Abstract
The ability to predict individual vulnerability to substance abuse would allow for a better understanding of the progression of the disease and development of better methods for prevention and/or early intervention. Here we use drug-induced devaluation of a saccharin cue in an effort to predict later addiction-like behavior in a model akin to that used by Deroche-Gamonet et al. (2004) and seek to link such vulnerability to changes in expression of various mu opioid receptor and D2 receptor-interacting proteins in brain. The results show that the greatest heroin-induced suppression of intake of a saccharin cue is associated with the greatest vulnerability to later addiction-like behavior and to differences in the expression of WLS, β-catenin, and NCS-1 in brain compared to rats that exhibited the least suppression of intake of the heroin-paired cue and/or saline controls. Finally, because the self-administration model employed produced no significant differences in drug intake between groups, overall, the resultant changes in protein expression can be more closely linked to individual differences in motivation for drug.
Keywords: Self administration, Opioid, Individual vulnerability, Wntless, Beta-catenin, NCS-1
1. Introduction
More than 15.3 million people worldwide have drug use disorders (World Health Organization, 2015a) and approximately 69,000 people die each year from opioid overdose (World Health Organization, 2015b). However, not everyone who takes a drug of abuse becomes addicted. Roughly 15% of drug users will eventually become drug abusers (Anthony et al., 1994). What contributes to the vulnerability of the 15% and what protects the other 85% of users from becoming addicted? Are there early behavioral or molecular indicators that can help identify those individuals that are vulnerable to addiction? The ability to identify such vulnerability could make early detection and intervention a reality.
Several rodent models have been developed to simulate behaviors that are exhibited by human addicts as described in the Diagnostic and Statistical Manual of Mental Disorders (DSM). Several measures of drug taking (escalation), seeking (signaled non-availability), working (progressive ratio), persistence in the face of adverse consequences (punishment), and relapse (reinstatement) are commonly used to study addiction-like behaviors in rat models. However, the self-administration paradigm itself can lead to differences in how animals perform on these measures. For example, rats that are given extended access (6 h) to drugs of abuse escalate the amount of drug intake over time (Ahmed and Koob, 1998). They also take large quantities at the beginning of each session (load up) in order to achieve a baseline high. In contrast, Piazza and colleagues have developed an intermittent access model (three 40 min drug access periods alternated with two 15 min periods of drug non-availability) for cocaine addiction (Deroche-Gamonet et al., 2004). This intermittent schedule of drug access does not lead to significant differences in the amount of drug taken across trials, but does result in differences in several other measures of addiction-like behavior including an increase in responding for drug during periods of signaled non-availability, an increase in the willingness to work for drug, and an increase in responding for drug in the face of adverse consequences (e.g., foot shock).
Difficulties may arise when choosing between these models when looking for differences in protein or epigenetic markers of vulnerability. In the extended access model, changes in gene and protein expression could be the result of differences in drug exposure and/or differences in motivation for drug. With the intermittent access model there is more confidence that molecular differences are not the result of differential drug exposure, but rather more directly linked to observed addictive behavior. That said, even with this model, several weeks of drug exposure are required before individual differences become evident in addiction-like behavior. Ideally, it would be advantageous to have an early indicator of future addiction-like behavior for drug, prior to a great deal of drug access.
Our laboratory has been using drug-induced avoidance of a natural reward (i.e., a saccharin solution) as an early indicator of vulnerability to addiction. As demonstrated by Huhn et al. (this issue), humans recovering from opioid addiction are less responsive to natural rewards and this is thought to predict increased vulnerability to relapse. A similar pattern is evidenced in a preclinical rodent model. Specifically, rats suppress intake of an otherwise palatable saccharin cue when paired with a drug of abuse. There are, however, robust individual differences whereby some rats, referred to as large suppressors, exhibit greater avoidance of the drug-paired cue than do others, referred to as small suppressors. This is true when the taste cue predicts access to cocaine or heroin (Grigson and Twining, 2002; Imperio and Grigson, 2015; Wise et al., 1976). Interestingly, in the saccharin-heroin extended access paradigm, this split occurs early (after only a few taste-drug pairings), before differences in drug-taking become evident. Further, with repeated taste-drug pairings, the large suppressors ultimately exhibited greater load up for drug, greater drug-taking, escalated intake over trials, marked willingness to work for drug, and greater seeking during extinction and during drug-induced reinstatement (see (Imperio and Grigson, 2015) and Imperio et al. (this issue)). This finding suggests that vulnerability for addiction, as evidenced by greater devaluation of a natural reward cue, links to many other measures of addiction, including escalated drug intake (Edwards and Koob, 2013). Here, we aimed to test whether conditioned avoidance of a heroin-paired saccharin cue following just three taste-drug pairings would predict addiction-like behavior in a variation of the intermittent access model employed by Deroche-Gamonet et al. (2004).
Another aim of this study was to determine whether changes in the expression of several protein mediators of synapse function could act as molecular markers of addiction vulnerability. We have previously performed protein interaction screens to identify novel modulators of mu-opioid receptor and D2 dopamine receptor (D2R) function that may contribute to the addiction phenotype. We demonstrated that opioids alter Wnt secretion, presumably through an interaction between the mu opioid receptor (the primary mediator of opioid reward) and WNTless (WLS), a regulator of Wnt release (Jin et al., 2010). Wnts are secreted molecules that act through cell surface receptors to stabilize β-catenin which, in turn, activates transcription of genes involved in embryonic development, synapse stability, and cancer. Also, we identified neuronal calcium sensor-1 (NCS-1) as a D2R interactor that serves to inhibit internalization and desensitization of the D2 receptor (Kabbani et al., 2002). If these proteins are altered as a function of vulnerability for addiction-like behavior, then they may serve as novel therapeutic targets for the prevention and/or treatment of addiction.
2. Materials and methods
2.1. Animals
The subjects were 48 naïve, male Sprague Dawley rats (Charles River, Wilmington, MA). Data from rats with catheters that did not remain patent for the entire study were eliminated. This study was conducted in 2 replications with final ns = 23 and 19, respectively. The rats were housed singly in suspended, wire mesh cages in a humidity-controlled environment under a 12/12 h light/dark cycle. Food (Teklad 2018, Harlan Industries, US) and water were available ad libitum, except where otherwise noted. Experiments were approved by the Penn State College of Medicine, Institutional Animal Care and Use Committee and were performed in accordance with National Institutes of Health specifications as outlined in the Guide for the Care and Use of Laboratory Animals.
