Abstract
Hydatid disease (HD) is endemic in many parts of the world. HD can affect virtually any organ system in body and should be kept as differential diagnosis of cystic lesion. HD is mostly asymptomatic; however, it demonstrates a variety of characteristic imaging findings depending on the site of involvement, stage of growth, mass effect, complications, or hematogenous spread, which helps in diagnosis. Radiography, ultrasonography (USG), computed tomography (CT), and magnetic resonance imaging (MRI) are commonly used imaging modalities. Radiography is helpful in chest and for demonstrating calcification. USG demonstrates characteristic findings such as cystic nature, daughter vesicles, membranes, septa, and hydatid sand. CT and MRI are modalities of choice for number, size, anatomic location, identification of local complications, and systemic spread. CT is, especially helpful for osseous involvement, and MRI is better for biliary and neurological involvement. Knowledge of these imaging findings helps in early diagnosis and timely initiation of appropriate therapy.
KEY WORDS: Computed tomography, hydatid disease, magnetic resonance imaging, ultrasonography
INTRODUCTION
Hydatid disease (HD) is a worldwide parasitic infection caused by the larval stage of Echinococcus tapeworm. The disease is endemic in many parts of the world, especially in cattle farming areas of South America, the Mediterranean region, the Middle East, Africa, Asia, and Australia.[1] HD is more commonly caused by Echinococcus granulosus and less commonly by Echinococcus multilocularis in its more aggressive form.
In its life cycle, the adult E. granulosus resides in the small bowel of its definite hosts, which are mostly dogs or other carnivorous animals, and then gravid proglottids release eggs that are passed in the feces. These eggs are ingested by intermediate host, which are commonly sheep or other ruminants. The eggs then release oncospheres in the small bowel and then migrate either through portal venous or lymphatic circulation to liver or less commonly lodged in other organs. At the organ site, the embryos either die or develop into hydatid cysts. Humans can be infected secondarily if they ingest substances such as water or vegetables contaminated with Echinococcus eggs.[2]
The liver acts as the first line of defense and, therefore, is the most common site of involvement in approximately 75% of cases by HD. Less commonly, hydatid cyst can involve lung, spleen, kidney, bones, brain, and other rare anatomic locations.[2,3]
Structurally, hydatid cyst is composed of three layers. The outer most layer is pericyst, which is formed by compressed host tissue and fibrous reaction; the middle laminated membrane, also referred to as ectocyst is an acellular structure; and the inner most germinative layer referred as endocyst produces daughter vesicles containing protoscolices.[2,4] The cystic fluid is antigenic in nature and can produce anaphylactic reaction if released into host circulation.
IMAGING OF HYDATID DISEASE
The imaging methods used for diagnosis and evaluation of the extent of HD are ultrasonography (USG), computed tomography (CT), magnetic resonance imaging (MRI), and less commonly radiography and urography. USG is screening modality of choice and is also used to monitor the efficacy of treatment. It clearly demonstrates the hydatid sand, floating membranes, daughter cysts, and vesicles inside the cyst. CT has high sensitivity and specificity for HD. CT is an important diagnostic modality in detecting cyst wall or septal calcification, demonstrating internal cystic structure posterior to calcification, assessing complications, depicting osseous lesions and in cases where USG has limitations (obesity, excessive bowel gases, abdominal wall deformities, and previous surgery). MRI is superior for demonstrating cyst wall defect, biliary communication, and neural involvement.[5,6] Recently, MRI has been shown to be important in differentiating liver hydatid cysts from other simple cysts using diffusion-weighted sequence.[7]
The imaging findings depend on the organ involved, host reaction, stage of evolution, and maturity of disease. The findings can range from purely cystic lesions to solid-appearing masses. The cysts may be solitary or multiple, unilocular or multivesicular, and with or without calcification. Presence of daughter vesicles and membranes within the cyst, peripheral cyst wall, or internal matrix calcification are important findings for differential diagnosis of HD. Various classifications are being used to describe hydatid cysts. More commonly, depending on the imaging appearance the hydatid cysts are classified into four types.[6,8]
Type I: Simple cyst with no internal architecture
During the initial stage of development, the hydatid cyst appears as a unilocular, well-defined cystic lesion with no internal architecture with or without hydatid sand or internal septa. On CT, there is a frequent postcontrast enhancement of cyst wall and septa [Figure 1]. On MRI, the hydatid cyst shows a characteristic hypointense rim on T2-weighted images representing the pericyst. MRI is useful in differentiating Type I hydatid cyst from simple cyst depending upon the apparent diffusion coefficient values, which are found to be lower in hydatid cysts (2.5 × 103 ± 0.9) in comparison to that of simple cyst (3.5 × 103 ± 0.5).[9]
Figure 1.
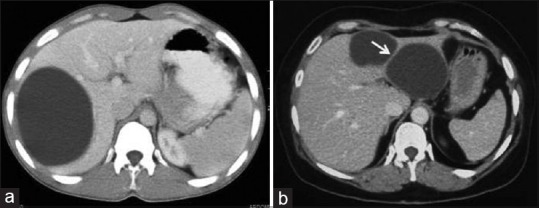
Contrast-enhanced computed tomography of abdomen in two different patients show unenhanced hypodense mass with well-defined borders and no internal architecture without (a) or with septa (arrow) (b) -Type I hydatid cysts
Type II: Cyst with daughter cyst(s) and/or matrix
Multivesicular cysts appear as fluid collection with multiple internal septae representing walls of daughter cysts, which are usually arranged at the periphery, having attenuation lower than that of the mother cyst [Figure 2]. Depending upon the maturity and arrangement of daughter cysts within the maternal cyst, a Type II cyst can be seen as Type IIA cyst containing multiple-rounded daughter cysts arranged at periphery with central high-density matrix (wheel spoke appearance); Type IIB cyst containing large irregular daughter cysts occupying almost the entire volume of maternal cyst (rosette appearance); or Type IIC cyst appearing as relatively high-attenuation lesion containing occasional calcification and daughter cyst suggesting degeneration of old cyst [Figure 3].[10]
Figure 2.
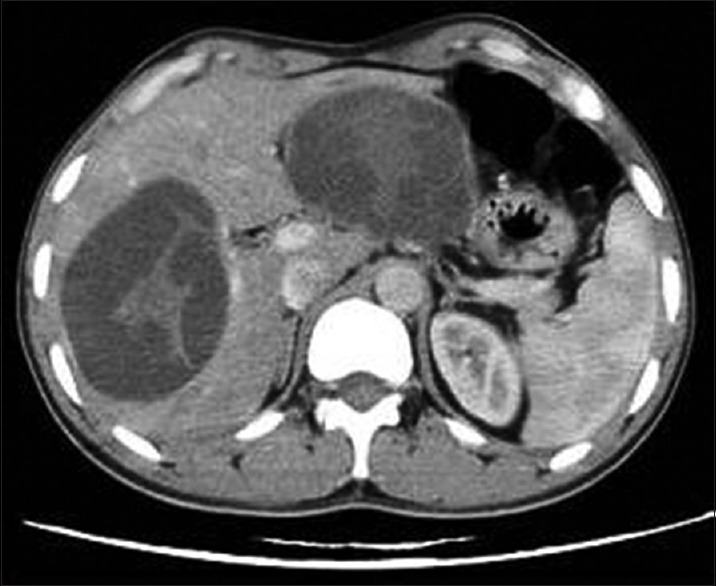
Contrast-enhanced computed tomography of abdomen shows Type II liver hydatid cysts with multiple irregularly shaped daughter cysts that occupy almost the entire volume of the mother cyst – “rosette appearance”
Figure 3.
