ABSTRACT
Approximately 30% of patients treated with ipilimumab will develop gastrointestinal toxicity. The immunological drivers that underpin the clinical observations in human tissues are poorly understood. We report here on the immune consequences of ipilimumab treatment in the colorectal mucosa of patients with treatment-related colitis. Using immunohistochemistry, we evaluated the immune infiltrate by CD8+, FoxP3, and granzyme B (GzmB) in colonic biopsies from 20 patients with ipilimumab-related colitis. We assessed 10 cases with normal colon biopsies for comparison. In eight cases (four on steroids only, four on steroids and infliximab), we evaluated two sequential biopsies. We observed that CD8+, FoxP3+, and GzmB T cell counts were significantly higher in patients with ipilimumab-related colitis compared to normal colon (p < 0.0001). Patients who required infliximab for the resolution of their colitis had a significantly higher CD8+/FoxP3 ratio than those treated only with steroids and this correlated with clinical severity. The analysis of repeat samples revealed that resolution of the colitis was associated with a decrease in CD8+ and FoxP3+ cells both in patients treated with steroids and infliximab. Our data suggest that counts of cytotoxic T cells and Tregs in the colonic mucosa from patients with ipilimumab-related colitis correlate with clinical findings and may predict severity and guide management.
KEYWORDS: Colitis, diarrhea, infliximab, ipilimumab, melanoma
Introduction
The treatment of advanced melanoma has dramatically changed in recent years as novel therapies have become available. A major breakthrough is the improved survival of patients with melanoma treated with immune modulatory agents. The first in this new class of immune therapies to be approved was the anti-cytotoxic T-lymphocyte-associated protein (CTLA)-4 antibody, ipilimumab; it demonstrated superiority to the previous standard of dacarbazine treatment, increasing both progression-free survival and overall survival. Strikingly, approximately 20% of patients achieve durable responses, lasting in excess of 5 y.1,2
Ipilimumab enhances immune responses against the tumor by activating cytotoxic T-cell activity and impairing the activity of regulatory T cells.3 It is usually a well-tolerated drug but 70% of patients experience at least one type of side effect during or after treatment and in 10–15% adverse events can be severe and, rarely, fatal.4 The organs most frequently affected by side effects are the skin, the gastrointestinal tract, and the liver. Other less common immune-related adverse events are hypophysitis and neuropathies. Recommendations regarding the management of these immune-related adverse events (irAEs) have been published.5 The most extensively used drugs to treat severe or persistent irAEs are steroids. These decrease the inflammatory response by targeting virtually all elements of the immune response from activating Tregs to inhibiting macrophages.6 Initial concerns about their possible deleterious effect in the outcome of patient treated with ipilimumab due to their immunosuppressant effect seem unjustified in the light of published data.7,8 Occasionally, side effects (mainly gastrointestinal) are not controlled with steroids and more potent immunosuppressants are recommended. Infliximab, an antitumor necrosis factor (TNF)-α agent, has been shown to suppress the immune system by upregulating Treg function, downregulating dendritic cells and therefore, inhibiting antigen presentation to T cells and their subsequent activity. 9 Additionally, TNFα blockade limits the effector function of T-cells directly. Infliximab is usually rapidly efficacious in controlling ipilimumab-related irAEs. However, to our knowledge, the modulation of immune cells through infliximab in cases with ipilimumab-related colitis remains to be characterized.
In the current work, we evaluated the immune cell infiltrate in the colonic mucosa in patients with ipilimumab-related colitis and its changes with different treatments.
Patients and methods
Patients
A list of patients treated with ipilimumab for melanoma was retrieved from the oncology pharmacy database. Clinical outcomes have been reported in a previous publication.10 Briefly, 113 patients with melanoma treated with ipilimumab 3 mg/kg for up to four cycles in the 2011–2014 period were reviewed, 29% experienced immune-related gastrointestinal symptoms. Those who had histological evaluation of the colitis (N = 20) were included in this study. Use of steroids and infliximab for toxicity management was recorded. Patient and treatment information were retrieved. Details on the endoscopy and histological grading of the colitis were recorded as well as C-reactive protein (CRP) blood levels. For comparison, 10 patients with histologically normal colonic biopsies (no inflammatory or malignant disease) were selected. Ethical approval was obtained for the study through the National Research Ethics Service (NRES; Rec No. 10/0504/32).