2.2. Catheters
In-dwelling intra-jugular catheters were custom made in our laboratory and implanted into rats as described previously (Grigson and Twining, 2002). After three days’ recovery, patency was maintained by daily flushing of catheters with heparinized saline (0.2 ml of 30 IU/ml heparin) and verified, when necessary, by using 0.2 ml of 1% propofol (Diprivan, APP Pharmaceuticals, Schaumburg, IL) administered through the catheter. After surgeries, rats were given one week to recover before the start of testing.
2.3. Apparatus
2.3.1. Self-administration chambers
Each rat was trained in one of 12 identical operant chambers (Med Associates, St. Albans, VT) measuring 30.5 × 24.0 × 29.0 cm and housed in light and sound attenuating cubicles as previously described (Puhl et al., 2012). All chambers have clear Plexiglas tops, fronts, and backs. Sidewalls are aluminum. The floors consist of 19 stainless steel rods (4.8 mm) spaced 1.6 cm apart center to center. Each chamber is equipped with three retractable operant sipper tubes (spouts) that enter the left side of the chamber through 1.3 cm diameter holes spaced at 8.0 cm center to center. A stimulus light is located 6 cm above each spout. A lickometer circuit is used to record spout licks (and contacts such as nose pokes). Each chamber is equipped with a house light (25 W), a tone generator (Sonalert Time Generator, 2900 Hz), and a white noise speaker (75 dB). Self-administration is controlled by an electronic circuit operating a syringe pump (Med Associates). Collection of the data and control of chamber events are performed on-line using a Windows-based computer running programs written in Medstate notation language (Med Associates). The lickometer circuit was used to monitor licking on the leftmost spout (saccharin), the middle spout (“inactive”, i.e. the spout upon which responding was measured but elicited no consequence), and the rightmost spout (“active”, i.e. the spout upon which responding typically elicited an intravenous (i.v.) infusion of heroin).
2.3.2. Coupling assembly
Before the start of each self-administration trial, a coupling assembly is attached to the cannula exiting through the rat’s back. The coupling assembly consists of a metal spring attached to a metal spacer with Tygon tubing inserted down the center to protect the tubing from interference by the rat. The tubing is attached to a counterbalanced swivel (Instech, Plymouth Meeting, PA) that in turn is attached to the syringe pump outside of the experimental chambers.
2.4. Drug preparation
Heroin HCl (generously provided by the National Institute on Drug Abuse, Research Triangle Park, NC) was dissolved in sterile, physiological (0.9%) saline at a concentration of 0.3 mg/ml. Each i.v. injection was a 0.06 mg/0.2 ml infusion delivered over 6 s (Kuntz et al., 2008).
2.5. Habituation and training
Rats were put on a water restriction regimen wherein they received 5 min access to water in the morning and one hour in the afternoon from a graduated cylinder with a stainless steel spout placed on each rat’s home cage. This occurred each day until morning intake of water stabilized (7 days). Rats were then habituated to the self-administration chambers for 5 min per day for 3 days prior to the start of drug access. During habituation, rats continued on the water restriction regimen but were then given 5 min morning access to water through one of the three chamber spouts each day for three days. Thereafter, water was returned to the rats ad libitum via lixits located at the back of each home cage. Rats were divided into heroin and saline groups counterbalanced based on their 5 min water intake averaged across the last two days of home cage water access.
2.5.1. Phase I: saccharin–heroin self-administration
After habituation, during Phase I of the experiment, rats were given 5 min access to a 0.15% saccharin solution from the left spout in the self-administration chamber, as described previously (Imperio and Grigson, 2015; Puhl et al., 2012). After 5 min, the left spout retracted, the center and right spouts advanced and the rats were given the opportunity to self-administer heroin or saline on a fixed ratio (FR) 10 schedule of reinforcement where 10 licks on the rightmost empty active spout resulted in a 6 s i.v. infusion of either saline (n = 11) or a 0.06 mg/0.2 ml of heroin (n = 31) as previously described by Kuntz et al. (2008). Drug or saline delivery was signaled by offset of the stimulus light and onset of the tone and house light, which remained on for 20 s (including the 6 s drug infusion). Further responding during this 20 s time out period was not reinforced. The access period for heroin was 6 h. There was one such taste-drug pairing per day for 3 days in succession.
2.5.2. Phase II: intermittent access heroin self-administration
Phase II of the experiment consisted of one trial per day, 5 days per week, for a total of 24 trials. Each daily trial included three 40 min periods of drug availability alternated with two 15 min periods of signaled drug non-availability (SNA) as described by Deroche-Gamonet et al. (2004). Rats remained on the FR 10 schedule of reinforcement. During periods of SNA, the active and inactive spouts were presented without the typical stimulus light, but a stimulus light on the opposite wall was illuminated.
2.5.3. Progressive ratio
After 24 trials of intermittent access, rats were given a single progressive ratio (PR) test wherein the first infusion of drug required 10 operant responses, and each subsequent infusion required an increasing number of responses (10, 12, 14, 16, 20, 24, 30, 36, 44, 52, 62, and continuing to increase by 10 thereafter) until no responses were emitted for 30 min or until six hours had elapsed. The breakpoint was defined as the number of spout responses completed for the last infusion received. This schedule was a slight modification of that used by (Deroche-Gamonet et al., 2004).
2.5.4. Extinction/reinstatement
The progressive ratio challenge was followed the next day by a single extinction/reinstatement challenge. This consisted of a 7 h period during which the first 6 h of operant activity was identical to that of Section 2.5.2 but responses were not rewarded with a heroin or saline infusion. At the end of 6 h, after drug seeking had extinguished, there was a single non-contingent, computer controlled i.v. infusion of heroin (0.06 mg/0.2 ml). Continued active spout responding (non-reinforced) was then measured in the h following this drug prime.
2.6. Addiction-like behavior score
To attain a score of each rat’s addiction-like behavior, we ranked rats by their performance based on behavioral correlates of three separate DSM-IV criteria for human substance abuse disorder (American Psychiatric Association, 2000). The first DSM criterion was difficulty stopping or limiting use. A measure of each rats’ persistence in drug seeking was calculated by the total number of responses on the active spout operant across the two periods of SNA on the terminal drug trial. The second DSM criterion used was motivation to take drug. A measure of each rats’ motivation to take drug, or willingness to work for drug, was calculated by the breakpoint spout responses achieved during the PR trial. The third behavior of the original scoring protocol used by Piazza and colleagues (Deroche-Gamonet et al., 2004), continued use despite harm, was not utilized in this study as the analgesic properties of opioids may confound the results of this measure. Instead, we chose to investigate another defining aspect of addiction: relapse. Thus, for the third criterion we used reinstatement of heroin seeking behavior based on the number of infusion attempts made during the 1 h reinstatement test. Deroche-Gamonet et al. (2004) showed that reinstatement behavior was highly correlated with SNA, PR, and punished responding (mild foot-shock), thus, reinstatement is a good replacement measure for foot-shock. Then, for each of the three criteria, data from all heroin rats were ranked from lowest to highest and each rat scoring in the highest third of rats in that criterion received a point. Consequently, rats received an addiction-like behavior score of 0, 1, 2, or 3.
