Highlight
ABA is required for plant acclimation to a combination of water deficit and heat stress regulating the accumulation of the key acclimation proteins APX1 and MBF1c.
Key words: aba1-1, abi1-1, abiotic stress, abscisic acid, acclimation, APX1, heat stress, MBF1c, stomata, stress combination, water deficit.
Abstract
Abscisic acid (ABA) plays a key role in plant acclimation to abiotic stress. Although recent studies suggested that ABA could also be important for plant acclimation to a combination of abiotic stresses, its role in this response is currently unknown. Here we studied the response of mutants impaired in ABA signalling (abi1-1) and biosynthesis (aba1-1) to a combination of water deficit and heat stress. Both mutants displayed reduced growth, biomass, and survival when subjected to stress combination. Focusing on abi1-1, we found that although its stomata had an impaired response to water deficit, remaining significantly more open than wild type, its stomatal aperture was surprisingly reduced when subjected to the stress combination. Stomatal closure during stress combination in abi1-1 was accompanied by higher levels of H2O2 in leaves, suggesting that H2O2 might play a role in this response. In contrast to the almost wild-type stomatal closure phenotype of abi1-1 during stress combination, the accumulation of ascorbate peroxidase 1 and multiprotein bridging factor 1c proteins, required for acclimation to a combination of water deficit and heat stress, was significantly reduced in abi1-1. Our findings reveal a key function for ABA in regulating the accumulation of essential proteins during a combination of water deficit and heat stress.
Introduction
Under natural conditions, or when grown in the field, plants are subjected to a combination of different abiotic stresses (Mittler, 2006; Mittler and Blumwald, 2010; Suzuki et al., 2014). Recent studies identified specific physiological and molecular responses of plants to a combination of different abiotic stresses, demonstrating the importance of studying stress combination (Mittler and Blumwald, 2010; Suzuki et al., 2014; Boeck et al., 2015; Hu et al., 2015; Liu et al., 2015; Zhang et al., 2015). Water deficit with high temperature represents one of the most frequent abiotic stress combinations occurring under natural conditions (Savin and Nicolas, 1996; Jiang and Huang, 2001; Mittler, 2006; Craufurd et al., 2008; Boeck et al., 2015). Previous studies have shown that the transcriptome of plants subjected to a combination of water deficit and heat stress is different from that of plants subjected to water deficit or heat stress alone (Rizhsky et al., 2002, 2004; Mittler, 2006), suggesting that the development of broad-spectrum abiotic stress-tolerant crops will require a more detailed study of the impact of multiple environmental conditions on plants and crops (Mittler and Blumwald, 2010).
Several different phytohormones play a pivotal role in the response of plants to abiotic stress (Peleg and Blumwald, 2011; De Ollas et al., 2013; Miura and Tada, 2014; Yoshida et al., 2014). Abscisic acid (ABA), for example, plays a key role in the response of plants to water deficit, salinity, and heat by regulating stomatal closure and the expression of different acclimation proteins (Bartels and Sunkar, 2005; Finkelstein, 2013). Guard cell ABA signalling has been extensively studied using the ABA-insensitive dominant mutant allele abi1-1, which severely reduces the catalytic activity of the ABI1 type 2C protein phosphatase (Koornneef et al., 1984; Merlot et al., 2001). Characterization of the redox sensitivity of ABI1 revealed strong enzymatic inactivation by H2O2 (Meinhard and Grill, 2001). Production of reactive oxygen species (ROS) by respiratory burst oxidase homologue proteins, activation of Ca2+ channels at the plasma membrane, and activation of SLAC1, required to drive stomatal closure, were all found to be impaired in the abi1-1 mutant (Wu et al., 2003; Nilson and Assmann, 2006). Jasmonic acid (JA) is also involved in stomatal responses during abiotic stresses (Munemasa et al., 2011; Daszkowska-Golec and Szarejko, 2013; Murata et al., 2015), and ROS production in guard cells is also dependent on jasmonates, which interact with the ABA pathway by increasing the influx of Ca2+ (Munemasa et al., 2011; Daszkowska-Golec and Szarejko, 2013). It has been reported that the stomata of the abi1-1 mutant are insensitive to jasmonates and that jasmonates might affect regulation of the ABA receptor complexes in guard cells (Murata et al., 2015). In addition to JA, salicylic acid (SA) can also induce stomatal closure, which is accompanied by extracellular and intracellular ROS accumulation and inward-rectifying K+ channel inactivation in guard cells (Khokon et al., 2011).
Aside from regulating stomatal responses, ABA is involved in transcriptional regulation, for example via the ABA-responsive element (ABRE) or dehydration-responsive element (DRE; Shinozaki and Yamaguchi-Shinozaki, 2007; Umezawa et al., 2010; Nakashima and Yamaguchi-Shinozaki, 2013). A recent genome‐wide search for ABRE and DRE cis‐motifs in Arabidopsis thaliana identified 2052 genes containing these elements (Mishra et al., 2014). In addition, about 1354 genes had impaired transcript accumulation in the abi1-1 mutant (Hoth et al., 2002).
Mutants impaired in ABA signalling (abi1-1) or ABA biosynthesis (aba1-1) were recently reported to be impaired in their acclimation to a combination of salt and heat stress (Suzuki et al., 2016), suggesting an important role for ABA in plant acclimation to abiotic stress combination. Nevertheless, it is not known whether this impairment is due to ABA’s role in regulating stomatal responses, transcript expression, or both (Suzuki et al., 2016).
Here we report that abi1-1 and aba1-1 plants are impaired in their acclimation to a combination of water deficit and heat stress. Focusing on the abi1-1 mutant, we found that although its stomata displayed an impaired response to water deficit stress, remaining significantly more open than wild type, its stomatal aperture was surprisingly reduced when plants were subjected to a combination of water deficit and heat stress, similar to wild type. This demonstrates a potentially unique stomatal regulation mechanism during stress combination. Stomatal closure in the abi1-1 mutant during stress combination was accompanied by higher levels of H2O2 in leaves, suggesting that H2O2 might play a role in this response. In contrast to the almost wild-type response of stomatal closure displayed by abi1-1 plants during the stress combination, the accumulation of ascorbate peroxidase 1 (APX1) and multiprotein bridging factor 1c (MBF1c), two proteins required for plant survival during a combination of water deficit and heat stress (Suzuki et al., 2005; Koussevitzky et al., 2008), was significantly reduced in the abi1-1 mutant compared to wild type. Our findings reveal a potential role for H2O2 in regulating stomatal responses during stress combination and point to a key role for ABA in regulating the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress.
