Abstract
Many laying hen producers are transitioning from conventional cages to new housing systems including multi-tier aviaries. Aviary resources, such as litter areas, are intended to encourage hens’ expression of natural behaviors to improve their welfare. Little research has examined the influence of laying hen strain on distribution and behavior inside aviaries, yet differences could influence a strain's suitability for an aviary design. This research examined how laying hens of 4 strains (Hy-Line Brown [HB], Bovans Brown [BB], DeKalb White [DW], and Hy-Line W36) distributed themselves among 3 enclosed aviary tiers and 2 litter areas at peak lay (25 to 28 wk of age) and after gaining access to litter on the floor (26 wk). Observations of hens’ spatial distribution were conducted immediately before and after, and 3 wk after hens gained access to litter. More HB and BB hens were in upper tiers in morning compared to DW and W36 (all P ≤ 0.05). However, DW and W36 hens roosted in upper tiers in larger numbers than HB and BB during evening (all P ≤ 0.05). More DW and W36 hens were on litter compared to BB and HB, particularly when litter was first accessible (all P ≤ 0.05). The number of hens on litter increased over time for all strains (P ≤ 0.06). White hens on litter occupied open areas in higher numbers (P ≤ 0.05), while more brown hens occupied litter under the aviary after acclimation (P ≤ 0.05). In the dark period, W36 and DW hens were present in higher numbers in upper tiers than HB and BB, while HB and BB showed higher tier-to-tier movement than DW and W36 (P ≤ 0.05). In general, more white hens roosted higher at night and explored litter sooner, while more brown hens were near or in nests in the morning and moved at night. Distinct strain differences indicate that attention should be paid to the match between configuration of the aviary design and strain of laying hen.
Keywords: aviary, laying hen, strain, spatial distribution, litter
INTRODUCTION
The distribution of laying hens within aviary housing environments is influenced by the behavior of the flock as well as by the available physical space and distribution of resources (e.g., the placement of perches, litter areas, nest boxes, solid ledges, feed, and water; Appleby and Hughes, 1991; Hansen, 1994; Abrahamsson et al., 1996; Lentfer et al., 2013). Certain management practices, such as giving hens access to floor litter areas after target levels of egg production are reached, may disrupt behavioral patterns as the birds adapt to the new conditions. Finally, it is also likely that different genetic strains of laying hens use space and resources in different ways (Klein et al., 2000; Schütz et al., 2001). These physical, management, and bird-based factors interact to create complex patterns of bird distribution in space and resource use over time.
Specific laying hen strains have been molded by a variety of selection pressures beyond higher egg production to include traits such as feed efficiency, egg quality, longevity, and behavior (Besbes et al., 2002; Wolc et al., 2012). As a result, the behavior of modern laying hens in general, as well as among strains, has been modified from that of the ancestral jungle fowl since their domestication approximately 10,000 years ago (Crawford, 1990). Anecdotal reports from producers and scientists studying laying hen behavior suggest substantial differences exist among strains in behavior and preferences, such as brown strains not using perches to the same degree that white strains do. However, there is limited scientific information on behavioral variability among the various strains of laying hens. For example, Schütz and Jensen (2001) observed that white Leghorns, selected primarily for egg production, were less social and performed less intensive foraging behavior than the wild, undomesticated ancestral red jungle fowl or Swedish bantams, a domesticated strain not selected for production traits. Further, Schütz and colleagues (2001) showed these Leghorns allocated more resources to egg production partly by reducing the frequency at which they performed energetically demanding behaviors such as exploration and foraging. Klein and colleagues (2000) noted differences in foraging behavior between Lohmann-selected Leghorns and DeKalb chicks, and Braastad and Katle (1989) concluded that layer strains selected for high feed conversion efficiency were less active, showed less foraging behavior, and were less aggressive compared to birds of low feed conversion efficiency. Ultimately, these behavioral differences among strains will influence hens’ use of space and resources in aviary systems, potentially making some strains better suited to certain aviary system designs than others.
Aviary housing systems are specifically designed to attempt to allow hens to fulfill their natural behavioral needs to nest, perch, forage, explore, and dust bathe by providing the birds with resources they have high motivation to use (as reviewed by Cooper and Albentosa (2003)). Aviaries also provide hens with more space for movement compared to conventional cages. The expectation is that provision of additional space and resources will improve hen welfare as compared to that of hens housed in conventional cages. However, both structural and bird-based factors may impair hens’ ability to use resources as intended. Birds’ freedom of movement may be restricted by the infrastructure of the housing environment, such as mesh walls between units or limited points of entry between litter and tiers. Such barrier effects hinder movement of individual birds between locations (Newberry and Hall, 1990; Estevez et al., 2005). Hindered movement, in turn, impairs inter-individual spacing (Estevez et al., 2007; Collins, 2008), which may lead to bird aggregation and possibly crowding. In addition, bird-based factors such as social facilitation (when performance by one hen of a certain behavior, such as dust bathing, encourages other hens to perform the behavior) (Webster and Hurnik, 1994; Duncan et al., 1998; Collins and Sumpter, 2007) and the hens’ internal biological rhythm can affect their distribution in the environment, leading to flock synchrony.
Internal diurnal rhythms, for example, dictate that hens will aggregate near and inside nests in the morning as they perform pre-lay behavior and oviposition. Insufficient space for simultaneous access to and use of nests by all hens may result in litter or system-laid eggs by individuals unable to access nests at this time. Furthermore, previous research has shown that more birds use the litter area in the afternoon than at other times of d (Channing et al., 2001; Campbell et al., 2016a,b), corresponding with hens’ internally driven preference to dust bathe in the afternoon (Vestergaard, 1982; Carmichael et al., 1999). Litter-area overcrowding at peak use can restrict hen movement (Carmichael et al., 1999) and might prevent some hens from performing dust bathing effectively (Odén et al., 2002). Finally, hens preferentially roost on higher perches at night (Schrader and Müller, 2009; Brendler et al., 2014; Campbell et al., 2016c), simulating the roosting of ancestral jungle fowl high in trees to avoid predators (Wood-Gush and Duncan, 1976; Wood-Gush et al., 1978); thus, hens perching lower in the system due to space limitations may be frustrated (Olsson and Keeling, 2002). In summary, diurnal rhythms of hens indicate times when high demand may potentially be placed on specific aviary resources. Subsequently, some individual hens may not be able to exhibit natural behavior or perform behavior in preferred ways if the target resource cannot accommodate the entire flock simultaneously.
Together, barrier effects, social aggregation, and flock synchrony can result in crowding during attempted simultaneous use of shared resources by hens in groups (Collins et al., 2011; Asher et al., 2013). All of these situations can lead to welfare concerns, such as overcrowding and subsequent aggression, frustration, as well as the economic concern of reduced productivity (Abrahamsson and Tauson, 1995; Odén et al., 2002; Freire et al., 2003). Thus, certain types of aviaries may fail to meet legislative and consumer expectations for improved hen welfare with respect to affective state and ability to perform natural behavior, and use of some aviaries also may reduce production efficiency (i.e., due to eggs laid outside the nests).
There have been studies looking at patterns of hen movement and resource use in commercial aviaries detailing where hens distribute over the aviary tiers and litter area throughout the d (Hansen, 1994; Carmichael et al., 1999; Channing et al., 2001; Odén et al., 2002; Campbell et al., 2016a,b,c). However, no studies have directly compared different laying hen strains or evaluated where all hens within a flock are in the various areas of an aviary system at different times of day. Further, no research has directly examined how hens in aviary systems respond to gaining access to additional space and another valued resource, in the form of a floor litter area, at peak lay. Thus, the main goal of this research was to examine how different strains (Hy-Line Brown, Bovans Brown, DeKalb White, and Hy-Line W36—common commercial strains likely to be used in alternative housing systems in North America) distributed themselves among aviary tiers and litter areas during and throughout light and dark periods at peak lay. A secondary objective was to examine the influence of hen strain on the birds’ response to litter access. It was predicted that white hens, which are anecdotally often considered to be more fearful and flighty, would roost at higher levels in the aviary at night and would require more time to acclimate to litter. Brown hens, on the other hand, were predicted to roost throughout the aviary at night and to initially access litter in larger numbers. Hens of both strains were predicted to use the nest area almost exclusively in the morning, and because of their larger body size, fewer brown hens were expected to occupy any given area of the system relative to white hens. This research aims to provide a better understanding of how considering hen's spatial distribution could help select an optimal strain to match a particular aviary design or to inform modification of resource presentation to allow fulfillment of hen behavioral needs.
MATERIALS AND METHODS
Ethics
All research protocols were approved by the Michigan State University Institutional Animal Care and Use Committee prior to the start of data collection.
