ABSTRACT
In cancer patients, immunosuppression through regulatory T cells (Treg) is a crucial component of tumor immune evasion and contributes to disease progression. Tumor-infiltrating Treg in particular suppress local effector T cell responses and are associated with poor prognosis in tumors such as human pancreatic cancer or hepatocellular carcinoma (HCC). The chemokine CCL22 is known to recruit Treg into the tumor tissue and many types of human tumors are known to express high levels of CCL22. The mechanisms leading to intratumoral secretion of CCL22 are so far unknown. We demonstrate here that intratumoral CCL22 is induced in tumor-infiltrating immune cells through cancer cell-derived interleukin-1 (IL-1α). In pancreatic cancer and HCC, CCL22 is produced by intratumoral dendritic cells, while the cancer cells themselves do not secrete CCL22 in vitro and in vivo. Incubation of human peripheral blood mononuclear cells (PBMC) or murine splenocytes with tumor cells or tumor cell supernatants strongly induced CCL22 secretion in vitro. Tumor cell supernatants contained IL-1 and CCL22 induction in PBMC could be specifically prevented by the IL-1 receptor antagonist anakinra or by transfection of tumor cell lines with IL-1 siRNA, leading to a suppression of Treg migration. In conclusion, we identify here tumor cell-derived IL-1α as a major inducer of the Treg attracting chemokine CCL22 in human cancer cells. Therapeutic blockade of the IL-1 pathway could represent a promising strategy to inhibit tumor-induced immunosuppression.
KEYWORDS: CCL22, chemokine, interleukin-1, regulatory T cells, tumor
Introduction
A key challenge in cancer immunotherapy is to overcome tumor-induced immunosuppression. Tumors are capable of shaping their immunologic microenvironment to counteract eradication by the host immune system. Different mechanisms contribute to the establishment of the tumor-associated immunosuppressive milieu, including the recruitment of suppressive cell populations as well as the secretion and upregulation of inflammatory or inhibitory cytokines and chemokines.1 The C-C-motif ligand 22 (CCL22) is a chemokine which has been known to play a role in tumor-associated immunosuppression.2 CCL22 is constitutively expressed in macrophages, monocytes and dendritic cells and is inducible in many other immune cells such as Th2 cells and B lymphocytes as well as in epithelial cells.3-6 CCL22 is the functional ligand for CCR4, a chemokine receptor expressed on the surface of Th2 cells and regulatory T cells (Treg).7,8 Elevated levels of CCL22 have been described in many human tumors, including B-CLL, Hodgkin lymphoma, breast, lung and gastrointestinal cancers.9-14
For many of these tumors, elevated intratumoral CCL22 concentrations are associated with a higher tumor infiltration by Treg. Treg are an inhibitory subset of T helper cells, whose function is to suppress cytotoxic T effector cell functions,15-17 involving cell-contact dependent and cell-contact independent mechanisms such as consumption of IL-2, expression of the cell-surface antigen CTLA-4 and secretion of inhibitory cytokines such as TGF-β and IL-10.18-21 Treg have been found to accumulate in tumor tissues as well as in tumor-draining lymph nodes, malignant ascites and peripheral blood of tumor patients.22-24 High intratumoral numbers of Treg as well as a high intratumoral ratio of Treg to T effector cells have in many cases been shown to have a negative prognostic impact and to be correlated with a higher stage of tumor disease.2,23-25 Thus, CCL22-mediated recruitment of Treg to the tumor site contributes to suppression of an effective antitumoral immune response.
In some types of cancer, infiltration by Treg seems to be particularly critical for tumor control and prognosis. In hepatocellular carcinoma (HCC) and pancreatic cancer, accumulation of Treg has been directly associated with poor prognosis.22,26-28
Little is known so far about the mechanisms leading to CCL22 expression in the cancer tissue. In our study, we show that tumor cell-derived IL-1α specifically induces CCL22 expression in tumor-infiltrating dendritic cells. We further demonstrate that CCL22 expression can be prevented by inhibition of the interleukin-1 (IL-1) pathway through the drug anakinra, which in consequence leads to a suppression of Treg migration.
Results
In hepatocellular carcinoma and pancreatic ductal adenocarcinoma CCL22 is expressed by tumor-infiltrating immune cells
In order to investigate, which cells are responsible for tumor-associated CCL22-expression in HCC and pancreatic ductal adenocarcinoma (PDAC), we examined paraffin-embedded tissue sections of HCC and PDAC for infiltration by CCL22-expressing cells. CCL22 was detected in nearly all tissue sections intratumorally as well as in peritumoral stroma. CCL22-positive cells in HCC and PDAC tissue sections showed typical cytoplasmic staining and were myeloid-shaped indicating that CCL22 is expressed by tumor-infiltrating immune cells rather than by tumor cells themselves (Fig. 1A and B). In HCC, high numbers of CCL22-expressing cells were located in the peritumoral stroma, whereas low levels were found in the tumor tissue (Fig. 1C). In PDAC, CCL22-expressing cells were found intratumorally in most sections analyzed; however, intratumoral numbers of CCL22-expressing cells were mostly lower than the numbers of CCL22-positive cells in peritumoral and intratumoral stroma (Fig. 1D). In summary, we found that CCL22 is expressed at high levels in human HCC and PDAC and expression is largely restricted to tumor-infiltrating immune cells of myeloid phenotype.
Figure 1.
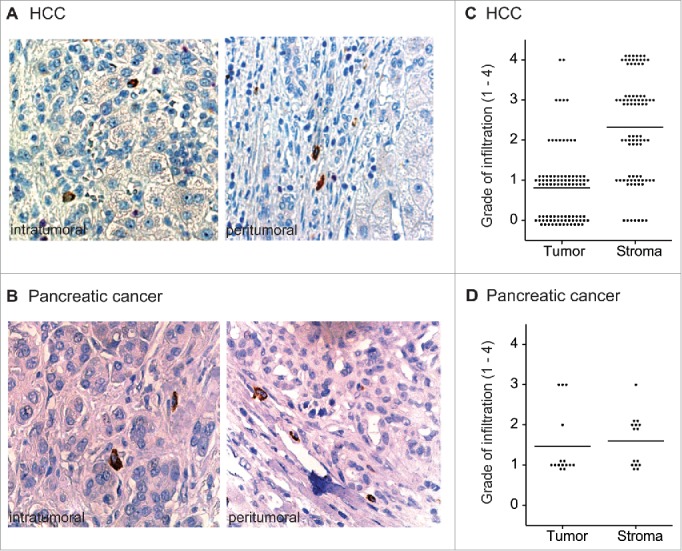
Infiltration of human HCC and PDAC by CCL22-expressing cells. Paraffin-embedded HCC and PDAC tissue sections were stained for CCL22 (brown). (A) and (B) Representative tissue sections of HCC and PDAC (40x) with intratumoral and stromal infiltration by CCL22-expressing, myeloid-shaped cells. (C), (D) Intratumoral and peritumoral infiltration by CCL22-expressing cells was assessed semiquantitatively in HCC (n = 95) and in PDAC TMA (n = 15) tissue sections. 0 = no infiltration; 1 = isolated infiltrating cells; 2 = few infiltrating cells; 3 = medium number of infiltrating cells; 4 = high number of infiltrating cells. Tumor = tumor epithelium, Stroma = peritumoral stroma. Lines indicate mean.
