Abstract
We have recently shown that repeated exposure of the peripheral terminal of the primary afferent nociceptor to the mu-opioid receptor (MOR) agonist DAMGO ([D-Ala2, N-Me-Phe4, Gly5-ol]-Enkephalin acetate salt) induces a model of the transition to chronic pain that we have termed Type II hyperalgesic priming. Similar to Type I hyperalgesic priming, there is a markedly prolonged response to subsequent administration of proalgesic cytokines, prototypically prostaglandin E2 (PGE2). However, Type II hyperalgesic priming differs from Type I in being rapidly induced, protein kinase A (PKA), rather than PKCε dependent, not reversed by a protein translation inhibitor, occurring in female as well as in male rats, and isolectin B4-negative neuron dependent. We report that as with the repeated injection of a MOR agonist, the repeated administration of an agonist at the A1-adenosine receptor, also a Gi-protein coupled receptor, N6-Cyclopentyladenosine (CPA), also produces priming similar to DAMGO-induced Type II hyperalgesic priming. In this study we demonstrate that priming induced by repeated exposure to this A1-adenosine receptor agonist shares the same mechanisms as MOR-agonist induced priming. However, the prolongation of PGE2 hyperalgesia induced by repeated administration of CPA depends on G-protein αi subunit activation, differently from DAMGO-induced Type II priming, in which it depends on the β/γ subunit. These data implicate a novel form of Gi-protein signaling pathway in the Type II hyperalgesic priming induced by repeated administration of an agonist at A1-adenosine receptor to the peripheral terminal of the nociceptor.
Keywords: hyperalgesic priming, hyperalgesia, A1-adenosine receptor, αi subunit, chronic pain
INTRODUCTION
We recently described a novel form of hyperalgesic priming, a model of transition to chronic pain, that we have termed Type II hyperalgesic priming [7]. The initially described form of priming, now referred to as Type I hyperalgesic priming [5; 8; 23; 24; 49], is induced in male but not female rats [33], by activation of cell surface receptors on the nociceptor that signal via the protein kinase Cε (PKCε) second messenger, or by direct activation of PKCε. Injection of prostaglandin E2 (PGE2) and other pronociceptive mediators into the peripheral receptive field of isolectin B4 (IB4)-positive primed nociceptors produces a marked, PKCε-dependent prolongation of PGE2 hyperalgesia [32], which can be permanently reversed by the administration of protein translation inhibitors to the peripheral terminal of the primed nociceptor [20].
In contrast to Type I priming, we recently reported that the repeated administration of a selective agonist for an inhibitory G-protein coupled receptor (Gi-GPCR), the mu-opioid receptor (MOR), to the peripheral terminal of the nociceptor, also induces marked prolongation of PGE2 hyperalgesia which, however, is PKA-rather than PKCε-dependent. The repeated administration of DAMGO alone also produces a decrease in nociceptive threshold, serving as a model of ‘opioid-induced hyperalgesia’ (OIH) [6; 40; 43], rather than the DAMGO-induced reversal of PGE2 hyperalgesia (anti-hyperalgesia) produced in the opioid naïve condition [1-3]. Furthermore, Type II hyperalgesic priming is not dependent on protein translation in the peripheral terminal of the nociceptor, occurs in IB4-negative nociceptors, and when produced by a MOR receptor agonist is similarly observed in female as well as in male rats [7]. In the present experiments we tested the hypothesis that the induction of Type II priming is shared by an agonist at another Gi-protein coupled receptor that is also expressed on the peripheral terminal of the nociceptor [25; 37] and in control nociceptors is anti-hyperalgesic, inhibiting PGE2 hyperalgesia [1; 3], the A1 subtype adenosine receptor.
METHODS
Animals
Experiments were performed on 230–280 g adult male and female Sprague–Dawley rats (Charles River Laboratories, Hollister, CA, USA). Experimental animals were housed in a controlled environment in the animal care facility at the University of California, San Francisco, under a 12-h light/dark cycle. Food and water were available ad libitum. The experimental protocols were approved by the Institutional Animal Care and Use Committee at UCSF and adhered to the guidelines of the American Association of Laboratory Animal Care, the National Institutes of Health (NIH), and the Committee for Research and Ethical Issues of the International Association for the Study of Pain (IASP), for the use of animals in research. In the design of experiments, all efforts were made to minimize the number of animals used and their suffering.
Mechanical nociceptive threshold testing
Mechanical nociceptive threshold was quantified using an Ugo Basile Analgesymeter® (Randall-Selitto paw-withdrawal test; Stoelting, Chicago, IL), which applies a linearly increasing mechanical force to the dorsum of the rat's hind paw, as previously described [26; 61; 62]. The nociceptive threshold was defined as the force in grams at which the animal withdrew its paw; baseline paw-pressure nociceptive mechanical threshold was defined as the mean of the 3 readings taken just before a test agent was injected. Each paw was treated as an independent measure and each experiment performed on a separate group of rats. Data are presented as mean change from baseline mechanical nociceptive threshold.
Drugs and their administration
The chemicals used in this study were: cordycepin 5’-triphosphate sodium salt (protein translation inhibitor), prostaglandin E2 (PGE2; a hyperalgesic agent that directly acts at nociceptors to sensitizes them), N6-cyclopentyl adenosine (CPA, an A1-adenosine receptor agonist); SU6656 (Src inhibitor), and pertussis toxin (Gi-protein inhibitor), all from Sigma-Aldrich (St. Louis, MO, USA); H-89 dihydrochloride (inhibitor of protein kinase A) and gallein (inhibitor of G- protein β/γ), both from Santa Cruz Biotechnology (Dallas, TX, USA); and PKCεV1–2 (PKCε-I, a PKCε-specific translocation inhibitor peptide) [28; 36], from Calbiochem (La Jolla, CA, USA).
The selection of doses used was based on previous studies that showed their effectiveness when injected intradermally on the dorsum of the rat hind paw [3; 4; 16; 19; 32; 61; 64]. The stock solution of PGE2 (1 μg/μL) was prepared in 10% ethanol, and additional dilutions made with physiological saline (0.9% NaCl), yielding a final ethanol concentration of less than 1%. CPA, cordycepin, and pertussis toxin were dissolved in saline. All other drugs were dissolved in 100% DMSO (Sigma-Aldrich) and further diluted in saline containing 2% Tween 80 (Sigma-Aldrich). The final concentration of DMSO and Tween 80 was 2%. All drugs were administered intradermally on the dorsum of the hind paw, in a volume of 5 μL, using a 30-gauge hypodermic needle adapted to a 50 μl Hamilton syringe (Reno, NV, USA). The administration of all drugs, except CPA and PGE2, was preceded by a hypotonic shock (2 μL of distilled water, separated by a bubble of air to avoid mixing in the same syringe), to facilitate the entry of compounds into the nerve terminal [10; 11].
Intrathecal administration of IB4-saporin
Isolectin B4 (IB4)-saporin, an IB4-positive nociceptor neurotoxin (Advanced Targeting Systems, San Diego, CA, USA), was diluted with saline, and a dose of 3.2 μg in a volume of 20 μL administered intrathecally, 10 days prior to priming experiments. The dose of IB4-saporin and time point were chosen based on previous reports from us and other groups [7; 30; 31; 45; 66]. Rats were briefly anesthetized with 2.5% isoflurane (Phoenix Pharmaceuticals, St. Joseph, MO, USA) in 97.5% O2. Then, a 30-gauge hypodermic needle was inserted, on the midline, into the subarachnoid space, between the L4 and L5 vertebrae. The control treatment consisted of intrathecal injection of saline (vehicle; 20 μL). Animals regained consciousness approximately 1 min after removal from the anesthetic chamber. There was no effect of IB4-saporin on the mechanical nociceptive threshold per se.
Oligodeoxynucleotide antisense to phospholipase C-beta 3 (PLC-β3) mRNA
The oligodeoxynucleotide (ODN) antisense (AS) sequence for PLC-β3 was directed against a unique region of the rat PLC-β3 mRNA sequence (GenBank Accession Number: NM_033350). The ODN AS sequence was 5’-CCTTCAAGACCTCACCCTAC-3’ and the mismatch [13] sequence (ODN MM) was designed by mismatching 8 bases (denoted by bold face) of the PLC-β3 ODN AS sequence, 5’-GGTTGAAGAGGTCACGCAAG-3’ [29]. We have previously shown that spinal intrathecal administration of ODN AS with this sequence decreases PLC-β3 protein in dorsal root ganglia [29] .
