Abstract
Background
The aim of this study was to investigate the possible associations of miRNA-27a and Leptin polymorphisms with the risk of recurrent spontaneous abortion (RSA).
Material/Methods
Between May 2013 and April 2015 at Shenzhen Longhua New District Central Hospital, we randomly recruited 138 RSA patients as the case group and another 142 normal pregnancy women as the control group. We used denaturing high-performance liquid chromatography (DHPLC) to determine the genotypes and allele frequencies of miRNA-27a rs895819 A/G and Leptin rs7799039 G/A.
Results
The GG genotype and G allele frequencies of miRNA-27a rs895819 A/G were higher in the case group than in the control group, and the AA genotype and A allele frequencies of Leptin rs7799039 G/A were also higher in the case group than in the control group (all P<0.05). MiRNA-27a rs895819 A/G and Leptin rs7799039 G/A polymorphisms increased the risk of RSA (Exp (B)=2.732, 95% CI=1.625~4.596, P=0.000; Exp (B)=4.081, 95% CI=1.817~9.164, P=0.001). GG-AA or AG-AA carriers had a higher risk of RSA. The miRNA-27a expression of AA carriers of miRNA-27a rs895819 was lower than that of AG+GG carriers both in the case and control groups (all P=0.024). The plasma leptin concentration of GG carriers was lower than that of GA+AA carriers in the case group (P=0.026).
Conclusions
The polymorphisms of miRNA-27a rs895819 A/G and Leptin rs7799039 G/A may contribute to an increased risk of RSA.
MeSH Keywords: Chromatography; DNA Copy Number Variations; Polymorphism, Genetic
Background
Recurrent spontaneous abortion (RSA) is defined as the miscarriage of two or more consecutive pregnancies with unknown origin within 20 weeks of pregnancy, affecting about 1–5% of reproductive age women [1,2], which is difficult to cure in clinical practice. Generally, RSA has been caused by recognized factors including genetics, endocrine, hormonal problems, infection, placental anomalies, smoking and alcohol consumption, exposure to environmental triggers, psychological trauma, and constant stressful emotion [3,4]. However, the cause for nearly half of the patients with RSA cannot be explained [5]. Many scientists have made trials on the treatment of RSA from many fields, such as genetics, endocrinology, and immunology [6–8]. With the rapid development of molecular biology technology, the focus has been shifted to the relationship between gene polymorphism and RSA, such as the relationships between microRNAs (miRNAs) and RSA [9,10]. Some previous studies suggest that miRNAs, by affecting endometrium, pre-eclampsia, and infertility, might be essential for the normal function of the reproductive system and provide noninvasively obtained diagnostic information [11,12].
MiRNAs are a class of small endogenous noncoding RNAs that negatively regulate target gene transcription through hybridization to incomplete complementary sequences in the 3′ untranslated region of their target mRNAs [13]. Previous evidence has shown the impact of miRNAs on human reproduction [14,15]. As an important member in the miRNA family, miRNA-27a is actively expressed in many cancers, such as breast cancer, colorectal cancer, and gastric cancer, and is considered as an effective indicator for early diagnosis and prognosis of tumors [16–18]. Accumulating evidence has demonstrated that single-nucleotide polymorphisms (SNPs) in miRNA precursors may influence the expression levels of miRNA. Recently, an important A to G transition SNP in pre-mir-27a (rs895819) was identified in common cancers [19,20]. Leptin is the gene product of the obese gene and may regulate body weight, satiety, and fertility [21]. The adipokine leptin is a lipostatic signal governing food intake and stimulating energy expenditure. It is also a pivotal metabolic regulator, which correlates with the pro-inflammatory Th1 immune response to energy balance and nutritional status [22]. In recent years, leptin regulation of immune response and inflammatory response has received much research attention due to its significant changes during infection and inflammation [21,23]. The human leptin gene is composed of three exons and two introns located on chromosome 7q31.3, which spans ~18 kb of genomic DNA [24]. Leptin rs7799039 G/A was shown to be a common SNP investigated in the association between leptin gene polymorphisms and plasma leptin level [25]. However, due to the limited amount of examined miRNA, and the scarcity of explorations into the effect of leptin on non-mammals, reports on the risks of RSA and its connection with gene polymorphism of miRNA-27a and leptin has received less attention. Therefore, our present study intended to explore the effect of gene polymorphisms of miRNA-27a and leptin on RSA, to evaluate the regulatory function of miRNA in the process of pregnancy, and to discuss the effect and significance of the miRNA-27a and leptin on RSA.
