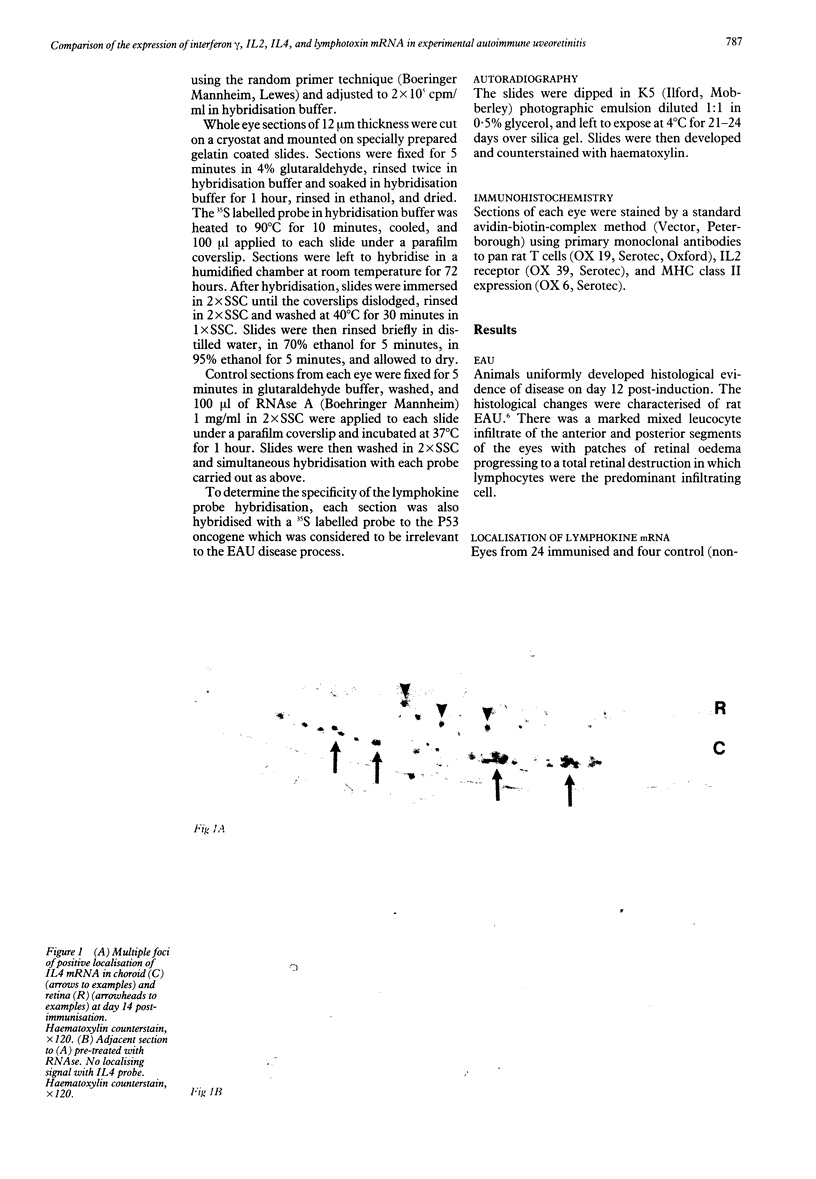
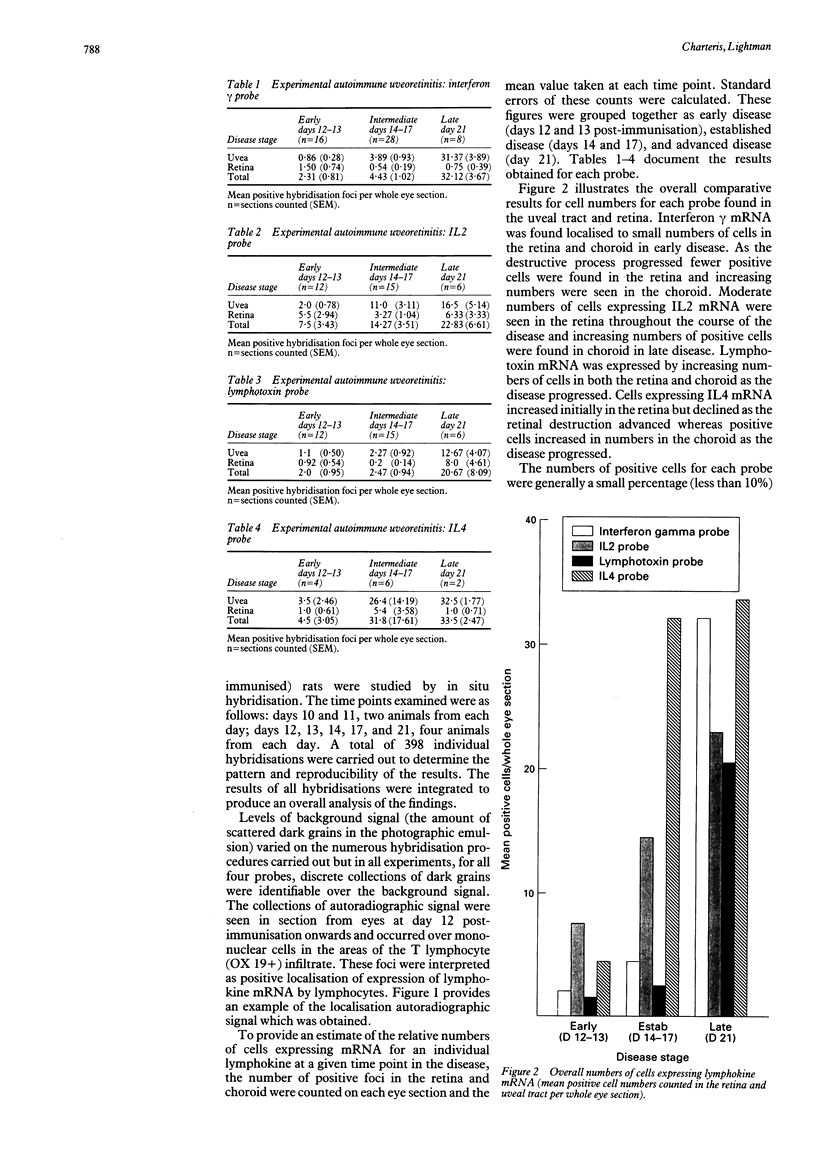
Abstract
The aim of this study was to investigate the T lymphocyte subsets involved in experimental autoimmune uveoretinitis (EAU) by quantifying the numbers of cells expressing mRNA for each of the lymphokines interferon gamma, interleukin 2, interleukin 4, and lymphotoxin throughout the disease process. Lewis rats were immunised with retinal S-antigen to provide a model of inflammatory eye disease. In situ hybridisation using cDNA probes specific for interferon gamma, IL2, IL4, and lymphotoxin mRNA were utilised to localise lymphokine mRNA expression by infiltrating cells and the numbers of positive cells counted. Localisation of mRNA for all four probes was found on increasing cell numbers as the disease process progressed. Similar numbers of cells expressed mRNA for each lymphokine, generally a small percentage of the T lymphocyte total. Activated cells within the eye express mRNA for interferon gamma, IL2, IL4, and lymphotoxin in EAU suggesting a mixed population of T lymphocyte subsets.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carding S. R., West J., Woods A., Bottomly K. Differential activation of cytokine genes in normal CD4-bearing T cells is stimulus dependent. Eur J Immunol. 1989 Feb;19(2):231–238. doi: 10.1002/eji.1830190203. [DOI] [PubMed] [Google Scholar]
- Caspi R. R., Roberge F. G., McAllister C. G., el-Saied M., Kuwabara T., Gery I., Hanna E., Nussenblatt R. B. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol. 1986 Feb 1;136(3):928–933. [PubMed] [Google Scholar]
- Chan C. C., Mochizuki M., Nussenblatt R. B., Palestine A. G., McAllister C., Gery I., BenEzra D. T-lymphocyte subsets in experimental autoimmune uveitis. Clin Immunol Immunopathol. 1985 Apr;35(1):103–110. doi: 10.1016/0090-1229(85)90083-2. [DOI] [PubMed] [Google Scholar]
- Chan C. C., Palestine A. G., Kuwabara T., Nussenblatt R. B. Immunopathologic study of Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol. 1988 Jun 15;105(6):607–611. doi: 10.1016/0002-9394(88)90052-9. [DOI] [PubMed] [Google Scholar]
