Highlight
Two suppressors of a weak abscission-deficient mutant were identified using genome sequencing. One suppressor was found to increase accumulation of the mutant hsl2-9 receptor compared with the parent genotype.
Key words: Abscission, EBS3, EBS4, ERAD, ER-associated degradation, genome sequencing, HAESA, HAESA-LIKE 2, suppressor.
Abstract
In Arabidopsis thaliana, the process of abscission, or the shedding of unwanted organs, is mediated by two genes, HAESA (HAE) and HAESA-LIKE 2 (HSL2), encoding receptor-like protein kinases (RLKs). The double loss-of-function mutant hae-3 hsl2-3 is completely deficient in floral abscission, but, interestingly, the hae-3 hsl2-9 mutant displays a less severe defect. This mutant was chosen for an ethyl methanesulfonate (EMS) screen to isolate enhancer and suppressor mutants, and two such suppressors are the focus of this study. Pooled DNA from the F2 generation of a parental backcross was analyzed by genome sequencing to reveal candidate genes, two of which complement the suppressor phenotype. These genes, EMS-MUTAGENIZED BRI1 SUPPRESSOR 3 (EBS3) and EBS4, both encode mannosyltransferases involved in endoplasmic reticulum (ER)-associated degradation (ERAD) of proteins. Further analysis of these suppressor lines revealed that suppressor mutations are acting solely on the partially functional hsl2-9 mutant receptor to modify the abscission phenotype. Expressing a hsl2-9–yellow fluorescent protein (YFP) transgene in ebs3 mutants yields a higher fluorescent signal than in EBS3/ebs3, suggesting that these mutants restore abscission by disrupting ERAD to allow accumulation of the hsl2-9 receptor, which probably escapes degradation to be trafficked to the plasma membrane to regain signaling.
Introduction
Abscission is a cell separation process that plants use to shed unwanted organs. This process has been best studied in the model plant Arabidopsis thaliana, which sheds its floral organs following pollination. Two receptor-like protein kinases, HAESA (HAE) and HAESA-LIKE 2 (HSL2), are required for abscission to occur, and the double loss-of-function mutant hae hsl2 is completely deficient in abscission (Cho et al., 2008; Stenvik et al., 2008). The secreted peptide INFLORESCENCE DEFICIENT IN ABSCISSION (IDA) is the ligand of HAE/HSL2, and ida mutants also do not abscise (Butenko et al., 2003, 2014; Meng et al., 2016). Several proteins belonging to the SOMATIC EMBRYOGENESIS RECEPTOR KINASE (SERK) family have also been shown to function as co-receptors of HAE/HSL2 (Meng et al., 2016; Santiago et al., 2016). HAE/HSL2 signaling activates a mitogen-activated protein kinase (MAPK) cascade consisting of MITOGEN-ACTIVATED PROTEIN KINASE KINASE 4 (MKK4) and MKK5, as well as MITOGEN-ACTIVATED PROTEIN KINASE 3 (MPK3) and MPK6 (Cho et al., 2008). One target of MAPK phosphorylation is the transcription factor AGAMOUS-like 15 (AGL15), which has been shown to bind to the promoter of HAE and regulate its transcription (Patharkar and Walker, 2015). Further, it has been shown that this signaling cascade constitutes part of a positive feedback loop to increase HAE expression just before abscission occurs (Patharkar and Walker, 2015). HAE/HSL2 signaling has also been shown to increase expression of cell wall-modifying and hydrolytic enzymes in a region of cells at the base of flowers called the abscission zone (AZ; Niederhuth et al., 2013b ). The AZ consists of a layer of cells attaching organs that will be abscised to the plant, and separation of these cells, caused by the breakdown of the middle lamella by hydrolytic enzymes, allows floral organs to be shed (Niederhuth et al., 2013a ). Abscission of the floral organs in Arbidopsis occurs at approximately floral position 7–9, where position 1 is defined as the youngest flower after anthesis (Cho et al., 2008). These positions correspond to floral developmental stage 16, which is defined by the withering and loss of petals, sepals, and stamen (Roeder and Yanofsky, 2006; Alvarez-Buylla et al., 2010).
Here, we connect the process of abscission to the protein quality control and degradation systems present in the endoplasmic reticulum (ER). ER-mediated protein quality control (ERQC) and ER-associated degradation (ERAD) are mechanisms allowing correctly folded proteins to be exported while retaining misfolded proteins to attempt refolding, or eventually to dispose of terminally misfolded proteins to prevent accumulation (Liu and Li, 2014; Lannoo and Van Damme, 2015). To achieve this, lipid-linked glycan precursors are transferred onto nascent polypeptides to provide a substrate that can be easily modified by ERQC/ERAD machinery to move polypeptides through the folding process and either export or degrade them, depending on whether it achieves its native state (Liu and Li, 2014; Lannoo and Van Damme, 2015). To date, ERQC and ERAD processes have been extensively studied in yeast, but relatively little is known about how these processes function in plants. The leucine-rich repeat (LRR) receptor kinase BRI1 has been adopted as a model protein for ERQC/ERAD studies in Arabidopsis, and genetic screens looking for suppressors of weak bri1 mutants have resulted in identifying several components of the ERQC and ERAD systems (Jin et al., 2007, 2009; Hong et al., 2009, 2012). Similarly, we demonstrate here that two mutants capable of suppressing the weak abscission defect of hae-3 hsl2-9 were found to have mutations in the genes EMS-MUTAGENIZED BRI1 SUPPRESSOR 3 (EBS3) and EBS4, both of which were shown to suppress the dwarf mutant brassinosteroid-insensitive 1 (bri1) by disrupting ERAD (Hong et al., 2009, 2012).
Genetic screens have identified a series of alleles for both hae and hsl2 mutants (Niederhuth et al., 2013a ). Of importance here are the double mutants hae-3 hsl2-3 (containing C222Y and G360R substitutions, respectively) and hae-5 hsl2-4 (containing W522* and Q165* substitutions, respectively). Both of these double mutants display completely abscission-deficient phenotypes. Interestingly, the hae-3 hsl2-9 mutant displays a weaker abscission defect (hsl2-9 contains a P404L substitution), and was chosen for an ethyl methanesulfonate (EMS) screen because it could result in isolating both enhancer and suppressor mutants. The lesions of all relevant hae/hsl2 mutant alleles are displayed in Supplementary Fig. S1A–E at JXB online. Through the course of this study, by identifying two mutants that suppress the weak abscission defect of hae-3 hsl2-9, we demonstrate that the HSL2 receptor is subject to the ERQC and ERAD systems. These two suppressor mutants further allowed us to elucidate that the hsl2-9 mutant receptor is biochemically functional and can signal to regain abscission in the suppressor mutants, illustrating that the ERQC and ERAD systems are often overly stringent in retaining and degrading misfolded proteins.
Materials and methods
Plant growth conditions
Plants were grown in a 16h light/8h dark cycle at temperatures of 20 °C during light and 18 °C during darkness, with 35–70% humidity. Columbia ecotype (Col-0) was used as the wild type. Mutations in the ERECTA (ER) and GLABROUS (GL) genes were introduced into several backgrounds throughout this study in order to identify contaminants easily.
Generation of mutagenized seed for the genetic screen
Approximately 10 000 seeds of the hae-3 hsl2-9 er gl mutant were mutagenized with EMS according to the protocol as described by Weigel and Glazebrook (2006). An M2 population was grown and screened for individuals exhibiting enhancement or suppression of the weak abscission defect.