2.7. Western blot
Within 24 h following behavioral testing, rats were sacrificed by rapid decapitation, brains were harvested immediately, and dissected on ice including the medial prefrontal cortex (mPFC), nucleus accumbens (NAc), ventral tegmental area (VTA), and hippocampus (HC). The rat atlas of coordinates (Paxinos and Watson, 2007) was used as a guide. Specimens were frozen on dry ice and stored at −80 °C until processing. Tissue was suspended in lysis buffer (50 mM Tris–HCl, 1 mM EDTA, 150 mM NaCl, 1% NP40, 0.25% deoxycholate, 5 mM NaF, 2 mM Na3VO4) containing protease inhibitors (cOmplete MINI EDTA free, Roche), homogenized using a microcentrifuge pestle for 2 min, sonicated using a probe sonicator, then centrifuged at 13,000 RPM to remove cellular debris. Bradford assay (BioRad) was used to determine protein concentration of supernatants. Samples were diluted to 2 μg/ul with lysis buffer and 4× loading dye (40% Glycerol, 240 mM Tris/HCl pH 6.8, 8% SDS, 0.04% bromophenol blue, 5% beta-mercaptoethanol). Finally, 10 μl (20 μg) of sample were separated on 4–20% TGX polyacrylamide gels (BioRad), transferred to PVDF membranes, ponceau stained to check for even loading, and subjected to western blotting. For each of the two behavioral replications, two blots per brain region (in order to analyze all rats’ samples) were run in triplicate. Saline controls were included on all gels to normalize expression across blots. Membranes were probed with the following antibodies: chicken anti-WLS (Jin et al., 2010, 1:5000), chicken anti-NCS-1 (Rockland 1:10,000), and rabbit anti-β-catenin (Cell Signaling 1:7500). Blots were scanned using a back-lit scanner and quantification was performed using IMAGEJ software. Expression was normalized to total protein (as measured by Ponceau stain), then to averaged saline controls (for comparison between blots), and then averaged between replicates. Expression levels were statistically analyzed for differences in expression related to drug taking, addiction score, and saccharin cue suppression.
2.8. Statistical analysis
All data were analyzed using mixed factorial ANOVAs with Statistica 7 (StatSoft Inc., Tulsa, OK). Newman–Keuls post hoc tests were conducted on significant ANOVAs with α = 0.05.
3. Results
3.1. Phase I: saccharin-heroin self-administration
3.1.1. Taste cue intake
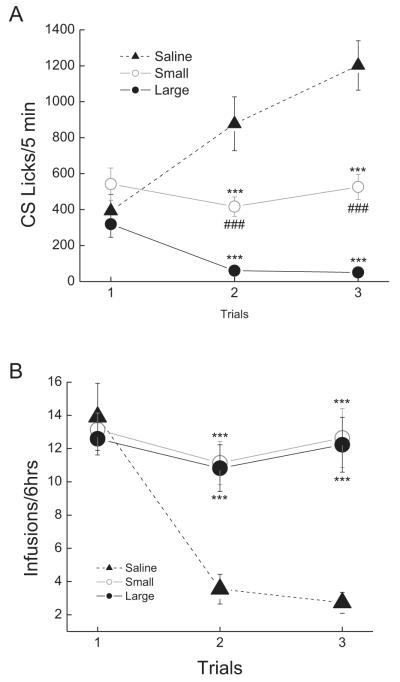
Compared to the saccharin-saline controls, intake of the saccharin cue was suppressed following saccharin-heroin pairings. However, some rats exhibited greater avoidance of the heroin-paired cue than did others. In an effort to address these individual differences, rats in the heroin group were separated by a 200 lick cutoff (which, in this case, is also a median split) into large suppressors (rats suppressing intake of the saccharin conditioned stimulus (CS) to a larger extent relative to controls), and small suppressors (rats suppressing intake of the conditioned stimulus (CS) to a smaller extent relative to controls), as described previously (Grigson and Twining, 2002; Imperio and Grigson, 2015), see Fig. 1A .
Fig. 1.
(A) Mean (±) SEM number of licks of the 0.15% saccharin CS as a function of acquisition trials 1–3 for saline controls (closed triangles), small suppressors (open circles, n = 13) and large suppressors (closed circles, n = 18). (B) Mean (±SEM) number of infusions/6 h across trials 1–3 for saline controls (closed triangles), large suppressor (closed circles, n-18), and small suppressor (open circles, n-13). (*difference from saline, #difference between large and small; *p < .05, ***p < .001).
This observation was supported by a significant 3 × 3 mixed factorial ANOVA varying group (saline, small and large suppressors) and trials (1–3), F(4,78) = 31.23, p < 0.001. Post hoc tests revealed that small suppressors decreased consumption of the CS solution compared to saline controls by the second and third taste-drug pairings (ps < 0.001). Large suppressors also made fewer licks of the saccharin cue than did the saline controls during trials 2 and 3 (ps < 0.001). Notably, large suppressors made fewer licks on the saccharin cue than did the small suppressors during both the second and third trials (ps < 0.001).
3.1.2. Drug intake
When examining drug intake (Fig. 1B), there was a significant main effect of group, with heroin-administering rats taking significantly more heroin infusions than saline rats took of saline, F(2,39) = 5.81, p < 0.01, overall, and a significant group × trials inter action, F(4,78) = 12.07, p < 0.001. Post hoc analysis of the two-way interaction confirmed that rats took more heroin than saline infusions on trials 2 and 3, ps < 0.001, with no differences found between small and large suppressors, p > 0.05.
3.2. Phase II: intermittent access heroin self-administration
3.2.1. Drug intake
During intermittent access, the number of heroin infusions/2 h trial was analyzed using a 2 × 24 mixed factorial ANOVA varying group (small, large) and trials (1–24)(Fig. 2). Not unexpectedly, there was no significant main effect of group or trials, Fs < 1, confirming that small and large suppressor rats self-administered the same amount of heroin overall. The group × trials interaction also did not attain statistical significance, F < 1, indicating that there were no differences in heroin self-administration across trials between the small and the large suppressors.
Fig. 2.
