Highlight
In barley, GIGANTEA and the PSEUDO RESPONSE REGULATOR clock genes respond rapidly to temperature in an EARLY FLOWERING 3-dependent manner.
Key words: Barley; circadian clock; elf3, flowering; temperature.
Abstract
An increase in global temperatures will impact future crop yields. In the cereal crops wheat and barley, high temperatures accelerate reproductive development, reducing the number of grains per plant and final grain yield. Despite this relationship between temperature and cereal yield, it is not clear what genes and molecular pathways mediate the developmental response to increased temperatures. The plant circadian clock can respond to changes in temperature and is important for photoperiod-dependent flowering, and so is a potential mechanism controlling temperature responses in cereal crops. This study examines the relationship between temperature, the circadian clock, and the expression of flowering-time genes in barley (Hordeum vulgare), a crop model for temperate cereals. Transcript levels of barley core circadian clock genes were assayed over a range of temperatures. Transcript levels of core clock genes CCA1, GI, PRR59, PRR73, PRR95, and LUX are increased at higher temperatures. CCA1 and PRR73 respond rapidly to a decrease in temperature whereas GI and PRR59 respond rapidly to an increase in temperature. The response of GI and the PRR genes to changes in temperature is lost in the elf3 mutant indicating that their response to temperature may be dependent on a functional ELF3 gene.
Introduction
The flowering time and yield of temperate cereals is strongly influenced by temperature (Fischer, 1985; Hemming et al., 2012). In barley (Hordeum vulgare), elevated ambient temperatures accelerate vegetative growth and reduce biomass (Hemming et al., 2012). Reproductive development is accelerated at higher temperatures in long days, resulting in shorter spikes, fewer total florets, and an overall decrease in grain yield (Fischer, 1985; Hemming et al., 2012; Rawson and Richards, 1993). By contrast, in short days, reproductive development is inhibited at higher temperatures; indicating an interaction between photoperiod and temperature in the control of reproductive development in barley (Hemming et al., 2012). Despite predicted global climate change and the negative impact of higher temperatures on cereal grain yield, little is known about the genes and molecular pathways controlling temperature-dependent growth and development in cereal crops.
The plant circadian clock comprises a network of interlocking gene loops that generate and maintain rhythmic gene expression over an approximately 24h period and, in turn, control many rhythmic biological processes (Harmer, 2009). The circadian clock also tracks changing day length to regulate photoperiod-dependent flowering (Imaizumi, 2010). An intrinsic property of the plant circadian clock is the ability to perceive and respond to temperature cues. Firstly, in a process termed ‘temperature entrainment’, the daily rhythmic expression of clock genes can be synchronized to daily temperature changes (Salome and McClung, 2005). Secondly, the plant circadian clock maintains rhythmic gene expression across a wide range of temperatures in a process termed ‘temperature compensation’ (Edwards et al., 2005; Gould et al., 2006). This ability of the plant circadian clock to perceive and integrate temperature cues and its role in photoperiod-dependent flowering make it a strong candidate for having a role in the control of temperature-dependent flowering in the cereals.
The plant circadian clock is best described in Arabidopsis thaliana. The key components of the plant circadian clock are the MYELOBLASTOSIS (MYB)-related genes CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY), the PSEUDO RESPONSE REGULATORS (PRRs), PRR5, PRR7, PRR9, TIMING OF CAB EXPRESSION 1 (TOC1 / PRR1), and the members of the evening complex; LUX ARRHYTHMO (LUX) / PHYTOCLOCK1 (PCL1), EARLY FLOWERING 3 (ELF3), and ELF4 (Schaffer et al., 1998; Somers et al., 1998; Wang and Tobin, 1998; Covington et al., 2001; Doyle et al., 2002; Hazen et al., 2005; Nakamichi et al., 2005a, b). Mathematical modelling of the plant circadian clock suggests that rhythmic gene expression is maintained by a three loop repressor model (Pokhilko et al., 2012). The morning-expressed CCA1 and LHY are repressed sequentially throughout the day by the protein products of PRR9, PRR7, and TOC1 which are, in turn, repressed in the evening by the evening complex (Pokhilko et al., 2012). Finally, the loop is closed with repression of the evening complex genes by CCA1 and LHY in the morning (Pokhilko et al., 2012). In addition, GIGANTEA (GI) is integral to maintaining circadian rhythms (Fowler et al., 1999; Park et al., 1999). CCA1, LHY, TOC1, PRR7, PRR9, ELF3, and GI have all been shown to have a role in either temperature entrainment or temperature compensation (Salome and McClung, 2005; Gould et al., 2006; Paltiel et al., 2006; Salome et al., 2010; Thines and Harmon, 2010).
A recent study has proposed a model to explain how temperature cues are integrated into the clock; warm temperatures inhibit the function of the evening complex, removing repression of PRR7, PRR9, GI, and LUX (Mizuno et al., 2014). The resultant increase in expression of these clock genes may contribute to temperature entrainment, temperature compensation, as well as temperature-dependent growth, development, and flowering (Mizuno et al., 2014). Consistent with this hypothesis, a prr5/prr7/prr9 triple mutant mimics normal low temperature responses (Nakamichi et al., 2009).
Many equivalent clock genes to those of Arabidopsis and rice (Oryza sativa) have been identified in barley (Campoli et al., 2012b ; Calixto et al., 2015). The similarity of their amino acid sequence, and daily expression patterns, suggest that the function of most of the barley and Arabidopsis clock genes may be conserved (Campoli et al., 2012b ; Calixto et al., 2015). Some significant differences do exist; in barley, there is no clear homologue of the Arabidopsis evening-expressed ELF4 gene, although two related ELF4-like genes are present (Calixto et al., 2015) and there is only one functionally equivalent morning-expressed gene that is more closely related to LHY than CCA1 (Calixto et al., 2015). In addition, unlike Arabidopsis, the barley circadian clock does not initiate robust rhythmic gene expression until it has been exposed to both a lights-on and a lights-off cue (Deng et al., 2015). It also responds rapidly to different photoperiods, adjusting the period of the clock to match the prevailing photoperiod conditions (Deng et al., 2015). Potentially, the barley circadian clock also responds differently to temperature cues.
Flowering time mutants have recently been identified as mutations of barley clock genes. The barley PPD-H1 gene is homologous to PRR3/PRR7 and mediates the acceleration of development in long-days. No similar role exists for PRR3 or PRR7 in Arabidopsis (Turner et al., 2005; Campoli et al., 2012b ). The barley elf3 (Mat.a8, eam8) mutant displays disrupted expression of clock genes and accelerated reproductive development (Faure et al., 2012; Zakhrabekova et al., 2012; Boden et al., 2014). The probable lux mutants in barley (eam10) and diploid wheat (Triticum monococcum) (Eps-3A) are also early flowering and the Eps-3A mutant displays a temperature-dependent variation in spikelet number (Campoli et al., 2013; Gawronski et al., 2014).