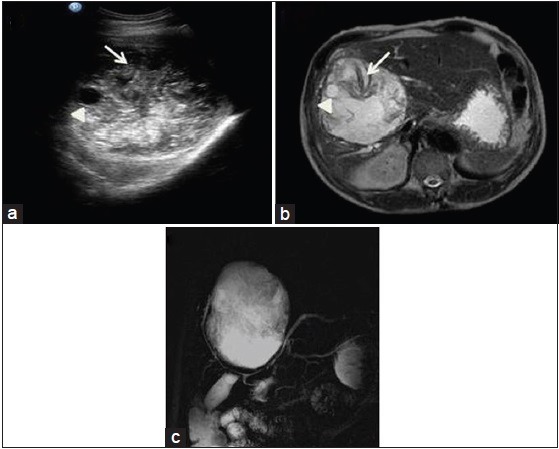
Ultrasound (a), magnetic resonance imaging axial T2-weighted (b) and magnetic resonance cholangiopancreatography (c) images show heterogeneous soft tissue lesion with internal membranes (arrow), few calcifications and daughter cysts (arrow head) causing mild intrahepatic biliary radical prominence due to mass effect
On MRI, the daughter cysts or brood capsules may appear hypointense or isointense relative to the maternal matrix on T1- and T2-weighted images with T2 hypointense pericyst [Figure 4]. Detached endocyst appears as twisted-linear structure within the cyst as the serpent-sign or snake-sign.[10]
Figure 4.
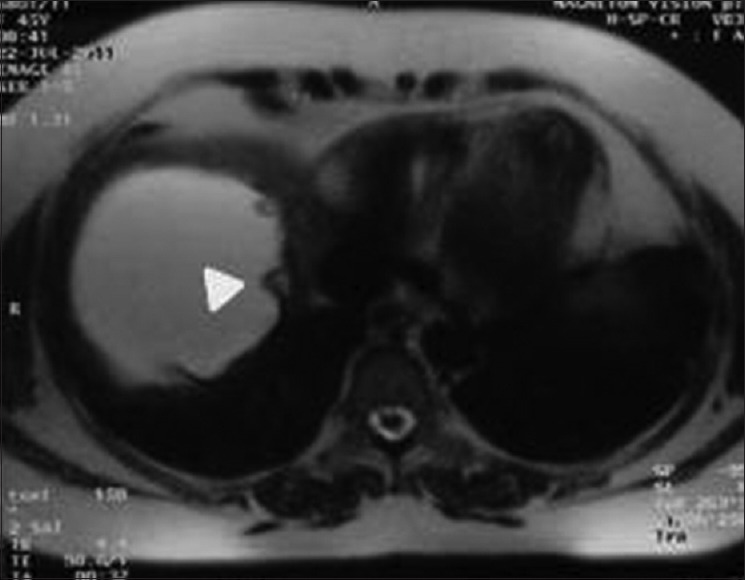
Magnetic resonance imaging of abdomen T2-weighted axial image shows liver hydatid with multiple peripherally arranged brood capsules (arrow head) and hypointense pericyst
Type III: Calcified cyst
Type III hydatid cysts are calcified cysts. CT is the preferred imaging method to evaluate calcified hydatid cysts as in USG, they show strong posterior acoustic shadowing. In CT, they appear as well-defined hyperattenuating lesions and in MRI as hypointense areas [Figure 5].
Figure 5.
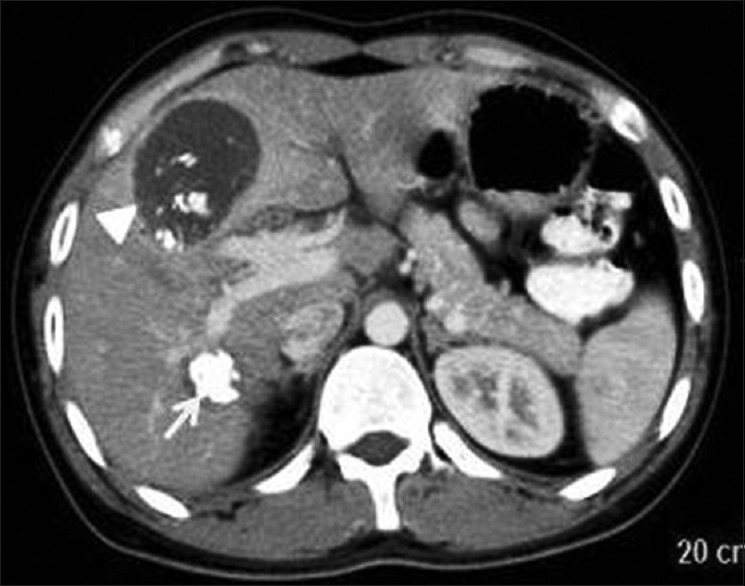
Contrast-enhanced computed tomography of abdomen shows calcified Type IIc (arrow head) and III (arrow) hydatid cysts showing calcification of wall, internal matrix, and membranes
Type IV: Complicated hydatid cyst
CT and MRI play an important role in imaging of complicated hydatid cysts, common complications include rupture and superinfection. Hydatid cyst rupture occurs in 50-90% cases, usually due to age, chemical reaction, or a host defense mechanism and can be of three types.[11,12] In contained rupture, the endocyst detaches from pericyst appearing as curvilinear structure or floating membranes, finding that is highly specific for HD [Figure 6]. In communicating rupture, the cyst ruptures into the structures incorporated in the pericyst usually in biliary and bronchial radicles. Direct rupture into peritoneal or pleural cavities or other structures is due to rupture of both the endocyst and the pericyst.[10]
Figure 6.
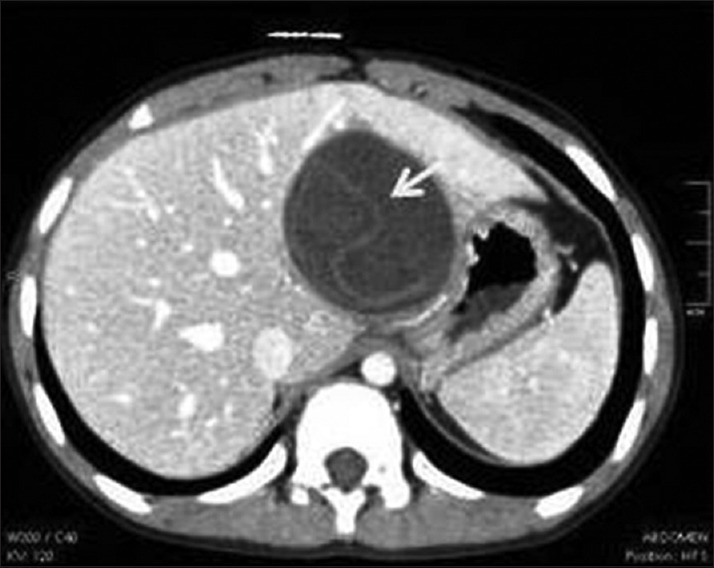
Contrast-enhanced computed tomography abdomen shows contained rupture of liver hydatid cyst with “floating membrane sign” (arrow) produced by detachment of the germinal membrane of the endocyst
Up to 25% cases of ruptured cyst may become secondarily infected. The cyst may show internal gas, fluid-fluid, or air-fluid level or fat as indirect signs of infection and/or communication with hollow viscera or biliary tree [Figure 7].
Figure 7.
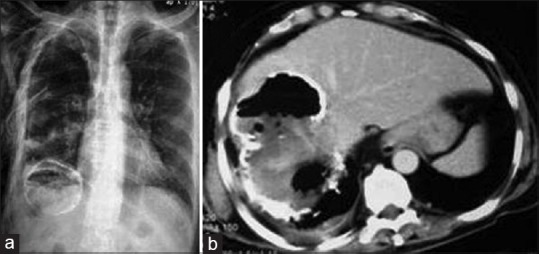
X-ray chest (a) and contrast-enhanced computed tomography of abdomen (b) show partially-calcified liver hydatid cyst with intracystic air and right pleural effusion, suggesting super-infection and rupture into the thoracic cavity
SITES OF INVOLVEMENT
Abdominal involvement
Liver
Liver is the most common site of involvement by HD; most cysts are located in the right lobe. They can be single or multiple in number with or without involvement of other organs. Differential diagnosis of Type I cyst includes simple epithelial cyst. Type II multivesicular cyst appears as well-defined fluid attenuation lesion with multiple septae or daughter cysts in a honeycomb pattern [Figure 8]. In advanced stage, cysts become partially or completely calcified, hydatid cyst showing complete dense calcification is assumed to be inactive cyst [Figure 5].[13]
Figure 8.