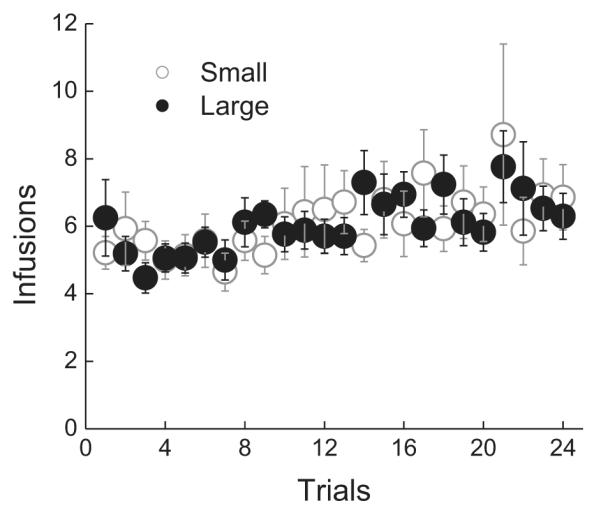
Mean (±SEM) number of infusions/2 h across trials 1–24 for small (open circles; n = 13) and large (closed circles; n = 18) suppressors.
3.2.2. SNA responding
Continued responding during signaled non-availability (SNA) was analyzed with a 2 × 24 mixed factorial ANOVA varying group (large and small suppressors) by trial (1–24). There was no significant main effect of group, F < 1, showing that overall there were no significant differences in SNA responding between the large and small suppressors. The group × trial interaction also was not significant, F < 1 (see Table 1).
Table 1.
Mean and standard error of the mean (SEM) responses generated in each criterion and contributing to the Addiction-like Behavior Score (ALB) for: all rats; small suppressors; large suppressors; smallest 5 suppressors; and largest 5 suppressors. Once ranked from lowest to highest, rats ranking in the highest third in each criterion received one point towards their ALB score.
| Range |
Mean (SEM) |
||||||
|---|---|---|---|---|---|---|---|
| Criterion | Lowest 2/3 | Highest 1/3 | All rats | Small suppressors | Large suppressors | Smallest 5 | Largest 5 |
| SNA | 0–51 | 65–263 | 49.6 (11.7) | 52.8 (14.0) | 47.3 (17.8) | 31.8 (18.9) | 109.2 (40.8) |
| PR breakpoint | 0–16 | 20–172 | 22.9 (5.4) | 14.8 (1.5) | 28.8 (9.2) | 8.8 (2.2) | 15.6 (4.9) |
| Reinstatement | 0–6 | 7–18 | 5.1 (0.9) | 5.5 (1.2) | 4.9 (1.3) | 2.2 (1.2) | 6.6 (3.2) |
3.2.3. Progressive ratio challenge
While there was a trend for large suppressor rats to work harder for drug during the progressive ratio challenge (Table 1), a one-way ANOVA revealed that the main effect of group was not significant, F(1,29) = 1.64, p > 0.20.
3.2.4. Reinstatement challenge
During the extinction phase, to confirm that seeking behavior had been extinguished before the non-contingent prime, Student’s t-tests demonstrated that, by the 5th and 6th hours of extinction testing, responding by large and small suppressors on the previously active spout was not significantly different from zero nor from each other (ps > 0.05). During the 7th hour, after the single delivery of a computer controlled, non-contingent priming dose of heroin, small and large suppressors increased seeking behavior, and a one way ANOVA confirmed that the levels were not significantly different from each other F < 1 (Table 1).
3.3. Addiction-like behavior score
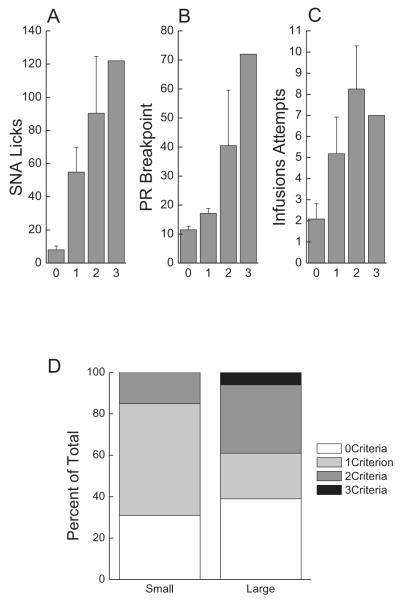
Although differences were not evident for SNA, PR, or reinstatement between small and large suppressors, overall, an addiction-like behavior score was determined for each individual rat. Thus, as described above, rats testing in the top third of each addiction-like criterion scored one point, for a total addiction-like behavior score of either zero (n = 11), one (n = 11), two (n = 8), or three (n = 1). As in the original model (Deroche-Gamonet et al., 2004), and even with only 1 subject having scored an ALB score of 3, there was a trend for the intensity of each of the addiction-like behaviors to be proportional to the number of criteria met (Fig. 3A–C).
Fig. 3.
(A–C) Rats testing in the top third of an addiction-like criterion scored a point, for a total addiction-like behavior score of either zero (n = 11), one (n = 11), two (n = 8), or three (n = 1). (A) Mean (±SEM) number of SNA responses on terminal trail 24 as a function of ALB score 0, 1, 2, or 3. (B) Mean (±SEM) PR breakpoint responding as a function of ALB score 0, 1, 2, or 3. (C) Mean (±SEM) infusion attempts/1 h as a function of ALB score 0, 1, 2, or 3. (D) Percent of small and large suppressor rats scoring an ALB of 0, 1, 2, or 3. The one rat scoring a 3 was a large suppressor.
3.3.1. Signaled non-availability
Responding during SNA was analyzed using a univariate test as a function of ALB score (0, 1, 2, 3) (trial 24). The results showed that SNA responding increased as a function of increasing ALB scores, F(3,27) = 3.77, p < 0.05 (Fig. 3A).
3.3.2. Breakpoint
Similarly, breakpoint during the PR challenge was analyzed using a univariate test varying the ALB score. The ALB score (0, 1, 2, or 3) did not significantly predict breakpoint, F(3,27) = 2.88, p > 0.05 (Fig. 3B).
3.3.3. Reinstatement
Relapse to seeking behavior in the 1 h reinstatement test was analyzed using a univariate test varying ALB score. Infusion attempts during the reinstatement test significantly increased with a greater ALB score, F(3,27) = 3.57, p < 0.05 (Fig. 3C).
3.3.4. Suppressor group and ALB
The percentage of small and large suppressors scoring in each addiction-like behavior range is shown in Fig. 3D. The one rat scoring a three was a large suppressor. In the small suppressor group, rats with a score of 2 (n = 2) or 3 (n = 0) made up only 15% of small suppressors, while 40% of the large suppressors had a score of 2 or 3 (n = 6 and n = 1).
