Abstract
Essential oils were obtained by hydrodistillation of the umbels+seeds and stems of Ferula akitschkensis (FAEOu/s and FAEOstm, respectively) and analyzed by gas chromatography and gas chromatography–mass spectrometry. Fifty two compounds were identified in FAEOu/s. The primary components were sabinene, α-pinene, β-pinene, terpinen-4-ol, eremophilene, and 2-himachalen-7-ol, while the primary components of FAEOstm were myristicin and geranylacetone. FAEOu/s, β-pinene, sabinene, γ-terpinene, geranylacetone, isobornyl acetate, and (E)-2-nonenal stimulated [Ca2]i mobilization in human neutrophils, with the most potent being geranylacetone (EC50 = 7.6 ± 1.9 µM) and isobornyl acetate 6.4 ± 1.7 (EC50 = 7.6 ± 1.9 µM). In addition, treatment of neutrophils with β-pinene, sabinene, γ-terpinene, geranylacetone, and isobornyl acetate desensitized the cells to N-formyl-Met-Leu-Phe (fMLF)- and interleukin-8 (IL-8)-induced [Ca2]i flux and inhibited fMLF-induced chemotaxis. The effects of β-pinene, sabinene, γ-terpinene, geranylacetone, and isobornyl acetate on neutrophil [Ca2+]i flux were inhibited by transient receptor potential (TRP) channel blockers. Furthermore, the most potent compound, geranylacetone, activated Ca2+ influx in TRPV1-transfected HEK293 cells. In contrast, myristicin inhibited neutrophil [Ca2+]i flux stimulated by fMLF and IL-8 and inhibited capsaicin-induced Ca2+ influx in TRPV1-transfected HEK293 cells. These findings, as well as pharmacophore modeling of TRP agonists, suggest that geranylacetone is a TRPV1 agonist, whereas myristicin is a TRPV1 antagonist. Thus, at least part of the medicinal properties of Ferula essential oils may be due to modulatory effects on TRP channels.
Keywords: Ferula akitschkensis, calcium flux, essential oil, neutrophil, transient receptor potential channel
Graphical Abstract
INTRODUCTION
The genus Ferula (Apiaceae) comprises ~ 185 species distributed throughout Central Asia, the Mediterranean, and Northern Africa. Various parts of these plants (e.g., roots, leaves, seeds) and resin prepared from the plant have been used historically in traditional herbal medicine for a variety of ailments, including diarrhea, hysteria, whooping cough, asthma, bronchitis, and ulcers1, 2. Asafetida, an oleo-gum-resin obtained from the roots of various Ferula spp., including F. assa-foetida, F. foetida, F. rubricaulis, F. rigidula, F. alliacea, and F. narthex, contains up to 17% of the essential oil and is used as a traditional phytomedicine for the treatment of asthma, epilepsy, intestinal parasites, weak digestion, stomach ache, and influenza1. Asafetida and Ferula essential oils have also been used as food additives (spices) and natural food preservatives1. Secondary metabolites isolated from Ferula spp., such as terpenoids and sesquiterpenes, have been shown to exhibit a variety of pharmacological properties, including anti-inflammatory, estrogenic, antitumor, antibacterial, and antiviral activities3. In addition, essential oils isolated from different parts of Ferula herbs, including stems, flowers, and seeds, have been reported as a rich source of monoterpenes, sesquiterpenes, and polysulfides4, 5, and biochemical studies of these chemical components have demonstrated desirable therapeutic properties, including antioxidant, antimutagenic, antimicrobial, and antifungal effects4, 6.
Despite the importance of Ferula spp. as a potential source of novel therapeutic compounds, the chemical composition and biological properties of many endemic Ferula spp. have not yet been evaluated. For example, Ferula akitschkensis B. Fedtschenko ex Koso-Poljansky grows on mountain slopes at an altitude of 900–2100 m and is endemic to northern China, Kazakhstan, Kyrgyzstan, and Russia. When mature, the plant produces numerous 2–4-inch-wide rounded yellow umbels on branched stems. Although several unique secondary metabolites have been isolated from F. akitschkensis, including akiferine, akiferidine, akiferidinine, and akichenin7, the chemical composition and biological properties of essential oils from F. akitschkensis have not yet been analyzed. While previous studies demonstrated that essential oils from a number of plants can have direct antimicrobial activity, the effects of essentail oils and their components on the innate immune system are not well understood.
Neutrophils are a key cellular component of the innate immune system and play a prominent role in the inflammatory response. These leukocytes are recruited to sites of infection or injury by a variety of factors, including N-formyl-Met-Leu-Phe (fMLF), a bacterial or mitochondria-derived peptide, and chemokines such as interleukin 8 (IL-8)8. IL-8 and fMLF activate G-protein coupled receptors (GPCR) to induce neutrophil chemotaxis and the release of various mediators, such as reactive oxygen species (ROS), cytokines, and proteases. Recently, we found that some essential oil components of Artemisia kotuchovii were able to modulate some of these neutrophil functional responses9. Thus, plant essential oils likely represent a source of novel therapeutics that could be developed to modulate innate immune responses and either enhance defense against microbial infection or control excessive inflammation.
In the present studies, we determined the composition of essential oils from umbels+seeds and stems of F. akitschkensis (designated as FAEOu/s and FAEOstm, respectively) and evaluated their immunomodulatory activity in human neutrophils. We found that FAEOu/s can activate Ca2+ mobilization and inhibit neutrophil migration. We also evaluated the main components of FAEOu/s and FAEOstm and found the primary bioactive constituents to be sabinene, β-pinene, γ-terpinene, geranylacetone, and isobornyl acetate, which were neutrophil agonists. We also found that one component, myristicin, inhibited neutrophil function. Using a set of pharmacological inhibitors and molecular modeling, the primary molecular targets for the agonists were determined to be transient receptor potential (TRP) channels. Indeed, the most potent compound, geranylacetone, directly activated Ca2+ flux in TRPV1-transfected HEK293 cells, whereas myristicin inhibited capsaicin-induced Ca2+ flux in TRPV1-transfected HEK293 cells. Thus, these data suggest that at least part of the medicinal properties of Ferula essential oils may be due to modulatory effects on TRP channels.
MATERIALS AND METHODS
Chemicals
The major and some minor compounds in the essential oils were obtained from commercial sources. Sabinene, p-cymen-8-ol, camphene, α-pinene oxide, α-terpineol, γ-terpinene, 4-carvomenthenol (terpinen-4-ol), myrtenol, and 3,7-dimethyl-1,3,6-octatriene (β-ocimene) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Myrcene, (+)-limonene, (−)-limonene, p-cymene, (−)-caryophyllene oxide, and α-terpinene were purchased from Acros Organics (Geel, Belgium). Elemicin was from Toronto Research Chemicals (Toronto, Canada). Geraniol and (E)-2-nonenal were from AK Scientific Inc (Union City, CA, USA). Terpinolene and α-pinene were from Santa Cruz Biotechnology (Dallas, TX, USA). (E/Z)-geranylacetone and (1S)-(−)-β-pinene were from Alfa Aesar (Ward Hill, MA, USA). Thymol, (−)-menthol, (R)-(−)-carvone, isobornyl acetate, carvacrol, (−)-fenchone, and capsaicin were from Tokyo Chemical Industry Co. Ltd. (Tokyo, Japan). Myristicin was from Cayman Chemical Co. (Ann Arbor, MI, USA). The compounds were dissolved in dimethyl sulfoxide (DMSO; 10 mM stock solutions) and stored at −20 °C.
Materials
DMSO, N-formyl-Met-Leu-Phe (fMLF), HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), and Histopaque 1077 were purchased from Sigma-Aldrich Chemical Co. Trp-Lys-Tyr-Met-Val-Met (WKYMVM), pertussis toxin (PTX) and phospholipase C inhibitor U73122 were from Tocris Bioscience (Ellisville, MO, USA). Capsazepine, SKF 96365, and N-(p-amylcinnamoyl) anthranilic acid (ACA) were from Cayman Chemical Co. 2-Aminoethyl diphenyl borate (2-APB) was from BioVision Inc. (Milpitas, CA, USA). Trp-Arg-Trp-Trp-Trp-Trp (WRW4) was from Phoenix Pharmaceuticals, Inc. (Burlingame, CA, USA). Cyclosporin H was from Toronto Research Chemicals. Stock solutions for the inhibitors/antagonists (except PTX) were prepared in DMSO; PTX was dissolved in H2O. Human IL-8 was purchased from Peprotech, Inc. (Rocky Hill, NJ, USA). Fura-2 acetoxy methylester (fura-2/AM) was from TEFLabs (Austin, TX, USA). Roswell Park Memorial Institute (RPMI) 1640 medium, Dulbecco’s Modified Eagle’s Medium (DMEM), and penicillin–streptomycin solution were purchased from Mediatech (Herndon, VA, USA). Fetal bovine serum (FBS) was purchased from Atlas Biologicals (Fort Collins, CO, USA). Hanks’ balanced salt solution (HBSS; 0.137 M NaCl, 5.4 mM KCl, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 4.2 mM NaHCO3, 5.56 mM glucose, and 10 mM HEPES, pH 7.4) and G418 were from Life Technologies (Grand Island, NY, USA). HBSS without Ca2+ and Mg2+ is designated as HBSS−; HBSS containing 1.3 mM CaCl2 and 1.0 mM MgSO4 is designated as HBSS+.
Plant Material
Ferula akitschkensis was collected at the fruiting stage (July 2015) in the Almaty region of Kazakhstan at an altitude of 1525 m. Voucher specimens were deposited at the Institute of Plant Biology and Biotechnology (Almaty, Kazakhstan). Umbels with seeds and stems were air-dried for 7–10 days at room temperature away from direct sunlight before hydrodistillation.
Essential Oil Extraction
Essential oil was obtained by hydrodistillation using a Clevenger type apparatus, as described previously9. For the hydrodistillation, we used conditions accepted by the European Pharmacopoeia (European Directorate for the Quality of Medicines, Council of Europe, Strasbourg, France, 2014) to avoid artifacts. Solutions of the essential oils were prepared in DMSO (10 mg/mL stock solutions) for biological evaluation, and in n-hexane (10 % w/v) for gas-chromatographic analysis.
Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
GC-MS analysis was performed with an Agilent 5975 GC-MSD system (Agilent Technologies, Santa Clara, CA, USA), as reported previously9. An Agilent Innowax FSC column (60 m × 0.25 mm, 0.25 µm film thickness) was used with He as the carrier gas (0.8 mL/min). The GC oven temperature was kept at 60 °C for 10 min, increased to 220 °C at a rate of 4 °C/min, kept constant at 220 °C for 10 min, and then increased to 240 °C at a rate of 1 °C/min. The split ratio was adjusted to 40:1, and the injector temperature was 250 °C. MS spectra were monitored at 70 eV with as mass range from m/z 35 to 450.
GC analysis was carried out using an Agilent 6890N GC system. To obtain the same elution order as with GC-MS, simultaneous injection was performed using the same column and appropriate operational conditions. Flame ionization detector (FID) temperature was 300 °C. The components of essential oils were identified by co-injection with standards (whenever possible), which were purchased from commercial sources or isolated from natural sources. In addition, compound identities were confirmed by comparison of their mass spectra with those in the Wiley GC/MS Library (Wiley, NY, USA), MassFinder software 4.0 (Dr. Hochmuth Scientific Consulting, Hamburg, Germany), Adams Library, and NIST Library. A C8–C40 n-alkane standard solution (Fluka, Buchs, Switzerland) was used to spike the samples for the determination of relative retention indices (RRI). Relative percentage amounts of the separated compounds were calculated from FID chromatograms.
Chiral GC/MS Analysis
Chromatographic separation on a chiral column was performed for α-pinene, β-pinene, and sabinene. GC-MS analysis of the enantiomers in the oil was performed with an Agilent 7890 GC (Agilent; SEM Ltd.) equipped with a FID and 5975 MSD with Triple-Axis Detector, and Agilent G 4513 autoinjector, integrated with a Gerstel CIS (Gerstel, Mülheim an der Ruhr, Germany; SEM Ltd., Istanbul, Turkey). Chiral separation was performed on a Lipodex G column (25 m × 0.25 mm × 0.125 m film thickness; Macherey-Nagel, Düren, Germany) with He as the carrier gas (65 min at 5 mL/min, average velocity 77.985 cm/sec). Injection quantity was 1 L (10% in hexane). The temperature program for separation of α-pinene, β-pinene, and sabinene enantiomers was 50 min at 35 °C, then increased 40 °C/min up to 200 °C for 10.875 min. Run time was 65 min. The split ratio was adjusted to 40:1, and the injector temperature was at 250 °C. FID temperature was 250 °C.