Immunohistochemical evaluation of colonic biopsies
For patients diagnosed clinically with ipilimumab-related diarrhea/colitis, 20 had histological evaluation of the colon with multiple colonic biopsies. All patients received steroids as part of the treatment and 11 subsequently received infliximab. Four patients treated only with steroids had two repeat biopsies and four patients treated with infliximab had a pre- and post-infliximab histological evaluation.
Hematoxilin and Eosins of these biopsies were reviewed and immunohistochemistry studies were performed to assess the presence of regulatory T cells (FoxP3; Ebioscience), granzyme B (GzmB) (Dako), and CD8+ lymphocytes (Novocastra); automated immunostaining (Ventana XT, Ventana) was performed by a clinical cellular pathology department using antibodies optimized to national diagnostic standards (NEQAS). Lymphocytes were quantified using a Zeiss AxioCam MRc5 microscope and Zeiss Axiovision software (version 4·8·1·0). An average tissue lymphocyte score/high-power field (X400) was calculated across 10 representative areas to allow for the heterogeneity of infiltrate.
Statistical analysis
To compare cumulative distributions between variables in the different groups, we used the non-parametric Mann–Whitney test for paired comparisons and the Kruskal–Wallis test for three group comparisons. All tests were conducted at the two-sided 0.05 level of significance. The GraphPad Prism statistical package was used to perform these analyses.
Results
Pathological evaluation of colonic biopsies
All patients (n = 20) who underwent endoscopy had been clinically diagnosed with ipilimumab-related gastrointestinal toxicity. Table 1 describes the tumor and treatment information for these patients. In 18/20 patients, mild to severe colitis was confirmed in the first histological evaluation, characterized by inflammatory infiltrate in the lamina propria (acute or chronic), with cryptitis and crypt abscess formation in some cases. Two patients showed no histological signs of colitis. One of these two had a grade 3 abdominal pain as a symptom with no diarrhea that was later confirmed to be due to disease progression. The other patient presented with diarrhea and abdominal pain and underwent a flexible sigmoidoscopy where only rectal biopsies were obtained due to poor bowel preparation. One month later a subsequent exploration of the bowel demonstrated a colitis.
Table 1.
Patient and treatment information for patients with ipilimumab-related colitis.
| Steroids only (n = 9) | Steroids+infliximab (n = 11) | ||
|---|---|---|---|
| Mean age (range) | 58 (38–75) | 65 (52–76) | |
| Primary | Cutaneous | 7 | 6 |
| Ocular | 2 | 4 | |
| Unknown | 0 | 1 | |
| Stage | M1a | 2 | 2 |
| M1b | 1 | 1 | |
| M1c | 6 | 8 | |
| Ipilimumab cycles | 1 | 2 | 0 |
| 2 | 0 | 4 | |
| 3 | 2 | 6 | |
| 4 | 5 | 1 | |
| Infliximab doses | 1 | NA | 7 |
| 2 | NA | 3 | |
| 3 | NA | 1 | |
| Worst grade diarrhea | 1 | 1 | 0 |
| 2 | 3 | 2 | |
| 3 | 5# | 8 | |
| 4 | 0 | 1 | |
| Endoscopic grading* | Normal | 1 | 2 |
| Mild | 2 | 3 | |
| Moderate | 6 | 1 | |
| Severe | 0 | 5 | |
| Histologic grading* | Normal | 1 | 1 |
| Mild | 2 | 2 | |
| Moderate | 6 | 4 | |
| Severe | 0 | 4 | |
| C-Reactive protein (Normal: 0–7.5) | Mean | 66.7 | 103.5 |
| Range | 2–211 | 10–330 | |
On the first endoscopy (pre-infliximab).
One patient had grade 3 abdominal pain with no diarrhea.