CCL22 is upregulated in immune cells upon co-culture with tumor cells
We next aimed to understand the mechanisms leading to CCL22 expression by tumor-infiltrating immune cells. To test whether CCL22 secretion in these cells may be induced by the tumor cells, we co-incubated human peripheral blood mononuclear cells (PBMC) with different human tumor cell lines. Strikingly, co-culture with tumor cells strongly increased CCL22 levels of PBMC (Fig. 2A). The tumor cell-mediated CCL22 induction was seen with a broad set of human tumor cell lines, comprising Hep3B (HCC), PaTu8988t (pancreatic cancer), SK-Mel23 (melanoma), A-375 (melanoma) or MDAMB-231 (breast cancer). Similar results were observed in the murine system, where co-cultures of splenocytes with a set of syngeneic tumor cells led to a strong upregulation of CCL22 (Fig. 2B). However, no CCL22 secretion was detected in any culture supernatant of a tumor cell line alone (Fig. 2C). To exclude that CCL22 induction in immune cells is caused by rapidly expanding cell lines in general, we next co-cultured PBMC or murine splenocytes with cell lines that lack a malignant background. Co-incubation of PBMC with HEK-293T cells, a fast-growing human embryonic kidney cell line did not lead to induction of CCL22, and similarly, murine splenocytes did not upregulate CCL22 secretion upon co-incubation with DC2.4 cells, an immature dendritic cell line with rapid expansion29 (Fig. 2A and B). Interestingly, we also observed an upregulation of CCL22 mRNA and protein in the splenocytes of Panc02 tumor-bearing mice as compared to tumor-free control mice (Fig. 2D). We observed similar results in 4T1 tumor and B16 tumor-bearing mice (data not shown). These results indicate that, in vivo, interaction of tumor cells with immune cells induces CCL22 not only locally, in the tumor tissue, but also systemically. Taken together, the interaction of leukocytes with tumor cells leads to a marked secretion of CCL22.
Figure 2.
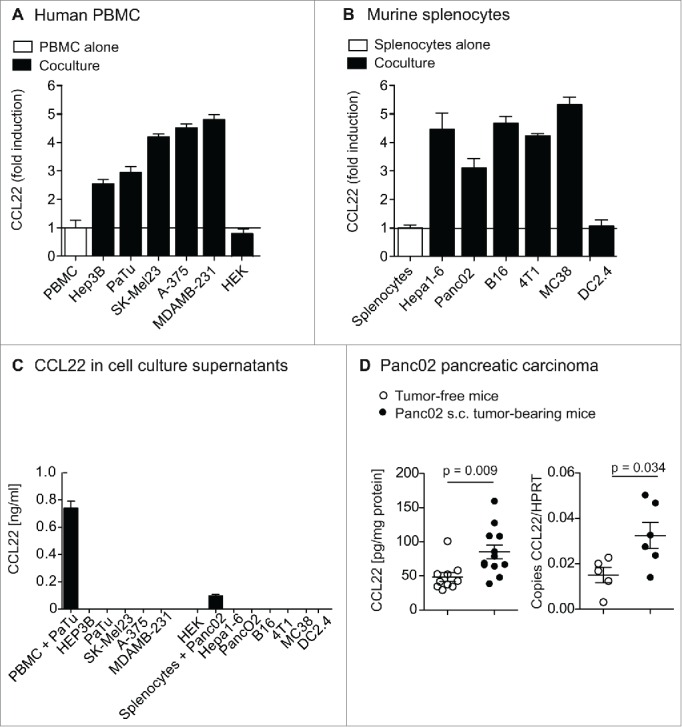
CCL22 upregulation in immune cells upon coculture with tumor cell lines. (A) and (B) Human PBMC and murine splenocytes were co-cultured with syngeneic tumor cell lines for 48 h and co-culture supernatants were analyzed for CCL22 protein levels by ELISA. Bars indicate fold induction of CCL22 in the supernatant. (C) Human PBMC, murine splenocytes or tumor cells were cultured for 48 h. Bars indicate CCL22 levels in culture supernatant. (D) Mice bearing s.c. Panc02 tumors (n = 12) were sacrificed and CCL22 levels in serum and splenocytes were measured by chemokine ELISA and compared to age-matched tumor-free control mice. Data of CCL22 protein levels in splenocytes pooled from two independent experiments, analysis of mRNA levels was only performed once (n = 6). Error bars indicate SEM.
CCL22 secretion in leukocytes is mediated by a tumor cell-derived soluble factor
As we observed systemic CCL22 upregulation in s.c. tumor-bearing mice, we next aimed at determining the mechanism of cancer cell-induced CCL22 expression. PBMC or splenocytes were cultured in cell-free supernatants of different tumor cell lines and CCL22 secretion was measured by ELISA. Interestingly, culture of PBMC in cell-free supernatants of PaTu, Hep3B, SK-Mel23, A-375 or MDAMB-231 cell lines resulted in a strong upregulation of CCL22 (Fig. 3A). Again, no CCL22 could be found in the tumor cell supernatants themselves. Correspondingly, culture of murine splenocytes in culture supernatants of the tumor cell lines B16, 4T1, MC38, Panc02 and HEPA1-6 lead to a significant induction of CCL22-production (Fig. 3B). To confirm CCL22 induction on mRNA level, we next performed quantitative rt-PCR of PBMC and splenocytes upon culture with cell-free tumor cell supernatants. As expected, high levels of CCL22 mRNA were detected in both human PBMC and murine splenocytes that were cultured in supernatants of MDAMB-231 or PancO2, respectively (Fig. S1A). To exclude that, inversely, CCL22 is induced on tumor cells through supernatants of PBMC, we incubated human tumor cell lines in PBMC culture supernatant. As expected, no upregulation of CCL22 in tumor cells was observed (Fig. S1B). We thus concluded that tumor cell lines secrete a soluble factor that induces CCL22 secretion in immune cells.
Figure 3.