Material and Methods
Subjects
From May 2013 to April 2015, a total of 138 RSA patients with a mean age of 28.83±4.57 years and menstrual cycle of 31.52±3.03 days, admitted to the obstetric clinic of Shenzhen Longhua New District Central Hospital, were recruited into our study as the case group. All RSA cases were confirmed according to the diagnostic criteria, which were: (1) women with two or more consecutive spontaneous abortions; (2) karyotypes of couples and chorionic villus sampling (CVS) after abortion showing normal; (3) no abnormal anatomy of the reproductive tract; (4) endocrine function such as sex hormone secretion and thyroid function presenting normal; (5) negative autoantibodies, including anticardiolipin antibody, antinuclear antibody, antipaternal complement-dependent antibody (APCA) and the Toxoplasma (TOX), Other (OTH), Rubella virus (RUV), Cytomegalovirus (CMV), and Herpes simplex virus-II (HSV-II) (TORCH); (6) no thrombotic disease or thrombotic tendency; and (7) no inflammatory response in the reproductive tract or systemic inflammatory response. During the same period, we also recruited 142 normal pregnancy women as the control group, with a mean age of 29.12±4.33 years and menstrual cycle of 32.04±3.17 days. The inclusion criteria for the control group were: (1) no history of spontaneous abortion; (2) karyotypes of couples presenting normal; and (3) at least one normal live birth and no pregnancy-related complications. Baseline characteristics of the subjects between the case and control groups show no significant differences (both P>0.05).
Also, we excluded patients with history of smoking, drinking, drug use, and family disease; patients with chronic pain, reproductive tract abnormality, or endocrine disorder; patients with severe cardio-cerebrovascular disease, liver and kidney dysfunction; patients with comorbidity of depression and other mental disorders; and patients with reoperation due to complications within the last three months. This study was approved by the Ethics Committee of Shenzhen Longhua New District Central Hospital. All subjects signed informed consents.
Detections of miRNA-27a and leptin polymorphisms
The peripheral blood (5 ml) from each subject was placed in a tube containing ethylenediamine tetraacetic acid (EDTA) and stored at −80°C. With addition of erythrocytic segment, DNA was extracted with the use of the Blood Genome DNA Extraction Kit (TaKaRa Biotech Co., Ltd., Dalian, China). Polymerase chain reaction (PCR) amplification was conducted with primers of rs895819 A/G in miRNA-27a and rs7799039 G/A in leptin, and the primers were designed by Prime 5.0 software and then synthesized by Shanghai Sangon Biotech Co., Ltd. (Table 1). The PCR reaction condition was: 94°C for 2 min; 35 cycles of 94°C for 1 min, 52°C for 1 min, and 72°C for 1 min; 72°C extension for 5 min. The products were stored at 4°C. All genotypes of the PCR products were examined using DHPLC (Transgenomic Inc., Omaha, NE, USA). With column temperature of 59.3°C and mobile phase flow rate of 0.9 ml/min, miRNA-27a rs895819 A/G was genotyped through two steps (Figure 1): (1) heterozygote AG exhibited bimodal DHPLC (Figure 1A); (2) PCR samples that exhibited unimodal DHPLC mixed with equivalent AA samples verified by sequencing, and then the mixture was subject to DHPLC analysis, with AA exhibiting unimodal and GG exhibiting bimodal (Figure 1B). A and G mutations were also confirmed by sequencing (Figure 1C). Leptin rs7799039 G/A was genotyped through two steps (Figure 2): (1) heterozygote GA displayed bimodal DHPLC (Figure 2A); (2) PCR samples that displayed unimodal DHPLC mixed with equivalent GG samples as verified by sequencing, and then the mixture underwent DHPLC analysis, with GG displaying unimodal and AA displaying bimodal (Figure 2B). G and A mutations were also confirmed by sequencing (Figure 2C).
Table 1.
The PCR primer sequences of miRNA-27a rs895819 A/G and Leptin rs7799039 G/A.
| Gene | Primer sequence | Annealing temperature | Annealing time | Cycle |
|---|---|---|---|---|
| miRNA-27a rs895819 A/G | F: 5′-ATATGAGAAAAGAGCTTCCCTGTG -3′ | 61°C | 45 s | 35 |
| R: 5′-CAAGGCCAGAGGAGGTGAG -3′ | ||||
| Leptin rs7799039 G/A | F: 5′-TTTCCTGTAATTTTCCCATGAG -3′ | 61°C | 45 s | 35 |
| R: 5′-AAAGCAAAGACAGGCATAAAA -3′ |
Figure 1.
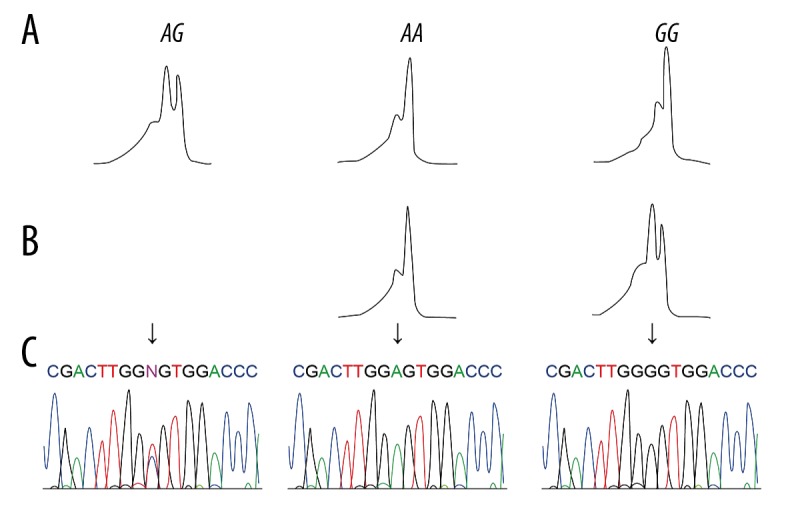
(A–C) Denaturing high-performance liquid chromatography (DHPLC) for miRNA-27a rs895819 A/G and sequencing of polymerase chain reaction (PCR) products.