- Charteris D. G., Barton K., McCartney A. C., Lightman S. L. CD4+ lymphocyte involvement in ocular Behçet's disease. Autoimmunity. 1992;12(3):201–206. doi: 10.3109/08916939209148460. [DOI] [PubMed] [Google Scholar]
- Charteris D. G., Champ C., Rosenthal A. R., Lightman S. L. Behçet's disease: activated T lymphocytes in retinal perivasculitis. Br J Ophthalmol. 1992 Aug;76(8):499–501. doi: 10.1136/bjo.76.8.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charteris D. G., Lee W. R. Multifocal posterior uveitis: clinical and pathological findings. Br J Ophthalmol. 1990 Nov;74(11):688–693. doi: 10.1136/bjo.74.11.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charteris D. G., Lightman S. L. In vivo lymphokine production in experimental autoimmune uveoretinitis. Immunology. 1993 Mar;78(3):387–392. [PMC free article] [PubMed] [Google Scholar]
- Charteris D. G., Lightman S. L. Interferon-gamma (IFN-gamma) production in vivo in experimental autoimmune uveoretinitis. Immunology. 1992 Mar;75(3):463–467. [PMC free article] [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Dallman M. J., Shiho O., Page T. H., Wood K. J., Morris P. J. Peripheral tolerance to alloantigen results from altered regulation of the interleukin 2 pathway. J Exp Med. 1991 Jan 1;173(1):79–87. doi: 10.1084/jem.173.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkema R., van der Meide P. H., Pouwels P. H., Caspers M., Dubbeld M., Schellekens H. Cloning and expression of the chromosomal immune interferon gene of the rat. EMBO J. 1985 Mar;4(3):761–767. doi: 10.1002/j.1460-2075.1985.tb03694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Roeder W. D., Laxer J. A., Townsend K. S., Weaver C. T., Hom J. T., Linton J., Torbett B. E., Glasebrook A. L. A new murine CD4+ T cell subset with an unrestricted cytokine profile. J Immunol. 1989 Jul 15;143(2):518–525. [PubMed] [Google Scholar]
- Forrester J. V., Liversidge J., Dua H. S., Towler H., McMenamin P. G. Comparison of clinical and experimental uveitis. Curr Eye Res. 1990;9 (Suppl):75–84. doi: 10.3109/02713689008999424. [DOI] [PubMed] [Google Scholar]