Quantification of abscission phenotypes
Abscission phenotypes were quantified by counting the number of petals and sepals retained on each flower after treatment with a homemade brush device (Supplementary Fig. S2A). This device was constructed using two 7.5cm wide paintbrushes (bristles ~3.5cm long) with a glass rod running through holes in the paintbrush handles. Brushes were spaced 8cm apart along the glass rod using tape to ensure only the bristles of the brush would touch at the bottom. Two 1cm spacers were taped to one brush to provide a space between brushes where inflorescences rest during treatment and prevent brushes from squashing or damaging the plant. The device essentially hinges open to allow placement of inflorescence between the brushes, then is closed so the bristles brush the entire inflorescence as the device is pulled upward (Supplementary Fig. S2B). This is then repeated once after rotating the device ~90° around the inflorescence (rotate the device ~90° when looking down at the top of the inflorescence) to ensure every flower was treated (Supplementary Fig. S2C). The number of petals and sepals still attached was recorded for every flower from position 1 to 15. The average number of petals and sepals retained for positions 8–15 were then summed. The rationale behind this is that abscission has occurred fully by position 8, and after this point very few additional floral organs will abscise. Summing the average number of petals and sepals retained also allows for straightforward statistical analysis using pairwise t-tests assuming unequal variance to determine if significant differences between phenotypes are present, and P-values were determined by Bonferonni correction (Dunn, 2012).
Genome sequencing
The two suppressor lines (lines 1.6 and 3.3) to be analyzed by genome sequencing were backcrossed to the hae-3 hsl2-9 er gl parent, and a segregating F2 population was grown. DNA was extracted using a DNeasy Plant Mini Kit (Qiagen, Cat. No. 69104) from pooled leaf tissue from 30 suppressed individuals for both suppressor lines, and also 30 non-suppressed individuals from the F2 of the line 1.6 backcross. DNA was sequenced on Illumina HiSeq 2500 and reads were aligned to the TAIR10 genome using Bowtie2 alignment software (Langmead and Salzberg, 2012). The resulting sequence alignment file was further analyzed using samtools and bcftools to create a list of variants between sequencing reads and the reference genome (Li et al., 2009). For more information on bioinformatics work flow, see Supplementary File S1. The list of variants was then refined by selecting only variants containing a base pair change from guanine to adenine or from cytosine to thymine, as these are the most common mutations caused by EMS in Arabidopsis. The number of reads containing each variant [i.e. single nucleotide polymorphism (SNP)] was then divided by the total number of reads aligning to that position to give the proportion of reads containing each SNP. These proportions were then plotted by chromosomal position to allow identification of a ‘linked region’ with a higher overall proportion of reads containing SNPs in the two suppressed pools when compared with non-suppressed pools, which would be expected to contain the causal mutation. This method of genome sequencing by parental backcross to identify EMS-generated SNPs was previously described by Allen et al. (2013).
Cloning
Constructs containing genomic fragments of EBS3 and EBS4 were obtained from the laboratory of Jianming Li, University of Michigan, and were transformed into the hae-3 hsl2-9 ebs3 er gl and hae-3 hsl2-9 ebs4 er gl mutants, respectively, by floral dip (Clough and Bent, 1998). T1s were selected on half-stength Murashige and Skoog (1/2 MS) plates (2.16g of MS basal medium per 1 liter, supplemented with 1% sucrose, w/v) containing 0.8% agar and kanamycin (K50), and then transplanted onto soil following selection. The HAEpr:HAE-YFP construct used was previously described (Taylor et al., 2016). This construct was then mutagenized to introduce the C222Y mutation of hae-3 by the Quick-Change site-directed mutagenesis method (Agilent Technologies, Santa Clara, CA, USA). To create HSL2–yellow fluorescent protein (YFP), a 6068bp fragment containing the entire HSL2 gene and 3002bp upstream promoter region was cloned into the NotI site of pE6c (Dubin et al., 2008). This construct was then mutagenized with primers to create the P404L substitution of hsl2-9 by the Quick-Change site-directed mutagenesis method (Agilent Technologies). Both the hae-3-YFP and hsl2-9-YFP constructs were then transferred to pGWB601 by Gateway recombination (Nakamura et al., 2010; Gateway LR Clonase II Enzyme Mix from Invitrogen Cat. No. 11791020), and then transformed separately into hae-3 hsl2-9 by floral dip (Clough and Bent, 1998). T1s were then selected with Basta.
Endo H assay
Tissue was frozen and ground in microcentrifuge tubes with pestles for each genotype. For hae-3 hsl2-3 HSL2-YFP, hae-3 hsl2-9 er gl hae-3-YFP ebs3, and hae-3 hsl2-9 er gl hsl2-9-YFP ebs3 plants, two whole flowers (stage 16 and late stage 16) were ground and resuspended in 30 µl of SDS sample buffer. All samples were then boiled for ~3min and centrifuged to pellet tissue debris. Half of the supernatant of each sample was then treated with 1000U of Endo Hf with 1× Glyco Buffer 3 (NEB Cat. No. P0703S) for 1h at 37 °C (according to the manufacturer’s recommendations). Half of each sample was also left untreated. Samples were then separated by SDS–PAGE and immunoblotted as described below.
Fluorescence microscopy and image quantification
Fluorescent images of stage 16 and late stage 16 flowers were obtained using a Canon EO5 6D camera in conjunction with a Zeiss Discovery.V12 fluorescent stereoscope. Images were then processed using Adobe Photoshop, and the fluorescent signal was quantified using ImageJ software (Schneider et al., 2012). This allowed highlighted pixels, or those giving off fluorescent signal, to be counted within an area defined by a border traced around AZs. These values were then corrected by subtracting the average of three background readings taken from the same photo, and then normalized to untransformed wild type. Statistical significance was determined by Student’s t-test, with a P-value <0.05.
Analysis of YFP fusion proteins by western blot
Two whole flowers (stage 16 and late stage 16) were frozen and ground in microcentrifuge tubes with pestles for each genotype (untransformed wild-type, hae-3 hsl2-9 hsl2-9-YFP er gl ebs3, and hae-3 hsl2-9 hsl2-9-YFP er gl EBS3/ebs3), resuspended in 30 µl of SDS sample buffer, and boiled for ~3min. A 10 µl aliquot of each sample was separated by SDS–PAGE on an 8% acrylamide gel, blotted to a nitrocellulose membrane, stained with Ponceau-S, and imaged. Blots were then blocked with 4% BSA in phosphate-buffered saline with 0.1% Tween-20 (PBS-T) for 1h, probed with anti-green fluorescent protein (GFP) antibody overnight at 4 °C, rinsed with PBS-T four times for 5min each, incubated with anti-rabbit–horseradish peroxidase (HRP) (1:2500 dilution in 1% BSA in PBS-T, Cell Signaling Technologies, Danvers, MA, USA) for 1h at room temperature, rinsed again with PBS-T four times for 5min each, incubated with chemiluminescent substrate (Super Signal West Pico, Life Technologies, Carlsbad, CA, USA), and imaged using BioRad Chemidoc.
RNA isolation and cDNA synthesis
Leaf RNA was isolated using TRIzol reagent (Ambion), each sample was split, and 2 µg of RNA was treated with DNase (Thermo Scientific) while 2 µg of RNA was left untreated. cDNA was then synthesized from RNA (both DNase treated and untreated) using SuperScript III (Invitrogen) and used in PCR (additional reactions were run with cDNA reactions lacking reverse transcriptase as a control) with primers flanking introns containing SNPs (as mapped by genome sequencing). For primer sequences and descriptions, see Supplementary File S3.