Before use, ODNs were lyophilized and reconstituted in 0.9% NaCl to a final concentration of 2 μg/μl. During each injection, rats were briefly anesthetized with isoflurane (2.5%) in O2 (97.5%). A 30-gauge hypodermic needle was then inserted, on the midline, into the subarachnoid space, between the L4 and L5 vertebrae. A total of 40 μg of ODN, in a volume of 20 μl, was then slowly injected. The intrathecal site of injection was confirmed by a sudden flick of the rat’s tail, a reflex that is evoked by subarachnoid space access and bolus injection [44]. Animals regained consciousness approximately 1 minute after completion of the injection. The use of ODN AS to manipulate the expression of proteins, important for their role in nociceptor sensitization, is well supported by previous studies by others [51; 55; 59; 60], as well as our group [8; 19; 21; 49].
CPA-induced changes in nociceptor function
We have previously shown that, while a single injection of the selective A1-adenosine receptor agonist CPA, alone has no effect on nociceptive threshold, and attenuates the mechanical hyperalgesia induced by PGE2 [41; 63], when injected repeatedly, it produces changes in nociceptor function, such as tolerance, no longer producing an anti-hyperalgesic effect, and producing mechanical hyperalgesia [1; 2]. The repeated injection of CPA, such as the repeated injection of selective mu-opioid receptor agonist DAMGO [7; 34], also induces a latent state of hyper-responsiveness to subsequent injection of pro-algesic mediators, referred to as Type II hyperalgesic priming [7]. To study the mechanism involved in the mechanical hyperalgesia produced by the repeated activation of the A1-adenosine receptor, 3 hourly intradermal injections of CPA (1 μg) were performed on the dorsum of the hind paw; mechanical hyperalgesia was observed starting after the 3rd injection of CPA (Fig. 1). To investigate the changes in nociceptor function produced by repeated injection of CPA — measured as prolonged response to a hyperalgesic mediator, at a point in time when the mechanical nociceptive threshold was not different from pre-CPA baseline levels — PGE2 (100 ng) was injected at the same site, and change in nociceptive threshold evaluated after 30 min and again at 4 h. Presence of hyperalgesia at the 4th h is characteristic of priming [5; 7; 23]. To evaluate the intracellular signaling pathways that play a role in priming induced by CPA, and to investigate the mechanisms that play a role in the induction of the changes in nociceptor function produced by the repeated activation of the A1-adenosine receptor, pharmacological agents were injected before CPA (prevention protocol). To investigate the second messengers involved in the expression of the neuroplasticity, the inhibitors were administered before the injection of PGE2 in the already primed paw (inhibition protocol), at a time when the mechanical nociceptive thresholds were not different from pre-CPA levels. And, to evaluate the role of messengers in the maintenance of the neuroplasticity, PGE2 was injected again, at a time point when the inhibitors were no longer present (reversal protocol).
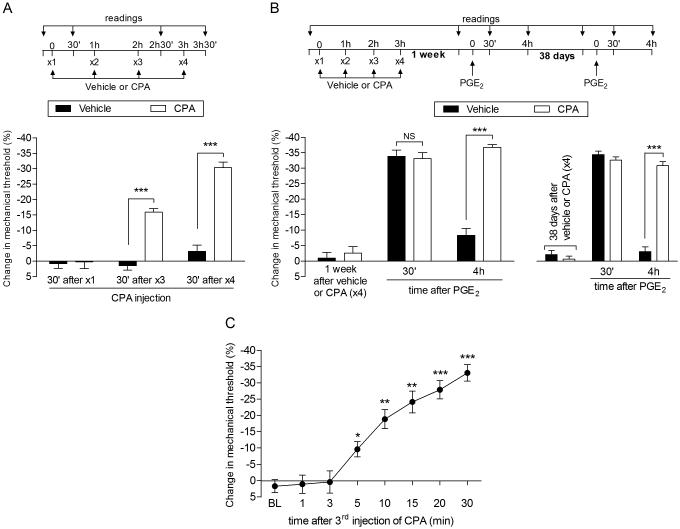
Fig. 1. Repeated exposure to CPA induces acute mechanical hyperalgesia and prolongation of PGE2 hyperalgesia in male rats.
A. Male rats received repeated (hourly x4) intradermal injections of vehicle (control, black bars) or CPA (1 µg, white bars) on the dorsum of the hind paw, and the mechanical nociceptive threshold was evaluated at the injection site 30 min after the 1st, 3rd and 4th administration, by the Randall-Sellitto paw withdrawal test. After 3 injections of CPA, significant mechanical hyperalgesia was observed, which increased in magnitude after a 4th injection (F1,20 = 77.41; ***p < 0.001, when both groups are compared; two-way repeated measures ANOVA followed by Bonferroni post hoc test), demonstrating that repeated administration of CPA produces changes in the function of the nociceptor; B (Left panel). Rats that were treated with 4 injections of vehicle (black bars) or CPA (white bars) one week before, received PGE2 (100 ng), injected at the same site. Mechanical nociceptive threshold was evaluated 30 min and 4 h later. Of note, at the time PGE2 was injected, the mechanical nociceptive thresholds were not different from pre CPA-injection control baseline (t5 = 0.3384; p = 0.7488, for the vehicle group; t5 = 1.112; p = 0.3165, for the CPA group; paired Student’s t-test). In both groups PGE2 induced significant hyperalgesia. However, while in the repeated vehicle-treated group the effect of PGE2 was no longer present at the 4th h, in the group previously treated with repeated injection of CPA (hourly x4) the hyperalgesia induced by PGE2 was still present, indicating the presence of priming (***p < 0.0001, when both groups are compared at the 4th h, two-way repeated measures ANOVA followed by Bonferroni post hoc test); B (Right panel). At 38 days after injection of the vehicle (x4) or CPA (x4) treatments [no difference in the average mechanical nociceptive thresholds from pre-treatments levels was observed: p = 0.0706 for the vehicle (t5 = 2.291) and p = 0.5717 for the CPA group (t5 = 0.6049), paired Student’s t-test], PGE2 was injected at the same site. We observed that it produced prolonged hyperalgesia in the group previously treated with CPA (x4), but not in the vehicle-treated control group (***p < 0.0001, when both groups are compared at the 4th h, two-way repeated-measures ANOVA followed by Bonferroni post hoc test), indicating that the repeated injection of CPA produced long-term plastic changes in nociceptors; C. Mechanical nociceptive threshold was evaluated 1, 3, 5, 10, 15, 20 and 30 min after a 3rd injection of CPA (1 µg) on the dorsum of the hind paw in male rats. Significant hyperalgesia was already observed 5 min after the 3rd injection (*p < 0.05, **p <0.005 and ***p < 0.0005, when compared with the baseline (BL), paired Student’s t-test). (n = 6 paws per group)
Statistics
In all experiments, the dependent variable was mechanical paw-withdrawal threshold, expressed as percentage change from baseline. The average paw withdrawal thresholds before and after the injections of CPA (2 or more days, depending on the experiment, when PGE2 was administered) were 119.9 ± 1.2 g and 120.8 ± 0.9 g, respectively; paired Student’s t-test showed no significant difference between these values (t179 = 0.8884, p = 0.3854). The total number of rats used in this study was 90 (180 paws). To compare the percentage change in the hyperalgesia induced by repeated injections of the neuroplasticity inducing agent (i.e., CPA) and to compare the effect of PGE2 in different groups, in the presence or absence of inhibitors, two-way repeated-measures ANOVA, followed by Bonferroni post hoc test, was performed. Graph Pad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA) was used to plot graphs and to perform the statistical analyses; a p-value of less than 0.05 was considered statistically significant. Data are presented as mean ± SEM.
RESULTS
A1-adenosine receptor agonist CPA-induced hyperalgesic priming
The mu-opioid receptor (MOR) is a Gi-protein coupled receptor (Gi-GPCR), whose downstream second messenger signaling pathway can undergo neuroplasticity after repeated administration of MOR agonists, such that agonists at the MOR alone now induce mechanical hyperalgesia, rather than the anti-hyperalgesia observed in the opioid naïve control animal [1-3; 34]. Since the A1-adenosine receptor, another Gi-coupled GPCR that is also located on nociceptors [1; 3; 25], mediates the prolongation of PGE2 hyperalgesia in Type I hyperalgesic priming [25], we first determined if the repeated exposure to the A1-adenosine receptor agonist, CPA, like the MOR agonist DAMGO [7; 34] induced marked prolongation of PGE2 hyperalgesia and itself induced hyperalgesia after repeated intradermal injection on the dorsum the rat’s hind paw. Similar to the effect of the repeated administration of the selective mu-opioid receptor agonist DAMGO, we found that following the repeated injection of CPA (hourly x3) it also induces hyperalgesia, with even more rapid onset than following repeated DAMGO-induced DAMGO hyperalgesia [i.e., following 3 rather than 4 hourly injections (Fig. 1A)]. When rats were subsequently injected with PGE2 (100 ng, i.d.), one week after the repeated injections of CPA, at the same site, this pronociceptive inflammatory mediator induced markedly prolonged mechanical hyperalgesia (Fig. 1B, left panel). This ability of PGE2 to produce prolonged mechanical hyperalgesia following repeated administration of CPA persisted, without attenuation, for more than 5 weeks (38 days) after the last injection of CPA (Fig. 1B, right panel).