Figure 2.
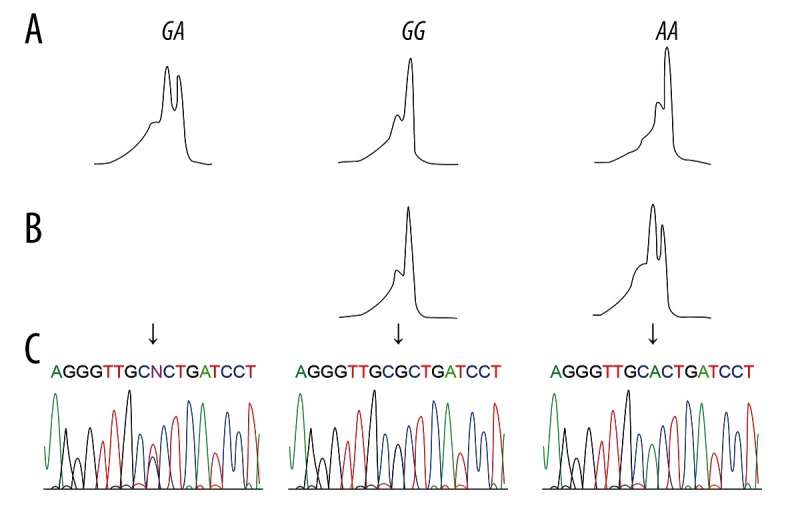
(A–C) Denaturing high-performance liquid chromatography (DHPLC) for Leptin rs7799039 G/A and sequencing of polymerase chain reaction (PCR) products.
Real-time quantitative PCR (RT-qPCR)
Serum separated from the whole blood by centrifugation at 3000 rpm for 10 min at 4°C was transferred into a centrifuge tube. Total RNA (tRNA) was extracted from the serum using the mirVana PARIS Kit (Ambion Inc., Austen, TX, USA), and then reversely transcribed to obtained cDNA using a reverse transcription system (Thermo Fisher Scientific Fermentas, MA, USA). With the cDNA as template, the primers of miRNA-27a (Forward: 5′ TGCGGTTCACAGTGGCTAAG 3′; Reverse: 5′ CTCAACTGGTGTCGTGGA 3′) were used for PCR amplification. Using the Taq-Man MicroRNA Kit (Ambion Inc., Austen, TX, USA), every cDNA sample obtained from RT-PCR was used to detect the expression level of miRNA-27a in a fluorescence-based RT-qPCR instrument (Model 7900; Applied Biosystems Inc., Foster City, CA, USA).
Enzyme-linked Immunosorbent Assay (ELISA)
Separated from whole blood by centrifugation at 3000 rpm for 10 min at 4°C, plasma was transferred into a centrifuge tube. The following procedures were all performed according to the kit (R&D Systems Inc, Minneapolis, MN, USA) manual. Samples were brought to 37°C, the ELISA plate was coated with diluted plasma for 30 min, then to each hole we added 50 μL enzyme liquid for 30 min. Subsequently, with the addition of 50 μL chromogenic reagent A and 50 μL reagent B, each hole was stored in the dark for 15 min. Consequently, each hole in the ELISA plate turned blue. It was appropriate to extend the coloration time if the color was too light. With 50 μL stopping solution added into each hole, the color changed to yellow immediately. The optical density (OD) value was measured at a wavelength of 450 nm at 15 min after the termination. The standard curve was drawn with OD value as the abscissa and concentration as the ordinate, and concentrations of the standard product were 25, 50, 100, 200, 400 ng/L successively. The optimal dilution ratio of other samples was predicted according to the concentration of plasma.
Statistical analysis
Continuous data are expressed as mean ± standard deviation (SD), with t test for comparison. Categorical data are exhibited as percentage or rate, with χ2 test for comparison. P<0.05 was considered as statistical significance. The χ2 test was also employed to identify whether samples between two groups met the Hardy-Weinberg equilibrium used to examine the representativeness of a study population. P≥0.05 indicated samples met the genetic equilibrium and had group representation. Odds ratio (OR) with 95% confidence interval (CI) was calculated to estimate association between disease and polymorphism in single-factor and multi-factor logistic regression analyses. Data analysis was conducted using SPSS 19.0 software (SPSS, Inc. IBM, Chicago, IL, USA).
