Abstract
Incorrect prescription and administration of medications account for a substantial proportion of medical errors in the USA, causing adverse drug events (ADEs) that result in considerable patient morbidity and enormous costs to the health-care system. Patients with chronic kidney disease or acute kidney injury often have impaired drug clearance as well as polypharmacy, and are therefore at increased risk of experiencing ADEs. Studies have demonstrated that recognition of these conditions is not uniform among treating physicians, and prescribed drug doses are often incorrect. Early interventions that ensure appropriate drug dosing in this group of patients have shown encouraging results. Both computerized physician order entry and clinical decision support systems have been shown to reduce the rate of ADEs. Nevertheless, these systems have been implemented at surprisingly few institutions. Economic stimulus and health-care reform legislation present a rare opportunity to refine these systems and understand how they could be implemented more widely. Failure to explore this technology could mean that the opportunity to reduce the morbidity associated with ADEs is missed.
Introduction
A report from the US Institute of Medicine in 2000 highlighted the staggering impact of medical errors on patient morbidity and mortality in the US health-care system.1 Findings pertaining to the common occurrence of adverse drug events (ADEs) and their undesirable effects on patient outcomes were particularly surprising. By some estimates, 770,000 people are injured or die every year from ADEs,2–4 with associated costs of between US$1.5 billion and $5.5 billion every year.5 The reasons for ADEs are numerous, but by far the most common is the prescription and administration of an incorrect drug dose.6
Because many parent compounds and their metabolites are cleared through a renal mechanism (either through glomerular filtration or tubular secretion), hospitalized patients with renal impairment are at particularly high risk of medication dosing errors.7 Furthermore, patients with chronic kidney disease (CKD) often have multiple co-morbidities and are commonly prescribed more than five medications with as many as 12 or more doses per day, leading to a high risk of adverse drug–drug interactions, dispensing errors and inadequate drug monitoring.8 Indeed, several studies have demonstrated that patients with CKD are at higher risk of medication errors and resulting complications than patients without kidney disease.9–11 Hug et al.10 examined the incidence of ADEs (defined as an injury attributable to a drug) among hospitalized patients with impaired kidney function (serum creatinine concentration >132.6 µmol/l [>1.5 mg/dl]) from six community hospitals. The results demonstrated a high rate of both ADEs (10 per 100 admissions) as well as potential ADEs (55.3 per 100 admissions). Importantly, 4.5% of the confirmed ADEs were classified as life-threatening and the majority of cases were preventable. In this study, antibiotics, analgesics and cardiovascular drugs were those most frequently involved in causing ADEs.10 Blix and colleagues reported that up to 62% of patients with CKD stages 3–5 were prescribed high-risk medications that led to a drug-related problem.11 The spectrum of ADEs in patients with CKD can be broad (Table 1), which raises the obvious question of what can be done to reduce the number of ADEs in this complex group of patients.
Table 1.
Spectrum of ADEs in patients with kidney dysfunction
| Study | ADE category | Example |
|---|---|---|
| Miller et al. (2009)46 Zand et al. (2010)47 |
Inappropriate medication dosing for level of kidney function |
Gabapentin 300 mg three times daily in a patient with an eGFR of 20 ml/min/1.73 m2, leading to central nervous system depression |
| Williams et al. (2004)48 Sica et al. (2004)49 |
Drug–drug interactions in patients with advanced CKD |
Increased risk of bradycardia with atenolol and diltiazem (drugs cleared via the kidney) in a patient with end-stage renal disease |
| Chen (2009)50 | Inappropriate prescribing | Use of morphine in a patient with stage 5 CKD, leading to accumulation of active metabolites |
| Bernstein et al. (1990)51 | Inadequate monitoring | High levels of aminoglycoside in a patient receiving antibiotics for a prolonged period of time |
| Connolly et al. (2009)52 | Failure to identify CKD | Reliance on serum creatinine concentration alone to assess renal function in an elderly patient |
| Perazella et al. (2009)53 | Inappropriate diagnostic test | Use of gadolinium for MRI contrast in a patient with stage 5 CKD |
Abbreviations: ADE, adverse drug event; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
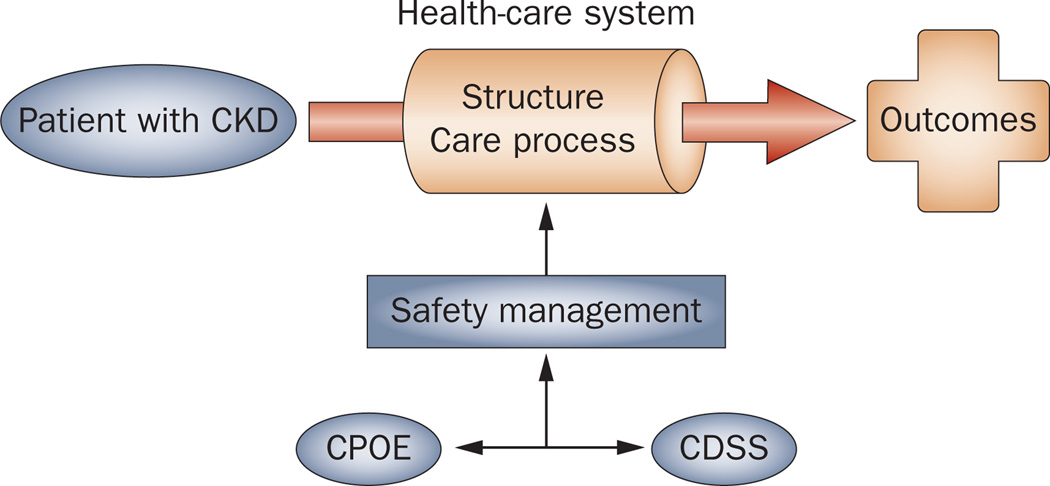
A plan that could potentially improve patient safety and outcomes through the increased use of electronic medical records was enacted in the American Recovery and Reinvestment Act of 2009, which provided $2 billion for discretionary health information technology funding and $18 billion in investments and incentives through Medicare and Medicaid.12 The Agency for Healthcare Research and Quality has proposed a framework in which efforts directed towards improved patient safety can be conceptualized and evaluated.13 This model focuses on structure (the physical and organizational properties of the settings in which care is provided), process (the treatment or service being provided to the patient), and outcomes (the result of the treatment). Use of computerized physician order entry (CPOE) and clinical decision support systems (CDSS) fit within this model and are important tools to improve outcomes, especially for preventing ADEs (Figure 1).