Results
Isolation of enhancers and suppressors of hae-3 hsl2-9
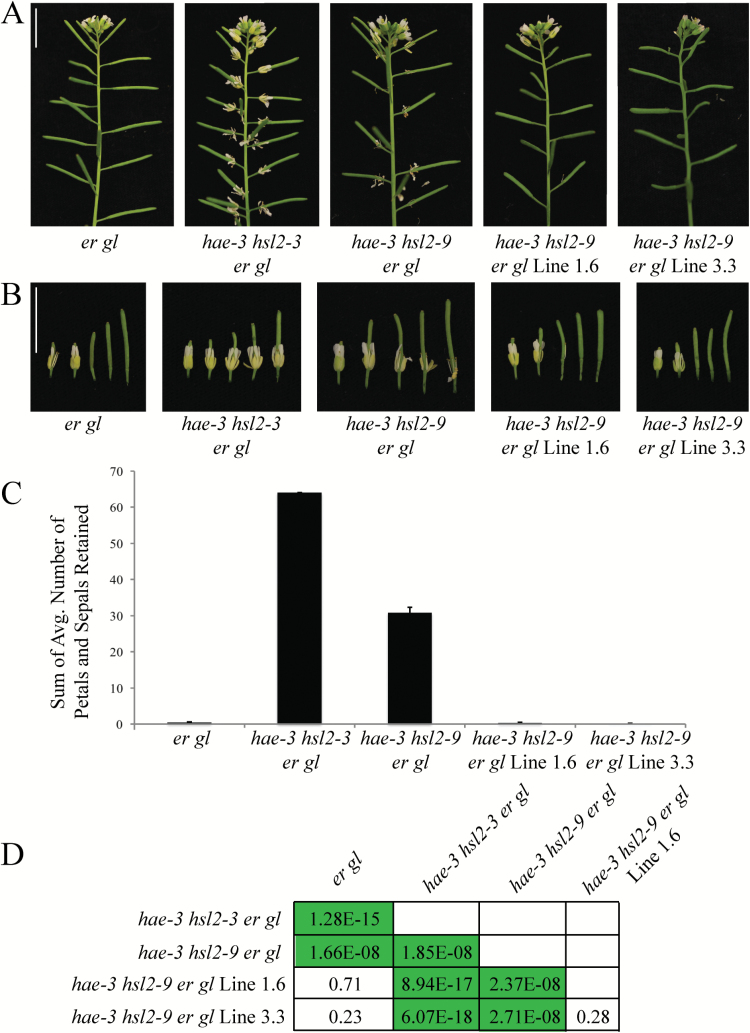
Seed from the hae-3 hsl2-9 er gl mutant was mutagenized with EMS to screen for enhancers and suppressors of the abscission-deficient phenotype. The hae-3 hsl2-9 mutant was chosen due to its weaker abscission deficiency, as compared with hae-3 hsl2-3, allowing for isolation of both enhancers and suppressors. Approximately 10 000 M1 seeds were grown, and progeny were harvested in 24 pools. Approximately 900 seeds were grown from each pool (~21 600 total plants) and screened in the M2 generation, resulting in isolation of 50 enhancers and 24 suppressors. These lines were scored based on the strength of their abscission phenotype, and those with the strongest abscission defect or rescue were regrown to confirm their phenotype in the M3 generation. Several enhancer and suppressor lines were backcrossed to the hae-3 hsl2-9 er gl parent to obtain a segregating F2 generation. Secondary mutations were found in the HSL2 gene of three backcrossed enhancer lines when Sanger sequenced in the F2 generation. From this, we inferred that the majority of the enhancer lines were probably HSL2 second site mutations. This prompted us to shift focus primarily to the suppressor lines, three of which were backcrossed as well. Of these three lines (named lines 1.6, 3.3, and 8.7), only lines 1.6 and 3.3 displayed the suppressor phenotype in the F2 generation (closely resembling wild-type abscission), and these lines became the focus of this study (Fig. 1A, B).
Fig. 1.
Mutant lines 1.6 and 3.3 suppress the abscission defect of hae-3 hsl2-9 er gl. (A) Photos of entire inflorescences showing abscission phenotypes of er gl, hae-3 hsl2-3 er gl, hae-3 hsl2-9 er gl, hae-3 hsl2-9 er gl Line 1.6, and hae-3 hsl2-9 er gl Line 3.3. (B) Stage 15 and the next four older flowers from the same inflorescences. (C) Quantification of abscission phenotypes. The number of petals and sepals retained was counted for floral positions 1–15 and averaged across 10 individuals. Averages of positions 8–15 were then summed for each genotype (SD error bars shown). (D) Table of P-values from statistical analysis using Student’s t-test. Values of statistical significance following Bonferroni correction (P<0.005) are highlighted. (This figure is available in colour at JXB online.)
The phenotypes of suppressor lines 1.6 and 3.3 were quantified by counting the number of petals and sepals retained at each floral position, from position 1 to 15, after treatment with a homemade brush device (Supplementary Fig. S2A; see the Materials and methods). Given that pre-abscission flowers have four petals and four sepals, the maximum number that could be retained on one flower post-abscission is eight. The wild type and er gl retain none of their floral organs post-abscission (after stage 16), while the hae-3 hsl2-3 er gl mutant retains all of its floral organs. These genotypes represent the low and high end of the range of abscission phenotypes, with hae-3 hsl2-9 er gl falling between them (Supplementary Fig. S1F). The number of petals and sepals retained at each floral position was averaged for 10 individuals of each genotype, and the averages for positions 8–15 were summed. This gives a good overall quantitative description of the total number of floral organs retained post-abscission, and clearly shows the differences between phenotypes (Fig. 1C). The average number of petals and sepals retained for individual floral positions is displayed in Supplementary Fig. S1G. Statistically significant differences between phenotypes were determined by pairwise t-tests assuming unequal variance (P-values determined by Bonferroni correction are displayed in Fig. 1D). Importantly, there are clear statistically significant differences between the hae-3 hsl2-9 er gl parent and the two suppressor lines.
Genome sequencing of lines 1.6 and 3.3 by parental backcross
Genome sequencing was employed to map EMS-generated SNPs and generate a list of candidate genes probably causing the suppressor phenotype. This was accomplished by crossing each suppressor line back to the parental hae-3 hsl2-9 er gl mutant. The F2 generation of these crosses was observed to segregate at an ~1:3 ratio of suppressed to non-suppressed individuals, suggesting a single recessive causal mutation. To identify the causal mutations, DNA was isolated from two pools of tissue (suppressed and non-suppressed individuals) from the F2 of the line 1.6 backcross. We chose to sequence a pool of non-suppressed 1.6 DNA in order to identify non-causal mutations that might have been present in the parental genome prior to mutagenesis. We also sequenced a single pool of tissue (suppressed individuals) from the F2 of the line 3.3 backcross.
