Abstract
The anterior commissure (AC) and the much smaller hippocampal commissure constitute the only interhemispheric pathways at the telencephalic level in birds. Since the degeneration study from Zeier and Karten (1973), no detailed description of the topographic organization of the AC has been performed. This information is not only necessary for a better understanding of interhemispheric transfer in birds, but also for a comparative analysis of the evolution of commissural systems in the vertebrate classes. We therefore examined the fiber connections of the AC by using choleratoxin subunit B (CTB) and biotinylated dextran amine (BDA). Injections into subareas of the arcopallium and posterior amygdala (PoA) demonstrated contralateral projection fields within the anterior arcopallium (AA), intermediate arcopallium (AI), PoA, lateral, caudolateral and central nidopallium, dorsal and ventral mesopallium, and medial striatum (MSt). Interestingly, only arcopallial and amygdaloid projections were reciprocally organized, and all AC projections originated within a rather small area of the arcopallium and the PoA. The commissural neurons were not GABA‐positive, and thus possibly not of an inhibitory nature. In sum, our neuroanatomical study demonstrates that a small group of arcopallial and amygdaloid neurons constitute a wide range of contralateral projections to sensorimotor and limbic structures. Different from mammals, in birds the neurons that project via the AC constitute mostly heterotopically organized and unidirectional connections. In addition, the great majority of pallial areas do not participate by themselves in interhemispheric exchange in birds. Instead, commissural exchange rests on a rather small arcopallial and amygdaloid cluster of neurons. J. Comp. Neurol. 524:343–361, 2016. © 2015 The Authors The Journal of Comparative Neurology Published by Wiley Periodicals, Inc.
Keywords: avian, eutheria, metatheria, tyrosine hydroxylase (RRID AB_2201526), GABA (RRID AB_477652), corpus callosum
ABBREVIATIONS
- AA
anterior arcopallium
- AC
anterior commissura
- AD
dorsal arcopallium
- AI
intermediate arcopallium
- AIdm
dorsomedial intermediate arcopallium
- AIvm
ventromedial intermediate arcopallium
- AM
medial arcopallium
- AV
ventral arcopallium
- Bas
nucleus Basorostralis pallii
- CC
corpus callosum
- HA
apical part of the hyperpallium
- HD
densocellular part of the hyperpallium
- HI
intercalated part of the hyperpallium
- HL
lateral hyperpallium
- LAD
dorsal arcopallial lamina
- LSt
lateral striatum
- MD
dorsal mesopallium
- MFD
frontodorsal mesopallium
- MFV
frontoventral mesopallium
- MID
dorsal intermediate mesopallium
- MSt
medial striatum
- MV
ventral mesopallium
- NCC
caudocentral nidopallium
- NCL
caudolateral nidopallium
- NFL
frontolateral nidopallium
- NFM
frontomedial nidopallium
- NFT
frontal trigeminal nidopallium
- NI
intermediate nidopallium
- NIL
lateral intermediate nidopallium
- OB
olfactory bulbus
- PoA
posterior pallial amygdala
- TnA
nucleus taeniae of the pallial amygdala
- TPO
temporo‐parieto‐occipital area
Integration of information from the left and the right side of the body is key for survival. This is accomplished by various commissural systems that interconnect the two halves of the nervous systems in all animals (Arendt et al., 2008; Semmler et al., 2010). In the brains of all vertebrates, the three main commissural systems at the telencephalic level are the anterior commissure (AC), the hippocampal commissure (HC), and the corpus callosum (CC). The CC is the evolutionarily most recent interhemispheric pathway, and probably developed in conjunction with the cortical expansion in eutherian mammals. In metatherian mammals, like wallabies and opossums, the AC and HC are relatively large, possibly to compensate for the absence of the CC. But the AC of metatheria is not only large, but also displays a connectivity pattern that is equivalent to a combination of the eutherian AC and CC (Martin, 1967; Granger et al., 1985).
Next to mammals, birds are the second major class of vertebrates that are able to process and coordinate complex behavioral and cognitive operations. Like metatherian mammals, birds do not possess a corpus callosum. Instead, interhemispheric exchange occurs at the telencephalic level via the AC and the small HC. Despite these limitations of commissural pathways, behavioral studies in pigeons demonstrated that not only color discrimination (Skiba et al., 2000; Letzner et al., 2014), but also cognitive inference information can be transferred between the hemispheres (Manns and Römling, 2012). However, pigeons are virtually unable to transmit interhemispherically conjoint spatial and visual information (Graves and Goodale, 1977; Watanabe 1980; Nottelmann et al., 2002). Thus, it is possible that the absence of a CC sets limits to what kind of information can be exchanged between hemispheres. Along these lines, the colloquial statement that birds are “natural split brains” could partly be true.