Figure 1.
CPOE and CDSS integrated within the structure–process–outcome framework of the Donabedian model of patient safety. CPOE and CDSS are key components of the health-care system that is focused on safety management. Abbreviations: CDSS, clinical decision support systems; CKD, chronic kidney disease; CPOE, computerized physician order entry. Reproduced with permission of Health Administration Press, from Definition of quality and approaches to its assessment: explorations in quality assessment and monitoring, Donabedian, A. Vol. 1 (1980); permission conveyed through Copyright Clearance Center, Inc.
In this Review, we discuss the effect of interventions designed to reduce the number of ADEs in patients with acute kidney injury (AKI) or CKD. A particular emphasis will be placed on CPOE and CDSS as strategies to reduce the occurrence of ADEs in these patients. We also discuss the barriers to widespread implementation of these technologies, and summarize the characteristics of successful CPOE and CDSS currently in use.
Pharmacist-based interventions
Before the implementation of CPOE systems and their increasing use in the clinical setting, efforts to ensure proper drug dosing in those with renal impairment relied on clinical pharmacists to alert physicians of abnormal renal function in patients (a practice that still occurs in many hospitals, either independently or in conjunction with CPOE). A study by Quartarolo et al.14 compared recognition of CKD in elderly inpatients (aged >65 years) by the physician before and after an intervention, which included documentation of the patient’s estimated glomerular filtration rate (eGFR) in a paper medical chart. Of 260 patients, only 10 (3%) were identified by the attending physician as having CKD before the intervention as compared with 12.6% after the intervention (P <0.001). As a secondary outcome, the investigators examined whether dosing of nonsteroidal anti-inflammatory drugs and antibiotics were affected by the intervention. Interestingly, no significant difference was seen between dosing before and after documentation of eGFR, and 40% of renally cleared antibiotics were dosed incorrectly at the time of hospital discharge. The investigators highlighted that even when physicians are aware of information that should change their behavior, they do not always do so.14
In a prospective cohort study, Falconnier et al.15 tested an intervention to help ensure proper dosing of medications in inpatients with AKI or CKD. A clinical pharmacist calculated the eGFR of a patient and placed a notation in the paper record alerting the physician if a given patient had an eGFR of <50 ml/min/1.73 m2. If no change in dosing occurred within 24 h, the pharmacist made explicit recommendations in the paper chart for medications requiring dose adjustment. Physicians in the control group received no such information. Overall, physicians in the control group adjusted doses of renally eliminated drugs in 33% of cases as compared with 81% in the intervention group (P <0.001). In patients with a creatinine clearance of <10 ml/min, only 8/26 (31%) received correct doses of renally excreted medications after the physician was notified of a patient’s eGFR. Once explicit drug dosing recommendations were given by the pharmacist, 19/26 (73%) of patients in the intervention group received appropriate doses. Reasons for 27% of physicians not following the explicit recommendation were not collected. No significant difference was found in length of hospital stay between the groups, and although the cost savings associated with the intervention were statistically significant (P <0.001), they were of questionable financial importance as a formal cost-effectiveness analysis was not carried out. The investigators concluded that renal dysfunction is often not considered when prescribing drugs, and immediate feedback that is highly specific could reduce the number of inappropriate doses and decrease the potential for ADEs.15
These two studies14,15 not only highlight the scope of problems relating to drug prescribing in the CKD population, but also emphasize the challenges involved in reducing ADEs in patients with AKI or CKD. Namely, even if providers are notified that a patient has renal impairment, this information does not necessarily translate into a change in clinical management. Understanding the factors that influence these decision-making processes is crucial in creating systems that can reduce the rate of ADEs. The increasing use of information technology in medicine will not only enable information gathering on user behavior, but could also help physicians provide improved care with avoidance of preventable ADEs. Furthermore, these automated systems have been proposed to be more cost-effective and less labor-intensive than clinical pharmacist-led initiatives.
Information technology in medicine
Over the past few years, various factors have resulted in the increased use of information technology in medicine. In particular, there has been a considerable growth in the use of CPOE, which is a system that enables direct entry of orders by an individual who has licensure and privileges to do so. CPOE results in a reduction in the number of ambiguous orders and errors owing to illegible handwriting and improper use of abbreviations.16 A systematic review indicated that CPOE results in a shorter length of stay in hospital, fewer ADEs and improved physician performance.17
CDSS, which are also being increasingly used in medical practice, are still much less common than CPOE and rarely exist independently of a CPOE system. Within CDSS, characteristics of individual patients generate patient-specific assessments or recommendations. Most knowledge-based CDSS are designed with three parts: the knowledge base, an inference engine, and a mechanism to communicate to the end-user.18 The knowledge base contains compiled data that usually take the form of ‘if–then’ rules. For example, a drug interaction alert from a CDSS would consist of a series of rules that would take the form: if drug A is taken and drug B is taken then alert end-user. Another example would be: if serum creatinine concentration increases by more than 26.5 µmol/l (0.3 mg/dl) then alert end-user to review medication dosing and appropriateness. These knowledge bases require continual editing and updating to add new drugs and interactions. The inference engine combines the rules from the knowledge base with actual patient data, and also includes a set of rules that could be derived by an evidence-based approach or through expert opinion (or occasionally through other sophisticated computational methods, such as neural networks, genetic algorithms, or Bayesian networks).19 The communication mechanism, such as an email or alert, displays the results to the user and, in some cases, can directly react to the information through new input into the system. The potential benefits of CDSS on patient care and outcomes are far-reaching, although it is important to also be aware of the potential drawbacks related to the use of CDSS (Box 1).