NA: not applicable
We next performed CD8+ and FoxP3 staining to identify cytotoxic T cells and Tregs, respectively. Fig. 1 illustrates the differences in the immune infiltration observed between normal and ipilimumab-related colonic biopsy findings. CD8+ T-cell counts were significantly higher in patients with ipilimumab-related colitis (median = 122; 95%CI: 99.6–155.9) compared to patients with no colitis (22; 18.7–31.6) (p < 0.0001). FoxP3 positive T-cell counts were also higher in patients with ipilimumab-induced colitis (24.8; 18.2–31.4) than in patients with no colitis (7.45; 5–9.2) (p < 0.0001). In 11/20 patients, the colitis failed to settle clinically on steroids alone and they required treatment with infliximab. We investigated whether there were differences in the immune infiltrate compared to patients (n = 9) who did not need treatment escalation (Fig. 1). Patients who required infliximab had higher CD8+ counts (145.3; 106.8–186.6) at first biopsy compared to those treated with steroids only (118.8; 82.2–149.2). In contrast, the median number of Tregs in the patients requiring infliximab appeared lower (16; 11.1–28.6), compared to a median of 30.6 (20.5–41.1), although neither individual measure reached significance. However, the difference became significant when the combined ratio of CD8+/FoxP3 was assessed (Fig. 1B). Additionally, the increased inflammation observed in patients who required infliximab linked clinically to a higher grade diarrhea, higher severity scores by endoscopy and histologically and higher levels of blood CRP (Table 1).
Figure 1.

Left column. CD8+ and FoxP3+ cell counts in colonic biopsies from patients without colitis (normal colon) and with ipilimumab-related colitis, Right column. CD8+ and FoxP3+ cell counts in colonic biopsies according to whether they received treatment with only steroids or steroids and infliximab. Statistical differences were assessed by the Mann–Whitney test (****p < 0.0001;***p < 0.001;**p < 0.01; *p <0.05) for paired comparisons (normal vs. infliximab, normal vs. steroids, infliximab vs. steroids) and Kruskal–Wallis test for the three group comparisons (normal vs. infliximab vs. steroids) (###p = 0.0001; #p = 0.037). Only significant associations are illustrated.
Finally, we analyzed the GzmB staining in the samples. In normal colon, we observed a small number of cell GzmB positive cells with a median of 0.3 (0–0.8). In cases with colitis, a significant increase of GzmB positive cells was found both for those treated with steroids only, median: 5 (0.8–19.4) or those who subsequently received infliximab, median: 5.4 (2.1–16.3) (with no significant differences within these groups) (Fig. 4 and Fig. S1). We attempted to also quantitate CD3 counts but the cell numbers were too dense for counting.
Figure 4.
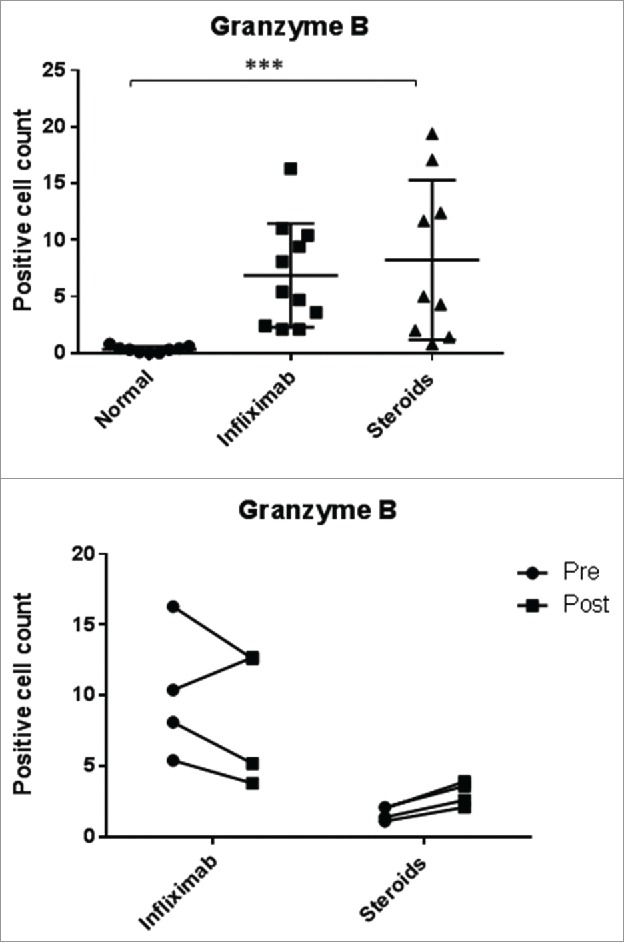
Granzyme B positive cell counts. Top: Normal mucosa compared to patient with ipilimumab-related colitis treated with steroids only (steroids) or with steroids and infliximab subsequently (infliximab) (***p <0.001 with Kruskal–Wallis test). Bottom: cell counts in serial colonic biopsies from patients with ipilimumab-related colitis treated with steroids only or steroids and infliximab (in the latter cases, samples were taken pre- and post-infliximab treatment). Rounds: first/pre; squares: second/post. Paired analyses were performed (Mann–Whitney test) and none of the differences were statistically significant.