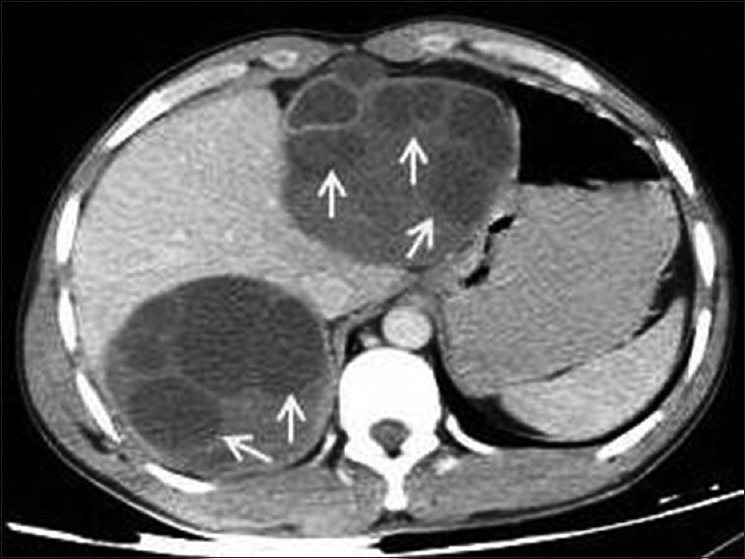
Contrast-enhanced computed tomography of abdomen shows Type II liver hydatids with multiple daughter cysts (arrows)
Complications of liver hydatid cyst
Common complications of hydatid cyst include cyst rupture and superinfection. In contained rupture, detached endocyst appears as floating membrane inside the cyst at USG, CT, or MRI [Figure 6]. On imaging, defect in the cyst wall can be identified in both communicating and direct rupture with or without partially collapsed cyst and passage of contents [Figure 7].
Communicating rupture can occur into adjacent biliary radicle, hepatic parenchyma, or sub-capsular hepatic space [Figure 9]. Biliary communication of HD has been demonstrated in up to 90% of hepatic cysts and can occur either through small fissures due to incorporation of biliary radicles (in 55% of cases) or through a wide direct perforation.[14,15] Daughter vesicles or floating membranes can be visualized within the biliary radicles besides the visualization of defect in the cyst wall [Figures 10 and 11]. CT and MRI are superior to USG in demonstrating hydatid contents in distal common bile duct.
Figure 9.
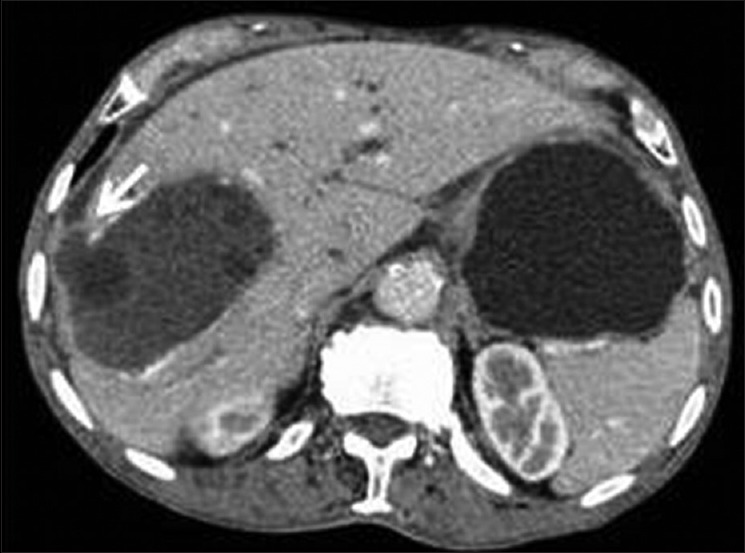
Contrast-enhanced computed tomography of abdomen shows communicating rupture of liver hydatid into surrounding hepatic parenchyma and sub-capsular hepatic space (arrow)
Figure 10.
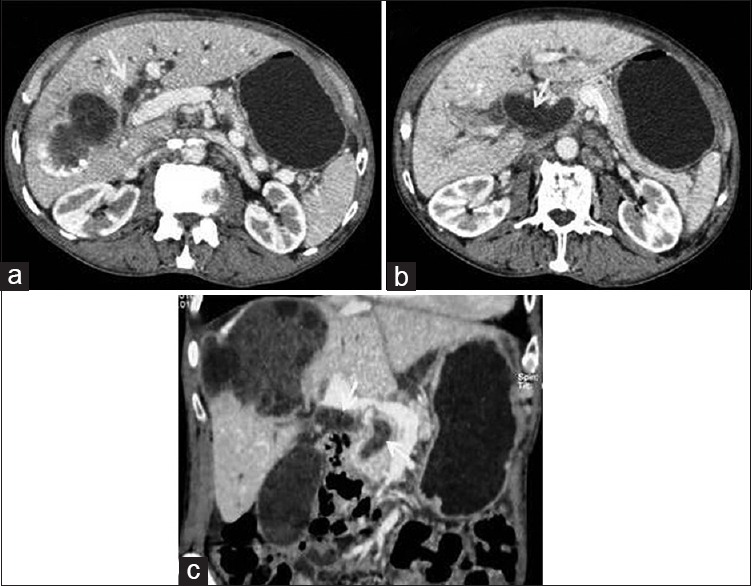
Contrast-enhanced computed tomography abdomen: Sequential axial (a and b) and reconstructed coronal (c) images show intrabiliary rupture of liver hydatid cyst visualized as dilated biliary system with intraluminal daughter vesicles and membranes (arrows) in a patient presented with jaundice
Figure 11.
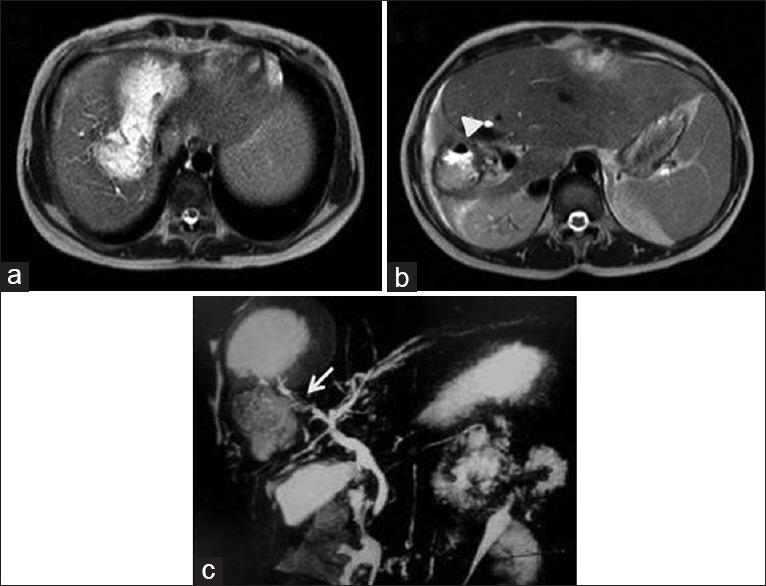
Magnetic resonance imaging axial T2-weighted (a and b) and magnetic resonance cholangiopancreatography (c) images show communication between ruptured hydatid cyst and biliary radicle (arrow) with air-foci within the cyst (arrow head) and biliary radicles. Right lobe of liver atrophy is noted
Direct rupture allows the passage of hydatid contents into the peritoneal cavity, pleural cavity, hollow viscera, or abdominal wall, and it is usually seen in peripherally located lesions [Figure 7]. Direct rupture can have important clinical consequences, including anaphylaxis, hydatid dissemination, or secondary bacterial infection.
Infection in hydatid cyst mimics hepatic abscess clinically and radiologically. Infected cyst demonstrates poorly defined margins with or without perilesional inflammation, presence of gas, or air-fluid level [Figure 12].[16]
Figure 12.