Results
Distributions of genotype and allele frequencies of miRNA-27a rs895819 A/G and Leptin rs7799039 G/A
Distributions of genotype and allele frequencies of miRNA-27a rs895819 A/G and Leptin rs7799039 G/A are presented in Table 2. The test for Hardy-Weinberg equilibrium revealed an agreement of P value to the law of genetic equilibrium, which demonstrated that the study population was representative.
Table 2.
Comparison of the distribution frequency of genotype polymorphism and allele gene on miRNA-27a rs895819 A/G and Leptin rs7799039 G/A.
| Genotype | Case group (n=138) | Control group (n=142) | χ2 | P | OR (95%CI) |
|---|---|---|---|---|---|
| miRNA-27a rs895819 | |||||
| AA | 56 (40.7%) | 78 (54.9%) | Ref. | ||
| AG | 60 (43.4%) | 51 (35.9%) | 3.662 | 0.056 | 1.639 (0.987~2.721) |
| GG | 22 (15.9%) | 13 (9.2%) | 4.956 | 0.026 | 2.357 (1.095~5.075) |
| AG + GG | 82 (59.3%) | 64 (45.1%) | 5.775 | 0.016 | 1.785 (1.111~2.867) |
| A | 172 (62.3%) | 207 (72.9%) | Ref. | ||
| G | 104 (37.7%) | 77 (27.1%) | 7.147 | 0.008 | 1.625 (1.137~2.324) |
| Leptin rs7799039 G/A | |||||
| GG | 11 (8.0%) | 25 (17.6%) | Ref. | ||
| GA | 61 (44.2%) | 71 (50.0%) | 2.831 | 0.092 | 1.953 (0.888~4.292) |
| AA | 66 (47.8) | 46 (32.4%) | 8.787 | 0.003 | 3.262 (1.461~7.279) |
| GA + AA | 127 (92.0%) | 117 (82.4%) | 5.798 | 0.016 | 2.467 (1.162~5.236) |
| G | 83 (30.1%) | 121 (42.6%) | Ref. | ||
| A | 193 (69.9%) | 163 (57.4%) | 9.494 | 0.002 | 1.726 (1.218~2.446) |
OR – odds ratio; Ref – reference; CI – confidence interval.
With respect to miRNA-27a rs895819 A/G, the frequencies of GG genotype and G allele were both significantly higher in the case group than in the control group (GG genotype: 15.9% vs. 9.2%, OR (95%CI)=2.357 (1.095~5.075), P=0.026; G allele: 37.7% vs. 27.1%, OR (95% CI)=1.625 (1.137~2.324), P=0.008). For Leptin rs7799039 G/A, the frequencies of AA genotype and A allele were also both higher in the case group than in the control group (AA genotype: 47.8% vs. 32.4%, OR (95% CI)=3.262 (1.461~7.279), P=0.003; A allele: 69.9% vs. 57.4%, OR (95% CI)=1.726 (1.218~2.446), P=0.002).
miRNA-27a rs895819 A/G and Leptin rs7799039 G/A polymorphisms and risk of RSA
With AA and non-AA genotypes of miRNA-27a rs895819 A/G and GG and non-GG genotypes of Leptin rs7799039 G/A as independent variables, and RSA patients as dependent variables, the bivariate Logistic regression analyses were performed. The results signified that non-AA genotypes of miRNA-27a rs895819 A/G exhibited 2.732 times higher risk of RSA compared with AA genotype (95% CI=1.625~4.596, P=0.000). Non-GG genotypes of Leptin rs7799039 G/A showed 4.081 times higher risk of RSA compared with GG genotype (95% CI=1.817~9.164, P=0.001) (Table 3).
Table 3.
Logistic regression analysis of gene locus of miRNA-27a rs895819 A/G and Leptin rs7799039 G/A.
| Variable | B | S.E. | Wald | df | Sig. | Exp(B) | 95% CI |
|---|---|---|---|---|---|---|---|
| miRNA-27a rs895819 A/G | 1.005 | 0.265 | 14.359 | 1 | 0.000 | 2.732 | 1.625~4.596 |
| Leptin rs7799039 G/A | 1.406 | 0.413 | 11.608 | 1 | 0.001 | 4.081 | 1.817~9.164 |
B – beta coeffient; S.E. – standard error; Wald – wald test statistic; df – difference; Sig. – significance; 95%CI – 95% confidence interval.
Gene combination of miRNA-27a rs895819 A/G and Leptin rs7799039 G/A and the risk of RSA
We found that, compared to individuals with AA-GG combined genotypes, GG-AA carriers had 4.714 times (95% CI=1.077~20.63, P=0.034) and AG-AA carriers had 3.480 times (95% CI=1.114~10.87, P=0.028) higher risk of RSA. However, other related gene combinations interacting with the risk of RSA exhibited no significant difference (Table 4).
