Abstract
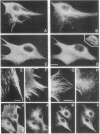
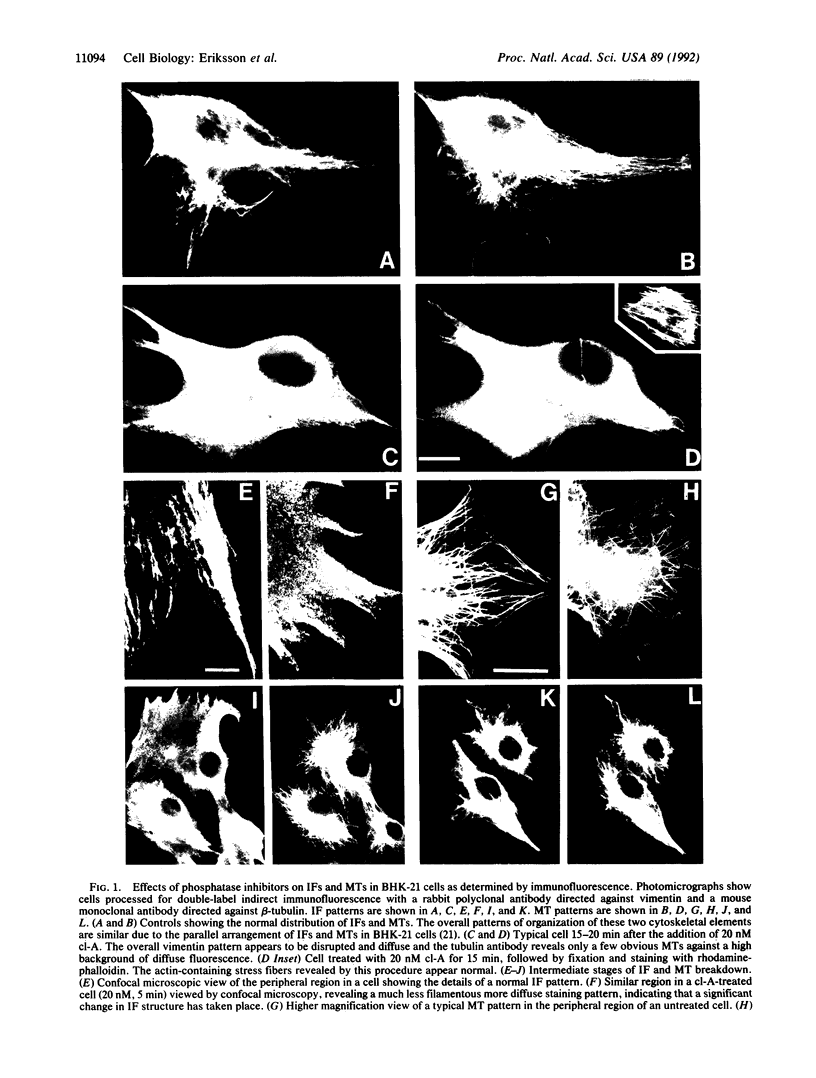
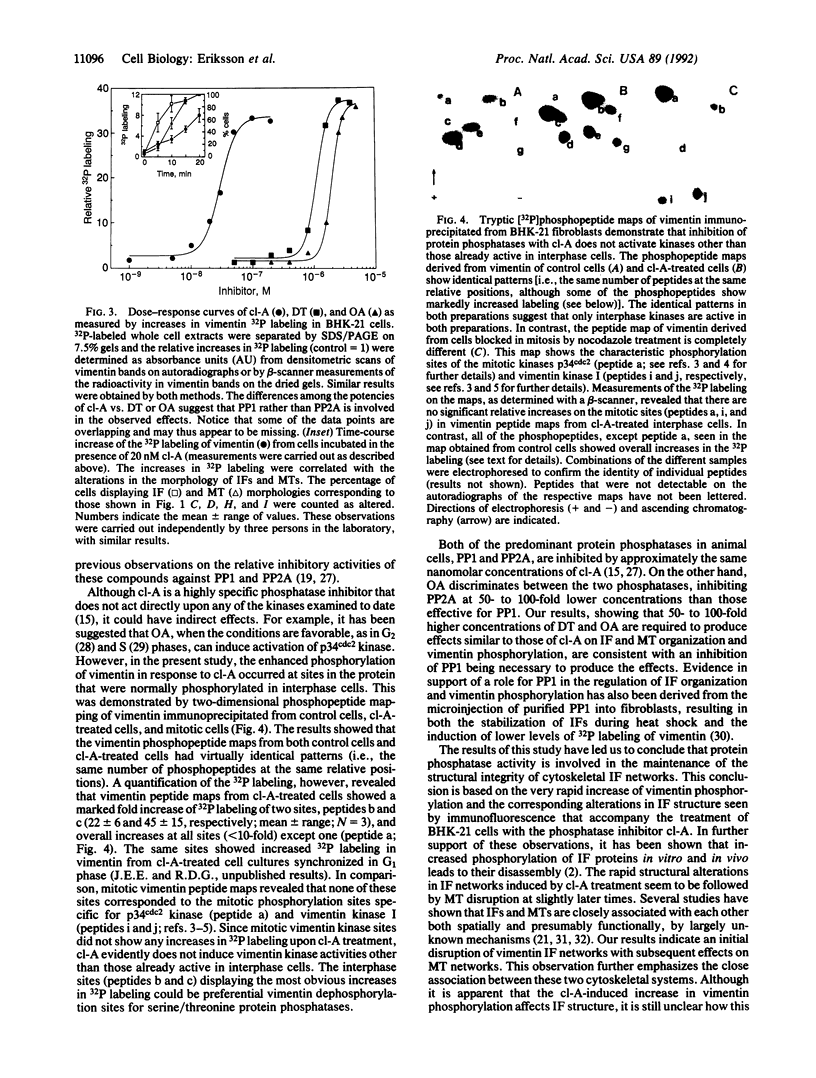
Phosphorylation by protein kinases has been established as a key factor in the regulation of cytoskeletal structure. However, little is known about the role of protein phosphatases in cytoskeletal regulation. To assess the possible functions of protein phosphatases in this respect, we studied the effects of the phosphatase inhibitors calyculin A, okadaic acid, and dinophysistoxin 1 (35-methylokadaic acid) on BHK-21 fibroblasts. Within minutes of incubation with these inhibitors, changes are seen in the structural organization of intermediate filaments, followed by a loss of microtubules, as assayed by immunofluorescence. These changes in cytoskeletal structure are accompanied by a rapid and selective increase in vimentin phosphorylation on interphase-specific sites, and they are fully reversible after removal of calyculin A. The results indicate that there is a rapid phosphate turnover on cytoskeletal intermediate filaments and further suggest that protein phosphatases are essential for the maintenance and structural integrity of two major cytoskeletal components.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bershadsky A. D., Gelfand V. I. ATP-dependent regulation of cytoplasmic microtubule disassembly. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3610–3613. doi: 10.1073/pnas.78.6.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. S., Luca F. C., Vallee R. B. Microtubule-associated protein 1B: identification of a major component of the neuronal cytoskeleton. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5404–5408. doi: 10.1073/pnas.82.16.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. S., Schoenfeld T. A., Vallee R. B. Widespread distribution of the major polypeptide component of MAP 1 (microtubule-associated protein 1) in the nervous system. J Cell Biol. 1984 Jan;98(1):320–330. doi: 10.1083/jcb.98.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blose S. H., Meltzer D. I., Feramisco J. R. 10-nm filaments are induced to collapse in living cells microinjected with monoclonal and polyclonal antibodies against tubulin. J Cell Biol. 1984 Mar;98(3):847–858. doi: 10.1083/jcb.98.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier L., Rankin L. L., Allen R. E., Kato Y., Fusetani N., Karaki H., Watabe S., Hartshorne D. J. Calyculin-A increases the level of protein phosphorylation and changes the shape of 3T3 fibroblasts. Cell Motil Cytoskeleton. 1991;18(1):26–40. doi: 10.1002/cm.970180104. [DOI] [PubMed] [Google Scholar]