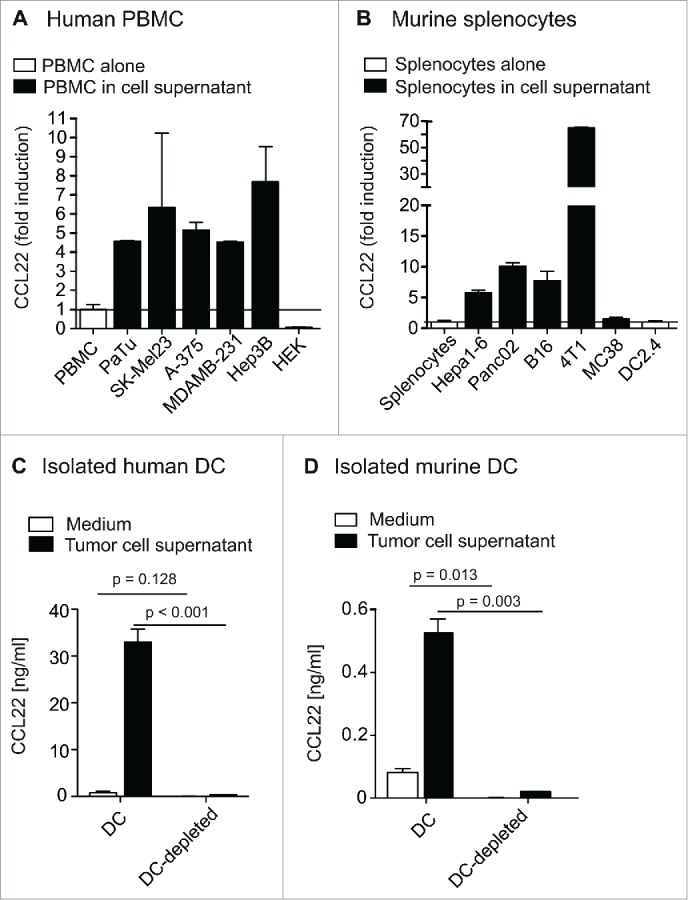
A soluble factor secreted by tumor cells leads to CCL22 induction in immune cells. (A) and (B) Human PBMC and murine splenocytes were cultured for 48 h in cell-free supernatant of indicated tumor cell lines. Tumor cell supernatant was collected after 48 h of tumor cell culture and centrifuged to remove remaining cells. CCL22 protein levels in culture supernatant were measured by ELISA. Bars indicate fold induction of CCL22. (C) Human DC were sorted from PBMC by magnetic-activated cell sorting using the Blood Dendritic Cell Isolation Kit II (Miltenyi). DC and DC-depleted fractions were cultured in medium or cell-free PaTu supernatant for 48 h. Bars indicate CCL22 levels. (D) Murine C57BL/6 splenocytes were enriched for CD11c-positive (CD11c+) cells by MACS using two separate columns. CD11c-positive and CD11c-negative fractions were cultured alone or with B16 melanoma cells for 48 h. Bars indicate CCL22 levels. All error bars indicate SEM.
We next aimed to determine the immune cell population responsible for tumor cell-induced CCL22 production. In humans and mice, dendritic cells have been described to be a major source of CCL22.5,6 We sorted human PBMC for DC using the markers CD304 (BDCA-4), CD141 (BDCA-3) and CD1c (BDCA-1) and analyzed the DC and the DC-depleted fraction for CCL22 secretion upon exposure to PaTu tumor cell supernatant. Indeed, CCL22 secretion upon exposure to tumor cell medium was highly restricted to DC (Fig. 3C). Also in mice, tumor cell-induced CCL22 secretion was observed only in CD11c+ DC (Fig. 3D). In summary, tumor cells produce a soluble factor that induces CCL22 secretion in DC.
CCL22-induction is mediated by tumor cell-derived interleukin-1α
Subsequently, we tried to identify the CCL22 inducing factor secreted by tumor cells. First, to exclude the influence of tumor cell-derived DNA or RNA molecules on CCL22 upregulation, we treated human tumor cell lysates with DNAse or Riboshredder RNAse (Epibio, Madison, USA). We observed no changes in CCL22 induction upon elimination of DNA and RNA from tumor cell supernatant (Fig. S2A). In contrast, heating of tumor cell culture supernatant at 65°C, resulted in a significant loss of their capacity to induce CCL22 in PBMC, suggesting that a protein mediates CCL22 induction (Fig. S2B). It is known that cytokines of the IL-1 family play a role in tumor-associated inflammation and most epithelial tumor cells produce these cytokines.30-32 We thus aimed to determine the role of IL-1α and β in tumor cell-mediated CCL22 induction. We analyzed tumor cells for IL-1α and β secretion and mRNA expression and compared it to the IL-1α and β expression in HEK cells, which do not induce CCL22. We could show that the human cancer cell lines used in our previous experiments expressed IL-1α or IL-1β or both cytokines (Fig. S2C). Within murine cells, IL-1 mRNA was detectable in the 4T1, but not in the DC2.4 cell line nor in other tumor cells. (Fig. S2C) The CCL22-inducing pancreatic cancer cell line PaTu expressed high levels of IL-1α but no IL-1β protein and mRNA, whereas both types of IL-1 were almost undetectable in HEK cells (Fig. 4A and B). To determine whether IL-1 was the tumor cell-derived CCL22-inducing factor, we blocked the IL-1 receptor 1 (IL-1R1) by adding anakinra to the culture of PBMC in tumor cell supernatant. Strikingly, addition of anakinra decreased CCL22 induction in a dose-dependent manner, with a complete loss of CCL22 induction at 5 ng/mL (Fig. 4C). To test whether IL-1α is sufficient to induce CCL22, we added recombinant human IL-1α (rhIL-1α) to the culture of PBMC. Indeed, we found that rhIL-1α clearly induced CCL22 secretion by PBMC (Fig. S2D). To confirm that tumor cell-derived IL-1α was required for CCL22 induction, we transfected PaTu tumor cells with IL-1α siRNA. SiRNA knockdown was functional, as confirmed by IL-1 qPCR (Fig. S2E). Indeed, IL-1 siRNA-treated PaTu cells were unable to induce CCL22 in PBMC, whereas supernatants of control siRNA-treated PaTu significantly induced CCL22 (Fig. 4D). Further, siRNA knockdown of IL-1α or IL-1β in murine 4T1 cells significantly reduced CCL22 induction in splenocytes (Fig. S3A and B). To demonstrate that IL-1α signaling directly leads to CCL22 induction in human PBMC, we designed a CCL22 reporter assay, cloning the human CCL22 promoter into a promoterless luciferase vector. HEK293 cells transfected with the CCL22-luciferase vector showed strongly increased luciferase activity upon addition of rhIL-1α (Fig. 4E). In contrast, no increase in luciferase signal was observed in cells transfected with the empty luciferase vector. We could thus demonstrate, that IL-1α induces CCL22 promoter activation. In vivo, treatment of 4T1 tumor-bearing mice with anakinra resulted in lowered (although not significantly reduced) CCL22 levels in tumors (Fig. S3). Taken together, our data demonstrate that tumor cells can secrete IL-1α, which induces CCL22 promoter activation and CCL22 secretion in immune cells.