Currently, there is little understanding of how temperature cues are integrated into the cereal circadian clock and how this might regulate temperature-dependent growth and development. Given the potential for reduced cereal yields in a warming environment, an in-depth study of temperature and the cereal circadian clock is required. This study examines the expression of core clock genes at high and low ambient temperatures in barley. We show that the barley circadian clock responds to temperature through significant changes in the expression of core clock genes.
Materials and methods
Plant material and growth conditions
Plant materials used in this study were the winter-type, photoperiod-sensitive, barley cultivar Sonja (VRN1, VRN2, PPD-H1) (Sasani et al., 2009), the barley elf3 (Mat.a8, eam8) mutant, and the isogenic spring-type, photoperiod-insensitive, wild-type parent cultivar of the elf3 mutant, Bonus (VRN1-1, ΔVRN2, ppd-H1) (Faure et al., 2012; Zakhrabekova et al., 2012). Barley seeds were sown on a mix of 50% perlite:50% vermiculite in 15ml Falcon tubes and saturated in water supplemented with 1.4g l-1 Thiram fungicide (Bayer Crop Science, www.bayercropscience.us) as previously described (Deng et al., 2015). Barley seeds of cultivar Sonja were vernalized in the dark at 4 °C for 28 d.
Temperature entrainment experiment
Temperature entrainment experiments were conducted in constant darkness in a temperature-controlled glasshouse with a temperature range of 16–25.5 °C. Temperature was monitored with a data logger with the temperature changing gradually over a 24h period (Fig. 1A). Five days after germination, barley seedlings (whole plant minus the roots) were harvested every 3h for 81h (Sonja) or 24h (Bonus and elf3).
Fig. 1.
Expression of circadian clock genes in barley cv. Sonja entrained to temperature cycles in constant dark. Relative expression of circadian clock genes in 5-d-old barley seedlings, cv. Sonja, grown in constant dark with a daily temperature range of 16–25.5 °C. (A). Daily temperature range for the duration of the experiment. (B–D). Expression of circadian clock genes. Values are means of three biological replicates ±standard error.
Twelve hour temperature shift from constant conditions
Temperature shift experiments were conducted on barley seedlings germinated and grown for 5 d in darkness at a constant 20 °C, in Ecotherm programmable temperature blocks. The growth temperature was then either increased to 25 °C or decreased to 15 °C for 12h before being returned to 20 °C. Gene expression from the temperature increase or decrease was compared with control seedlings maintained at 20 °C. Data from Sonja control seedlings has previously been presented in Deng et al. (2015). Individual barley seedlings were harvested every 3h from the initial temperature increase or decrease for 24h.
Constant temperature treatments in long days
Long-day experiments were performed in controlled environment cabinets (Conviron CMP6050) set to 16/8h light/dark with a light intensity of 250 µmol m−2 s−1. Barley seedlings of cv. Sonja were germinated and grown for 5 d at a constant 15, 20, 25 or 30 °C in Ecotherm programmable temperature blocks. For analysis of daily gene expression at a constant 15 °C or 25 °C, individual barley seedlings of cv. Sonja were harvested every 3h for 24h. For analysis of the dosage response to temperature at a constant 15, 20, 25 or 30 °C, individual barley seedlings of cv. Sonja were germinated and grown for 5 d and then harvested at 1, 4, 7, 10, and 16h after lights on.
Bonus and elf3 seedlings were germinated and grown at a constant 15 °C or 25 °C in Ecotherm programmable temperature blocks, in 16/8h light/dark, for 4 d to allow for entrainment of the barley circadian clock. Constant dark conditions were then imposed just prior to the light period at the start of day 5 by covering the tubes in foil. Seedlings were grown for a further 24h in constant dark and then individual seedlings were harvested every 4h for 24h.
Six hour temperature increase in long days
Barley seedlings cv. Sonja were germinated and grown for 5 d in long-day conditions 16/8h light/dark at a constant 15 °C in Ecotherm programmable temperature blocks. The temperature was then increased to 25 °C for 6h on day 6 at 1, 7, and 15h, after lights on. Individual barley seedlings were harvested immediately prior to and after the 6h temperature increase.
Seedling growth experiment
Seedlings of the barley cultivar Sonja were germinated and grown as previously described at a constant 15, 20, 25 or 30 °C in Ecotherm programmable temperature blocks in long days. Seedlings were harvested every day after germination for 3 d for measurement of seedling length.
Gene expression analyses
To assess gene expression patterns, individual barley seedlings (whole plant minus the roots) were harvested, with seedlings sampled during dark periods harvested in the dark. RNA was extracted using the Spectrum™ Plant Total RNA Kit (Sigma-Aldrich) as per the manufacturer’s instructions or by the method of Chang et al. (1993). Total RNA (2 µg) was DNase treated using the On-column DNase I Digestion set (Sigma-Aldrich) or RQ1 DNase (Promega) as per the manufacturers’ instructions. First strand cDNA synthesis primed with Oligo dT(18) was performed using Maxima H Minus Reverse Transcriptase (Thermo-Scientific) as per the manufacturer’s instructions. Quantitative real time reverse transcriptase PCR (qRT-PCR) was performed using the 7900HT Fast Real-Time PCR System (Applied Biosystems), using Platinum Taq DNA polymerase (Life Technologies) and SYBR green. ACTIN was used as the reference gene as it is the standard reference gene for barley clock studies and has been previously shown to be stable at different temperatures (Campoli et al., 2012b , 2013; Faure et al., 2012; Hemming et al., 2012; Deng et al., 2015). GAPDH was also used as a reference gene on a small subset of experiments with results similar to those obtained using ACTIN (see Supplementary Fig. S1 at JXB online). Relative transcript levels were calculated using the ΔΔCt method allowing for primer amplification efficiencies as previously described by Deng et al. (2015). Appropriate no template controls were included and melt curve analysis conducted for all qRT-PCR experiments. qRT-PCR results are the mean values of at least three biological replicates. Sequences of qRT-PCR primers used in this study have previously been described (Deng et al., 2015). Additional primers used in this study are listed in see Supplementary Table S1 at JXB online. Barley circadian clock gene sequences (CCA1, PRR59, PRR73, PRR95, TOC1, and GI) have previously been described (Campoli et al., 2012b ). Sequences of VRN1, VRN2, PPD1, FT1, FT2, FT4, FT5, TFL1, FPF1-like1, FPF1-like2, FPF1-like3, ELF3, CO1, CO2, and LUX have previously been described (Yan et al., 2003, 2004, 2006; Turner et al., 2005; Faure et al., 2007, 2012; Kikuchi et al., 2009; Greenup et al., 2010; Campoli et al., 2012a , 2013; Zakhrabekova et al., 2012).