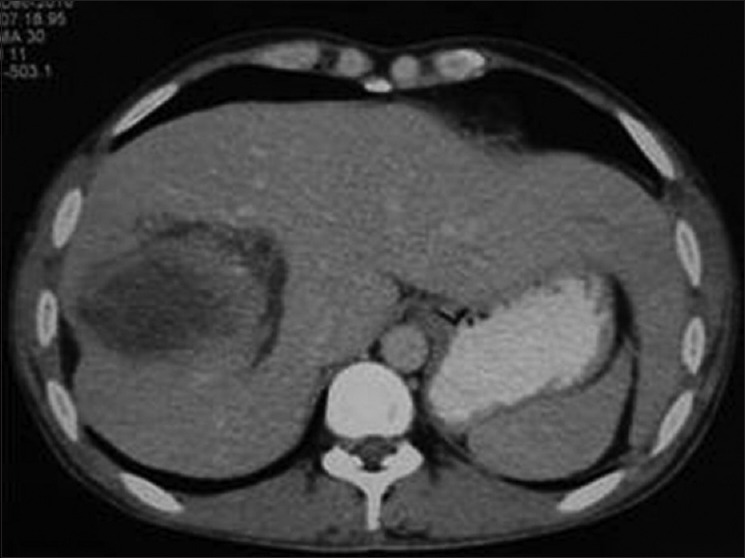
Contrast-enhanced computed tomography of abdomen shows infected hydatid cyst with peripheral enhancement and perilesional inflammatory changes
Compression of portal vein and thrombosis is a rare complication of hepatic hydatid and usually seen in hydatid cysts located in the caudate lobe and at hepatic bifurcation.[17,18] Lack of flow within the portal venous system with or without visualization of cyst material may be demonstrated on imaging. Multiple venous collaterals at hepatic hilum, secondary cavernomatous transformation, or secondary Budd-Chiari syndrome can be seen [Figure 13].
Figure 13.
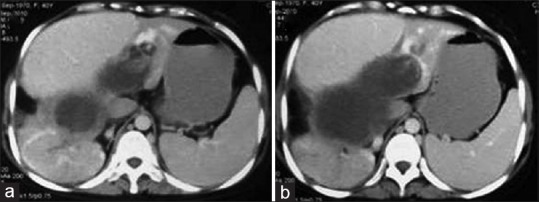
Contrast-enhanced computed tomography of abdomen: Sequential axial images (a and b) show liver hydatid cyst leading to secondary Budd-Chiari syndrome due to compression of inferior vena cava and hepatic veins
Spleen
Involvement of spleen by HD has been reported from 0.9% to 8% of cases, and it is found to be the third most common location after liver and lungs in some series.[19] Splenic involvement is commonly secondary to systemic dissemination or intraperitoneal spread from ruptured liver hydatid cysts, isolated primary splenic HD is rare and constitutes for <2% of cases.[20] Imaging appearances are similar to those of hydatid cysts in liver [Figure 14a and b]. Complications are rare and include infection; rupture into pleural or peritoneal cavity; fistula formation into hollow organs, such as colon or stomach; rupture into the bronchial tree; splenothoracic fistula; pleural effusion; calcification or hypersplenism [Figures 15 and 16].
Figure 14.
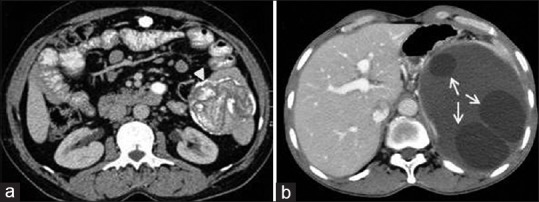
Contrast-enhanced computed tomography of abdomen in two different patients shows splenic hydatid cysts (a) well-defined lesion with peripheral wall calcification and membranes appear as lamellated calcification (arrow head) and (b) well-defined multivesicular mass with daughter cysts (arrow)
Figure 15.
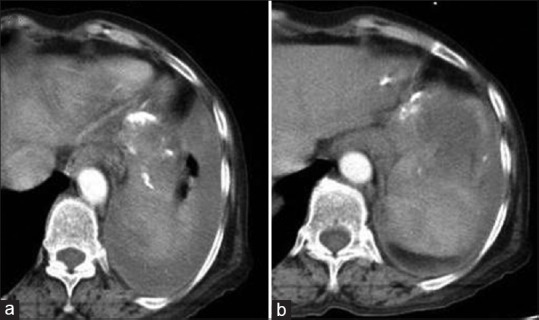
Contrast-enhanced computed tomography of abdomen (a and b) shows rupture of splenic hydatid causing left pleural effusion
Figure 16.
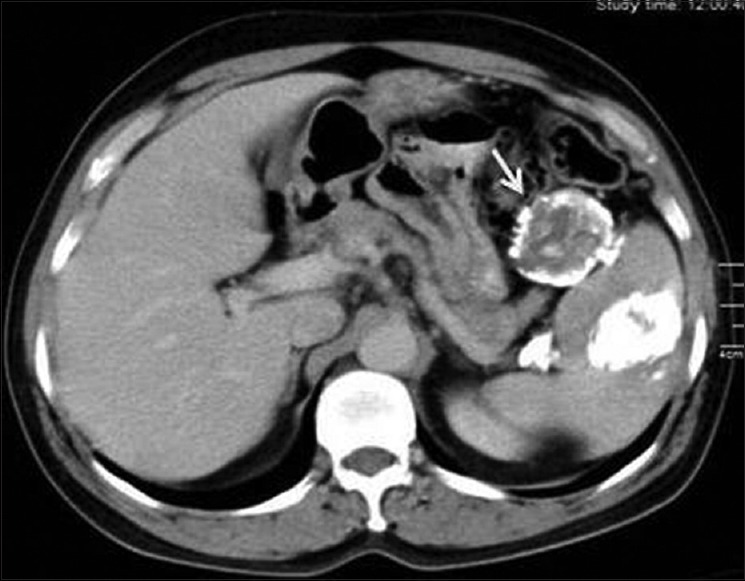
Contrast-enhanced computed tomography of abdomen shows calcified splenic hydatid cyst with peritoneal seedling (arrow)
Kidneys
Renal involvement of HD is rare and has been reported in 2–3% of cases.[21,22] Renal hydatid cysts are usually solitary and located in cortex, at upper or lower pole [Figure 17] and can reach up to 10 cm before they become symptomatic.[21] Renal hydatid may appear as uni- or multi-locular well-defined cystic lesion with or without calcification or curvilinear structure. Hydatid cyst may infect secondarily or rupture into collecting system or perinephric tissues. Ruptures into the collecting system have been reported in up to 18% of cases. Differential diagnoses are renal cyst, cystic nephroma, and necrotic renal cell carcinoma. HD of adrenal gland is rare (0.06-0.18%).[23]
Figure 17.
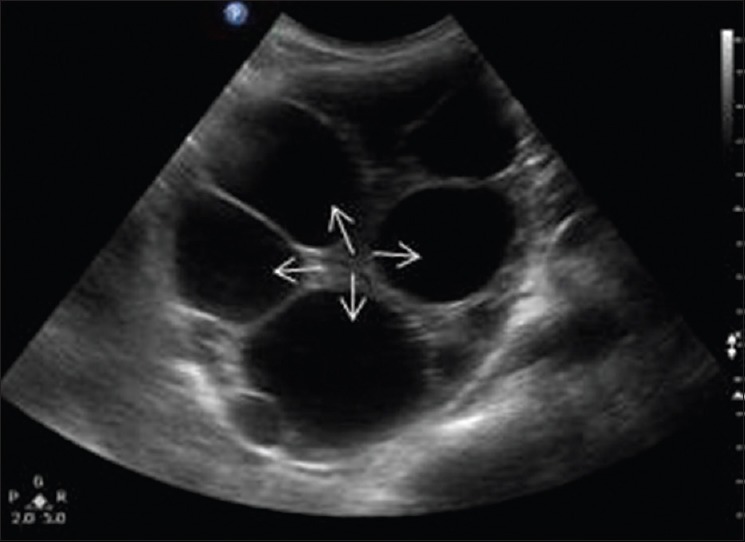
Ultrasonography abdomen shows well-capsulated multivesicular cystic lesion at upper pole of right kidney with multiple daughter cysts (arrows)
Pancreas
Primary pancreatic involvement of HD is very rare (0.25%), and it is often secondary to hepatic disease.[24] The diagnosis of pancreatic hydatid cyst is possible with high index of suspicion as it is commonly confused with pancreatic pseudocyst or cystic neoplasm. They appear as single or multiple, well-defined cystic lesion with thickened wall with or without calcification.
