Abstract
Leishmaniasis is caused by parasitic protozoa of the genus Leishmania. Cutaneous leishmaniasis (CL) is endemic in Sri Lanka with over 3000 cases during the last decade and numbers are increasing. Treatment options available in Sri Lanka for CL include intralesional/intramuscular sodium stibogluconate and cryotherapy. Eight cases of treatment failure with standard therapy are reported from the Dermatology Clinic, Teaching Hospital Anuradhapura. Therapeutic regimes aim for clinical healing, these patients responded poorly to anti-leishmanial therapy, indicating the need for close monitoring, explore alternative treatment options and to investigate for drug resistance in parasites.
KEY WORDS: Leishmania donovani, skin lesions, sodium stibogluconate
INTRODUCTION
The first autochthonous case of cutaneous leishmaniasis (CL) was reported in Sri Lanka in 1992,[1] but now it is an established disease in this country with over 3000 cases seen throughout the country and is rising. The causative organism of CL isolated in Sri Lanka is Leishmania donovani MON-37,[2] which also has the potential to invade the viscera and cause life threatening visceral leishmaniasis. In Sri Lanka intra-lesional or intra-muscular sodium stibogluconate (SSG) and cryotherapy are standard therapy.[3] However the rising resistance to SSG is posing a major global treatment challenge.[4,5,6] Here we report for the first time a case series of delayed response or failure to respond to standard CL therapy in Sri Lanka.
STUDY
Clinical records of patients seen at the Anuradhapura Teaching Hospital, Dermatology Clinic, from April 2013 to February 2014 were studied. Out of the 396 patients who presented to the clinic during this period, eight patients continued to attend clinic due to poor response to treatment. “Delayed or lack of response to therapy” was defined as <50% reduction of the size of the lesion or degree of re-epithelialization of the lesion in case of ulcers, or persistence of inflammation and swelling at the borders of the lesion in spite of several sessions of intralesional SSG or intramuscular SSG and/or cryotherapy administered over a period of 10 weeks to 6 months. Response to treatment was judged by an experienced consultant dermatologist through clinical examination.
Case series of eight patients who conformed to the definition of poor clinical response are described here. Informed consent was obtained from all study subjects to answer the interview schedule, to obtain samples from the lesions, and to take photographs. Lesion materials (aspirate and scrapings) were obtained under sterile conditions, following the standard procedure. Samples were inoculated into capillary cultures and examined for promastigotes; smears were stained with Giemsa to identify amastigotes. Samples were also stored at −70°C for molecular studies.
The study was approved by the Ethics Review Committee, Faculty of Medicine, Colombo.
RESULTS
Case reports and follow-up details of the patients are given below:
Patient 1
A 59-year-old male presented in 2012 with an ulcerated nodule on the right cheek of 6 weeks duration. The nodule was 3.2 cm in size and the central ulcer was 1 cm. CL was confirmed by smear examination and he was treated with 18 doses of weekly intralesional SSG, and since there was <50% improvement, he was given further 18 doses of intralesional SSG every other week. Lesion persisted and repeat smear and culture done after 4 months were positive for parasites.
Patient 2
A 63-year-old male presented with a 2.3 cm × 1.5 cm nodule over his nose of 3 years duration. CL was confirmed by smear examination and he was treated initially with 21 daily doses of intramuscular SSG; since he had <50% response to treatment, further weekly doses of intralesional SSG was given for 12 weeks with 5 sessions of cryotherapy once in 2 weeks. Repeat smear and culture done 6 months later showed the persistence of parasites [Figure 1].
Figure 1.
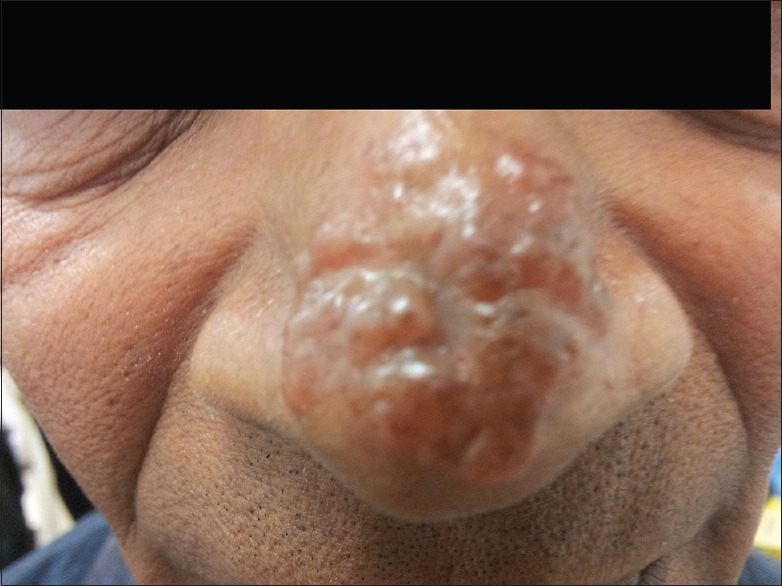
Lesion image of cutaneous leishmaniasis patient 2 after 21 doses of intramuscular sodium stibogluconate and 12 doses of intralesional sodium stibogluconate
Patient 3
A 53-year-old male presented with a 2 cm × 3 cm nodule over the left elbow of 3 months duration. CL was confirmed by smear examination and he was treated with 11 intralesional doses of SSG weekly. Since he showed <50% response to treatment, this was followed by 9 sessions of cryotherapy. Lesion persisted with no clinical improvement. Repeat smear and culture done 6 weeks after cessation of therapy remained positive for parasites.