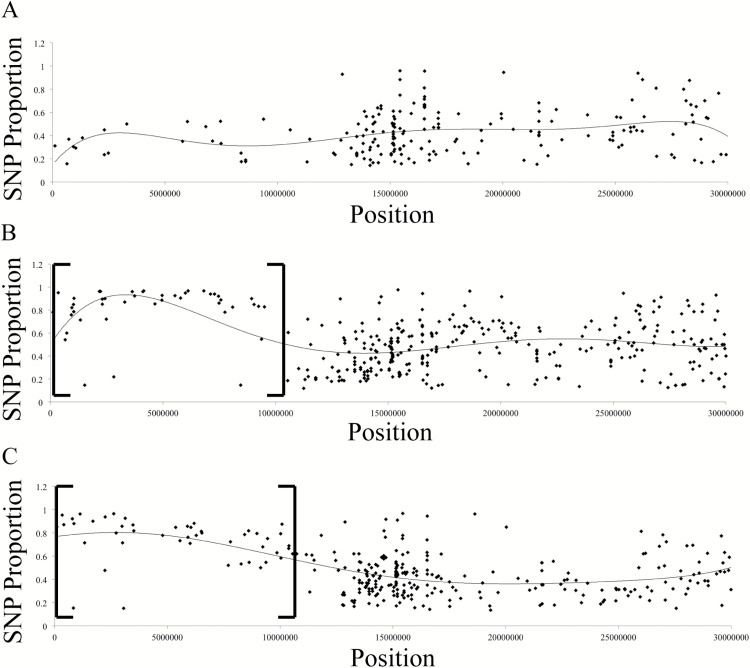
Reads were aligned to the TAIR10 reference genome using Bowtie2 alignment software, and alignment files were further analyzed using samtools and bcftools to generate a list of variants between the sequenced genome and the TAIR10 reference genome; for work flow and commands used, see Supplementary File S1 (Li et al., 2009; Langmead and Salzberg, 2012). For each variant, the number of reads containing the alternative called-base (SNP) was divided by the total number of reads aligning to that position to give the proportion of reads containing the SNP. This number was termed ‘SNP Proportion’, and means that if every read contained an alternative called-base at one position, then the SNP Proportion would be 1. SNP Proportion was plotted for every variant by chromosomal position to reveal a linked region on chromosome 1 for both suppressor pools containing variants with a higher SNP Proportion than in the non-suppressed pool (Fig. 2A–C). Graphs for chromosomes 1–5 are presented in Supplementary Fig. S3. SNPs in this region are linked to the causal mutation and are present in a higher proportion of reads due to the selection for the suppressor phenotype when pooling F2 tissue. We expected the SNP Proportion to increase closer to the causal mutation, with the SNP causing the suppressor phenotype to have an SNP Proportion of 1; however, we observed in the linked regions that the SNP proportion only approached 0.9. This is probably due to incorrectly scoring suppressed F2 individuals when pooling tissue. There are sometimes subtle differences between the suppressed phenotypes of lines 1.6 or 3.3 and the partial abscission defect of the hae-3 hsl2-9 er gl parent, and it is not unreasonable to think that one or two individuals included in the suppressed pools were not homozygous for the causative mutations. This may be due to variation in abscission that occurs due to other unknown endogenous factors, or these mutations may exhibit weak semi-dominance with incomplete penetrance.
Fig. 2.
Genome sequencing of suppressor lines 1.6 and 3.3. Proportion of reads containing SNPs on chromosome 1 for (A) pool of non-suppressed individuals from a line 1.6 backcross, (B) pool of suppressed individuals from a line 1.6 backcross, and (C) pool of suppressed individuals from a line 3.3 backcross. Brackets indicate the region on chromosome 1 with a higher overall SNP Proportion that is linked to the suppressor phenotype.
The linked regions for line 1.6 and line 3.3 allowed us to narrow the number of variants present in the whole genome down to a reasonable number, with 46 variants present at an SNP Proportion >0.85 in line 1.6 and 37 variants in line 3.3. This number was further reduced by selecting only non-synonymous variants in genic regions and mutations at exon–intron junctions, leaving only 16 candidates in line 1.6 and 13 candidates in line 3.3 (all candidate genes are listed in Supplementary File S2). Searching the Arabidopsis genome by the chromosomal position of these variants allowed us to determine the genes containing these SNPs. From here, we noticed the candidate genes EMS-MUTAGENIZED BRI1 SUPPRESSOR 3 (EBS3) and EMS-MUTAGENIZED BRI1 SUPPRESSOR 4 (EBS4) in line 1.6 and line 3.3, respectively, and these genes, both being mannosyltransferases involved in the same biochemical pathway, became the most likely candidates. Further investigation revealed that these genes have both been previously described to suppress the semi-dwarf phenotype of weak BRASSINOSTEROID-INSENSITIVE 1 (bri1) mutants (Hong et al., 2009, 2012). The mannosyltransferase activity of both EBS3 and EBS4 is involved in the assembly of N-glycans in the ER, which are heavily utilized in the ERQC and ERAD systems of proteins.
Interestingly, the SNPs present in both of these genes are located in splice sites of introns. PCR using cDNA derived from the mRNA of line 1.6 leaf tissue, as well as primers flanking the intron containing the SNP, results in a larger product than that amplified from cDNA of the hae-3 hsl2-9 er gl mutant (Supplementary Fig. S4A). This suggests that this mutation interferes with splicing and results in retention of the intron containing the SNP (Supplementary Fig. S4C). Repeating this experiment for line 3.3 results in a slightly smaller product from line 3.3 compared with the hae-3 hsl2-9 er gl mutant (Supplementary Fig. S4B). Sequencing this product reveals that the mutated intron, as well as 22bp of the following exon, is not contained in the product, and suggests activation of a cryptic splice site downstream of the mutation (Supplementary Fig. S4D). In both cases, the coding region would be shifted out of frame, resulting in premature stop codons.
gEBS3 and gEBS4 transgenes complement lines 1.6 and 3.3, respectively
To confirm that the suppression of the abscission-deficient phenotype in line 1.6 is caused by the ebs3 mutation, a genomic version was transformed into the suppressor line 1.6. Several independent T1 lines were generated (Fig. 3A, B) and clearly show an abscission defect. Similarly, a genomic version of the EBS4 gene was transformed into suppressor line 3.3. Independent T1 lines were again observed to have an abscission defect similar to the hae-3 hsl2-9 er gl parent (Fig. 3C, D), suggesting that the mutation in ebs4 in line 3.3 is causing the suppression of the abscission-deficient phenotype. Abscission phenotypes for complemented lines were quantified as described above and the average number of petals and sepals retained for individual floral position is displayed in Supplementary Fig. S5A, B. The sum of the average number of petals and sepals retained for positions 8–15 is also shown (Supplementary Fig. S5C, D), with statistically significant differences present between both suppressors and complemented lines (determined by t-test assuming unequal variance, P-value <0.05).
Fig. 3.
gEBS3 and gEBS4 transgenes complement suppressor lines 1.6 and 3.3, respectively. (A) Photos of entire inflorescences of hae-3 hsl2-9 er gl, hae-3 hsl2-9 er gl ebs3, hae-3 hsl2-9 er gl ebs3 gEBS3 Line 6, and hae-3 hsl2-9 er gl ebs3 gEBS3 Line 10. (B) Stage 15 and the next four older flowers from the same inflorescences. (C) Photos of entire inflorescences of hae-3 hsl2-9 er gl, hae-3 hsl2-9 er gl ebs4, hae-3 hsl2-9 er gl ebs4 gEBS4 Line 1, and hae-3 hsl2-9 er gl ebs4 gEBS4 Line 2. (D) Stage 15 and the next four older flowers from the same inflorescences. (This figure is available in colour at JXB online.)
Identifying which receptor acts to regain abscission
The assembly of N-glycans is critical for the proper function of ERQC and ERAD (Lannoo and Van Damme, 2015). N-Glycans are transferred onto nascent polypeptides in the lumen of the ER, and are recognized by the protein folding machinery, quality control systems, and ultimately the signal for ERAD if proteins never achieve proper folding. This is probably the case with the hae-3 and hsl2-9 mutant receptors in hae-3 hsl2-9 er gl, as their mutations would cause structural imperfections prohibiting proper folding and causing them to be degraded. The hae-3 hsl2-9 ebs3 er gl and hae-3 hsl2-9 ebs4 er gl mutants, however, would not assemble the necessary N-glycans, allowing hae-3 and/or hsl2-9 to escape degradation and be trafficked to the plasma membrane, as was previously reported to be the case with the ebs3-1 bri1-9 and ebs4 bri1-9 mutants (Hong et al., 2009, 2012). If this were the case in the suppressor mutants, then one or both of the receptors would have to be biochemically functional to be able to bind ligand and signal to regain abscission. We hypothesized that the hsl2-9 receptor is probably functional due to the change in severity of the abscission defect between hae-3 hsl2-3 er gl and hae-3 hsl2-9 er gl (Fig. 1).