Potential benefits and drawbacks of CDSS20.
Benefits
Improved patient safety by reducing the number of adverse events resulting from inappropriate prescribing of medication
Improved quality of care by increasing the use of protocols and guidelines
Improved efficiency in health-care delivery by faster order processing and reductions in the use of unnecessary diagnostic testing
Automatic provision of relevant care recommendations based on best practice knowledge
Supports medical education and training by ensuring best practice use in trainees
Drawbacks
Over-reliance on CDSS
Potential threat to the clinical judgment of the user
Lack of flexibility and freedom in decision making
Potentially time-consuming to use and integrate into clinical practice
Uncertainty regarding legal implications of decision support that leads to errors
Requires constant updating as medical knowledge evolves
Alert fatigue, which could lead to users overriding important alerts
Abbreviation: CDSS, clinical decision support systems.
The term CPOE can refer to an integrated system, including decision aids such as CDSS, whereas other publications refer to CPOE and CDSS as separate entities. What is important to note is that many of the improved outcomes attributed to CPOE can be ascribed to CDSS embedded within these systems.
Evaluation of CDSS in medical practice
CDSS have varied uses in medical practice,17,19 including the following: administrative functions, such as supporting clinical coding and documentation, as well as authorization of procedures and referrals; management of clinical complexity, such as chemotherapy protocols, tracking orders, and preventative care; cost–control measures, such as the monitoring of medication orders with the substitution of generic medications, and avoidance of duplicate or unnecessary testing; decision support, such as aiding differential diagnosis and determination of treatment plans based on condition-specific guidelines; and patient safety functions, such as avoidance of drug–drug interactions, inappropriate medication or dose selection, and alerting clinicians to high-risk situations.20
Several systematic reviews have examined the effects of CPOE and CDSS on the rates of ADEs and on improvements in drug dosing.17,21–25 A discussion of these studies is beyond the scope of this Review, but most data indicate that CPOE and CDSS can have a substantial impact on decreasing the rate of ADEs as well as determining the correct dose of medications.21–25 A Cochrane review on computerized dosing advice demonstrated a moderate, but significant, reduction in length of hospital stay of 0.35 days (95% CI 0.52–0.17 days).26 Specific examples of the benefit of CPOE and CDSS include a drug allergy alert that decreased allergy error events by 56%,27 a drug–condition alert that increased prescribing for venous thromboembolism prophylaxis in patients at risk,28 and providing default dosing guidance that resulted in a reduction of dosing errors in two studies of 23%27 and 71%.28 Other types of drug alerts triggered by CDSS are shown in Table 2.
Table 2.
Drug alerts provided by clinical decision support systems
| Type of alert | Definition |
|---|---|
| Drug allergy alert | Generated when a medication is ordered to which the patient has an electronically documented allergy |
| Drug–drug interaction alert | Generated when the mode of action of one drug is known to be affected by the simultaneous prescribing of another drug |
| Duplicate medication or therapeutic duplication alert |
Generated when the patient is already receiving the medication ordered or a drug in the same therapeutic category (e.g. two β-blockers) |
| Basic medication order guidance | Generated to provide dosing strings with a default dosing given that is the most appropriate initial dose |
| Drug–laboratory alert | Generated when administration of a drug (or test) requires close monitoring of laboratory parameters before and/or after administration |
| Drug–condition alert | Generated to raise awareness of specific prescribing for certain conditions |
| Drug–disease contraindication alert | Generated to warn against prescribing of certain drugs in specific disease states |
| Drug–condition alerts aimed at appropriate prescribing |
Generated to encourage prescribing of a certain drug in a specific disease state |
| Drug–age alert | Generated to discourage prescribing of a certain drug in the elderly |
| Drug–formulary alert | Generated when a drug is not included or recommended in the local formulary |
| Dosing guidelines | Generated when complex patient characteristics, such as age, renal function, pregnancy and liver function should be considered |
Adapted from J. Am. Med. Inform. Assoc. Schedlbauer, A. et al. 16, 531–538 © 2009, with permission from BMJ Publishing Group Ltd.
Use of CDSS in clinical nephrology
Within nephrology practice, CDSS have the promise to offer considerable improvements in patient care, which could occur through several mechanisms utilizing various triggers such as: identification of patients at risk of nephrotoxicity (through specific risk factor determination algorithms); identification of patients with CKD with alerts to care algorithms (such as avoidance of gadolinium, or other diagnostic or therapeutic interventions that prompt physicians to order specific laboratory tests such as measurement of vitamin D levels); alerting providers to changes in renal function that might require medication dose changes or make certain procedures contraindicated; determination of correct drug dosing and drug–drug interactions; and implementation of national quality standards and protocols.