Evaluation of repeat biopsies
In eight patients, repeat biopsies were taken to evaluate changes in the histological appearances. Of these, four had been given steroids only and four required infliximab treatment escalation. In the latter group, samples were evaluated pre- and post-infliximab. Fig. 2 shows the CD8+ and FoxP3 counts and their ratio. In 3/4 cases, treatment with infliximab moderately decreased CD8+ infiltration and FoxP3+ counts (Fig. 3), resulting in an increase in the CD8+/FoxP3 ratios. In the remaining case, the post-infliximab biopsy was performed 2 d after the pre-infliximab biopsy and 1 d after the infliximab administration due to rapid deterioration and the patient required a colectomy. In the group of those treated with steroids 3/4 showed a decrease in both cell types. The outlier was the patient cited above who had an initial normal colonoscopy but was rebiopsied due to lack of steroid response; the repeat biopsy revealed colitis and, compared to baseline, increase in CD8+ and Treg counts.
Figure 2.
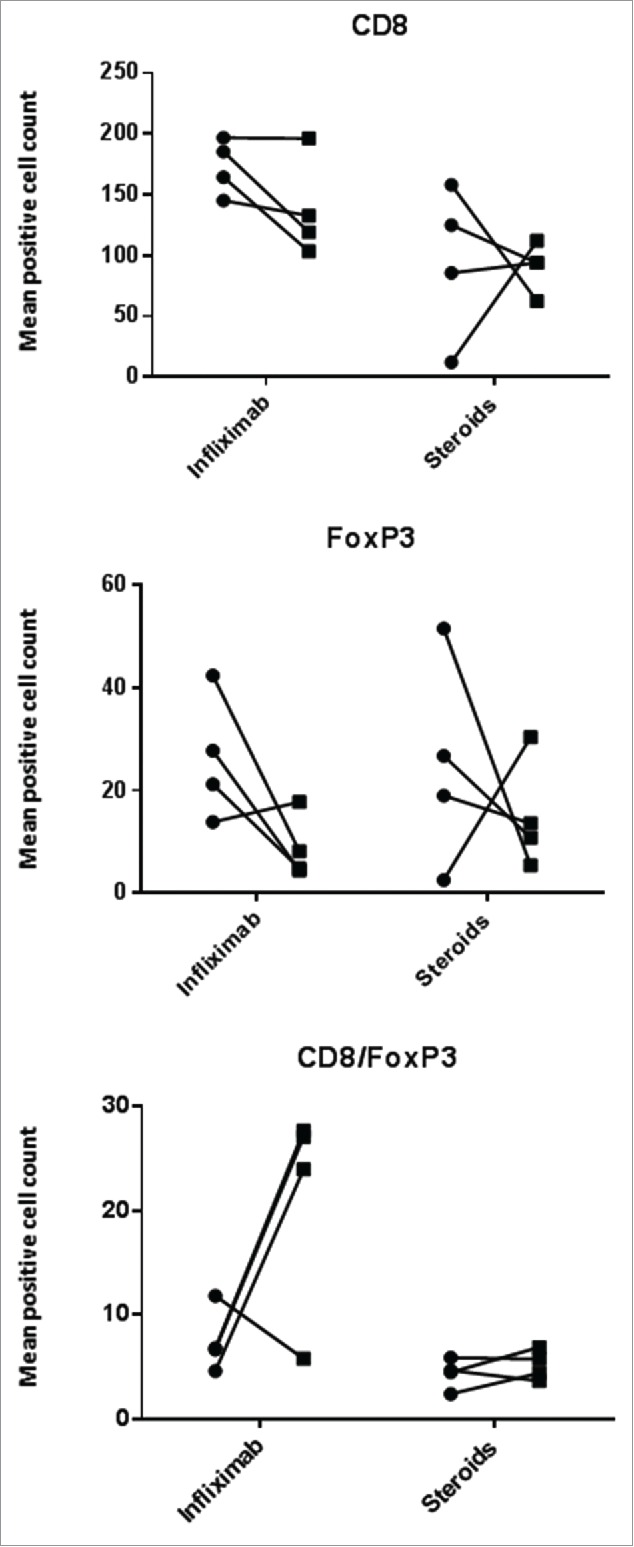
CD8+ and FoxP3+ cell counts in serial colonic biopsies from patients with ipilimumab-related colitis treated with steroids only or steroids and infliximab (in the latter cases, samples were taken pre- and post-infliximab treatment). Rounds: first/pre; squares: second/post. Paired analyses were performed (Mann–Whitney test) and none of the differences were statistically significant.