In the setting of Type I priming, PGE2 induces the release of cAMP from the peripheral terminal of the nociceptor that is then metabolized to adenosine [25], which acts at the A1-adenosine receptor to induce mechanical hyperalgesia, with onset of ~ 5 min. The latency to onset of the hyperalgesia induced by CPA, in the CPA primed nociceptor, is also ~5 min, and it takes ~30 minutes after administration of CPA to produce peak hyperalgesia (Fig. 1C).
Nociceptor subset mediating CPA-induced Type II priming
Type I hyperalgesic priming is mediated by IB4-positive nociceptors; it is not detectable in rats that have been pre-treated with IB4-saporin, an IB4-nociceptor selective neurotoxin [32]. Type II priming, induced by the MOR agonist DAMGO is, however, not attenuated in IB4-saporin treated rats [7]. In the present study we found that, as for DAMGO-induced Type II priming, IB4-saporin pretreatment did not attenuate CPA-induced hyperalgesia. In fact, we observed an increase in the mechanical hyperalgesia after the 3rd CPA injection, when compared to the vehicle injected control group. Also, the pretreatment with IB4-saporin did not inhibit the prolongation of PGE2-induced hyperalgesia produced by repeated administration of CPA (Fig. 2A). These data support the suggestion of shared mechanisms in Type II hyperalgesic priming induced by agonists at both the MOR and A1-adenosine receptors. Thus, the similarity in the mechanisms of DAMGO- and CPA-induced Type II priming extend to the subset of nociceptors that mediate Type II priming induced by repeated administration of DAMGO or CPA (i.e., the IB4-negative nociceptor) and both differ from the subset of nociceptors that mediate Type I hyperalgesic priming, the IB4-positive population of nociceptors.
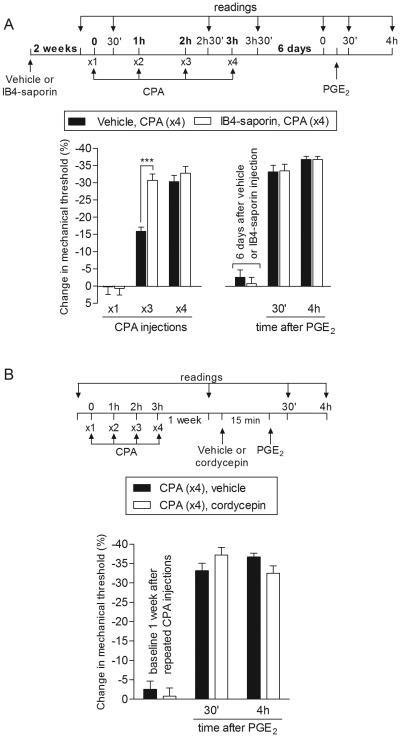
Fig. 2. Type II priming induced by repeated exposure to CPA is not dependent on IB4-positive neurons or local protein translation.
A. (Left panel). Male rats were treated with vehicle (control, black bars) or IB4-saporin (3.2 µg/20 µL; white bars), by intrathecal injection. 2 weeks later, CPA (1 µg) was injected (hourly, x4) on the dorsum of the hind paw. Significant mechanical hyperalgesia was observed in both groups after the 3rd injection of CPA. However, in the IB4-saporin-treated group the magnitude of the CPA-induced hyperalgesia was significantly higher than in the vehicle-treated group (F1,20 = 6.94; ***p < 0.001, when both groups are compared; two-way repeated-measures ANOVA followed by Bonferroni post hoc test); A. (Right panel). Six days later, when the mechanical thresholds were not different from pre-CPA baseline, (t5 = 1.419; p = 0.2150, for the control group; t5 = 0.4732; p = 0.6560, for the IB4-saporin group, paired Student’s t-test), PGE2 (100 ng) was injected intradermally at the same site on the dorsum of the hind paw, and the mechanical hyperalgesia evaluated 30 min and 4 h later. Two-way repeated-measures ANOVA followed by Bonferroni post hoc test showed PGE2-induced hyperalgesia at 30 min, that was still present at the 4th h after injection, in both groups, with no significant difference between the groups (p > 0.05, two-way repeated-measures ANOVA followed by Bonferroni post hoc test), indicating that IB4-positive nociceptors do not contribute to the prolonged hyperalgesia induced by PGE2 observed in the type II priming; B. Male rats that were treated with repeated intradermal administration of CPA (1 µg, hourly, x4) on the dorsum of the hind paw received, one week later, an injection of PGE2 (100 ng) at the same site, in the presence of vehicle (control, black bars) or the inhibitor of protein translation, cordycepin (1 µg, white bars), administered 15 min before. Mechanical nociceptive threshold was evaluated 30 min and 4 h after injection of PGE2. We observed no difference between the groups in the prolongation of the PGE2-induced hyperalgesia (F1,20 = 0.19; p = 0.6737, when both groups are compared; two-way repeated-measures ANOVA followed by Bonferroni post hoc test). (n = 6 paws per group)
Reversal of CPA-induced Type II priming
Another feature that distinguishes DAMGO-induced Type II from PKCε-induced Type I hyperalgesic priming is the ability of the peripheral administration of a protein translation inhibitor, such as cordycepin, to permanently reverse PKCε-induced, and mediated, Type I [20], but not DAMGO Type II [7] hyperalgesic priming. When rats that had received repeated injection of CPA one week before were treated with the protein translation inhibitor, cordycepin, at the site of nociceptor testing in the periphery, no difference in the prolongation of PGE2 hyperalgesia was observed, when compared to the control group (Fig. 2B), demonstrating that local protein translation does not play a role in CPA-induced Type II hyperalgesic priming.
CPA-induced priming in female rats
While Type I priming, induced by receptor agonists (e.g., TNFα, IL-6 and MCP-1) or PKCε activator, ψεRACK, occurs in male but not in gonad intact females, Type II priming, induced by DAMGO, develops in female as well as male rats [7]. Therefore, we next determined if CPA would induce priming in the female rat. The repeated injection of CPA induced hyperalgesic priming as indicated by a marked prolongation of PGE2 hyperalgesia and CPA hyperalgesia in the female rat (Fig. 3).
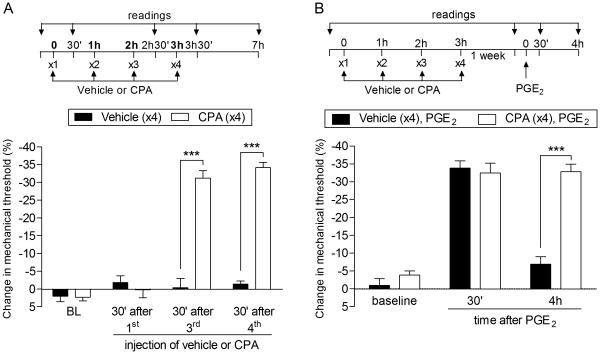
Fig. 3. Repeated exposure to CPA induces acute mechanical hyperalgesia and prolongation of PGE2 hyperalgesia in female rats.