Table 4.
Relativity of risk of RSA and combined genotype on the locus of miRNA-27a rs895819 A/G and Leptin rs7799039 G/A.
| Combined genotype | Case group (n=138) | Control group (n=142) | OR | 95%CI | P | |
|---|---|---|---|---|---|---|
| miRNA-27a rs895819 A/G | Leptin rs7799039 G/A | |||||
| AA | GG | 7 (17.0) | 11 (35.7) | Ref. | ||
| AG | GA | 27 (17.0) | 26 (35.7) | 1.632 | 0.549~4.855 | 0.376 |
| GG | AA | 12 (21.8) | 4 (24.8) | 4.714 | 1.077~20.63 | 0.034 |
| AA | GA | 26 (10.9) | 39 (14.7) | 1.048 | 0.359~3.054 | 0.932 |
| AG | AA | 31 (34.7) | 14 (41.9) | 3.480 | 1.114~10.87 | 0.028 |
| GG | GG | 1 (17.7) | 3 (24.8) | 0.524 | 0.045~6.095 | 0.601 |
| AA | AA | 22 (23.1) | 28 (17.1) | 1.235 | 0.411~3.710 | 0.707 |
| AG | GG | 3 (17.0) | 11 (35.7) | 0.429 | 0.087~2.102 | 0.291 |
| GG | GA | 9 (21.8) | 6 (24.8) | 2.357 | 0.580~9.579 | 0.227 |
OR – odds ratio; 95%CI – 95% confidence interval; Ref – reference.
Polymorphism of miRNA-27a rs895819 A/G and Leptin rs7799039 G/A and clinical features of RSA
We analyzed the clinical data collected from the 138 patients with RSA, and found that, compared with patients with AA genotype of miRNA-27a rs895819 A/G, patients with AG + GG genotype suffered more intense pregnant reactions, shorter duration of miscarriage in pregnancy, more frequent abortion, and longer interval to next pregnancy (all P<0.05), indicating that there were significant differences in clinical features between patients with G allele and those with non-G allele. Also, compared to patients with GG genotype of Leptin rs7799039 G/A, patients with GA + AA genotype suffered more intense pregnant reactions, shorter duration of miscarriage in pregnancy, more frequent abortion, and lengthened interval to next pregnancy (all P<0.05), revealing that there were also significant differences in clinical features between patients with A allele and those with non-A allele (Table 5).
Table 5.
Association between clinical features of RSA and genotype of miRNA-27a rs895819 A/G and Leptin rs7799039 G/A n (%).
| Genotype | Case group | Severe pregnancy reaction | Abortion time in pregnancy (≥3 months) | Number of abortion (≥3 times) | Duration to next pregnancy (≥6 months) |
|---|---|---|---|---|---|
| miRNA-27a rs895819 | |||||
| AA | 56 (40.7%) | 5 (8.9%) | 2 (3.6%) | 9 (16.1%) | 1 (1.8%) |
| AG + GG | 82 (59.3%) | 23 (28.0%) | 14 (17.1%) | 32 (39.0%) | 13 (4.8%) |
| χ2 | 5.171 | 4.815 | 4.743 | 6.063 | |
| P | 0.023 | 0.028 | 0.029 | 0.014 | |
| Leptinrs7799039 | |||||
| GG | 11 (8.0%) | 6 (54.5%) | 4 (36.4%) | 8 (72.7%) | 5 (45.5%) |
| GA + AA | 127 (92.0%) | 22 (17.3%) | 12 (9.4%) | 33 (26.0%) | 9 (7.1%) |
| χ2 | 4.586 | 4.729 | 4.438 | 10.39 | |
| P | 0.032 | 0.030 | 0.035 | 0.001 | |
MiRNA-27a and leptin level in plasma and RSA
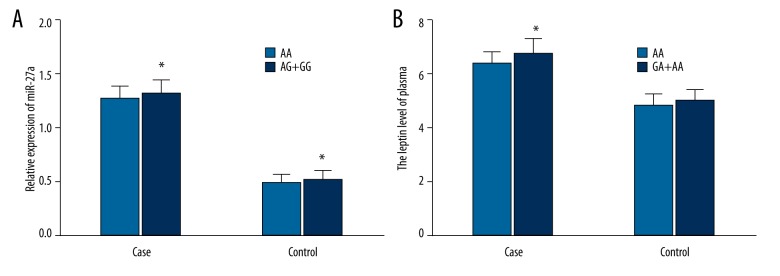
MiRNA-27a was expressed in the patients with AA genotype and AG + GG genotype of rs895819 A/G in both case group and control group, with a significant difference in its total expression volume between the control group (0.50±0.09) and the case group (1.30±0.13) (P<0.01) (Figure 3A). In the control group, subjects with AA genotype had miRNA-27a expression of 0.49±0.08, lower than the 0.52±0.09 (P=0.037) in those with AG + GG genotype. In the case group, miRNA-27a expression of patients with AA genotype was 1.27±0.12, lower than the 1.32±0.13 (P=0.024) in patients with AG + GG genotype.