3.4. Western analysis
We have chosen three proteins to analyze for potential expression changes that may be related to drug-induced suppression of intake of a natural reward and ALBs in rats: WLS (Fig. 5), β-catenin (Fig. 6), and NCS-1 (Fig. 7). Western blots were performed on lysates from PFC, NAc, hippocampus, and VTA. Proteins were then analyzed for differences based on the animal’s heroin exposure (saline vs. heroin), saccharin cue suppression of intake (saline vs. small suppressors vs. large suppressors), and ALBs (saline vs. ALB score of 0, 1, 2, or 3). When examining the protein data for all rats, no significant differences were found in any of the proteins in any of the brain regions between large and small suppressors overall. To obtain a better assessment of possible differences between small and large suppressing rats, protein expression data were then analyzed across all saline rats (n = 11), the 5 largest suppressors (i.e. those rats that drank the least saccharin on the third taste-drug pairing), and the five smallest suppressors (i.e. those rats that drank the most saccharin on the third taste-drug pairing). The five smallest suppressors (Fig. 4) had addiction-like behavior scores of 0, 0, 0, 1, and 2, and the five largest suppressors had addiction-like behavior scores of 1, 1, 2, 2, and 3. Mean (±SEM) responses on each of the three measures is shown for the 5 smallest and the 5 largest suppressors in Table 1.
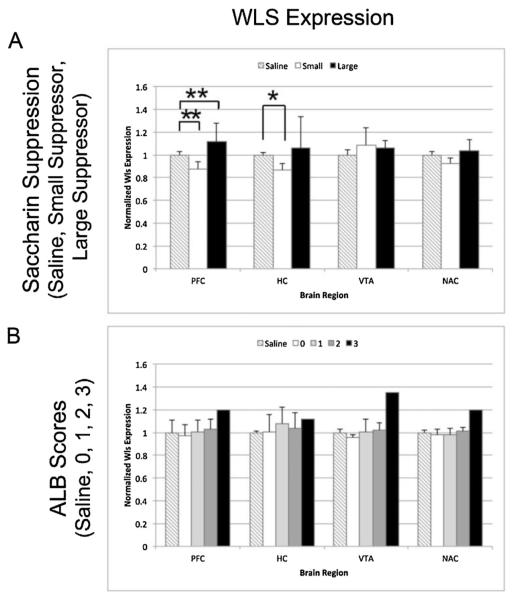
Fig. 5.
WLS protein expression in heroin self-administering rats in the prefrontal cortex (PFC), hippocampus (HC), ventral tegmental area (VTA), and nucleus accumbens (NAc). Bar graphs represent normalized band intensities across three Western blots for individual rats averaged within each behavioral category. (A). Normalized WLS expression (±SEM) for saline animals (n = 7), the largest saccharin suppressors (n = 5), and the smallest saccharin suppressors (n = 5.) (B). Normalized WLS expression (±SEM) for the saline animals (n = 7) and subjects with an ALB score of 0 (n = 11), 1 (n = 9), 2 (n = 10), and 3 (n = 1) *p < 0.05 and **p < 0.01.
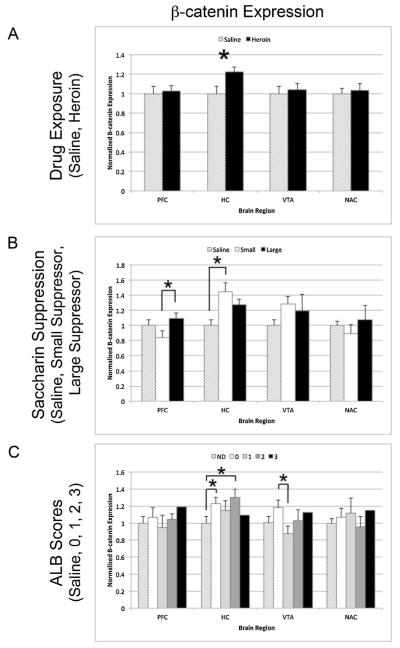
Fig. 6.
β-catenin protein expression in heroin self-administering rats in the medial prefrontal cortex (PFC), hippocampus (HC), ventral tegmental area (VTA), and nucleus accumbens (NAc). Bar graphs represent normalized band intensities across three Western blots for individual rats averaged within behavioral categories. (A) Normalized β-catenin expression (±SEM) for saline animals (n = 7) and heroin self-administering animals (n = 31). (B) Normalized β-catenin expression (±SEM) for saline animals (n = 7), the largest saccharin suppressors (n = 5), and the smallest saccharin suppressors (n = 5) (C). Normalized β-catenin expression (+/− SEM) for saline animals (n=7) and ALB scores of 0 (n = 11), 1 (n = 9), 2 (n = 10), and 3 (n = 1). *p < 0.05.
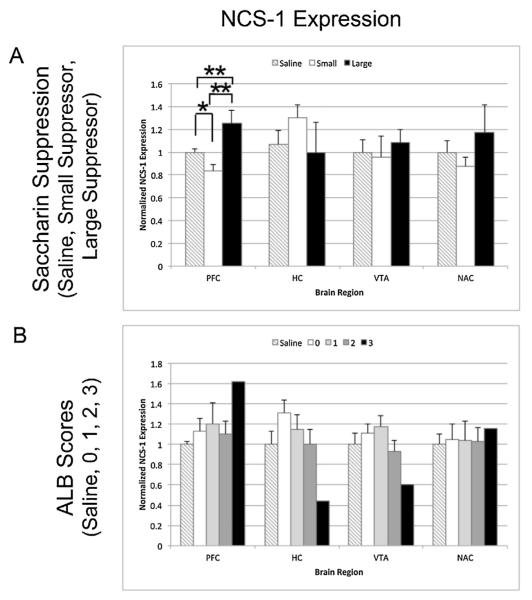
Fig. 7.
NCS-1 protein expression in heroin self-administering rats in the prefrontal cortex (PFC), hippocampus (HC), ventral tegmental area (VTA) and nucleus accumbens (NAc). Bar graphs represent normalized band intensities across three Western blots for each individual rat averaged within behavioral categories. (A) Normalized NCS-1 (±SEM) expression for saline animals (n = 7), largest saccharin suppressors (n = 5), and smallest saccharin suppressors (n = 5) (B). Normalized NCS-1 expression (±SEM) for into saline animals (n = 7) and ALB scores of 0 (n = 11), 1 (n = 9), 2 (n = 10), and 3 (n = 1). *p < 0.05 and **p < 0.01.
Fig. 4.