Materials and methods
Plant material, growth conditions, and stress treatments
Arabidopsis thaliana Ler (cv Landsberg erecta), aba1-1, and abi1-1 (Assmann et al., 2000) plants were grown in 240-cm2 inserts on soil mixture (MetroMix 200, SUN GRO) under controlled conditions: 21°C, 10-h light cycle, 100 μmol m−2 s−1, and relative humidity of 70% (AR-66, Percival Scientific) as described in Suzuki et al. (2016). All stress treatments were performed in parallel as described in Rizhsky et al. (2004) with the following modifications: A water deficit was applied by withdrawing water from 10-day-old plants until reaching 40% of control soil weight, typically within 20–25 days. Heat stress was imposed by transferring 30-day-old plants to 38°C for 8h as follows: 06:00–08:00, 21°C; 08:00–16:00, 38°C. The water deficit and heat stress combination was performed by applying heat stress to 30-day-old plants under water deficit (Supplementary Fig. S1). Rosettes from Ler and abi1-1 plants were sampled at the same time and all measurements were performed in parallel after each stress condition (Supplementary Fig. S1). Following the stress treatments, plants were recovered under controlled conditions for 5 days and scored for survival. Temperature and relative humidity were recorded regularly with a portable USB datalogger (OM-EL-USB-2-LCD-PLUS, OMEGA Engineering, Inc., Stamford, CT, USA; Supplementary Fig. S1B). All experiments were repeated at least three times.
Growth characteristics
Fresh weight (FW), dry weight (DW), and plant diameter were measured as described in Suzuki et al., (2005, 2016). Relative water content (RWC) was measured using rosettes, which were immediately weighed after stress treatments to obtain FW. Rosettes were then placed in a beaker of water overnight in the dark, allowing them to become fully hydrated. They were then reweighed to obtain turgid weight (TW) and dried at 40ºC for 4 days to obtain DW. Finally, RWC (%) was calculated as [(FW − DW) × (TW − DW)−1] × 100. For plant survival, plants were recovered for 5 days under controlled conditions (well-watered and 21°C) and the percentage survival of plants was scored and calculated as described in Koussevitzky et al. (2008) and Suzuki et al. (2016).
Stomatal conductance
Stomatal conductance (gs) was measured in parallel on aba1-1 and abi1-1 plants of each treatment using an LCpro+ portable infrared gas analyser (ADC BioScientific Ltd., Hoddesdon, UK). After instrument stabilization, at least 10 measurements were taken on three leaves in three replicate plants from each mutant and stress treatment.
Plant hormone analysis
Hormone extraction and analysis were carried out as described in Durgbanshi et al. (2005) with few modifications. Briefly, 0.1g of dry tissue was extracted in 2mL of ultrapure water after spiking with 50ng of [2H6]-ABA, [C13]-SA, and dihydrojasmonic acid in a ball mill (MillMix20, Domel, Železniki, Slovenija). After centrifugation at 4000 g at 4ºC for 10min, supernatants were recovered and pH adjusted to 3 with 30% acetic acid. The water extract was partitioned twice against 2mL of diethyl ether and the organic layer recovered and evaporated under vacuum in a centrifuge concentrator (Speed Vac, Jouan, Saint Herblain Cedex, France). Once dried, the residue was resuspended in a 10:90 MeOH:H2O solution by gentle sonication. The resulting solution was filtered through 0.22 µm polytetrafluoroethylene membrane syringe filters (Albet S.A., Barcelona, Spain) and directly injected into an ultra performance LC system (Acquity SDS, Waters Corp., Milford, MA, USA). Chromatographic separations were carried out on a reversed-phase C18 column (Gravity, 50×2.1mm, 1.8-µm particle size, Macherey-Nagel GmbH, Germany) using a MeOH:H2O (both supplemented with 0.1% acetic acid) gradient at a flow rate of 300 µL min−1. Hormones were quantified with a TQS triple quadrupole mass spectrometer (Micromass, Manchester, UK) connected online to the output of the column though an orthogonal Z-spray electrospray ion source.
Stomatal aperture
Stomatal aperture analysis was performed as described in Morillon and Chrispeels (2001). Briefly, three leaves of Ler and abi1-1 from each plant were cut and the lower surface was immediately stuck to a microspore slide with a medical adhesive (Hollister, Libertyville, IL, USA). After 1–2min, the leaf was peeled away under distilled water. The lower epidermis stuck to the glass was visualized under the microscope and stomatal images were recorded. Measurements of stomatal aperture were performed using the imaging software Image J, version 6.
H2O2 measurement
H2O2 accumulation in rosette tissues was measured using the Amplex Red Hydrogen Peroxide-Peroxidase Assay kit (Molecular Probes, Invitrogen, Carlsbad, CA, USA) as described in Suzuki et al. (2015). In brief, 500 µL of 50-mM sodium phosphate buffer (pH 7.4) containing 50 µM Amplex Red and 0.05 U mL−1 horseradish peroxidase was added to ground, frozen tissues. Samples were centrifuged at 12 000g for 12min at 4°C. Next, 450 µL of supernatant was transferred into fresh tubes and incubated for 30min at room temperature in the dark. Absorbance at 560nm was measured using a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The concentration of H2O2 in each sample was determined from a standard curve consisting of 0, 0.5, 1, 3, 6, and 9 µM of H2O2. Following the measurement of absorbance, tissue samples were completely dried using a speed vacuum concentrator for 90min and H2O2 accumulation per gram of dry weight was calculated.
H2O2 and ABA treatments
H2O2 and ABA treatments were conducted by spraying 1mM H2O2 or 30 µM ABA on 30-day-old Ler and abi1-1 plants. Control plants were simultaneously sprayed with distilled water. Stomatal aperture for control and treated leaves was measured after 30 and 60min of each treatment in plants kept in the light or in the dark.
Protein blot analysis
Protein was isolated, quantified, and analysed by protein blot as previously described (Miller et al., 2007). Coomassie Blue staining of protein gels was used to control for protein loading.
Statistical analysis
Genotypic differences were discriminated by a one-tailed Student’s t-test. Results are presented as the mean ± SD (P < 0.05). Stress acclimation data were subjected to a two-way ANOVA with the interaction genotype × stress followed by a Tukey post hoc test (P < 0.05) when a significant difference was detected (Supplementary Table S1).