Left panel. Female rats received repeated hourly (x4) intradermal injections of CPA (1 µg, white bars) or vehicle (black bars) on the dorsum of the hind paw. The mechanical nociceptive threshold was evaluated before and 30 min after the 1st, 3rd and 4th administration. Significant hyperalgesia was observed after the 3rd injection of CPA (F1,30 = 203.98; ***p < 0.0001, when both groups are compared, two-way repeated measures ANOVA followed by Bonferroni post hoc test); Right panel. One week later, when the mechanical thresholds were not different from pre-vehicle or pre-CPA baseline levels (t5 = 0.8155; p = 0.4519, for the vehicle group; t5 = 1.112; p = 0.3165, for the CPA group, paired Student’s t-test), PGE2 (100 ng) was injected at the same site, and the mechanical hyperalgesia evaluated 30 min and 4 h later. One way ANOVA followed by Bonferroni post hoc test showed that PGE2 induced significant hyperalgesia that was still present 4 h after its injection (F1,20 = 24.99; ***p < 0.001, when compared to the vehicle group). Taken together, these data demonstrate that, as in males, repeated injection of CPA also produces neuroplasticity in female rats. (n = 6 paws per group)
Role of PKA in the expression of A1-adenosine receptor agonist-induced Type II priming
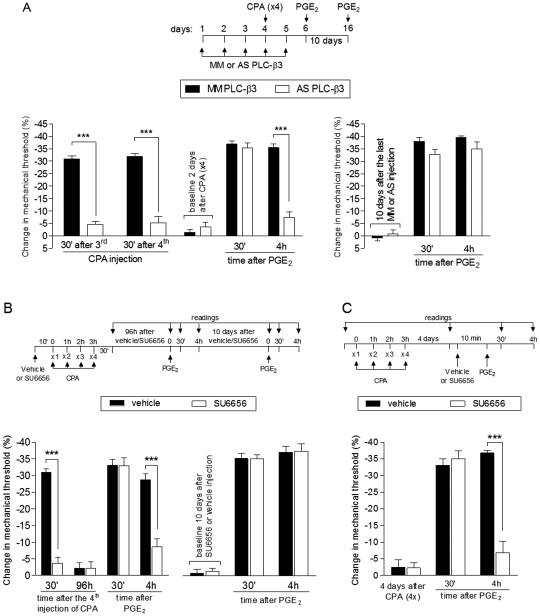
Type II and I hyperalgesic priming are also distinguished from each other by the second messenger that mediates the prolongation of PGE2 hyperalgesia, PKCε in Type I [5; 48] and PKA in DAMGO-induced Type II [7]. We now report that while in CPA-induced priming the treatment with the PKCε inhibitor PKCεV1-2 did not inhibit the prolongation of PGE2-induced hyperalgesia induced by previous repeated injection of CPA (Fig. 4A), the PKA inhibitor H-89 [7, 38] markedly attenuated both CPA-induced hyperalgesia and the prolongation of PGE2 hyperalgesia (Fig. 4B, left side). However, injection of PGE2 10 days after H-89 produced prolonged hyperalgesia (Fig. 4B, right side) demonstrating that the effect of H-89 is reversible, and that PKA is involved in the expression but not the induction or maintenance of Type II priming. Injection of PGE2 in the presence of H-89 in rats that had been primed with repeated injection of CPA 4 days prior, induced a significantly less intense hyperalgesia when compared to the control group (Fig. 4C), confirming a role of PKA in the prolongation of PGE2 hyperalgesia in Type II priming produced by repeated injection of CPA. Thus, the second messenger that mediates repeated DAMGO-induced prolongation of PGE2 hyperalgesia and opioid-induced hyperalgesia, PKA, also mediates repeated CPA-induced prolongation of PGE2 hyperalgesia and CPA-induced hyperalgesia, similarly distinguishing it from the PKCε dependence of the prolongation of PGE2 hyperalgesia in Type I priming.
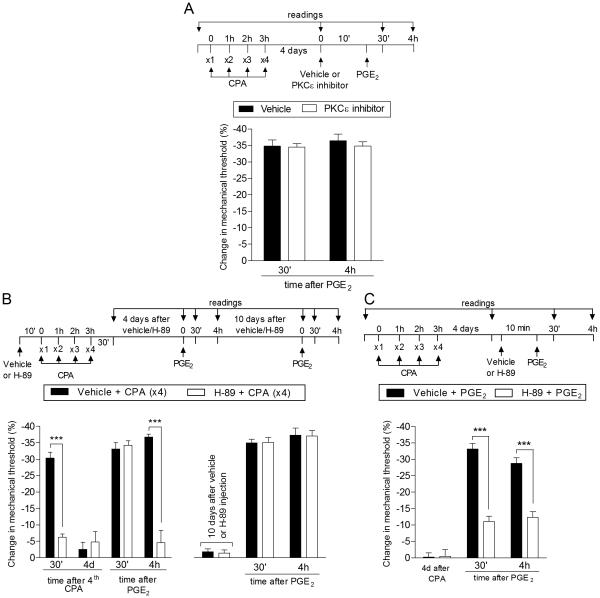
Fig. 4. PKA but not PKCε plays a role in the expression of Type II priming induced by repeated exposure to CPA.
A. Male rats that were treated with repeated intradermal administration of CPA (1 µg, hourly, x4) on the dorsum of the hind paw received, 4 days later, an injection of PGE2 (100 ng) at the same site, in the presence of vehicle (control, black bars) or the PKCε inhibitor PKCεV1-2 (1 µg, white bars), administered 10 min before. Mechanical nociceptive threshold was evaluated 30 min and 4 h after PGE2. We observed no difference between the groups in the prolongation of the PGE2-induced hyperalgesia (F1,10 = 0.27; p = 0.6167, two-way repeated-measures ANOVA followed by Bonferroni post hoc test), indicating that the prolongation of PGE2-induced hyperalgesia in Type II priming produced by CPA is not dependent on PKCε; B (Left panel). Rats were treated with intradermal injection of vehicle (control, black bars) or H-89 (1 µg, white bars) on the dorsum of the hind paw. 10 min later, 4 injections of CPA (1 µg, hourly) were performed in both groups of rats. We observed, in the group pretreated with H-89, significant attenuation of the mechanical hyperalgesia induced by the 4th injection of CPA, when compared with the control group (F1,10 = 22.76; ***p < 0.001, two-way repeated-measures ANOVA followed by Bonferroni post hoc test). Four days later, a time point when the mechanical nociceptive threshold was not different from the pre-treatment levels (t5 = 0.3384; p = 0.7488, for the control group; t5 = 0.5547; p = 0.6030, for the H-89 group; paired Student’s t-test), PGE2 (100 ng) was injected at the same site, and the mechanical hyperalgesia evaluated 30 min and 4 h later. Although the hyperalgesia induced by PGE2 was present 30 min after injection in the group previously treated with H-89, it was significantly inhibited at the 4th h (F1,20 = 35.11, ***p < 0.001, when both groups are compared at the 4th h; two-way repeated-measures ANOVA followed by Bonferroni post hoc test); B (Right panel). To determine if Type II priming was permanently prevented by H-89, PGE2 was injected again, at the same site, 10 days later. At this time we observed that the hyperalgesia induced by PGE2 was prolonged in both groups; C. Rats received repeated (hourly, x4) intradermal injection of CPA (1 µg) on the dorsum of the hind paw. Four days later, when the mechanical thresholds were not different from pre-CPA levels (t5 = 0.3953; p = 0.7089, for the control group; t5 = 0.4060; p = 0.7015, for the H-89 group; paired Student’s t-test), vehicle (control, black bars) or H-89 (1 µg, white bars) was injected at the same site, followed, 10 min later, by PGE2 (100 ng). While PGE2-induced hyperalgesia was still present 4 h later in the group that received vehicle, in the group treated with H-89 it was attenuated at both time points (F1,20 = 119.89 ***p < 0.0001, two-way repeated-measures ANOVA followed by Bonferroni post hoc test). (n = 6 paws per group)
Role of the G-protein αi subunit
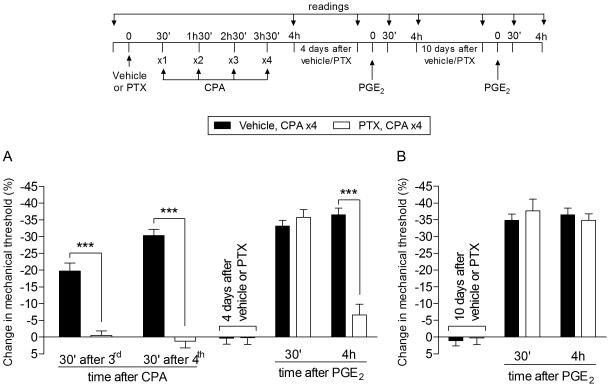
Since the A1-adenosine receptor is a Gi-protein coupled receptor (Gi-GPCR), to determine which known signaling pathways downstream of the A1-adenosine receptor contribute to the prolongation of PGE2 hyperalgesia and CPA-induced hyperalgesia induced by repeated administration of CPA, we tested the effect of the treatment with the αi inhibitor, pertussis toxin (PTX), administered before CPA. We found that both CPA hyperalgesia and the prolongation of PGE2 hyperalgesia were inhibited by PTX (Fig. 5, left side). However, this inhibition was reversible, since 10 days after PTX the injection of PGE2 at the same site produced prolonged hyperalgesia (Fig. 5, right side).
Fig. 5. Role of inhibitory G-protein αi subunit in CPA-induced Type II priming.