- Chou Y. H., Bischoff J. R., Beach D., Goldman R. D. Intermediate filament reorganization during mitosis is mediated by p34cdc2 phosphorylation of vimentin. Cell. 1990 Sep 21;62(6):1063–1071. doi: 10.1016/0092-8674(90)90384-q. [DOI] [PubMed] [Google Scholar]
- Chou Y. H., Ngai K. L., Goldman R. The regulation of intermediate filament reorganization in mitosis. p34cdc2 phosphorylates vimentin at a unique N-terminal site. J Biol Chem. 1991 Apr 25;266(12):7325–7328. [PubMed] [Google Scholar]
- Chou Y. H., Rosevear E., Goldman R. D. Phosphorylation and disassembly of intermediate filaments in mitotic cells. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1885–1889. doi: 10.1073/pnas.86.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Eriksson J. E., Opal P., Goldman R. D. Intermediate filament dynamics. Curr Opin Cell Biol. 1992 Feb;4(1):99–104. doi: 10.1016/0955-0674(92)90065-k. [DOI] [PubMed] [Google Scholar]
- Eriksson J. E., Paatero G. I., Meriluoto J. A., Codd G. A., Kass G. E., Nicotera P., Orrenius S. Rapid microfilament reorganization induced in isolated rat hepatocytes by microcystin-LR, a cyclic peptide toxin. Exp Cell Res. 1989 Nov;185(1):86–100. doi: 10.1016/0014-4827(89)90039-6. [DOI] [PubMed] [Google Scholar]
- Eriksson J. E., Toivola D., Meriluoto J. A., Karaki H., Han Y. G., Hartshorne D. Hepatocyte deformation induced by cyanobacterial toxins reflects inhibition of protein phosphatases. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1347–1353. doi: 10.1016/s0006-291x(05)80936-2. [DOI] [PubMed] [Google Scholar]
- Fernandez A., Brautigan D. L., Lamb N. J. Protein phosphatase type 1 in mammalian cell mitosis: chromosomal localization and involvement in mitotic exit. J Cell Biol. 1992 Mar;116(6):1421–1430. doi: 10.1083/jcb.116.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A., Brautigan D. L., Mumby M., Lamb N. J. Protein phosphatase type-1, not type-2A, modulates actin microfilament integrity and myosin light chain phosphorylation in living nonmuscle cells. J Cell Biol. 1990 Jul;111(1):103–112. doi: 10.1083/jcb.111.