Statistical analysis
All data presented are mean values ±the standard error of the mean. For all experiments where temperature treatments were compared (Figs 2, 3, 5–8; Supplementary Figs S1; 3–6) differences between mean values at every time point were tested by a Student’s t test assuming a two-tailed distribution and equal variance. Where greater than two means were compared, an ANOVA test was conducted prior to the Student’s t test.
Fig. 2.
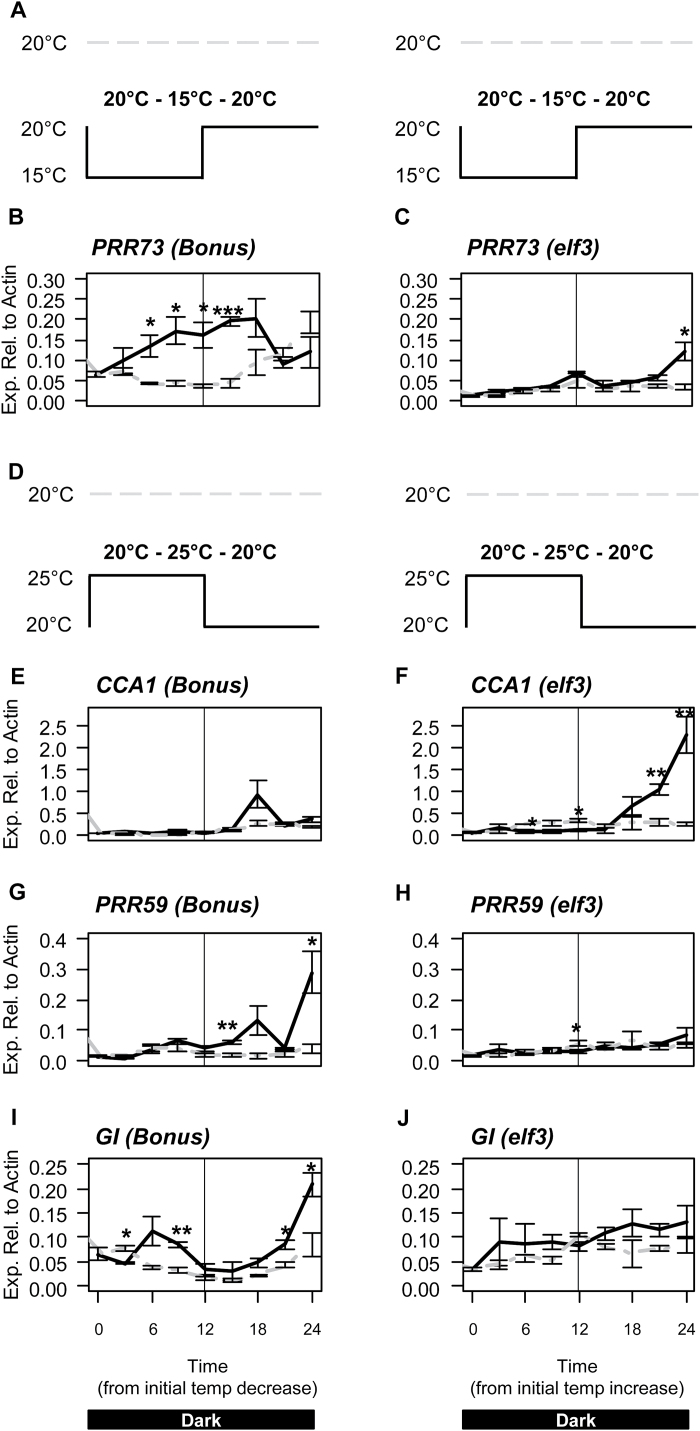
Response of circadian clock genes to temperature change from constant conditions in barley cv. Sonja. Relative expression of circadian clock genes in 5-d-old barley seedlings of cv. Sonja grown at a constant 20 °C (grey dashed line) in constant darkness. At time=0, temperature was either decreased to 15 °C (black line, left panels) or increased to 25 °C (black line, right panels) for 12h and then returned to 20 °C. Values are means of three biological replicates ±standard error. Significant differences are indicated by asterisks (*P<0.05, **P<0.01, ***P<0.001); where no asterisk is present the result is not significant.
Fig. 3.
Expression of circadian clock genes in cv. Sonja at 15 °C and 25 °C in long days.Relative expression of circadian clock genes in 5-d-old barley seedlings of cv. Sonja grown at 15 °C (blue dashed line) or 25 °C (red line) in long day (16/8h light/dark) conditions. The labels on the horizontal axis indicate time from lights on. Values are means of three biological replicates ±standard error. Significant differences are indicated by asterisks (*P<0.05, **P<0.01); where no asterisk is present the result is not significant.
Fig. 5.
Response of PRR73, CCA1, PRR59, and GI to temperature change from constant conditions in cv. Bonus and elf3 mutant. The relative expression of PRR73, CCA1, PRR59, and GI in 5-d-old cv. Bonus and elf3 mutant seedlings grown at a constant 20 °C (grey dashed line) in constant darkness. (A–C) At time=0, the temperature was decreased to 15 °C (black line) for 12h and then returned to 20 °C. (D–J) At time=0, the temperature was increased to 25 °C (black line) for 12h and then returned to 20 °C. Values are means of three biological replicates ±standard error. Significant differences are indicated by asterisks (*P<0.05, **P<0.01); where no asterisk is present the result is not significant.
Fig. 8.
Expression of genes associated with the long-day. promotion of flowering at 15 °C and 25 °C in barley cv. Bonus and elf3 mutants. The relative expression of circadian clock genes in barley seedlings cv. Bonus and elf3 mutant grown at 15 °C (blue dashed line) or 25 °C (red line). Bonus and elf3 seedlings were germinated and grown for 4 d in long-day conditions (16/8h light/dark) to entrain the barley clock. Seedlings were then moved into darkness for 24h and gene expression was sampled every 4h over the next 24h period. The labels on the horizontal axis indicate the time from lights on (from preceding entraining conditions). Values are means of at least three biological replicates ±standard error. Significant differences are indicated by asterisks (*P<0.05, **P<0.01, ***P<0.001); where no asterisk is present the result is not significant.
Results
Temperature entrainment of the barley circadian clock
To determine if the barley circadian clock can be entrained by thermo-cycles, transcript levels of clock genes were examined in barley seedlings cv. Sonja. Seedlings were germinated and grown for 5 d in constant darkness with a daily temperature cycle of approximately 16–25.5 °C (Fig. 1A). Expression of CCA1, TOC1, GI, PRR73, PRR95, and PRR59 showed a rhythmic pattern with a period of approximately 24h (Fig. 1B–D).