A (Left panel). Rats received an intradermal injection of vehicle (control, black bars) or pertussis toxin (PTX, 1 µg, white bars) on the dorsum of the hind paw. 30 min later, CPA (1 µg) was injected (hourly x4) in the same site. We observed significant inhibition of the mechanical hyperalgesia induced by the repeated injection of CPA in the group treated with PTX, compared with the vehicle group (F1,20 = 135.49; ***p < 0.0001; two-way repeated-measures ANOVA followed by Bonferroni post hoc test), indicating a role of the αi subunit in the hyperalgesia induced by repeated injection of CPA; A (Right panel). Four days later, when the mechanical thresholds were not different from the pre-CPA injections levels, rats received intradermal injection of PGE2 (100 ng) in the same site, and the mechanical hyperalgesia evaluated 30 min and 4 h later. A significant difference was observed in the mechanical hyperalgesia induced by PGE2 at 4th h after injection (F1,20 = 14.43; ***p < 0.001, when comparing both groups; two-way repeated-measures ANOVA followed by Bonferroni post hoc test), indicating that the αi subunit plays a role in the prolongation of PGE2 hyperalgesia in the type II priming; B. In order to evaluate if the reversal of Type II priming by treatment with PTX was permanent, PGE2 was again injected, in the same site, 10 days after the PTX injection. We observed that PGE2-induced mechanical hyperalgesia was still present at the 4th h, indicating the presence of Type II priming. (n = 6 paws per group)
Of note, the ability of PTX to inhibit the expression of Type II priming induced by repeated administration of an A1-adenosine receptor agonist contrasts with DAMGO induced Type II priming, where PTX failed to inhibit DAMGO hyperalgesia or the prolongation of PGE2 hyperalgesia induced by DAMGO [7]. PTX also inhibits the prolongation of PGE2-induced hyperalgesia in Type I hyperalgesic priming [16; 25; 35].
Role of the G-protein β/γ subunit
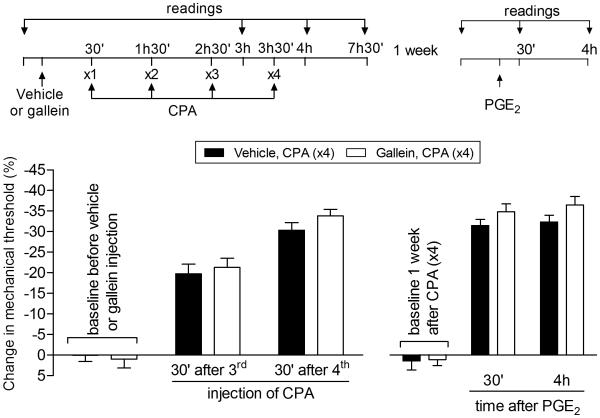
We next determined if the β/γ subunit of the Gi heterotrimeric G-protein also contributes to the prolongation of PGE2 hyperalgesia and to CPA-induced hyperalgesia produced by the repeated administration of CPA. Unlike the inhibitory effect of PTX, the β/γ subunit inhibitor, gallein, did not inhibit CPA hyperalgesia (Fig. 6, left side) or the prolongation of PGE2 hyperalgesia (Fig. 6, right side) in the setting of CPA-induced priming. Of note, the inability of gallein to inhibit the expression of Type II priming induced by repeated administration of an A1-adenosine receptor contrasts with DAMGO-induced Type II priming, where gallein does inhibit DAMGO hyperalgesia and the prolongation of PGE2 hyperalgesia [7]. The inability of gallein to inhibit the expression of Type II priming induced by repeated administration of an A1-adenosine receptor agonist also contrasts with Type I hyperalgesic priming, where prolongation of PGE2 hyperalgesia is inhibited by gallein (unpublished data).
Fig. 6. Type II priming induced by repeated exposure to CPA is not dependent on the G-protein β/γ subunit.
Rats were treated with intradermal injection of vehicle (control, black bars) or the G-protein β/γ inhibitor, gallein (1 µg, white bars), on the dorsum of the hind paw. 30 min later, repeated injections of CPA (1 µg, hourly, x4) were performed at the same site, and the mechanical nociceptive threshold evaluated 30 min after the 3rd and 4th administration. We observed significant mechanical hyperalgesia, with no significant difference, in both groups (F1,20 = 0.69, p = 0.4261, when both groups are compared, two-way repeated-measures ANOVA followed by Bonferroni post hoc test). One week later, a time point when the mechanical thresholds were not different from pre-injection levels (t5 = 1.661; p = 0.1576, for the vehicle group, and t5 = 0.7559; p = 0.4838, for the gallein group; paired Student’s t-test), rats received an intradermal injection of PGE2 (100 ng), at the same site, and the mechanical nociceptive threshold was evaluated 30 min and 4 h later. In both groups, PGE2 induced mechanical hyperalgesia was still present 4 h after injection (F1,20 = 3.78, p = 0.0808, when control and gallein groups are compared; two-way repeated-measures ANOVA followed by Bonferroni post hoc test), indicating that the inhibition of the β/γ subunit did not affect the induction of Type II priming by repeated injection of CPA. (n = 6 paws per group)
Role of PLC-β3 and Src
Two other second messengers that have been implicated in DAMGO-induced Type II hyperalgesic priming are PLC-β3 and Src [7]. To investigate if these second messengers also participate in Type II priming induced by CPA, we used intrathecal administration of ODN AS against PLC-β3 mRNA or intradermal injection of the Src inhibitor SU6656. We observed that both treatments, performed before the repeated injection of CPA, inhibited the CPA hyperalgesia and the prolongation of PGE2 hyperalgesia (Fig. 7A and 7B, left side). However, since 10 days after the last administration of ODN AS or SU6656, the injection of PGE2 produced prolonged hyperalgesia (Fig. 7A and 7B, right side), the inhibition of Type II priming was only temporary, indicating that, PLC-β3 and Src play a role in the expression of Type II priming, and not in its induction. Moreover, the injection of SU6656 in paws primed with CPA 4 days before, significantly inhibited the prolongation of PGE2-induced (Fig. 7C), also indicating a role of Src in the expression of priming.
Fig. 7. Second messengers involved in Type II priming: PLC-β3 and Src.
A (Left panel). Rats were treated with daily spinal intrathecal injections of ODN MM (black bars) or AS (white bars) for PLC-β3 mRNA, for 3 consecutive days. On the 4th day, repeated (hourly x4) intradermal injections of CPA (1 µg) on the dorsum of the hind paw were performed, and the mechanical nociceptive threshold evaluated 30 min after the 3rd and 4th CPA injection. We observed that in the AS-treated group, but not in the MM-treated group, after the 3rd and 4th injection of CPA the mechanical hyperalgesia was significantly inhibited (F1,20 = 164.20; ***p < 0.0001, when both groups are compared, two-way repeated-measures ANOVA followed by Bonferroni post hoc test), suggesting a role for PLC-β3 in Type II hyperalgesic priming. The ODN treatment continued for 2 more days, and, at a time point when the mechanical thresholds were not different from the pre-CPA levels (t5 = 0.6956; p = 0.5177, for the ODN MM group; t5 = 0.5423; p = 0.6109, for the ODN AS group; paired Student’s t-test), PGE2 (100 ng) was administered in both groups. Evaluation of the mechanical thresholds 30 min and 4 h after injection showed that, while PGE2 induced hyperalgesia that was still present 4 h later in the group treated with MM, in the group treated with AS it was significantly attenuated at the 4th h (F1,20 = 33.23; ***p < 0.001, two-way repeated-measures ANOVA followed by Bonferroni post hoc test); A (Right panel). In order to evaluate if the induction of Type II priming was prevented by the treatment with the PLC-β3 ODN AS, PGE2 was injected, in the same site, 10 days after the last ODN injection. We observed that, in both groups, PGE2 induced mechanical hyperalgesia was still present 4 h after its injection, indicating the presence of priming; B (Left panel). Rats were treated with intradermal injection of vehicle (black bars) or SU6656 (1 µg, white bars). 10 min later, repeated injections of CPA (1 µg) were performed on the dorsum of the hind paw. We observed inhibition of the mechanical hyperalgesia induced by repeated injection of CPA in the group previously treated with SU6656, when compared with vehicle group (F1,20 = 107.42; ***p < 0.0001, two-way repeated-measures ANOVA followed by Bonferroni post hoc test). Four days later, when the mechanical thresholds were not different from pre-CPA levels (t5 = 0.7559; p = 0.4838, for the control group; t5 = 0.3152; p = 0.7654, for the SU6656 group; paired Student’s t-test), PGE2 (100 ng) was injected at the same site and the mechanical hyperalgesia evaluated 30 min and 4 h later. In the group previously treated with SU6656, PGE2-induced hyperalgesia was significantly attenuated at the 4th h (F1,20 = 16.92; ***p < 0.001, when both groups are compared at the 30 min and 4th h, respectively; two-way repeated-measures ANOVA followed by Bonferroni post hoc test); B (Right panel). To determine if CPA-induced Type II priming was permanently prevented by SU6656, PGE2 was injected again, at the same site, 10 days later. At this time we observed that the hyperalgesia induced by PGE2 was prolonged in both groups; C. Male rats received repeated (hourly, x4) intradermal injections of CPA (1 µg) on the dorsum of the hind paw. Four days later, when the mechanical thresholds were not different from the pre-CPA levels (t5 = 0.1152; p = 0.9128, for the control group; t5 = 0.8305; p = 0.4441, for the SU6656 group; paired Student’s t-test), vehicle (black bars) or the Src inhibitor SU6656 (1 µg, white bars) was injected at the same site, followed by, 10 min later, PGE2 (100 ng). In the group treated with SU6656, PGE2-induced hyperalgesia was almost completely inhibited at the 4th h (F1,20 = 30.71; ***p < 0.001, two-way repeated-measures ANOVA followed by Bonferroni post hoc), indicating a role of Src in the prolongation of PGE2 hyperalgesia in CPA-induced Type II priming. (n = 6 paws per group)
DISCUSSION
The A1-adenosine, a Gi-protein coupled receptor (Gi-GPCR), is found on the peripheral terminal of the primary afferent nociceptor [1; 3; 17; 25; 63] where it exerts an anti-hyperalgesic, peripheral analgesic effect. As do most Gi-coupled receptors, the A1-adenosine receptor normally couples to the inhibition of adenylyl cyclase, attenuating intracellular cAMP levels [18; 25; 65; 67], and can reverse nociceptor sensitization and mechanical hyperalgesia induced by pronociceptive inflammatory mediators that act at stimulatory G-protein (Gs) coupled GPCRs [1; 3; 27; 39; 53; 56; 57; 68], the latter stimulate adenylyl cyclase to increase cAMP levels in the cell [37; 47; 50; 64]. Since the repeated administration of the A1-adenosine receptor agonist CPA to the peripheral receptive field of the primary afferent nociceptor produces tolerance and dependence [1; 3], similar to the effect of MOR agonists [1-3; 12; 15; 34; 46], which induce Type II hyperalgesic priming [7], a proposed model of opioid-induced hyperalgesia (OIH) [6; 40; 43], we determined if the A1-adenosine receptor agonist also produced Type II hyperalgesic priming, and whether priming-induced by repeated activation of this receptor shared mechanisms with Type II hyperalgesic priming induced by a MOR agonist.