Contrasting these assumptions, the classic study of Zeier and Karten (1973) demonstrated that the AC of pigeons interconnects a wide network of forebrain structures and differs in part substantially from the metatherian organization. Unfortunately, the degenerating tracing technique employed by Zeier and Karten (1973) does not allow for a detailed identification of the topographic organization with regard to cells of origin and partly also with regard to termination areas. However, such knowledge is necessary to compare the AC of birds with those of other vertebrates and especially with the organization in methateria and eutheria. Therefore, more than 40 years after the publication of Zeier and Karten (1973), we decided to reexamine the fiber connections and the chemoarchitecture of the commissura anterior of pigeons with modern tract‐tracing methods.
MATERIALS AND METHODS
Overall, 35 pigeons (Columba livia) of unknown sex were used for the study. In five of them the AC was transected before tracer application, while in a further 30 animals the AC was left intact. The animals received unilateral injections of either the predominantly anterogradely transported tracer biotinylated dextran amine (BDA, 10,000 molecular weight, Molecular Probes, Leiden, Netherlands) or the tracer choleratoxin subunit B (CTB, Sigma, Deisenhofen, Germany) into subunits of the left or right arcopallium to analyze the termination areas of the AC. Because CTB is transported both in anterograde as well as retrograde direction, this tracer was also injected into the termination areas to analyze the cells of origin of the AC. All experiments were carried out according to the specifications of German law for the prevention of cruelty to animals and, hence, the European Communities Council Directive of November 24, 1986 (86/609/EEC). All efforts were made to minimize the number of animals used and their suffering.
Transection of the AC and tracer application
Pigeons were anesthetized with a 7:3 mixture of ketamine (100 mg/ml) and xylaxine (20 mg/ml). The skull was opened and the sinus sagittalis along the midline of the brain was gently pulled sideward. A surgical microknife was inserted under stereotaxic guidance between the forebrain hemispheres at coordinates A 7.75, L 0.00 according to the pigeon brain atlas by Karten and Hodos (1967). The blade was slowly lowered for 9.0 mm, dissecting the AC in the process. After 1 week recovery the tracer application was performed. Pigeons were anesthetized as described above. For arcopallial tracer injections, a modified device was used, which allowed lateral rotation of the head along the longitudinal axis over 100° to the left and right (Hellmann and Güntürkün, 1999). Both tracers (BDA, 10% in 3% dimethyl sulfoxide [DMSO] and CTB, 1% in deionized water) were injected through a glass micropipette (inner tip diameter 15–20 μm) with a mechanic pressure device (WPI Nanoliterinjector; World Precision Instruments, Berlin, Germany). CTB was also injected into the nidopallium (A 6.50), the intercalate hyperpallium (A 12.00), and the medial striatum (A 11.00). For every area the injection volume was between 200 to 400 nl (for overview of injection sites, see Table 1). Following a survival time of 2 days (for CTB) and 7 days (for BDA), the animals were deeply anesthetized with equithesin (0.45 ml per 100 g body weight) and perfused with 0.9% sodium chloride followed by ice‐cold 4% paraformaldehyde (PFA) in 0.12M phosphate‐buffered saline (PBS), pH 7.4. Brains were removed and postfixed for 2 hours in PFA with a supplement of 30% sucrose. Subsequently, the brains were cryoprotected overnight in a solution of 30% sucrose in PBS. Brains were cut in the frontal plane at 30 μm on a freezing microtome (Leica Microsystems, Wetzlar, Germany), and the slices were collected in PBS containing 0.1% sodium azide.
Table 1.