Figure 3.
The expression of plasma miRNA-27a influenced by miRNA-27a rs895819 A/G and the level of plasma leptin influenced by Leptin rs7799039 G/A. (A) Refers to the expression of plasma miRNA-27a; * refers to P<0.05 when AA genotype was compared with AG + GG genotype; (B) Refers to the level of plasma leptin; * refers to P<0.05 when GG genotype was compared with AG + AA genotype.
Plasma leptin level of patients with GG genotype and GA + AA genotype rs7799039 G/A was much different (P<0.01) between the case group (6.74±0.56) ng/ml and control group (4.99±0.39) ng/ml (Figure 3B). In the control group, leptin level in the subjects with GG genotype was (4.87±0.36) ng/ml, slightly lower than the 5.01±0.39 ng/ml in subjects with GA + AA genotype (P=0.101). In the case group, leptin level in the patients with GG genotype was (6.38±0.45) ng/ml, lower than the 6.77±0.56 ng/ml (P=0.026) in patients with GA + AA genotype (Table 6).
Table 6.
Expression of miRNA-27a and level of Leptin in plasma (mean ±SD).
| Genotype | Case group | Control group |
|---|---|---|
| miRNA-27a | ||
| AA | 1.27±0.12 | 0.49±0.08 |
| AG + GG | 1.32±0.13 | 0.52±0.09 |
| P | 0.024 | 0.037 |
| Leptin (ng/ml) | ||
| GG | 6.38±0.45 | 4.87±0.36 |
| GA + AA | 6.77±0.56 | 5.01±0.39 |
| P | 0.026 | 0.101 |
SD – standard deviation.
Discussion
The etiology of RSA is complicated, involving genetics, endocrine, hormonal problems, placental anomalies, psychological trauma, and constant stressful emotion [3,4]. In recent years, some new ideas were put forward from the perspective of immunology and genetics, considering that the causes of RSA are related with gene polymorphism [24]. Due to the scarcity of literature on the relationship between miRNA and leptin, and the risk of RSA, we expected to make a comprehensive evaluation on the relationship between RSA and gene polymorphism of miRNA and leptin.
Our results revealed the case group had a significantly higher frequency of G allele gene and GG genotype distributed on miRNA-27a rs895819 A/G in comparison with the control group. There was also a significant up-regulation in the frequency of A allele gene and AA genotype of Leptin rs7799039 G/A. Consequently, we concluded that mutation of G gene of miRNA-27a rs895819 A/G and mutation of gene A of Leptin rs7799039 G/A might increase the risk of RSA. As an important component of the miRNA class, miRNA-27a is closely related to the development of diseases, notably tumors, cardiovascular diseases, and metabolic diseases [25,26]. Recent studies have shown that the irregular expression of miRNAs might lead to recurrent miscarriage [9,10]. As a kind of protein mainly secreted by fat cells and involved in energy intake and balance of protein, leptin can influence the female reproductive endocrine system, regulate the hypothalamus-pituitary-gonadal axis, affect reproductive function and female ovulation, and participate in pubertal behavior, which plays a complex role in physiological regulation in connection with vegetative and reproductive metabolism [27,28]. Mounzih et al. found that leptin may directly or indirectly regulate the formation of blood vessels by affecting vascular permeability and formation of endothelial cells and trophoblastic cells in the process of forming placenta [29].
Further research deepened insights into the impact of miRNA and leptin gene polymorphisms on cancers, and Yang et al. has found that the protective effect of single-nucleotide polypeptide rs895819 distributed on the end ring of miRNA-G allele gene might decrease the possibility of gene mutation of miRNA-27a with carcinogenesis, thus reducing the risk for familial breast cancer and presents possible hormone-related effects [30]. The polymorphism of Leptin rs7799039 G/A had a significant association with antipsychotic drug-induced weight gain [31]. Hoffstedt et al. has reported that a common polymorphism in Leptin rs7799039 G/A has an effect on the expression of leptin, possibly at the transcriptional level, and therefore also adipose secretion levels of the hormone [32]. It is also reported by Mammès et al. that the A allele of Leptin rs7799039 G/A polymorphism was demonstrated to be associated with higher leptin levels before diet and lower BMI loss in women [35]. A previous study found that the AA genotype of rs7799039 G/A polymorphism and higher circulating leptin level may be associated with preeclampsia/pregnancy-induced hypertension, further leading to the occurrence of RSA [36]. Additionally, the secretion of leptin is positively correlated with body mass, and Winnie et al. reported that maternal obesity is an independent factor related to a higher risk of RSA, suggesting that leptin polymorphism may directly or indirectly contribute to the development of RSA [37]. Interestingly, reports on the risks of RSA and its connection with gene polymorphism of miRNA-27a and leptin remain scarce. Our logistic regression analysis showed that compared with individuals with combined AA-GG genotype distributed on miRNA-27a rs895819 A/G locus and Leptin rs7799039 G/A locus, patients with combined genotype of GG-AA and AG-AA had a higher risk of RSA, which further demonstrates the close ties between RSA and gene polymorphism of miRNA-27a and leptin.