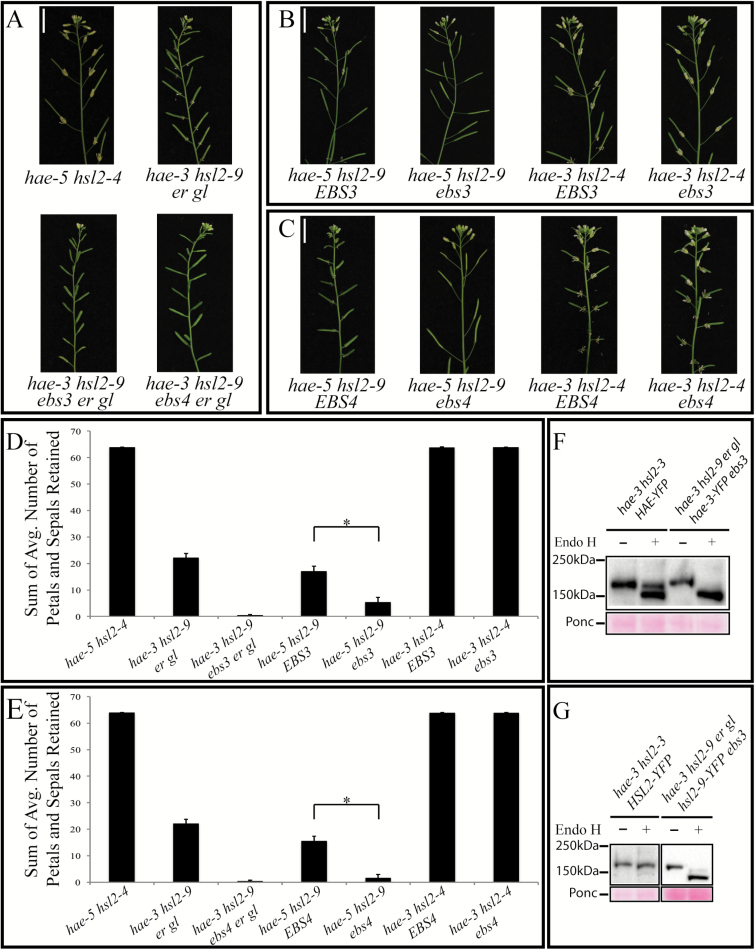
To test this hypothesis, we crossed both suppressor mutants to the double null mutant hae-5 hsl2-4. The F2 generation of these crosses was genotyped looking for hae-3 or hsl2-9 paired with the alternative null receptor (i.e. hae-3 paired with hsl2-4 and hae-5 paired with hsl2-9). Each of these pairs also needed either homozygous EBS3 or ebs3 (or EBS4 and ebs4), meaning we needed four different triple homozygous mutants for each suppressor. All of these mutants were successfully genotyped in the F2 generation (Fig. 4A–C), and further analyzed in the F3 to quantify their abscission phenotypes (Fig. 4D, E). Again, the sum of the average number of petals and sepals for positions 8–15 is shown here (individual positions 1–15 are shown in Supplementary Fig. S6). The hae-3 hsl2-4 pair of receptors does not abscise at all when present with homozygous mutant or wild-type EBS3 or EBS4. On the other hand, the hae-5 hsl2-9 pair of receptors does abscise when present with homozygous mutant ebs3 or ebs4, but only partially abscise when present with homozygous wild-type EBS3 or EBS4 (Fig. 4B, D, and C, E, respectively). These results suggest that the hae-3 receptor is not responsible for the suppressor phenotype, but the hsl2-9 receptor is probably biochemically functional and is being trafficked to the plasma membrane in the hae-3 hsl2-9 ebs3 er gl and the hae-5 hsl2-9 ebs3 mutants (as well as the hae-3 hsl2-9 ebs4 er gl and the hae-5 hsl2-9 ebs4 mutants).
Fig. 4.
The hsl2-9 receptor is functioning to regain abscission. (A) Photos of entire inflorescences of hae-5 hsl2-4 (both null alleles), hae-3 hsl2-9 er gl, hae-3 hsl2-9 ebs3 er gl, and hae-3 hsl2-9 ebs4 er gl. (B) Partial abscission defect of hae-5 hsl2-9 EBS3, no abscission defect of hae-5 hsl2-9 ebs3, and complete abscission defect of hae-3 hsl2-4 EBS3 and hae-3 hsl2-4 ebs3. (C) Partial abscission defect of hae-5 hsl2-9 EBS4, no abscission defect of hae-5 hsl2-9 ebs4, and complete abscission defect of hae-3 hsl2-4 EBS4 and hae-3 hsl2-4 ebs4. (D, E) Quantification of abscission phenotypes (by the same method as described above) for ebs3 mutants (D) and ebs4 mutants (E). Statistical significance determined by Student’s t-test (P-value <0.05, SD error bars shown). (F) Western blots showing sensitivity and mobility shift of HAE–YFP and hae-3–YFP receptors to Endo H digestion (blots probed with anti-GFP antibody). Ponceau stain of non-specific band for loading control. (G) Western blots showing the sensitivity of HSL2–YFP and hsl2-9–YFP receptors to Endo H digestion (blots probed with anti-GFP antibody). Ponceau stain of non-specific band for loading control. (This figure is available in colour at JXB online.)
HAE and HSL2 are glycoproteins
To test whether HAE and HSL2 are indeed glycoproteins with N-glycans, an Endo H assay was used. Endo H is an endoglycosidase capable of cleaving high-mannose-type (H-type) N-glycans present on glycoproteins localized in the ER. Endo H cannot, however, cleave Golgi-processed complex-type (C-type) N-glycans. Both wild-type HAE and HSL2 receptors were tagged with a C-terminal YFP tag and transformed separately into the hae-3 hsl2-3 mutant. Tagged wild-type receptors were then treated with Endo H, as well as tagged versions of the mutant receptors hae-3 and hsl2-9 (Fig. 4F, G). Treatment of HAE–YFP with Endo H resulted in the appearance of two distinct bands of slightly different molecular weights (Fig. 4F). This could possibly indicate both an ER-localized receptor (lower molecular weight band) and also a receptor that has been processed by the Golgi (higher molecular weight band). The higher molecular weight band only has a small mobility shift compared with untreated HAE–YFP probably due to the presence of a few incompletely processed N-glycans, which has been reported with wild-type BRI1 (Hong et al., 2009). Endo H treatment of HSL–YFP is somewhat less clear, but results in a majority of the protein having a small mobility shift and shows significantly less protein at a lower molecular weight that probably corresponds to ER-localized receptor (Fig. 4G). Both hae-3 and hsl2-9 receptors were tagged with C-terminal YFP and transformed into the hae-3 hsl2-9 ebs3 er gl suppressor mutant. Both of these mutant receptors show nearly complete Endo H sensitivity and a greater mobility shift in immunoblot assays, meaning that these mutant receptors are almost completely localized to the ER. Interestingly, a small amount of hsl2-9–YFP was detected at a slightly higher molecular weight than the majority of the Endo H-sensitive ER-localized protein (Fig. 4G). This is likely to indicate that a small amount of receptor is able to escape the ER and be processed by the Golgi, which has also been shown to be the case with bri1-9 in both ebs3 and ebs4 suppressor mutants (Hong et al., 2009, 2012). Predictive software was also used to count the number of Asn-Xaa-Ser/Thr sequons present and estimate the number of asparagines likely to be glycosylated on both HAE and HSL2 (R. Gupta et al., unpublished results). Six of the nine sequons in the extracellular domain of HAE were predicted to be glycosylated, and 10 of 14 sequons were predicted to be glycosylated in HSL2 (Supplementary Fig. S7).