Figure 3.
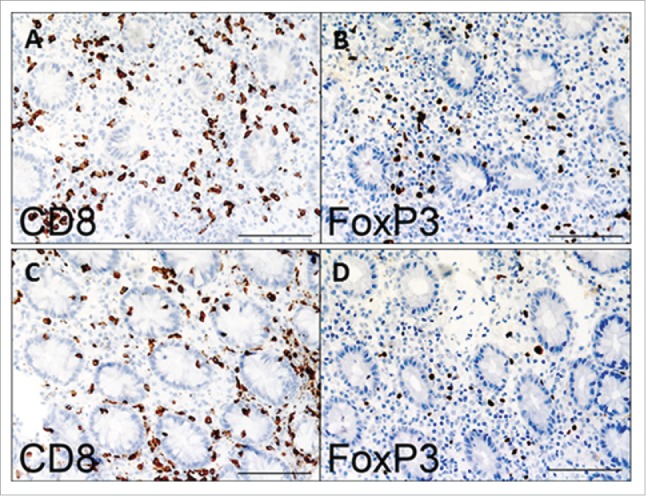
Micrographs illustrating immunohistochemical staining of colonic biopsies with FoxP3 and CD8+ antibodies (see Methods). (A and B) Pre-infliximab treatment; (C and D) post-infliximab treatment. Scale bar: 100 microns.
Regarding GzmB positive cells, in patients treated with infliximab the count of positive cells decreased in three cases (two when the ratio with CD8+ cells was considered) (Fig. 4 and Fig. S1). For the cases for whom steroids were the only treatment, an increase between the first and the second biopsies was observed. When the ratio with CD8+ was applied, the case that shows a decrease is the same outlier cited above (Fig. S1).
Discussion
We observed an increase of T cells, both CD8+ and Foxp3+ cells in colonic biopsies from patients with ipilimumab-related colitis, when compared to biopsies from normal colon. The normal colonic samples were not obtained from aged-matched or cancer patients, and it is therefore possible that some of the differences observed might reflect the changes in the intestinal immune cells due to aging or advanced cancer.11,12 The levels of the effects observed in our study are unlikely to be reflective of such natural variation.