Conclusions
Our study revealed polymorphisms of MiRNA-27a rs895819 A/G and Leptin rs7799039 G/A may contribute to an increased risk of RSA. Given the complicated etiology of RSA, other external triggers might have interfered with our results. Therefore, further exploration into the genetic mechanisms is needed to prevent RSA.
Footnotes
Competing interests
We declare that we have no conflicts of interest.
Source of support: Departmental sources
References
- 1.Pang L, Wei Z, Li O, et al. An increase in vascular endothelial growth factor (VEGF) and VEGF soluble receptor-1 (sFlt-1) are associated with early recurrent spontaneous abortion. PLoS One. 2013;8:e75759. doi: 10.1371/journal.pone.0075759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sierra S, Stephenson M. Genetics of recurrent pregnancy loss. Semin Reprod Med. 2006;24:17–24. doi: 10.1055/s-2006-931797. [DOI] [PubMed] [Google Scholar]
- 3.Kolte AM, Olsen LR, Mikkelsen EM, et al. Depression and emotional stress is highly prevalent among women with recurrent pregnancy loss. Hum Reprod. 2015;30:777–82. doi: 10.1093/humrep/dev014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shang W, Wang A, Lv L, et al. Individualized Hormone Adjustment in the Treatment of Recurrent Spontaneous Abortions. Cell Biochem Biophys. 2015 doi: 10.1007/s12013-015-0539-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Musters AM, Taminiau-Bloem EF, van den Boogaard E, et al. Supportive care for women with unexplained recurrent miscarriage: patients’ perspectives. Hum Reprod. 2011;26:873–77. doi: 10.1093/humrep/der021. [DOI] [PubMed] [Google Scholar]
- 6.Pereza N, Volk M, Zrakic N, et al. Genetic variation in tissue inhibitors of metalloproteinases as a risk factor for idiopathic recurrent spontaneous abortion. Fertil Steril. 2013;99:1923–29. doi: 10.1016/j.fertnstert.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Ke RW. Endocrine basis for recurrent pregnancy loss. Obstet Gynecol Clin North Am. 2014;41:103–12. doi: 10.1016/j.ogc.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Wu L, Luo LH, Zhang YX, et al. Alteration of Th17 and Treg cells in patients with unexplained recurrent spontaneous abortion before and after lymphocyte immunization therapy. Reprod Biol Endocrinol. 2014;12:74. doi: 10.1186/1477-7827-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Li B, Wang J, et al. Evidence that miR-133a causes recurrent spontaneous abortion by reducing HLA-G expression. Reprod Biomed Online. 2012;25:415–24. doi: 10.1016/j.rbmo.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Jeon YJ, Choi YS, Rah H, et al. Association study of microRNA polymorphisms with risk of idiopathic recurrent spontaneous abortion in Korean women. Gene. 2012;494:168–73. doi: 10.1016/j.gene.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 11.Pan Q, Luo X, Toloubeydokhti T, et al. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol Hum Reprod. 2007;13:797–806. doi: 10.1093/molehr/gam063. [DOI] [PubMed] [Google Scholar]
- 12.Pickering MT, Stadler BM, Kowalik TF. miR-17 and miR-20a temper an E2F1-induced G1 checkpoint to regulate cell cycle progression. Oncogene. 2009;28:140–45. doi: 10.1038/onc.2008.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 14.Wu MF, Tian Q, Reed JW. Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression, and regulates both female and male reproduction. Development. 2006;133:4211–18. doi: 10.1242/dev.02602. [DOI] [PubMed] [Google Scholar]
- 15.Carletti MZ, Christenson LK. MicroRNA in the ovary and female reproductive tract. J Anim Sci. 2009;87:E29–38. doi: 10.2527/jas.2008-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mertens-Talcott SU, Chintharlapalli S, Li X, et al. The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67:11001–11. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- 17.Bian Q, Chen JJ, Gu JP, et al. Association between pre-miR-27a functional polymorphism and risk of colorectal cancer in north Chinese Han population. Onco Targets Ther. 2015;8:3003–7. doi: 10.2147/OTT.S89754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang D, Wang H, Liu R, et al. miRNA27a is a biomarker for predicting chemosensitivity and prognosis in metastatic or recurrent gastric cancer. J Cell Biochem. 2014;115:549–56. doi: 10.1002/jcb.24689. [DOI] [PubMed] [Google Scholar]