Response of clock genes to a transient increase in temperature from a non-oscillating state
To determine which barley clock genes respond rapidly to changes in temperature, the responses of barley clock genes to a transient shift in temperature were examined. Barley seedlings of cv. Sonja were germinated and grown in constant darkness at 20 °C; conditions in which the clock is not oscillating and normal feedback loops are absent (Deng et al., 2015). The temperature was then either increased to 25 °C or decreased to 15 °C for 12h, before being returned to 20 °C (Fig. 2A, F). Gene expression after the temperature shifts was compared with expression in Sonja seedlings grown in constant dark at 20 °C, previously presented in Deng et al. (2015). Increased expression of PRR73 was observed when the temperature decreased from 20 °C to 15 °C and from 25 °C to 20 °C (after the 12h transient increase to 25 °C) (Fig. 2C, H). CCA1 expression also increased when the temperature was decreased from 25 °C to 20 °C (Fig. 2G). Expression of PRR59 also increased when the temperature was decreased from 20 °C to 15 °C (Fig. 2D). Transcript levels of GI, PRR73, and PRR59 were significantly higher, 6h after the temperature increase from 20 °C to 25 °C and expression began to decline before any other temperature change (Fig. 2H–J). The overall expression pattern of CCA1, GI, and PRR59 in response to the temperature increase from 20 °C to 25 °C, appeared to be rhythmic (Supplementary Fig. S2). Expression of PRR95, TOC1, and ELF3 were unaffected by changes in temperature (Supplementary Fig. S3).
Expression profiles of barley clock genes at high versus low ambient temperatures in light/dark cycles
To determine the effect of different temperatures on the expression of clock genes in long-day conditions, where the clock is oscillating and temperature impacts growth and development, cv. Sonja seedlings were germinated and grown for 5 d in 16/8h light/dark (long-days) at a constant 15 °C or 25 °C. Under these conditions all clock genes assayed displayed a rhythmic expression profile over the 24h period similar to those described in previous studies (Campoli et al., 2012b ; Deng et al., 2015). Peak expression of CCA1, PRR73, PPD1, PRR95, and GI was significantly higher at 25 °C compared with 15 °C (Fig. 3A–D, G). The PRR genes did not all behave the same way; there was little difference between the expression of PRR59 and TOC1 at 15 °C compared with 25 °C (Fig. 3E, F). At 25 °C the peak of expression of LUX was increased and shifted to the start of the dark period, whereas only minor changes in ELF3 expression were observed (Fig. 3H, I).
To ensure that differences in the expression of clock genes observed in long days were not an artefact of the temperature treatments chosen, barley seedlings were grown as described above, but at 15, 20, 25, and 30 °C. To maximize any potential differences between the treatments, expression of clock genes was assayed at the time of day when expression was highest (at 25 °C), as previously determined (Fig. 3). Consistent with the previous findings, there was no significant difference in the expression of TOC1, PRR59 or ELF3 between any of the temperature treatments Supplementary (Fig. S4E, F, H). Although expression of PRR95 appeared to follow a pattern of increase with increasing temperature, this was not statistically significant (Supplementary Fig. S4D). Expression of CCA1 increased in a dosage-dependent manner from 15 °C to 25 °C (Supplementary Fig. S4A). PRR73, PPD1, GI, and LUX all responded in a similar manner to CCA1, although the differences in expression were only statistically significant between the 15 °C and 25 °C temperature treatments (Supplementary Fig. S4B, C, G, I). Expression of all genes, except LUX, was lower at 30 °C than at 25 °C and this correlates with a reduction in seedling growth at 30 °C (Supplementary Figs S4; S5). This may indicate that the barley seedlings were experiencing mild heat stress at 30 °C.
To examine which clock genes respond directly to temperature when the clock is oscillating in long-day conditions, barley seedlings were grown at 15 °C and then a subset of seedlings were shifted to 25 °C for 6h. Gene expression in seedlings shifted to 25 °C was compared with expression in seedlings maintained at 15 °C. The temperature shift was timed to coincide approximately with the 6-h period immediately prior to the peak of expression for each gene (Supplementary Fig. S6A). As PRR59, TOC1, and ELF3 did not show any significant change in expression in response to temperature (Fig. 3; Supplementary Fig. S4) they were not included in this analysis. Of the clock genes analysed, only PRR95 showed a significant difference in gene expression between 15 °C and 25 °C within the 6-h treatment (Supplementary Fig. S6B).
ELF3 is required for temperature-dependent differences in clock gene expression
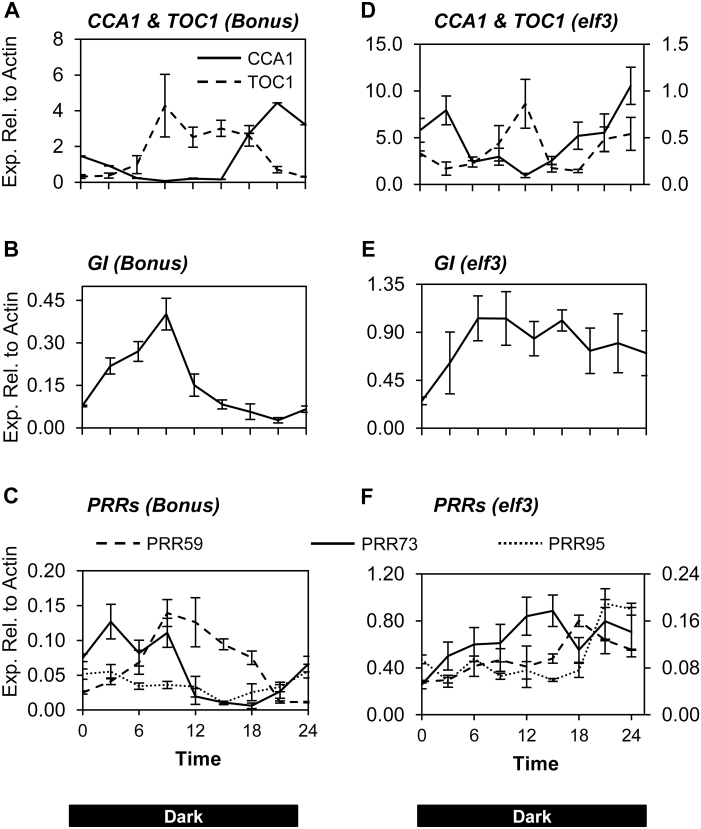
The Arabidopsis ELF3 gene may play a role in integrating temperature cues into the circadian clock (Thines and Harmon, 2010). To examine whether ELF3 might play such a role in barley, the effect of temperature on the barley elf3 loss-of-function mutant and the isogenic wild-type parent cv. Bonus were determined. Bonus and elf3 were germinated and grown in constant darkness with a daily temperature range of approximately 16–25.5 °C (Fig. 1A). Gene expression in cv. Bonus was rhythmic and similar to that observed for cv. Sonja (Fig. 4A–C). In the elf3 mutant, rhythmic gene expression of CCA1 and TOC1 was observed, but the rhythmic expression of GI, PRR73, PRR95, and PRR59 was severely dampened compared with cv. Bonus (Fig. 4D–F). Expression levels of CCA1, TOC1, GI, and PRR73 were increased in the elf3 loss-of-function mutant compared with cv. Bonus (Fig. 4D–F).