Peritoneum and retroperitoneum
Primary intra- or retro-peritoneal hydatid cysts are very rare.[25] Peritoneal hydatid cysts are prevalent in approximately 13% of abdominal hydatid cysts and are usually secondary to spontaneous, traumatic, or surgical rupture of hepatic, liver, or mesenteric hydatid cysts [Figure 18]. Retroperitoneal cysts are also secondary to involvement of other organs, most common being liver. On imaging, they look similar to hydatid cyst at any other location. Peritoneal hydatid cysts are usually multiple and can be seen anywhere [Figure 19]. Presence of daughter cysts helps in differentiating hydatid cyst from mesenteric or intestinal duplication cyst.
Figure 18.
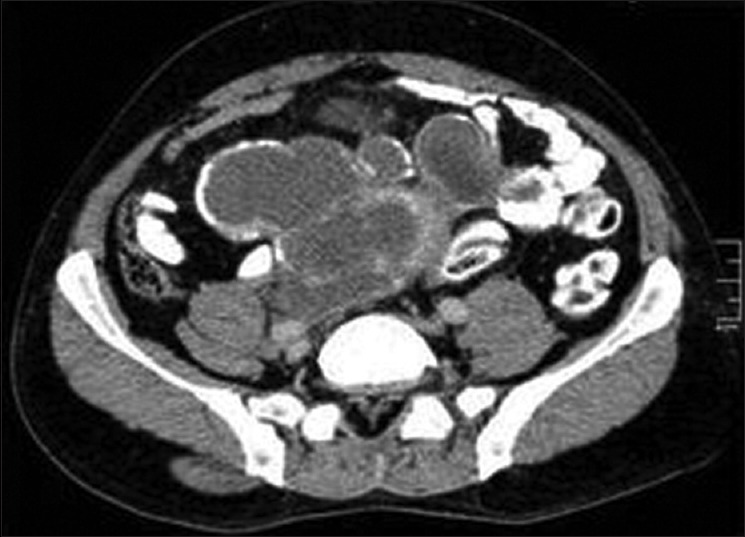
Contrast-enhanced computed tomography of abdomen shows peritoneal seeding of liver hydatid with daughter cysts and rim like mural calcification
Figure 19.
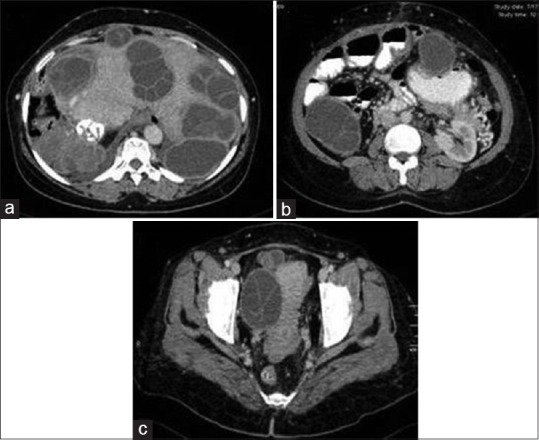
Contrast-enhanced computed tomography of abdomen: serial axial images (a-c) show liver hydatidosis with peritoneal seeding
An isolated hydatid cyst in the pelvic cavity in retrovesical location has also been reported causing urinary symptoms due to mass effects [Figure 20]. In such cases, the hydatid embryo gains access to pelvis by hematogenous or lymphatic route.[26,27]
Figure 20.
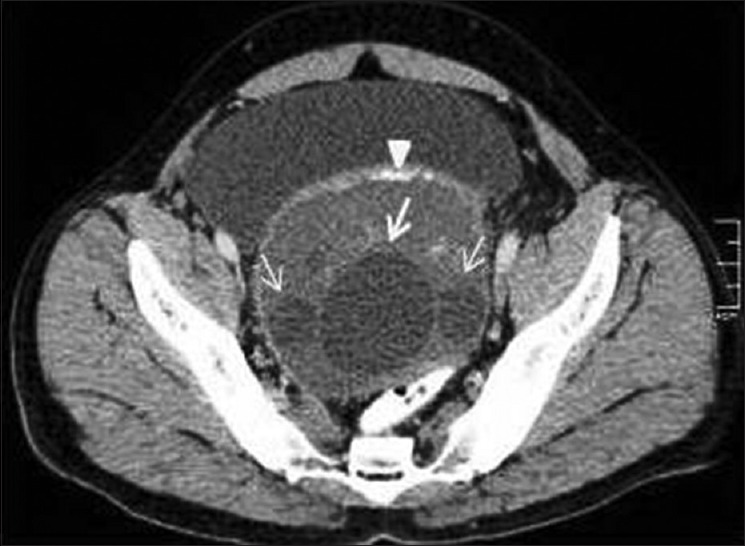
Contrast-enhanced computed tomography of pelvis shows isolated retrovesical multivesicular hydatid cyst with daughter cysts (arrows) and rim like mural calcification (arrow head)
Extraabdominal involvement
Brain
Involvement of central nervous system by HD is rare and has been reported in 1-2% of cases.[28] Cerebral HD is more commonly seen in children and young adults. Brain hydatid cysts are usually single and unilocular, most common location being cerebral hemispheres, particularly in the territory of the middle cerebral artery. The less common sites of involvement are cerebellum, pons, ventricles, cavernous sinus, extradural, skull, and eye ball.[29] Cystic lesions such as porencephalic cyst, arachnoid cyst, cystic tumor of the brain, and pyogenic abscess are included as differential diagnosis of intracerebral hydatid cyst.
The cerebral hydatid cysts grow slowly and present late generally due to the compression of adjacent structures. In CT and MRI, cerebral hydatid cyst appears as well-defined, cerebrospinal fluid (CSF) signal density, or intensity lesion with or without brood capsules or daughter cysts [Figure 21]. Thin rim of peripheral enhancement can be visualized due to fibrous capsule or secondary to superadded infection. Calcification of brain hydatid cyst is rare (<1%). Perilesional edema is usually not seen as compare to brain abscesses or cystic neoplasms.
Figure 21.
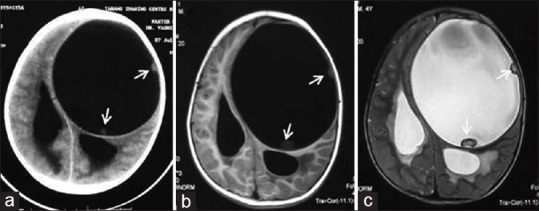
Computed tomography (a) and magnetic resonance imaging T1-weighted (b) and T2-weighted (c) images of brain show a well-circumscribed cystic lesion in left cerebral hemisphere with peripheral daughter cysts (arrows) sugg
Hydrocephalous due to ventricular compression can be seen. Rarely, hydatid cyst may rupture into the ventricular system or subarachnoid spaces with demonstration of floating membranes or fluid-CSF level [Figure 22]. Peripherally located hydatid cyst can protrude into the meninges and calvaria or can cause bone erosions. Sutural separation or unilateral enlargement of vault can be seen in childhood.
Figure 22.
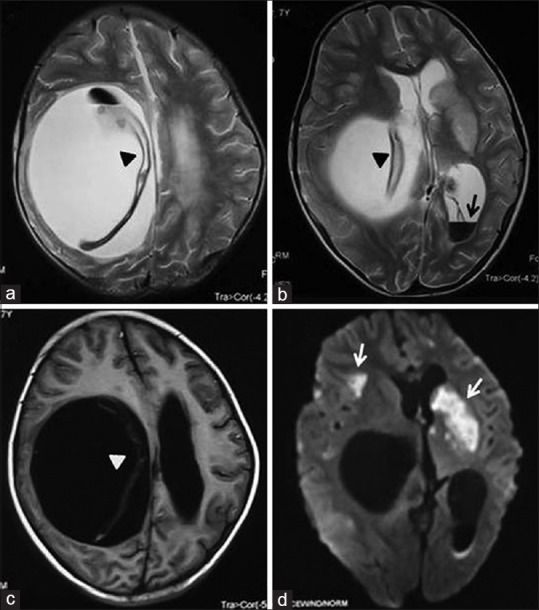
Magnetic resonance imaging axial T2-weighted (a and b), T1-weighted (c) and diffusion-weighted (d) images of brain show a well-circumscribed cystic lesion in right cerebral hemisphere with detached germinal membrane (arrow heads) and intraventricular rupture (black arrow) causing mass effect and multiple brain infarcts (white arrow)
Spinal cord hydatid cysts are rare (<1%), thoracic spine being most common location.[29] They are usually multiple and can be intramedullary, intradural extramedullary, or extradural intraspinal in location. Spinal cord hydatid cyst usually appears as cystic lesion of CSF signal intensity without postcontrast enhancement.