Results
Growth of wild-type (Ler), aba1-1, and abi1-1 plants subjected to a combination of water deficit and heat stress
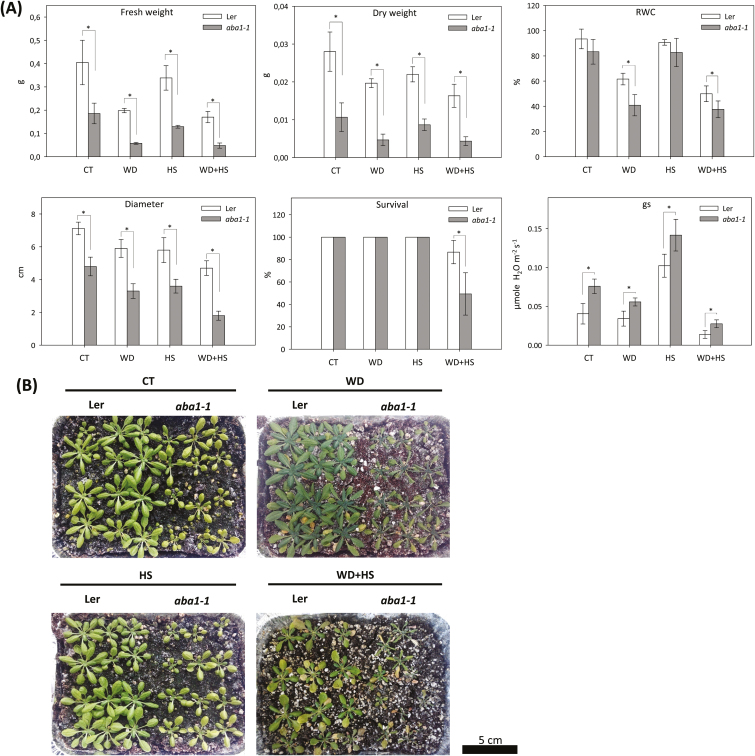
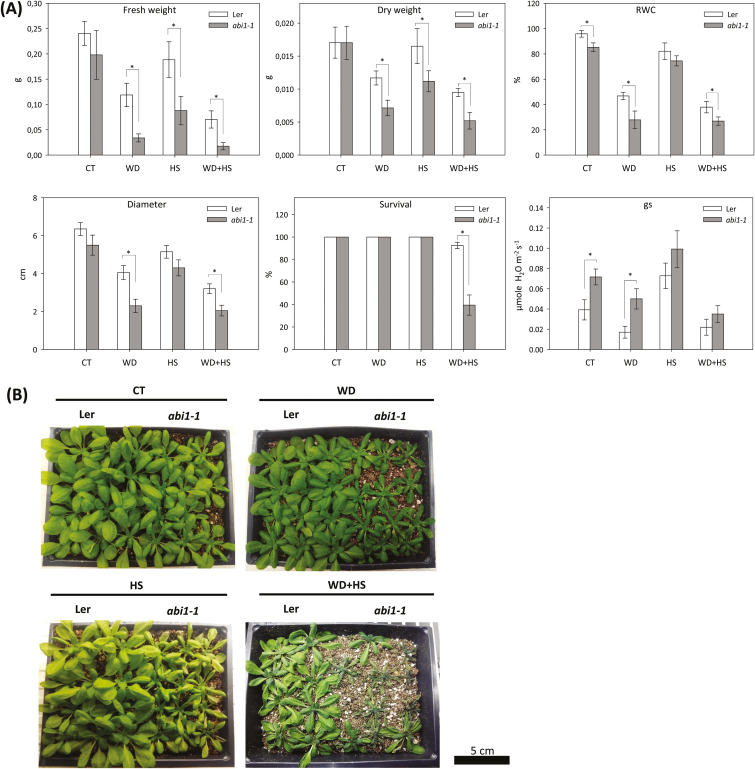
Rosette FW and DW, RWC, plant diameter, survival and gs of Ler, aba1-1, and abi1-1 plants subjected to water deficit, heat stress, and a combination of water deficit and heat stress were characterized (Figs 1, 2). Compared to wild-type Ler plants, aba1-1 plants showed a decrease in FW, DW, and rosette diameter in response to all stress treatments. In addition, water deficit and a combination of water deficit and heat stress significantly reduced RWC, whereas gs increased in aba1-1 in response to all stress treatments. Plant survival under combined stress conditions was 90% in wild-type plants and 50% in aba1-1 plants (Fig. 1A). Compared to wild type, abi1-1 plants showed a significant reduction in FW and DW in response to all stress treatments. In contrast, a decreased diameter and RWC were only observed in abi1-1 plants in response to water deficit or water deficit combined with heat stress (Fig. 2A). Whereas 95% of wild-type plants survived the stress combination, only about 40% of abi1-1 survived exposure to a combination of water deficit and heat stress (Fig. 2A). Increased gs was observed in abi1-1 plants compared to wild-type plants in control conditions as well as in response to water deficit (Fig. 2A). The results shown in Figs 1 and 2 demonstrate that although the growth of abi1-1 and aba1-1 plants was negatively impacted by water deficit or heat stress compared to wild type, these treatments had no adverse effect on survival. In contrast, the combination of water deficit and heat stress significantly impacted the survival of abi1-1 and aba1-1 plants, demonstrating that mutants impaired in ABA biosynthesis or signalling are impaired in their acclimation to this stress combination. A genotype × stress interaction analysis further confirmed the dependency of plant survival on ABA function with a P value ≤ 0.001 (Supplementary Table S1).
Fig. 1.
Growth, biomass, survival, RWC, and stomatal conductance of wild-type and aba1-1 plants subjected to a combination of water deficit and heat stress. (A) Shoot fresh and dry weight (g; average of five individual rosettes), RWC, rosette diameter, survival, and stomatal conductance (gs) of plants subjected to water deficit (WD), heat stress (HS), and a combination of water deficit and heat stress (WD+HS). * Student’s t-test significant at P < 0.05. Error bars represent SD. (B) Representative images of wild-type and aba1-1 plants subjected to the different stresses. CT, control.
Fig. 2.
Growth, biomass, survival, RWC, and stomatal conductance of wild-type and abi1-1 plants subjected to a combination of water deficit and heat stress. (A) Shoot fresh and dry weight (g; average of five individual rosettes), RWC, rosette diameter, survival, and stomatal conductance (gs) of plants subjected to water deficit (WD), heat stress (HS), and a combination of water deficit and heat stress (WD+HS). * Student’s t-test significant at P < 0.05. Error bars represent SD. (B) Representative images of wild-type and abi1-1 plants subjected to the different stresses. CT, control.