Fig. 4.
Expression of circadian clock genes in barley cv. Bonus and the elf3 mutant entrained to temperature cycles in constant darkness. The relative expression of circadian clock genes in 5-d-old barley seedlings, cv. Bonus and elf3 loss-of-function mutant in Bonus background, grown in constant dark with a daily temperature range of 16–25.5 °C. In elf3, the relative expression of TOC1 and PRR73 are plotted against the left vertical axis and CCA1, PRR59, and PRR95 are plotted against the right vertical axis. Values are means of three biological replicates ±standard error.
In cv. Sonja, expression of CCA1, GI, PRR59, and PRR73 responded rapidly to a change in temperature from constant conditions (Fig. 2). To determine if these temperature-dependent changes require a functional ELF3 gene, seedlings of the elf3 loss-of-function mutant and the isogenic wild-type parent cv. Bonus were germinated and grown for 5 d in constant darkness at 20 °C. The temperature was then either increased to 25 °C or decreased to 15 °C for 12h, before being returned to 20 °C (Fig. 5A, D). In cv. Bonus, PRR73 expression increased in response to a decrease in temperature from 20 °C to 15 °C similar to that observed in cv. Sonja (Fig. 5B). In the elf3 mutant, no statistically significant changes in transcript levels were observed for PRR73 in response to the temperature decrease (Fig. 5C). In cv. Bonus, GI expression increased in response to the temperature increase from 20 °C to 25 °C similar to that observed in cv. Sonja, but no significant difference in expression of GI was detected in the elf3 background (Fig. 5I, J). In cv. Bonus and elf3, CCA1 expression increased after the temperature was decreased from 25 °C to 20 °C similar to that observed in cv. Sonja, although the response in cv. Bonus was not as strong as in elf3 or cv. Sonja (Fig. 5E, F). The response of PRR59 to the temperature increase from 20 °C to 25 °C observed in cv. Sonja was not present in cv. Bonus, indicating that the genotypic differences between Sonja and Bonus may affect PRR59 expression (Fig. 5G).
To determine whether the observed differences in expression of clock genes in normal oscillating conditions at different temperatures are dependent on a functional circadian clock, gene expression was analysed in the elf3 loss-of-function mutant. Seedlings of the elf3 loss-of-function mutant and the isogenic wild-type parent cv. Bonus were germinated and grown at a constant 15 °C or 25 °C in 16/8h light/dark for 4 d to allow for entrainment of the barley circadian clock. Bonus and elf3 seedlings were then shifted to constant dark, where cv. Bonus maintains rhythmic expression of clock genes, but the barley clock is arrhythmic in the elf3 background (Faure et al., 2012; Deng et al., 2015). The expression of the core clock genes CCA1, PRR73, PPD1, PRR95, GI, and LUX in cv. Bonus, all showed a rhythmic expression pattern with increased expression at 25 °C, consistent with the changes observed in cv. Sonja (Fig. 6A–F). The expression of the same genes in the elf3 mutant did not show a rhythmic diurnal expression pattern and there were no significant differences in gene expression between the temperature treatments (Fig. 6G–L).
Fig. 6.
Expression of circadian clock genes at 15 °C and 25 °C in barley cv. Bonus and the elf3 mutant entrained in long-day conditions. The relative expression of circadian clock genes in barley seedlings cv. Bonus and the elf3 mutant grown at 15 °C (blue dashed line) or 25 °C (red line). Bonus and elf3 seedlings were germinated and grown for 4 d in long-day conditions (16/8h light/dark) to entrain the barley clock. Seedlings were then shifted to darkness for 24h and gene expression was sampled every 4h over the next 24h period. The labels on the horizontal axis indicate the time from lights on (from preceding entraining conditions). Values are means of at least three biological replicates ±standard error. Significant differences are indicated by asterisks (*P<0.05, **P<0.01); where no asterisk is present the result is not significant.
Temperature affects expression of genes associated with the long-day promotion of flowering
To establish if the changes in expression of core clock genes observed at 25 °C correlates with the changes in expression of genes known to promote flowering, the expression level of genes associated with the long-day promotion of flowering was examined at 3-h time intervals in barley seedlings cv. Sonja grown in long-days at constant 25 °C or 15°C. There was no difference in the expression of CONSTANS 1 (CO1) at 25 °C compared with 15 °C (Fig. 7A). A small but statistically significant increase in CO2 expression was detected at 25 °C early in the light period (Fig. 7B). Barley has five members of the FLOWERING LOCUS T (FT) gene family (FT1, 2, 3, 4, and 5) and one TERMINAL FLOWERING 1 (TFL1) homologue. FT3 is deleted in cv. Sonja and expression of FT5 and TFL1 could not be detected. Expression of FT1 and FT2 was detectable. There was no significant difference in expression of FT1 at 15 °C compared with 25 °C but small differences in expression of FT2 were observed (Fig. 7C, D). Expression of FT4 was significantly increased at 25 °C, especially in the dark (Fig. 7E). Next, the expression of three FLOWERING PROMOTING FACTOR 1-like (FPF1-like) genes that respond to high temperature were determined (Hemming et al., 2012). FPF1-like 1, 2, and 3 all had significantly higher expression at 25 °C (Fig. 7F–H).
Fig. 7.
Expression of genes in cv. Sonja associated with the long-day promotion of flowering at 15 °C and 25 °C. The relative expression of flowering time genes in 5-d-old barley seedlings of cv. Sonja grown at 15 °C (blue dashed line) or 25 °C (red line) in long-day (16/8h light/dark) conditions. The labels on the horizontal axis indicate the time from lights on of gene sampling. Values are means of three biological replicates ±standard error. Significant differences are indicated by asterisks (*P<0.05, **P<0.01, ***P<0.001); where no asterisk is present the result is not significant.