Cell Culture
Wild-type human promyelocytic leukemia HL-60 cells and HL-60 cells stably transfected with human N-formyl peptide receptor (FPR) 1 (FPR1-HL60 cells) or human FPR2 (FPR2-HL60 cells) (kind gift from Dr. Marie-Josephe Rabiet, INSERM, Grenoble, France) were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated FBS, 10 mM HEPES, 100 µg/mL streptomycin, and 100 U/mL penicillin, as described9. Transfected HL-60 cells were cultured in the presence of G418 (1 mg/mL).
Cultured HEK293 cells were plated in a 6-well plate for 24 hours after which, the cells were transfected with human TRPV1 (transient receptor potential vanniloid receptor type 1) cDNA using TransIT®-2020 (Mirus Bio LLC, Madison, WI, USA) according to the manufacturer’s protocol. Following transfection, the cells were then suspended in DMEM supplemented with 10% fetal bovine serum at 37°C and used for intracellular Ca2+ measurements.
Neutrophil Isolation
For isolation of human neutrophils, blood was collected from healthy donors in accordance with a protocol approved by the Institutional Review Board at Montana State University. Neutrophils were purified from the blood using dextran sedimentation, followed by Histopaque 1077 gradient separation and hypotonic lysis of red blood cells, as described previously9. Isolated neutrophils were washed twice and resuspended in HBSS. Neutrophil preparations were routinely >95% pure, as determined by light microscopy, and >98% viable, as determined by trypan blue exclusion. Neutrophils were obtained from multiple different donors (n=8); however, the cells from different donors were never pooled during experiments.
Ca2+ Mobilization Assay
Changes in intracellular Ca2+ ([Ca2+]i) in neutrophils and HL-60 cells were measured with a FlexStation 3 scanning fluorometer (Molecular Devices, Sunnyvale, CA, USA) using fluorescent dye Fluo-4AM (Invitrogen, Carlsbad, CA, USA). Human neutrophils, wild-type HL-60 cells, or transfected HL-60 cells were suspended in HBSS, loaded with Fluo-4AM dye (final concentration, 1.25 µg/mL) and incubated for 30 min in the dark at 37 °C. After dye loading, the cells were washed with HBSS−, resuspended in HBSS+, separated into aliquots, and aliquotted into the wells of flat-bottom, half-area well black microtiter plates (2 × 105 cells/well). Essential oils or pure compounds diluted in DMSO were added to the wells (final concentration of DMSO was 1%), and changes in fluorescence were monitored (λex = 485 nm, λem = 538 nm) every 5 s for 240 s at room temperature and normal atmospheric conditions immediately after an addition of the test compound/oil. The maximum change in fluorescence, expressed in arbitrary units over baseline, was used to determine agonist response. Responses were normalized to the response induced by 5 nM fMLF, which was assigned a value of 100%. Curve fitting (at least five or six points) and calculation of median effective concentration values (EC50 or IC50) were performed by nonlinear regression analysis of the dose–response curves generated using Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). To examine the inhibitory effects of PTX, FPR1-HL60 cells were treated with PTX (100 ng/mL) for 18 h.
Transient Ca2+ influx in HEK293 cells (transfected with TRPV1 and non-transfected cells) was evaluated as previously described10. HEK293 cells were incubated in growth medium containing fura-2 (2 µM) for 30 min at 37 °C and 5% CO2. Coverslips containing the fura-2-loaded HEK293 cells were mounted on the stage of an Olympus IX-81 inverted fluorescence microscope (Olympus America, Lake Success, NY, USA), and the cells were superfused continuously with serum-free medium at a flow rate of 2 mL/min. Cells were exposed to compounds under investigation by switching from control medium to compound-containing medium for 10 sec unless noted otherwise. Ca2+ influx measurements and data acquisition were simultaneously performed on multiple individual cells using a fluorescence imaging system (Easy Ratio Pro, Photon Technology International, Lawrenceville, NJ, USA) equipped with a multi-wavelength spectrofluorometer (DeltaRAM X, Photon Technology International Inc.) and a QuantEM 512SC electron multiplying charge-coupled device camera (Photometrics, Tucson, AZ, USA), as previously described10. Representative traces were acquired using an alternating excitation wavelength protocol (340, 380 nm/20 Hz) and emission wavelength of 510 nm.
Neutrophil Migration Assay
Human neutrophils were suspended in HBSS+ containing 2% (v/v) heat-inactivated FBS (2 × 106 cells/mL), and cell migration was analyzed in 96-well ChemoTx chemotaxis chambers (Neuroprobe, Gaithersburg, MD, USA). In brief, neutrophils were preincubated with the indicated concentrations of the test sample (oil or pure compound) or 1% DMSO for 30 min at room temperature and added to the upper wells of the ChemoTx chemotaxis chambers. The lower wells were loaded with 30 µL of HBSS+ containing 2% (v/v) FBS and the indicated concentrations of test sample, 1% DMSO as a negative control, or 1 nM fMLF as a positive control. Neutrophils were added to the upper wells and allowed to migrate through the 5.0 µm pore polycarbonate membrane filter for 60 min at 37 °C and 5% CO2. The number of migrated cells was determined by measuring ATP in lysates of transmigrated cells using a luminescence-based assay (CellTiter-Glo; Promega, Madison, WI, USA). Chemiluminescence was recorded using a Fluoroskan Ascent FL fluorescence microplate reader (Thermo Scientific, Rockford, IL, USA), and the signal was converted to absolute cell numbers by comparison of the values with standard curves obtained with known numbers of neutrophils. Curve fitting (at least six points) and calculation of median effective concentration values (IC50) were performed by nonlinear regression analysis of the dose–response curves generated using Prism 5.
Compound Cytotoxicity
Cytotoxicity was analyzed with a CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega), according to the manufacturer’s protocol. Briefly, HL-60 cells were cultured at a density of 1 × 105 cells/well with different concentrations of essential oil or compound (final concentration of DMSO was 1%) for 4 or 24 h at 37 °C and 5% CO2. Following treatment, the cells were allowed to equilibrate to room temperature for 30 min, substrate was added, and the samples were analyzed with a Fluoroscan Ascent FL microplate reader.
Molecular Modeling
We used a ligand-based approach for molecular modeling employing field point methodology11. The structures of (−)-β-pinene, (−)-sabinene, and γ-terpinene in Tripos MOL2 format were imported into the FieldTemplater program (FieldTemplater Version 2.0.1; Cresset Biomolecular Discovery Ltd., Hertfordshire, UK) for the construction of Template A. Similarly, the structures of (−)-carveol, 6-tert-butyl-m-cresol, carvacrol, and thymol were imported to construct Template B; and capsaicin, N-geranyl cyclopropylcarboxamide (NGCC), and (Z)-N-(2,7-dimethylocta-2,6-dienyl)-2-phenylacetamide (compound 2k)12, 13 were imported to construct Template C. Using built-in capabilities of FieldTemplater software, representative sets of conformations for the compounds were generated corresponding to local energy minima calculated within the extended electron distribution force field11. To lower the number of rotatable bonds, “force amides trans” option was applied to the conformational search. For the generation of field point patterns, probe atoms having positive, negative, and zero charge were placed in the vicinity of a given conformation, and the energy of their interaction with the molecular field was calculated using the extended electron distribution parameter set. Positions of energy extrema for positive probes give “negative” field points, whereas energy extrema for negative and neutral probe atoms correspond to “positive” and steric field points, respectively. Hydrophobic field points were also generated with neutral probes capable of penetrating into the molecular core and reaching extrema in the centers of hydrophobic regions. A detailed description of the field point calculation procedure has been published elsewhere11. A clique-matching algorithm with further simplex optimization was applied to obtain the conformations of three (Templates A and C) or four molecules (for Template B) giving good mutual overlays in terms of geometric and field similarity (S). The best overlays were taken as templates representative of the bioactive conformations.
The structures of geranylacetone and isobornyl acetate were imported into FieldAlign program (FieldAlign Version 2.0.1; Cresset Biomolecular Discovery Ltd., Hertfordshire, UK) in Tripos MOL2 format. Conformational search and field point calculation were performed as described above for template building. Conformations with the best fit to the geometry and field points of Template A (for geranylacetone and isobornyl acetate) and of Template C (for geranylacetone) were identified, and their superimpositions were refined by the simplex optimization algorithm and visualized using instruments incorporated in FieldAlign.
Statistical Analysis
To calculate median effective concentration values (EC50 or IC50), curve fitting (at least five or six points) was performed by nonlinear regression analysis of the dose–response curves generated using Prism 7 (GraphPad Software, Inc., San Diego, CA, USA). One way analysis of variance (ANOVA) was performed on the data sets, followed by Tukey’s pair-wise comparisons. Pair-wise comparisons with differences at P < 0.05 were considered to be statistically significant.
RESULTS
Composition of the Essential Oils from F. akitschkensis
Essential oils were obtained by conventional hydrodistillation of the dried stems or umbels with seeds of F. akitschkensis (designated FAEOstm and FAEOu/s, respectively) and analyzed by GC and GC-MS simultaneously to determine their chemical compositions. Hydrodistillation of the umbels+seeds and stems produced 0.7 and 0.02% (v/w on the basis of the weight of dried material) essential oils, respectively. Table 1 summarizes the identified compounds, their retention times, and percentage composition.
Table 1.
Composition of the Volatile Compounds Identified in F. akitschkensis Essential Oils
| Compd # |
RRI | Compound Name | A (%) |
B (%) |
Compd # |
RRI | Compound Name | A (%) |
B (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1032 | α-pineneb | 15.4 | 1.3 | 32 | 1661 | α-himachalenec | 0.3 | 0.7 |
| 2 | 1035 | α-thujenec | 0.6 | - | 33 | 1662 | trans-pinocarvyl acetateb | - | 0.7 |
| 3 | 1076 | campheneb | 0.1 | - | 34 | 1706 | α-terpineolb | 0.1 | - |
| 4 | 1118 | β-pineneb | 8.5 | 1.6 | 35 | 1711 | γ-himachalenec | 0.3 | 0.9 |
| 5 | 1132 | sabineneb | 58.7 | 1.4 | 36 | 1739 | β-himachalenec | 0.4 | 0.9 |
| 6 | 1174 | myrceneb | 0.6 | - | 37 | 1743 | eremophileneb | 1.4 | 2.1 |
| 7 | 1188 | α-terpineneb | 0.3 | - | 38 | 1755 | dauca-8,11-dienec | 0.1 | - |
| 8 | 1203 | limoneneb | 0.4 | - | 39 | 1758 | cis-piperitolb | 0.1 | - |
| 9 | 1218 | β-phellandrenec | 0.3 | - | 40 | 1771 | (E)-γ-bisaboleneb | tr | - |
| 10 | 1255 | γ-terpineneb | 0.8 | 0.7 | 41 | 1783 | β-sesquiphellandrenec | tr | - |
| 11 | 1266 | (E)-β-ocimeneb | tr | - | 42 | 1786 | ar-curcumenec | tr | - |
| 12 | 1280 | p-cymeneb | 0.9 | - | 43 | 1792 | p-mentha-1(7),5-dien-2-olb | tr | - |
| 13 | 1290 | terpinoleneb | 0.2 | - | 44 | 1804 | myrtenolb | tr | - |
| 14 | 1384 | α-pinene oxidec | tr | - | 45 | 1854 | germacrene Bb | 0.1 | - |
| 15 | 1474 | trans-sabinene hydratec | 1.0 | 0.2 | 46 | 1864 | p-cymen-8-olb | tr | - |
| 16 | 1482 | longipineneb | 0.1 | - | 47 | 1868 | (E)-geranylacetoneb | - | 4.0 |
| 17 | 1493 | α-ylangenec | 0.2 | - | 48 | 1888 | ar-himachalenec | tr | - |
| 18 | 1504 | dauceneb | 0.1 | - | 49 | 1941 | α-calacoreneb | tr | - |
| 19 | 1512 | longicycleneb | tr | - | 50 | 1985 | γ-calacoreneb | tr | - |
| 20 | 1548 | (E)-2-nonenalc | - | 0.3 | 51 | 2008 | caryophyllene oxideb | - | 1.0 |
| 21 | 1549 | β-cubebeneb | 0.2 | - | 52 | 2028 | junenolc | - | 0.4 |
| 22 | 1556 | cis-sabinene hydratec | 0.6 | - | 53 | 2045 | β-himachalene oxidec | tr | - |
| 23 | 1571 | trans-p-menth-2-en-1-olb | 0.3 | - | 54 | 2066 | p-mentha-1.4-dien-7-olb | tr | - |
| 24 | 1586 | pinocarvonec | tr | - | 55 | 2180 | 6-epi-cubenolb | tr | - |
| 25 | 1594 | α-trans-β-bergamotenec | tr | - | 56 | 2245 | elemicinec | - | 0.8 |
| 26 | 1604 | isobornyl acetatec | - | 0.3 | 57 | 2254 | 2-himachalen-7-olc | 1.3 | 7.9 |
| 27 | 1611 | terpinen-4-olb | 3.9 | 1.6 | 58 | 2296 | myristicineb | - | 67.9 |
| 28 | 1638 | cis-p-menth-2-en-1-olc | tr | - | 59 | 2300 | tricosaneb | - | 1.1 |
| 29 | 1645 | (Z)-β-santaleneb | 0.1 | - | 60 | 2500 | pentacosaneb | - | 0.5 |
| 30 | 1651 | sabinaketoneb | tr | - | 61 | 2900 | nonacosaneb | 0.1 | - |
| 31 | 1653 | γ-elemeneb | tr | - | 62 | 2931 | hexadecanoic acidb | tr | - |
The data are presented as relative % by weight for components of FAEOu/s (A) and FAEOstm (B). RRI: Relative retention index calculated based on retention of n-alkanes; %, calculated from flame ionization detector data. Trace amounts (tr) were present at <0.1 %.