To further study the role of ABA in plant acclimation to a combination of water deficit and heat stress, we focused our studies on the abi1-1 mutant that is impaired in ABA signalling (Assmann et al., 2000; Wu et al., 2003; Nilson and Assmann, 2006).
Stomatal aperture of Ler and abi1-1 plants subjected to a combination of water deficit and heat stress
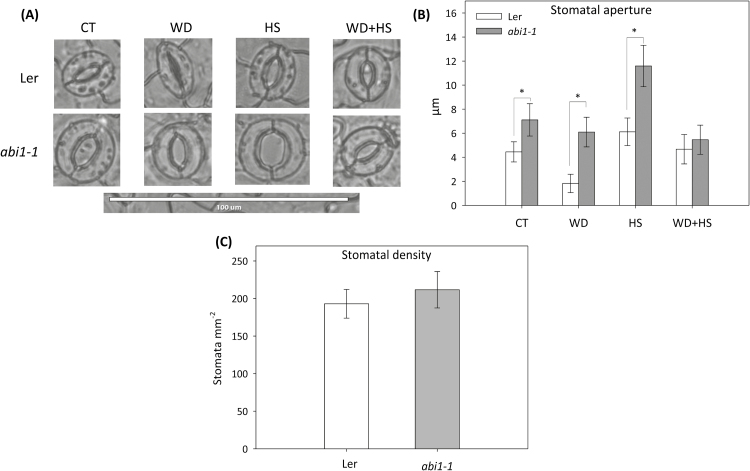
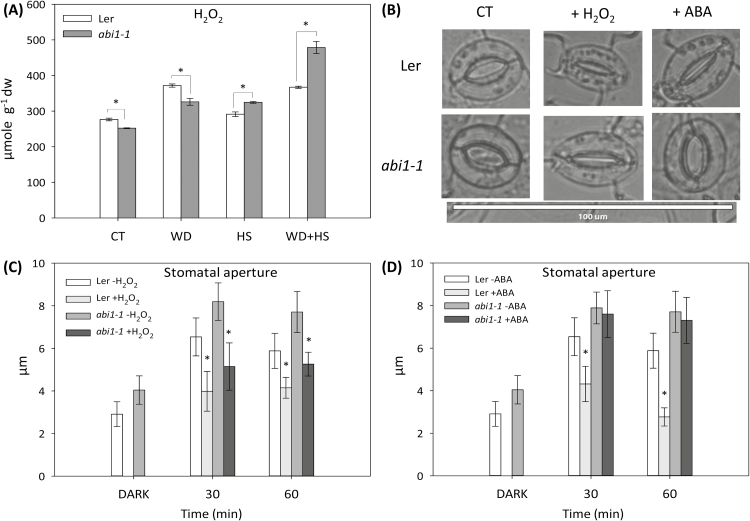
To determine whether the differences observed between the survival of abi1-1 and wild-type plants in response to the stress combination (Fig. 2) were related to the impaired stomatal responses of abi1-1 (Wu et al., 2003; Nilson and Assmann, 2006), the stomatal aperture of Ler and abi1-1 plants was measured under water deficit, heat stress, and a combination of water deficit and heat stress (Fig. 3A, B). Under controlled conditions, the stomatal aperture of abi1-1 plants was larger than that of wild type (Fig. 3A, B). The stomatal aperture of abi1-1 plants did not decrease in response to water deficit, reflecting the impairment of abi1-1 in stomatal responses (Fig. 3A, B; Wu et al., 2003; Nilson and Assmann, 2006). In contrast, the stomatal aperture of abi1-1 increased in response to heat stress and was larger than that of wild type, showing that the stomata of abi1-1 could open in response to heat stress (Fig. 3A, B). Surprisingly, and in contrast to the differences in stomatal responses and stomatal aperture observed between wild-type and abi1-1 plants under controlled conditions, heat stress, or water deficit (Fig. 3A, B), in response to the stress combination the stomatal aperture of abi1-1 was reduced to levels similar to that of wild-type plants (Fig. 3A, B). The measurements of stomatal aperture in control and abi1-1 plants during stress combination (Fig. 3A, 3B) were in agreement with the gs measurements of control and abi1-1 subjected to the stress combination (Fig. 2A), providing further confidence in this finding. To determine whether the density of stomata in the abi1-1 mutant would be a factor in affecting overall transpiration during the different stresses, we also measured the stomatal density of wild-type and abi1-1 plants. As shown in Fig. 3C, no significant difference was observed between wild-type and abi1-1 plants, both harbouring about 200 stomata per mm2. The results shown in Fig. 3 could explain the differences between the reduced growth, biomass, and RWC of abi1-1 and wild-type plants shown in Fig. 2, but likely not the differences in abi1-1 survival in response to the stress combination (Fig. 2).
Fig. 3.
Stomatal aperture of wild-type and abi1-1 plants subjected to a combination of water deficit and heat stress. (A) Representative images of stomata from wild-type and abi1-1 plants subjected to water deficit (WD), heat stress (HS), and a combination of water deficit and heat stress (WD+HS). (B) Measurements of stomatal aperture of wild-type and abi1-1 plants subjected to the different stresses. (C) Stomatal density of wild-type and abi1-1 plants. * Student’s t-test significant at P < 0.05. Error bars represent SD. CT, control.
Accumulation of ABA, JA, and SA in wild-type and abi1-1 plants subjected to a combination of water deficit and heat stress
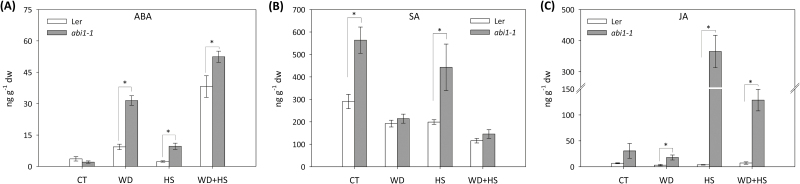
To examine whether the differences in stomatal responses and plant survival observed between wild-type and abi1-1 plants in response to the stress combination (Figs 2, 3) were related to the accumulation of ABA, JA, and/or SA in leaves, we measured the levels of these hormones in wild-type and abi1-1 plants subjected to the different stresses (Fig. 4). As shown in Fig. 4A, water deficit and a combination of water deficit and heat stress was accompanied by an accumulation of ABA, but not SA or JA, in wild-type plants. In addition, JA and SA did not accumulate in wild-type leaves in response to water deficit, heat stress, or their combination (Fig. 4B, C). In contrast, abi1-1 plants accumulated high levels of ABA in response to all stress treatments (Fig. 4A; this observation was further supported by correlation analysis between RWC and ABA in plants subjected to the different stresses; Supplementary Fig. S2, Supplementary Table S1), high levels of SA under control conditions and in response to heat stress (Fig. 4B), and high levels of JA in response to heat stress and a combination of water deficit and heat stress (Fig. 4C). The combination of water deficit and heat stress resulted in the highest accumulation of ABA in both abi1-1 and wild-type plants, suggesting that ABA is important for plant acclimation to this stress combination. The results shown in Fig. 4 could implicate JA-related pathways in the stomatal aperture reduction response of abi1-1 plants during the stress combination (Fig. 3). In addition, they reflect the deficiency in ABA signalling in abi1-1 plants that results in higher accumulation of ABA in response to stress (Fig. 4A).