Measuring abundance of hsl2-9 receptor by fluorescent microscopy
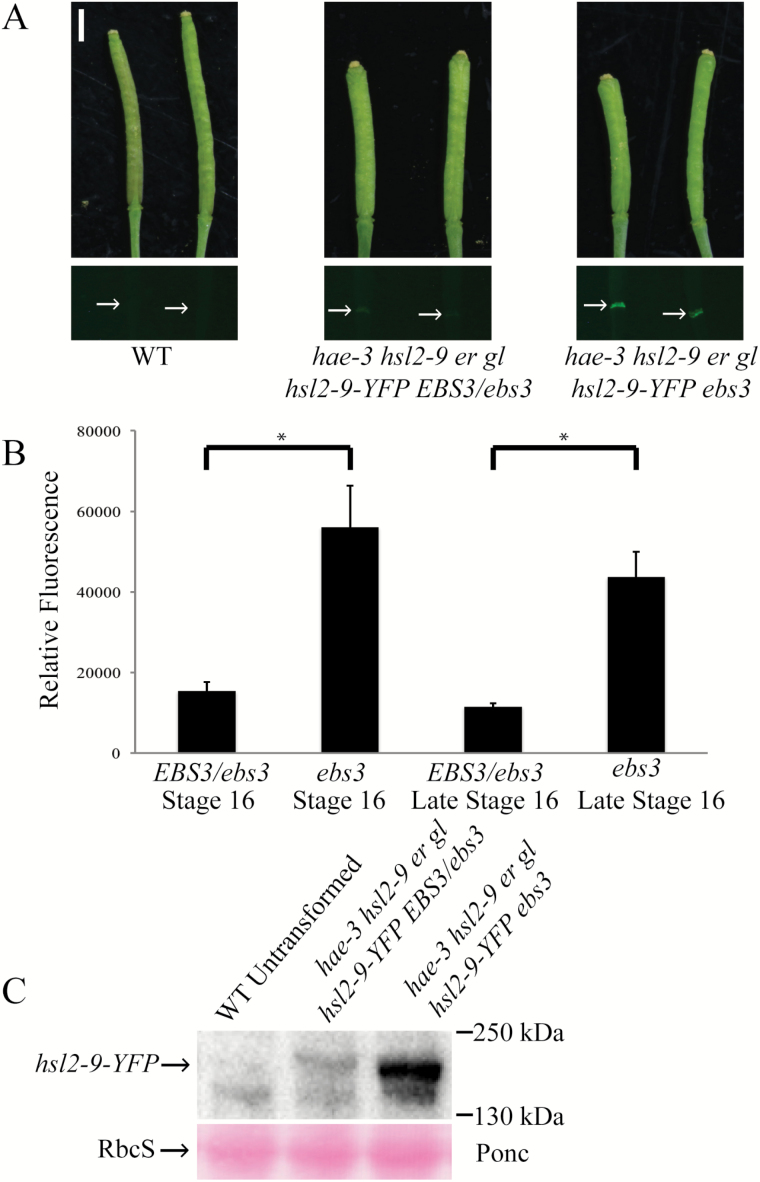
To test further the hypothesis that the hsl2-9 mutant receptor is able to escape degradation in the suppressor mutant, but not in the hae-3 hsl2-9 er gl parent, we sought to measure the abundance of hsl2-9 protein in both mutant backgrounds by fluorescent microscopy. We added a C-terminal YFP tag to the hsl2-9 mutant receptor and drove expression by the native HSL2 promoter. This was then transformed into hae-3 hsl2-9 ebs3 er gl. This experiment was only done in the ebs3 mutant partly due to time constraints, but primarily because it has already been demonstrated that both ebs3 and ebs4 mutants accumulate more bri1-9, and both of these suppressors were expected to behave similarly in regards to disrupting ERAD and accumulating protein (Hong et al., 2012). Expressing T1 lines were then crossed to the hae-3 hsl2-9 er gl parent and to hae-3 hsl2-9 ebs3 er gl. The F1 progeny of these crosses, being either EBS3/ebs3 (containing one functional copy of EBS3) or ebs3 (homozygous for the suppressor mutation), were checked for YFP expression. Only F1 progeny that came from a single expressing T1 plant were compared to control for any differences in expression that could occur between T1s. Photographs of YFP expression in the abscission zones of stage 16 and late stage 16 siliques show a clear difference in signal between the two crosses (Fig. 5A), with the ebs3 progeny having significantly higher YFP signal than EBS3/ebs3, which is consistent with our hypothesis that hsl2-9 receptor can escape degradation in the ebs3 suppressor mutant, but not in the hae-3 hsl2-9 er gl parent (EBS3). Both stage 16 and late stage 16 siliques were measured in order to give two time points during the process of abscission. Photographs of YFP expression were then quantified using ImageJ software, which assigns values to pixels depending on the color displayed (i.e. amount of fluorescent signal), and averages the values of pixels within a defined region. For these images, this region was carefully traced around abscission zones of individual siliques, allowing for signal to be measured and then normalized to the wild type (Fig. 5B). Samples were then analyzed by western blot to confirm that full-length hsl2-9–YFP receptor was present. Probing with anti-GFP antibody revealed bands close to the expected size of hsl2-9–YFP (estimated to be 167kDa), and showed a similar pattern of protein levels, with ebs3 having a higher level of hsl2-9–YFP than EBS3/ebs3 (Fig. 5C). This experiment was repeated using YFP-tagged hae-3 receptor. Fluorescent microscopy revealed an identical pattern of signal, with the EBS3/ebs3 mutant showing significantly less YFP signal than ebs3 (Supplementary Fig. S8), suggesting that hae-3 is also likely to be subject to ERAD. Because the hae-3–YFP mutant receptor is non-functional in both the mutant ebs3 background and the EBS3/ebs3 background, this suggests that the hae-3 mutation causes a negative impact on receptor function in addition to ERAD (Fig. 4).
Fig. 5.
Levels of the hsl2-9–YFP receptor are higher in the ebs3 mutant. (A) Photographs (white light and YFP fluorescence) of stage 16 and late stage 16 siliques of the wild type, hae-3 hsl2-9 er gl hsl2-9-YFP EBS3/ebs3, and hae-3 hsl2-9 er gl hsl2-9-YFP ebs3. Floral organs were forcibly removed for all siliques. (B) Quantification of the YFP signal present in abscission zones. Significant differences between EBS3/ebs3 and ebs3 for both stage 16 and late stage 16 siliques determined by Student’s t-test (P-value <0.05, SD error bars shown). (C) Western blot with anti-GFP antibody showing higher detection of hsl2-9–YFP in the ebs3 mutant than in the EBS3/ebs3 mutant. Bands detected are close to the expected size of hsl2-9–YFP (estimated at 167kDa). Ponceau stain (bottom) for loading control. (This figure is available in colour at JXB online.)