Hens and Housing
A total of 2,304 laying hens of 4 genetic strains (n = 576 each: DeKalb White [DW], Hy-Line Brown [HB], Bovans Brown [BB] and Hy-Line W36) were used in this study. Strains were chosen based on breeder recommendations as being likely to be used in the United States as alternative housing systems are adopted. Prior to placement in the aviary, chicks were reared from hatch in environmentally controlled, windowless houses containing 6 pens per side (n = 12 total pens) at the Michigan State University Poultry Teaching and Research Center (East Lansing, MI). Each pen housed 225 to 250 chicks, with 3 pens per strain (n = 675 to 750 chicks/strain). Adequate feeding space and nipple drinkers were provided as per industry breeder management guidelines. From 3 wk of age, chicks were given access to a floor covered with wood shavings and a roosting area.
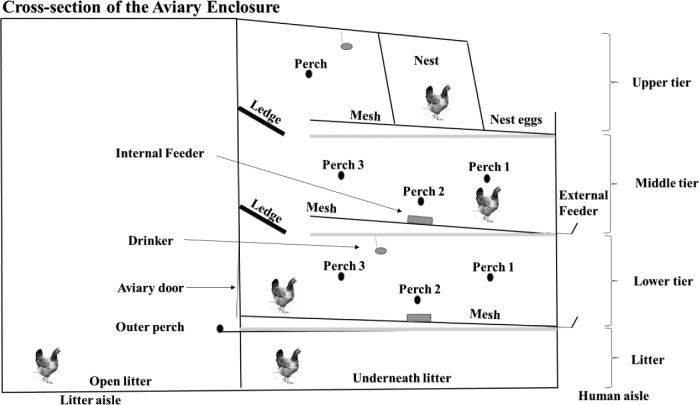
At 17 wk of age (March 2015), pullets were placed into a commercial-style aviary system (NATURA60, Big Dutchman, Holland, MI) in the Laying Hen Facility at the Michigan State University Poultry Teaching and Research Center. The facility included 4 rooms and each room contained 4 aviary units (one unit per strain x 4 strains x 4 rooms = 16 units total) initially populated with 144 birds per unit. To ensure that each hen housing unit contained a mix of birds from each rearing room, pullets from each of the 3 rearing pens per strain were randomly allocated to each of the 4 hen housing units per strain. Strains were placed into units within rooms in a balanced fashion to ensure that across the 4 rooms, each strain occupied each of the 4 different unit locations to account for possible effects of units being near the door or at ends versus the center of rows. As shown in Figure 1, each aviary unit was composed of a 3-level, tiered, wire-mesh enclosure (each level with 61 cm internal ceiling height) and a litter area (divided into an open litter area in front of the tiered enclosure and a litter area underneath the tiered enclosure). As measured from the center of the enclosure, the mesh floor of the lower tier was 50.80 cm from the aviary floor, while the mesh floors of the middle tier and the upper tiers were 112 and 173 cm from the aviary floor, respectively. Each tiered enclosure contained perches at all levels (Figure 1). Two solid, metal ledges, intended to help hens transition between tiers within the enclosure, ran the full length of the unit in front of the middle and upper tiers. Feeders (one feeder with external and internal branches, on both the lower and middle tiers) provided 5 cm feeder space per bird, while water lines in the lower and upper tiers provided water access at a rate of 9 hens per pin-metered drinker (nipple).
Figure 1.
A cross-sectional diagram of the tiered aviary unit, showing litter areas, perches, and ledges, human and litter aisles, and location of the colony nest, drinkers (gray ovals), and external and internal feeders (gray boxes).
Perch allowance per hen exceeded United Egg Producers (2016) guidelines specifying that at least 55% of hens be able to perch at once (with 15 cm/hen). When hens had litter access, floor space per hen exceeded United Egg Producers (2016) guidelines, which specify that hens in multi-tier systems should have 929 cm2/hen. However, when access to litter was restricted during 17 to 25 wk of age and between 01:00 and 11:30 each d (as described below in System management), there were 551 cm2/hen of floor space per hen, falling below recommended floor space allowances. Table 1 describes the number of hens that could be accommodated in the aviary based on United Egg Producers (2016) and 563 cm2 space required for a standing Hy-Line W36 hen as determined via kinematic analysis by Mench and Blatchford (2014). These calculations were computed in order to later compare maximum numbers of hens counted in each area to common industry space recommendations and scientific assessment of the physical space occupied by a hen's body.
Table 1.
Comparison of capacity of the aviary system to accommodate hens in the various levels with the maximum number of white (DW or W36) and brown (BB or HB) hens observed at different times of the day.
| Capacity1 (# hens) | Maximum # of hens observed | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Floor | Perches | Ledges | Nests | Combined | MORNING | MIDDAY | EVENING | DARK | ||||
| White | Brown | White | Brown | White | Brown | White | Brown | ||||||
| Lower tier | 37 (61) | 48 (48) | – | – | 85 (109) | 68a,* | 49a,† | 58a,* | 67a,† | 48a,§ | 74a,† | 38a,* | 50a,† |
| 62b,§ | 61b,† | 51b,§ | 43b,† | 29b,§ | 69b,† | 39b,* | 60b,† | ||||||
| 68c,* | 71c,† | 41c,§ | 44c,† | 23c,§ | 59c,† | 44c,* | 73c,† | ||||||
| Middle tier | 31 (51) | 48 (48) | 8 (14) | – | 87 (113) | 57a,* | 59a,† | 59a,§ | 67a,† | 71a,* | 56a,† | 57a,§ | 63a,† |
| 62b,* | 57b,† | 31b,§ | 73b,† | 54b,§ | 48b,† | 51b,§ | 57b,† | ||||||
| 60c,§ | 48c,† | 27c,§ | 38c,† | 37c,* | 32c,† | 53c,§ | 49c,† | ||||||
| Upper tier | 10 (17) | 16 (16) | 8 (14) | 13 (21) | 47 (68) | 42a,§ | 72a,† | 50a,§ | 40a,† | 47a,* | 33a,† | 96a,§ | 53a,† |
| 34 (47)2 | 43b,§ | 66b,‡ | 31b,* | 25b,† | 36b,§ | 23b,† | 106b,§ | 54b,† | |||||
| 37c,* | 59c,† | 26c,§ | 18c,† | 37c,§ | 21c† | 96c,* | 49c,† | ||||||
| Litter | 90 (133) | 16 (16) | – | – | 106 (149) | – | – | 110b,* | 48b,† | 69b,§ | 43b,† | – | – |
| 89c,* | 74c,† | 84c,* | 70c,† | ||||||||||
1Perching space was calculated at 15 cm/hen following the United Egg Producers (2016) guidelines, while the space required to accommodate a standing hen was calculated at 929 cm2/hen as per United Egg Producers (2016) recommendations for multi-tier systems and (presented in parentheses) at 563 cm2/standing hen following kinematic analysis of hen space requirements for Hy-Line W36 by Mench and Blatchford (2014). Note that United Egg Producers specifies that only floor area and tiers should be counted at usable space when calculating stocking density (United Egg Producers, 2016).
2Nest areas closed automatically 2 h before lights off, and this space was not available during the dark period, further reducing the floor space available in the upper tier. Bold numbers indicate hen numbers above capacity. Different letter superscripts represent different periods of time relative to hens gaining litter access:
aPRE is the period before hens had litter access;
bIMM is the period immediately after hens had litter access, and
cACC is the period 3 wk after hens had gained access to litter. Different symbol superscripts represent different strains of hens:
*DeKalb White hens (DW);
†Hyline brown hens (HB);
‡Bovans brown (BB). and
§Hyline W36 hens (W36);
In the aviary unit, each hen was provided with 1,132 cm2 of useable floor area. This space was divided into 581 cm2/hen of litter area and 551 cm2/hen of tiered enclosure space (439 cm2/hen wire mesh flooring plus 112 cm2/hen via solid metal ledges). Each unit had 8 round metal perches (3.1 cm diameter; 3 internal perches in both the lower and middle tiers and one perch in the upper tier, plus one outer perch in front of the lower tier) that extended the full length of the unit (244 cm). Using 15 cm/hen, 128 hens (89%) could perch simultaneously in each unit (Table 1). A colony nest with one central divider (creating 2 compartments) ran the length of the unit in the upper tier. The colony nest was 52 cm wide and each compartment was 122 cm long. In total the nests provided 88 cm2 nesting space per hen at the initial stocking of 144 hens/unit, which meets United Egg Producer (2016) recommendations of 83.6 cm2/hen. Nesting areas automatically closed 2 h before lights off and this space was not available at night.