Identification based on comparison with co-injected standards.
Tentatively identified using Wiley and MassFinder mass spectra libraries and published RRI. Compounds selected for further biological screening are indicated in bold.
Fifty two compounds were identified in FAEOu/s, representing around 98% of the total oil composition. The main components of the FAEOu/s were sabinene (58.7%), α-pinene (15.4%), β-pinene (8.5%), terpinen-4-ol (3.9%), eremophilene (1.4%), 2-himachalen-7-ol (1.3%), and trans-sabinene hydrate (1.0%). Twenty four compounds were present at concentrations from 0.1 to <1.0%. The remaining 21 volatile compounds were identified in trace amounts (<0.1%). Thus, monoterpene hydrocarbons dominated (85.7%) over all other compound groups. Among the other significant compounds, 14 oxygenated monoterpenes were present (6.0%), with terpinen-4-ol (3.9%) as the major representative. Small amounts of sesquiterpene hydrocarbons (20 compounds) and oxygenated sesquiterpenes (3 compounds) were also present (3.3% and 1.3%, respectively), with eremophilene (1.4%) as the major sesquiterpene constituent. Only trace amounts of alkanes and fatty acids were detected in FAEOu/s. Finally, we observed that FAEOu/s contained a series of himachalene-type compounds (α-, β-, γ-himachalene, ar-himachalene, β-himachalene oxide, and 2-himachalen-7-ol).
Gas-chromatographic analysis of the volatiles on a Lipodex G chiral column revealed the existence of enantiomeric pairs of α-pinene, β-pinene and sabinene in the FAEOu/s. We found (1S)-(−)-α-pinene (95%), (1S)-(−)-β-pinene (94%), and (1R,5R)-(+)-sabinene (97%).
Twenty one compounds were identified in FAEOstm, representing 96.6% of the total oil composition. FAEOstm was distinguished by a high percentage of phenylpropanoids, such as myristicin (67.9%) and elemicine (0.8%). Mono- and sesquiterpenoids were detected in the oil in small amounts (5.0 and 4.6%, respectively), although oxygenated sesquiterpenes were present in higher amounts (9.3%) than in FAEOu/s. Interestingly, six himachalene-type sesquiterpenoids were present in relatively high amounts (10.4%).
Effect of the Essential Oils on Ca2+ Mobilization in Neutrophils
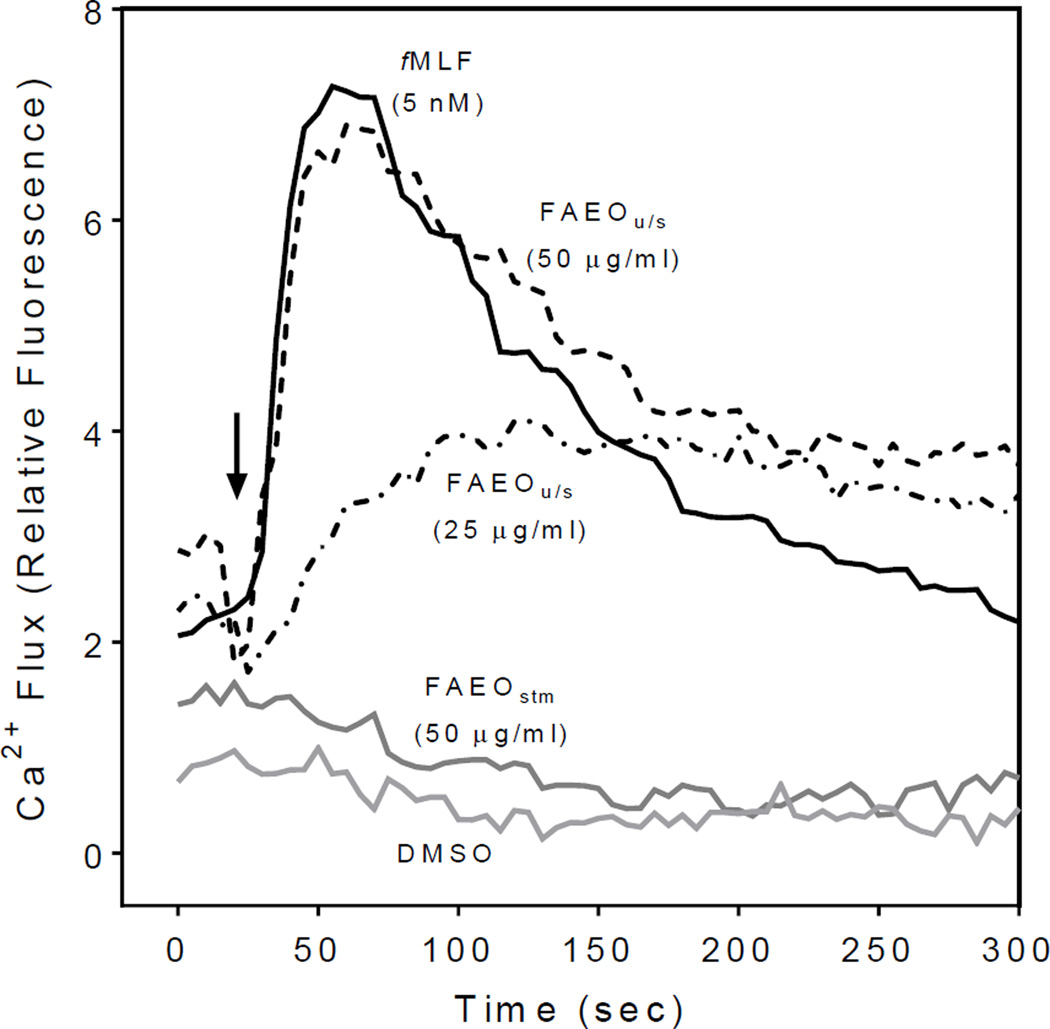
Essential oils and their components have been reported previously to modulate [Ca2+]i levels in neutrophils and other cells9. We found that FAEOstm did not activate human neutrophil Ca2+ flux, whereas FAEOu/s stimulated the cells with an EC50 of 16.5 µg/ml (Table 2). As shown in Figure 1, kinetics of the [Ca2+]i response activated by 50 µg/ml of FAEOu/s resembled the response stimulated by our positive control, fMLF, which is an agonist of FPR1, a chemotactic receptor expressed on neutrophils and other leukocytes14. Treatment with fMLF or FAEOu/s caused a rapid increase in [Ca2+]i that peaked by ~40 sec and gradually declined to basal levels, reflecting clearance of Ca2+ from the cytosol. Because essential oils and individual test compounds were suspended in DMSO, we also evaluated the effects of DMSO on our assay system and confirmed that 1% DMSO did not induce a [Ca2+]i flux in human neutrophils (Figure 1).
Table 2.
Effect of F. akitschkensis Essential Oils and Selected Components on Ca2+ Flux in Human Neutrophils and HL-60 Cells
| Essential Oil | Neutrophils | HL-60 Cells |
|---|---|---|
| EC50, µg/ml (efficacy, %)a | ||
| FAEOu/s | 16.5 ± 3.6 (90) | 5.0 ± 1.2 |
| FAEOstm | N.A.b | N.A. |
| Compound | EC50, µM (efficacy, %) | |
| camphene | N.A. | N.A. |
| caryophyllene oxide | N.A. | N.A. |
| p-cymen-8-ol | N.A. | N.A. |
| p-cymene | N.A. | N.A. |
| elemicine | N.A. | N.A. |
| (E/Z)-geranylacetone | 7.6 ± 1.9 (105) | 6.5 ± 1.9 |
| isobornyl acetate | 6.4 ± 1.7 (100) | 4.3 ± 0.9 |
| myrcene | N.A. | N.A. |
| α-terpinene | N.A. | N.A. |
| (R)-(+)-limonene | N.A. | N.A. |
| (S)-(−)-limonene | N.A. | N.A. |
| myristicin | N.A. | N.A |
| myrtenol | N.A. | N.A. |
| (E)-2-nonenal | 33.7 ± 4.7 (70) | 31.3 ± 4.2 |
| (E/Z)-β-ocimene | N.A. | N.A. |
| (+/−)-α-pinene | N.A. | N.A. |
| (1S)-(−)-β-pinene | 24.6 ± 3.4 (70) | 16.4 ± 2.4 |
| α-pinene oxide | N.A. | N.A. |
| (+/−)-sabinene | 49.4 ± 6.3 (55) | 22.7 ± 3.5 |
| terpinen-4-ol | N.A. | N.A. |
| γ-terpinene | 23.2 ± 3.5 (30) | 9.3 ± 1.6 |
| α-terpineol | N.A. | N.A. |
| terpinolene | N.A. | N.A. |
EC50 values are presented as the mean ± S.D. of three independent experiments, and efficacy (in parentheses) is expressed as % of the response induced by a positive control (5 nM fMLF).
N.A.: no activity was observed, even at the highest concentration tested (50 µM).
Figure 1.
Effect of Ferula essential oils on Ca2+ mobilization in human neutrophils. Human neutrophils were treated with the indicated concentrations of FAEOu/s, FAEOstm, and fMLF (positive control), or 1% DMSO (negative control), and [Ca2+]i flux was monitored for the indicated times (arrow indicates when treatment was added). The data are from one experiment that is representative of three independent experiments.
Effect of Essential Oil Components on Ca2+ Mobilization in Neutrophils
Because FAEOu/s activated Ca2+ flux in human neutrophils, we focused on analysis of the effects of its constituents to possibly identify the active compound(s). Sixteen commercially available compounds, including four of the major compounds (sabinene, α-pinene, β-pinene, and terpinen-4-ol) and twelve of the minor compounds, were tested. All tested compounds, representing >89.5% of the FAEOu/s composition, were monoterpenes or their oxygenated forms. The terpenes were first evaluated for agonist effects on [Ca2+]i in human neutrophils. Consistent with our previous studies9, we found that β-pinene and sabinene can directly activate neutrophil Ca2+ flux. In addition, we found that γ-terpinene, a minor component of FAEOu/s also had agonist activity (Table 2). Thus, these three compounds, representing 68.0% of FAEOu/s, directly activated a neutrophil [Ca2+]i flux similarly to FAEOu/s itself.