A number of studies have examined the role of CPOE and CDSS in either reducing renal events, such as medication-associated renal deterioration, or in preventing ADEs in patients with CKD or AKI. All of these studies showed considerable, but variable, improvements in drug dosing in patients with impaired renal function when CDSS were implemented, and several studies showed improvements in specific outcomes (Table 3). Chertow et al.28 made explicit use of CDSS to guide drug dosing in patients with CKD or AKI. The CDSS interfaced with the laboratory data system to detect changes in kidney function in real time. When a medication order was placed, the system presented the user with the default dose and frequency together with an alert suggesting necessary modifications in the case of patients with CKD or AKI. The user could view the calculations that prompted these alterations if they desired. The physician then either chose to accept the modified dose or to override this recommendation. The system noted how many dose recommendations were accepted or rejected for later analysis. The effect of this intervention was assessed during a control period, where the system recorded prompts that were triggered, but did not display them to the user, and during an intervention period where prompts were prominently displayed to the user. Comparing control and intervention periods, appropriate doses were prescribed in 54% versus 67% of cases (P <0.001), respectively. Dosing frequency was appropriate in 35% of cases during the control period versus 59% during the intervention period (P <0.001). No significant differences in hospital or pharmacy costs were found between the two periods. Furthermore, there were no differences in renal function changes between the groups. Although the intervention reduced the number of inappropriate drug doses, approximately 49% of drug doses in the intervention period were still considered inappropriate. Reasons for physicians not accepting the suggested action were not recorded.28
Table 3.
Studies of CDSS for improving renal outcomes
| Study | Intervention and study goal | Results |
|---|---|---|
| Rind et al. (1994)29 |
Prospective time-series (before and after implementation) to evaluate the effects of computerized alerts on physicians regarding serum creatinine levels in inpatients receiving potentially nephrotoxic or renally excreted medications |
Medication doses changed or discontinued an average of 21.6 h sooner during the intervention period than before implementation of the alert system For patients receiving nephrotoxic medications, the relative risk of renal impairment was 0.45 (95% CI 0.22–0.94) |
| Chertow et al. (2001)28 |
Prospective time-series (multiple control and intervention intervals) to evaluate whether CDSS could improve correct drug prescribing (dose and frequency) and outcomes in an inpatient setting |
15% of orders were modified by the computer based on renal function Appropriate prescriptions increased during the intervention period: correct dose was 67% vs 54% during the intervention vs control periods, respectively, and correct frequency was 59% vs 35% during the intervention vs control periods, respectively |
| Nash et al. (2005)54 |
Prospective cohort study to assess the effectiveness of a computerized alert system for reducing excessive drug dosing in inpatients with CKD Feedback was provided to the prescriber by nurses or pharmacists who reviewed the alerts |
Rates of excessive medication dosing at baseline were 23.2%, which decreased to 17.3% with nurse feedback, and to 16.8% with pharmacist feedback |
| Colpaert et al. (2006)55 |
Prospective trial to evaluate whether a computer system (Centricity® Critical Care Clinisoft, GE Healthcare, Waukesha, WI, USA) could reduce the incidence and severity of medication prescription errors in an ICU setting |
Patients with renal insufficiency assessed with a computer-based system experienced less dosing errors than patients in a paper-based unit (12 vs 35 errors, respectively) |
| Field et al. (2009)56 |
Randomized trial to evaluate whether real-time CDSS could improve adherence to drug-dosing guidelines in elderly patients with CKD or AKI in a long-term care facility, and detect excessive medication dosing |
Appropriate drug orders were significantly more common with CPOE/CDSS than in the control group (relative risk 1.2, 95% CI 1.0–1.4) |
| Evans et al. (1998)57 |
Prospective study to determine whether CDSS using an anti-infective management program could reduce excess doses of antibiotics in the ICU setting |
Excess drug dosing significantly decreased in patients with CKD or AKI during and before the intervention period (87 vs 405, respectively; P <0.01) |
| Roberts et al. (2010)58 |
Prospective trial to study whether CDSS alerts independent of CPOE could improve drug dosing in an inpatient setting CDSS were used to calculate and update measurements of renal function and adjust drug doses accordingly, as well as for reporting clinically important changes in renal function |
Improvements were made in dosing of key medications (enoxaparin, vancomycin and gentamicin), and in therapeutic monitoring of gentamicin During episodes of acute renal impairment, renally cleared drugs were withheld on 38% of cases before the intervention period compared with 62% after the intervention period (P = 0.01) |
| Matsumura et al. (2009)59 |
Prospective time-series to study whether CDSS could monitor data from a CPOE system and detect excessive medication dosing and reduce the prescription of medications contraindicated in patients with CKD in the inpatient setting |
24% of patients had medication changes before implementation of the alert system compared with 54% after implementation (P <0.01) |
| Galanter et al. (2005)60 |
Prospective times-series to study whether CPOE/CDSS could detect excessive medication dosing and reduce the likelihood of receiving renally contraindicated medicine in the inpatient setting |
Likelihood of receiving contraindicated medication after implementation of the CPOE/CDSS decreased from 89% to 47% (P <0.01) |
Abbreviations: AKI, acute kidney injury; CDSS, clinical decision support systems; CKD, chronic kidney disease; CPOE, computerized physician order entry; ICU, intensive care unit.
Only one study has examined the impact of CDSS on the prevention of AKI, and demonstrated one of the greatest reductions in the rate of AKI seen in any similar intervention study so far.29 In this study, an alert was generated for a patient prescribed a potentially nephrotoxic medication when there was an increase in serum creatinine of >44.2 µmol/l (>0.5 mg/dl) from baseline after starting the medication. This alert system resulted in a significant decrease in renal impairment in the group that received these alerts as compared with the group that did not receive alerts (relative risk 0.45, 95% CI 0.22–0.94).
Drug dosing of immunosuppressive medications, such as calcineurin inhibitors, is complex yet critical in ensuring both short-term and long-term allograft survival after kidney transplantation. Computer-assisted dosing of calcineurin inhibitors with the use of complex pharmacokinetic modeling (such as nonlinear mixed effects modeling) has been assessed in several small studies and shown to be useful in determining dosing parameters and predicting drug levels.30–32 However, these systems have not been used to assess allograft outcomes and will need to be validated before routine use can be recommended.