System Management
Hens did not have litter access between 17 and 25 wk of age. At 26 wk of age (when the target of ∼90% of egg production was achieved), doors on the lower tier of the aviary enclosures began opening automatically each morning at 11:30. These doors allowed hens daily access to litter-covered floor areas following egg laying. The doors automatically closed at 01:00, approximately 5 h after lights off. In the first weeks following hens’ access to litter, farm personnel walked through the barn immediately after lights off and placed any hens remaining on litter into the lower tier of the aviary. Lights were turned on every d at 05:15, 05:15, and 05:00 and turned off at 19:30, 19:45, and 20:00 during 25, 26, and 28 wk of age, respectively. (The lights off times represent the beginning of a 30 min period of gradual overhead light dimming followed by dimming of a rope light in the middle tier. The rope light began to dim 15 min later than the overhead light.) The feed belts ran at 6:00, 14:00, and 19:30 to deliver feed to hens. At 09:00 and 16:45 the feed belts ran for approximately 10 s to stimulate hens to feed. Eggs were collected by hand each morning.
Observation of Hen Spatial Distribution and Resource Use
Direct observations and video recording of hens’ spatial distribution were conducted over 3 consecutive d at each of 3 time periods relative to hens gaining access to litter. Pre-opening observations (PRE: hens = 25 wk of age) occurred starting 3 d before hens accessed the litter area. Thus, observations during PRE were made of hens inside the tiered enclosures only. Immediate post-opening observations (IMM: hens = 26 wk of age) started one d following initial litter access to capture hens’ immediate reaction to gaining access to litter. (As hens were reared on litter, this IMM period is actually a re-introduction of hens to litter following 9 wk without litter access rather than an entirely novel experience.) Acclimated post-opening observations (ACC: hens = 28 wk of age) occurred 3 wk following initial litter access. Observations during IMM and ACC periods captured hens’ distribution throughout the tiered enclosures as well as in the open and underneath litter areas.
For each time period of interest relative to litter access (PRE, IMM, and ACC), observations were done during both d (i.e., lights on = light period) and night (i.e., lights completely off = dark period). In the dark periods, observations were done 30 min after full darkness (DARK PM: 20:45 for 25 wk, 21:15 for 26 wk, and 21:30 for 28 wk) and 2 h before lights on (DARK AM: 3:15 for 25 wk and 26 wk, and 3:00 for 28 wk). In the light periods, observations were done 15 min after lights on (MORNING: 5:30 for 25 wk and 26 wk and 5:15 for 28 wk), during the middle of the lights on period (MIDDAY: 11:30 for 25 wk, 12:00 for 26 wk, and 12:15 for 28 wk) and 2 h before lights off (EVENING: 17:30 for 25 wk, 17:45 for 26 wk, and 18:00 for 28 wk). The observations done during MIDDAY after the aviary doors opened at 11:30 at 26 wk (IMM) and 28 wk (ACC) were started 30 min later than the exact midpoint of the light period to allow hens to fully distribute throughout the tiers and litter areas of the units.
Prior to the start of data collection, 3 observers were trained for 3 d to establish synchrony within observer pairs and ensure a high level of inter-observer reliability. All direct observations were performed by a pair of observers (composed of 2 of the 3 previously trained observers). Observations were completed by the pair of observers visiting each room in a different randomized order across the 3 d of each time period (PRE, IMM, and ACC). One observer was located on each side of the tiered enclosure to allow for simultaneous recording of birds’ distribution from both human and litter aisles (Figure 1). Each observer counted the number of hens per location throughout the aviary unit, starting from the litter area or bottom tier, depending on whether hens had litter access, and working upward. During the light period, hens in the upper tier and open colony nest were recorded only by the observer in the litter aisle (Figure 1); at night the human aisle observer was able climb onto the aviary to look down on hens without disturbing them. A single count of all hens in an aviary unit took approximately 90 seconds. Two counts were made for each unit at each time of d; the second count was made about one h after the first count, after hens in all units had been observed the first time.
To minimize disturbance, counts of hens made from the litter aisle were done when the observer was positioned either at the end of the row or in the preceding unit to the one being observed. Observers used slow and calm movement to avoid bird disturbance as they moved between units. During the observer-training period, hens also become acclimated to the presence of observers in the room performing the data collection routine. At least one h elapsed before scans were repeated within each time of da, allowing birds to redistribute following any disturbance that occurred as observers moved past and through the units. Direct observations were paused for 5 min if the feed belt ran during data collection, to allow hens to regain their normal distribution. Disturbance of hens during dark observations was minimized by use of green headlamps, which allowed observers to see hens without rousing them to movement (Campbell et al., 2016c). No large-scale hen movements in the aviary were noted during observation sessions.
Observations that occurred during the light period while aviary doors were closed (i.e., MORNING), were done by observers performing counts of the number of birds within each tiered enclosure location as described above. Observations that occurred when aviary doors were open (MIDDAY and EVENING of IMM and ACC) also included use of ceiling-mounted high-resolution digital video cameras (VF450, Clinton Electronics, Loves Park, IL) to record number of hens on the open litter area. Hens on litter underneath the enclosure were counted by the observer in the human aisle and simultaneously recorded using a hand-held video camera (VIXIA, HFM41, Canon, Tokyo, Japan). To ensure accurate counts were made of hens underneath the enclosure (as this was difficult due to the narrow opening available for observers to look through), direct counts were later confirmed using video footage. Dark observations were performed by observers making direct counts of the number of hens within each location in the system (i.e., Tier 1: mesh, perch 1, 2, and 3; Tier 2: mesh, ledge, perch 1, 2, and 3; and Tier 3: mesh, ledge, perch, and colony nest; Figure 1).
Hen Mortality
Hen mortalities were recorded daily for each unit within the 4 rooms. At the end of each observation period, the total number of hens within each unit was summed, and cumulative mortality was calculated as a percentage of the 144 hens originally placed in that unit. Cumulative mortality per strain was as follows: PRE = HB: 0.87%, BB: 0.52%, DW: 1.04%, W36: 0.87%; IMM = HB: 0.87%, BB: 0.69%, DW: 1.04%, W36: 0.87%; and ACC = HB: 1.04%, BB: 1.04%, DW: 1.04%, W36: 1.22%.
Data Processing and Statistical Analyses
Prior to analysis, all count data obtained from direct observations and video recordings were collated. There were 4 aviary unit replicates for each of the 4 hen strains, and aviary unit was the subject of analysis for all statistical tests. Each observation set was composed of 2 counts of hen distribution within each unit for each of the 5 times of d (light period: MORNING, MIDDAY, and EVENING; dark period: DARK PM and DARK AM) across 3 time periods (PRE, IMM, and ACC).
Firstly, the average number of birds per each tier, per strain, for the whole light and dark periods were calculated and compared among different strains. Counts of hens per tier were then compared among strains for each specific time of d (MORNING, MIDDAY, EVENING, DARK PM, and DARK AM), and comparisons within each strain were made across the 3 periods of data collection (PRE, IMM, and ACC). Initial comparisons of DARK PM and DARK AM observations showed similar distribution patterns within each strain and, thus, the DARK PM and DARK AM observations were averaged to create a single DARK hen count for the entire dark period. There were 24 total observations for each strain during each individual time of d (e.g., for light: MORNING, MIDDAY, and EVENING) or for averaged times of d (e.g., light and dark). This was calculated as follows: 24 = 2 counts/unit per observation set * 3 d of observation for each time period of data collection (PRE, IMM, and ACC) * 4 units per strain. All analyses were conducted in SPSS Statistics for Windows (Version 22.0. IBM Corp., Armonk, NY), with α set at 0.05. As the data did not meet assumptions of normality, non-parametric statistics were used for analysis. First, Kruskal-Wallis tests were applied to determine differences in the distribution among the 4 strains, across the aviary tiers and litter area, and throughout different times of the d at each time period of data collection. Then, Mann-Whitney's U- tests were applied to the significant results to perform multiple comparisons between corresponding groups.
Cohen's Kappa Statistic for Measuring Agreement (K) was used following Landis and Koch (1977) to measure inter-observer reliability for direct observations and video decoding in order to determine consistency among observers. Coefficients for both interobserver reliability and hen movement in the dark were calculated as follows: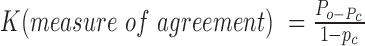 , where Po was the proportion of observed agreements and Pc was the proportion of agreements expected by chance (where Pc was hypothesized to be 0). The Kappa coefficient was then interpreted following Landis and Koch (1977) as follows: K = 0, poor agreement; 0.0 ≥ K ≤ 0.2, slight agreement; 0.21 ≥ K ≤ 0.4, fair agreement; 0.41 ≥ K ≤ 0.6, moderate agreement; 0.61 ≥ K ≤ 8, substantial agreement; and 0.81 ≥ K ≤ 1, almost perfect agreement. Inter-observer reliability was calculated during the training period when trainees had viewed the same areas of the aviary simultaneously, and was Kappa = 0.94 (P < 0.001), 95% CI (0.504, 0.848), throughout all direct observations and video decoding work.