In the present experiments we demonstrated that, similar to the effect of the repeated administration of the agonist for the Gi-protein coupled mu-opioid receptor, DAMGO, the repeated administration of an A1-adenosine receptor agonist, CPA produces a very similar, but not identical neuroplastic change in primary afferent nociceptor function. Following administration of CPA there is a change in A1-adenosine receptor signaling, such that the A1-adenosine receptor agonist comes to induce mechanical hyperalgesia, and also alters the hyperalgesia induced by PGE2, which is now markedly prolonged [5; 7; 8; 23; 24; 48]. In this regard the expression of priming induced by CPA is similar to that induced by DAMGO. Importantly, we have previously shown that agonism at the A1-adenosine receptor also contributes to the prolongation of PGE2-induced hyperalgesia in the setting of Type I hyperalgesic priming [25]. However, in contrast to Type I hyperalgesic priming, where the prolongation of PGE2 hyperalgesia induced by activation of the A1-adenosine receptor is mediated by activation of PKCε, the prolongation of PGE2 hyperalgesia in CPA-induced priming, as well as the early phase, first 30 min, are both PKA mediated [7; 25], and the time course of the onset and peak hyperalgesia markedly differ. How this may be related to differences in coupling to second messengers downstream of the A1-adenosine receptor in these two types of priming is discussed below.
The prolongation of PGE2 hyperalgesia in Type I priming is mediated by an autocrine mechanism, the release of cAMP from the peripheral terminal of the primary afferent nociceptor, its metabolism to adenosine by two ectonucleotidases (i.e., ecto-5’-phosphodiesterase and ecto-5’-nucleotidase [14; 25]), and the action of the end product of this metabolic pathway, adenosine, at the A1-adenosine receptor on the peripheral terminal of the nociceptor, to produce PKCε-dependent prolonged mechanical hyperalgesia [25]. Thus, it would appear that the coupling of the A1-adenosine receptor to hyperalgesic second messengers differs between Type I and Type II priming, coupling to PKCε in Type I hyperalgesic priming and to PKA in Type II. Determining exactly how this difference in coupling of the A1-adenosine receptor to produce PKCε-dependent hyperalgesia in Type I priming and PKA-dependent hyperalgesia in Type II priming remains to be elucidated. However, we do know that Type I and Type II priming occur in different populations of nociceptors, Type I in IB4-positive nociceptors [31] and Type II in IB4-negative nociceptors [7]. Studies of differences in A1-adenosine receptor signaling in IB4-positive and IB4-negative nociceptors will be required to elucidate how this receptor couples to different downstream second messenger signaling pathways in these two classes of nociceptors.
In the present experiments we have shown that hyperalgesia induced by the repeated administration of CPA is mediated by αi, as demonstrated by its inhibition by PTX, but not by the β/γ subunit since its inhibitor, gallein failed to attenuate CPA hyperalgesia. This contrasts with the role of the heterotrimeric G-proteins in Type I priming, where prolongation of PGE2 hyperalgesia is inhibited by PTX [16; 25; 35] and also gallein (unpublished data). Taken together with previous studies of Type I hyperalgesic priming, the present experiments support the suggestion of different roles of the subunits of the heterotrimeric Gi-protein, that is the αi and β/γ subunits, in the hyperalgesia induced by adenosine, in the setting of Type I and CPA-induced Type II priming. Thus, in Type I priming the PGE2-induced prolonged hyperalgesia is signaled via the β/γ subunit [as well as Type II priming induced by repeated exposure to DAMGO], whereas αi mediates the prolonged hyperalgesia in CPA-induced Type II priming and Type I priming. In this context it is important to note that Type I priming occurs in IB4-positive nociceptors while CPA-induced priming occurs in IB4-negative nociceptors. However, how the adenosine receptor couples to different second messengers to produce prolonged mechanical hyperalgesia, following neuroplastic changes in IB4-positive versus IB4-negative nociceptors, remains to be flushed out. In addition to their differential staining by the plant lectin IB4, which binds the V2 isoform of versican, made by IB4-positive nociceptors [9], these two populations of nociceptors also differ in neuropeptide content [42; 54] and ion channel composition [58].
Also, since we have previously shown that the Src inhibitor, SU6656, and antisense to mRNA for PLC-β3 inhibited PKA-dependent prolongation of PGE2 hyperalgesia in rats primed by the transient reduction of GRK2- [19] or DAMGO-induced Type II priming [19], we evaluated their effect on the hyperalgesia induced by injection of PGE2 in the setting of CPA-induced priming. In rats submitted to repeated injection of CPA, both SU6656 and antisense to PLC-β3 inhibited the prolongation of PGE2-induced hyperalgesia and the hyperalgesia induced by CPA.
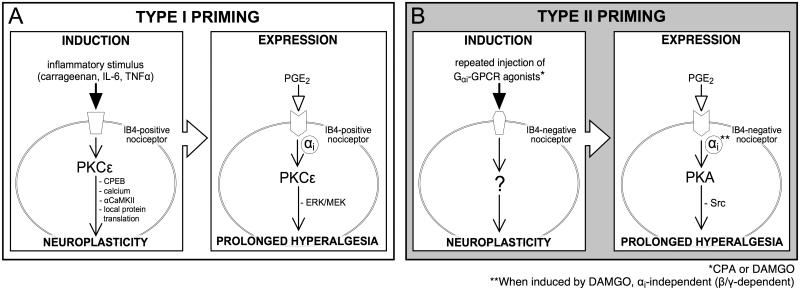
Finally, with respect to the mechanisms underlying Type I and Type II hyperalgesic priming, we have divided both forms of priming into 3 major components, induction, expression and maintenance. While for Type I priming we have elucidated mechanisms involved in each of the 3 components [20; 23; 24], to date we have only elucidated mechanisms involved in expression of Type II priming, induced by either DAMGO or CPA. Thus, attempts to prevent the induction or permanently reverse the Type II priming with the protein translator inhibitor cordycepin, which prevented and reversed Type I priming, failed to inhibit the induction of Type II priming by CPA. Additionally, the PKCε inhibitor failed to prevent Type II priming and cordycepin also failed to reverse it. The mechanisms of induction and maintenance of Type II hyperalgesic priming induced by DAMGO or CPA remain to be established. In Fig. 8 the differences in the signaling pathways that participate in Type I and Type II priming are illustrated.
Fig. 8. Schematic representation of the differences in the signaling pathways in Type I and Type II hyperalgesic priming.