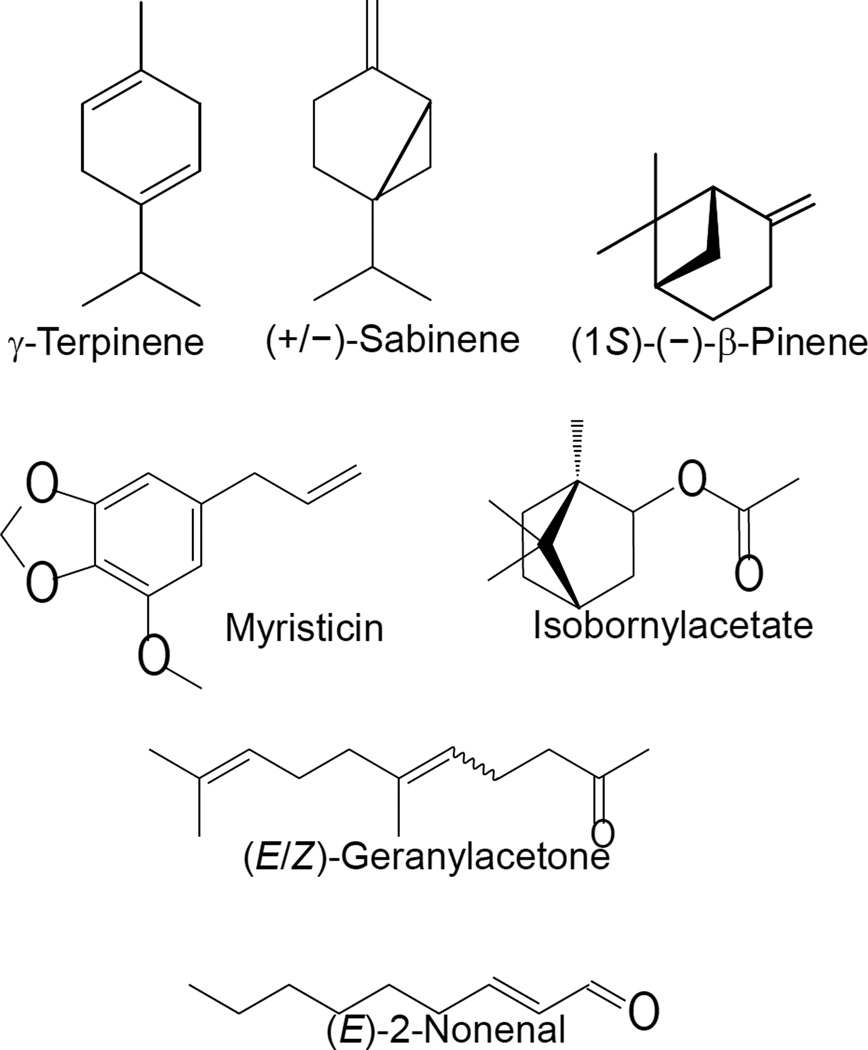
Although FAEOstm did not stimulate Ca2+ flux in neutrophils, we also evaluated several of its components that were not present in FAEOu/s. The commercially available compounds included myristicin, isobornyl acetate, elemicine, (E)-2-nonenal, (E/Z)-geranylacetone, and caryophyllene oxide. These compounds, together with the above tested compounds that were included in both oils, represented 80.9% of the FAEOstm composition. In addition to the active compounds shared by FAEOstm and FAEOu/s, we found that 2-nonenal, isobornyl acetate, and geranylacetone also stimulated neutrophil Ca2+ flux (Table 2). Thus, the active constituents with agonist potential represent 8.3% of the FAEOstm composition. Chemical structures of the active compounds identified in FAEOu/s and FAEOstm are shown in Figure 3. 2-Nonenal is a known product of lipid peroxidation with a broad spectrum of biological properties (e.g., see15). Because this bioactive aldehyde can easily conjugate with proteins and was previously reported to activate phospholipase C and stimulate neutrophils15, we did not further evaluate properties of this minor oil component.
Figure 3.
Chemical structures of the compounds evaluated for biological activity. For sabinene and geranylacetone, mixtures of stereoisomers were studied; general structures are shown for these compounds.
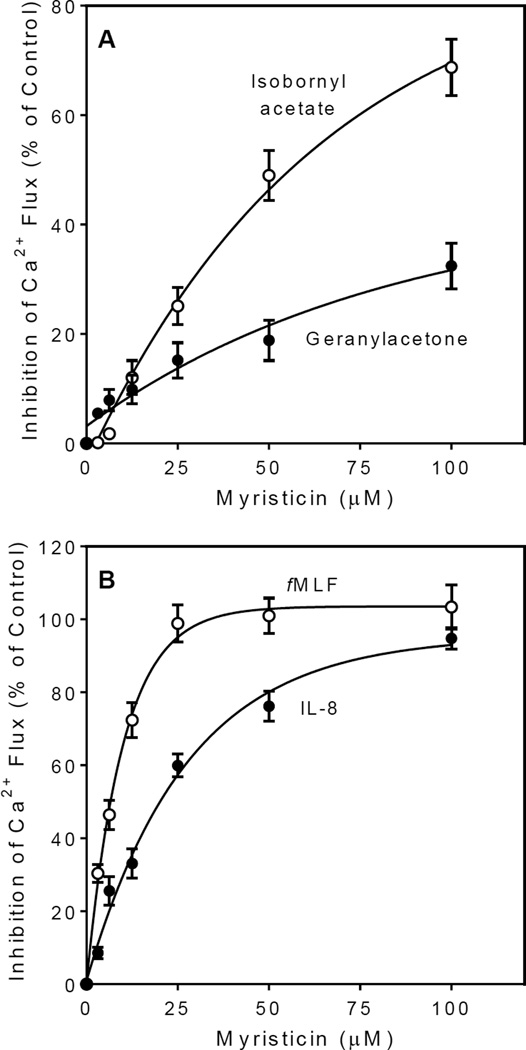
Myristicin, a major component of FAEOstm, was reported previously to inhibit [Ca2+]i flux in murine RAW 264.7 macrophages16. Therefore, we tested if myristicin also inhibited [Ca2+]i flux in human neutrophils activated by some of the active components, including β-pinene, sabinene, isobornyl acetate, and geranylacetone, and found that myristicin dose-dependently inhibited [Ca2+]i flux induced by these compounds, indicating that myristicin can suppress agonist effects of other components. As an example, Figure 2A shows myristicin inhibition of the neutrophil [Ca2+]i flux induced by geranylacetone and isobornyl acetate.
Figure 2.
Effect of myristicin on Ca2+ mobilization in human neutrophils. Panel A. Human neutrophils were preincubated with the indicated concentrations of myristicin for 5 min and then stimulated with 50 µM isobornyl acetate (○) or geranylacetone (●). Panel B. Neutrophils were preincubated with the indicated concentrations of myristicin for 5 min and then stimulated with 5 nM of fMLF (○) or 25 nM of IL-8 (●). For Panels A and B, the response induced by an agonist alone in neutrophils that were preincubated for 5 min with control 1% DMSO was assigned a value of 100%. Values are the mean ± SD of triplicate samples from one experiment that is representative of three independent experiments.
Since it was apparent that FAEOstm contained both agonist and antagonist compounds, we evaluated a mixture of the major agonist component of FAEOstm (geranylacetone; 4% by weight of the oil, mol. weight 194.3 Da) with the major antagonist compound (myristicin, 67.9% by weight of the oil, mol. weight 192.2 Da) at a molar ratio of 1:17, which corresponds approximately to the percentage ratio of these constituents in the original oil. Treatment of human neutrophils with this mixture at a final concentration of geranylacetone equal to its EC50 value (7.6 µM) did not activate [Ca2+]i flux (data not shown). Thus, these findings explain why FAEOstm, which contains neutrophil activating compounds, appeared to be inactive. Indeed, myristicin also inhibited fMLF- and IL-8-induced [Ca2+]i mobilization in neutrophils (Figure 2B) with IC50 values of 5.4 and 17.3 µM, respectively. These data indicate that myristicin can modulate intracellular signaling pathways that are common to both FPR1 and CXCR1/2 chemokine receptors.
Effect of the Ferula Essential Oils and Components on Neutrophil Migration and Cell Viability
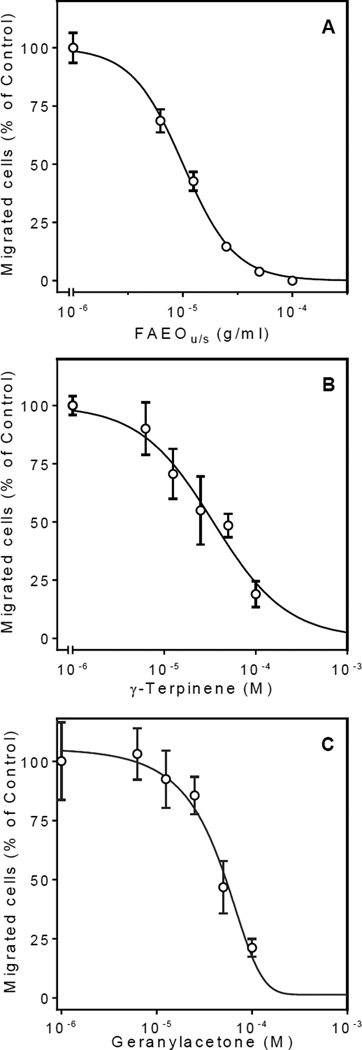
Various essential oils and their components have been reported previously to inhibit leukocyte migration (e.g., see17). We found that pretreatment with FAEOu/s for 30 min dose-dependently attenuated fMLF-induced chemotaxis in human neutrophils, with an IC50 of 10.1 µg/ml, but FAEOstm and myristicin had low activity in this assay. Even at the highest concentrations tested, FAEOstm (100 µg/ml) and myristicin (100 µM) inhibited cell migration only by 20–30%. Other components of the oils, including β-pinene, sabinene, γ-terpinene, isobornyl acetate, and geranylacetone, were also evaluated for their effects on fMLF-induced cell migration. In agreement with our previous studies on Artemisia essential oils9, we found that pretreatment with β-pinene and sabinene inhibited neutrophil migration with IC50 values of 23.9 and 39.1 µM, respectively. Likewise, γ-terpinene, isobornyl acetate, and geranylacetone also inhibited fMLF-induced neutrophil migration with IC50 values of 32.5, 47.5, and 37.2 µM, respectively. As examples, Figure 4 shows the effects of FAEOu/s, γ-terpinene, and geranylacetone on fMLF-induced neutrophil migration.
Figure 4.
Inhibition of human neutrophil migration by FAEOu/s and selected components. Human neutrophils were preincubated with the indicated concentrations of FAEOu/s (Panel A), γ-terpinene (Panel B), and geranylacetone (Panel C) for 30 min, and migration toward 1 nM fMLF was analyzed, as described. In each experiment, positive control cells were pretreated with 1% DMSO, and cell migration toward 1 nM fMLF was analyzed. The results are expressed as % of positive control (1 nM fMLF). The background level of cell migration was 3,258 ± 1,809 cells/well, and migration toward control fMLF resulted in 15,946 ± 1,421 cells/well. Values are the mean ± SD of triplicate samples from one experiment that is representative of three independent experiments.
To ensure that the results on inhibition of neutrophil migration were not influenced by possible compound toxicity, we evaluated cytotoxicity of sabinene, β-pinene, γ-terpinene, isobornyl acetate, geranylacetone, and myristicin at various concentrations (up to 50 µM) in undifferentiated HL-60 cells during a 4 h incubation. This incubation period is consistent with the relatively short time used to measure cell migration (up to 90 min). All compounds tested did not affect cell viability, thereby verifying that they are not cytotoxic, at least during the 4 h incubation period (data not shown).
Desensitization of Neutrophils by Essential Oil Active Components
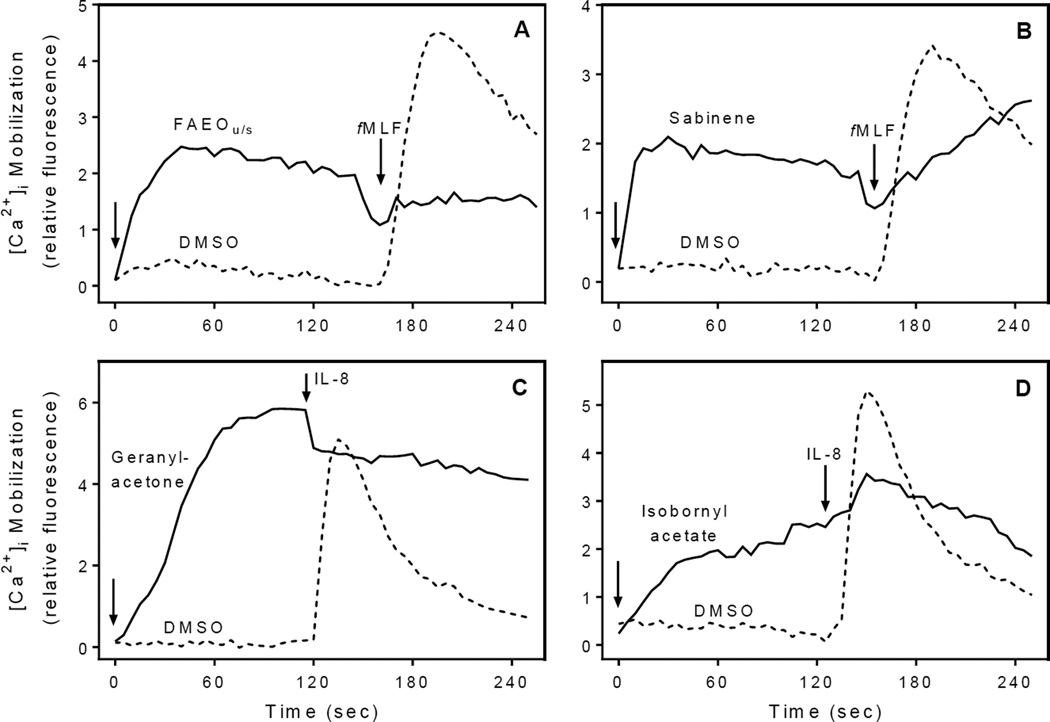
Activation of Ca2+ channels, specific receptors, or other unidentified molecular targets by agonists can result in their desensitization and subsequent downregulation of neutrophil responses18. To evaluate this issue, we examined whether neutrophil pretreatment with the active components of Ferula essential oils could cross-desensitize the neutrophil response to fMLF and IL-8. After pretreatment with various concentrations of FAEOu/s, β-pinene, sabinene, γ-terpinene, isobornyl acetate, or geranylacetone, neutrophils were treated with fMLF or IL-8, and [Ca2+]i flux was evaluated. We found that FAEOu/s and the individual compounds tested all attenuated fMLF- and IL-8-induced [Ca2+]i flux in neutrophils. As examples, Figure 5 shows that pretreatment with FAEOu/s, sabinene, isobornyl acetate, and geranylacetone markedly attenuated neutrophil [Ca2+]i mobilization induced by 5 nM fMLF or 25 nM IL-8, indicating that these treatments did indeed cross-desensitize the cells to subsequent agonist activation.