Patient 4
A 17-year-old female presented with a 2 cm × 3.5 cm plaque over her left knee of 6 months duration. She was treated with 11 intralesional doses of SSG weekly, followed with one session of cryotherapy. Lesion remained with no clinical improvement and the left leg was swollen. Smears and culture remained positive for Leishmania parasites 14 weeks after the last treatment.
Patient 5
A 36-year-old male presented with a nodule 1.5 cm × 3 cm over the right arm of 2 years duration. CL was confirmed by microscopy and he was treated with 13 intralesional doses of SSG weekly. As he had <50% response, he had 5 sessions of cryotherapy once in 2 weeks. Following which the patient defaulted treatment for over a year and returned, he was worried about the persisting lesion. Repeat smear and culture were done and both remained positive for parasites.
Patient 6
A 38-year-old female patient with a previous diagnosis of rheumatoid arthritis and on long-term steroid treatment presented having a 3 cm nodule with a 1.2 cm central ulcer on her left leg of 18 months duration. CL was confirmed by smear and she was treated with 15 weekly doses of intralesional SSG weekly. Since she responded poorly, this was followed by 4 sessions of cryotherapy once in 2 weeks. Repeat smear and culture done 3 months after the last cryotherapy session remained positive.
Patient 7
A 32-year-old man from Anuradhapura presented with a plaque of 5.6 cm over the left side of his forehead, which he had for 18 months, a small papule over his nose of 0.2 cm, and three other nodules on his back for 3 months. CL was confirmed by smear. He was administered intramuscular SSG for 21 days, and since response to treatment was poor, he was treated with 8 doses of intralesional SSG for the lesion on the forehead and with 3 doses of cryotherapy for the lesions on the back. Oral fluconazole 200 mg daily for 1 month was also given as well. The response still remained poor. Repeat smear and culture done after 1 month following treatment remained positive for parasites [Figure 2].
Figure 2.
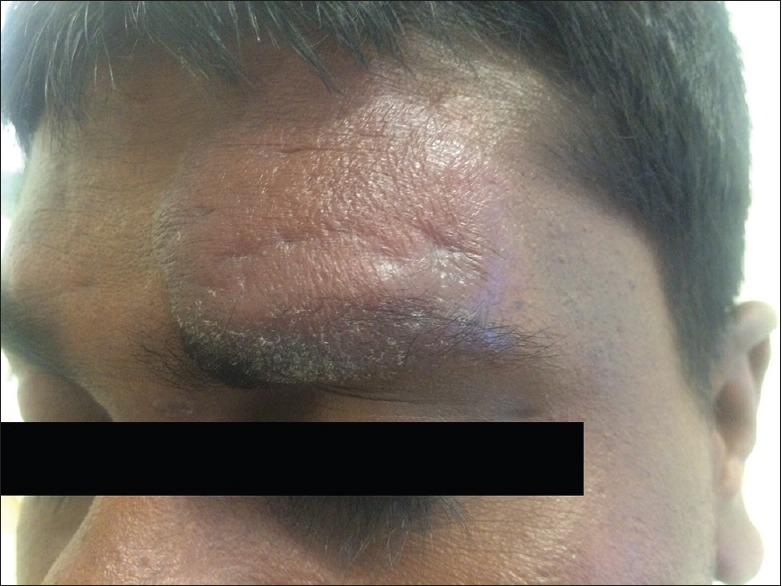
Lesion image of cutaneous leishmaniasis patient 7 after 21 doses of intramuscular sodium stibogluconate and 8 doses of intralesional sodium stibogluconate
Patient 8
A 28-year-old female homemaker presented in 2012 with a periorbital plaque of 2 cm × 3.5 cm of 2 months duration. CL was confirmed by smear and culture. She was treated with 21 doses of intramuscular SSG; since she showed poor response, this was followed up with 11 doses of intralesional SSG weekly. Repeat smears done after 3 months following treatment were positive for parasites and lesion persisted [Figure 3].