- 19.Zhang N, Huo Q, Wang X, et al. A genetic variant in pre-miR-27a is associated with a reduced breast cancer risk in younger Chinese population. Gene. 2013;529:125–30. doi: 10.1016/j.gene.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 20.Song B, Yan G, Hao H, et al. rs11671784 G/A and rs895819 A/G polymorphisms inversely affect gastric cancer susceptibility and miR-27a expression in a Chinese population. Med Sci Monit. 2014;20:2318–26. doi: 10.12659/MSM.892499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–46. [PubMed] [Google Scholar]
- 22.Abdel Hay RM, Rashed LA. Association between the leptin gene 2548G/A polymorphism, the plasma leptin and the metabolic syndrome with psoriasis. Exp Dermatol. 2011;20:715–19. doi: 10.1111/j.1600-0625.2011.01299.x. [DOI] [PubMed] [Google Scholar]
- 23.Carlton ED, Demas GE, French SS. Leptin, a neuroendocrine mediator of immune responses, inflammation, and sickness behaviors. Horm Behav. 2012;62:272–79. doi: 10.1016/j.yhbeh.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Tucholski K, Otto-Buczkowska E. The role of leptin in the regulation of carbohydrate metabolism. Endokrynol Pol. 2011;62:258–62. [PubMed] [Google Scholar]
- 25.Yang Y, Liu P, Guo F, et al. Genetic G2548A polymorphism of leptin gene and risk of cancer: a meta-analysis of 6860 cases and 7956 controls. J BUON. 2014;19:1096–104. [PubMed] [Google Scholar]
- 26.Daher S, Shulzhenko N, Morgun A, et al. Associations between cytokine gene polymorphisms and recurrent pregnancy loss. J Reprod Immunol. 2003;58:69–77. doi: 10.1016/s0165-0378(02)00059-1. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee N, Talcott S, Safe S, et al. Cytotoxicity of pomegranate polyphenolics in breast cancer cells in vitro and vivo: potential role of miRNA-27a and miRNA-155 in cell survival and inflammation. Breast Cancer Res Treat. 2012;136:21–34. doi: 10.1007/s10549-012-2224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeh CH, Chen TP, Wang YC, et al. MicroRNA-27a regulates cardiomyocytic apoptosis during cardioplegia-induced cardiac arrest by targeting interleukin 10-related pathways. Shock. 2012;38:607–14. doi: 10.1097/SHK.0b013e318271f944. [DOI] [PubMed] [Google Scholar]
- 29.Elzein AO, Ali AA, Hamdan HZ, et al. Materno-foetal leptin and insulin-like growth factor in low birth weight neonates. J Obstet Gynaecol. 2015 doi: 10.3109/01443615.2015.1030607. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Bajoria R, Sooranna SR, Ward BS, et al. Prospective function of placental leptin at maternal-fetal interface. Placenta. 2002;23:103–15. doi: 10.1053/plac.2001.0769. [DOI] [PubMed] [Google Scholar]
- 31.Mounzih K, Qiu J, Ewart-Toland A, et al. Leptin is not necessary for gestation and parturition but regulates maternal nutrition via a leptin resistance state. Endocrinology. 1998;139:5259–62. doi: 10.1210/endo.139.12.6523. [DOI] [PubMed] [Google Scholar]
- 32.Yang R, Schlehe B, Hemminki K, et al. A genetic variant in the pre-miR-27a oncogene is associated with a reduced familial breast cancer risk. Breast Cancer Res Treat. 2010;121:693–702. doi: 10.1007/s10549-009-0633-5. [DOI] [PubMed] [Google Scholar]
- 33.Templeman LA, Reynolds GP, Arranz B, et al. Polymorphisms of the 5-HT2C receptor and leptin genes are associated with antipsychotic drug-induced weight gain in Caucasian subjects with a first-episode psychosis. Pharmacogenet Genomics. 2005;15:195–200. doi: 10.1097/01213011-200504000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Hoffstedt J, Eriksson P, Mottagui-Tabar S, et al. A polymorphism in the leptin promoter region (−2548 G/A) influences gene expression and adipose tissue secretion of leptin. Horm Metab Res. 2002;34:355–59. doi: 10.1055/s-2002-33466. [DOI] [PubMed] [Google Scholar]
- 35.Mammes O, Betoulle D, Aubert R, et al. Novel polymorphisms in the 5′ region of the LEP gene: association with leptin levels and response to low-calorie diet in human obesity. Diabetes. 1998;47:487–89. doi: 10.2337/diabetes.47.3.487. [DOI] [PubMed] [Google Scholar]
- 36.Sugathadasa BH, Tennekoon KH, Karunanayake EH, et al. Association of −2548 G/A polymorphism in the leptin gene with preeclampsia/pregnancy-induced hypertension. Hypertens Pregnancy. 2010;29:366–74. doi: 10.3109/10641950903214617. [DOI] [PubMed] [Google Scholar]
- 37.Lo W, Rai R, Hameed A, et al. The effect of body mass index on the outcome of pregnancy in women with recurrent miscarriage. J Family Community Med. 2012;19:167–71. doi: 10.4103/2230-8229.102316. [DOI] [PMC free article] [PubMed] [Google Scholar]