Figure 4.
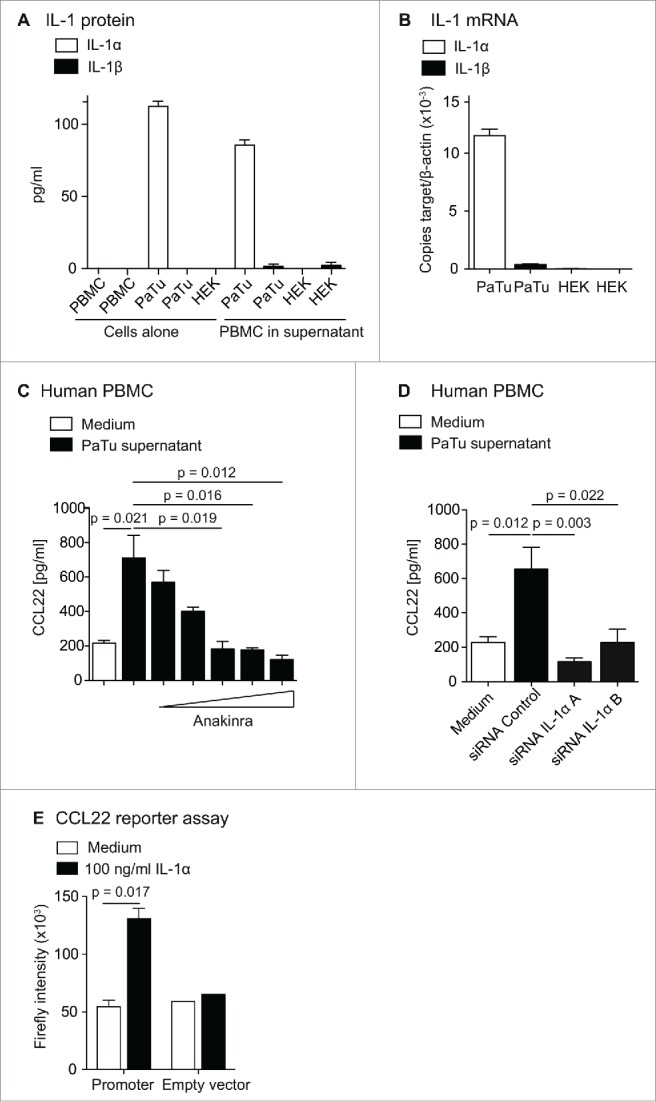
CCL22 upregulation is mediated by IL-1α. (A) IL-1α and IL-1β protein levels in PaTu and HEK-293 cell culture supernatants were determined by cytokine ELISA. B, IL-1α and IL-1β mRNA levels in PaTu and HEK-293T cells were determined by reverse transcriptase quantitative real-time PCR. (C) PBMC were cultured in control medium or cell-free culture supernatant of PaTu cells. The IL-1R1-antagonist anakinra was added to the culture in the following concentrations: 0.05 ng/mL, 0.5 ng/mL, 5 ng/mL, 50 ng/mL and 500 ng/mL. CCL22 protein levels in culture supernatant were determined after 48 h by ELISA. Error bars indicate SEM. (D) PaTu cells were transfected with control siRNA or two different siRNAs against IL-1α, respectively. Medium was changed 48 h after transfection, afterwards cell culture supernatant was collected for another 48 h. PBMC were incubated in supernatants for 48 h and CCL22 protein levels in PBMC culture supernatants were determined by ELISA. Error bars indicate SEM. Data displayed were obtained in two independent experiments. (E) The full promoter sequence of the CCL22 gene was cloned into a promoterless luciferase vector and transfected into HEK293T cells. After transfection, recombinant human IL-1α (100 ng/mL) was added to the culture medium. Luciferase activity was analyzed after 24 h using a Berthold Orion II Microplate Luminometer. Data displayed were obtained in two independent experiments.
Suppression of CCL22 by IL-1 receptor blockade inhibits treg migration
We next aimed to evaluate whether the inhibition of CCL22 induction through IL-1 receptor blockade influenced the migration of Treg. We cultured PBMC in medium or PaTu culture supernatant with or without addition of anakinra. As previously observed, CCL22 induction in PBMC upon culture in PaTu supernatant was abrogated by addition of anakinra (Fig. 5A). The supernatants were collected after 48 h of culture and transferred into the lower well of a transwell migration chamber. Whole freshly isolated PBMC were then transferred into the upper well and left to migrate at 37°C for 6 h. As expected, we observed a significantly increased migration of Treg toward the supernatants of PBMC cultured in PaTu supernatant compared to PBMC cultured in control medium. Strikingly, this induction of migration was abrogated in the anakinra-treated PBMC supernatants (Fig. 5B). In conclusion, migration of Treg through tumor cell-derived IL-1α-induced CCL22 can be inhibited by the IL-1 receptor blocker anakinra.
Figure 5.

Anakinra blocks human Treg migration toward PaTu supernatant. (A) Human PBMC were cultured in medium or cell-free supernatant of PaTu cells for 48 h with or without addition of 500 ng/mL anakinra. CCL22 levels in culture supernatant were measured by ELISA. (B) Supernatants of cultures described in A were transferred into the lower well of a 3 µm migration chamber. Human PBMC were loaded into the upper chamber and left to migrate for 6 h at 37°C. Migrated cells in the lower chamber were analyzed by flow cytometry with addition of counting beads. Bars show total numbers of migrated CD3+ CD4+ FoxP3+ Treg in the lower chamber. Error bars indicate SEM.
Discussion
Tumor cells exhibit several mechanisms to escape from the control of the immune system. One major component in the creation of an immunosuppressive microenvironment is the recruitment of inhibitory Treg to the tumor site, which may be attracted by intratumorally expressed CCL22. HCC and PDAC are tumors in which several mechanisms of immune escape have so far been described.28,33 In HCC, infiltration by Treg has been observed both in the tumor tissue, in the blood and ascites26,28,34 and tumor infiltration by Treg was clearly associated with poor clinical outcome.26 In the present study, we found that the Treg-attracting chemokine CCL22 is expressed in the majority of human HCC samples suggesting that CCL22 is involved in the recruitment of Treg to these tumors. Indeed, it has been reported previously that, in HCC, CCL22 promotes tumor growth and the formation of portal vein thrombosis through the recruitment of Treg.35 High numbers of Treg have also been found in the tumor tissue of PDAC patients and, as in HCC patients, correlate with poor clinical outcome.22,25 In pancreatic cancer, tissue accumulation of Treg has so far been associated mainly with expression of TGF-β. Nothing is known about the role of the Treg-attracting chemokine CCL22 in these tumors.36,37
We found that the majority of HCC and all pancreatic cancer samples were infiltrated with CCL22-expressing immune cells. We show here for the first time that tumor cells potently induce CCL22 secretion in leukocytes. We further identified secretion of tumor cell-derived IL-1α as the paracrine mechanism for this induction. Importantly, our finding was not restricted to HCC or pancreatic cancer as several other tumor cell lines—derived from breast cancer or melanoma—also strongly induced CCL22 in PBMC. CCL22 induction by tumor cells was also shown in the murine immune system, where tumor cell lines upregulated CCL22 in splenocytes. Only a minority of the murine cell lines however expressed IL-1, suggesting that in mice additional tumor cell-derived factors may contribute to CCL22 induction in tumor-infiltrating immune cells. In conclusion, we suggest here a novel pathway of tumor immune suppression, namely the induction of the Treg attracting chemokine CCL22 through tumor cell-derived factors and notably IL-1.