, where Po was the proportion of observed agreements and Pc was the proportion of agreements expected by chance (where Pc was hypothesized to be 0). The Kappa coefficient was then interpreted following Landis and Koch (1977) as follows: K = 0, poor agreement; 0.0 ≥ K ≤ 0.2, slight agreement; 0.21 ≥ K ≤ 0.4, fair agreement; 0.41 ≥ K ≤ 0.6, moderate agreement; 0.61 ≥ K ≤ 8, substantial agreement; and 0.81 ≥ K ≤ 1, almost perfect agreement. Inter-observer reliability was calculated during the training period when trainees had viewed the same areas of the aviary simultaneously, and was Kappa = 0.94 (P < 0.001), 95% CI (0.504, 0.848), throughout all direct observations and video decoding work.
Though there were no significant differences observed within a strain in the average number of hens in each tier between the 2 dark observations for any period, lack of perfect agreement between counts of hens after lights out (DARK PM) and before lights on (DARK AM) suggested hens were still moving between tiers in the dark. Therefore, to examine the degree of hens’ movement from one tier to another between DARK PM and DARK AM, Kappa coefficients of agreement (K coefficients) were calculated. For each strain at each time period (PRE, IMM, and ACC), the difference between the 2 DARK PM observations and the 2 DARK AM observations for each tier within the unit were used to calculate the K coefficient.
RESULTS AND DISCUSSION
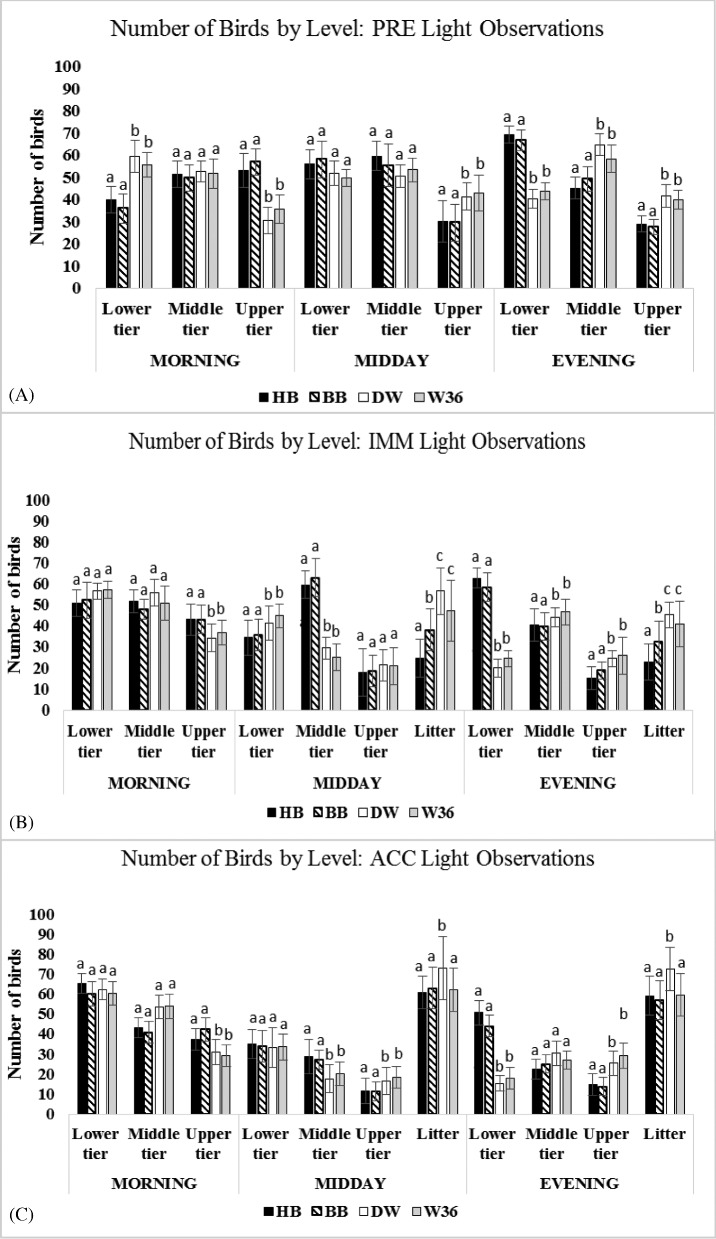
At first glance, during the light period, hens of different strains appeared to distribute themselves similarly throughout the 3 vertical tiers of the aviary system studied here. For example, in the PRE period, before hens had litter access, there were similar numbers of hens of the 4 strains found in each tier when the entire light period was examined (i.e., MORNING, MIDDAY, and EVENING observations averaged together; P ≥ 0.05). However, when the light period was subdivided into MORNING, MIDDAY, and EVENING, times of d that are typically linked to hens’ performance of key behaviors (e.g., egg laying, dust bathing, and preparing to roost), differences between the strains became evident (Figure 2). Generally, hens of the 2 brown strains (HB and BB) showed similar distribution patterns in the system, while the distribution patterns of the hens of the 2 white strains (W36 and DW) was generally similar to each other and were often different from that of the brown hens.
Figure 2.
Number of hens of each of the 4 strains found in the 3 tiers of the aviary enclosure during the PRE period before hens had any access to litter (2A); the IMM period when hens first gained access to litter (2B); and the ACC period after 3 wk of access to litter (2C). Data are presented separately for MORNING, MIDDAY, and EVENING. All parameters are expressed as mean bird counts ± standard deviation. Different superscripts indicate differences (P < 0.05) among different strains in that tier level at that time of day.
Tier Occupancy in MORNING and MIDDAY
In all periods, more brown hens than white hens were observed in the upper tier during MORNING observations (i.e., the 2 h immediately after lights on; P ≤ 0.05; Figure 2). As the colony nests were located in the upper tier of this system, brown hens could have been displaying a stronger internally driven circadian pattern to egg lay in the morning (Yeates, 1963; Vestergaard, 1982; Channing et al., 2001). Paradoxically, more white hens were seen in the upper tier compared to brown hens during MIDDAY PRE and ACC observations (P ≤ 0.05). Such a distribution suggests that white hens were laying across more h of the light period compared to the brown birds. This interpretation agrees with counts of eggs laid by hens of the different strains at different times of d in this system (Villanueva et al., unpublished data).
The greatest number of hens recorded in the upper tier during MORNING occurred in the PRE period (Figure 2A), when 69 HB and 72 BB hens were counted, exceeding the combined capacity of 47 to 68 hens for the upper tier (Table 1). MORNING occupancy of the upper tier by brown hens dropped somewhat during the ACC period, even though hens could not go down to the litter until MIDDAY (i.e., during ACC MORNING observations, a maximum of 59 HB and 50 BB hens were seen in the upper tier; Figure 2C, Table 1).
Hence, in the morning, hens appear to have experienced overcrowding in the upper tier, which contains the colony nests. Hens may end up laying eggs outside the nest if they cannot get into a nest during times of peak demand (Kruschwitz et al., 2008). Kruschwitz and colleagues (2008) postulated that overcrowding of nests might prevent some hens from performing pre-lay behavior or laying in the correct location, thus impairing hen welfare by causing frustration and increasing the probability of floor laid eggs. Moreover, examination of egg laying by hens in a separate part of this study suggests that brown hens did lay more often outside the colony nests, and that more damaged eggs were seen in units housing brown strains compared to white strains (Villanueva et al., unpublished data).
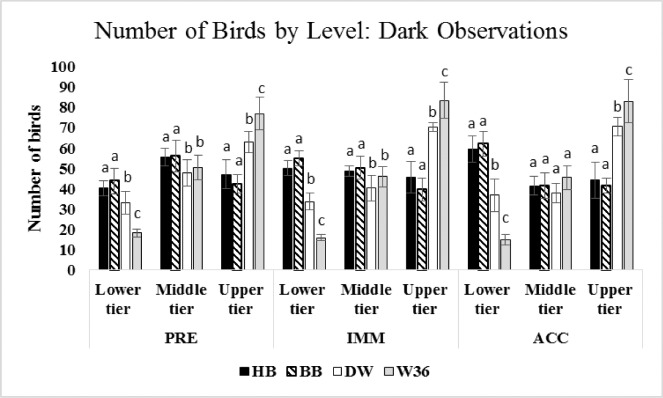
Tier Occupancy in EVENING and DARK
In the EVENING of all observation periods, more brown hens were counted in lower tiers while more white hens were observed in upper tiers (P ≤ 0.05; Figure 2). This pattern also was seen during DARK observations (P ≤ 0.05; Figure 3), but was even more pronounced. Further, in DARK observations, differences in tier occupancy also arose between the white strains, with more W36 hens counted in the upper tier compared to DW hens and more DW hens seen in the lower tier compared to W36 hens (P ≤ 0.05). More white hens occupied the middle tiers in EVENING PRE and IMM periods (P ≤ 0.05), but more brown hens occupied the middle tiers in DARK PRE and IMM periods (P ≤ 0.05). No strain difference in middle tier use was observed in either EVENING or DARK ACC periods (P ≥ 0.25).