The literature clearly shows that there is great potential for CDSS in improving outcomes in patients with kidney disease. However, many of the studies conducted so far lack details on outcome measures, such as reductions in mortality rates, cost reductions, or changes in length of hospital stay. As CDSS with increased functionality are developed, it is hoped that the benefits seen in preventing ADEs and in guiding drug dosing can be broadened to include improvement in protocol and guideline adherence as well as in the prevention of AKI.
Barriers to widespread implementation
Despite mounting evidence that CPOE and CDSS might improve patient care and safety, adoption of these systems remain the exception rather than the rule. Multiple reasons have been put forward to explain this phenomenon, but the most widely cited are financial and cultural reasons.
Because current reimbursement favors precise billing and not necessarily patient care and safety, most commercial CPOE systems utilize tools that ensure correct coding of patient encounters.33 Systems that focus on patient safety and quality have usually been developed by individual institutions with little commercial or governmental support. Formal published cost-effectiveness estimates remain rare, and institutions are therefore hesitant to make the initial large investment in these systems without understanding of the cost–savings ratio. Field et al.34 found that the cost of developing CDSS for medication dosing in patients with kidney disease (94 alerts for 62 drugs) was $48,668 and required 924.5 man hours. Furthermore, continual maintenance of CDSS is required to adjust decision tools to new data. However, it is highly likely that this cost would be easily offset by the reduction in ADEs. Commercial vendors might hesitate to invest financial resources into the development of CDSS when the extent of their legal liability in medical malpractice cases remains open-ended (for example, if an erroneous alert led to a medical error and harm to a patient).
Substantial public financial support will be required to design and implement these CDSS. The American Recovery and Reinvestment Act of 2009 presents an important first step in supporting these endeavors. This legislation allocated approximately $26 billion to health information technology (Health Information Technology for Economic and Clinical Health Act).35 The Patient Protection and Affordable Care Act of 2010 also proposed substantial spending in the area of health information technology.36 Through these initiatives, an increasing number of hospitals and clinics will integrate electronic medical records and CPOE systems within their environment of care. The decision to implement CDSS might be driven, in part, by payments linked to performance outcomes. In any case, this US legislation marks an important turning-point in creating financial support for implementation of CPOE and CDSS.
In addition to financial barriers, the culture of medical practice presents considerable obstacles to the adoption of CPOE and CDSS. Physicians feel that their clinical experience cannot be replaced by these systems. These beliefs persist, despite evidence that these tools can improve patient safety and physician performance. Specific barriers exist that are unique to nephrology. Nephrologists practice across many different care settings, such as intensive care units, hospital wards, dialysis units, and office practices, with patients who have complex and challenging diseases. Development of CDSS that meet the varied requirements across these settings is a considerable, but not insurmountable, challenge. Furthermore, drug metabolism in a patient with kidney disease is not as simple as correcting for dose based on changes in eGFR. Other factors, such as protein binding, presence of the nephrotic syndrome (with loss of protein-bound drugs in the urine), volume of drug distribution, blood pH, and extracellular volume all affect drug disposition, and currently available CDSS do not address these complex factors. Thus, current CDSS are limited by their over-reliance on drug dosing focused on eGFR.
Another barrier to the widespread implementation of CDSS is the difficulty in the creation of seamless integration of workflow. In the past, CDSS have been designed with a strict focus on the functional decision-making process, with less emphasis on how the system functions in real time.23 CDSS that require additional steps that disrupt workflow and decrease efficiency of the physician are likely to be underutilized. Another problem associated with some CDSS is ‘alert fatigue’. For example, the first time a user is informed that a certain drug should not be prescribed to patients with CKD, they might be impressed. However, when they have received this alert 10–20 times, they might become frustrated and tend to ignore alerts in an effort to maintain workflow.37 Alert fatigue is the most common complaint about CPOE and CDSS, and alerts are overridden 49–96% of the time.38,39 Reducing alert fatigue is a critical aspect of CDSS that must be addressed in order to maximize implementation.32
Creating effective support tools
If resources are available to develop and implement these systems, what characteristics might increase their success and acceptance by busy practitioners? Bates et al.40 have extensive experience with CPOE and CDSS, and have synthesized what they have learned over more than 10 years with Partners HealthCare System (Boston, MA, USA). First and foremost, speed and efficiency are critical. This parameter is the one most valued by users, even above other quality improvement measures.41 If decision support takes too long to appear, busy providers will ignore or override these systems, rendering them useless. Systems must also be capable of presenting information when the user needs it. In other words, systems must gather and make associations between data sources that providers might miss because of the sheer volume of data.41 For example, a system might strongly emphasize the monitoring of potassium levels in a patient on digoxin who is also receiving six other medications. These systems should also anticipate the future needs of a patient that the user might not remember, such as recommending therapeutic drug level monitoring after prescribing a nephrotoxic medication to a patient with kidney disease.
Another crucial characteristic of successful CDSS is that these systems are integrated into the workflow and practice pattern of a user.42 Stand-alone guidelines, no matter how strongly backed by evidence, are rarely used.43 Users must be presented with supporting data and alternative actions in order for these aids to change behavior as physicians often strongly resist changing their course of action if they are not provided with an alternative, even if their action might be counterproductive or repetitive.43 An ideal system would thus anticipate the needs of the user, without being burdensome.
Successful systems must actively monitor the impact of their interventions, ask users for feedback and respond with changes. Systems that continue to generate alerts with low specificity lead to alert fatigue, which results in providers overriding both important and unimportant reminders that could compromise the desired safety effect.39 Many interventions have a lower impact on improving safety than expected, often for reasons that were never considered. Information technology makes it possible to collect data on provider behavior to better determine why an intervention has not achieved its expected aim. This process must be carried out regularly to tailor systems to the changing needs of the providers, patients and institutions. Responding to user feedback also leads to greater buy-in and utilization of these systems by providers.