High expression of CCL22 in tumors has been demonstrated extensively by many authors, the reports about the cellular source of tumor-associated CCL22 are however inconsistent. In breast cancer, both tumor cells themselves and tumor-infiltrating immune cells can secrete CCL22,38,39 in most other types of cancer, however, CCL22 is secreted by tumor-infiltrating immune cells.40 In HCC, CCL22 upregulation was shown in HBV-infected HCC cell lines and HBV+ primary HCC tumors on mRNA level.35 In HCC, using immunohistology, we did not observe any CCL22 expression by tumor cells in vivo. Thus, our data indicate that in vivo, in HCC, CCL22 expression is restricted to tumor-infiltrating immune cells. Also in pancreatic tumors, CCL22 was expressed exclusively by tumor infiltrating myeloid-shaped cells. Further, no CCL22 secretion was detected in vitro in several other human and murine cancer cell lines. In contrast, the cell-free supernatant of these malignant cells induced significant upregulation of CCL22 in immune cells. These findings are seemingly contradictory to a former study reporting CCL22 upregulation in human breast cancer cell lines upon culture in PBMC supernatants.39 This may, however, be explained by the addition of IFNγ in incubation protocols of that study, which is known to induce CCL22 in epithelial cells.41,42 Although the regulation of CCL22 has been investigated in several further studies,3,6,35,43 we describe here for the first time its induction through IL-1α by cancer cells. Further, we show that IL-1α-induced CCL22 leads to the recruitment of Treg and this can be blocked by the IL-1 receptor antagonist anakinra. As anakinra blocks both IL-1α and IL-1β signaling, we cannot exclude a role for IL-1β in tumor-mediated CCL22 induction. In the human pancreatic cancer cell line PaTu, which we used for the present studies, we found high expression of IL-1α on mRNA and protein level whereas IL-1β—both mRNA and protein—was nearly undetectable. However, the role of IL-1β produced by tumor cells or tumor-associated immune cells in other cancer types remains to be investigated.
High levels of IL-1α expression in cancers have been described to play a role in enhanced malignancy, dedifferentiation, lymphangiogenesis and metastasis.44-48 IL-1 has already been described to induce CCL22 expression.6 Our data suggest a novel role of tumor cell-derived IL-1α, mediating CCL22 induction in immune cells and thus fostering the formation of an immunosuppressive micromilieu. The results of our study raise the question whether therapeutic blockade of IL-1α may be useful for cancer therapy. In our murine 4T1 mouse tumor model anakinra treatment lowered intratumoral CCL22, but did not affect intratumoral Treg numbers (data not shown). Many murine tumor cells did however not express IL-1 and we propose that additional mechanisms are responsible for CCL22 induction in mice. Another limitation is that CCL22 is only one out of many factors that contribute to Treg recruitment. Interestingly, in a recent clinical trial, IL-1α was neutralized with a blocking antibody in end-stage cancer patients, resulting in an increase in lean body mass and enhanced survival.31 Recent achievements in cancer immunotherapy such as the clinical approval of the immune checkpoint blockade antibodies ipilimumab and nivolumab have impressively proven that immunomodulation is a potent weapon in antitumor therapy.49,50 It seems possible that anticancer treatment with anakinra also promotes antitumor immunity and this approach may be of particular interest when combined with other immunostimulatory and conventional therapeutic regimens.
Material and methods
Mice, cell lines and reagents
Female BALB/c or C57BL/6 mice were purchased from Janvier. Mice were 5 to 12 weeks of age at the onset of experiments. All animal studies were approved by the local regulatory agency (Regierung von Oberbayern). The human cell lines A-375, HEK-293T, HEP3B, MDAMB-231 and SK-Mel23 and the murine cell lines 4T1, CT26, Hepa1-6, MC38 and B16-F10 were obtained from American Type Culture Collection and were used within 6 mo after resuscitation (ATCC, Manassas, VA, USA). PaTu was kindly provided by Prof. Michl (Marburg, Germany) and Panc02 by Prof. Bruns. The murine C57BL/6 immortalized dendritic cell line DC2.4 was kindly provided by Kenneth Rock (University of Massachusetts, Worcester, USA). Cell lines were cultured in complete DMEM or RPMI medium (PAA Laboratories) and routinely tested for mycoplasma contamination by MycoAlert™ Mycoplasma Detection Kit (LONZA). For in vivo tumor models, syngeneic tumor cells were injected s.c. into the flank. Tumor growth was supervised every second day. Mice were sacrificed when tumors had reached or exceeded a size of 120 mm2. Anakinra (Kineret→) was purchased from Swedish Orphan Biovitrum (Stockholm, Sweden).
Co-culture of tumor cells and immune cells
Murine splenocytes were isolated by passing the spleen through a 40 µm cell strainer and erythrocytes were lysed with lysis buffer. Human PBMCs were obtained from healthy donors using Biocoll Separating Solution (Biochrom, Darmstadt, Germany). After centrifugation at 1,000 g for 20 min, mononuclear cells were transferred into a new tube. For cell culture experiments, 1 to 5 × 105 splenocytes or PBMC per well were transferred into a 96-well plate. For experiments with sorted human DC, 5 × 104 DC were incubated in 96-well plates. For experiments with sorted human DC, 5 × 104 DC were incubated in 96-well plates. For co-culture, 5 × 104 syngeneic tumor cells were added and cultured for 48 h at 37°C. Cell-free tumor cell supernatant was obtained after 48 h to 72 h of culture of 5 × 106 tumor cells in 25 mL medium. For culture of immune cells in tumor cell supernatant, supernatant was centrifuged at 400 g for 7 min in order to remove remaining cells. 150 µL per well of supernatant were added to 50 µL of PBMC or splenocytes (4 × 106/mL) in a 96-well plate and culture was left at 37°C for 48 h.