Fig. 4.
Accumulation of ABA, JA, and SA in wild-type and abi1-1 plants subjected to a combination of water deficit and heat stress. ABA (A), SA (B), and JA (C) accumulation in wild-type and abi1-1 plants subjected to water deficit (WD), heat stress (HS), and a combination of water deficit and heat stress (WD+HS). * Student’s t-test significant at P < 0.05. Error bars represent SD. CT, control.
H2O2 accumulation in wild-type and abi1-1 plants in response to a combination of water deficit and heat stress
H2O2 plays an important role in abiotic stress and stomatal responses (Qiao et al., 2014; Song et al., 2014). To determine whether H2O2 plays a role in the regulation of stomatal aperture during a combination of water deficit and heat stress, we measured the levels of H2O2 in leaves from plants subjected to the different stresses. As shown in Fig. 5A, H2O2 accumulated in wild-type plants in response to water deficit and a combination of water deficit and heat stress, but not to heat stress alone. In the abi1-1 mutant, H2O2 accumulated in response to all stress treatments, with the highest levels obtained in abi1-1 plants subjected to a combination of water deficit and heat stress (Fig. 5A). The high levels of H2O2 measured in the leaves of the abi1-1 mutant in response to the stress combination (Fig. 5A) could explain the closure of stomata in the abi1-1 mutant during a combination of water deficit and heat stress (Fig. 3). We therefore examined how H2O2 or ABA application would affect the stomatal aperture of wild-type and abi1-1 plants. As shown in Fig. 5B, D, application of H2O2 (1mM), but not ABA (30 µM), resulted in a significant reduction of stomatal aperture in the abi1-1 mutant. The results presented in Fig. 5 could implicate H2O2 as an important signalling molecule that promotes stomatal closure in wild type and the abi1-1 mutant during abiotic stress combination.
Fig. 5.
H2O2 accumulation in wild-type and abi1-1 plants in response to a combination of water deficit and heat stress. (A) H2O2 accumulation in wild-type and abi1-1 plants subjected to water deficit (WD), heat stress (HS), and a combination of water deficit and heat stress (WD+HS). (B) Representative images of stomata of Arabidopsis plants 60min after the application of H2O2 or ABA. (C) Measurements of stomatal aperture of wild-type and abi1-1 plants following application of 1mM H2O2. (D) Measurements of stomatal aperture of wild-type and abi1-1 plants following application of 30 µM ABA. * Student’s t-test significant at P < 0.05. Error bars represent SD. CT, control.
Accumulation of key acclimation proteins involved in the response of plants to a combination of water deficit and heat stress in wild-type and abi1-1 plants
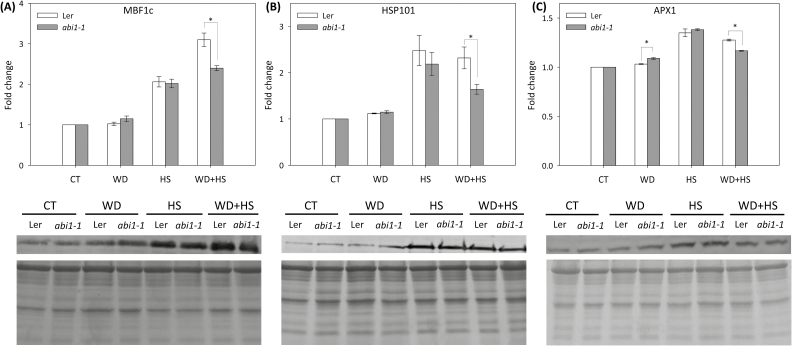
Although the abi1-1 mutant was more susceptible to a combination of water deficit and heat stress than wild type (Fig. 2), this susceptibility was not reflected in its stomatal responses to the stress combination (Fig. 3), and therefore may not be explained by excessive water loss of the abi1-1 mutant during the stress combination. ABA plays a dual role during the response of plants to abiotic stress, controlling stomatal responses as well as stress-response transcript and protein expression. We thus measured the accumulation of three key proteins important for plant acclimation to osmotic, heat, or oxidative stress (HSP101, APX1, and MBF1c; Queitsch et al., 2000; Arce et al., 2010; Suzuki et al., 2011), or a combination of water deficit and heat stress (MBF1c and APX1; Suzuki et al., 2005; Koussevitzky et al., 2008), in wild-type plants and abi1-1 mutants subjected to water deficit, heat stress, and their combination. As shown in Fig. 6, compared to wild-type plants, the accumulation of all three proteins was suppressed in the abi1-1 mutant in response to the stress combination. Because the expression of APX1 and MBF1c is required for plant acclimation to a combination of water deficit and heat stress (Suzuki et al., 2005; Koussevitzky et al., 2008), the results shown in Fig. 6 could explain the higher susceptibility of the abi1-1 mutant to the stress combination (Fig. 2), as well as highlight the important role ABA could play in the regulation of acclimation mechanisms during abiotic stress combination.
Fig. 6.
Accumulation of key acclimation proteins involved in the response of plants to a combination of water deficit and heat stress in wild-type and abi1-1 plants. Protein blot analysis of MBF1c (A), HSP101 (B), and APX1 (C) accumulation in leaves of wild-type and abi1-1 plants subjected to water deficit (WD), heat stress (HS), and a combination of water deficit and heat stress (WD+HS). Top: Quantification bar graphs for fold change in protein accumulation. Bottom: Protein blots and Coomassie-stained gels of total protein. Quantification of protein expression was performed per total protein for three different experiments. * Student’s t-test significant at P < 0.05. Error bars represent SD. CT, control.