Kuperman et al.44 argue that overcoming the barriers and complexities of CDSS would best be approached by a two-stage implementation strategy: the first stage would involve implementation of basic decision support (checking for drug allergy, dosing guidance and drug–drug interactions), followed by adoption of more-advanced decision support (guiding drug-triggered laboratory testing, dosing of medications for complex patients, and drug–disease state contraindications).
Conclusions
ADEs represent a large proportion of the medical errors made annually in the USA, and result in substantial morbidity and mortality. Patients with kidney disease are at particularly high risk of experiencing ADEs because of their limited ability to excrete drugs and metabolites that are harmful, and also because of the polypharmacy that is common in this patient population. Early studies of interventions to reduce ADEs in patients with kidney disease have indicated that providers are often not aware of the renal status of their patients and even when they are aware, incorrect doses have still often been prescribed. CPOE and CDSS help reduce these errors by presenting real-time information about a patient’s status and making patient-specific recommendations. Ensuring that all users follow a similar management procedure could reduce the variability in care and decrease the number of ADEs experienced by patients. Although these systems are intended to augment rather than usurp the decision-making ability of the provider, we have to move beyond the current culture that is focused on provider autonomy to one focused on patient safety.
Despite evidence that these systems can improve patient care and safety, they have not been widely adopted. Institutions are hesitant to invest considerable resources into these systems without proof that they will be cost-effective. However, an analysis of the return on investment from the Brigham and Women’s Hospital in Boston, MA, USA revealed a net saving of $16.7 million over 10 years, strongly indicating that such an investment is financially sound.45 Many providers remain skeptical that these systems will improve patient care without overburdening them with additional tasks. In order to address these concerns, additional resources must be devoted to studying these questions. Public funds are likely to be needed initially to provide a stimulus for more research and innovation in this area. Other incentives to further explore this technology would be to provide merit-based reimbursement based on quality targets achieved by using these systems. Institutions, industry and providers must be involved in this process so that these systems reflect their interests. The complexities of human disease make the elimination of medical error an unlikely possibility. However, evaluating tools such as CPOE and CDSS, which enable us to reduce the incidence of these errors, and utilizing them to their full potential, is essential. These systems require refinement, but offer great promise as a cost-effective solution to improving the safety and quality of our patient care.
Key points.
Adverse drug events (ADEs) are an important cause of morbidity among patients with chronic kidney disease or acute kidney injury, with considerable financial costs to the health-care system
The use of information technology in the form of computerized physician order entry and clinical decision support systems has the potential to reduce incorrect drug dosing and ADEs
Despite the obvious benefits of computer-based strategies, both financial and cultural barriers prevent the more-widespread adaptation of these systems
Substantial resources that have been allocated to promote the use of information technology in medicine should be utilized to develop this technology and decide how to implement it more widely
Components of an ideal clinical decision support system that is tailored to maximize usage and efficiency need to be explored
Review criteria.
Information for this Review was obtained by searching the PubMed database using combinations of the terms “renal insufficiency”, “chronic kidney disease”, “acute kidney injury”, “acute renal failure”, “drug dosing”, “computer”, “clinical decision support systems”, “renal”, “kidney”, and “adverse drug events”. No filters were applied. All papers were manually reviewed for relevance, and additional material was obtained by searching the reference lists of identified articles.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
J. Chang and M. H. Rosner researched data for the article and wrote the article. J. Chang, C. Ronco and M. H. Rosner contributed equally to discussion of content for the article and reviewing/editing of the manuscript before submission.
Contributor Information
Jamison Chang, Division of Nephrology, University of Virginia Health System, Box 80013, 1215 Lee Street, Charlottesville, VA 22908, USA.
Claudio Ronco, Division of Nephrology, San Bortolo Hospital, Viale Rodolfi 37, 36100 Vicenza, Italy.
Mitchell H. Rosner, Division of Nephrology, University of Virginia Health System, Box 80013, 1215 Lee Street, Charlottesville, VA 22908, USA
References
- 1.Institute of Medicine of the National Academies. To err is human: building a safer health system. 1999 [online], http://www.iom.edu/Reports/1999/To-Err-is-Human-Building-A-Safer-Health-System.aspx. [Google Scholar]
- 2.Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277:301–306. [PubMed] [Google Scholar]
- 3.Cullen DJ, et al. Preventable adverse drug events in hospitalized patients: a comparative study of intensive care and general care units. Crit. Care. Med. 1997;25:1289–1297. doi: 10.1097/00003246-199708000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Cullen DJ, et al. The incident reporting system does not detect adverse drug events: a problem for quality improvement. Jt Comm. J. Qual. Improv. 1995;21:541–548. doi: 10.1016/s1070-3241(16)30180-8. [DOI] [PubMed] [Google Scholar]
- 5.Bates DW, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA. 1997;277:307–311. [PubMed] [Google Scholar]
- 6.Bates DW, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274:29–34. [PubMed] [Google Scholar]
- 7.Hu KT, Matayoshi A, Stevenson FT. Calculation of the estimated creatinine clearance in avoiding drug dosing errors in the older patient. Am. J. Med. Sci. 2001;322:133–136. doi: 10.1097/00000441-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Hassan Y, Al-Ramahi RJ, Abd Aziz N, Ghazali R. Drug use and dosing in chronic kidney disease. Ann. Acad. Med. Singapore. 2009;38:1095–1103. [PubMed] [Google Scholar]
- 9.Jick H. Adverse drug effects in relation to renal function. Am. J. Med. 1977;62:514–517. doi: 10.1016/0002-9343(77)90406-5. [DOI] [PubMed] [Google Scholar]
- 10.Hug BL, et al. Occurrence of adverse, often preventable, events in community hospitals involving nephrotoxic drugs or those excreted by the kidney. Kidney Int. 2009;76:1192–1198. doi: 10.1038/ki.2009.353. [DOI] [PubMed] [Google Scholar]
- 11.Blix HS, Viktil KK, Moger TA, Reikvam A. Use of renal risk drugs in hospitalized patients with impaired renal function—an underestimated problem? Nephrol. Dial. Transplant. 2006;21:3164–3171. doi: 10.1093/ndt/gfl399. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Department of Human Health & Services. The Official Web Site for the Medicare and Medicaid Electronic Health Records (EHR) Incentive Programs. 2011 [online], http://www.cms.gov/EHRIncentivePrograms/01_Overview.asp#TopOfPage.
