0404.2002.01313.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Peng Z, Xiao B, Houser CR. Activation of ERK by spontaneous seizures in neural progenitors of the dentate gyrus in a mouse model of epilepsy. Exp Neurol. 2010;224:133–145. doi: 10.1016/j.expneurol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TN, Te J, Lee M, Sun GY, Hsu CY. Induction of basic fibroblast growth factor (bFGF) expression following focal cerebral ischemia. Mol Brain Res. 1997;49:255–265. doi: 10.1016/s0169-328x(97)00152-6. [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Sharp F. BrdU uptake into dividing microglia/macrophages occurs in striatum prior to hippocampus following global ischemia in the gerbil. J Cerebr Blood F Met. 1997;17:422–422. [Google Scholar]
- Longair MH, Baker DA, Armstrong JD. Simple Neurite Tracer: open source software for reconstruction, visualization and analysis of neuronal processes. Bioinformatics. 2011;27:2453–2454. doi: 10.1093/bioinformatics/btr390. [DOI] [PubMed] [Google Scholar]
- Matsumori Y, Hong SM, Fan Y, Kayama T, Hsu CY, Weinstein PR, Liu J. Enriched environment and spatial learning enhance hippocampal neurogenesis and salvages ischemic penumbra after focal cerebral ischemia. Neurobiology of disease. 2006a;22:187–198. doi: 10.1016/j.nbd.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Matsumori Y, Hong SM, Fan Y, Kayama T, Hsu CY, Weinstein PR, Liu J. Enriched environment and spatial learning enhance hippocampal neurogenesis and salvages ischemic penumbra after focal cerebral ischemia. Neurobiol Dis. 2006b;22:187–198. doi: 10.1016/j.nbd.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Guthrie PB, Kater S. Intrinsic factors in the selective vulnerability of hippocampal pyramidal neurons. Prog Clin Biol Res. 1989;317:333. [PubMed] [Google Scholar]
- McCoy C, Campbell D, Deak M, Bloomberg G, ARTHUR J. MSK1 activity is controlled by multiple phosphorylation sites. Biochem J. 2005;387:507–517. doi: 10.1042/BJ20041501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard C, Hein P, Paquin A, Savelson A, Yang XM, Lederfein D, Barnabé-Heider F, Mir AA, Sterneck E, Peterson AC. An essential role for a MEK-C/EBP pathway during growth factor-regulated cortical neurogenesis. Neuron. 2002;36:597–610. doi: 10.1016/s0896-6273(02)01026-7. [DOI] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S-i, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Niv F, Keiner S, Krishna K, Witte OW, Lie DC, Redecker C. Aberrant Neurogenesis After Stroke A Retroviral Cell Labeling Study. Stroke. 2012;43:2468–2475. doi: 10.1161/STROKEAHA.112.660977. [DOI] [PubMed] [Google Scholar]
- Nozaki K, Nishimura M, Hashimoto N. Mitogen-activated protein kinases and cerebral ischemia. Mol Neurobiol. 2001;23:1–19. doi: 10.1385/MN:23:1:01. [DOI] [PubMed] [Google Scholar]
- Okuyama N, Takagi N, Kawai T, Miyake-Takagi K, Takeo S. Phosphorylation of extracellular-regulating kinase in NMDA receptor antagonist-induced newly generated neurons in the adult rat dentate gyrus. J Neurochem. 2004;88:717–725. doi: 10.1046/j.1471-4159.2003.02215.x. [DOI] [PubMed] [Google Scholar]
- Ortega FJ, Jolkkonen J. Restorative therapies to enhance sensorimotor recovery following cerebral ischemia. Acta Neurobiol Exp. 2013;73:66–78. doi: 10.55782/ane-2013-1922. [DOI] [PubMed] [Google Scholar]
- Pacey LK, Stead S, Gleave J, Tomczyk K, Doering L. Neural Stem Cell Culture: Neurosphere generation, microscopical analysis and cryopreservation. Nat Protoc. 2006;215:1–14. [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Raber J, Fan Y, Matsumori Y, Liu Z, Weinstein PR, Fike JR, Liu J. Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Ann Neurol. 2004;55:381–389. doi: 10.1002/ana.10853. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, Berardi N. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. European Journal of Neuroscience. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda N, Han F, Fukunaga K. Role of Akt and ERK signaling in the neurogenesis following brain ischemia. Int Rev Neurobiol. 2009;85:375–387. doi: 10.1016/S0074-7742(09)85026-5. [DOI] [PubMed] [Google Scholar]