Figure 3.
Number of hens of each of the 4 strains found in the 3 tiers of the aviary during DARK observations. Data are presented separately for PRE, IMM, and ACC. (The PRE period occurred before hens had access to litter, the IMM period occurred when hens were first given access to litter, and data for the ACC period were recorded 3 wk after hens initial access to litter.) All parameters are expressed as mean bird counts ± standard deviation. Different superscripts indicate differences (P < 0.05) among different strains in that tier level at that time of day.
Nest areas in the aviary system automatically closed 2 h prior to lights off, further reducing the already limited space available for roosting in the upper tier (Table 1). For all 3 observation periods, the maximum number of W36 and DW hens recorded in the upper tier during DARK observations exceeded the combined capacity of 47 to 68 birds for that tier by 16 to 125% (Table 1; i.e., PRE: 96 W36 and 79 DW hens; IMM: 106 W36 and 95 DW hens; and ACC: 96 W36 and 95 DW hens). Brown hens were generally evenly distributed among the 3 tiers or occupied lower and middle tiers at higher rates at night. These patterns suggest that hens of the white strains had a stronger preference for elevated roosts, and that this preference was most marked in W36 hens. Such a preference is not surprising as ancestral jungle fowl and feral chickens also prefer to roost high in trees to avoid predators (Wood-Gush and Duncan, 1976; Wood-Gush et al., 1978; EFSA AHAW Panel, 2015). These findings are also consistent with the typical night roosting behavior patterns recorded in previous studies (Yeates, 1963; Vestergaard, 1982; Channing et al., 2001; Campbell et al., 2016c). It is interesting, therefore, that the 2 brown strains studied here did not show such preference for roosting at height at night, and further study is needed to determine why this is the case.
Impact of Gaining Access to Litter
Laying hens in certain types of aviary systems may be restricted to tiered mesh enclosures until they have reached a certain level of production to encourage hens to lay their eggs in designated nests. At this point, hens are permitted to go down to floor litter areas, but litter access may remain somewhat restricted with hens confined during the morning and perhaps overnight. Beside these restrictions, the attractiveness of the nest itself and presence of other hens inside it should stimulate hens to lay eggs in the nest as desired by the producer (Riber, 2010, 2012; Lentfer et al., 2013).
In the present study, hens received litter access once they reached 90% of their expected maximum production, which occurred at 26 wk of age. When hens were initially granted litter access in the IMM period, more white hens than brown hens were counted on litter at MIDDAY and EVENING (P ≤ 0.05; Figure 2B), and fewer HB hens were observed on litter compared to BB hens at both times of d (P ≤ 0.05). As hens acclimated, numbers of HB and BB hens on the litter during both MIDDAY and EVENING doubled or tripled between the IMM and ACC periods (P ≤ 0.05 in all cases; Figure 2B,C). Numbers of W36 hens also increased in the EVENING between IMM and ACC (P = 0.04) and showed a tendency to increase at MIDDAY (P = 0.06). However, there was also a tendency for more DW hens to be on litter in the ACC period compared to the IMM period (P ≥ 0.05), with the result that there were more DW hens on the litter compared to hens of the 3 other strains during ACC (P ≤ 0.05; Figure 2C).
It is known that individual hens can have different motivational strengths or preferences for accessing litter (Hughes, 1976), and hens can prefer one type of litter over another for dust bathing or foraging (Olsson and Keeling, 2005; Guinebretière et al., 2014). However, though Odén and colleagues (2002) examined behavior of white and brown hens in aviaries, they did not report an impact of strain related to use of litter, and no previous work has examined how different strains of hens initially react to litter access in aviaries. The present results suggest that white hens, DW in particular, were more motivated to access litter following a period of deprivation compared to brown hens (Figure 2B) or that BB and HB hens required more time to acclimate to traveling from enclosures to the litter area.
The variable responses by the strains of hens to litter access are consistent with findings reported by Klein and colleagues (2000) who found that different strains of laying hens show varying behavioral reactions to the changes in their environment. It is possible that brown hens were more fearful of entering a novel environment than were hens of the white strains. However, brown hens generally react less fearfully to stressful or novel situations than white hens (Odén et al., 2002; Fraisse and Cockrem, 2006; de Haas et al., 2013), and all hens in this study were reared on similar wood shaving litter. Alternatively, is possible that the brown hens were less agile or heavier than white hens and found movement down to the litter more difficult (Moinard et al., 2004).
The litter area of the aviary system used in this study consisted of an open litter area and an area under the tiered aviary enclosure, which accounted for 59 and 41% of the litter area, respectively. In the IMM period immediately following initial litter access, the majority of brown hens on litter were in the open litter area during both MIDDAY (57%) and EVENING (55%). However, in the ACC period this proportion changed so that more brown hens on litter were counted under the tiered enclosure (MIDDAY: 62%; EVENING: 65%, P ≤ 0.05). In contrast, white hens on litter were found in greater numbers in the open litter areas in both IMM (MIDDAY: 65.96%; EVENING: 66.56%) and ACC periods (MIDDAY: 69.63%; EVENING: 70.56%; P ≤ 0.05). Thus, neither strain was distributed in proportion with the available space, though neither strain would likely have been overcrowded based on the capacity of the litter area (Table 1). Brown hens’ preference for litter under the enclosure, like their slower acclimation to litter, seems to contradict findings of previous work showing less fear in brown hens (Odén et al., 2002; Fraisse and Cockrem, 2006; de Haas et al., 2013). It is also possible that the brown hens’ distribution on the litter area reflects a preference by the hens to maintain a certain inter-bird distance appropriate for behaviors they are performing (Keeling, 1994). If brown hens were performing more wing flapping or dust bathing on the open litter areas, fewer hens might be expected in this area as these behaviors require more space than standing, sitting, or preening (Mench and Blatchford, 2014). Previous observations in litter-based systems also have shown that while hens disperse across the space provided to them, they do not always do so uniformly (Channing et al., 2001; Odén et al., 2002). As hens in the present study were not explicitly fear tested and behaviors on litter were not recorded, it is not possible to say with any certainty whether desire for greater inter-hen space or fear influenced their occupancy of litter areas.
Movement at Night
Finally, a considerable degree of tier-to-tier movement between DARK PM and DARK AM was recorded, with brown hens consistently showing more tier-to-tier movement than white hens (Table 2). Hens of all strains moved least in the dark period during the PRE observation period, most during IMM (Table 2), and hens showed intermediate levels of movement in the ACC dark period. Increased movement in the IMM period could indicate hens were unsettled by changes to their routine resulting from litter access, including learning to return to the aviary at night before lights went out. Further investigation is required to distinguish whether tier-to-tier movements in the dark are a result of voluntary behavior, or are due to birds slipping from ledges or mesh edges. Movement occurring between levels at night might be a contributing factor to the high level of body injuries, keel damage, and leg bones fractures that have been recorded in aviary-housed laying hens (Stratmann et al., 2015) and is a subject worthy of further study.
Table 2.
Amount of hen movement in the dark period observation as indicated by the Kappa coefficient (K).
| Strain1 | HB | BB | DW | W36 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Observation2 | PRE | IMM | ACC | PRE | IMM | ACC | PRE | IMM | ACC | PRE | IMM | ACC |
| Lower tier | 0.45 | 0.18 | 0.43 | 0.49 | 0.28 | 0.40 | 0.60 | 0.39 | 0.53 | 0.61 | 0.38 | 0.57 |
| Middle tier | 0.39 | 0.20 | 0.28 | 0.46 | 0.27 | 0.34 | 0.64 | 0.38 | 0.58 | 0.59 | 0.30 | 0.59 |
| Upper tier | 0.46 | 0.23 | 0.27 | 0.45 | 0.26 | 0.41 | 0.66 | 0.41 | 0.45 | 0.73 | 0.41 | 0.53 |
1HB (Hy-Line Brown); BB (Bovans Brown); DW (DeKalb White); W36 (Hy-Line W36).
2PRE (pre-opening); IMM (immediate post opening); ACC (acclimated post opening).
Results are expressed as K coefficients (degree of agreement), with higher values indicating higher agreement among counts and, therefore, less movement of hens to or from a particular tier.