- 13.Donabedian A. Definition of quality and approaches to its assessment: explorations in quality assessment and monitoring. Ann Arbor: Health Administration Press; 1980. [Google Scholar]
- 14.Quartarolo JM, Thoelke M, Schafers SJ. Reporting of estimated glomerular filtration rate: effect on physician recognition of chronic kidney disease and prescribing practices for elderly hospitalized patients. J. Hosp. Med. 2007;2:74–78. doi: 10.1002/jhm.172. [DOI] [PubMed] [Google Scholar]
- 15.Falconnier AD, Haefeli WE, Schoenenberger RA, Surber C, Martin-Facklam M. Drug dosage in patients with renal failure optimized by immediate concurrent feedback. J. Gen. Intern. Med. 2001;16:369–375. doi: 10.1046/j.1525-1497.2001.016006369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bates DW. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280:1311–1316. doi: 10.1001/jama.280.15.1311. [DOI] [PubMed] [Google Scholar]
- 17.Hunt DL, Haynes RB, Hanna SE, Smith K. Effects of computer-based clinical decision support systems on physician performance and patient outcomes: a systematic review. JAMA. 1998;280:1339–1346. doi: 10.1001/jama.280.15.1339. [DOI] [PubMed] [Google Scholar]
- 18.Perreault L, Metzger J. A pragmatic framework for understanding clinical decision support. J. Healthc. Inf. Manag. 1999;13:5–21. [Google Scholar]
- 19.Fieschi M, Dufour JC, Staccini P, Gouvernet J, Bouhaddou O. Medical decision support systems: old dilemmas and new paradigms? Methods Inf. Med. 2003;42:190–198. [PubMed] [Google Scholar]
- 20.Brender J, Ammenwerth E, Nykänen P, Talmon J. Factors influencing success and failure of health informatics systems—a pilot Delphi study. Methods Inf. Med. 2006;45:125–136. [PubMed] [Google Scholar]
- 21.Schedlbauer A, et al. What evidence supports the use of computerized alerts and prompts to improve clinicians’ prescribing behavior. J. Am. Med. Inform. Assoc. 2009;16:531–538. doi: 10.1197/jamia.M2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolfstadt JI, et al. The effect of computerized order entry with clinical decision support on the rates of adverse drug events: a systematic review. J. Gen. Intern. Med. 2008;23:451–458. doi: 10.1007/s11606-008-0504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eslami S, de Keizer NF, Abu-Hanna A. The impact of computerized physician medication order entry in hospitalized patients—a systematic review. Int. J. Med. Inform. 2008;77:365–376. doi: 10.1016/j.ijmedinf.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Garg AX, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293:1223–1238. doi: 10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- 25.Walton R, Dovey S, Harvey E, Freemantle N. Computer support for determining drug dose: systematic review and meta-analysis. BMJ. 1999;318:984–990. doi: 10.1136/bmj.318.7189.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durieux P, et al. Computerized advice on drug dosage to improve prescribing practice. Cochrane Database of Systematic Reviews. 2001;(16) doi: 10.1002/14651858.CD002894.pub3. Art. No.: CD002894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teich JM, et al. Effects of computerized physician order entry on prescribing practices. Arch. Intern. Med. 2000;160:2741–2747. doi: 10.1001/archinte.160.18.2741. [DOI] [PubMed] [Google Scholar]
- 28.Chertow GM, et al. Guided medication dosing for inpatients with renal insufficiency. JAMA. 2001;286:2839–2844. doi: 10.1001/jama.286.22.2839. [DOI] [PubMed] [Google Scholar]
- 29.Rind DM, et al. Effect of computer-based alerts on the treatment and outcomes of hospitalized patients. Arch. Intern. Med. 1994;154:1511–1517. [PubMed] [Google Scholar]
- 30.Asberg A, et al. Computer-assisted cyclosporine dosing performs better than traditional dosing in renal transplant recipients: results of a pilot study. Ther. Drug Monit. 2010;32:152–158. doi: 10.1097/FTD.0b013e3181d3f822. [DOI] [PubMed] [Google Scholar]
- 31.Camps-Valis G, et al. Prediction of cyclosporine dosage in patients after kidney transplantation using neural networks. IEEE Trans. Biomed. Eng. 2003;50:442–448. doi: 10.1109/TBME.2003.809498. [DOI] [PubMed] [Google Scholar]
- 32.van Hest R, Mathot R, Vulto A, Weimar W, van Gelder T. Predicting the usefulness of therapeutic drug monitoring of mycophenolic acid: a computer stimulation. Ther. Drug Monit. 2005;27:163–167. doi: 10.1097/01.ftd.0000158083.45954.97. [DOI] [PubMed] [Google Scholar]
- 33.Bates DW, Gawande AA. Improving safety with information technology. N. Engl. J. Med. 2003;348:2526–2534. doi: 10.1056/NEJMsa020847. [DOI] [PubMed] [Google Scholar]
- 34.Field TS, et al. Costs associated with developing and implementing a computerized clinical decision support system for medication dosing for patients with renal insufficiency in the long-term care setting. J. Am. Med. Inform. Assoc. 2008;15:466–472. doi: 10.1197/jamia.M2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American Society for Gastrointestinal Endoscopy. Financial incentives available in 2011 for physicians and hospitals adopting electronic health records. 2009 [online], http://www.asge.org/uploadedFiles/Members_Only/Advocacy/HITECH%20ACT%2004-01-2009%20v2.pdf. [Google Scholar]
- 36.GovTrack. Patient protection and affordable care act. 2010 [online], www.govtrack.us/congress/bill.xpd?bill=h111–3590.