Tregs (FoxP3+cells) express high levels of CTLA-4, and mouse models of colitis support that CTLA-4 is involved in the mechanisms by which Treg cells control intestinal inflammation.13,14 The effect of CTLA-4 blockade on Treg in patients could be mediated through Treg depletion, inhibition of Treg function, and/or an increase in the number of effector T cells, resulting in an increased CD8+/Treg ratio. This in turn would lead to an impaired ability of Tregs to counteract the pro-inflammatory effects of CD8+ cells and clinically link to diarrhea. Although Treg depletion by anti-CTLA-4 treatment has been elegantly demonstrated in the tumor microenvironment,15,16 we did not observe depletion in the colonic biopsies suggesting that the effect on Treg numbers may be context specific. For example, the tumor microenvironment is enriched for FcγR-bearing cells that are required for Treg depletion.17 However, our data suggest that the ipilimumab-induced colitis results from an excess in CD8+ T-cells, with only a relative paucity in Tregs. Unexpectedly, we observe that in parallel to an influx of CD8+ T cells, absolute Treg numbers were also elevated, compared to the numbers in normal bowel. This is consistent with data from other small series18,19 where no depletion of colonic Tregs was observed following ipilimumab treatment. Also consistent with our observation, previous data show that Treg function may be impaired even in the absence of a numerical decrease of these cells.13,20 Treg suppressive function is likely to be mediated by the blockade of the interaction between CTLA-4 in Tregs and its ligands (i.e. ,B7-1/B7-2) on antigen presenting cells13 and blockade of this pathway will further augment the global immune activation in the bowel. Interestingly, the relative increase of CD8+ cells was greater in patients who subsequently required treatment with infliximab compared to those whose diarrhea resolved with steroids only, and the data further suggest that the density of CD8+ infiltration is mirrored in the severity of the resulting clinical effect.
GzmB is involved in the apoptosis of target cells by natural killer (NK) and CD8+ cells and its expression is associated with the cytolytic potential of these cells.21 In normal colonic mucosa, the low number of cells observed in our study is consistent with the literature and possibly correspond to NK cells.22 The observed increase of GzmB positive cells in cases with ipilimumab-related colitis might reflect the mechanism of antigen-specific cytotoxicity by CD8+ cells, as reported for Crohn's disease.22
We included in the study two patients with a clinical presentation consistent with immune-related colitis, but in whom histological examination did not confirm this. In one case, the patient developed severe abdominal pain and she eventually developed disease progression that might explain the symptomatology. In the other patient, the first examination showed normal appearances both endoscopically and histologically. However, the examination was limited by poor preparation, perhaps leading to a false negative result. Significant variation in the baseline T-cell distribution along the healthy human gastrointestinal tract has been reported 23 and this variability would affect detection where sampling is guided by endoscopic findings. This patient continued to have symptoms and a repeat examination 5 weeks later demonstrated moderate colitis histologically with increased immune infiltration, post hoc suggesting that the negative histology at baseline was likely a false negative.
Following treatment with infliximab, rebiopsy showed a decrease of FoxP3 positive cells with a less pronounced decrease in the CD8+ cell count. A number of studies have evaluated the modulation of Tregs following treatment with infliximab, the majority from patients with inflammatory bowel disease (IBD). A consistent increase has been observed in circulating Tregs in the blood of patients with IBD. 24,25 This suggests that TNFα blockade is associated with a release of Treg from the tissue into the blood. In contrast, a significant decrease of Foxp3 positive cells in the colonic mucosa in IBD patients treated with infliximab was found in clinical responders. 24 These data are concordant with our own findings in non-IBD colitis, where the gut has been perturbed by ipilimumab. In contrast, Veltkamp et al. 26 demonstrate that the loss of Treg in IBD is, at least partly, explained by increased apoptosis. Interruption of the pathophysiological process with infliximab relieves this effect over time, 26 leading to reaccumulation of Tregs in parallel to inflammatory recovery. This is a dynamic process and in our patients ipilimumab may still have been present (1/2 life of 15 days) and therefore Treg recovery may simply not yet become measurable. The decrease observed in some cases treated with infliximab in the GzmB staining has previously been observed and might reflect the ability of the anti-TNFα agent to target these cytotoxic population and thus decrease inflammation effectively.27
Globally, our data demonstrate that CD8+ and FoxP3+ T-cell absolute counts and their ratio in colonic biopsies correlate with clinical severity as illustrated by higher grade symptomatology (diarrhea), increased scores in the endoscopic and histologic assessments, and inflammation biomarkers such as CRP. Although we evaluated a limited number of samples, it seems that the anti-inflammatory effect of infliximab does not depend on Treg presence as postulated for IBD patients, as both steroids and infliximab seem to reduce Treg counts over time. Quantifying CD8+ T-cells and Treg would be a relatively simple biomarker to predict for the need for more intense immunosuppressive treatment in addition to steroids (i.e., infliximab) early on. Further prospective analysis of these cell populations may allow us to validate whether numerical cutoffs can be set for clinical management of ipilimumab-related colitis.