- Shioda N, Han F, Morioka M, Fukunaga K. Bis (1-oxy-2-pyridinethiolato) oxovanadium (IV) enhances neurogenesis via phosphatidylinositol 3-kinase/Akt and extracellular signal regulated kinase activation in the hippocampal subgranular zone after mouse focal cerebral ischemia. Neuroscience. 2008;155:876–887. doi: 10.1016/j.neuroscience.2008.05.056. [DOI] [PubMed] [Google Scholar]
- Sugino T, Nozaki K, Takagi Y, Hattori I, Hashimoto N, Moriguchi T, Nishida E. Activation of mitogen-activated protein kinases after transient forebrain ischemia in gerbil hippocampus. The Journal of Neuroscience. 2000;20:4506–4514. doi: 10.1523/JNEUROSCI.20-12-04506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Nozaki K, Takahashi J, Yodoi J, Ishikawa M, Hashimoto N. Proliferation of neuronal precursor cells in the dentate gyrus is accelerated after transient forebrain ischemia in mice. Brain Res. 1999;831:283–287. doi: 10.1016/s0006-8993(99)01411-0. [DOI] [PubMed] [Google Scholar]
- Tian H-P, Huang B-S, Zhao J, Hu X-H, Guo J, Li L-X. Non-receptor tyrosine kinase Src is required for ischemia-stimulated neuronal cell proliferation via Raf/ERK/CREB activation in the dentate gyrus. BMC Neurosci. 2009;10:139. doi: 10.1186/1471-2202-10-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türeyen K, Vemuganti R, Sailor KA, Bowen KK, Dempsey RJ. Transient focal cerebral ischemia-induced neurogenesis in the dentate gyrus of the adult mouse. J Neurosurg. 2004;101:799–805. doi: 10.3171/jns.2004.101.5.0799. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Kempermann G, Gage FH. Neural consequences of enviromental enrichment. Nature Reviews Neuroscience. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Vize PD, McCoy KE, Zhou X. Multichannel wholemount fluorescent and fluorescent/chromogenic in situ hybridization in Xenopus embryos. Nature Protocols. 2009;4:975–83. doi: 10.1038/nprot.2009.69. [DOI] [PubMed] [Google Scholar]
- Walter C, Murphy BL, Pun RY, Spieles-Engemann AL, Danzer SC. Pilocarpine-induced seizures cause selective time-dependent changes to adult-generated hippocampal dentate granule cells. J Neurosci. 2007;27:7541–7552. doi: 10.1523/JNEUROSCI.0431-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggin GR, Soloaga A, Foster JM, Murray-Tait V, Cohen P, Arthur JSC. MSK1 and MSK2 are required for the mitogen-and stress-induced phosphorylation of CREB and ATF1 in fibroblasts. Molecular and cellular biology. 2002;22:2871–2881. doi: 10.1128/MCB.22.8.2871-2881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D-C, Ye W, Che X-M, Yang G-Y. Activation of mitogen-activated protein kinases after permanent cerebral artery occlusion in mouse brain. Journal of Cerebral Blood Flow & Metabolism. 2000a;20:1320–1330. doi: 10.1097/00004647-200009000-00007. [DOI] [PubMed] [Google Scholar]
- Wu D-C, Ye W, Che X-M, Yang G-Y. Activation of mitogen-activated protein kinases after permanent cerebral artery occlusion in mouse brain. J Cerebr Blood F Met. 2000b;20:1320–1330. doi: 10.1097/00004647-200009000-00007. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Kong Y, Yang S, Li M, Wen J, Li L. Upregulation of Flk-1 by bFGF via the ERK pathway is essential for VEGF-mediated promotion of neural stem cell proliferation. Cell Res. 2007;17:73–79. doi: 10.1038/sj.cr.7310126. [DOI] [PubMed] [Google Scholar]
- Yagita Y, Kitagawa K, Ohtsuki T, Takasawa K-i, Miyata T, Okano H, Hori M, Matsumoto M. Neurogenesis by progenitor cells in the ischemic adult rat hippocampus. Stroke. 2001;32:1890–1896. doi: 10.1161/01.str.32.8.1890. [DOI] [PubMed] [Google Scholar]
- Yan X-B, Hou H-L, Wu L-M, Liu J, Zhou J-N. Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology. 2007;53:487–495. doi: 10.1016/j.neuropharm.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Ming G-l, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu DY, Lau L, Liu SH, Wei JS, Lu YM. Activation of cAMP-response-element-binding protein (CREB) after focal cerebral ischemia stimulates neurogenesis in the adult dentate gyrus. Proc Natl A Sci USA. 2004;101:9453–9457. doi: 10.1073/pnas.0401063101. [DOI] [PMC free article] [PubMed] [Google Scholar]