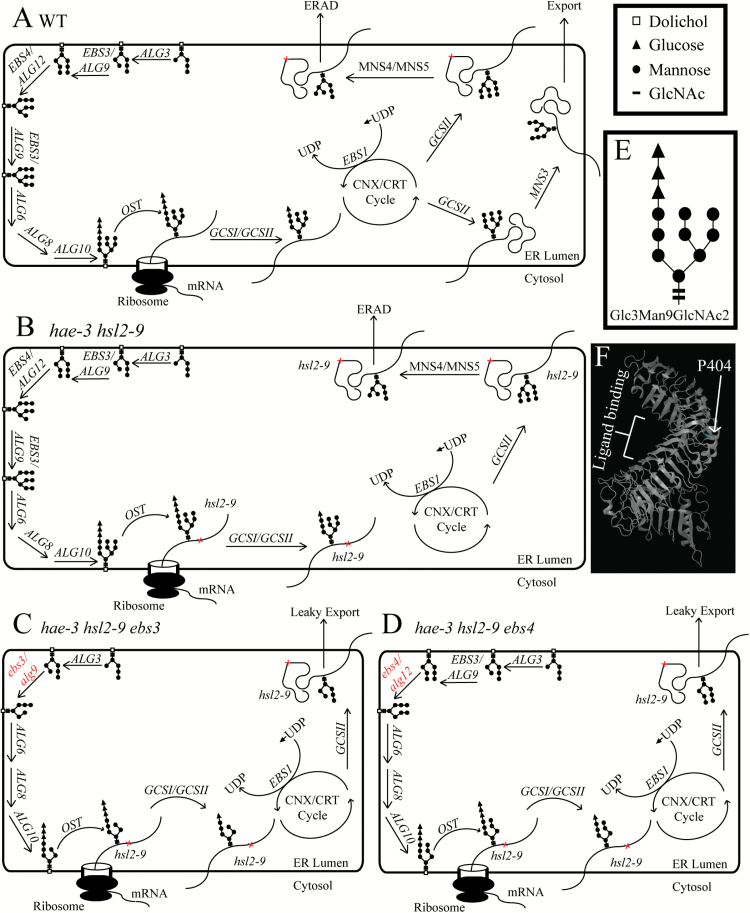
Hypothetical model of ERQC and ERAD
Figure 6A illustrates normal ERQC and ERAD systems. Note that this model is partially based on data from studies in yeast, as ERQC and ERAD have not been studied in plants as extensively. In wild-type cells, the glycan precursor Glc3Man9GlcNAc2 (where Glc is glucose, Man is mannose, and GlcNAc is N-acetylglucosamine, shown in Fig. 6E) is assembled in the ER and transferred onto asparagine residues of nascent polypeptides contained in the Asn-X-Ser/Thr motif (where X represents any residue except proline). Two glucose molecules are then trimmed from Glc3Man9GlcNAc2 by α-glucosidase I (GCSI) and α-glucosidase II (GCSII), and polypeptides now carry Glc1Man9GlcNAc2, which is recognized by calnexin (CNX) and calreticulin (CRT) to enter the protein folding cycle. Proteins are released from this cycle when GCSII cleaves the final glucose, leading to either export or re-entry into the protein folding cycle if correct folding was not achieved. In the case of re-entry and continued folding, N-glycans are re-glucosylated by UDP-Glc:glycoprotein glucosyltransferase (UGGT, or EBS1 in Arabidopsis) to allow CNX/CRT to re-bind the protein and attempt to fold correctly. This cycle of attempted protein folding will continue for a time; however, if proper folding is never achieved, proteins will have the remaining glucose and two mannose molecules trimmed from Glc1Man9GlcNAc2 by GCSII and MNS4/MNS5, respectively. The resulting N-glycans have an exposed α1,6-linked mannose present on the C-branch, which is the signal for degradation (Clerc et al., 2009). This is the case with mutant receptors that are structurally compromised (i.e. hae-3, hsl2-9, or bri1-9), and they will only be sent for degradation, as they never achieve proper folding (Fig. 6B). Note that in Fig. 6B–D, only hsl2-9 is shown to be misfolded. The hae-3 receptor is also probably misfolded and subject to ERAD (Supplementary Fig. S8), but is not shown because only the hsl2-9 receptor is involved in regaining abscission (Fig. 4).
Fig. 6.
Hypothetical model of N-glycan assembly and protein folding. (A) Wild-type cells sequentially add mannose and glucose molecules to assemble the N-glycan Glc3Man9GlcNAc2 in the ER. Glycans are then transferred onto nascent polypeptides before entering the CNX/CRT cycle of protein folding. Correctly folded proteins are eventually exported, while terminally misfolded proteins are sent for degradation (both of these paths are dependent upon trimming N-glycans to expose specific mannose residues). (B) The hae-3 hsl2-9 mutant correctly assembles Glc3Man9GlcNAc2, and polypeptides enter the protein folding cycle, but structural imperfections in hsl2-9 prevent correct folding (note that hae-3 is also likely to be misfolded as well). These proteins will be sent for degradation. (C, D) The hae-3 hsl2-9 ebs3 (C) and hae-3 hsl2-9 ebs4 (D) mutants do not assemble complete N-glycans, preventing α1,6-linked mannose residues from being exposed to signal receptors for degradation. This allows mutant hsl2-9 receptor to leak out of the ER and be trafficked to the plasma membrane to signal to regain abscission. Note that the ebs3 mutation prevents downstream action of EBS4, and assembly of the glycan is halted (C). Similarly, the ebs4 mutation prevents EBS3 from adding a second mannose residue, also halting glycan assembly (D). (E) Structure of Glc3Man9GlcNAc2. (F) Theoretical structure of the HSL2 receptor showing the P404 residue and putative ligand-binding region. (This figure is available in colour at JXB online.)
The ebs3 and ebs4 suppressor mutants (Fig. 6C, D) differ in these processes because the glycans they assemble are incomplete (Hong et al., 2012). The ebs3 mutation halts the assembly of glycans and prevents downstream action of EBS4, resulting in incomplete glycans. Likewise, the ebs4 mutation prevents the downstream action of EBS3, and glycan assembly cannot continue. In both of these mutants, glycans are still transferred onto polypeptides, which then enter the CNX/CRT cycle to attempt folding. Mutant receptors will never achieve proper folding due to structural imperfections, but here they cannot be sent for degradation because their glycans cannot be properly trimmed to expose the α1,6-linked mannose. This will hypothetically result in retention and accumulation of these proteins in the ERQC/protein folding machinery until this system is oversaturated and some protein is likely to be able to escape the ER and be sent to its proper destination. Here, these proteins can bind ligand and regain signaling if biochemically functional, as is probably the case with hsl2-9. A theoretical structure of HSL2 (Fig. 6F) based on the known structure of BRI1 maps the P404 residue to the opposite side of the extracellular domain to the putative ligand-binding region, and supports the hypothesis that hsl2-9 can still function because it is unlikely that the hsl2-9 lesion (P404L) would interfere with ligand binding.
Discussion
In this study, we identify two mutant lines that show suppression of the abscission defect present in the parental hae-3 hsl2-9 er gl mutant. Both of these lines were isolated from an EMS screen of hae-3 hsl2-9 er gl. The genomes of both of these suppressors were sequenced to reveal the most likely candidate genes, EBS3 and EBS4, causing the suppressor phenotype. It was then confirmed by complementation that the suppressor phenotypes of these mutants are caused by mutations in the genes EBS3 and EBS4. EBS3 encodes the Arabidopsis ortholog of the yeast gene ASPARAGINE-LINKED GLYCOSYLATION 9 (ALG9) (Hong et al., 2012), and catalyzes the addition of two α1,2 mannose (man) residues during the assembly of N-glycans in the lumen of the ER. EBS4, also involved in the ER luminal assembly of N-glycans, encodes the ortholog of the yeast gene ASPARAGINE-LINKED GLYCOSYLATION 12 (ALG12) (Hong et al., 2009), and catalyzes the addition of an α1,6 man residue. These genes are only two components of the highly specific assembly of the three branched N-glycans (Glc3Man9GlcNAc2) that are heavily utilized in ERQC and ERAD. Failure at any one of the steps in assembling Glc3Man9GlcNAc2 can result in incomplete or incorrectly constructed N-glycans that are incapable of being recognized by ERQC and ERAD machinery (Liu and Li, 2014; Lannoo and Van Damme, 2015).