Distribution of Hens Throughout the System
Overcrowding occurs when hens aggregate in space and time and perform certain behaviors synchronously (McLean et al., 1986; Odén et al., 2002; Febrer et al., 2006; Collins and Sumpter, 2007). This synchrony is most apparent in egg laying, feeding, dust bathing, and perching (Lill, 1968; Hughes, 1971; McLean et al., 1986). Behavioral synchrony under natural conditions was proposed to be important to hens (Odén et al., 2002; Riber et al., 2007) and has been suggested to increase individual fitness (Powell, 1974; Krause and Ruxton, 2002). At the most basic level, overcrowding and areas of high local stocking density do not allow hens to experience the recommended space allowance per hen, and can, in some cases, cause situations in which the number of birds that can be safely accommodated in an area is exceeded (Febrer et al., 2006). Overcrowding might also have adverse effects on health and welfare of laying hens such as reduced ability to move inside the housing environment (Febrer et al., 2006); increased chance for physical injury (Frankenhuis et al., 1991); reduced ability to access resources and, in turn, increased competition over those resources (Arnould et al., 2001); and might predispose hens to develop stereotypies and injurious behavior such as feather pecking (Bestman et al., 2009).
Overcrowding and pockets of high local stocking densities were observed at certain times in the current study despite the provision of 1,131.8 cm2/bird when hens had access to litter during the day. High concentrations of hens were particularly noticeable for brown hens in upper tiers during PRE and IMM MORNING observations (Figure 2, Table 1). White hens also crowded the litter area, particularly during MIDDAY IMM observations, when litter access was first granted (Figure 2B). However, the most dramatic cases of overcrowding occurred in the upper tier in the dark period, when the maximum number of hens counted in that level exceeded the combined capacity for all strains (Table 1). Furthermore, for all strains of hens, the average number of hens occupying the upper tier in the dark period was at or above the combined capacity (Figure 3), suggesting that overcrowding of the upper tier at night was a regular phenomenon. Therefore, this system design does not adequately accommodate hens’ preference for roosting at night (Schrader and Müller, 2009; Brendler et al., 2014; Campbell et al., 2016c), and it could be expected that hens perching lower in the system due to space limitations might be frustrated (Olsson and Keeling, 2002).
Overcrowding of the upper tier in the MORNING by brown hens and high occupancy from MORNING to MIDDAY in white hens was likely due to internal diurnal rhythms related to pre-lay behavior and oviposition (Cooper and Albentosa, 2003; Hunniford et al., 2014). Insufficient space for simultaneous access to and use of nests by all hens during peak times of demand may result in litter or system-laid eggs by individuals unable to access nests at this time (Riber, 2010; Lentfer et al., 2013) as well as in increased aggression (Hunniford et al., 2014). The degree to which eggs are laid outside the nest area may be influenced by strain, and it has previously been suggested that this is due to less motivation to use nests in some strains (Singh et al., 2009; Wall, 2011). However, the high occupancy rates of the upper tier by brown hens in the morning in this study (Figure 2, Table 1) were accompanied by more frequent egg laying outside the nest during this time (Villanueva et al., unpublished); thus an alternative explanation is that some strains may be more sensitive to crowding near nests.
When hens received access to litter at IMM, the amount of space available per hen was doubled (581.19 cm2/hen of litter area + 550.69 cm2/hen of tiered enclosure space = 1,131.88 cm2/hen). Based on Keeling's (1994) work, it might be expected that hens would increase their inter-bird space in response to more area, particularly when performing active behaviors such as walking or dust bathing, and would perform such active behaviors more frequently. In the present study, inter-bird distance was not explicitly measured or associated with activity types. However, prior to litter access, distribution of hens of all strains throughout the space available in the tiered enclosure was relatively proportionate over the course of the d (Figure 2A). After acclimation to litter, hens of all strains often displayed a proportionate distribution between litter and enclosure tiers (Figure 2C), with roughly half of the hens in each unit observed on litter and half in the enclosure. However, immediately following access to litter, as hens adjusted to this new area, hens of white strains and brown strains showed different patterns of distribution in both enclosure and litter areas. Specifically, when hens first had access to litter during IMM MIDDAY, more brown hens remained in the middle tier compared to white hens (P ≤ 0.05), and more white hens were present in the lower tier compared to brown hens (P ≤ 0.04). In fact, nearly half of brown hens were counted in the middle tier during MIDDAY IMM observations, with only 30 to 40 hens of HB or BB strains on litter. This pattern resulted in a higher number of brown birds in the enclosure space and lower numbers on the litter compared to white hens (P ≤ 0.05). The higher numbers of white hens in the lower tier during IMM might be due to frequent transitions between the tiered enclosure and open litter area, when hens would use the lower tier and external perch as a transition area. In a commercial scale study of the same aviary style, Campbell and colleagues (2016b) reported large numbers of white hens moving between the lower tier of the enclosure and the litter area immediately after opening the aviary enclosure door.
As noted earlier, during both MIDDAY and EVENING of the IMM period when litter access was granted to hens, large numbers of DW and W36 hens were counted in the litter area (Figure 2B). In fact, the maximum numbers of white hens recorded in the litter area during MIDDAY IMM observations were 110 DW and 105 W36 hens (Table 1). This means that about 75% of the white birds in the aviary unit were occupying 51.35% of the total available area in the aviary. In effect, this disproportionate distribution reduced the available litter space per hen from 581.19 cm2 to 390.70 cm2, which could prevent hens from performing activities litter provision aims to encourage. The amount of physical space needed by a hen increases for dynamic behaviors, such as dust bathing and wing flapping (Mench and Blatchford, 2014); however, this does not necessarily imply that group-housed hens must be stocked at a rate that provides each hen with the space needed by the most space-demanding behavior. The size of the group must be considered, as larger groups require larger spaces for housing, and within these spaces, the spatial distribution of hens would not be uniform as they tend to cluster in small groups rather than disperse evenly (Channing et al., 2001). Such clustering would provide pockets of space that hens could use for more dynamic behaviors. Further, not all hens would be performing space-demanding dynamic behaviors simultaneously. Thus, hens in flocks of 100 individuals or greater could be predicted to need approximately 600 cm2 of space to perform both static postures and dynamic behaviors (Mench and Blatchford, 2014).
Implications and Limitations
The laying hen industry is transitioning from conventional cages to complex indoor, tiered aviary systems, intended to provide more space and resources to hens. Several studies have recorded differences in behavior among strains and attributed this phenomenon to the process of selection of the strains for different production traits (e.g., Braastad and Katle, 1989; Schütz and Jensen, 2001; Schütz et al., 2001; Odén et al., 2002). Hence, behavior and distribution of various laying hen strains when housed in similar facilities should not be expected to be similar, which could give rise to concern that some hen strains might not match well with certain housing systems (Hansen, 1994). Therefore, addressing the lack of available literature describing the genetic influence on laying hen distribution and behavior inside the modern housing system was a primary reason for conducting the current study. Observations were conducted during the peak lay period, when resource demand is at its greatest and focused on the time hens were given litter access, in order to investigate the additional influence of management alteration on the birds’ distribution and behavior.
This study was conducted using a single aviary design. The design offered limited perch space in the upper tier, nest boxes located in the top level of the aviary, and less than the recommended floor space per hen in the PRE period and at night when hens did not have litter access (551 cm2/hen of enclosure space vs. 1132 cm2/hen of enclosure + litter space). Comparison of the number of hens observed in various areas of the aviary was made against both capacities derived from producer guidelines (United Egg Produces, 2016) and static space requirements of standing hens from a scientific study on one strain of hen (W36; Mench and Blatchford, 2014). The first is a frequently referenced and relied upon guide when determining how much space a laying hen should be given, and the second could be considered the minimum amount of physical space a hen's body occupies—without accounting for the fact that hens do more than simply stand. Thus, neither of these is definitive standards for how much space a group-housed laying hen needs to perform highly motivated behaviors nor ensures good quality of life. Nonetheless, the present study provides evidence that strains respond differently to a similar environment and to change in available space.
Additional strain comparisons within aviaries of different configurations are needed to complete the picture sketched by the current study. For example, would more BB and HB hens roost at the highest levels at night if sufficient perch and floor space was provided to accommodate all hens or would they continue to disperse throughout levels of the aviary? Would DW and W36 hens continue to increase their occupancy of the upper level of the aviary if space in that level increased? Further, hens in this study were not given access to the litter areas on the floor until they were approaching peak production in an attempt to reduce later floor laying, and after having been reared on litter as pullets, such a restriction could have caused frustration. Therefore it will be important to examine how various strains’ distributions on litter might differ if access was never restricted and whether the restriction was actually effective in reducing floor eggs for all strains. Examining the attractiveness of open litter versus areas under systems and whether performance of behavioral activities differ between the areas also will be important for evaluating whether underneath areas are less desirable. Additionally, studies in aviaries with nests provided at different heights — including near the ground in keeping with ancestral jungle fowls’ preference for ground nesting, configurations or space per hen could examine what specific strains prefer. Such information could elucidate whether crowding would still occur near nests in the morning among BB and HB strains or if W36 and DW strains would lay earlier in the d rather than continue into the afternoon.