Figure 5.
Effect of selected compounds on fMLF- and IL-8-induced [Ca2+]i mobilization. Human neutrophils were loaded with calcium dye and pretreated with 50 µg/ml FAEOu/s (Panel A), 50 µM sabinene (Panel B), 50 µM geranylacetone (Panel C), 50 µM isobornyl acetate (Panel D), or 1% DMSO (Panels A–D) followed by a second addition of 5 nM fMLF (Panels A and B) or 25 nM IL-8 (Panels C and D). [Ca2+]i mobilization was monitored, as described, and the data are presented as relative fluorescence intensity. Data from two representative experiments are shown.
Elucidation of Molecular Targets/Pathways for Ferula Essential Oil and Active Components
Human neutrophils and undifferentiated human HL-60 cells express a different spectrum of receptors and ion channels19 and can be evaluated in comparison to identify ligand targets. Thus, we tested if the active oil compounds could also stimulate [Ca2+]i flux in HL-60 cells. Similar to their effects in neutrophils, the active essential oil (FAEOu/s), β-pinene, sabinene, γ-terpinene, isobornyl acetate, and geranylacetone all stimulated a [Ca2+]i flux in HL-60 cells, with EC50 values in the low/mid micromolar range (Table 2).
Based on similarity of the [Ca2+]i flux kinetics in neutrophils treated with fMLF and FAEOu/s, we considered whether FPR1 and/or FPR2 might be involved in the response to Ferula essential oils or its components since these receptors are known to be highly promiscuous14. Although undifferentiated HL-60 cells do not express FPRs, these cells can be transfected to express either human FPR1 (FPR1-HL60 cells) or FPR2 (FPR2-HL60 cells), and receptor-specific antagonists can be used to assess their involvement in a given response20. While [Ca2+]i flux was induced in FPR1-HL60 and FPR2-HL60 cells treated with FAEOu/s, β-pinene, sabinene, γ-terpinene, isobornyl acetate, or geranylacetone, this response was not inhibited by pretreatment of the cells with the specific FPR1 and FPR2 antagonists, cyclosporin H and WRW4, respectively (data not shown). In control experiments, cyclosporin H and WRW4 inhibited fMLF- and WKYMVM-induced Ca2+ flux in FPR1-HL60 and FPR2-HL60 cells, respectively (data not shown). Thus, these results indicate that FAEOu/s and the active constituents of FAEOstm and FAEOu/s stimulated molecular targets other than FPR1/FPR2.
Various constituents of essential oils (e.g., monoterpenes) have been previously reported to be agonists/antagonists of temperature-sensitive transient receptor potential (TRP) channels TRPA1, TRPV1/3, and TRPM821. Thus, we evaluated the effects of TRP channel blockers, including 2-APB, ACA, capsazepine, and SKF 96365 (reviewed in22), on neutrophil [Ca2+]i flux stimulated by FAEOu/s and the active Ferula essential oil components. As shown in Table 3, most of the TRP blockers tested dose-dependently inhibited neutrophil [Ca2+]i flux induced by these treatments, suggesting that TRPs may be at least one of the cellular targets for these compounds. Indeed, several essential oil-derived monoterpenes have been previously reported as TRP agonists, including thymol, menthol, carvacrol, geraniol, (±)-camphor, (−)-carvone, (−)-fenchone, and capsaicin (Table 4)23, 24. Evaluation of these compounds in neutrophils showed that the mixed TRPA1/TRPV3/TRPM8 agonists thymol and menthol activated [Ca2+]i flux with EC50 values in the mid-micromolar range, whereas the other tested monoterpenes were inactive. Furthermore, the TRPV1 agonist capsaicin also stimulated [Ca2+]i flux in human neutrophils (Table 4), which is consistent with previous studies25. Together, these results suggest a role of TRP channels in neutrophil responses to essential oil-derived monoterpenes, such as those found in essential oils from Ferula and other plant species.
Table 3.
Effect of TRP Channel Blockers and a Phospholipase C inhibitor on [Ca2+]i Flux in Human Neutrophils Treated with FAEOu/s and Active Components
| Essential Oil/ Compound |
2-APB | ACA | Capsazepine | SKF 96365 | U73122 |
|---|---|---|---|---|---|
| IC50 (µM) | |||||
| FAEOu/s | 12.6 ± 2.3 | 9.4 ± 2.4 | 27.4 ± 3.4 | 23.6 ± 4.1 | N.A. |
| β-pinene | 13.7 ± 2.5 | 8.0 ± 1.6 | 27.2 ± 3.1 | 17.8 ± 2.1 | N.A. |
| sabinene | 24.4 ± 2.6 | 17.0 ± 3.1 | 32.7 ± 3.5 | 25.4 ± 3.8 | N.A. |
| γ-terpinene | 18.6 ± 2.9 | 6.1 ± 1.8 | 22.6 ± 2.4 | 15.6 ± 2.2 | N.A. |
| isobornyl acetate | N.A. | 8.9 ± 1.1 | N.A. | 26.7 ± 3.6 | N.A. |
| geranylacetone | 39.5 ± 4.3 | 22.5 ± 2.8 | 14.2 ± 3.1 | 17.3 ± 3.1 | N.A. |
Neutrophils were preincubated with various concentrations of the indicated TRP inhibitors (up to 50 µM) for 10 min. The cells were stimulated with 25 µg/ml FAEOu/s or the indicated compound (100 µM), and Ca2+ flux was measured, as described.
N.A.: no activity was observed, even at the highest concentration tested (50 µM).
Table 4.
Reported Effects of Essential Oil Components and Capsaicin on TRP Channels23, 24 and Evaluation of their Effects on [Ca2+]i Flux in Human Neutrophils.
| Compound | Previously reported effects on TRP channels | [Ca2+]i flux in neutrophilsa EC50 (µM) |
|---|---|---|
| carvacrol | TRPA1, TRPV3, TRPM8 agonist; TRPM7 antagonist | N.A. |
| thymol | TRPA1, TRPV3, and TRPM8 agonist | 29.5 ± 3.4 |
| geraniol | TRPV3 and TRPM8 agonist | N.A. |
| (−)-menthol | TRPA1, TRPV3, and TRPM8 agonist | 30.8 ± 3.6 |
| (±)-camphor | TRPV1/3 and TRPM8 agonist, TRPA1 antagonist | N.A. |
| (−)-carvone | TRPV1/3 agonist | N.A. |
| (−)-fenchone | TRPV3 agonist | N.A. |
| capsaicin | TRPV1 agonist | 17.5 ± 2.1 |
Evaluated as described under Materials and Methods.
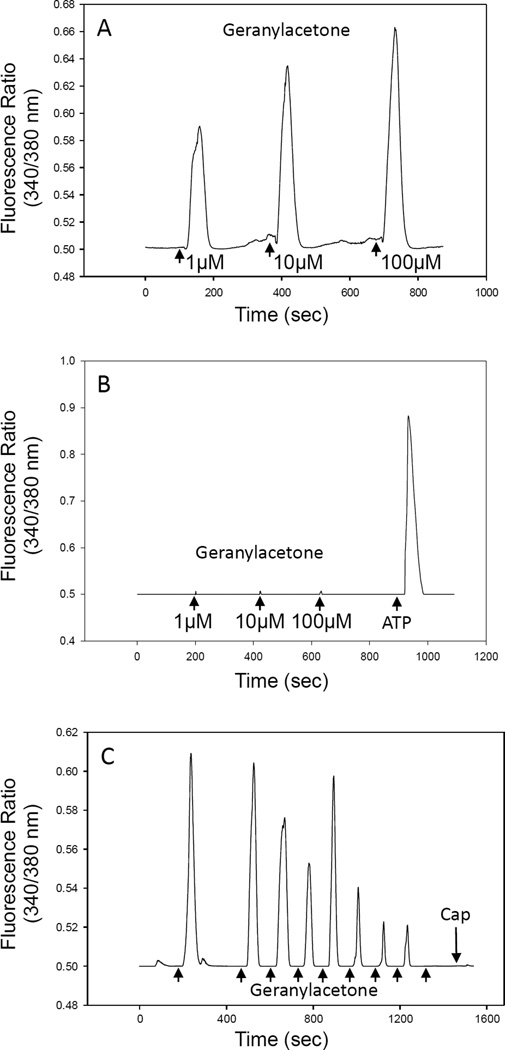
Based on results of our TRP channel inhibitor studies (Table 4) and the structural similarity of geranylacetone to capsaicin12, 13 (see below), we evaluated the effects of the neutrophil activing compounds (sabinene, β-pinene, γ-terpinene, geranylacetone, and isobornyl acetate) on transient Ca2+ influx in HEK293 cells transfected with TRPV1. Interestingly, only geranylacetone dose-dependently induced a transient Ca2+ influx in TRPV1-HEK293 cells (Figure 6A), while sabinene, γ-terpinene, and isobornyl acetate had no effect, and β-pinene only induced a small Ca2+ influx at the highest concentration tested (100 µM) (data not shown). Importantly, geranylacetone did not induce a Ca2+ influx in non-transfected HEK293 cells at all tested concentrations (1, 10, and 100 µM) (Figure 6B), demonstrating the specific requirement of TRPV1 for this response. Furthermore, repetitive application of 10 µM geranylacetone desensitized TRPV1, and no response to capsaicin was observed post desensitization (Figure 6C).
Figure 6.
Activation of TRPV1-dependent Ca2+ influx by geranylacetone. TRPV1-HEK293 cells (Panel A) and non-transfected HEK293 cells (Panel B) were treated with the indicated concentrations of geranylacetone, and transient Ca2+ influx was monitored, as described. In Panel B, cells were treated with 10 M ATP as a positive control to verify receptor activity. Panel C. TRPV1-HEK293 cells were treated with repetitive applications of 10 µM geranylacetone at the indicated times (arrows) to evaluate response desensitization. After full desensitization by geranylacetone, the cells were treated with 100 nM capsaicin (Cap) to evaluate the post-desensitization response. Data from three representative experiments are shown.
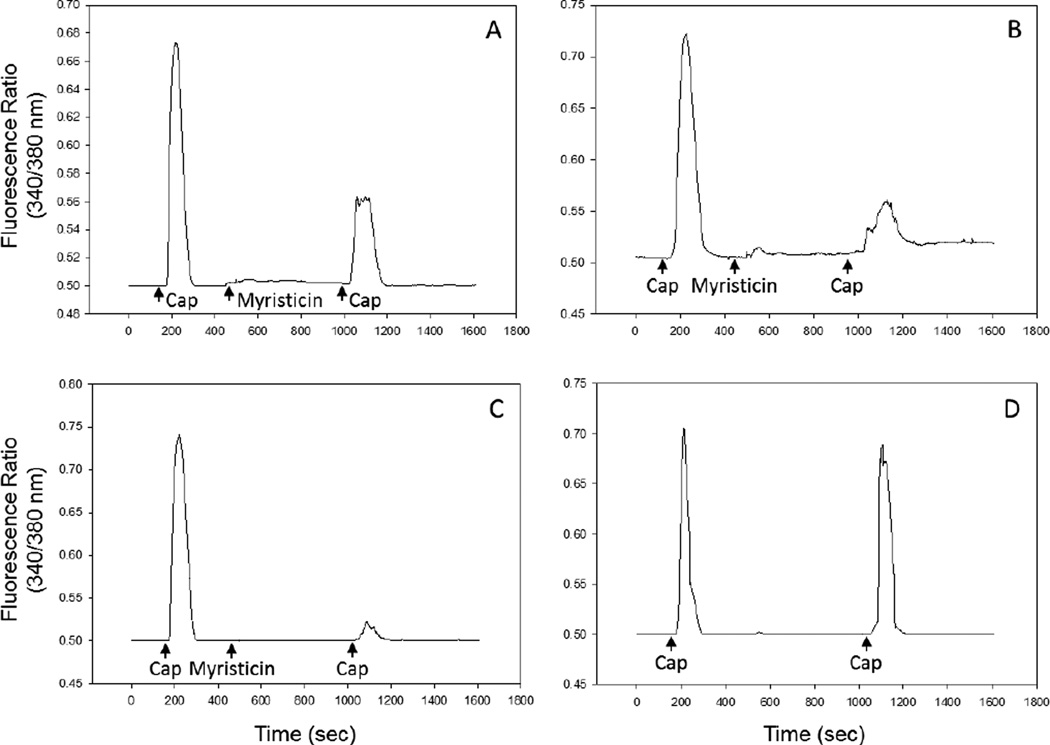
We also evaluated the effect of myristicin on Ca2+ influx in TRPV1-HEK293 cells and found that it dose-dependently inhibited the capsaicin-induced a transient Ca2+ influx in TRPV1 expressing cells (Figure 7). Additionally, myristicin did not induce Ca2+ influx in TRPV1-HEK293 cells.