List of Injected Brain Areas and the Respective Coordinates
| Injected area | Anteroposterior position |
|---|---|
| Anterior arcopallium (AA) | A 7.50 |
| Intermediate arcopallium (AI) | A 7.50; A 6.50; A 6.25 |
| Medial arcopallium (AM) | A 6.50 |
| Dorsal arcopallium (AD) | A 6.50 |
| Posterior pallial amygdala (PoA) | A 6.25; A 5.00 |
| Caudolateral nidopallium (NCL) | A 6.50 |
| Caudocentral nidopallium (NCC) | A 6.50 |
| Medial striatum (MST) | A 11.00 |
| Apical hyperpallium (HA) | A 12.00 |
| Intercalate hyperpallium (HI) | A 12.50; A 11.00; A 10.00 |
Immunohistochemical staining of CTB and BDA
Both tracers were immunohistochemically visualized by using 3′3‐diaminobenzidine (DAB; Sigma, Steinheim, Germany). Slices were pretreated with 0.3% H2O2 for 30 minutes. After washing in PBS, they were blocked in 10% normal rabbit serum for 1 hour, followed by overnight incubation in PBS containing a goat anti‐CTB antibody (Calbiochem, Darmstadt, Germany; Cat no. 227040, RRID AB_211712; 1:10,000; Table 2) and 0.3% Triton X‐100 at 4°C. After being rinsed in PBS, the sections were incubated for 60 minutes at room temperature in the biotinylated rabbit antigoat IgG (Vectastain ABC‐Elite kit, Linaris, Dossenheim, Germany; 1:200). Finally, the sections for CTB and BDA visualization were incubated in avidin‐biotin‐peroxidase solution (Vectastain ABC‐Elite kit, Linaris, Dossenheim, Germany; 1:100) for 60 minutes at room temperature. After washing, the peroxidase activity was detected using a heavy metal intensified DAB‐reaction, modified by the use of b‐D‐glucose/glucose‐oxidase (Sigma‐Aldrich, Taufkirchen, Germany; 1 mg/ml). Sections were mounted on gelatin‐coated slides, dehydrated, and coverslipped with DPX.
Table 2.
Table of Primary Antibodies Used
| Antigen | Immunogen | Manufacturer | Dilution |
|---|---|---|---|
| Choleratoxin subunit B |
Nondenatured CTB isolated from Vibria cholerae |
Calbiochem, goat polyclonal, Cat no. 227040, RRID: AB_211712 | 1:10,000 |
| Choleratoxin subunit B |
Nondenatured CTB isolated from Vibria cholerae |
Sigma‐Aldrich, rabbit polyclonal, Cat no. C3062, RRID: AB_258833 | 1:1,000 |
| Tyrosine Hydroxylase | Purified tyrosine hydroxylase from rat pheochromocytoma | Millipore, mouse monoclonal, Cat no. MAB5280, RRID: AB_2201526 | 1:2,000 |
| γ‐aminobutyric acid | GABA conjugated to BSA | Sigma‐Aldrich, rabbit polyclonal, Cat no. A2052, RRID: AB_477652 | 1:1,000 |
Fluorescence double staining CTB‐GABA and CTB‐TH (tyrosine hydroxylase)
For CTB‐TH double staining, the slices were incubated for 30 minutes in 10% normal goat serum. After being rinsed, the slices were incubated for 3 days at 4°C in PBS containing a rabbit anti‐CTB antibody (Sigma‐Aldrich, Munich, Germany; Cat no. C3062, RRID AB_258833, 1:1,000; Table 2), a mouse anti‐TH antibody (Millipore, Schwalbach, Germany; Cat no. MAB5280, RRID AB_2201526; 1:2,000; Table 2), and 0.3% Triton X‐100, followed by incubation in goat antirabbit Alexa‐488 IgG (Invitrogen, Darmstadt, Germany; 1:200) and goat antimouse Alexa‐594 IgG (Invitrogen; 1:200). Sections were then mounted on glass slides and coverslipped with fluoromount (SouthernBiotech, Eching, Germany).
For CTB‐GABA double staining, a sequential staining was performed, starting with CTB labeling. Slices were incubated for 30 minutes in 10% normal horse serum, followed by incubation in PBS containing a goat anti‐CTB antibody (1:1,000) and 0.3% Triton X‐100 for 3 days at 4°C. After rinsing in PBS, the sections were incubated in PBS containing donkey antigoat Alexa‐594 IgG (Invitrogen; 1:200) and 0.3% Triton X‐100. For the GABA staining the slices were incubated for 3 days at 4°C in PBS containing rabbit anti‐GABA antibody (Sigma‐Aldrich, Steinheim, Germany; Cat no. A2052, RRID AB_477652; 1:1,000; Table 2) and 0.3% Triton X‐100. The GABA‐antibody was diluted in an incubation solution (IS; consisting of 2% NaCl, 0.3% Triton, 4% BSA, 5% normal horse serum in 0.05M Tris‐buffered saline, pH 7,4). The slices were washed in PBS and incubated in PBS containing donkey antirabbit Alexa‐488 IgG (Invitrogen; 1:500 diluted in IS) for 1 hour at room temperature. After rinsing in PBS, the incubation in primary antibody solution was repeated for 1 day at 4°C, followed by incubation in secondary antibody solution for 1 hour at room temperature. The sections were mounted on glass slides and coverslipped with fluoromount with DAPI (SouthernBiotech).