Figure 3.
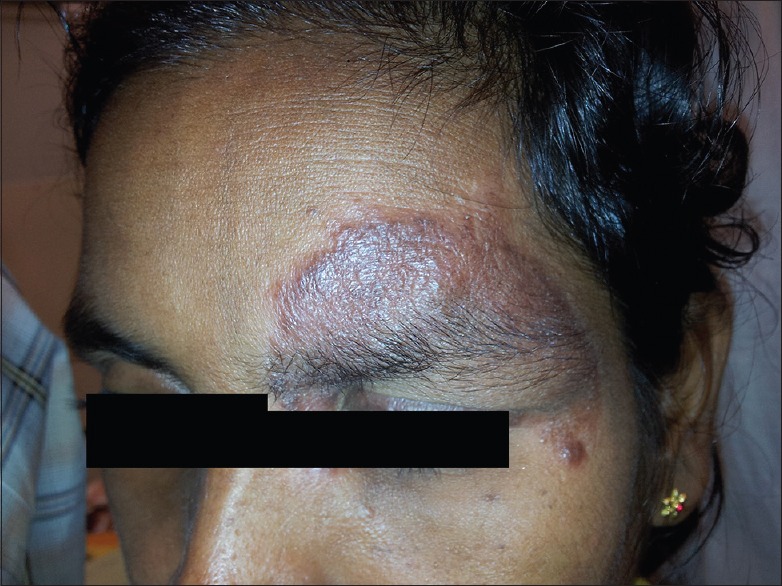
Lesion image of cutaneous leishmaniasis patient 8 after 21 doses of intramuscular sodium stibogluconate and 11 doses of intralesional sodium stibogluconate
DISCUSSION
The case series confirms failure of CL lesions to respond to standard SSG therapy and cryotherapy.
Response to the treatment in CL is difficult to tackle because of multitude factors that influence the efficacy of drugs such as intrinsic and acquired variations in drug susceptibility of the different Leishmania species, host factors such as immunity, variable clinical response to treatment, doses used and technique of instillation, age of the lesions, coinfections, factors associated with drugs, and variations in compliance.[5,6]
A possible reason for treatment failure could be either due to intrinsic or due to acquired resistance to SSG. Intrinsic difference in species sensitivity to SSG is well documented. Studies using the amastigote-macrophage model has demonstrated that L. donovani and Leishmania braziliensis to be 3-5-fold more sensitive to SSG than Leishmania major, Leishmania tropica, and Leishmania mexicana.[7] Similar findings have been observed in a controlled clinical trial in Guatemala, which compared the cure rate to antimonials in CL caused by different species.[8] It was investigated in Iran whether the increased incidence of glucantime-unresponsive CL correlates with parasite resistance to the drug and reported that treatment failure for CL in Iran is due to glucantime-resistant parasites. The unresponsive isolates were mainly L. tropica.[9]
The causative parasite of CL in Sri Lanka L. donovani MON-37[2] is a species that usually causes visceral leishmaniasis.
Samples obtained from these patients for molecular studies identified the strain to belong to a distinctly different cluster within the Sri Lankan L. donovani group (unpublished data). In India acquired resistance of Leshmania donovani was due to inadequate doses of SSG, which has been well documented.[6] In vitro amastigote-macrophage assay of L. donovani isolates taken from responders and nonresponders has shown that isolates from patients who did respond to SSG treatment were 3-fold more sensitive, compared to isolates from patients who did not respond.[10] The significant differences in amastigote sensitivity support the concept of acquired resistance in India.[7]
There is still no definite evidence on the level of efficacy of SSG based on standardized controlled clinical trials in CL caused by the species L. donovani. The treatment guidelines given by the WHO advisory committee for CL are focused on the standard parasites that cause CL, namely, L. major, L. tropica, Leishmania aethiopica, and Leishmania infantum. Recognized treatment options for old world CL include systemic therapy such as oral fluconazole, combination therapy of intravenous/intramuscular SSG with oral pentoxifylline or allopurinol or intravenous paromomycin, and local therapy with 15% paromomycin + 12% methyl benzethonium chloride ointment or thermotherapy.[11] A clinical trial was carried out using thermotherapy as a novel mode of treatment for L. donovani CL in patients by the authors, and it was found to be a safe and efficient mode of treatment (personal communication). Thermotherapy may be the answer to patients with poorly responding lesions. Further studies regarding this are ongoing. It could also be argued that ineffectiveness of SSG might be due to the generic versions of SSG used in Sri Lanka. However, no differences were seen in studies, done on American CL, though the numbers were small.[12]
CONCLUSIONS
CL is an emerging public health problem in Sri Lanka and is now presented also with a treatment challenge. Although SSG still remains the gold standard for treatment of CL in Sri Lanka, incomplete response to treatment and drug resistance of the causative parasite is a major concern. It is recommended policy for monitoring and standardization of therapy be urgently implemented for early recognition and effective control of the spread of drug resistance. There is also a need for well-designed clinical trials on all available treatment options for CL to identify the most appropriate treatment regimens for CL caused by the local parasite, L. donovani.