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki H., Suganuma M., Suguri H., Yoshizawa S., Takagi K., Uda N., Wakamatsu K., Yamada K., Murata M., Yasumoto T. Diarrhetic shellfish toxin, dinophysistoxin-1, is a potent tumor promoter on mouse skin. Jpn J Cancer Res. 1988 Oct;79(10):1089–1093. doi: 10.1111/j.1349-7006.1988.tb01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen G. G., Kalnoski M. H., Bulinski J. C. Distinct populations of microtubules: tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell. 1984 Oct;38(3):779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- Gyoeva F. K., Gelfand V. I. Coalignment of vimentin intermediate filaments with microtubules depends on kinesin. Nature. 1991 Oct 3;353(6343):445–448. doi: 10.1038/353445a0. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Martin B. L., Brautigan D. L., Karaki H., Ozaki H., Kato Y., Fusetani N., Watabe S., Hashimoto K., Uemura D. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989 Mar 31;159(3):871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller R. K., Vikstrom K., Goldman R. D. Keratin incorporation into intermediate filament networks is a rapid process. J Cell Biol. 1991 May;113(4):843–855. doi: 10.1083/jcb.113.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J. B. Non-motor microtubule-associated proteins. Curr Opin Cell Biol. 1991 Feb;3(1):52–58. doi: 10.1016/0955-0674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- Parysek L. M., Asnes C. F., Olmsted J. B. MAP 4: occurrence in mouse tissues. J Cell Biol. 1984 Oct;99(4 Pt 1):1309–1315. doi: 10.1083/jcb.99.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M., Nakagawa J., Dorée M., Labbé J. C., Nigg E. A. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990 May 18;61(4):591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- Picard A., Labbé J. C., Barakat H., Cavadore J. C., Dorée M. Okadaic acid mimics a nuclear component required for cyclin B-cdc2 kinase microinjection to drive starfish oocytes into M phase. J Cell Biol. 1991 Oct;115(2):337–344. doi: 10.1083/jcb.115.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers J. R. Regulation of cytoplasmic and smooth muscle myosin. Curr Opin Cell Biol. 1991 Feb;3(1):98–104. doi: 10.1016/0955-0674(91)90171-t. [DOI] [PubMed] [Google Scholar]
- Skalli O., Goldman R. D. Recent insights into the assembly, dynamics, and function of intermediate filament networks. Cell Motil Cytoskeleton. 1991;19(2):67–79. doi: 10.1002/cm.970190202. [DOI] [PubMed] [Google Scholar]