Elisa
Levels of CCL22, IL-1α and IL-1β in murine and human cell culture supernatants and in organ lysates were quantified by ELISA according to the manufacturer's protocol. ELISA sets were purchased from BD Biosciences and RnD Systems (CCL22). For analysis of chemokine levels in tumors, tumors were frozen in liquid nitrogen and mechanically pulverized. Organ powder was resolved in Biorad Cell Lysis Buffer, incubated and mixed for several times at room temperature and finally centrifuged at 13,000 g for 30 min. For analysis of CCL22 levels in splenocytes, cells were lysed by repeated freeze-thaw cycles and dissolved in Biorad Cell Lysis Buffer, followed by centrifugation as described above. Supernatant was collected and stored at – 80°C. Protein levels in organ lysates were quantified by Bradford assay according to manufacturer's protocol (Protein Assay Kit, Biorad, Hercules, USA).
Quantitative real-time PCR
CCL22 mRNA in murine splenocytes, lymph node single cell suspensions and human PBMC was quantified by real-time PCR. RNA from single cell suspensions was extracted using Trizol Reagent (Life Technologies, Carlsbad, USA) and RNA was quantified photometrically. Reverse transcription of RNA into cDNA was performed with the RevertAid First Strand cDNA Synthesis Kit (Fermentas, St. Leon-Rot, Germany). Each reaction contained 4 µL 5x Reaction Buffer, 1 µL 20 U/µL RNase Inhibitor, 2 µL 10 mM dNTP Mix and 1 µL 200 U/µL Reverse Transcriptase. CCL22 cDNA copies were quantified by real-time PCR and were normalized to a reference gene. Real-time PCR was performed with the LightCycler 480 (Roche) and the LightCycler 480 Probes Master. Each reaction contained 3.5 µL DEPC-treated water, 0.2 µL primer sense, 0.2 µL primer antisense, 5.0 µL Probes Master, 0.1 µL Probe and 1 µL cDNA. Primers were designed with the Roche Universal ProbeLibrary Assay Design Center and used with the according probes from the Universal ProbeLibrary Set (Roche, Basel, Switzerland).
Purification of murine dendritic cells and human dendritic cells
Murine dendritic cells and human dendritic cells were purified from whole C57BL/6 splenocytes or whole human PBMC, respectively, by magnetic-activated cell sorting (MACS) following the manufacturer's protocol. CD11c MicroBeads (Miltenyi Biotech, Bergisch Gladbach, Germany) were incubated with whole splenocytes, washed off and cells were separated in a magnetic column. Purity was detected by flow cytometry and was around 70%. For purification of human DC, freshly isolated PBMC were sorted by MACS using the Blood Dendritic Cell Isolation Kit II (Miltenyi Biotech, Bergisch Gladbach) following the manufacturer's protocol.
Treg migration assay
Treg migration assays were performed using Corning→ Transwell Plates (3.0 µm pore size). Culture supernatants of PBMC stimulated with PaTu conditioned medium or DMEM alone with or without anakinra were transferred into the lower chamber. 1 × 106 freshly isolated PBMC were resuspended in T cell medium containing 3% FCS and loaded into the upper chamber. Migration was allowed for 6 h at 37°C. After removing the transwell inserts, cells in the lower chamber were collected and stained for CD3, CD4+, CD8+, FoxP3 and analyzed by flow cytometry. Absolute numbers of migrated cells were calculated by addition of CountBright→ Absolute Counting Beads (LifeTechnologies).
Transfection of tumor cells with siRNA
1–2 × 105 PaTu-8988T cells were transfected with 25 pmol of siRNA targeting IL-1α (Origene, IL1A (ID 3552) Trilencer-27 Human siRNA) using Lipofectamine RNAiMAX following the standard protocol. 24 h and 48 h after siRNA transfection, culture medium was exchanged. After 96 h, supernatants of the transfected cells were harvested for further analysis. Functional siRNA knockdown was confirmed by IL-1α qPCR. For murine siRNA knockdown, 1–2 × 105 4T1 cells were transfected with 200 pmol of siRNA targeting IL-1α (Origene, Il1α (ID 16175) Trilencer-27 Mouse siRNA) or Il-1β (Origene, Il1β (ID 16176) Trilencer-27 Mouse siRNA,) using Lipofectamine RNAiMAX following the standard protocol. 24 h and 48 h after siRNA transfection, culture medium was exchanged. After 72 h, supernatants of the transfected cells were harvested for further analysis. Functional siRNA knockdown was confirmed by IL-1α and IL1-β qPCR.
CCL22 reporter Assay
The full promoter sequence of the CCL22 gene, which has been published previously51 was cloned in the promoterless pGL4.17 [luc2/Neo] vector (Promega, Madison,WI) and transfected into HEK293T cells using Lipofectamine 2000 following the standard protocol. After 6 h, the transfection medium was replaced by fully supplemented growth medium with or without IL-1α (100 ng/mL). After 24 h, cells were lysed and luciferase signal was determined using the Dual-Luciferase Assay System (Promega, Madison, WI). Plates were analyzed using a Berthold Orion II Microplate Luminometer.
Immunohistochemistry
Tissue sections from 101 patients with HCC and tissue microarrays (TMA) of 15 patients with PDAC were stained for CCL22 according to standard protocols. For quantification of infiltration with CCL22-positive cells, the whole tissue section surface was screened with a light microscope. Analysis of infiltration was performed in a semi-quantitative way using the following system: 0 = no infiltration; 1 = single infiltrating cells; 2 = few infiltrating cells; 3 = medium amount of infiltrating cells; 4 = high number of infiltrating cells. Liver tissue was divided into the following areas according to histomorphological aspects: tumor, peri- and intratumoral stroma, periportal field, liver epithelium. TMA of pancreatic cancers was divided into the following areas: tumor, peri- and intratumoral stroma.
Statistics
Statistical analysis was performed with GraphPad Prism Software 5.0. Chemokine and cytokine levels were analyzed with two-tailed Student's t-test. Error bars indicate SEM.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by grants from LMUexcellent research professorship (SE), from the Deutsche Forschungsgemeinschaft Graduiertenkolleg 1202 (D.A., M.R., R.T., S.H., S.E) and DFG AN 801/2–1 (D.A., SE), the Deutsche Krebshilfe 111326 (D.A., M.R.).