Figure 7.
Inhibition of capsaicin induced Ca2+ influx in TRPV1-HEK293 cells by myristicin. TRPV1-HEK293 cells were treated with 100 nM capsaicin (Cap) to establish the basal transient Ca2+ influx response. The cells were then treated with 1 µM (Panel A), 10 µM (Panel B), and 100 µM (Panel C) myristicin followed by 100 nM capsaicin (Cap) to evaluate response desensitization, as described. Panel D. To confirm that the initial application of capsaicin did not desensitize TRPV1, cells were treated with 100 nM capsaicin (Cap) followed by a second treatment of 100 nM capsaicin (Cap) at the same interval that was used in panels A–C. Data from three representative experiments are shown.
Although limonene, p-cymene, α-pinene, α-pinene oxide, and α-terpineol are components of FAEOu/s and/or FAEOstm (see Table 1) and have previously been reported to weakly activate TRPV3-tranfected HEK293 cells, as determined by patch-clamp techniques23, these monoterpenes did not activate neutrophil [Ca2+]i flux (Table 2). In addition, one of the most potent TRPV3 agonists identified, carvacrol (EC50=490 nM in TRPV3-tranfected HEK293 cells)23 was also inactive in human neutrophils (Table 4), indicating that TRPV3 is likely not a target for Ferula essential oils/components in human neutrophils.
It should be noted that the TRP blocker 2-APB is also an inositol triphosphate (IP3) receptor antagonist26. To evaluate involvement of IP3, the phospholipase C inhibitor U73122 was used to inhibit IP3 production. In agreement with previous reports (e.g.,27), U73122 dose-dependently inhibited the fMLF-induced [Ca2+]i flux. In contrast, the neutrophil [Ca2+]i flux stimulated by FAEOu/s or its active components was not affected by U73122 at concentrations up to 50 µM (Table 3), indicating that the phospholipase C pathway is not involved in the neutrophil response to these compounds.
Activation of TRP channels can be mediated by Gi/o proteins (e.g.,28). Although we did not find a direct involvement of FPR1/FPR2 in [Ca2+]i flux stimulated by the active compounds, TRP channels could be activated by other Gi/o-coupled GPCR. PTX, a Gi inhibitor, was used to assess whether these G-proteins were involved in the [Ca2+]i mobilization stimulated by FAEOu/s and essential oil components. While the [Ca2+]i flux induced by fMLF in FPR1-HL60 transfected cells was significantly inhibited by PTX, as expected, no inhibition was found in PTX-treated cells activated with FAEOu/s, β-pinene, sabinene, γ-terpinene, isobornyl acetate, or geranylacetone (data not shown). Thus, neutrophil activation by Ferula essential oils also does not appear to involve Gi-coupled GPCRs.
Pharmacophore Modeling
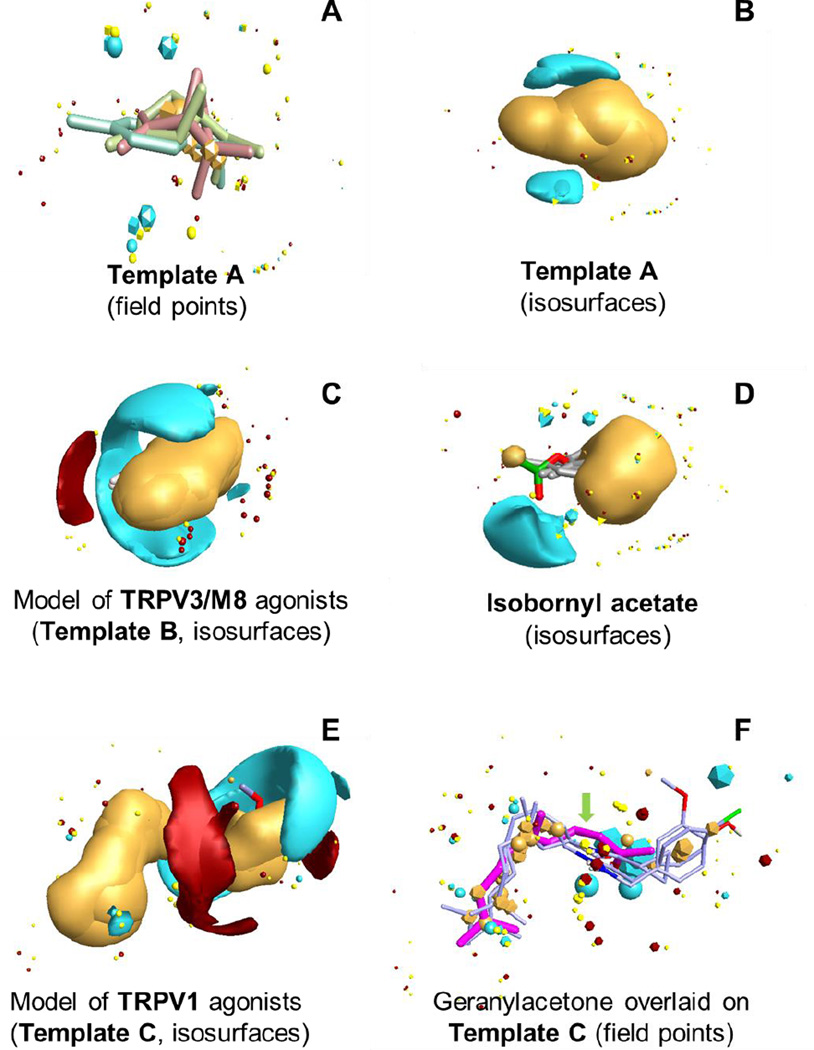
Molecular docking experiments and virtual screening can be helpful in defining or confirming the molecular target of an active molecule. However, crystal structures for only two (TRPV1 and TRPA1) of the 28 known TRP members have been resolved29, 30. In the absence of a defined target structure, ligand-based methods for compound evaluation can be utilized. Here, we evaluated molecular conformations of the active components in Ferula essential oils [(−)-β-pinene, (−)-sabinene, γ-terpinene, geranylacetone, and isobornyl acetate] using the FieldTemplater program11 to try to develop a pharmacophore template with optimal alignment of the molecules. This software is based on field point methodology and performs superimposition of compounds with the best volume overlap and coincidence of molecular fields. We found that only three of these compounds [(−)-β-pinene, (−)-sabinene, and γ-terpinene] could be overlaid simultaneously with good correspondence of their molecular fields (Figure 8A), suggesting that they likely have different cellular targets than geranylacetone and isobornyl acetate.
Figure 8.
Pharmacophore modeling based on active constituents of essential oils and known agonists of TRP channels. Panel A. Three-molecule template A created by alignment of (−)-β-pinene (magenta), (−)-sabinene (light-green), and γ-terpinene (light-blue). Panel B. Field isosurfaces for template A. Panel C. Field isosurfaces for the four-molecule pharmacophore model of TRPV3/TRPM8 agonists, which includes carveol, 6-tert-butyl-m-cresol, carvacrol, and thymol (template B). Panel D. Field isosurfaces for the best alognment of isobornyl acetate onto template A. Panel E. Field isosurfaces for the three-molecule pharmacophore model of TRPV1 agonists, which includes capsaicin, NGCC, and compound 2k (template C). Panel F. Geranylacetone (magenta) overlaid on the pharmacophore model of TRPV1 agonists. In all panels, negative, positive, hydrophobic, and van der Waals field points and corresponding isosurfaces are colored in blue, red, orange, and yellow, respectively (van der Waals isosurfaces on the panels are not shown). The field isosurfaces were calculated at a threshold of 3 kcal/mol.
Although molecular templates are usually represented as sets of field points, we also analyzed shapes of the molecular fields scanned by negative, positive, and neutral probe atoms around the template and in the vicinity of individual compounds. Template A has two small areas of negative field and a large central hydrophobic region, which is consistent with the hydrocarbon nature of the compounds involved in this model (Figure 8B). This result indicates that (−)-β-pinene, (−)-sabinene, and γ-terpinene are likely recognized by the same or a similar molecular target, such as one of the TRP channel members.
Although a comparative analysis of the pharmacophore features of Template A and all 28 known TRP channel members lies out of scope of the present work, we created two pharmacophore models on the basis of previous studies showing that essential oil-derived compounds targeted TRPRPV3/TRPM8, as well as our biological results implicating TRPV1. The molecular conformations of (−)-carveol, 6-tert-butyl-m-cresol, carvacrol, and thymol were used for the model of TRPV3/TRPM8 agonists23, whereas conformations of capsaicin, N-geranyl cyclopropylcarboxamide (NGCC)12, and compound 2k13 were used for the model of TRPV1 agonists.
In the four-molecule pharmacophore model of TRPV3/TRPM8 channel agonists (Template B), the molecules overlap by four methyl groups and hydrophobic isopropyl, isopropenyl, and tert-butyl substituents. Their hydroxyl groups are all oriented in the same direction, forming a prolonged isosurface of negative field embracing the central hydrophobic region (Figure 8C). Template B also includes a small area of positive field near sterically non-hindered hydroxyl hydrogens. Its location, shown in red in Figure 8C, may correspond to H-bond acceptor(s) in TRPV3/TRPM8 channels. The substantial differences between Templates A and B indicate that (−)-β-pinene, (−)-sabinene, and γ-terpinene target TRP channels other than TRPV3/TRPM8, which is consistent with our studies above suggesting that TRPV3 is likely not a relevant target for Ferula essential oils.
Consistent with the differences observed in biological activities, geranylacetone and isobornyl acetate had relatively low similarity with Template A (S=0.591 and 0.625, respectively) and Template B (S=0.621 and 0.622, respectively) in their best alignments determined by the FieldAlign program. Indeed, the shapes of their molecular fields are quite different from Templates A and B (e.g., see Figure 8D), confirming that geranylacetone and isobornyl acetate do not interact with the same molecular targets reflected by Templates A and B. On the other hand, the molecular structure of geranylacetone resembles such TRPV1 agonists as capsaicin and its analogs12, 13. Indeed, the pharmacophore model of TRPV1 agonists based on capsaicin, NGCC, and compound 2k12, 13 (Template C) differs substantially from pharmacophore Templates A and B and contains two different hydrophobic regions. A large area of negative field (blue area in Figure 8E) surrounds the overlapping carbonyl groups and electron acceptor substituents in the aromatic rings. The blue field in the template corresponds to the protein's positively charged regions or to amino and hydroxyl groups in the TRPV1 binding site that are capable of forming H-bonds with electronegative atoms of the agonist. In addition, a positive field (red area in Figure 8E) is located near NH-groups of the three molecules, where potential H-bond acceptors could be present in the protein. Superimposition of the geranylacetone molecule onto this template using the FieldAlign program shows a relatively high quality of alignment and similarity (S=0.644). Thus, the proposed bioactive conformation of geranylacetone has a molecular field distribution close to that of other known TRPV1 agonists (Figure 6F) and provides support for our biological data showing that geranylacetone is indeed a TRPV1 agonist.
DISCUSSION
Essential oils from Ferula species are an important source of bioactive monoterpenes, phenylpropanoids, sesquiterpenes, and organosulfur compounds with a broad spectrum of pharmacological properties4, 6, 31. In the present study, we defined the chemical profile of essential oils extracted from F. akitschkensis umbels+seeds and stems (FAEOu/s and FAEOstm), evaluated the effect of these oils and their pure constituents on human neutrophil functions, and investigated their cellular targets.