Supplementary Material
Disclosure of potential conflicts of interest
MW declares non-financial support from Bristol Myers Squibb, outside the submitted work; GT reports personal fees from Bristol Myers Squibb, outside the submitted work; CO reports grants and personal fees from Bristol Myers Squibb, outside the submitted work; EA and MAL declare no conflicts of interest.
Funding
This study was funded by Southampton NIHR and CR UK Experimental Cancer Medicine Center, UK.
References
- 1.Maio M, Grob JJ, Aamdal S, Bondarenko I, Robert C, Thomas L, Garbe C, Chiarion-Sileni V, Testori A, Chen TT et al.. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol 2015; 33:1191-6; PMID:25713437; http://dx.doi.org/ 10.1200/JCO.2014.56.6018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 2015; 33:1889-94; PMID:25667295; http://dx.doi.org/ 10.1200/JCO.2014.56.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol 2008; 26:5275-83; PMID:18838703; http://dx.doi.org/ 10.1200/JCO.2008.17.8954 [DOI] [PubMed] [Google Scholar]
- 4.Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist 2013; 18:733-43; PMID:23774827; http://dx.doi.org/ 10.1634/theoncologist.2012-0483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber JS. Practical management of immune-related adverse events from immune checkpoint protein antibodies for the oncologist. Am Soc Clin Oncol Educ Book 2012; 174-7; PMID:24451730; http://dx.doi.org/ 10.14694/EdBook_AM.2012.32.174 [DOI] [PubMed] [Google Scholar]
- 6.Zen M, Canova M, Campana C, Bettio S, Nalotto L, Rampudda M, Ramonda R, Iaccarino L, Doria A. The kaleidoscope of glucorticoid effects on immune system. Autoimmun Rev 2011; 10:305-10; PMID:21224015; http://dx.doi.org/ 10.1016/j.autrev.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 7.Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, Ridolfi R, Assi H, Maraveyas A, Berman D et al.. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res 2009; 15:5591-8; PMID:19671877; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-1024 [DOI] [PubMed] [Google Scholar]
- 8.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ et al.. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A 2003; 100:8372-7; PMID:12826605; http://dx.doi.org/ 10.1073/pnas.1533209100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong M, Ziring D, Korin Y, Desai S, Kim S, Lin J, Gjertson D, Braun J, Reed E, Singh RR. TNFalpha blockade in human diseases: mechanisms and future directions. Clin Immunol 2008; 126:121-36; PMID:17916444; http://dx.doi.org/ 10.1016/j.clim.2007.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arriola E, Wheater M, Karydis I, Thomas G, Ottensmeier C. Infliximab for IPILIMUMAB-Related Colitis-Letter. Clin Cancer Res 2015; 21:5642-3; PMID:26672088; http://dx.doi.org/ 10.1158/1078-0432.CCR-15-2471 [DOI] [PubMed] [Google Scholar]
- 11.Saffrey MJ. Aging of the mammalian gastrointestinal tract: a complex organ system. Age 2014; 36:9603; PMID:24352567; http://dx.doi.org/ 10.1007/s11357-013-9603-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bindels LB, Neyrinck AM, Claus SP, Le Roy CI, Grangette C, Pot B, Martinez I, Walter J, Cani PD, Delzenne NM. Synbiotic approach restores intestinal homeostasis and prolongs survival in leukaemic mice with cachexia. The ISME journal 2015; 10:1456-70; PMID:26613342; http://dx.doi.org/ 10.1038/ismej.2015.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, Sharpe AH, Powrie F. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol 2006; 177:4376-83; PMID:16982872; http://dx.doi.org/11722634 10.4049/jimmunol.177.7.4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh B, Read S, Asseman C, Malmstrom V, Mottet C, Stephens LA, Stepankova R, Tlaskalova H, Powrie F. Control of intestinal inflammation by regulatory T cells. Immunol Rev 2001; 182:190-200; PMID:11722634; http://dx.doi.org/ 10.1034/j.1600-065X.2001.1820115.x [DOI] [PubMed] [Google Scholar]