Thoracic involvement
Pulmonary
Lungs are the second most frequent site of hematogenous spread in adults and have been reported as the most common site in children (15-25% of cases).[2,11] Concurrent involvement of the liver and lungs is seen in approximately 6% of all patients.[30] The size of the cyst may vary from 1 to 20 cm due to the compressibility of lungs.[11] Cysts are more commonly located in lower lobes (60% cases) and can be multiple (30% cases) and bilateral (20% cases).[10,31] Complications of pulmonary hydatid cyst include rupture (50-90% cases), superinfection, and rarely hepato bronchial fistula or acute pulmonary embolism secondary to rupture.
Uncomplicated cysts appear as round, oval, or polycyclic well-defined mass lesion. Calcification of the pericyst is very rare (0.7%) in pulmonary hydatid cysts. Daughter cysts are rarely seen in lung HD. As the cyst grows, it may cause erosions in the incorporated bronchioles allowing introduction of air within the cyst, appearing either as thin crescent of air between the pericyst and the laminated membrane (the “crescent sign” or “meniscus sign”) or air-fluid level within the endocyst. Sometimes, collapsed membranes may be seen floating on dependent portion of the cyst or on the surface of the cyst also known as the “water-lily sign” [Figure 23].
Figure 23.
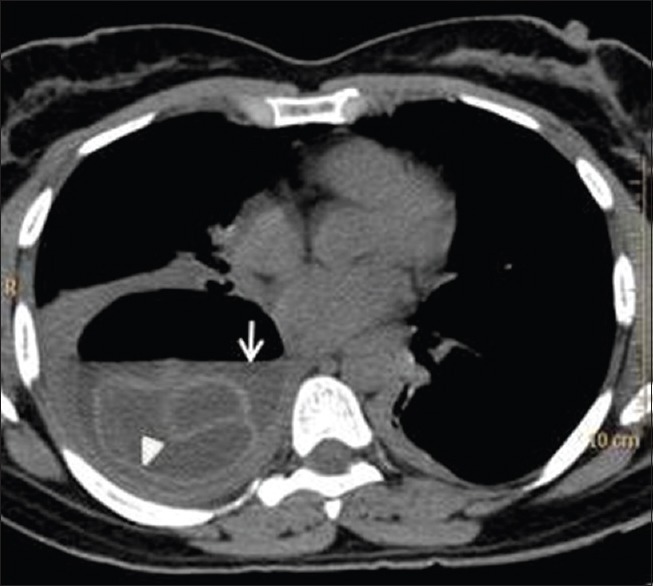
Non-contrast computed tomography of thorax shows a cystic lesion with air-fluid level (arrow) and collapsed endocyst (arrow head) lying in the dependent part of the cyst ("Water-lily sign")
The “air bubble sign” seen as the presence of air bubbles in regions surrounding the cyst may develop as a consequence of cyst rupture, secondary to bacterial infections with presence of consolidation in adjacent lung parenchyma [Figure 24]. The “air bubble sign” is reported to be very sensitive and specific (85.7% sensitivity and 96.6% specificity) in establishing the diagnosis of complicated hydatid cyst.[32] Cysts may rupture directly into the pleural or peritoneal cavity. Rarely, during the medical treatment, pulmonary hydatid would be seen as thin-walled air-filled cavitary lesion [Figure 25].
Figure 24.
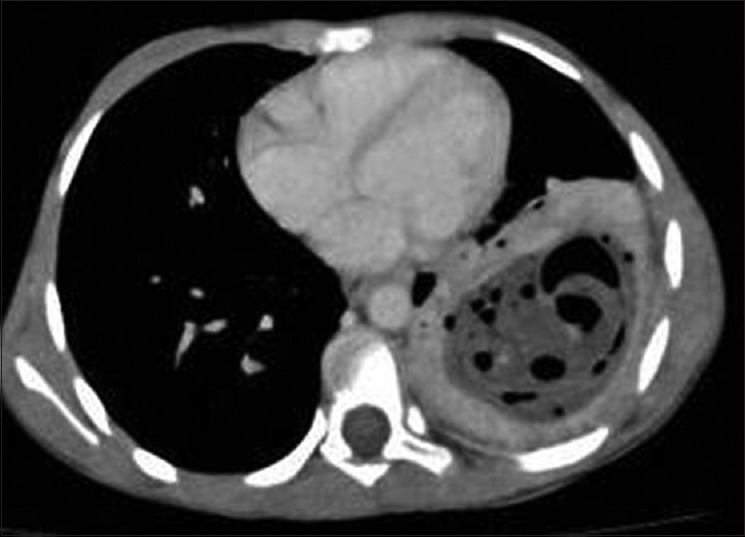
Contrast-enhanced computed tomography of chest (mediastinal window) shows hypodense lesion in left lower lobe with thick-enhancing wall with air bubbles and surrounding infection- The “air bubble” sign
Figure 25.
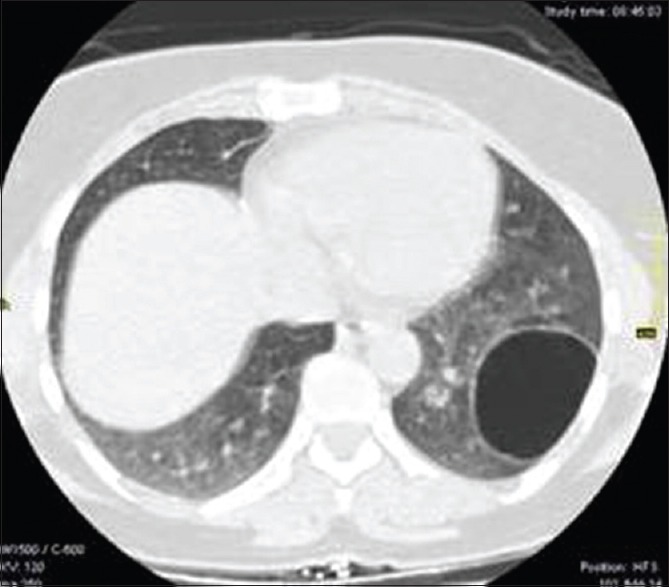
Contrast-enhanced computed tomography of chest shows thin walled air-filled cavitary lesion in left lower lobe during the course of pulmonary hydatid disease
Intrathoracic extrapulmonary
Intrathoracic but extrapulmonary hydatid cysts are rare and can be located in the fissures, pleural cavity, chest wall, mediastinum, myocardium, and diaphragm. They can be solitary or multiple and appearance can vary from Type I to Type III cysts.[33] CT and MRI are the main diagnostic tools in such cases. Pleural and mediastinal involvement are generally secondary to lung, hepatic, or splenic involvement [Figures 26 and 27].
Figure 26.
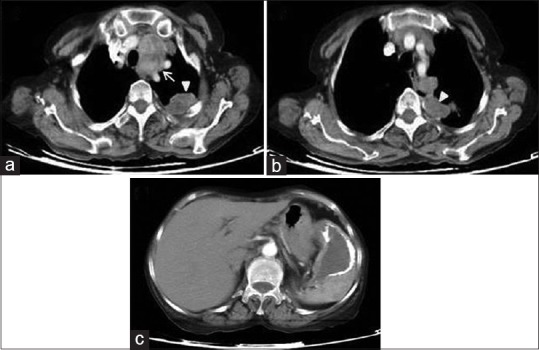
Contrast-enhanced computed tomography of thorax shows mediastinal (a – arrow) and pleural (a and b – arrow heads) dissemination of (c) splenic hydatid cyst
Figure 27.