References
- 1.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331:1565-70; PMID:21436444; http://dx.doi.orgsol; 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 2.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M et al.. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10:942-9; PMID:15322536; http://dx.doi.orgsol; 10.1038/nm1093 [DOI] [PubMed] [Google Scholar]
- 3.Iellem A, Colantonio L, Bhakta S, Sozzani S, Mantovani A, Sinigaglia F, D'Ambrosio D. Inhibition by IL-12 and IFN-alpha of I-309 and macrophage-derived chemokine production upon TCR triggering of human Th1 cells. Eur J Immunol 2000; 30:1030-9; PMID:10760790; http://dx.doi.orgsol; [DOI] [PubMed] [Google Scholar]
- 4.Godiska R, Chantry D, Raport CJ, Sozzani S, Allavena P, Leviten D, Mantovani A, Gray PW. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med 1997; 185:1595-604; PMID:9151897; http://dx.doi.orgsol; 10.1084/jem.185.9.1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaniel C, Pardali E, Sallusto F, Speletas M, Ruedl C, Shimizu T, Seidl T, Andersson J, Melchers F, Rolink AG et al.. Activated murine B lymphocytes and dendritic cells produce a novel CC chemokine which acts selectively on activated T cells. J Exp Med 1998; 188:451-63; PMID:9687523; http://dx.doi.orgsol; 10.1084/jem.188.3.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vulcano M, Albanesi C, Stoppacciaro A, Bagnati R, D'Amico G, Struyf S, Transidico P, Bonecchi R, Del Prete A, Allavena P et al.. Dendritic cells as a major source of macrophage-derived chemokine/CCL22 in vitro and in vivo. Eur J Immunol 2001; 31:812-22; PMID:11241286; http://dx.doi.orgsol; [DOI] [PubMed] [Google Scholar]
- 7.Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D'Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med 2001; 194:847-53; PMID:11560999; http://dx.doi.orgsol; 10.1084/jem.194.6.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imai T, Chantry D, Raport CJ, Wood CL, Nishimura M, Godiska R, Yoshie O, Gray PW. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J Biol Chem 1998; 273:1764-8; PMID:9430724; http://dx.doi.orgsol; 10.1074/jbc.273.3.1764 [DOI] [PubMed] [Google Scholar]
- 9.Ghia P, Transidico P, Veiga JP, Schaniel C, Sallusto F, Matsushima K, Sallan SE, Rolink AG, Mantovani A, Nadler LM et al.. Chemoattractants MDC and TARC are secreted by malignant B-cell precursors following CD40 ligation and support the migration of leukemia-specific T cells. Blood 2001; 98:533-40; PMID:11468146; http://dx.doi.orgsol; 10.1182/blood.V98.3.533 [DOI] [PubMed] [Google Scholar]
- 10.Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D et al.. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res 2009; 69:2000-9; PMID:19244125; http://dx.doi.orgsol; 10.1158/0008-5472.CAN-08-2360 [DOI] [PubMed] [Google Scholar]
- 11.Ishida T, Ishii T, Inagaki A, Yano H, Komatsu H, Iida S, Inagaki H, Ueda R. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Res 2006; 66:5716-22; PMID:16740709; http://dx.doi.orgsol; 10.1158/0008-5472.CAN-06-0261 [DOI] [PubMed] [Google Scholar]
- 12.Mailloux AW, Clark AM, Young MR. NK depletion results in increased CCL22 secretion and Treg levels in Lewis lung carcinoma via the accumulation of CCL22-secreting CD11b+CD11c+ cells. Int J Cancer 2010; 127:2598-611; PMID:20198623; http://dx.doi.orgsol; 10.1002/ijc.25281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer 2008; 122:2286-93; PMID:18224687; http://dx.doi.orgsol; 10.1002/ijc.23392 [DOI] [PubMed] [Google Scholar]
- 14.Wagsater D, Dienus O, Lofgren S, Hugander A, Dimberg J. Quantification of the chemokines CCL17 and CCL22 in human colorectal adenocarcinomas. Mol Med Reports 2008; 1:211-7; PMID:21479399; http://dx.doi.org/ 10.3892/mmr.1.2.211 [DOI] [PubMed] [Google Scholar]
- 15.Goyne HE, Stone PJ, Burnett AF, Cannon MJ. Ovarian tumor ascites CD14+ cells suppress dendritic cell-activated CD4+ T-cell responses through IL-10 secretion and indoleamine 2,3-dioxygenase. J Immunother (Hagerstown, Md : 1997) 2014; 37:163-9; PMID:24598451; http://dx.doi.orgsol; 10.1097/CJI.0000000000000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell 2000; 101:455-8; PMID:10850488; http://dx.doi.orgsol; 10.1016/S0092-8674(00)80856-9 [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi S, Powrie F. Emerging challenges in regulatory T cell function and biology. Science 2007; 317:627-9; PMID:17673654; http://dx.doi.orgsol; 10.1126/science.1142331 [DOI] [PubMed] [Google Scholar]
- 18.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 1999; 190:995-1004; PMID:10510089; http://dx.doi.orgsol; 10.1084/jem.190.7.995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boden E, Tang Q, Bour-Jordan H, Bluestone JA. The role of CD28 and CTLA4 in the function and homeostasis of CD4+CD25+ regulatory T cells. Novartis Found Symp 2003; 252:55-63; discussion -6, 106-14; PMID:14609212; http://dx.doi.orgsol; 10.1002/0470871628.ch5 [DOI] [PubMed] [Google Scholar]
- 20.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med 2002; 196:851-7; PMID:12235217; http://dx.doi.orgsol; 10.1084/jem.20020190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fahlen L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med 2005; 201:737-46; PMID:15753207; http://dx.doi.orgsol; 10.1084/jem.20040685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS et al.. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol (Baltimore, Md : 1950) 2002; 169:2756-61; PMID:12193750; http://dx.doi.orgsol; 10.4049/jimmunol.169.5.2756 [DOI] [PubMed] [Google Scholar]
- 23.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C et al.. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 2005; 102:18538-43; PMID:16344461; http://dx.doi.orgsol; 10.1073/pnas.0509182102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer 2003; 98:1089-99; PMID:12942579; http://dx.doi.orgsol; 10.1002/cncr.11618 [DOI] [PubMed] [Google Scholar]
- 25.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 2006; 12:5423-34; PMID:17000676; http://dx.doi.orgsol; 10.1158/1078-0432.CCR-06-0369 [DOI] [PubMed] [Google Scholar]
- 26.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C et al.. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 2007; 132:2328-39; PMID:17570208; http://dx.doi.orgsol; 10.1053/j.gastro.2007.03.102 [DOI] [PubMed] [Google Scholar]
- 27.Tang Y, Xu X, Guo S, Zhang C, Tang Y, Tian Y, Ni B, Lu B, Wang H. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PloS One 2014; 9:e91551; PMID:24637664; http://dx.doi.orgsol; 10.1371/journal.pone.0091551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology (Baltimore, Md) 1998; 27:407-14; PMID:9462638; http://dx.doi.orgsol; 10.1002/hep.510270214 [DOI] [PubMed] [Google Scholar]