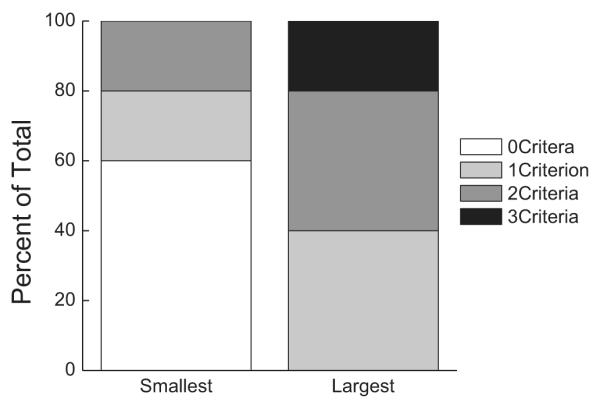
The percent of rats scoring a 0, 1, 2, or 3 on the ALB score for the 5 Smallest and 5 Largest suppressors.
3.4.1. WLS expression
Previous studies by our laboratory and others have suggested that WLS expression decreases with opioid use in the brains of rats, mice, and zebrafish (Herrero-Turrion et al., 2014; Petko et al., 2013;Tacelosky et al., 2015). Recently, using a similar intermittent access model as that used in this study (albeit without saccharin suppression training), Tacelosky et al. 2015 found a significant correlation such that lower levels of WLS expression in the PFC were associated with increasing addiction-like behavior scores for heroin in rats. In the current study, when looking at all of the experimental subjects tested, we did not observe a similar decrease in WLS expression with increasing addiction score (Fig. 5B). However, because only one animal received an addiction score of 3, no statistical inferences can be made for this category. It is worth noting that this individual rat actually displayed higher WLS expression in the PFC, Hippocampus, NAc, and VTA compared to saline animals and those scoring 0, 1, or 2.
In terms of saccharin suppression, there was a trend in the PFC and hippocampus for a decrease in WLS expression in the smallest saccharin suppressor group and an increase for rats in the largest saccharin suppressor group as compared to the saline controls (Fig. 5A). Significant changes in WLS expression, however, were only reached in the PFC for a decrease in the smallest suppressors vs. saline animals (p = 0.03) and, in the hippocampus, an increase in the largest suppressors vs. saline (p = 0.007) and vs. the smallest suppressors (p = 0.007). Finally, as in our previous study (Tacelosky et al., 2015), no differences in WLS expression were observed between saline animals and those receiving heroin, overall (graphs not shown), indicating that heroin exposure, alone, did not alter WLS expression. Because WLS is essential for Wnt secretion, we next chose to look at a downstream component of the Wnt pathway to determine whether Wnt signaling was altered in the brains of rats displaying ALB for heroin.
3.4.2. β-catenin expression
β-catenin is a downstream effector of the Wnt signaling pathway. Upon Wnt activation of Frizzled receptors on Wnt receiving cells, the β-catenin protein becomes stabilized and is able to enter the nucleus where it exerts its effects on the TCF/LEF transcription factor. Changes in β-catenin expression that correlate with ALBs could indicate a change in Wnt signaling, which may or may not be dependent on altered WLS function or expression. Interestingly, β-catenin is the only protein in this study that showed a significant change in expression between drug and no drug groups (Fig. 6A). In the hippocampus, β-catenin levels were significantly increased in self-administering rats compared to saline controls (p = 0.049). Upon further division of the heroin self-administering animals into different behavioral categories, no significant differences in hippocampal β-catenin expression were found between largest and smallest suppressors (Fig. 6B) and limited differences were found in the hippocampus and VTA as a function of the ALB scores (Fig. 6C). This pattern of data provides further indication that this effect is due to heroin exposure rather than to individual vulnerability for ALB. Significant changes in β-catenin expression were seen in the PFC between the smallest and largest suppressors and in the hippocampus between the saline group and the smallest suppressors (Fig. 6B).
3.4.3. NCS-1 expression
We analyzed the expression of NCS-1, a D2 dopamine receptor (D2R) interacting protein that prevents GRK2 induced internalization and desensitization of the D2R (Kabbani et al., 2002). Changes in NCS-1 expression might indicate altered stability of D2R localization and signaling at the plasma membrane. Expression of NCS-1 was highly variable between individuals in comparison to the other proteins analyzed (Fig. 7). No significant changes in expression were detected when analyzing effects of drug administration (i.e., saline vs. heroin animals). However, in terms of saccharin suppression, a similar pattern of alterations in NCS-1 expression levels was observed in the PFC as was seen for WLS and β-catenin. In the PFC, NCS-1 was decreased in the smallest saccharin suppressors (p = 0.02) and increased in the largest saccharin suppressors (p = 0.008) when compared to the saline controls. There also was a significant difference between the smallest and the largest suppressors (p = 0.003). Altogether, the expression data for these proteins suggest that the PFC and hippocampus are areas where altered Wnt signaling or D2R desensitization may affect addiction vulnerability but perhaps not severity.
4. Discussion
4.1. Early suppression of intake of the taste cue and addiction-like behavior
Rats avoided intake of a saccharin cue when paired with the opportunity to self-administer heroin in the 6 h extended access paradigm of Phase I. Individual differences were immediately evident whereby some rats greatly avoided the saccharin cue (the large suppressors), while others (the small suppressors) did not. Even so, conditioned avoidance of the drug-paired cue occurred immediately for all rats following a single saccharin-heroin pairing, although the effect was greater in the large suppressors.
At this point, early in training, drug intake was identical between the small and the large suppressors. Drug-taking continued to be equal for the small and the large suppressor rats when switched in Phase II to the intermittent access paradigm. There was a tendency for the number of infusions per 2 h to increase slightly across trials 1–24, overall, but this trend was not significant. Thus, unlike the extended access model where escalation is the norm (Imperio and Grigson, 2015; Koob and Kreek, 2007), rats failed to evidence escalation of drug self-administration across trials here, as is typical for this paradigm (Deroche-Gamonet et al., 2004; Puhl et al., 2011; Tacelosky et al., 2015).
Despite having taken about the same amount of drug, rats were differentially positive for 0, 1, 2, or 3 addiction-like behaviors. Surprisingly, out of 31 rats, only 1 rat (3.2%) scored a 3 and 8 rats (25.8%) scored a 2. This is less than Deroche-Gamonet et al., 2004 reported with cocaine, less than we reported with cocaine (Puhl et al., 2011), and less than we found using a similar regimen with heroin (Tacelosky et al., 2015). When examined as a function of the small and large suppressors, the large suppressor group accounted for the only subject to score a 3 and for 6/8 (75%) of the subjects that scored a 2. That said, clear differences were not evident in the behaviors that served as the component parts of the ALB score—i.e., as a group, small and large suppressor rats did not significantly differ from one another in their SNA, PR, or reinstatement behavior.