As shown in “A” (left side), inflammatory stimuli, such as carrageenan, IL-6 or TNFα, applied at the terminal of the IB4-positive nociceptor, triggers the events that will lead to the development of Type I hyperalgesic priming. Activation of PKCε stimulates CPEB, release of calcium, activation of αCaMKII and local protein translation [5; 8; 20; 22; 52], ultimately producing neuroplastic changes that are expressed as prolongation of the PGE2-induced hyperalgesia (A, right side). PGE2 hyperalgesia, which is dependent only on PKA in the normal state [26], in the primed state is prolonged due to activation of an additional pathway involving Gαi-protein, PKCε and ERK/MEK [23]. In “B” (left side) the induction of Type II hyperalgesic priming by repeated activation of Gαi-protein coupled receptor in IB4-negative nociceptors is illustrated. The repeated administration of CPA (an A1-adenosine receptor agonist) or DAMGO (a mu-opioid receptor agonist)(*) [7], stimulates a yet-to-be determined pathway that produces neuroplastic changes in the nociceptor, also expressed as prolongation of PGE2-induced hyperalgesia (B, right side). However, in contrast to Type I, in Type II priming the prolongation of PGE2-induced hyperalgesia involves the G-protein subunits αi or β/γ, depending on the inducer (**), PKA and Src. Abbreviations: IL-6, Interleukin 6; TNFα, tumor necrosis factor alpha; PKCε, protein kinase C epsilon; CPEB, cytoplasmic polyadenylation element binding protein; αCaMKII, α calmodulin-dependent protein kinase II; ERK/MEK, extracellular signal-related kinase (ERK)⁄mitogen-activated protein kinase (MEK); GPCR, G-protein coupled receptor; PGE2, prostaglandin-E2; PKA, protein kinase A; Src, Src tyrosine kinase.
In summary, this study further investigates the intracellular mechanisms involved in the plastic changes in the nociceptor produced by repeated activation of an inhibitory G-protein receptor. We show that the repeated exposure to the A1-adenosine receptor agonist CPA produces hyperalgesic priming, similar to the repeated injection of DAMGO [7]. However, while in the latter the prolongation of PGE2-induced hyperalgesia depends on the G-protein β/γ subunit, in the type II priming induced by repeated administration of CPA it depends on the αi subunit.
Acknowledgements
This study was funded by a grant from the National Institutes of Health (NIH), NS084545.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
Contributions: designed and performed research, analyzed data, wrote the paper
Contributions: performed research, wrote the paper
Contributions: designed research, wrote the paper
REFERENCES
- [1].Aley KO, Green PG, Levine JD. Opioid and adenosine peripheral antinociception are subject to tolerance and withdrawal. J Neurosci. 1995;15(12):8031–8038. doi: 10.1523/JNEUROSCI.15-12-08031.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aley KO, Levine JD. Dissociation of tolerance and dependence for opioid peripheral antinociception in rats. J Neurosci. 1997;17(10):3907–3912. doi: 10.1523/JNEUROSCI.17-10-03907.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Aley KO, Levine JD. Multiple receptors involved in peripheral alpha 2, mu, and A1 antinociception, tolerance, and withdrawal. J Neurosci. 1997;17(2):735–744. doi: 10.1523/JNEUROSCI.17-02-00735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Aley KO, Martin A, McMahon T, Mok J, Levine JD, Messing RO. Nociceptor sensitization by extracellular signal-regulated kinases. J Neurosci. 2001;21(17):6933–6939. doi: 10.1523/JNEUROSCI.21-17-06933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20(12):4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104(3):570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- [7].Araldi D, Ferrari LF, Levine JD. Repeated Mu-Opioid Exposure Induces a Novel Form of the Hyperalgesic Priming Model for Transition to Chronic Pain. J Neurosci. 2015;35(36):12502–12517. doi: 10.1523/JNEUROSCI.1673-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bogen O, Alessandri-Haber N, Chu C, Gear RW, Levine JD. Generation of a pain memory in the primary afferent nociceptor triggered by PKCepsilon activation of CPEB. J Neurosci. 2012;32(6):2018–2026. doi: 10.1523/JNEUROSCI.5138-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bogen O, Bender O, Lowe J, Blenau W, Thevis B, Schroder W, Margolis RU, Levine JD, Hucho F. Neuronally produced versican V2 renders C-fiber nociceptors IB4 -positive. J Neurochem. 2015;134(1):147–155. doi: 10.1111/jnc.13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Borle AB, Snowdowne KW. Measurement of intracellular free calcium in monkey kidney cells with aequorin. Science. 1982;217(4556):252–254. doi: 10.1126/science.6806904. [DOI] [PubMed] [Google Scholar]
- [11].Burch RM, Axelrod J. Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A. 1987;84(18):6374–6378. doi: 10.1073/pnas.84.18.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen Y, Yang C, Wang ZJ. Ca2+/calmodulin-dependent protein kinase II alpha is required for the initiation and maintenance of opioid-induced hyperalgesia. J Neurosci. 2010;30(1):38–46. doi: 10.1523/JNEUROSCI.4346-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cheng X, Ma Y, Moore M, Hemmings BA, Taylor SS. Phosphorylation and activation of cAMP-dependent protein kinase by phosphoinositide-dependent protein kinase. Proc Natl Acad Sci U S A. 1998;95(17):9849–9854. doi: 10.1073/pnas.95.17.9849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chiavegatti T, Costa VL, Jr., Araujo MS, Godinho RO. Skeletal muscle expresses the extracellular cyclic AMP-adenosine pathway. Br J Pharmacol. 2008;153(6):1331–1340. doi: 10.1038/sj.bjp.0707648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008;24(6):479–496. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- [16].Dina OA, Khasar SG, Gear RW, Levine JD. Activation of Gi induces mechanical hyperalgesia poststress or inflammation. Neuroscience. 2009;160(2):501–507. doi: 10.1016/j.neuroscience.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dowd E, McQueen DS, Chessell IP, Humphrey PP. Adenosine A1 receptor-mediated excitation of nociceptive afferents innervating the normal and arthritic rat knee joint. Br J Pharmacol. 1998;125(6):1267–1271. doi: 10.1038/sj.bjp.0702185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Duarte T, Menezes-Rodrigues FS, Godinho RO. Contribution of the extracellular cAMP-adenosine pathway to dual coupling of beta2-adrenoceptors to Gs and Gi proteins in mouse skeletal muscle. Journal Pharmacol Exp Ther. 2012;341(3):820–828. doi: 10.1124/jpet.112.192997. [DOI] [PubMed] [Google Scholar]
- [19].Ferrari LF, Bogen O, Alessandri-Haber N, Levine E, Gear RW, Levine JD. Transient decrease in nociceptor GRK2 expression produces long-term enhancement in inflammatory pain. Neuroscience. 2012;222:392–403. doi: 10.1016/j.neuroscience.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ferrari LF, Bogen O, Chu C, Levine JD. Peripheral administration of translation inhibitors reverses increased hyperalgesia in a model of chronic pain in the rat. J Pain. 2013;14(7):731–738. doi: 10.1016/j.jpain.2013.01.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ferrari LF, Bogen O, Levine JD. Nociceptor subpopulations involved in hyperalgesic priming. Neuroscience. 2010;165(3):896–901. doi: 10.1016/j.neuroscience.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ferrari LF, Bogen O, Levine JD. Role of nociceptor alphaCaMKII in transition from acute to chronic pain (hyperalgesic priming) in male and female rats. J Neurosci. 2013;33(27):11002–11011. doi: 10.1523/JNEUROSCI.1785-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ferrari LF, Bogen O, Levine JD. Second messengers mediating the expression of neuroplasticity in a model of chronic pain in the rat. J Pain. 2014;15(3):312–320. doi: 10.1016/j.jpain.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ferrari LF, Bogen O, Reichling DB, Levine JD. Accounting for the delay in the transition from acute to chronic pain: axonal and nuclear mechanisms. J Neurosci. 2015;35(2):495–507. doi: 10.1523/JNEUROSCI.5147-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ferrari LF, Levine E, Levine JD. Role of a novel nociceptor autocrine mechanism in chronic pain. Eur J Neurosci. 2013;37(10):1705–1713. doi: 10.1111/ejn.12145. [DOI] [PubMed] [Google Scholar]