- 37.Cash JJ. Alert fatigue. Am. J. Health Syst. Pharm. 2009;66:2098–2101. doi: 10.2146/ajhp090181. [DOI] [PubMed] [Google Scholar]
- 38.Ash JS, Sittig DF, Campbell EM, Guappone KP, Dykstra RH. Some unintended consequences of clinical decision support systems. AMIA Annu. Symp. Proc. 2007;2007:26–30. [PMC free article] [PubMed] [Google Scholar]
- 39.Van der Sijs H, Aarts J, van Gelder T, Berg M, Vulto A. Turning off frequently overridden drug alerts: limited opportunities for doing it safely. J. Am. Med. Inform. Assoc. 2008;15:439–448. doi: 10.1197/jamia.M2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bates DW, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J. Am. Med. Inform. Assoc. 2003;10:523–530. doi: 10.1197/jamia.M1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee F, Teich JM, Spurr CD, Bates DW. Implementation of physician order entry: user satisfaction and self-reported usage patterns. J. Am. Med. Inform. Assoc. 1996;3:42–55. doi: 10.1136/jamia.1996.96342648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maviglia SM, et al. Automating complex guidelines for chronic disease: lessons learned. J. Am. Med. Inform. Assoc. 2003;10:154–165. doi: 10.1197/jamia.M1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glassman PA, Simon B, Belperio P, Lanto A. Improving recognition of drug interactions: benefits and barriers to using automated drug alerts. Med. Care. 2002;40:1161–1171. doi: 10.1097/00005650-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Kuperman GJ, et al. Medication-related clinical decision support in computerized provider order entry systems: a review. J. Am. Med. Inform. Assoc. 2007;14:29–40. doi: 10.1197/jamia.M2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaushal R, et al. Return on investment for a computerized physician order entry system. J. Am. Med. Inform. Assoc. 2006;13:261–266. doi: 10.1197/jamia.M1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller A, Price G. Gabapentin toxicity in renal failure: the importance of dose adjustment. Pain. Med. 2009;10:190–192. doi: 10.1111/j.1526-4637.2008.00492.x. [DOI] [PubMed] [Google Scholar]
- 47.Zand L, McKian KP, Qian Q. Gabapentin toxicity in patients with chronic kidney disease: a preventable cause of morbidity. Am. J. Med. 2010;123:367–373. doi: 10.1016/j.amjmed.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 48.Williams SG, Bird M, Currie P. A 67 year old female with renal failure and sinus bradycardia. Postgrad. Med. J. 2004;80:48. doi: 10.1136/pmj.2002.003400q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sica DA, Gehr TW. Calcium-channel blockers in end-stage renal disease: pharmacokinetic and pharmacodynamic considerations. Curr. Opin. Nephrol. Hypertens. 2004;12:123–131. doi: 10.1097/00041552-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Chen E. Morphine overdose in a patient with renal failure. Clinical cases and images. 2009 [online], http://clinicalcases.org/2003/10/morphine-overdose-in-renal-failure.html. [Google Scholar]
- 51.Bernstein JM, Erk SD. Choice of antibiotics, pharmacokinetics and dose adjustments in acute and chronic renal failure. Med. Clin. North. Am. 1990;74:1059–1076. doi: 10.1016/s0025-7125(16)30536-3. [DOI] [PubMed] [Google Scholar]
- 52.Connolly JO, Woolfson RG. A critique of clinical guidelines for detection of individuals with chronic kidney disease. Nephron Clin. Pract. 2009;111:c69–c73. doi: 10.1159/000180122. [DOI] [PubMed] [Google Scholar]
- 53.Perazella MA. Advanced kidney disease, gadolinium and nephrogenic systemic fibrosis: the perfect storm. Curr. Opin. Nephrol. Hypertens. 2009;18:519–525. doi: 10.1097/MNH.0b013e3283309660. [DOI] [PubMed] [Google Scholar]
- 54.Nash IS, et al. Reducing excessive medication administration in hospitalized adults with renal dysfunction. Am. J. Med. Qual. 2005;20:64–69. doi: 10.1177/1062860604273752. [DOI] [PubMed] [Google Scholar]
- 55.Colpaert K, et al. Impact of computerized physician order entry on medication prescription errors in the intensive care unit: a controlled cross-sectional trial. Crit. Care. 2006;10:R21. doi: 10.1186/cc3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Field TS, et al. Computerized clinical decision support during medication ordering for long-term care residents with renal insufficiency. J. Am. Med. Inform. Assoc. 2009;16:480–485. doi: 10.1197/jamia.M2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Evans RS, et al. A computer-assisted management program for antibiotics and other antiinfective agents. N. Engl. J. Med. 1998;338:232–238. doi: 10.1056/NEJM199801223380406. [DOI] [PubMed] [Google Scholar]
- 58.Roberts GW, et al. Clinical decision support implemented with academic detailing improves prescribing of key renally cleared drugs in the hospital setting. J. Am. Med. Inform. Assoc. 2010;17:308–312. doi: 10.1136/jamia.2009.001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsumura Y, et al. Alert system for inappropriate prescriptions relating to patients’ clinical condition. Methods Inf. Med. 2009;48:566–573. doi: 10.3414/ME9244. [DOI] [PubMed] [Google Scholar]
- 60.Galanter WL, Didomenico RJ, Polikaitis A. A trial of automated decision support alerts for contraindicated medications using computerized physician order entry. J. Am. Med. Inform. Assoc. 2005;12:269–274. doi: 10.1197/jamia.M1727. [DOI] [PMC free article] [PubMed] [Google Scholar]