Meta-analysis of transcriptomics data from ABA-treated abi1-1 and wild-type plants, and wild-type plants subjected to a combination of water deficit and heat stress
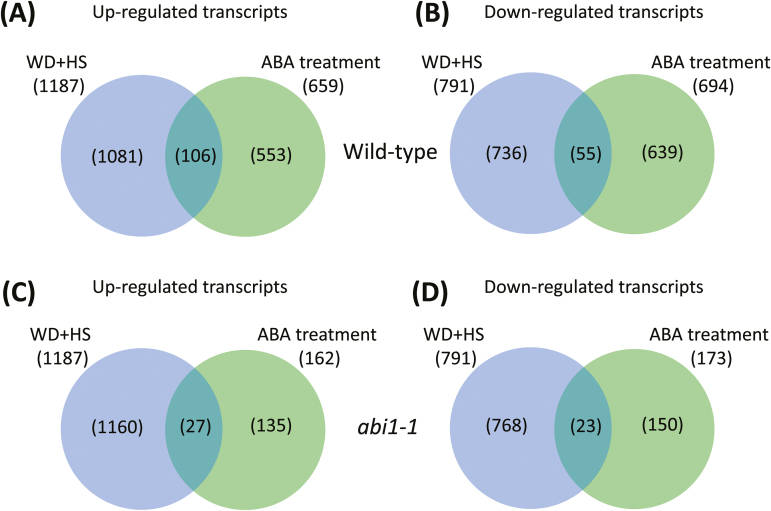
The results presented in Fig. 6 strongly support a role for ABA in the accumulation of different acclimation proteins during water deficit and heat stress combination. To examine how broad this role might be, we compared the transcriptome of wild-type plants (Col) subjected to a combination of water deficit and heat stress (Rizhsky et al., 2004) with that of wild-type plants (Ler) treated with 50 µM ABA (Hoth et al., 2002). As shown in Fig. 7A, 106 transcripts were common between the 1187 up-regulated transcripts during water deficit and heat stress combination (Rizhsky et al., 2004) and the 659 transcripts up-regulated following ABA treatment of unstressed wild-type plants (Hoth et al., 2002). An overlap of 55 transcripts was found between the 791 down-regulated transcripts during water deficit and heat stress combination and the 694 transcripts down-regulated following ABA treatment of unstressed wild-type plants (Fig. 7B). When the same overlap was tested between transcripts up- or down-regulated in wild-type (Col) plants in response to a combination of water deficit and heat stress (Rizhsky et al., 2004), and transcripts up- or down-regulated in the abi1-1 mutant (Ler) in response to ABA treatment of unstressed plants (50 µM; Hoth et al., 2002) (Fig. 7C, D), it was found that the number of overlapped transcripts between the two groups decreased by about 75% (up-regulated) and 50% (down-regulated). This finding could suggest that in addition to HSP101, MBF1c, and APX1 (Fig. 6), abi1-1 could be involved in the regulation of several other acclimation pathways in plants in response to a combination of water deficit and heat stress. A list of the transcripts that could be under the control of abi1-1 in response to the stress combination is included in Supplementary Table S2.
Fig. 7.
Meta-analysis of transcriptomics data from ABA-treated abi1-1 and wild-type (Ler) plants, and wild-type (Col) plants subjected to a combination of water deficit and heat stress. Top: Venn diagrams showing the overlap between transcripts specifically up-regulated (A) or down-regulated (B) in response to a combination of water deficit and heat stress or to ABA treatment in wild-type plants. Bottom: Venn diagrams showing the overlap between transcripts specifically up-regulated (C) or down-regulated (D) in response to a combination of water deficit and heat stress in wild-type plants and ABA treatment in abi1-1 plants. References used for the meta-analysis are Hoth et al. (2002) and Rizhsky et al. (2004).
Discussion
We recently conducted transcriptome analysis of Arabidopsis plants subjected to a combination of salinity and heat stress and identified many ABA-response transcripts within the group of transcripts that were specifically expressed in response to the stress combination (Suzuki et al., 2016). We subsequently determined that mutants impaired in ABA synthesis (aba1-1) or ABA signalling (abi1-1) were more susceptible than wild-type plants to a combination of salinity and heat stress (Suzuki et al., 2016). Nonetheless, whether this enhanced susceptibility to the stress combination was due to impaired stomatal responses, deficiency in the expression of different acclimation transcripts and proteins, or both, was unclear.
To deepen our understanding of ABA’s role in the response of plants to a combination of different abiotic stresses, we focused in the current study on the acclimation of plants to a combination of water deficit and heat stress. Our findings that the abi1-1 and aba1-1 mutants are susceptible to this stress combination (Figs 1, 2; Supplementary Table S1), that the stress combination was accompanied by elevated accumulation of ABA in wild-type and abi1-1 plants (Fig. 4), and that many transcripts involved in the response of plants to a combination of water deficit and heat stress are ABA-response transcripts (Fig. 7), demonstrate that ABA is required for the acclimation of plants to yet another type of stress combination, that is, water deficit and heat stress. These findings underscore a possible general role for ABA in the acclimation of plants to abiotic stress combinations. Further studies focused on the acclimation of mutants deficient in ABA metabolism and signalling to additional abiotic stress combinations would reveal how broad the role of ABA is in the response of plants to stress combination.
We further focused on the abi1-1 mutant and examined whether ABA is required for stomatal responses, expression of different acclimation transcripts and proteins, or both (Figs 3, 6). Surprisingly, we found that the stomata of abi1-1 plants, although impaired in their responses to water deficit (Figs 2, 3), reduced their aperture to levels that are similar to that of wild-type plants in response to the combination of heat stress and water deficit (Fig. 3). This finding suggested that ABI1 might not be required for stomatal closure during the stress combination, and that the decreased survival of abi1-1 plants subjected to the stress combination (Fig. 2) can not be simply explained by enhanced water loss during the stress combination due to impaired stomatal responses (although some loss of RWC was observed in abi1-1 plants during the stress combination; Fig. 2A). In contrast to the almost wild-type stomatal phenotype of abi1-1 plants under the stress combination (Fig. 3), the accumulation of proteins important for plant acclimation to heat stress (HSP101 and MBF1c) and a combination of water deficit and heat stress (MBF1c and APX1) was attenuated in abi1-1 plants during the stress combination (Fig. 6). Taken together, the findings shown in Figs 3 and 6 suggest that the cause of the enhanced susceptibility of abi1-1 plants to the stress combination (Fig. 2) could be an outcome of the inability of these plants to mount an acclimation response involving the accumulation of MBF1c and APX1 (Fig. 6), as opposed to their inability to close their stomata (Fig. 3). ABA may therefore be required for the accumulation of key proteins required for the acclimation of plants to a combination of different stresses (Fig. 6).