Pentostam® Injection (GlaxosmithKline) – Sodium Stibogluconate.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
This study was supported by the National Institute of Allergy and Infectious Disease of The National Institute of Health, USA, under the award number R01AI099602. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Drugs were supplied to the Teaching Hospital Anuradhapura by the Medical Supplies Division (MSD), Health Ministry, Sri Lanka.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We are grateful to Dr. B. Sumanasena for identifying patients with poor response to treatment with sodium stibogluconate, and Mr. Sudath Weerasingha and Ms. K.K.G.D.U.L. Kariyawasam for their laboratory assistance and for helping with sample collection.
REFERENCES
- 1.Athukorale DN, Seneviratne JK, Ihalamulla RL, Premaratne UN. Locally acquired cutaneous leishmaniasis in Sri Lanka. J Trop Med Hyg. 1992;95:432–3. [PubMed] [Google Scholar]
- 2.Karunaweera ND, Pratlong F, Siriwardane HV, Ihalamulla RL, Dedet JP. Sri Lankan cutaneous leishmaniasis is caused by Leishmania donovani zymodeme MON-37. Trans R Soc Trop Med Hyg. 2003;97:380–1. doi: 10.1016/s0035-9203(03)90061-7. [DOI] [PubMed] [Google Scholar]
- 3.Sirimanna GMP, Seneviratne JKK, Samaraweera ESN, Ranawaka RR, Hulangamuwa CS, de Silva VNH, et al. Guidelines on management of leishmaniasis. Sri Lanka College of Dermatologists. 2013:7. [Google Scholar]
- 4.Roychoudhury J, Ali N. Sodium stibogluconate: Therapeutic use in the management of leishmaniasis. Indian J Biochem Biophys. 2008;45:16–22. [Google Scholar]
- 5.Sundar S, Thakur BB, Tandon AK, Agrawal NR, Mishra CP, Mahapatra TM, et al. Clinicoepidemiological study of drug resistance in Indian Kala-azar. BMJ. 1994;308:307. doi: 10.1136/bmj.308.6924.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakravarty J, Sundar S. Drug resistance in leishmaniasis. J Glob Infect Dis. 2010;2:167–76. doi: 10.4103/0974-777X.62887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen S, Neal RA. The in vitro Susceptibility of Macrophages Infected with Amastigotes of Leishmania spp. to Pentavalent Antimonial Drugs and Other Compounds with Special Relevance to Cutaneous Isolates [Internet] In: Hart DT, editor. Leishmaniasis. Boston, MA: Springer US; 1989. [Cited 2016 Sep 07]. pp. 711–20. Available from: http://link.springer.com/10.1007/978-1-4613-1575-9_88 . [Google Scholar]
- 8.Navin TR, Arana BA, Arana FE, Berman JD, Chajón JF. Placebo-controlled clinical trial of sodium stibogluconate (Pentostam) versus ketoconazole for treating cutaneous leishmaniasis in Guatemala. J Infect Dis. 1992;165:528–34. doi: 10.1093/infdis/165.3.528. [DOI] [PubMed] [Google Scholar]
- 9.Hadighi R, Mohebali M, Boucher P, Hajjaran H, Khamesipour A, Ouellette M. Unresponsiveness to glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med. 2006;3:e162. doi: 10.1371/journal.pmed.0030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lira R, Sundar S, Makharia A, Kenney R, Gam A, Saraiva E, et al. Evidence that the high incidence of treatment failures in Indian Kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J Infect Dis. 1999;180:564–7. doi: 10.1086/314896. [DOI] [PubMed] [Google Scholar]
- 11.Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases. Control of the leishmaniases. World Health Organ Tech Rep Ser. 2010;949:69–70. [Google Scholar]
- 12.Soto J, Valda-Rodriquez L, Toledo J, Vera-Navarro L, Luz M, Monasterios-Torrico H, et al. Comparison of generic to branded pentavalent antimony for treatment of new world cutaneous leishmaniasis. Am J Trop Med Hyg. 2004;71:577–81. [PubMed] [Google Scholar]