- Grabstein K., Dower S., Gillis S., Urdal D., Larsen A. Expression of interleukin 2, interferon-gamma, and the IL 2 receptor by human peripheral blood lymphocytes. J Immunol. 1986 Jun 15;136(12):4503–4508. [PubMed] [Google Scholar]
- Jakobiec F. A., Marboe C. C., Knowles D. M., 2nd, Iwamoto T., Harrison W., Chang S., Coleman D. J. Human sympathetic ophthalmia. An analysis of the inflammatory infiltrate by hybridoma-monoclonal antibodies, immunochemistry, and correlative electron microscopy. Ophthalmology. 1983 Jan;90(1):76–95. doi: 10.1016/s0161-6420(83)34602-9. [DOI] [PubMed] [Google Scholar]
- Li C. B., Gray P. W., Lin P. F., McGrath K. M., Ruddle F. H., Ruddle N. H. Cloning and expression of murine lymphotoxin cDNA. J Immunol. 1987 Jun 15;138(12):4496–4501. [PubMed] [Google Scholar]
- Mason D. Subsets of CD4+ T cells defined by their expression of different isoforms of the leucocyte-common antigen, CD45. Biochem Soc Trans. 1992 Feb;20(1):188–190. doi: 10.1042/bst0200188. [DOI] [PubMed] [Google Scholar]
- McKnight A. J., Barclay A. N., Mason D. W. Molecular cloning of rat interleukin 4 cDNA and analysis of the cytokine repertoire of subsets of CD4+ T cells. Eur J Immunol. 1991 May;21(5):1187–1194. doi: 10.1002/eji.1830210514. [DOI] [PubMed] [Google Scholar]
- McKnight A. J., Mason D. W., Barclay A. N. Sequence of rat interleukin 2 and anomalous binding of a mouse interleukin 2 cDNA probe to rat MHC class II-associated invariant chain mRNA. Immunogenetics. 1989;30(2):145–147. doi: 10.1007/BF02421547. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Powrie F., Mason D. Phenotypic and functional heterogeneity of CD4+ T cells. Immunol Today. 1988 Sep;9(9):274–277. doi: 10.1016/0167-5699(88)91309-6. [DOI] [PubMed] [Google Scholar]
- Yokoyama A., Evavold B., Dunn D. E., Quintans J. Production of IL-2 and IFN by TH2 clones. Immunol Lett. 1989 May;21(2):119–125. doi: 10.1016/0165-2478(89)90047-3. [DOI] [PubMed] [Google Scholar]