To determine if any of the observed changes in flowering time genes were under circadian control, the expression of FT4, FPF1-like 1, 2, and 3 was examined in the elf3 loss-of-function mutant in conditions where the clock is arrhythmic. As described previously, the isogenic wild-type parent cv. Bonus and elf3 mutant seedlings were entrained in long-day conditions at a constant 15 °C or at 25 °C and then transferred to constant darkness, where elf3 is arrhythmic. An initial increase in FT4 expression at 25 °C was observed in cv. Bonus, similar to the initial peak of expression observed in cv. Sonja (Fig. 8A). In the elf3 background there was also a significant increase in FT4 expression, although peak expression occurred 6h earlier than observed in cv. Bonus (Fig. 8E). Expression of FPF1-like 3 was also increased in both cv. Bonus and the elf3 mutant (Fig. 8D, H).The increased expression of FPF1-like 1 and 2 previously seen in cv. Sonja at 25 °C was not observed in either cv. Bonus or the elf3 mutant (Fig. 8B, C, F, G).
Discussion
The barley circadian clock can be entrained by temperature and maintain rhythmic gene expression at different temperatures
A key feature of the plant circadian clock is the capacity to be entrained to thermo-cycles (‘temperature entrainment’). Here we show that when barley seedlings are germinated and grown in constant darkness with oscillating temperature, core clock genes display robust rhythms with a period of approximately 24h (Fig. 1). These data show that, as in other plant species, the barley clock is competent to be entrained by thermo-cycles. Interestingly, a 12h temperature shift was not sufficient to initiate robust rhythms in all clock genes, although the differences in the peaks and troughs of CCA1, PRR59, and GI expression, detected after the temperature was increased from 20 °C to 25 °C, appear to resemble normal clock rhythms (Fig. 2; Supplementary Fig. S2). This is different from the response to light where one light period was sufficient to initiate clock rhythms in all clock genes (Deng et al., 2015).
Another key feature of the plant circadian clock is the capacity to maintain the period of rhythmic gene expression across a range of temperatures. In this study, we show that there was no difference in the timing of daily peaks and troughs of clock gene expression in barley seedlings, grown for 5 d, in long days, at either 15 °C or 25 °C (Fig. 3). This demonstrates that the barley clock appears to be able to maintain the same period of expression of clock genes at different temperatures.
Despite evidence implicating the circadian clock in the control of flowering time in cereals, the way in which temperature affects the cereal clock and its involvement in temperature-related growth and development is not well understood. In this study, we demonstrate that four clock genes, GI, CCA1, PRR59, and PRR73, show rapid and significant changes in expression levels from a non-oscillating state when exposed to changes in temperature (Fig. 2). GI and CCA1 have previously been shown to be important for light entrainment of the barley circadian clock (Deng et al., 2015). Expression of GI increases rapidly in response to a transition from dark to light (Deng et al., 2015) and in response to a transition from a cool to a warm temperature (Fig. 2), both of which are indicative of a dawn cue. Similarly, CCA1 expression increases rapidly in response to a transition from light to dark (Deng et al., 2015) and to a transition from warm to cool (Fig. 2), both of which are indicative of a dusk cue. The expression of GI, CCA1, and PRR59 also appeared to become rhythmic after the temperature was increased from 20 °C to 25 °C (Fig. 2; Supplementary Fig. S2). Given the common response of GI and CCA1 to both temperature and light cues, these genes may play a role in setting daily circadian rhythms in barley.
In long-day conditions, expression levels of CCA1, PRR73, PPD1, PRR95, GI, and LUX were all increased at 25 °C compared with 15 °C, but rhythmic gene expression was maintained (Fig. 3). To determine which genes might be important for this transcriptional response to temperature, barley seedlings were grown at 15 °C and the temperature was increased to 25 °C for 6h. Of all the clock genes analysed, only PRR95 showed an increase in expression in response to a temperature increase in long-day conditions (Supplementary Fig. S6). PRR95 has also been shown to respond strongly to light (Deng et al., 2015), suggesting that it may be important for integrating temperature and light cues into the barley circadian clock.
It is important to note that the clock genes that respond to a change in temperature from constant conditions are different to those from oscillating conditions. In constant conditions, CCA1, PRR59, PRR73, and GI all responded rapidly to changes in temperature (Fig. 2). In long-day oscillating conditions only PRR95 responded to a change in temperature (Supplementary Fig. S6). The response of clock genes to changes in temperature may be dependent on the state of the clock or light. Given the limited response of clock genes to temperature changes in oscillating conditions, constant (non-oscillating) conditions may be best to analyse the affects of temperature on the barley clock. A common factor across all temperature experiments was the responsiveness of CCA1, PRR59, PRR73, PRR95, and GI to different temperature treatments. This implies that CCA1, GI, and the PRR genes have an important role in the temperature responses of the barley clock.
Genes associated with the long-day promotion of flowering respond to temperature in a clock-independent manner
In cv. Sonja, expression of FT4, FPF1-like 1, 2, and 3 was increased at 25 °C compared with 15 °C (Fig. 7). In cv. Bonus, the significant increase in expression of FPF1-like 1 and 2 was not detected (Fig. 8). This may be due to differences in the genetic backgrounds of these genotypes. Similar to cv. Sonja, increased expression of FT4 and FPF1-like 3 was detected at higher temperature in cv. Bonus and in elf3 (Fig. 8). under these conditions, normal rhythmic expression and the temperature response of clock genes is lost in the elf3 mutant (Figs 6, 8). This suggests that the up-regulation of FT4 and FPF1-like 3, while potentially important in temperature-dependent flowering, may be independent of the circadian clock.
Expression of PPD1, the major determinant of photoperiod-dependent flowering in barley, is increased at 25 °C, although no increase in expression was observed in the downstream floral promoting gene FT1 (Fig. 7). This is similar to previous work that showed no difference in the transcript levels of FT1 in early-flowering barley plants grown at 25 °C compared with those grown at 15 °C (Hemming et al., 2012). The strong up-regulation of PPD1 at 25 °C may be important for accelerating flowering at warm temperatures, but potential downstream targets are still to be identified.