- 29.Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol (Baltimore, Md : 1950) 1997; 158:2723-30; PMID:9058806 [PubMed] [Google Scholar]
- 30.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454:436-44; PMID:18650914; http://dx.doi.orgsol; 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 31.Hong DS, Hui D, Bruera E, Janku F, Naing A, Falchook GS, Piha-Paul S, Wheler JJ, Fu S, Tsimberidou AM et al.. MABp1, a first-in-class true human antibody targeting interleukin-1alpha in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol 2014; 15:656-66; PMID:24746841; http://dx.doi.orgsol; 10.1016/S1470-2045(14)70155-X [DOI] [PubMed] [Google Scholar]
- 32.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 2012; 11:633-52; PMID:22850787; http://dx.doi.orgsol; 10.1038/nrd3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Bernstorff W, Voss M, Freichel S, Schmid A, Vogel I, Johnk C, Henne-Bruns D, Kremer B, Kalthoff H. Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res 2001; 7:925s-32s; PMID:11300493 [PubMed] [Google Scholar]
- 34.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res 2005; 65:2457-64; PMID:15781662; http://dx.doi.orgsol; 10.1158/0008-5472.CAN-04-3232 [DOI] [PubMed] [Google Scholar]
- 35.Yang P, Li QJ, Feng Y, Zhang Y, Markowitz GJ, Ning S, Deng Y, Zhao J, Jiang S, Yuan Y et al.. TGF-beta-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell 2012; 22:291-303; PMID:22975373; http://dx.doi.orgsol; 10.1016/j.ccr.2012.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hinz S, Pagerols-Raluy L, Oberg HH, Ammerpohl O, Grussel S, Sipos B, Grutzmann R, Pilarsky C, Ungefroren H, Saeger HD et al.. Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res 2007; 67:8344-50; PMID:17804750; http://dx.doi.orgsol; 10.1158/0008-5472.CAN-06-3304 [DOI] [PubMed] [Google Scholar]
- 37.Moo-Young TA, Larson JW, Belt BA, Tan MC, Hawkins WG, Eberlein TJ, Goedegebuure PS, Linehan DC. Tumor-derived TGF-beta mediates conversion of CD4+Foxp3+ regulatory T cells in a murine model of pancreas cancer. J Immunother (Hagerstown, Md : 1997) 2009; 32:12-21; PMID:19307989; http://dx.doi.orgsol; 10.1097/CJI.0b013e318189f13c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anz D, Eiber S, Scholz C, Endres S, Kirchner T, Bourquin C, Mayr D. In breast cancer, a high ratio of tumour-infiltrating intraepithelial CD8+ to FoxP3+ cells is characteristic for the medullary subtype. Histopathology 2011; 59:965-74; PMID:22092408; http://dx.doi.orgsol; 10.1111/j.1365-2559.2011.04040.x [DOI] [PubMed] [Google Scholar]
- 39.Faget J, Biota C, Bachelot T, Gobert M, Treilleux I, Goutagny N, Durand I, Leon-Goddard S, Blay JY, Caux C et al.. Early detection of tumor cells by innate immune cells leads to T(reg) recruitment through CCL22 production by tumor cells. Cancer Res 2011; 71:6143-52; PMID:21852386; http://dx.doi.orgsol; 10.1158/0008-5472.CAN-11-0573 [DOI] [PubMed] [Google Scholar]
- 40.Anz D, Rapp M, Eiber S, Koelzer VH, Thaler R, Haubner S, Knott M, Nagel S, Golic M, Wiedemann GM et al.. Suppression of intratumoral CCL22 by type I interferon inhibits migration of regulatory T cells and blocks cancer progression. Cancer Res 2015; 75(21):4483-93; PMID:26432403; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-3499 [DOI] [PubMed] [Google Scholar]
- 41.Berin MC, Dwinell MB, Eckmann L, Kagnoff MF. Production of MDC/CCL22 by human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 2001; 280:G1217-26; PMID:11352815 [DOI] [PubMed] [Google Scholar]
- 42.Bonecchi R, Sozzani S, Stine JT, Luini W, D'Amico G, Allavena P, Chantry D, Mantovani A. Divergent effects of interleukin-4 and interferon-gamma on macrophage-derived chemokine production: an amplification circuit of polarized T helper 2 responses. Blood 1998; 92:2668-71; PMID:9763548 [PubMed] [Google Scholar]
- 43.Xia S, Wei J, Wang J, Sun H, Zheng W, Li Y, Sun Y, Zhao H, Zhang S, Wen T et al.. A requirement of dendritic cell-derived interleukin-27 for the tumor infiltration of regulatory T cells. J Leukoc Biol 2014; 95(5):733-742;PMID:24443555 [DOI] [PubMed] [Google Scholar]
- 44.Ling J, Kang Y, Zhao R, Xia Q, Lee DF, Chang Z, Li J, Peng B, Fleming JB, Wang H et al.. KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell 2012; 21:105-20; PMID:22264792; http://dx.doi.orgsol; 10.1016/j.ccr.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singer CF, Hudelist G, Gschwantler-Kaulich D, Fink-Retter A, Mueller R, Walter I, Czerwenka K, Kubista E. Interleukin-1alpha protein secretion in breast cancer is associated with poor differentiation and estrogen receptor alpha negativity. Int J Gynecol Cancer 2006; 16(Suppl 2):556-9; PMID:17010072; http://dx.doi.orgsol; 10.1111/j.1525-1438.2006.00695.x [DOI] [PubMed] [Google Scholar]
- 46.Singer CF, Kronsteiner N, Hudelist G, Marton E, Walter I, Kubista M, Czerwenka K, Schreiber M, Seifert M, Kubista E. Interleukin 1 system and sex steroid receptor expression in human breast cancer: interleukin 1alpha protein secretion is correlated with malignant phenotype. Clin Cancer Research 2003; 9:4877-83; PMID:14581361 [PubMed] [Google Scholar]
- 47.Tomimatsu S, Ichikura T, Mochizuki H. Significant correlation between expression of interleukin-1alpha and liver metastasis in gastric carcinoma. Cancer 2001; 91:1272-6; PMID:11283926; http://dx.doi.orgsol; [DOI] [PubMed] [Google Scholar]
- 48.Watari K, Shibata T, Kawahara A, Sata K, Nabeshima H, Shinoda A, Abe H, Azuma K, Murakami Y, Izumi H et al.. Tumor-derived interleukin-1 promotes lymphangiogenesis and lymph node metastasis through M2-type macrophages. PloS One 2014; 9:e99568; PMID:24924428; http://dx.doi.orgsol; 10.1371/journal.pone.0099568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al.. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.orgsol; 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr., Lao CD et al.. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015; 16:375-84; PMID:25795410; http://dx.doi.orgsol; 10.1016/S1470-2045(15)70076-8 [DOI] [PubMed] [Google Scholar]
- 51.Nakayama T, Hieshima K, Nagakubo D, Sato E, Nakayama M, Kawa K, Yoshie O. Selective induction of Th2-attracting chemokines CCL17 and CCL22 in human B cells by latent membrane protein 1 of Epstein-Barr virus. J Virol 2004; 78:1665-74; PMID:14747532; http://dx.doi.orgsol; 10.1128/JVI.78.4.1665-1674.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