4.2. Protein expression and Addiction-like behavior
Brain changes also differed from earlier findings. Tacelosky et al. 2015 found WLS levels to be reduced in the PFC and lower levels of WLS were significantly correlated with greater addiction-like behavior for heroin, in particular with a greater willingness to work for drug on the PR schedule of reinforcement. Here, small differences were found in WLS protein expression in brain, with levels being lower in the PFC of the smallest suppressors, and higher in the PFC of the largest suppressors vs. saline controls, respectively. Differences also were found in β-catenin levels in the HC and in the PFC. Higher levels in the HC were associated with heroin vs. saline self-administration; while higher levels in the PFC were associated with being among the largest vs. the smallest suppressors. NCS-1 also was elevated in the PFC in the largest suppressors relative to both the smallest suppressors and the saline controls.
There is no evidence from this study to suggest that WLS, β-catenin, or NCS-1 differ with severity of ALBs including SNA, PR, and reinstatement measures (i.e., with scoring a 0, 1, 2, or a 3). This is in contrast to other studies by our laboratory that demonstrated decreased WLS levels with increasing ALB scores in the PFC (Tacelosky et al., 2015) in heroin self-administering rats and decreased WLS expression in the midbrain and striatum of animals exposed to non-contingent morphine (Petko et al., 2013). The paradigm used in the study by Tacelosky et al. 2015 utilized both SNA and PR when calculating the addiction score, but instead of using reinstatement (as done here), the third measure looked at activity during time out periods (periods after heroin infusions during which heroin cannot be delivered) as a measure of disinhibition. However, one could argue that time out activity is a similar measure to SNA that models drug seeking. Therefore, we chose to incorporate a different measure of addictive behavior, reinstatement, which models the human condition of drug relapse and was found by Deroche-Gamonet et al. (2004) to correlate with each of their measures of ALB.
Another difference between the present study and the Tacelosky et al. 2015 study is the antibody used to detect WLS. The current study utilized a non-commercial antibody that specifically detects the mature (glycosylated) form of WLS that can be detected as a cluster of bands at 50 kDA (Jin et al., 2010). In contrast, Tacelosky et al. 2015 utilized a commercially available antibody that detects a band of about 37 kDA, the size of the core WLS protein. The mature form of WLS is stable and can be recycled to the Golgi to perform multiple rounds of Wnt trafficking (Yang et al., 2008). It would not be surprising if levels of immature WLS change in order to maintain a constant amount of mature WLS in the cell. In addition, we have shown in cell culture that morphine can affect WLS function through a direct interaction with the mu-opioid receptor, the primary mediator of opioid reward. This effect is due to a sequestering of WLS at the plasma membrane rather than a change in WLS protein levels. Therefore, opioids can likely alter Wnt signaling through mechanisms that do not require changes in WLS gene expression. It was for this reason that we also examined β-catenin, a downstream effecter of Wnt signaling.
Changes in Wnt signaling within the brain should consequently alter β-catenin levels. Interestingly, as summarized, we observed a significant increase in β-catenin levels in the hippocampus of those rats self-administering heroin compared to those who self-administered saline, hinting at an increase in Wnt signaling in this brain region. It would be interesting to determine whether transcript levels of β-catenin are changed in the hippocampus of heroin self-administering rats to assess whether this increase is due to a change in gene expression or protein stability. It is of note that the changes in WLS and β-catenin protein levels in the PFC of the largest and smallest saccharin suppressors were very similar to one another. One could argue, therefore, that changes in Wnt signaling, caused by alterations in functional levels of WLS, similarly affect β-catenin levels in Wnt receiving cells. That said, unlike WLS, levels of β-catenin in the PFC were higher in the largest suppressors vs. the smallest suppressors. Again, assessing mRNA levels for β-catenin could potentially support or refute this hypothesis.
Also, as described, in this study NCS-1 expression (like WLS and β-catenin) in the PFC was significantly decreased in the smallest saccharin suppressors and increased in the largest saccharin suppressors compared to the saline controls. It is known that, NCS-1 blocks D2R phosphorylation and internalization (Kabbani et al., 2002). The D2R is considered the major mediator of reward-associated behaviors not only in the NAc but also in the PFC (Shumay et al., 2012; Volkow et al., 2013). Tacelosky et al. 2015 have observed decreases in D2R expression in the PFC of heroin self-administering rats. It is tempting to speculate that an increase in NCS-1 in the largest suppressing animals boosts signaling through the reduced number of D2Rs in the PFC of these animals leading to their vulnerability to ALB.
4.3. Protective effects of sweet cue
Another possible explanation for the apparent reduction in ALB in this study relates to the potential protective effects of having had prior experience with the sweet cue. Sweets can be a highly protective form of enrichment. Of course, environmental enrichment is very protective in both cocaine (Puhl et al., 2012) and heroin (Imperio et al., in preparation) self-administering rats. Regarding a sweet, in a microdialysis study, we found that presentation of the saccharin cue before a morphine injection fully blunted the dopamine response to morphine (Grigson and Hajnal, 2007). The availability of a sweet cue also reverses the trend whereby female rats generally take more drug than male rats (Cason and Grigson, 2013). Indeed, with the taste cue, female rats actually take less cocaine than male rats. Ahmed finds that the concurrent availability of a sweet can completely prevent cocaine (Lenoir et al., 2007) and heroin (Madsen and Ahmed, 2015) self-administration in a large portion of rats. Additionally, Ahmed finds that the protective effect of a sweet over heroin increases with shorter access periods such as that used in the present study (Lenoir et al., 2013). So, the availability of the sweet cue, along with the limited time of daily access, may have contributed to the lack of animals exhibiting an addiction score of 3 (the percent of 3s in Tacelosky et al. 2015 was nearly 12% vs. 3% here). While there are alternative explanations, prior exposure to the sweet cue also may have contributed to the failure to find reduced WLS expression in the PFC with increasing addiction-like behavior. Future studies must test the merits of this hypothesis.