Findings from the current study suggest that white hens in particular should be housed in aviaries with more space in the upper levels to accommodate their preference for roosting at height at night while BB and HB hens should have more nest space per hen to allow them to lay during the morning hours. DW hens, which were present in the highest numbers on the litter, may be strongly motivated to use this resource and could need continuous access to litter to ensure good welfare. It also will be important to consider whether more space may be needed to accommodate the larger-sized brown birds when determining management practices and minimum space requirements. Finally, it could be recommended that allowing laying hens to exhibit their behavioral synchrony, while preventing or even decreasing the incidence of overcrowding, should be achieved by designing a housing environment that guarantees constant access to all the resources by all individual hens throughout the production cycle. Thus, while this research indicates that strain differences in space use do exist that could impact welfare, more information is needed describing hens’ use of available space and resources. Such research is crucial for matching strains of hens to aviary configurations or for proposing modifications to aviary system designs to ensure that these systems fulfill behavioral needs of different laying hen strains and, in turn, provide good welfare as intended.
CONCLUSION
In conclusion, the present study found distinct strain influences over the distribution pattern of laying hens throughout a tiered aviary. More brown than white hens were recorded regularly in the upper tier during MORNING observations, which resulted in occasional incidents of overcrowding, as the number of brown hens counted in the upper tier exceeded its capacity. On the other hand, more white than brown hens were observed regularly in the upper tier during both EVENING and DARK observations, and in the DARK the upper tier was regularly occupied beyond combined capacity by hens of all strains. This distribution pattern resulted in incidents of overcrowding, particularly during the DARK for DW and W36 hens in the upper tier. When litter was first accessible, white hens accessed it in larger numbers than brown hens, and more white hens on litter occupied open areas while more brown hens on litter were seen under the enclosure after acclimation. Large numbers of white hens in the litter area resulted in a reduction of the available litter space per hen, particularly during MIDDAY. Unexpectedly, a considerable incidence of tier-to-tier hen movement during dark periods by brown hens, particularly immediately after litter provision, was recorded in the current study, which could have implications for injuries if the movement is resulting from slips and falls. Ultimately, because there are differences among strains in how they use space within an aviary, consideration must be given to system design and hen preference to ensure a good match for improved hen welfare.
Acknowledgments
This study was supported by the Michigan Alliance for Animal Agriculture. The authors thank Nicholas Newsome and Silvia Villanueva for their assistance with onsite data collection and video decoding. We would also like to thank Angelo Napolitano and Michigan State University Poultry Teaching and Research Center personnel for their assistance with and contribution to this research.
Footnotes
Research support provided in part by a grant from the Michigan Alliance for Animal Agriculture (East Lansing, MI).
Now at University of New England and CSIRO, Armidale, NSW, Australia.
REFERENCES
- Abrahamsson P., Tauson R. Aviary systems and conventional cages for laying hens: Effects on production, egg quality, health and bird location in three hybrids. Acta Agric. Scand. Sec. A – Animal Sci. 1995;45:191–203. [Google Scholar]
- Abrahamsson P., Tauson R., Appleby M. Behaviour, health and integument of four hybrids of laying hens in modified and conventional cages. Br. Poult. Sci. 1996;37:521–540. doi: 10.1080/00071669608417882. [DOI] [PubMed] [Google Scholar]
- Appleby M. C., Hughes B. O. Welfare of laying hens in cages and alternative systems: environmental, physical and behavioural aspects. World Poult. Sci. J. 1991;47:109–128. [Google Scholar]
- Arnould C., Fraysse V., Mirabito L. Use of pen space by broiler chickens reared in commercial conditions: access to feeders and drinkers. Br. Poult. Sci. 2001;42:S7–S7. [Google Scholar]
- Asher L., Collins L. M., Pfeiffer D. U., Nicol C. J. Flocking for food or flockmates? Appl. Anim. Behav. Sci. 2013;147:94–103. [Google Scholar]
- Besbes B., Ducrocq V., Protais M. Proceedings of the 7th World Congress on Genetics Applied to Livestock Production, Montpellier, France, August, 2002. Session 20. Institut National de la Recherche Agronomique (INRA); 2002. An approximate total merit index combining linear traits, a survival trait and a categorical trait in laying hens; pp. 1–4. [Google Scholar]
- Bestman M., Koene P., Wagenaar J. P. Influence of farm factors on the occurrence of feather pecking in organic reared hens and their predictability for feather pecking in the laying period. Appl. Anim. Behav. Sci. 2009;121:120–125. [Google Scholar]
- Braastad B., Katle J. Behavioural differences between laying hen populations selected for high and low efficiency of food utilisation. Br. Poult. Sci. 1989;30:533–544. doi: 10.1080/00071668908417177. [DOI] [PubMed] [Google Scholar]
- Brendler C., Kipper S., Schrader L. Vigilance and roosting behaviour of laying hens on different perch heights. Appl. Anim. Behav. Sci. 2014;157:93–99. [Google Scholar]
- Campbell D. L. M., Makagon M. M., Swanson J. C., Siegford J. M. Litter use by laying hens in a commercial aviary: Dust bathing and piling. Poult. Sci. 2016a;95:164–175. doi: 10.3382/ps/pev183. [DOI] [PubMed] [Google Scholar]
- Campbell D. L. M., Makagon M. M., Swanson J. C., Siegford J. M. Laying hen movement in a commercial aviary: Enclosure to floor and back again. Poult. Sci. 2016b;95:176–187. doi: 10.3382/ps/pev186. [DOI] [PubMed] [Google Scholar]
- Campbell D. L. M., Makagon M. M., Swanson J. C., Siegford J. M. Perch use by laying hens in a commercial aviary. Poult. Sci. 2016c doi: 10.3382/ps/pew111. In press. doi: 10.3382/ps/pew111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael N., Walker W., Hughes B. Laying hens in large flocks in a perchery system: Influence of stocking density on location, use of resources and behaviour. Br. Poult. Sci. 1999;40:165–176. doi: 10.1080/00071669987566. [DOI] [PubMed] [Google Scholar]
- Channing C., Hughes B., Walker A. Spatial distribution and behaviour of laying hens housed in an alternative system. Appl. Anim. Behav. Sci. 2001;72:335–345. doi: 10.1016/s0168-1591(00)00206-9. [DOI] [PubMed] [Google Scholar]
- Collins L., Sumpter D. The feeding dynamics of broiler chickens. J. R. Soc. Interface. 2007;4:65–72. doi: 10.1098/rsif.2006.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L. M. Non-intrusive tracking of commercial broiler chickens in situ at different stocking densities. Appl. Anim. Behav. Sci. 2008;112:94–105. [Google Scholar]
- Collins L. M., Asher L., Pfeiffer D. U., Browne W. J., Nicol C. J. Clustering and synchrony in laying hens: The effect of environmental resources on social dynamics. Appl. Anim. Behav. Sci. 2011;129:43–53. [Google Scholar]
- Cooper J. J., Albentosa M. J. Behavioural priorities of laying hens. Av. Poult. Biol. Rev. 2003;14:127–149. [Google Scholar]
- Crawford R. D. Poultry Breeding and Genetics. CAB International; Oxfordshire, UK: 1990. [Google Scholar]
- De Haas E. N., Kemp B., Bolhuis J. E., Groothuis T., Rodenburg T. B. Fear, stress, and feather pecking in commercial white and brown laying hen parent-stock flocks and their relationships with production parameters. Poult. Sci. 2013;92:2259–2269. doi: 10.3382/ps.2012-02996. [DOI] [PubMed] [Google Scholar]
- Duncan I. J., Widowski T. M., Malleau A. E., Lindberg A. C., Petherick J. C. External factors and causation of dustbathing in domestic hens. Behav. Proc. 1998;43:219–228. doi: 10.1016/s0376-6357(98)00017-5. [DOI] [PubMed] [Google Scholar]