- [26].Ferrari LF, Levine JD. Plasma membrane mechanisms in a preclinical rat model of chronic pain. J Pain. 2015;16(1):60–66. doi: 10.1016/j.jpain.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hurt JK, Zylka MJ. PAPupuncture has localized and long-lasting antinociceptive effects in mouse models of acute and chronic pain. Mol Pain. 2012;8:28. doi: 10.1186/1744-8069-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Johnson JA, Gray MO, Chen CH, Mochly-Rosen D. A protein kinase C translocation inhibitor as an isozyme-selective antagonist of cardiac function. J Biol Chem. 1996;271(40):24962–24966. doi: 10.1074/jbc.271.40.24962. [DOI] [PubMed] [Google Scholar]
- [29].Joseph EK, Bogen O, Alessandri-Haber N, Levine JD. PLC-beta 3 signals upstream of PKC epsilon in acute and chronic inflammatory hyperalgesia. Pain. 2007;132(1-2):67–73. doi: 10.1016/j.pain.2007.01.027. [DOI] [PubMed] [Google Scholar]
- [30].Joseph EK, Chen X, Bogen O, Levine JD. Oxaliplatin acts on IB4-positive nociceptors to induce an oxidative stress-dependent acute painful peripheral neuropathy. J Pain. 2008;9(5):463–472. doi: 10.1016/j.jpain.2008.01.335. [DOI] [PubMed] [Google Scholar]
- [31].Joseph EK, Levine JD. Hyperalgesic priming is restricted to isolectin B4-positive nociceptors. Neuroscience. 2010;169(1):431–435. doi: 10.1016/j.neuroscience.2010.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Joseph EK, Levine JD. Multiple PKCepsilon-dependent mechanisms mediating mechanical hyperalgesia. Pain. 2010;150(1):17–21. doi: 10.1016/j.pain.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Joseph EK, Parada CA, Levine JD. Hyperalgesic priming in the rat demonstrates marked sexual dimorphism. Pain. 2003;105(1-2):143–150. doi: 10.1016/s0304-3959(03)00175-1. [DOI] [PubMed] [Google Scholar]
- [34].Joseph EK, Reichling DB, Levine JD. Shared mechanisms for opioid tolerance and a transition to chronic pain. J Neurosci. 2010;30(13):4660–4666. doi: 10.1523/JNEUROSCI.5530-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28(22):5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24(1):253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Khasar SG, Wang JF, Taiwo YO, Heller PH, Green PG, Levine JD. Mu-opioid agonist enhancement of prostaglandin-induced hyperalgesia in the rat: a G-protein beta gamma subunit-mediated effect? Neuroscience. 1995;67(1):189–195. doi: 10.1016/0306-4522(94)00632-f. [DOI] [PubMed] [Google Scholar]
- [38].Konopka KH, van Wijhe M. Opioid-induced hyperalgesia: pain hurts? Br J Anaesth. 2010;105(5):555–557. doi: 10.1093/bja/aeq286. [DOI] [PubMed] [Google Scholar]
- [39].Korboukh I, Hull-Ryde EA, Rittiner JE, Randhawa AS, Coleman J, Fitzpatrick BJ, Setola V, Janzen WP, Frye SV, Zylka MJ, Jin J. Orally active adenosine A(1) receptor agonists with antinociceptive effects in mice. J Med Chem. 2012;55(14):6467–6477. doi: 10.1021/jm3004834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14(2):145–161. [PubMed] [Google Scholar]
- [41].Levine JD, Taiwo YO. Involvement of the mu-opiate receptor in peripheral analgesia. Neuroscience. 1989;32(3):571–575. doi: 10.1016/0306-4522(89)90279-0. [DOI] [PubMed] [Google Scholar]
- [42].Mantyh PW, Hunt SP. Hot peppers and pain. Neuron. 1998;21(4):644–645. doi: 10.1016/s0896-6273(00)80575-9. [DOI] [PubMed] [Google Scholar]
- [43].Mao J. Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain. 2002;100(3):213–217. doi: 10.1016/S0304-3959(02)00422-0. [DOI] [PubMed] [Google Scholar]
- [44].Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 1994;32(4):197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- [45].Nishiguchi J, Sasaki K, Seki S, Chancellor MB, Erickson KA, de Groat WC, Kumon H, Yoshimura N. Effects of isolectin B4-conjugated saporin, a targeting cytotoxin, on bladder overactivity induced by bladder irritation. Eur J Neurosci. 2004;20(2):474–482. doi: 10.1111/j.1460-9568.2004.03508.x. [DOI] [PubMed] [Google Scholar]
- [46].Ossipov MH, Lai J, King T, Vanderah TW, Porreca F. Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers. 2005;80(2-3):319–324. doi: 10.1002/bip.20254. [DOI] [PubMed] [Google Scholar]
- [47].Parada CA, Reichling DB, Levine JD. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCepsilon second messenger pathways. Pain. 2005;113(1-2):185–190. doi: 10.1016/j.pain.2004.10.021. [DOI] [PubMed] [Google Scholar]
- [48].Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci. 2003;17(9):1847–1852. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- [49].Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience. 2003;120(1):219–226. doi: 10.1016/s0306-4522(03)00267-7. [DOI] [PubMed] [Google Scholar]
- [50].Pierre S, Eschenhagen T, Geisslinger G, Scholich K. Capturing adenylyl cyclases as potential drug targets. Nat Rev Drug Discov. 2009;8(4):321–335. doi: 10.1038/nrd2827. [DOI] [PubMed] [Google Scholar]
- [51].Quanhong Z, Ying X, Moxi C, Tao X, Jing W, Xin Z, Li W, Derong C, Xiaoli Z, Wei J. Intrathecal PLC(beta3) oligodeoxynucleotides antisense potentiates acute morphine efficacy and attenuates chronic morphine tolerance. Brain Res. 2012;1472:38–44. doi: 10.1016/j.brainres.2012.06.030. [DOI] [PubMed] [Google Scholar]
- [52].Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32(12):611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rittiner JE, Korboukh I, Hull-Ryde EA, Jin J, Janzen WP, Frye SV, Zylka MJ. AMP is an adenosine A1 receptor agonist. J Biol Chem. 2012;287(8):5301–5309. doi: 10.1074/jbc.M111.291666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20(4):629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- [55].Song MJ, Wang YQ, Wu GC. Additive anti-hyperalgesia of electroacupuncture and intrathecal antisense oligodeoxynucleotide to interleukin-1 receptor type I on carrageenan-induced inflammatory pain in rats. Brain Res Bull. 2009;78(6):335–341. doi: 10.1016/j.brainresbull.2008.10.009. [DOI] [PubMed] [Google Scholar]
- [56].Sowa NA, Taylor-Blake B, Zylka MJ. Ecto-5'-nucleotidase (CD73) inhibits nociception by hydrolyzing AMP to adenosine in nociceptive circuits. J Neurosci. 2010;30(6):2235–2244. doi: 10.1523/JNEUROSCI.5324-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Street SE, Walsh PL, Sowa NA, Taylor-Blake B, Guillot TS, Vihko P, Wightman RM, Zylka MJ. PAP and NT5E inhibit nociceptive neurotransmission by rapidly hydrolyzing nucleotides to adenosine. Mol Pain. 2011;7:80. doi: 10.1186/1744-8069-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Stucky CL, Lewin GR. Isolectin B(4)-positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;19(15):6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Su L, Wang C, Yu YH, Ren YY, Xie KL, Wang GL. Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci. 2011;12:120. doi: 10.1186/1471-2202-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sun JL, Xiao C, Lu B, Zhang J, Yuan XZ, Chen W, Yu LN, Zhang FJ, Chen G, Yan M. CX3CL1/CX3CR1 regulates nerve injury-induced pain hypersensitivity through the ERK5 signaling pathway. J Neurosci Res. 2013;91(4):545–553. doi: 10.1002/jnr.23168. [DOI] [PubMed] [Google Scholar]
- [61].Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience. 1989;32(3):577–580. doi: 10.1016/0306-4522(89)90280-7. [DOI] [PubMed] [Google Scholar]
- [62].Taiwo YO, Levine JD. Prostaglandin effects after elimination of indirect hyperalgesic mechanisms in the skin of the rat. Brain Res. 1989;492(1-2):397–399. doi: 10.1016/0006-8993(89)90928-1. [DOI] [PubMed] [Google Scholar]
- [63].Taiwo YO, Levine JD. Direct cutaneous hyperalgesia induced by adenosine. Neuroscience. 1990;38(3):757–762. doi: 10.1016/0306-4522(90)90068-f. [DOI] [PubMed] [Google Scholar]
- [64].Taiwo YO, Levine JD. Further confirmation of the role of adenyl cyclase and of cAMP-dependent protein kinase in primary afferent hyperalgesia. Neuroscience. 1991;44(1):131–135. doi: 10.1016/0306-4522(91)90255-m. [DOI] [PubMed] [Google Scholar]
- [65].van Calker D, Muller M, Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]
- [66].Vulchanova L, Olson TH, Stone LS, Riedl MS, Elde R, Honda CN. Cytotoxic targeting of isolectin IB4-binding sensory neurons. Neuroscience. 2001;108(1):143–155. doi: 10.1016/s0306-4522(01)00377-3. [DOI] [PubMed] [Google Scholar]
- [67].Zahn PK, Straub H, Wenk M, Pogatzki-Zahn EM. Adenosine A1 but not A2a receptor agonist reduces hyperalgesia caused by a surgical incision in rats: a pertussis toxin-sensitive G protein-dependent process. Anesthesiology. 2007;107(5):797–806. doi: 10.1097/01.anes.0000286982.36342.3f. [DOI] [PubMed] [Google Scholar]
- [68].Zylka MJ. Pain-relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol Med. 2011;17(4):188–196. doi: 10.1016/j.molmed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]