- Starger J. M., Brown W. E., Goldman A. E., Goldman R. D. Biochemical and immunological analysis of rapidly purified 10-nm filaments from baby hamster kidney (BHK-21) cells. J Cell Biol. 1978 Jul;78(1):93–109. doi: 10.1083/jcb.78.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf W. E., Vallee R. B. Molecular characterization of the cAMP-dependent protein kinase bound to microtubule-associated protein 2. J Biol Chem. 1982 Mar 25;257(6):3284–3290. [PubMed] [Google Scholar]
- Vale R. D. Severing of stable microtubules by a mitotically activated protein in Xenopus egg extracts. Cell. 1991 Feb 22;64(4):827–839. doi: 10.1016/0092-8674(91)90511-v. [DOI] [PubMed] [Google Scholar]
- Vandré D. D., Wills V. L. Inhibition of mitosis by okadaic acid: possible involvement of a protein phosphatase 2A in the transition from metaphase to anaphase. J Cell Sci. 1992 Jan;101(Pt 1):79–91. doi: 10.1242/jcs.101.1.79. [DOI] [PubMed] [Google Scholar]
- Vikstrom K. L., Lim S. S., Goldman R. D., Borisy G. G. Steady state dynamics of intermediate filament networks. J Cell Biol. 1992 Jul;118(1):121–129. doi: 10.1083/jcb.118.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Goldman R. D. Functions of cytoplasmic fibers in intracellular movements in BHK-21 cells. J Cell Biol. 1978 Dec;79(3):708–726. doi: 10.1083/jcb.79.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S., Matsumura F. Mitosis-specific phosphorylation of caldesmon: possible molecular mechanism of cell rounding during mitosis. Bioessays. 1991 Nov;13(11):563–568. doi: 10.1002/bies.950131103. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Yasuda H., Pines J., Yasumoto K., Nishitani H., Ohtsubo M., Hunter T., Sugimura T., Nishimoto T. Okadaic acid, a potent inhibitor of type 1 and type 2A protein phosphatases, activates cdc2/H1 kinase and transiently induces a premature mitosis-like state in BHK21 cells. EMBO J. 1990 Dec;9(13):4331–4338. doi: 10.1002/j.1460-2075.1990.tb07882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. Y., Lieska N., Goldman A. E., Goldman R. D. A 300,000-mol-wt intermediate filament-associated protein in baby hamster kidney (BHK-21) cells. J Cell Biol. 1985 Feb;100(2):620–631. doi: 10.1083/jcb.100.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunami J., Fujiki H., Suganuma M., Yoshizawa S., Eriksson J. E., Olson M. O., Goldman R. D. Vimentin is hyperphosphorylated in primary human fibroblasts treated with okadaic acid. Biochem Biophys Res Commun. 1991 Jun 28;177(3):1165–1170. doi: 10.1016/0006-291x(91)90662-q. [DOI] [PubMed] [Google Scholar]