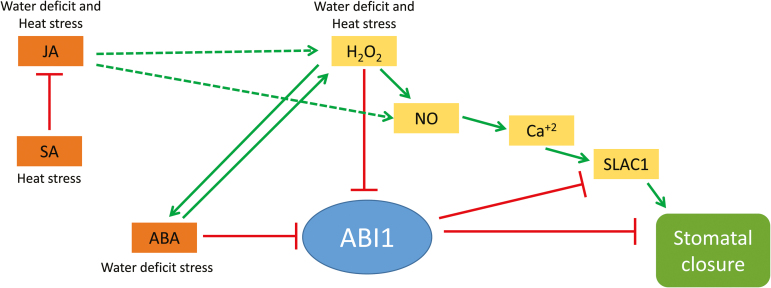
In an attempt to address the question of how the stomata of abi1-1 had reduced aperture during the stress combination, we measured the levels of ABA, JA, SA, and H2O2 in plants subjected to the stress combination. As shown in Figs 4 and 5, the stress combination was accompanied by a unique combination of high H2O2, high JA, and low SA in leaves of abi1-1 plants. Under this combination, H2O2 and JA can signal stomatal closure, independent of ABA signalling, by enhancing nitric oxide (NO) levels and triggering Ca2+ and SLAC1 function (Fig. 8; Daszkowska-Golec and Szarejko, 2013; Murata et al., 2015). In contrast, during heat stress when both SA and JA are enhanced (Fig. 4), SA could antagonize JA function and stomata will open in abi1-1 (Figs 3, 8; Thaler et al., 2012; Caarls et al., 2015). In support of the proposed role of H2O2 in mediating stomatal closer in abi1-1 plants, the application of H2O2, but not ABA, was able to induce stomatal closer in unstressed abi1-1 plants (Fig. 5B, D). Our findings point to an alternative pathway that may be involved in the stomatal responses of plants subjected to stress combination, involving JA and/or H2O2 (Fig. 8). Further studies are of course needed to address this possibility, including the analysis of additional mutants in ABA, JA, and ROS signalling and direct measurements of H2O2, JA, SA, NO, and ABA in the stomata of plants subjected to stress combination. Because ABA is required for different physiological acclimation responses, as well as for the regulation of protein and transcript accumulation during different stresses and their combination, ABA could function as an overall regulator that tailors the plant response to the different environmental conditions.
Fig. 8.
A hypothetical model for the signalling role of JA, SA, ABA, and H2O2 in the regulation of stomatal aperture in abi1-1 during water deficit, heat stress, and a combination of water deficit and heat stress. Dotted lines indicate hypothetical interactions. Solid lines and arrows indicate positive and negative regulation based on published literature, respectively. Abbreviations: ABA, abscisic acid; JA, jasmonic acid; NO, nitric oxide; SA, salicylic acid; SLAC1, SLOW ANION CHANNEL-ASSOCIATED 1.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Experimental design, and temperature and humidity measurements.
Figure S2. Correlation analysis between RWC and hormonal concentrations (ABA, SA, and JA) obtained for Ler and abi1-1 plants under water deficit, heat stress, and a combination of water deficit and heat stress.
Table S1. Analysis of variance of growth characteristic parameters and hormonal concentrations for aba1-1 and abi1-1 plants.
Table S2. List of the transcripts that could be under the control of abi1-1 in response to the stress combination.
Acknowledgements
This paper was supported by funding from the National Science Foundation (IOS-0639964, IOS-0743954, IOS-0820188, and IOS-1353886), and University of North Texas College of Arts and Sciences. SIZ was supported by Universitat Jaume I of Castellón (mobility grant E-2015-05) and Ministerio de Economía y Competitividad (Spain) (AGL2013-42038R).
References
- Arce DP, Godoy AV, Tsuda K, Yamazaki K, Valle EM, Iglesias MJ, Di Mauro MF, Casalongué CA. 2010. The analysis of an Arabidopsis triple knock-down mutant reveals functions for MBF1 genes under oxidative stress conditions. Journal of Plant Physiology 167, 194–200. [DOI] [PubMed] [Google Scholar]
- Assmann SM, Snyder JA, Lee YJ. 2000. ABA-deficient (aba1) and ABA-insensitive (abi1-1, abi2-1) mutants of Arabidopsis have a wild-type stomatal response to humidity. Plant, Cell and Environment 23, 387–395. [Google Scholar]
- Bartels D, Sunkar R. 2005. Drought and salt tolerance in plants. Critical Reviews in Plant Sciences 24, 23–58. [Google Scholar]
- Boeck HJ De, Bassin S, Verlinden M, Zeiter M, Hiltbrunner E. 2015. Simulated heat waves affected alpine grassland only in combination with drought. New Phytologist 209, 531–541. [DOI] [PubMed] [Google Scholar]
- Caarls L, Pieterse CMJ, Van Wees SCM. 2015. How salicylic acid takes transcriptional control over jasmonic acid signaling. Frontiers in Plant Science 6, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craufurd PQ, Flower DJ, Peacock JM. 2008. Effect of heat and drought stress on sorghum (Sorghum bicolor). I. Panicle development and leaf appearance. Experimental Agriculture 29, 61–76. [Google Scholar]
- Daszkowska-Golec A, Szarejko I. 2013. Open or close the gate – stomata action under the control of phytohormones in drought stress conditions. Frontiers in Plant Science 4, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ollas C, Hernando B, Arbona V, Gómez-Cadenas A. 2013. Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiologia Plantarum 147, 296–306. [DOI] [PubMed] [Google Scholar]
- Durgbanshi A, Arbona V, Pozo O, Miersch O, Sancho JV, Gómez-Cadenas A. 2005. Simultaneous determination of multiple phytohormones in plant extracts by liquid chromatography-electrospray tandem mass spectrometry. Journal of Agricultural and Food Chemistry 53, 8437–8442. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. 2013. Abscisic acid synthesis and response. The Arabidopsis Book 11, 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth S, Morgante M, Sanchez JP, Hanafey MK, Tingey S, Chua NH. 2002. Genome-wide gene expression profiling in Arabidopsis thaliana reveals new targets of abscisic acid and largely impaired gene regulation in the abi1-1 mutant. Journal of Cell Science 115, 4891–4900. [DOI] [PubMed] [Google Scholar]
- Hu X, Wu L, Zhao F, Zhang D, Li N, Zhu G, Li C, Wang W. 2015. Phosphoproteomic analysis of the response of maize leaves to drought, heat and their combination stress. Frontiers in Plant Science 6, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Huang B. 2001. Drought and heat stress injury to two cool-season turfgrasses in relation to antioxidant metabolism and lipid peroxidation. Crop Science 41, 436–442. [Google Scholar]
- Khokon MAR, Okuma E, Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC, Murata Y. 2011. Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant, Cell and Environment 34, 434–443. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Reuling G, Karssen CM. 1984. The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana . Physiologia Plantarum 61, 377–383. [Google Scholar]