- 15.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Investig 2006; 116:1935-45; PMID:16778987; http://dx.doi.org/ 10.1172/JCI27745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, Korman AJ. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res 2013; 1:32-42; PMID:24777248; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0013 [DOI] [PubMed] [Google Scholar]
- 17.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD et al.. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 2013; 210:1695-710; PMID:23897981; http://dx.doi.org/ 10.1084/jem.20130579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lord JD, Hackman RC, Moklebust A, Thompson JA, Higano CS, Chielens D, Steinbach G, McDonald GB. Refractory colitis following anti-CTLA4 antibody therapy: analysis of mucosal FOXP3+ T cells. Dig Dis Sci 2010; 55:1396-405; PMID:19507029; http://dx.doi.org/ 10.1007/s10620-009-0839-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I et al.. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A 2008; 105:3005-10; PMID:18287062; http://dx.doi.org/ 10.1073/pnas.0712237105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol 2005; 175:7746-54; PMID:16301685; http://dx.doi.org/9120391 10.4049/jimmunol.175.11.7746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi L, Mai S, Israels S, Browne K, Trapani JA, Greenberg AH. Granzyme B (GraB) autonomously crosses the cell membrane and perforin initiates apoptosis and GraB nuclear localization. J Exp Med 1997; 185:855-66; PMID:9120391; http://dx.doi.org/ 10.1084/jem.185.5.855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins D, Seth R, Kummer JA, Scott BB, Hawkey CJ, Robins RA. Differential levels of granzyme B, regulatory cytokines, and apoptosis in Crohn's disease and ulcerative colitis at first presentation. J Pathol 2000; 190:184-9; PMID:10657017; http://dx.doi.org/ 10.1002/(SICI)1096-9896(200002)190:2%3c184::AID-PATH531%3e3.0.CO;2-E [DOI] [PubMed] [Google Scholar]
- 23.Tauschmann M, Prietl B, Treiber G, Gorkiewicz G, Kump P, Hogenauer C, Pieber TR. Distribution of CD4(pos) -, CD8(pos) - and regulatory T cells in the upper and lower gastrointestinal tract in healthy young subjects. PloS one 2013; 8:e80362; PMID:24265815; http://dx.doi.org/ 10.1371/journal.pone.0080362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Arijs I, De Hertogh G, Vermeire S, Noman M, Bullens D, Coorevits L, Sagaert X, Schuit F, Rutgeerts P et al.. Reciprocal changes of Foxp3 expression in blood and intestinal mucosa in IBD patients responding to infliximab. Inflamm Bowel Dis 2010; 16:1299-310; PMID:20196149; http://dx.doi.org/ 10.1002/ibd.21229 [DOI] [PubMed] [Google Scholar]
- 25.Boschetti G, Nancey S, Sardi F, Roblin X, Flourie B, Kaiserlian D. Therapy with anti-TNFalpha antibody enhances number and function of Foxp3(+) regulatory T cells in inflammatory bowel diseases. Inflamm Bowel Dis 2011; 17:160-70; PMID:20848510; http://dx.doi.org/ 10.1002/ibd.21308 [DOI] [PubMed] [Google Scholar]
- 26.Veltkamp C, Anstaett M, Wahl K, Moller S, Gangl S, Bachmann O, Hardtke-Wolenski M, Langer F, Stremmel W, Manns MP et al.. Apoptosis of regulatory T lymphocytes is increased in chronic inflammatory bowel disease and reversed by anti-TNFalpha treatment. Gut 2011; 60:1345-53; PMID:21459928; http://dx.doi.org/ 10.1136/gut.2010.217117 [DOI] [PubMed] [Google Scholar]
- 27.Van den Brande JM, Braat H, van den Brink GR, Versteeg HH, Bauer CA, Hoedemaeker I, van Montfrans C, Hommes DW, Peppelenbosch MP, van Deventer SJ. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn's disease. Gastroenterology 2003; 124:1774-85; PMID:12806611; http://dx.doi.org/ 10.1016/S0016-5085(03)00382-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