5. Conclusions
It would be highly advantageous to be able to predict individual vulnerability to substance use disorder. This is a primary goal of addiction research. Rats quickly avoid intake of an otherwise palatable saccharin cue when paired with a drug of abuse and greater avoidance is associated with greater drug-taking. As such, early avoidance of the cue potentially can be used to predict later vulnerability to drug. In accordance, here we tested whether early avoidance of a heroin-paired saccharin cue would predict later addiction-like behavior using a variation of the intermittent access model (Deroche-Gamonet et al., 2004). The results found somewhat less robust addiction-like behavior than expected and, while there was a relationship between avoidance of the drug-paired saccharin cue and addiction-like behavior, the relationship was evident only when looking at the extreme small and large suppressors. Avoidance of the heroin-paired saccharin cue also was not associated with a reduction in WLS, as previously found (Tacelosky et al., 2015), but it was associated with an elevation in both β-catenin and NCS-1 in the PFC. The reduction in ALB and the failure to find reduced levels of WLS in the PFC in high ALB rats are not likely due to small differences in the behavioral methodology used here vs. other papers (e.g., Deroche-Gamonet et al., 2004; Tacelosky et al., 2015), but it may relate to the use of a different antibody for WLS or to the fact that the rats in this study had prior exposure to a sweet, which can be highly protective (Cason and Grigson, 2013; Freet et al., 2009; Grigson and Hajnal, 2007; Lenoir et al., 2013). Future studies will test whether these proteins differ early in training, prior to differences in drug exposure, and whether ALB can be reduced by relatively brief prior exposure to a sweet. Such plasticity, albeit in the opposite direction, has been evidenced before when ALB was greatly augmented in rats with a history of having binged on fat (Puhl et al., 2011).
Acknowledgements
Support for this research was provided by NIH grant DA009815 (PSG), NIH grant DA035608 (RL), and by a grant from the Pennsylvania Department of Health, Commonwealth Universal Research Enhancements SAP#4100055576 (PSG and RL). The Department specifically disclaims responsibility for any analyses, interpretations or conclusions.
Abbreviations
- ALB
addiction-like behavior
- CS
conditioned stimulus
- CTA
conditioned taste aversion
- D2R
D2 dopamine receptor
- i.v.
intravenous
- NCS-1
neuronal calcium sensor-1
- NAc
nucleus accumbens
- PFC
prefrontal cortex
- PR
progressive ratio
- SNA
signaled non-availability
- US
unconditioned stimulus
- VTA
ventral tegmental area
- WLS
Wntless
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. A.P.A.T.F.o.D.-IV . Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. ISBN 9780125476126. [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp. Clin. Psychopharmacol. 1994;2:25. [Google Scholar]
- Cason AM, Grigson PS. Prior access to a sweet is more protective against cocaine self-administration in female rats than in male rats. Physiol. Behav. 2013;112–113:96–103. doi: 10.1016/j.physbeh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Edwards S, Koob GF. Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav. Pharmacol. 2013;24:356–362. doi: 10.1097/FBP.0b013e3283644d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freet CS, et al. Overexpression of DeltaFosB is associated with attenuated cocaine-induced suppression of saccharin intake in mice. Behav. Neurosci. 2009;123:397–407. doi: 10.1037/a0015033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behav. Neurosci. 2002;116:321–333. [PubMed] [Google Scholar]
- Grigson PS, Hajnal A. Once is too much: conditioned changes in accumbens dopamine following a single saccharin–morphine pairing. Behav. Neurosci. 2007;121:1234–1242. doi: 10.1037/0735-7044.121.6.1234. [DOI] [PubMed] [Google Scholar]
- Herrero-Turrion MJ, et al. Whole-genome expression profile in zebrafish embryos after chronic exposure to morphine: identification of new genes associated with neuronal function and mu opioid receptor expression. BMC Genomics. 2014;15:874. doi: 10.1186/1471-2164-15-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperio CG, Grigson PS. Greater avoidance of a heroin-paired taste cue is associated with greater escalation of heroin self-administration in rats. Behav. Neurosci. 2015;129:380–388. doi: 10.1037/bne0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, et al. Interaction of the mu-opioid receptor with GPR177 (Wntless) inhibits Wnt secretion: potential implications for opioid dependence. BMC Neurosci. 2010;11:33. doi: 10.1186/1471-2202-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbani N, et al. Interaction with neuronal calcium sensor NCS-1 mediates desensitization of the D2 dopamine receptor. J. Neurosci. 2002;22:8476–8486. doi: 10.1523/JNEUROSCI.22-19-08476.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am. J. Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz KL, et al. Heroin self-administration: II. CNS gene expression following withdrawal and cue-induced drug-seeking behavior. Pharmacol. Biochem. Behav. 2008;90:349–356. doi: 10.1016/j.pbb.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, et al. Intense sweetness surpasses cocaine reward. PLoS One. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, et al. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology. 2013;38:1209–1220. doi: 10.1038/npp.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen HB, Ahmed SH. Drug versus sweet reward: greater attraction to and preference for sweet versus drug cues. Addict. Biol. 2015;20:433–444. doi: 10.1111/adb.12134. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press/Elsevier; Amsterdam, Boston: 2007. [Google Scholar]
- Petko J, et al. MOR is not enough: identification of novel mu-opioid receptor interacting proteins using traditional and modified membrane yeast two-hybrid screens. PLoS One. 2013;8:e67608. doi: 10.1371/journal.pone.0067608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, et al. A history of bingeing on fat enhances cocaine seeking and taking. Behav. Neurosci. 2011;125:930–942. doi: 10.1037/a0025759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, et al. Environmental enrichment protects against the acquisition of cocaine self-administration in adult male rats, but does not eliminate avoidance of a drug-associated saccharin cue. Behav. Pharmacol. 2012;23:43–53. doi: 10.1097/FBP.0b013e32834eb060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumay E, et al. Repeat variation in the human PER2 gene as a new genetic marker associated with cocaine addiction and brain dopamine D2 receptor availability. Transl. Psychiatry. 2012;2:e86. doi: 10.1038/tp.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacelosky DM, Alexander DN, Morse M, Hajnal A, Berg A, Levenson R, Grigson PS. Low expression of D2R and Wntless correlates with high motivation for heroin. Behav Neurosci. 2015 Dec;129(6):744–55. doi: 10.1037/bne0000104. 2015. Epub 2015 Oct 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, et al. Predominance of D2 receptors in mediating dopamine’s effects in brain metabolism: effects of alcoholism. J. Neurosci. 2013;33:4527–4535. doi: 10.1523/JNEUROSCI.5261-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Yokel RA, Dewit H. Both Positive reinforcement and conditioned aversion from amphetamine and from apomorphine in rats. Science. 1976;191:1273–1275. doi: 10.1126/science.1257748. [DOI] [PubMed] [Google Scholar]
- World Health Organization [Retrieved October 23, 2015];Facts and figures. 2015 from www.who.int/substance_abuse/facts/en/
- World Health Organization [Retrieved October 23, 2015];Information sheet on opioid overdose. 2015 from www.who.int/substance_abuse/information-sheet/en/
- Yang PT, et al. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev. Cell. 2008;14:140–147. doi: 10.1016/j.devcel.2007.12.004. [DOI] [PubMed] [Google Scholar]