A total of 52 constituents were identified in FAEOu/s, with sabinene, α-pinene, β-pinene, and terpinen-4-ol being the major components. Although α-pinene and β-pinene are common monoterpenes in Ferula oils4–6, 31, high levels of sabinene were previously found only in F. behboundiana31. Other primary components present at lower concentrations (1–1.5% total weight) were trans-sabinene hydrate and sesquiterpenoids eremophilene and 2-himachalen-7-ol. FAEOstm had a high content of phenylpropanoids, with myristicin as the major component. Among Ferula spp., myristicin was previously found only in F. glauca6.
Neutrophils are principal effectors of the innate immune response to injury or infection and are essential for elimination of invading bacterial pathogens8. Thus, it is significant that FAEOu/s activated Ca2+ mobilization in human neutrophils. To the best of our knowledge, only a few studies have reported that essential oils can directly activate neutrophil functions. For example, essential oils from Citrus aurantium (bergamot) stimulated ROS production in human neutrophils32. These oils have some common components with FAEOu/s, including monoterpenes, such as α-pinene, β-pinene, γ-terpinene, and limonene. Given the critical role played by neutrophils in innate immunity against pathogens, our data support the possibility that at least part of the observed therapeutic effects of essential oils with similar primary constituents are due to enhancement of innate immune responses.
To further define the bioactive component(s) in Ferula essential oils, we evaluated 22 of their constituents and found that sabinene, β-pinene, γ-terpinene, isobornyl acetate, geranylacetone, and 2-nonenal directly activated [Ca2+]i flux in human neutrophils. Although the agonist effects of β-pinene, sabinene, and 2-nonenal in neutrophils were previously reported9, 15, this is the first report that γ-terpinene, isobornyl acetate, and geranylacetone can directly modulate human neutrophil responses, including [Ca2+]i mobilization and chemotaxis (in vitro). Note that γ-terpinene treatment reduced neutrophil migration in a carrageenan-induced peritonitis model (in vivo)33. Whether this was due to direct effects on neutrophils is not known; however, our current studies support this possibility. Eosinophil migration was inhibited by essential oils from Syzygium cumini and Psidium guajava, which have relatively high levels of β-pinene34. The current studies are the first to report inhibitory effects of isobornyl acetate and geranylacetone on leukocyte migration.
TRP channels are functionally expressed in various cells of the immune system, including neutrophils, and elevation of intracellular Ca2+ through activation of these channels may regulate various aspects of the inflammatory response35. In addition, it has been reported that TRPM2 channels can be activated by neutrophil-generated ROS36. Several essential oil-derived monoterpenes have been evaluated for their effects on TRP channels, including TRPA1, TRPV1/3, and TRPM8, which are molecular detectors of thermal and chemical stimuli21, 23, 37. For example, TRPV3 is activated by camphor, menthol, 6-tert-butyl-m-cresol, carvacrol, dihydrocarveol, eucalyptol, thymol, carveol, and (+)-borneol23. Camphor and carvone, which is a major compound in spearmint essential oil, can also activate TRPV1 and TRPA1 channels37. Borneol, one of major constituents of rosemary oil, inhibited TRPA1 mediated cationic currents38, whereas carvacrol, which is abundant in oregano oil, activated TRPA1 but not TRPV1 channels39. Finally, menthol, one of major constituents of peppermint essential oil, is a ligand of TRPM8 channels40. Although α-pinene oxide, (−)-α-pinene, p-cymene had weak agonist activity for TRPV3 channels23, we found that these monoterpenes did not activate human neutrophils (Table 2).
Evaluation of the effects of TRP channel blockers showed that they inhibited neutrophil [Ca2+]i flux stimulated by FAEOu/s, β-pinene, sabinene, γ-terpinene, and geranylacetone. However, 2-APB and capsazepine did not block [Ca2+]i flux stimulated by isobornyl acetate (Table 3). Capsazepine is relatively specific for TRPV1 and TRPM8 channels24. It should be noted that capsazepine had the lowest inhibitory activity on [Ca2+]i flux in human neutrophils activated by Ferula essential oil-derived agonists, with the exception of geranylacetone. Although geranylacetone does not possess a vanillyl group like capsaicin, its structure exhibits spatial similarity with capsaicin and other TRPV1 agonists, such as compound 2k and NGCC12, 13. Indeed, the proposed bioactive conformation of geranylacetone fits well with the TRPV1 pharmacophore template and has hydrophobic, negative, and positive molecular field distributions similar to those of the template (Figure 8F). This channel is expressed in neutrophils35, and capsaicin, a potent TRPV1 agonist, can activate neutrophil [Ca2+]i flux (Table 4 and25). We also found that geranylacetone activated a transient Ca2+ influx in transfected TRPV1-HEK293 cells, but not in non-transfected cells. Together, these data demonstrate that geranylacetone is a TRPV1 agonist and can activate TRPV1 channels in human neutrophils.
The other three TRP channel blockers, 2-APB, ACA, and SKF 96365, can inhibit a broad range of TRP channels (for review22) and even other molecular targets. For example, 2-APB blocks TRPC3/6/7 and several non-classical TRP channels (TRPM2/7 and TRPV6)41. ACA directly blocks TRPC6, TRPM2/8, and phospholipase A241. SKF 96365 is believed to be a non-selective blocker of TRPC3/6/7 channels, but not TRPV1 channels41. Thus, we propose that the SKF 96365-blockable [Ca2+]i flux in neutrophils activated by FAEOu/s and its active components (β-pinene, sabinene, γ-terpinene) is not mediated by TRPV1 but more likely via TRPM and/or TRPC channels; however, further studies will be necessary to address this issue. In support of this conclusion, TRPM2/7 and TRPC6 channels are expressed in neutrophils and implicated in various inflammatory responses (e.g., see42). Moreover, neutrophils from Trpc6−/− knockout mice have modestly decreased Ca2+ flux in response to fMLF43. In addition, our molecular modeling showed that the ligand-based pharmacophore for β-pinene, sabinene, and γ-terpinene (template A) was substantially different that the pharmacophore models for TRPV3/TRPM8 (template B) and TRPV1 (template C) agonists, indicating that these compounds target TRP channels other than TRPV1/3 and TRPM8. Finally, sabinene and γ-terpinene, and β-pinene did not induce a significant Ca2+ influx in transfected TRPV1-HEK cells, confirming they are not TRPV1 agonists.
Monoterpenes are hydrophobic small-molecule compounds with relatively high LogP values, suggesting that they could allosterically interact with a hydrophobic pocket(s) within the TRP transmembrane bundle and regulate these gates to control channel opening and closing44. Moreover, TRP channels can have several sites for pharmacological intervention41. Thus, the exact nature of TRP channel interactions and/or other additional targets for essential oil-derived constituents in human neutrophils still remains unclear, and further studies will be required to elucidate the molecular mechanisms of how such target(s) could be involved in modulation of neutrophil functions by monoterpenes and other essential oil constituents.
Based on our neutrophil Ca2+ flux assays, sabinene, β-pinene, and γ-terpinene are the main bioactive components of FAEOu/s. On the basis of the molecular weight of these compounds (136.2 Da), their relative levels in FAEOu/s, and the EC50 value for this oil in Ca2+ flux activation assays (16.5 µg/ml), the effective concentration of these compounds would be in the range of 80 µM, which is close to the EC50 value for sabinene (49.4 µM), the major component (58.7%) of this oil. It should be noted that natural essential oils are mixtures of monoterpenes, which could have opposing agonist and antagonist activities that could modify or even suppress agonist effects of the primary component(s)45. This could explain some discrepancies between EC50 values for pure compounds and their partial activity in a mixture. In addition, activation of TRP channels by agonists can result in their desensitization (e.g.,46) and inhibition of [Ca2+]i flux induced by other neutrophil agonists. Although there are no reports on habitual intake and/or medicinal application of any essential oil preparations from F. akitschkensis, its active components are common constituents or even active principles of some essential oils with well-known therapeutic properties (e.g.,4, 33, 47). Interaction of these constituents with various ion channels, including TRP channels in neutrophils may be an additional mechanism-based explanation for the immunomodulatory and anti-inflammatory properties of these oils43.
Myristicin is the major component in parsley, carrot and the essential oils of mace and nutmeg48. This phenylpropanoid has anti-cholinergic, antibacterial, anti-inflammatory, and hepatoprotective effects16, 49. Here, we found that myristicin inhibited both fMLF- and IL-8-induced [Ca2+]i flux in neutrophils. Moreover, myristicin dose-dependently inhibited capsaicin-induced transient Ca2+ influx in TRPV1-HEK293 cells, indicating that it is indeed a TRPV1 channel antagonist. Whether it can also inhibit other pathways will need to be investigated in future studies. In any case, our findings support the anti-inflammatory potential of myristicin. Note that myristicin had low activity in the neutrophil migration assay. The discrepancy in this effect could be explained by the relatively long incubation time in the cell migration assay (90 min) compared with the [Ca2+]i flux assay (<5 min). Indeed, myristicin, like other allyl-benzenes, can form covalent conjugates with macromolecules during its enzymatic bioconversion50. In addition, the activation of neutrophil migration via FPR1 and CXCR1/2 could be independent of TRPV1 channels. Nevertheless, further studies will be necessary to determine the precise molecular mechanisms for the inhibitory effects of this phenylpropanoid in human neutrophils and other cells, as well as its applicability as an anti-inflammatory agent in preventive or therapeutic animal models.
In conclusion, we showed that Ferula essential oils (FAEOu/s and FAEOstm) and several components are agonists of human neutrophils but can also attenuate neutrophil migration via the likely desensitization of TRP channels. Our experimental and molecular modeling studies suggest that geranylacetone can activate TRPV1 channels in human neutrophils. Overall, these results suggest a potential new strategy for developing novel medicines based on components of essential oils that could effectively modulate neutrophil functional responses. Further detailed studies are warranted to define the structure and pharmacological effects of Ferula essential oil-derived constituents to understand their potential as therapeutic remedies for various disorders.
Acknowledgments
Funding Information
This research was supported in part by National Institutes of Health IDeA Program COBRE Grant GM110732; Grants 0504/GF3 and 2117/GF4 from The Ministry of Education and Science, Kazakhstan; a USDA National Institute of Food and Agriculture Hatch project; Montana University System Research Initiative 51040-MUSRI2015-03; and the Montana State University Agricultural Experiment Station.