The barley ELF3 gene plays a role in the response of clock genes to warm temperature cues
The barley circadian clock was able to be entrained by temperature in cvs Sonja and Bonus but not in the elf3 loss-of-function mutant, indicating that ELF3 is important for temperature entrainment (Figs 1, 4). ELF3’s role in temperature entrainment appears to be conserved, as the Arabidopsis ELF3 gene has also been shown to be important for temperature entrainment (Thines and Harmon, 2010). In cv. Sonja, temperature changes resulted in altered expression of GI and the PRR genes. In the barley elf3 loss-of-function mutant, in which functioning of the circadian clock is compromised, changes in expression of GI, PRR59, and PRR73, observed in response to a rapid temperature shift from constant conditions were lost (Fig. 5). The increased expression of GI, PRR73, and PRR95, at 25 °C in normal oscillating conditions were also lost in the elf3 mutant (Fig. 6). This indicates a potential role for ELF3 in mediating these transcriptional responses to temperature. As shown previously, and in this study, expression of PRR genes and GI is constitutively increased in the barley elf3 mutant, suggesting that these genes are negatively regulated by ELF3 (Faure et al., 2012; Zakhrabekova et al., 2012; Deng et al., 2015). The increase in expression of GI, LUX, and the PRR genes at 25 °C observed in this study, may be due to the removal of ELF3-mediated repression. Interestingly, no changes in expression of ELF3 were observed in response to changes in temperature indicating that ELF3 may be post-transcriptionally regulated by temperature. In Arabidopsis, ELF3 together with ELF4 and LUX form the evening protein complex that represses GI and the PRR genes (Dixon et al., 2011; Helfer et al., 2011; Nusinow et al., 2011; Mizuno et al., 2014). Warm temperatures have also been proposed negatively to regulate the evening complex in Arabidopsis (Mizuno et al., 2014). A similar mechanism may exist in barley, although this has yet to be confirmed.
Warm temperatures are known to alter the vegetative and reproductive development of barley (Hemming et al., 2012). In addition, ELF3 has been shown to regulate photoperiod-dependent flowering and gibberellic acid (GA) synthesis, altering growth and development (Turner et al., 2005; Faure et al., 2012; Hemming et al., 2012; Zakhrabekova et al., 2012; Campoli et al., 2013;Boden et al., 2014). The elf3-dependent changes in the expression of barley clock genes identified in this study may be a potential mechanism controlling warm temperature-dependent growth and development in barley.
Conclusion
In this study we examined the relationship between temperature and expression of core genes in the barley circadian clock. We show that the barley circadian clock can integrate temperature cues to entrain, and compensate for, changes in temperature. In barley, the expression levels of the core clock genes CCA1, GI, PRR59, PRR73, PRR95, PPD1, and LUX change in response to changes in temperature. These data show that some clock genes are temperature responsive at the transcript level in barley. In barley, the responses of the circadian clock genes and floral promoting genes analysed in this study do not appear to be linked. If a temperature- and clock-dependent flowering mechanism exists in cereals, further investigation into the significance of the increase in PPD1 expression at high temperature may prove a good starting point for identifying such a mechanism.
Yields of the world’s major cereal crops are predicted to fall as global temperatures rise; these data will underpin future studies on cereal development, flowering, and grain yield under increasing temperature.
Supplementary data
Figure S1. Comparison of ACTIN and GAPDH reference genes.
Figure S2. Expression of CCA1, GI, and PRR59 after a 12h temperature increase from constant conditions.
Figure S3. Response of PRR95, TOC1, and ELF3 to a 12h temperature change in cv. Sonja.
Figure S4. Expression of circadian clock genes in cv. Sonja at 15, 20, 25, and 30 °C.
Figure S5. Barley seedling growth at different temperatures.
Figure S6. Expression of circadian clock genes in cv. Sonja after a 6-h temperature pulse.
Table 1. qRT-PCR primers used in this study.
Acknowledgements
We acknowledge the excellent technical support from Kerrie Ramm, Sandra Stops, and Tanya Phongkham (Commonwealth Scientific and Industrial Research Organization). We also thank Jose Barrero Sanchez and Saul Newman (Commonwealth Scientific and Industrial Research Organization) for critical reading of the manuscript.
References
- Boden SA, Weiss D, Ross JJ, Davies NW, Trevaskis B, Chandler PM, Swain SM. 2014. EARLY FLOWERING3 regulates flowering in spring barley by mediating gibberellin production and FLOWERING LOCUS T expression. The Plant Cell 26, 1557–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto CPG, Waugh R, Brown JWS. 2015. Evolutionary relationships among barley and Arabidopsis core circadian clock and clock-associated genes. Journal of Molecular Evolution 80, 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campoli C, Drosse B, Searle I, Coupland G, von Korff M. 2012. a Functional characterisation of HvCO1, the barley (Hordeum vulgare) flowering time ortholog of CONSTANS . The Plant Journal 69, 868–880. [DOI] [PubMed] [Google Scholar]
- Campoli C, Pankin A, Drosse B, Casao CM, Davis SJ, von Korff M. 2013. HvLUX1 is a candidate gene underlying the early maturity 10 locus in barley: phylogeny, diversity, and interactions with the circadian clock and photoperiodic pathways. New Phytologist 199, 1045–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campoli C, Shtaya M, Davis SJ, von Korff M. 2012. b Expression conservation within the circadian clock of a monocot: natural variation at barley Ppd-H1 affects circadian expression of flowering time genes, but not clock orthologs. BMC Plant Biology 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. 1993. A simple and efficient method for isolating RNA from pine trees. Plant Molecular Biology Reporter 11, 113–116. [Google Scholar]
- Covington MF, Panda S, Liu XL, Strayer CA, Wagner DR, Kay SA. 2001. ELF3 modulates resetting of the circadian clock in Arabidopsis. The Plant Cell 13, 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng WW, Clausen J, Boden S, Oliver SN, Casao MC, Ford B, Anderssen RS, Trevaskis B. 2015. Dawn and dusk set states of the circadian oscillator in sprouting barley (Hordeum vulgare) seedlings. PLOS One 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon LE, Knox K, Kozma-Bognar L, Southern MM, Pokhilko A, Millar AJ. 2011. Temporal repression of core circadian genes is mediated through EARLY FLOWERING 3 in Arabidopsis. Current Biology 21, 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MR, Davis SJ, Bastow RM, McWatters HG, Kozma-Bognar L, Nagy F, Millar AJ, Amasino RM. 2002. The ELF4 gene controls circadian rhythms and flowering time in Arabidopsis thaliana . Nature 419, 74–77. [DOI] [PubMed] [Google Scholar]
- Edwards KD, Lynn JR, Gyula P, Nagy F, Millar AJ. 2005. Natural allelic variation in the temperature-compensation mechanisms of the Arabidopsis thaliana circadian clock. Genetics 170, 387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure S, Higgins J, Turner A, Laurie DA. 2007. The FLOWERING LOCUS T-like gene family in barley (Hordeum vulgare). Genetics 176, 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure S, Turner AS, Gruszka D, Christodoulou V, Davis SJ, von Korff M, Laurie DA. 2012. Mutation at the circadian clock gene EARLY MATURITY 8 adapts domesticated barley (Hordeum vulgare) to short growing seasons. Proceedings of the National Academy of Sciences, USA 109, 8328–8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer RA. 1985. Number of kernels in wheat crops and the influence of solar-radiation and temperature. Journal of Agricultural Science 105, 447–461. [Google Scholar]