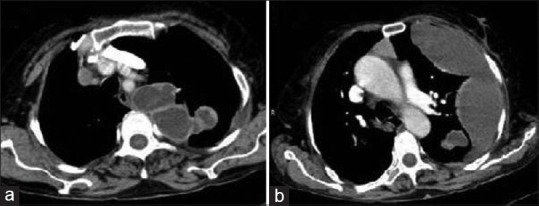
Contrast-enhanced computed tomography of chest: Serial axial images (a and b) show pleural dissemination of liver hydatid cyst
Cardiac HD is rare (0.02-2% cases) and may be due to hematogenous spread or rupture of a lung hydatid cyst.[32] Most common location being left ventricle followed by interventricular septum, right ventricle, pericardium, and right or left atrium.[34,35] CT and MRI demonstrate unilocular or multivesicular cystic lesion in relation to cardiac chambers [Figure 28]. Pericardial hydatid cysts are usually seen in costophrenic recess, at hila and in superior mediastinum at the level of aortic arch [Figure 29].
Figure 28.
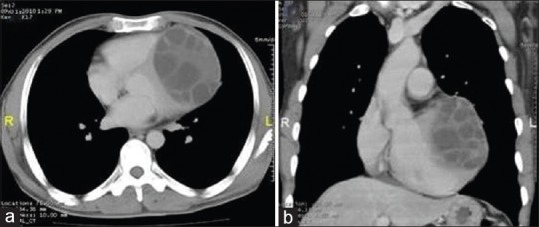
Contrast-enhanced computed tomography of chest: axial (a) and coronal reconstructed (b) images show well capsulated multivesicular cystic lesion in myocardium of left ventricle with multiple daughter cysts
Figure 29.
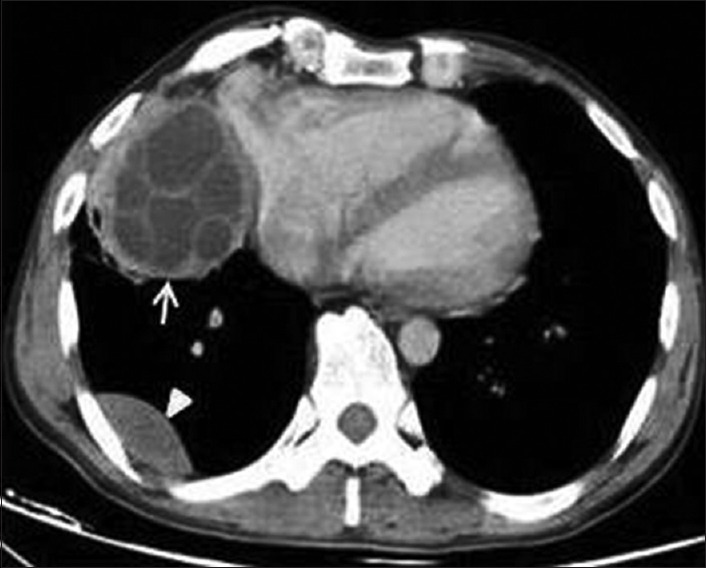
Contrast-enhanced computed tomography of thorax shows Type II hydatid cysts with multiple daughter cysts in right cardiophrenic angle that connects to the pericardium (arrow) and a unilocular pleural-based cyst (arrow head)
Bones and soft tissues
Osseous involvement has been reported in 0.5-2% of cases, most common location being spine followed by pelvis, long bones, skull, and ribs.[36] On imaging, bone hydatid appears as a well-defined, multiloculated expansile lytic lesion with irregular branching. Pathological fracture can also be seen. As the lesion grows, it causes thinning and breach of cortex and extension into the adjacent soft tissue. Intraosseous cyst rarely demonstrates calcification; however, extraosseous component can calcify.
Primary soft-tissue involvement is rare and represents 0.5-4.7% of cases.[37] Muscular HD preferentially involves muscles of the neck and trunk and at the root of the limbs. At imaging, they may appear as unilocular or multilocular cystic lesion or complex solid lesion depending upon growth of the cyst [Figure 30]. Differential diagnoses include abscess, synovial cyst, chronic hematoma, and necrotic soft tissue tumor.
Figure 30.
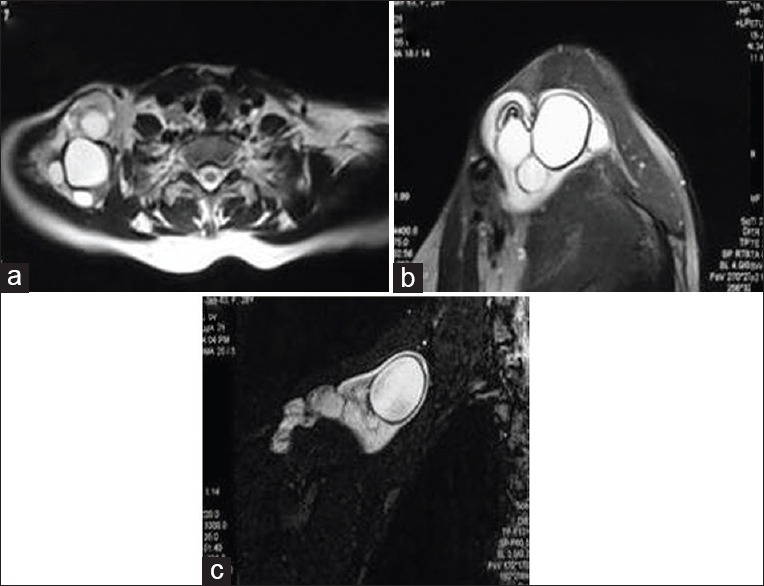
Magnetic resonance imaging of shoulder, axial T2-weighted (a) sagittal T2-weighted (b) and fat-suppressed Short tau inversion recovery (c) images of a patient with painless swelling in shoulder region showing multiple hydatid cysts with internal daughter cysts and membrane
CONCLUSION
HD can affect virtually any organ system in body. Commonly, it demonstrates characteristic imaging findings that help to differentiate HD from other entities. However, at times, it can present itself with atypical manifestations due to secondary complications or at atypical sites. Familiarity with these imaging findings helps in early diagnosis and timely initiation of appropriate therapy, thereby reducing patient morbidity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Nathani Sanjay MD, Consultant Radiologist, SKG Scan (Unit of Goyal Hospital and Research Centre PVT., Ltd.,), Jodhpur, Rajasthan, India.