- EFSA AHAW Panel Scientific opinion on welfare aspects of the use of perches for laying hens. EFSA Journal. 2015;13 [Google Scholar]
- Estevez I., Andersen I. L., Nævdal E. Group size, density and social dynamics in farm animals. Appl. Anim. Behav. Sci. 2007;103:185–204. [Google Scholar]
- Estevez I., Freed M., Christman M. Effects of density on movement and use of space in broilers. Poult. Sci. 2005;84:63–S1. [Google Scholar]
- Febrer K., Jones T. A., Donnelly C. A., Dawkins M. S. Forced to crowd or choosing to cluster? Spatial distribution indicates social attraction in broiler chickens. Anim. Behav. 2006;72:1291–1300. [Google Scholar]
- Fraisse F., Cockrem J. F. Corticosterone and fear behaviour in white and brown caged laying hens. Brit. Poult. Sci. 2006;47:110–119. doi: 10.1080/00071660600610534. [DOI] [PubMed] [Google Scholar]
- Frankenhuis M., Vertommen M., Hemminga H. Influence of claw clipping, stocking density and feeding space on the incidence of scabby hips in broilers. Br. Poult. Sci. 1991;32:227–230. doi: 10.1080/00071669108417344. [DOI] [PubMed] [Google Scholar]
- Freire R., Wilkins L., Short F., Nicol C. Behaviour and welfare of individual laying hens in a non-cage system. Br. Poult. Sci. 2003;44:22–29. doi: 10.1080/0007166031000085391. [DOI] [PubMed] [Google Scholar]
- Guinebretière M., Beyer H., Arnould C., Michel V. The choice of litter material to promote pecking, scratching and dustbathing behaviours in laying hens housed in furnished cages. Appl. Anim. Behav. Sci. 2014;155:56–65. [Google Scholar]
- Hansen I. Behavioural expression of laying hens in aviaries and cages: frequencies, time budgets and facility utilisation. Br. Poult. Sci. 1994;35:491–508. doi: 10.1080/00071669408417715. [DOI] [PubMed] [Google Scholar]
- Hughes B. Allelomimetic feeding in the domestic fowl. Br. Poult. Sci. 1971;12:359–366. doi: 10.1080/00071667108415891. [DOI] [PubMed] [Google Scholar]
- Hughes B. O. Preference decisions of domestic hens for wire or litter floors. Appl. Anim. Ethol. 1976;2:155–165. [Google Scholar]
- Hunniford M. E., Torrey S., Bédécarrats G., Duncan I. J., Widowski T. M. Evidence of competition for nest sites by laying hens in large furnished cages. Appl. Anim. Behav. Sci. 2014;161:95–104. [Google Scholar]
- Keeling L. J. Inter-bird distances and behavioural priorities in laying hens: The effect of spatial restriction. Appl. Anim. Behav. Sci. 1994;39:131–140. [Google Scholar]
- Klein T., Zeltner E., Huber-Eicher B. Are genetic differences in foraging behaviour of laying hen chicks paralleled by hybrid-species differences in feather pecking? Appl. Anim. Behav. Sci. 2000;143:155–170. doi: 10.1016/s0168-1591(00)00147-7. [DOI] [PubMed] [Google Scholar]
- Krause J., Ruxton G. D. Living in Groups. Oxford University Press; Oxford, UK: 2002. [Google Scholar]
- Kruschwitz A., Zupan M., Buchwalder T., Huber-Eicher B. Prelaying behaviour of laying hens (Gallus gallus domesticus) in different free range settings. Arch. Geflügelk. 2008;72:84–89. [Google Scholar]
- Landis J. R., Koch G. G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Lentfer T. L., Gehbardt-Henrich S. G., Frölich E. K. F., von Borrell E. Nest use is influence by positions of the nests and drinkers in aviaries. Poult. Sci. 2013;92:1433–1442. doi: 10.3382/ps.2012-02718. [DOI] [PubMed] [Google Scholar]
- Lill A. Spatial organisation in small flocks of domestic fowl. Behaviour. 1968;32:258–290. doi: 10.1163/156853968x00225. [DOI] [PubMed] [Google Scholar]
- McLean K. A., Baxter M. R., Michie W. Comparison of the welfare of laying hens in battery cages and in a perchery. Res. Dev. Agric. 1986;3:93–98. [Google Scholar]
- Mench J. A., Blatchford R. A. Determination of space use by laying hens using kinematic analysis. Poult. Sci. 2014;93:794–798. doi: 10.3382/ps.2013-03549. [DOI] [PubMed] [Google Scholar]
- Moinard C., Statham P., Haskell M. J., McCorquodale C., Jones R. B., Green P. R. Accuracy of laying hens in jumping upwards and downwards between perches in different light environments. Appl. Anim. Behav. Sci. 2004;85:77–92. [Google Scholar]
- Newberry R., Hall J. Use of pen space by broiler chickens: Effects of age and pen size. Appl. Anim. Behav. Sci. 1990;25:125–136. [Google Scholar]
- Odén K., Keeling L., Algers B. Behaviour of laying hens in two types of aviary systems on 25 commercial farms in Sweden. Br. Poult. Sci. 2002;43:169–181. doi: 10.1080/00071660120121364. [DOI] [PubMed] [Google Scholar]
- Olsson I. A. S., Keeling L. J. The push-door for measuring motivation in hens: Hens are motivated to perch at night. Anim. Welf. 2002;11:11–19. [Google Scholar]
- Olsson I. A. S., Keeling L. J. Why in earth? Dustbathing behaviour in jungle and domestic fowl reviewed from a Tinbergian and animal welfare perspective. Appl. Anim. Behav. Sci. 2005;93:259–282. [Google Scholar]
- Powell G. Experimental analysis of the social value of flocking by starlings (Sturnus vulgaris) in relation to predation and foraging. Anim. Behav. 1974;22:501–505. [Google Scholar]
- Riber A. B. Development with age of nest box use and gregarious nesting in laying hens. Appl. Anim. Behav. Sci. 2010;123:24–31. [Google Scholar]
- Riber A. B. Gregarious nesting—An anti-predator response in laying hens. Appl. Anim. Behav. Sci. 2012;138:70–78. [Google Scholar]
- Riber A. B., Nielsen B. L., Ritz C., Forkman B. Diurnal activity cycles and synchrony in layer hen chicks (Gallus gallus domesticus) Appl. Anim. Behav. Sci. 2007;108:276–287. [Google Scholar]
- Schrader L., Müller B. Night-time roosting in the domestic fowl: The height matters. Appl. Anim. Behav. Sci. 2009;121:179–183. [Google Scholar]
- Schütz K. E., Jensen P. Effects of resource allocation on behavioural strategies: A comparison of red junglefowl (Gallus gallus) and two domesticated breeds of poultry. Ethology. 2001;107:753–765. [Google Scholar]
- Schütz K. E., Forkman B., Jensen P. Domestication effects on foraging strategy, social behaviour and different fear responses: A comparison between the red junglefowl (Gallus gallus) and a modern layer strain. Appl. Anim. Behav. Sci. 2001;74:1–14. [Google Scholar]
- Singh R., Cheng K. M., Silversides F. G. Production performance and egg quality of four strains of laying hens kept in conventional cages and floor pens. Poult. Sci. 2009;88:256–264. doi: 10.3382/ps.2008-00237. [DOI] [PubMed] [Google Scholar]
- Stratmann A., Konrad E., Fröhlich F., Gebhardt-Henrich S., Harlander-Matauschek A., Würbel H., Toscano M. Modification of aviary design reduces incidence of falls, collisions and keel bone damage in laying hens. Appl. Anim. Behav. Sci. 2015;165:112–123. [Google Scholar]
- United Egg Producers . Animal Husbandry Guidelines for U.S. Laying Flocks. 2016 Edition. 2016. http://uepcertified.com/wp-content/uploads/2015/08/2016-UEP-Animal-Welfare-Guidelines-2016-Cage-Free-Edit-002.pdf, Accessed April 2016. [Google Scholar]
- Vestergaard K. Dust-bathing in the domestic fowl—diurnal rhythm and dust deprivation. Appl. Anim. Ethol. 1982;8:487–495. [Google Scholar]
- Wall H. Production performance and proportion of nest eggs in layer hybrids housed in different designs of furnished cages. Poult. Sci. 2011;90:2153–2161. doi: 10.3382/ps.2011-01495. [DOI] [PubMed] [Google Scholar]
- Webster A., Hurnik J. Synchronization of behavior among laying hens in battery cages. Appl. Anim. Behav. Sci. 1994;40:153–165. [Google Scholar]
- Wolc A., Arango J., Settar P., Fulton J., O'Sullivan N., Preisinger R., Habier D., Fernando R., Garrick D., Hill W. Genome‐wide association analysis and genetic architecture of egg weight and egg uniformity in layer chickens. Anim. Genet. 2012;43:87–96. doi: 10.1111/j.1365-2052.2012.02381.x. [DOI] [PubMed] [Google Scholar]
- Wood-Gush D., Duncan I. Some behavioural observations on domestic fowl in the wild. Appl. Anim. Ethol. 1976;2:255–260. [Google Scholar]
- Wood-Gush D. G., Duncan I. J., Savory C. Observations on the social behaviour of domestic fowl in the wild. Biol. Behav. 1978;3:193–205. [Google Scholar]
- Yeates N. The activity pattern in poultry in relation to photoperiod. Anim. Behav. 1963;11:287–289. [Google Scholar]