- Fowler S, Lee K, Onouchi H, Samach A, Richardson K, Coupland G, Putterill J. 1999. GIGANTEA: a circadian clock-controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membrane-spanning domains. The EMBO Journal 18, 4679–4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawroński P, Ariyadasa R, Himmelbach A, et al. 2014. A distorted circadian clock causes early flowering and temperature-dependent variation in spike development in the Eps-3A m mutant of einkorn wheat. Genetics 196, 1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould PD, Locke JCW, Larue C, et al. 2006. The molecular basis of temperature compensation in the arabidopsis circadian Clock. The Plant Cell 18, 1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenup AG, Sasani S, Oliver SN, Talbot MJ, Dennis ES, Hemming MN, Trevaskis B. 2010. ODDSOC2 is a MADS box floral repressor that is down-regulated by vernalization in temperate cereals. Plant Physiology 153, 1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer SL. 2009. The circadian system in higher plants. Annual Review of Plant Biology 60, 357–377. [DOI] [PubMed] [Google Scholar]
- Hazen SP, Schultz TF, Pruneda-Paz JL, Borevitz JO, Ecker JR, Kay SA. 2005. LUX ARRHYTHMO encodes a Myb domain protein essential for circadian rhythms. Proceedings of the National Academy of Sciences, USA 102, 10387–10392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer A, Nusinow DA, Chow BY, Gehrke AR, Bulyk ML, Kay SA. 2011. LUX ARRHYTHMO encodes a nighttime repressor of circadian gene expression in the Arabidopsis core clock. Current Biology 21, 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming MN, Walford SA, Fieg S, Dennis ES, Trevaskis B. 2012. Identification of high-temperature-responsive genes in cereals. Plant Physiology 158, 1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T. 2010. Arabidopsis circadian clock and photoperiodism: time to think about location. Current Opinion in Plant Biology 13, 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi R, Kawahigashi H, Ando T, Tonooka T, Handa H. 2009. Molecular and functional characterization of PEBP genes in barley reveal the diversification of their roles in flowering. Plant Physiology 149, 1341–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Nomoto Y, Oka H, Kitayama M, Takeuchi A, Tsubouchi M, Yamashino T. 2014. Ambient temperature signal feeds into the circadian clock transcriptional circuitry through the EC night-time repressor in Arabidopsis thaliana . Plant and Cell Physiology 55, 958–976. [DOI] [PubMed] [Google Scholar]
- Nakamichi N, Kita M, Ito S, Sato E, Yamashino T, Mizuno T. 2005. a The Arabidopsis pseudo-response regulators, PRR5 and PRR7, coordinately play essential roles for circadian clock function. Plant and Cell Physiology 46, 609–619. [DOI] [PubMed] [Google Scholar]
- Nakamichi N, Kita M, Ito S, Yamashino T, Mizuno T. 2005. b. PSEUDO-RESPONSE REGULATORS, PRR9, PRR7 and PRR5, together play essential roles close to the circadian clock of Arabidopsis thaliana . Plant and Cell Physiology 46, 686–698. [DOI] [PubMed] [Google Scholar]
- Nakamichi N, Kusano M, Fukushima A, Kita M, Ito S, Yamashino T, Saito K, Sakakibara H, Mizuno T. 2009. Transcript profiling of an Arabidopsis PSEUDO RESPONSE REGULATOR arrhythmic triple mutant reveals a role for the circadian clock in cold stress response. Plant and Cell Physiology 50, 447–462. [DOI] [PubMed] [Google Scholar]
- Nusinow DA, Helfer A, Hamilton EE, King JJ, Imaizumi T, Schultz TF, Farre EM, Kay SA. 2011. The ELF4-ELF3-LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature 475, 398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paltiel J, Amin R, Gover A, Ori N, Samach A. 2006. Novel roles for GIGANTEA revealed under environmental conditions that modify its expression in Arabidopsis and Medicago truncatula . Planta 224, 1255–1268. [DOI] [PubMed] [Google Scholar]
- Park DH, Somers DE, Kim YS, Choy YH, Lim HK, Soh MS, Kim HJ, Kay SA, Nam HG. 1999. Control of circadian rhythms and photoperiodic flowering by the Arabidopsis GIGANTEA gene. Science 285, 1579–1582. [DOI] [PubMed] [Google Scholar]
- Pokhilko A, Fernandez AP, Edwards KD, Southern MM, Halliday KJ, Millar AJ. 2012. The clock gene circuit in Arabidopsis includes a repressilator with additional feedback loops. Molecular Systems Biology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson HM, Richards RA. 1993. Effects of high-temperature and photoperiod on floral development in wheat isolines differing in vernalization and photoperiod genes. Field Crops Research 32, 181–192. [Google Scholar]
- Salome PA, McClung CR. 2005. PSEUDO-RESPONSE REGULATOR 7 and 9 are partially redundant genes essential for the temperature responsiveness of the Arabidopsis circadian clock. The Plant Cell 17, 791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salome PA, Weigel D, McClung CR. 2010. The role of the Arabidopsis morning loop components CCA1, LHY, PRR7, and PRR9 in temperature compensation. The Plant Cell 22, 3650–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasani S, Hemming MN, Oliver SN, et al. 2009. The influence of vernalization and daylength on expression of flowering-time genes in the shoot apex and leaves of barley (Hordeum vulgare). Journal of Experimental Botany 60, 2169–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer R, Ramsay N, Samach A, Corden S, Putterill J, Carre IA, Coupland G. 1998. The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell 93, 1219–1229. [DOI] [PubMed] [Google Scholar]
- Somers DE, Webb AAR, Pearson M, Kay SA. 1998. The short-period mutant, toc1-1, alters circadian clock regulation of multiple outputs throughout development in Arabidopsis thaliana . Development 125, 485–494. [DOI] [PubMed] [Google Scholar]
- Thines B, Harmon FG. 2010. Ambient temperature response establishes ELF3 as a required component of the core Arabidopsis circadian clock. Proceedings of the National Academy of Sciences, USA 107, 3257–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A, Beales J, Faure S, Dunford RP, Laurie DA. 2005. The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 310, 1031–1034. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Tobin EM. 1998. Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell 93, 1207–1217. [DOI] [PubMed] [Google Scholar]
- Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Yasuda S, Dubcovsky J. 2006. The wheat and barley vernalization gene VRN3 is an orthologue of FT . Proceedings of the National Academy of Sciences, USA 103, 19581–19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J. 2003. Positional cloning of the wheat vernalization gene VRN1 . Proceedings of the National Academy of Sciences, USA 100, 6263–6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LL, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V, Dubcovsky J. 2004. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303, 1640–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhrabekova S, Gough SP, Braumann I, et al. 2012. Induced mutations in circadian clock regulator Mat-a facilitated short-season adaptation and range extension in cultivated barley. Proceedings of the National Academy of Sciences, USA 109, 4326–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.