REFERENCES
- 1.Marsden PD. The cestodes. In: Beeson PD, McDermott W, editors. Textbook of Medicine. Philadelphia, PA: WB Saunders; 1975. pp. 510–1. [Google Scholar]
- 2.Beggs I. The radiology of hydatid disease. AJR Am J Roentgenol. 1985;145:639–48. doi: 10.2214/ajr.145.3.639. [DOI] [PubMed] [Google Scholar]
- 3.Engin G, Acunas B, Rozanes I, Acunas G. Hydatid disease with unusual localization. Eur Radiol. 2000;10:1904–12. doi: 10.1007/s003300000468. [DOI] [PubMed] [Google Scholar]
- 4.Dahniya MH, Hanna RM, Ashebu S, Muhtaseb SA, el-Beltagi A, Badr S, et al. The imaging appearances of hydatid disease at some unusual sites. Br J Radiol. 2001;74:283–9. doi: 10.1259/bjr.74.879.740283. [DOI] [PubMed] [Google Scholar]
- 5.Gharbi HA, Hassine W, Brauner MW, Dupuch K. Ultrasound examination of the hydatic liver. Radiology. 1981;139:459–63. doi: 10.1148/radiology.139.2.7220891. [DOI] [PubMed] [Google Scholar]
- 6.Marrone G, Crino’ F, Caruso S, Mamone G, Carollo V, Milazzo M, et al. Multidisciplinary imaging of liver hydatidosis. World J Gastroenterol. 2012;18:1438–47. doi: 10.3748/wjg.v18.i13.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oruç E, Yıldırım N, Topal NB, Kılıçturgay S, Akgöz S, Savcı G. The role of diffusion-weighted MRI in the classification of liver hydatid cysts and differentiation of simple cysts and abscesses from hydatid cysts. Diagn Interv Radiol. 2010;16:279–87. doi: 10.4261/1305-3825.DIR.2807-09.2. [DOI] [PubMed] [Google Scholar]
- 8.Precetti S, Gandon Y, Vilgrain V. Imaging of cystic liver diseases. J Radiol. 2007;88:1061–72. doi: 10.1016/s0221-0363(07)89919-7. [DOI] [PubMed] [Google Scholar]
- 9.Inan N, Arslan A, Akansel G, Anik Y, Sarisoy HT, Ciftci E, et al. Diffusion-weighted imaging in the differential diagnosis of simple and hydatid cysts of the liver. AJR Am J Roentgenol. 2007;189:1031–6. doi: 10.2214/AJR.07.2251. [DOI] [PubMed] [Google Scholar]
- 10.Polat P, Kantarci M, Alper F, Suma S, Koruyucu MB, Okur A. Hydatid disease from head to toe. Radiographics. 2003;23:475–94. doi: 10.1148/rg.232025704. [DOI] [PubMed] [Google Scholar]
- 11.Pedrosa I, Saíz A, Arrazola J, Ferreirós J, Pedrosa CS. Hydatid disease: Radiologic and pathologic features and complications. Radiographics. 2000;20:795–817. doi: 10.1148/radiographics.20.3.g00ma06795. [DOI] [PubMed] [Google Scholar]
- 12.Lewall DB, McCorkell SJ. Rupture of echinococcal cysts: Diagnosis, classification, and clinical implications. AJR Am J Roentgenol. 1986;146:391–4. doi: 10.2214/ajr.146.2.391. [DOI] [PubMed] [Google Scholar]
- 13.Shah DS, Parikh H, Shah B, Banuprakash S, Shah J. Imaging appereances of hydatid cyst. Indian J Radiol Imaging. 2006;16:533–5. [Google Scholar]
- 14.Mendez Montero JV, Arrazola Garcia J, Lopez Lafuente J, Antela Lopez J, Mendez Fernandez R, Saiz Ayala A. Fat-fluid level in hepatic hydatid cyst: A new sign of rupture into the biliary tree? AJR Am J Roentgenol. 1996;167:91–4. doi: 10.2214/ajr.167.1.8659428. [DOI] [PubMed] [Google Scholar]
- 15.Moguillanski SJ, Gimenez CR, Villavicencio RL. Radiologiae imagen diagnostica terapeutica: Abdomen. Vol 2. Philadelphia, Pa: Lippincott Williams and Wilkins; 1999. Radiologia de la hidatidosis abdominal; pp. 47–72. [Google Scholar]
- 16.von Sinner WN. New diagnostic signs in hydatid disease; radiography, ultrasound, CT and MRI correlated to pathology. Eur J Radiol. 1991;12:150–9. doi: 10.1016/0720-048x(91)90119-g. [DOI] [PubMed] [Google Scholar]
- 17.Khaldi F, Braham N, Ben Chehida F, Ben Jaballah N, Bennaceur B. Hepatic hydatidosis and portal hypertension in children. Is it the Budd-Chiari syndrome? Ann Pediatr (Paris) 1993;40:631–4. [PubMed] [Google Scholar]
- 18.Gil-Egea MJ, Alameda F, Girvent M, Riera R, Sitges-Serra A. Hydatid cyst in the hepatic hilum causing a cavernous transformation in the portal vein. Gastroenterol Hepatol. 1998;21:227–9. [PubMed] [Google Scholar]
- 19.Franquet T, Montes M, Lecumberri FJ, Esparza J, Bescos JM. Hydatid disease of the spleen: Imaging findings in nine patients. AJR Am J Roentgenol. 1990;154:525–8. doi: 10.2214/ajr.154.3.2106214. [DOI] [PubMed] [Google Scholar]
- 20.Durgun V, Kapan S, Kapan M, Karabiçak I, Aydogan F, Goksoy E. Primary splenic hydatidosis. Dig Surg. 2003;20:38–41. doi: 10.1159/000068864. [DOI] [PubMed] [Google Scholar]
- 21.Odev K, Kilinc M, Arslan A, Aygün E, Güngör S, Durak AC, et al. Renal hydatid cysts and the evaluation of their radiologic images. Eur Urol. 1996;30:40–9. doi: 10.1159/000474143. [DOI] [PubMed] [Google Scholar]
- 22.Von Sinner WN, Hellström M, Kagevi I, Norlen BJ. Hydatid disease of the urinary tract. J Urol. 1993;149:577–80. doi: 10.1016/s0022-5347(17)36153-0. [DOI] [PubMed] [Google Scholar]
- 23.Akçay MN, Akçay G, Balik AA, Böyük A. Hydatid cysts of the adrenal gland: Review of nine patients. World J Surg. 2004;28:97–9. doi: 10.1007/s00268-003-6901-3. [DOI] [PubMed] [Google Scholar]
- 24.Khiari A, Mzali R, Ouali M, Kharrat M, Kechaou MS, Beyrouti MI. Hydatid cyst of the pancreas. Apropos of 7 cases. Ann Gastroenterol Hepatol (Paris) 1994;30:87–91. [PubMed] [Google Scholar]
- 25.Karavias DD, Vagianos CE, Kakkos SK, Panagopoulos CM, Androulakis JA. Peritoneal echinococcosis. World J Surg. 1996;20:337–40. doi: 10.1007/s002689900054. [DOI] [PubMed] [Google Scholar]
- 26.Ouadfel J, Assem A, Errougani A, Jalil A, Belkacem R, Balafrej S. Isolated retroperitoneal hydatid cyst. Apropos of a case. Ann Chir. 1990;44:243–5. [PubMed] [Google Scholar]
- 27.Fernández Larrañaga A, Silmi-Moyano A, Rodríguez-Vallejo JM, Usón-Calvo A. Retrovesical hydatidosis. Actas Urol Esp. 1983;7:165–74. [PubMed] [Google Scholar]
- 28.Ersahin Y, Mutluer S, Güzelbag E. Intracranial hydatid cysts in children. Neurosurgery. 1993;33:219–24. [PubMed] [Google Scholar]
- 29.Tüzün M, Hekimoglu B. Hydatid disease of the CNS: Imaging features. AJR Am J Roentgenol. 1998;171:1497–500. doi: 10.2214/ajr.171.6.9843277. [DOI] [PubMed] [Google Scholar]
- 30.Pomelov VS, Karimov SH, Nishanov KH. Surgical tactics in associated echinococcosis of the liver and lung. Khirurgiia (Mosk) 1991;11:69–74. [PubMed] [Google Scholar]
- 31.Aytac A, Yurdakul Y, Ikizler C, Olga R, Saylam A. Pulmonary hydatid disease: Report of 100 patients. Ann Thorac Surg. 1977;23:145–51. doi: 10.1016/s0003-4975(10)64088-x. [DOI] [PubMed] [Google Scholar]
- 32.Yuncu G, Ors KS, Sevinc S, Karabulut N, Alper H. The diagnostic value of the ‘air bubble sign’ in complicated pulmonary hydatid cysts. J Thorac Cardiovasc Surg. 2007;133:1524–74. [Google Scholar]
- 33.Mathur RK, Doda SS, Buxi TB, Talwar JR. Primary mediastinal echinococcosis. J Comput Tomogr. 1985;9:195–7. doi: 10.1016/0149-936x(85)90060-8. [DOI] [PubMed] [Google Scholar]
- 34.Yekeler I, Koçak H, Aydin NE, Basoglu A, Okur A, Senocak H, et al. A case of cardiac hydatid cyst localized in the lungs bilaterally and on anterior wall of right ventricle. Thorac Cardiovasc Surg. 1993;41:261–3. doi: 10.1055/s-2007-1013868. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan M, Demirtas M, Cimen S, Ozler A. Cardiac hydatid cysts with intracavitary expansion. Ann Thorac Surg. 2001;71:1587–90. doi: 10.1016/s0003-4975(01)02443-2. [DOI] [PubMed] [Google Scholar]
- 36.Kizilkaya E, Silit E, Basekim C, Karsli AF. Hepatic, extrahepatic soft tissue and bone involvement in hydatid disease. Turk J Diagn Interv Radiol. 2002;8:101–4. [Google Scholar]
- 37.Gossios KJ, Kontoyiannis DS, Dascalogiannaki M, Gourtsoyiannis NC. Uncommon locations of hydatid disease: CT appearances. Eur Radiol. 1997;7:1303–8. doi: 10.1007/s003300050293. [DOI] [PubMed] [Google Scholar]