- Koussevitzky S, Suzuki N, Huntington S, Armijo L, Sha W, Cortes D, Shulaev V, Mittler R. 2008. Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. The Journal of Biological Chemistry 283, 34197–34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Xin M, Qin J, Peng H, Ni Z, Yao Y, Sun Q. 2015. Temporal transcriptome profiling reveals expression partitioning of homeologous genes contributing to heat and drought acclimation in wheat (Triticum aestivum L.). BMC Plant Biology 15, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhard M, Grill E. 2001. Hydrogen peroxide is a regulator of ABI1, a protein phosphatase 2C from Arabidopsis. FEBS Letters 508, 443–446. [DOI] [PubMed] [Google Scholar]
- Merlot S, Gosti F, Guerrier D, Vavasseur A, Giraudat J. 2001. The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. The Plant Journal 25, 295–303. [DOI] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Rizhsky L, Hegie A, Koussevitzky S, Mittler R. 2007. Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiology 144, 1777–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Shukla A, Upadhyay S, et al. 2014. Identification, occurrence, and validation of DRE and ABRE Cis-regulatory motifs in the promoter regions of genes of Arabidopsis thaliana . Journal of Integrative Plant Biology 56, 388–399. [DOI] [PubMed] [Google Scholar]
- Mittler R. 2006. Abiotic stress, the field environment and stress combination. Trends in Plant Science 11, 15–19. [DOI] [PubMed] [Google Scholar]
- Mittler R, Blumwald E. 2010. Genetic engineering for modern agriculture: challenges and perspectives. Annual Review of Plant Biology 61, 443–462. [DOI] [PubMed] [Google Scholar]
- Miura K, Tada Y. 2014. Regulation of water, salinity, and cold stress responses by salicylic acid. Frontiers in Plant Science 5, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillon R, Chrispeels MJ. 2001. The role of ABA and the transpiration stream in the regulation of the osmotic water permeability of leaf cells. Proceedings of the National Academy of Sciences of the United States of America 98, 14138–14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Hossain MA, Nakamura Y, Mori IC, Murata Y. 2011. The Arabidopsis calcium-dependent protein kinase, CPK6, functions as a positive regulator of methyl jasmonate signaling in guard cells. Plant Physiology 155, 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Mori IC, Munemasa S. 2015. Diverse stomatal signaling and the signal integration mechanism. Annual Review of Plant Biology 66, 369–392. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Yamaguchi-Shinozaki K. 2013. ABA signaling in stress-response and seed development. Plant Cell Reports 32, 959–70. [DOI] [PubMed] [Google Scholar]
- Nilson SE, Assmann SM. 2006. The control of transpiration. Insights from Arabidopsis. Plant Physiology 143, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg Z, Blumwald E. 2011. Hormone balance and abiotic stress tolerance in crop plants. Current Opinion in Plant Biology 14, 290–295. [DOI] [PubMed] [Google Scholar]
- Qiao W, Li C, Fan L-M. 2014. Cross-talk between nitric oxide and hydrogen peroxide in plant responses to abiotic stresses. Environmental and Experimental Botany 100, 84–93. [Google Scholar]
- Queitsch C, Hong SW, Vierling E, Lindquist S. 2000. Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. The Plant Cell 12, 479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Mittler R. 2002. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiology 130, 1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R. 2004. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiology 134, 1683–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savin R, Nicolas M. 1996. Effects of short periods of drought and high temperature on grain growth and starch accumulation of two malting barley cultivars. Australian Journal of Plant Physiology 23, 201–210. [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. 2007. Gene networks involved in drought stress response and tolerance. Journal of Experimental Botany 58, 221–227. [DOI] [PubMed] [Google Scholar]
- Song Y, Miao Y, Song CP. 2014. Behind the scenes: the roles of reactive oxygen species in guard cells. New Phytologist 201, 1121–1140. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Basil E, Hamilton JS, et al. 2016. ABA is required for plant acclimation to a combination of salt and heat stress. PloS One 11, e0147625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Devireddy AR, Inupakutika MA, Baxter A, Miller G, Song L, Shulaev E, Azad RK, Shulaev V, Mittler R. 2015. Ultra-fast alterations in mRNA levels uncover multiple players in light stress acclimation in plants. The Plant Journal 84, 760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R. 2014. Abiotic and biotic stress combinations. New Phytologist 203, 32–43. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Rizhsky L, Liang H, Shuman J, Shulaev V, Mittler R. 2005. Enhanced tolerance to environmental stress in transgenic plants expressing the transcriptional coactivator multiprotein bridging factor 1c. Plant Physiology 139, 1313–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Sejima H, Tam R, Schlauch K, Mittler R. 2011. Identification of the MBF1 heat-response regulon of Arabidopsis thaliana . The Plant Journal 66, 844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JS, Humphrey PT, Whiteman NK. 2012. Evolution of jasmonate and salicylate signal crosstalk. Trends in Plant Science 17, 260–270. [DOI] [PubMed] [Google Scholar]
- Umezawa T, Nakashima K, Miyakawa T, Kuromori T, Tanokura M, Shinozaki K, Yamaguchi-Shinozaki K. 2010. Molecular basis of the core regulatory network in ABA responses: sensing, signaling and transport. Plant and Cell Physiology 51, 1821–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Sanchez J, Lopez-Molina L, Himmelbach A, Grill E, Chua N. 2003. The abi1-1 mutation blocks ABA signaling downstream of cADPR action. The Plant Journal 34, 307–315. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Mogami J, Yamaguchi-Shinozaki K. 2014. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Current Opinion in Plant Biology 21, 133–139. [DOI] [PubMed] [Google Scholar]
- Zhang YP, E ZG, Jiang H, Wang L, Zhou J, Zhu DF. 2015. A comparative study of stress-related gene expression under single stress and intercross stress in rice. Genetics and Molecular Research 14, 3702–3717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.