ABBREVIATIONS USED
- ACA
N-(p-amylcinnamoyl)anthranilic acid
- 2-APB
2-aminoethyl diphenyl borate
- FAEO
essential oils of F. akitschkensis
- DMSO
dimethyl sulfoxide
- FBS
fetal bovine serum
- FID
flame ionization detector
- FPR
N-formyl peptide receptor
- GC
gas chromatography
- GPCR
G-protein coupled receptors
- HBSS
Hanks’ balanced salt solution
- IL
interleukin
- IP3
inositol triphosphate
- MS
mass spectrometry
- NGCC
N-geranyl cyclopropylcarboxamide
- PTX
pertussis toxin
- ROS
reactive oxygen species
- RRI
relative retention indices
- TRP
transient receptor potential
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Iranshahy M, Iranshahi M. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin)-a review. J. Ethnopharmacol. 2011;134:1–10. doi: 10.1016/j.jep.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 2.Mahendra P, Bisht S. Ferula asafoetida: Traditional uses and pharmacological activity. Pharmacogn. Rev. 2012;6:141–146. doi: 10.4103/0973-7847.99948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zamani Taghizadeh Rabe S, Iranshahi M, Mahmoudi M. In vitro anti-inflammatory and immunomodulatory properties of umbelliprenin and methyl galbanate. J. Immunotoxicol. 2015:1–8. doi: 10.3109/1547691X.2015.1043606. [DOI] [PubMed] [Google Scholar]
- 4.Kavoosi G, Rowshan V. Chemical composition, antioxidant and antimicrobial activities of essential oil obtained from Ferula assa-foetida oleo-gum-resin: effect of collection time. Food Chem. 2013;138:2180–2187. doi: 10.1016/j.foodchem.2012.11.131. [DOI] [PubMed] [Google Scholar]
- 5.Kavoosi G, Tafsiry A, Ebdam AA, Rowshan V. Evaluation of antioxidant and antimicrobial activities of essential oils from Carum copticum seed and Ferula assafoetida latex. J. Food. Sci. 2013;78:T356–T361. doi: 10.1111/1750-3841.12020. [DOI] [PubMed] [Google Scholar]
- 6.Maggi F, Cecchini C, Cresci A, Coman MM, Tirillini B, Sagratini G, Papa F. Chemical composition and antimicrobial activity of the essential oil from Ferula glauca L (F. communis L. subsp. glauca) growing in Marche (central Italy) Fitoterapia. 2009;80:68–72. doi: 10.1016/j.fitote.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Kushmuradov AYKASh, Saidkhodzhaev AI, Malikov VM. The structure of akiferidine and akiferidinine. Chem. Nat. Compd. 1978;6:725–727. [Google Scholar]
- 8.Bokoch GM. Chemoattractant signaling and leukocyte activation. Blood. 1995;86:1649–1660. [PubMed] [Google Scholar]
- 9.Schepetkin IA, Kushnarenko SV, Ozek G, Kirpotina LN, Utegenova GA, Kotukhov YA, Danilova AN, Ozek T, Baser KH, Quinn MT. Inhibition of Human Neutrophil Responses by the Essential Oil of Artemisia kotuchovii and Its Constituents. J. Agric. Food Chem. 2015;63:4999–5007. doi: 10.1021/acs.jafc.5b01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinharoy P, Zhang H, Sinha S, Prudner BC, Bratz IN, Damron DS. Propofol restores TRPV1 sensitivity via a TRPA1-, nitric oxide synthase-dependent activation of PKCepsilon. Pharmacol Res Perspect. 2015;3:e00153. doi: 10.1002/prp2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheeseright T, Mackey M, Rose S, Vinter A. Molecular field technology applied to virtual screening and finding the bioactive conformation. Expert Opin. Drug Discov. 2007;2:131–144. doi: 10.1517/17460441.2.1.131. [DOI] [PubMed] [Google Scholar]
- 12.Kim MJ, Son HJ, Kim Y, Kweon HJ, Suh BC, Lyall V, Rhyu MR. Selective activation of hTRPV1 by N-geranyl cyclopropylcarboxamide, an amiloride-insensitive salt taste enhancer. PLoS One. 2014;9:e89062. doi: 10.1371/journal.pone.0089062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortar G, Schiano Moriello A, Morera E, Nalli M, Di Marzo V, De Petrocellis L. Effect of acyclic monoterpene alcohols and their derivatives on TRP channels. Bioorg. Med. Chem. Lett. 2014;24:5507–5511. doi: 10.1016/j.bmcl.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, Serhan CN, Murphy PM. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the Formyl Peptide Receptor (FPR) Family. Pharmacol. Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi MA, Fidale F, Di Mauro C, Esterbauer H, Dianzani MU. Effect of 4-hydroxy-2,3-trans-nonenal and related aldehydes on phospholipase C activity of rat neutrophils. Int. J. Tissue React. 1993;15:201–205. [PubMed] [Google Scholar]
- 16.Lee JY, Park W. Anti-inflammatory effect of myristicin on RAW 264.7 macrophages stimulated with polyinosinic-polycytidylic acid. Molecules. 2011;16:7132–7142. doi: 10.3390/molecules16087132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fachini-Queiroz FC, Kummer R, Estevao-Silva CF, Carvalho MD, Cunha JM, Grespan R, Bersani-Amado CA, Cuman RK. Effects of Thymol and Carvacrol, Constituents of Thymus vulgaris L. Essential Oil, on the Inflammatory Response. Evid. Based Complement. Alternat. Med. 2012;2012:657026. doi: 10.1155/2012/657026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson RM, Ali H, Tomhave ED, Haribabu B, Snyderman R. Cross-desensitization of chemoattractant receptors occurs at multiple levels. Evidence for a role for inhibition of phospholipase C activity. J. Biol. Chem. 1995;270:27829–27833. doi: 10.1074/jbc.270.46.27829. [DOI] [PubMed] [Google Scholar]
- 19.Klinker JF, Wenzel-Seifert K, Seifert R. G-protein-coupled receptors in HL-60 human leukemia cells. Gen. Pharmacol. 1996;27:33–54. doi: 10.1016/0306-3623(95)00107-7. [DOI] [PubMed] [Google Scholar]
- 20.Stenfeldt AL, Karlsson J, Wennerås C, Bylund J, Fu H, Dahlgren C. Cyclosporin H, Boc-MLF and Boc-FLFLF are antagonists that preferentially inhibit activity triggered through the formyl peptide receptor. Inflammation. 2007;30:224–229. doi: 10.1007/s10753-007-9040-4. [DOI] [PubMed] [Google Scholar]
- 21.Premkumar LS. Transient receptor potential channels as targets for phytochemicals. ACS Chem. Neurosci. 2014;5:1117–1130. doi: 10.1021/cn500094a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pottosin I, Delgado-Enciso I, Bonales-Alatorre E, Nieto-Pescador MG, Moreno-Galindo EG, Dobrovinskaya O. Mechanosensitive Ca(2)(+)-permeable channels in human leukemic cells: pharmacological and molecular evidence for TRPV2. Biochim. Biophys. Acta. 2015;1848:51–59. doi: 10.1016/j.bbamem.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Vogt-Eisele AK, Weber K, Sherkheli MA, Vielhaber G, Panten J, Gisselmann G, Hatt H. Monoterpenoid agonists of TRPV3. Br. J. Pharmacol. 2007;151:530–540. doi: 10.1038/sj.bjp.0707245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br. J. Pharmacol. 2004;141:737–745. doi: 10.1038/sj.bjp.0705652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kose SA, Naziroglu M. N-acetyl cysteine reduces oxidative toxicity, apoptosis, and calcium entry through TRPV1 channels in the neutrophils of patients with polycystic ovary syndrome. Free Radic. Res. 2015;49:338–346. doi: 10.3109/10715762.2015.1006214. [DOI] [PubMed] [Google Scholar]
- 26.Anderson R, Steel HC, Tintinger GR. Inositol 1,4,5-triphosphate-mediated shuttling between intracellular stores and the cytosol contributes to the sustained elevation in cytosolic calcium in FMLP-activated human neutrophils. Biochem. Pharmacol. 2005;69:1567–1575. doi: 10.1016/j.bcp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Smith RJ, Sam LM, Justen JM, Bundy GL, Bala GA, Bleasdale JE. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J. Pharmacol. Exp. Ther. 1990;253:688–697. [PubMed] [Google Scholar]
- 28.Jeon JP, Hong C, Park EJ, Jeon JH, Cho NH, Kim IG, Choe H, Muallem S, Kim HJ, So I. Selective Galphai subunits as novel direct activators of transient receptor potential canonical (TRPC)4 and TRPC5 channels. J. Biol. Chem. 2012;287:17029–17039. doi: 10.1074/jbc.M111.326553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504:107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paulsen CE, Armache JP, Gao Y, Cheng Y, Julius D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature. 2015;520:511–517. doi: 10.1038/nature14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanani MR, Rahiminejad MR, Sonboli A, Mozaffarian V, Kazempour Osaloo S, Nejad Ebrahimi S. Chemotaxonomic significance of the essential oils of 18 Ferula species (Apiaceae) from Iran. Chem. Biodivers. 2011;8:503–517. doi: 10.1002/cbdv.201000148. [DOI] [PubMed] [Google Scholar]
- 32.Cosentino M, Luini A, Bombelli R, Corasaniti MT, Bagetta G, Marino F. The essential oil of bergamot stimulates reactive oxygen species production in human polymorphonuclear leukocytes. Phytother. Res. 2014;28:1232–1239. doi: 10.1002/ptr.5121. [DOI] [PubMed] [Google Scholar]
- 33.Ramalho TR, Oliveira MT, Lima AL, Bezerra-Santos CR, Piuvezam MR. Gamma-Terpinene Modulates Acute Inflammatory Response in Mice. Planta Med. 2015;81:1248–1254. doi: 10.1055/s-0035-1546169. [DOI] [PubMed] [Google Scholar]
- 34.Siani AC, Souza MC, Henriques MG, Ramos MF. Anti-inflammatory activity of essential oils from Syzygium cumini and Psidium guajava. Pharm. Biol. 2013;51:881–887. doi: 10.3109/13880209.2013.768675. [DOI] [PubMed] [Google Scholar]
- 35.Heiner I, Eisfeld J, Luckhoff A. Role and regulation of TRP channels in neutrophil granulocytes. Cell Calcium. 2003;33:533–540. doi: 10.1016/s0143-4160(03)00058-7. [DOI] [PubMed] [Google Scholar]
- 36.Naziroglu M. Molecular role of catalase on oxidative stress-induced Ca(2+) signaling and TRP cation channel activation in nervous system. J. Recept. Signal Transduct. Res. 2012;32:134–141. doi: 10.3109/10799893.2012.672994. [DOI] [PubMed] [Google Scholar]
- 37.Kang Q, Jiang CY, Fujita T, Kumamoto E. Spontaneous L-glutamate release enhancement in rat substantia gelatinosa neurons by (-)-carvone and (+)-carvone which activate different types of TRP channel. Biochem. Biophys. Res. Commun. 2015;459:498–503. doi: 10.1016/j.bbrc.2015.02.135. [DOI] [PubMed] [Google Scholar]
- 38.Sherkheli MA, Schreiner B, Haq R, Werner M, Hatt H. Borneol inhibits TRPA1, a proinflammatory and noxious pain-sensing cation channel. Pak. J. Pharm. Sci. 2015;28:1357–1363. [PubMed] [Google Scholar]
- 39.Luo QT, Fujita T, Jiang CY, Kumamoto E. Carvacrol presynaptically enhances spontaneous excitatory transmission and produces outward current in adult rat spinal substantia gelatinosa neurons. Brain. Res. 2014;1592:44–54. doi: 10.1016/j.brainres.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 40.Journigan VB, Zaveri NT. TRPM8 ion channel ligands for new therapeutic applications and as probes to study menthol pharmacology. Life. Sci. 2013;92:425–437. doi: 10.1016/j.lfs.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 41.Harteneck C, Gollasch M. Pharmacological modulation of diacylglycerol-sensitive TRPC3/6/7 channels. Curr Pharm Biotechnol. 2011;12:35–41. doi: 10.2174/138920111793937943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang CH, Rong MY, Wang L, Ren Z, Chen LN, Jia JF, Li XY, Wu ZB, Chen ZN, Zhu P. CD147 up-regulates calcium-induced chemotaxis, adhesion ability and invasiveness of human neutrophils via a TRPM-7-mediated mechanism. Rheumatology (Oxford) 2014;53:2288–2296. doi: 10.1093/rheumatology/keu260. [DOI] [PubMed] [Google Scholar]
- 43.Damann N, Owsianik G, Li S, Poll C, Nilius B. The calcium-conducting ion channel transient receptor potential canonical 6 is involved in macrophage inflammatory protein-2-induced migration of mouse neutrophils. Acta Physiol (Oxf) 2009;195:3–11. doi: 10.1111/j.1748-1716.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- 44.Bandell M, Dubin AE, Petrus MJ, Orth A, Mathur J, Hwang SW, Patapoutian A. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat. Neurosci. 2006;9:493–500. doi: 10.1038/nn1665. [DOI] [PubMed] [Google Scholar]
- 45.Anderson EM, Jenkins AC, Caudle RM, Neubert JK. The effects of a co-application of menthol and capsaicin on nociceptive behaviors of the rat on the operant orofacial pain assessment device. PLoS One. 2014;9:e89137. doi: 10.1371/journal.pone.0089137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iannotti FA, Hill CL, Leo A, Alhusaini A, Soubrane C, Mazzarella E, Russo E, Whalley BJ, Di Marzo V, Stephens GJ. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem. Neurosci. 2014;5:1131–1141. doi: 10.1021/cn5000524. [DOI] [PubMed] [Google Scholar]
- 47.Guzman-Gutierrez SL, Gomez-Cansino R, Garcia-Zebadua JC, Jimenez-Perez NC, Reyes-Chilpa R. Antidepressant activity of Litsea glaucescens essential oil: identification of beta-pinene and linalool as active principles. J. Ethnopharmacol. 2012;143:673–679. doi: 10.1016/j.jep.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 48.Ozaki Y, Soedigdo S, Wattimena YR, Suganda AG. Antiinflammatory effect of mace, aril of Myristica fragrans Houtt. and its active principles. Jpn. J. Pharmacol. 1989;49:155–163. doi: 10.1254/jjp.49.155. [DOI] [PubMed] [Google Scholar]
- 49.Morita T, Jinno K, Kawagishi H, Arimoto Y, Suganuma H, Inakuma T, Sugiyama K. Hepatoprotective effect of myristicin from nutmeg (Myristica fragrans) on lipopolysaccharide/d-galactosamine-induced liver injury. J. Agric. Food Chem. 2003;51:1560–1565. doi: 10.1021/jf020946n. [DOI] [PubMed] [Google Scholar]
- 50.Yang AH, He X, Chen JX, He LN, Jin CH, Wang LL, Zhang FL, An LJ. Identification and characterization of reactive metabolites in myristicin-mediated mechanism-based inhibition of CYP1A2. Chem. Biol. Interact. 2015;237:133–140. doi: 10.1016/j.cbi.2015.06.018. [DOI] [PubMed] [Google Scholar]