It has been previously shown that making loss-of-function mutations in many of the mannosyltransferases involved in assembling Glc3Man9GlcNAc2 can suppress mutant phenotypes, most notably the semi-dwarf phenotype of weak bri1 alleles (Jin et al., 2007, 2009; Hong et al., 2009, 2012). Here, we demonstrate that ebs3 and ebs4 mutants are able to suppress the partial abscission defect of hae-3 hsl2-9 er gl in a similar manner, which connects ERQC, ERAD, and the strict regulation of protein folding to the process of abscission. As the receptors HAE and HSL2 are being produced, these methods of regulation are in place to prevent toxic or non-functional proteins from accumulating in the cell. We also demonstrate that these methods are often overly stringent in sending incompletely or incorrectly folded proteins for degradation, as many mutant receptors are biochemically functional despite minor imperfections or lesions affecting their structure. This is clearly the case with hsl2-9, which can function solely to regain abscission (Fig. 4B, C) when not impeded by degradation. We also further characterized the ebs3 mutant by looking at protein levels of fluorescently tagged hsl2-9 receptor. Fluorescent signal was observed to have a significant increase in the ebs3 mutant compared with EBS3/ebs3 (Fig. 5A, B), suggesting that hsl2-9 is able to escape degradation and accumulate in the suppressor, but that much of the receptor is degraded when at least one functional copy of EBS3 is present and ERAD is still functional.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Allele diagrams, range of abscission phenotypes, and average number of petals and sepals retained for er gl, hae-3 hsl2-3 er gl, hae-3 hsl2-9 er gl, hae-3 hsl2-9 er gl Line 1.6, and hae-3 hsl2-9 er gl Line 3.3.
Figure S2. Pictures of the brush device used for quantification of abscission phenotypes, including dimensions and method of use.
Figure S3. Graphs showing the proportion of reads containing an SNP for each variant across all five chromosomes and all pools of DNA sequenced.
Figure S4. PCR flanking introns with a suppressor mutation shows incorrect splicing.
Figure S5. Average number of petals and sepals retained for complemented lines containing the gEBS3 or gEBS4 transgene.
Figure S6. Average number of petals and sepals retained for ebs3 and ebs4 mutants crossed to the double null mutant hae-5 hsl2-4.
Figure S7. Predicted number and location of N-glycans in HAE and HSL2 extracellular domains.
Figure S8. Levels of hae-3–YFP receptor are higher in the ebs3 suppressor mutant.
File S1. General work flow for genome sequencing data alignment and analysis, resulting in a list of variants. Includes commands entered with descriptions.
File S2. List of candidate genes from linked region of chromosome 1 for line 1.6 and line 3.3.
File S3. List of primer sequences and descriptions.
Acknowledgements
This work was supported in part by a National Science Foundation Grant MCB-0743955 (to JCW). We thank the laboratory of Jianming Li for supplying complementation constructs.
References
- Allen RS, Nakasugi K, Doran RL, Millar AA, Waterhouse PM. 2013. Facile mutant identification via a single parental backcross method and application of whole genome sequencing based mapping pipelines. Frontiers in Plant Science 4, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla ER, Benítez M, Corvera-Poiré A, et al. 2010. Flower development. Arabidopsis Book 8, e0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenko MA, Patterson SE, Grini PE, Stenvik G-E, Amundsen SS, Mandal A, Aalen RB. 2003. Inflorescence deficient in abscission controls floral organ abscission in Arabidopsis and identifies a novel family of putative ligands in plants. The Plant Cell 15, 2296–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenko MA, Wildhagen M, Albert M, Jehle A, Kalbacher H, Aalen RB, Felix G. 2014. Tools and strategies to match peptide–ligand receptor pairs. The Plant Cell 26, 1838–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SK, Larue CT, Chevalier D, Wang H, Jinn T-L, Zhang S, Walker JC. 2008. Regulation of floral organ abscission in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 105, 15629–15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc S, Hirsch C, Oggier DM, Deprez P, Jakob C, Sommer T, Aebi M. 2009. Htm1 protein generates the N-glycan signal for glycoprotein degradation in the endoplasmic reticulum. Journal of Cell Biology 184, 159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method forAgrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Dubin MJ, Bowler C, Benvenuto G. 2008. A modified Gateway cloning strategy for overexpressing tagged proteins in plants. Plant Methods 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn OJ. 2012. Multiple comparisons among means. Journal of the American Statistical Association 56, 52–64. [Google Scholar]
- Hong Z, Jin H, Fitchette A-C, Xia Y, Monk AM, Faye L, Li J. 2009. Mutations of an alpha1,6 mannosyltransferase inhibit endoplasmic reticulum-associated degradation of defective brassinosteroid receptors in Arabidopsis. The Plant Cell 21, 3792–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Kajiura H, Su W, Jin H, Kimura A, Fujiyama K, Li J. 2012. Evolutionarily conserved glycan signal to degrade aberrant brassinosteroid receptors in Arabidopsis. Proceedings of the National Academy of Sciences, USA 109, 11437–11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Hong Z, Su W, Li J. 2009. A plant-specific calreticulin is a key retention factor for a defective brassinosteroid receptor in the endoplasmic reticulum. Proceedings of the National Academy of Sciences, USA 106, 13612–13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Yan Z, Nam KH, Li J. 2007. Allele-specific suppression of a defective brassinosteroid receptor reveals a physiological role of UGGT in ER quality control. Molecular Cell 26, 821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nature Methods 9, 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannoo N, Van Damme EJM. 2015. Review/N-glycans: the making of a varied toolbox. Plant Science 239, 67–83. [DOI] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li J. 2014. Endoplasmic reticulum-mediated protein quality control in Arabidopsis. Frontiers in Plant Science 5, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Zhou J, Tang J, Li B, de Oliveira MV, Chai J, He P, Shan L. 2016. Ligand-induced receptor-like kinase complex regulates floral organ abscission in Arabidopsis. Cell Reports 14, 1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Mano S, Tanaka Y, et al. 2010. Gateway binary vectors with the bialaphos resistance gene, bar, as a selection marker for plant transformation. Bioscience, Biotechnology, and Biochemistry 74, 1315–1319. [DOI] [PubMed] [Google Scholar]
- Niederhuth CE, Cho SK, Seitz K, Walker JC. 2013. a Letting go is never easy: abscission and receptor-like protein kinases. Journal of Integrative Plant Biology 55, 1251–1263. [DOI] [PubMed] [Google Scholar]
- Niederhuth CE, Patharkar OR, Walker JC. 2013. b Transcriptional profiling of the Arabidopsis abscission mutant hae hsl2 by RNA-Seq. BMC Genomics 14, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patharkar OR, Walker JC. 2015. Floral organ abscission is regulated by a positive feedback loop. Proceedings of the National Academy of Sciences, USA 112, 2906–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder AHK, Yanofsky MF. 2006. Fruit development in Arabidopsis. Arabidopsis Book 4, e0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago J, Brandt B, Wildhagen M, Hohmann U, Hothorn LA, Butenko MA, Hothorn M. 2016. Mechanistic insight into a peptide hormone signaling complex mediating floral organ abscission. eLife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvik G-E, Tandstad NM, Guo Y, Shi C-L, Kristiansen W, Holmgren A, Clark SE, Aalen RB, Butenko MA. 2008. The EPIP peptide of INFLORESCENCE DEFICIENT IN ABSCISSION is sufficient to induce abscission in arabidopsis through the receptor-like kinases HAESA and HAESA-LIKE2. The Plant Cell 20, 1805–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor I, Wang Y, Seitz K, et al. 2016. Analysis of phosphorylation of the receptor-like protein kinase HAESA during Arabidopsis floral abscission. PLoS One 11, e0147203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Glazebrook J. 2006. EMS mutagenesis of Arabidopsis seed. CSH Protocols 2006, pdb.prot4621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.