Histological analysis
DAB stained sections were analyzed using a Zeiss Axio Imager M1 Microscope (Carl Zeiss MicroImaging, Göttingen, Germany), equipped with an AxioCam MRM (Carl Zeiss MicroImaging) and the software AxioVison 4.8 (Carl Zeiss MicroImaging). The fluorescence staining was analyzed using a Zeiss LSM 510 Meta Confocal Microscope (Carl Zeiss MicroImaging) and the software AxioVison 4.8.
Nomenclature used in the present study is based on the Avian Brain Nomenclature Forum, Reiner et al. (2004) and Karten and Hodos (1967).
RESULTS
Injections into subareas of the arcopallium and posterior pallial amygdala
CTB retrograde labeling
The AC in the pigeon brain is localized within the telencephalon and extends in the anteroposterior direction from A 7.50 to A 8.00 (Fig. 1A,B). To visualize the neurons of origin of the AC, a CTB injection was placed at A 7.50 below the pia mater into the rostral portion of the left arcopallium (case P‐798) (Fig. 2A). Diffusion was restricted to the rostral portion of the arcopallium, including AA and AI and partly innervated PoA. Numerous CTB‐labeled neurons were found around the injection site, and ipsilateral arcopallial cell labeling extended between A 7.25 and A 7.75. Contralaterally, a moderate number of labeled neurons were seen in the dorsal AI at A 7.50 and more rostral in AA at A 7.75 close to the border of the dorsal arcopallium (AD) but never crossing this border (Fig. 2B,C,J). From A 7.25 to A 7.00 this cluster of CTB‐labeled neurons shifted to the ventral part of AI (Fig. 2J). Furthermore, caudal to the injection site, at A 6.75 and within the contralateral telencephalon, a few CTB‐labeled neurons were scattered within PoA (Fig. 2J).
Figure 1.
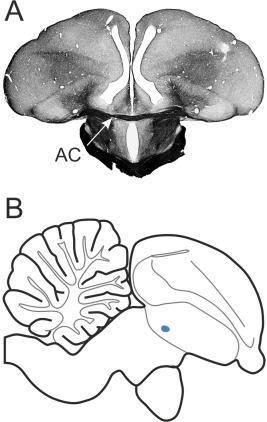
Location of the AC in the pigeon brain. A: Frontal section at A 7.75 of a Gallyas fiber staining. Arrow indicates the AC. B: Sagittal view of a schematic drawing at L 0.00. The blue dot marks the location of the AC.
Figure 2.
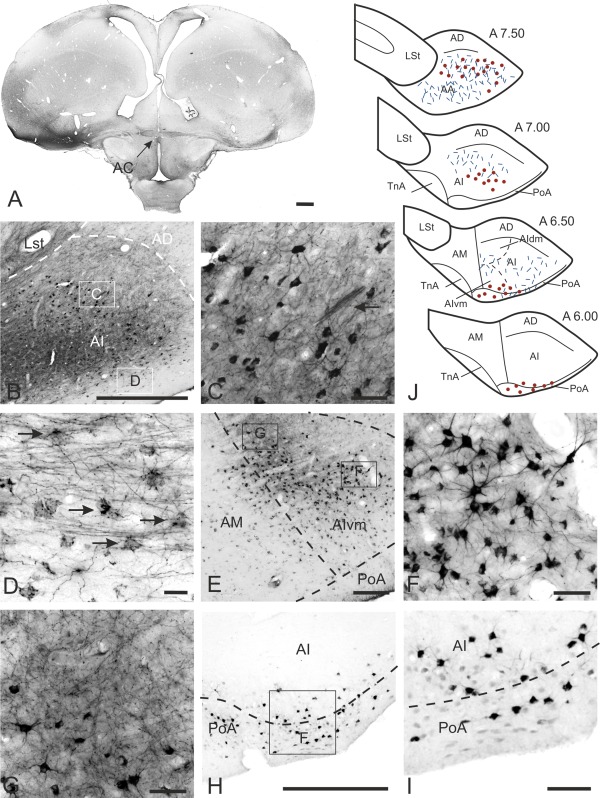
Cells of origin and termination areas within the arcopallium. A: Injection site into AA at A 7.75 (left) with labeled fibers within the AC leading towards the neurons of origin in the contralateral side (right). B: CTB labeling within the contralateral AI at A 7.50. C: Enlargement of the box in B. Within a network of fibers retrogradely labeled neurons are found. Arrow indicates a bundle of fibers crossing through AI. D: Enlargement of the box in B. Axosomatic‐like fibers coil around perikarya within the ventral AI. E: CTB labeling within the contralateral AI at A 6.75. F: Enlargement of the box in E showing many CTB‐labeled cells within AI. G: Enlargement of the box in E displaying the fine network of terminating fibers. H: CTB labeling within the contralateral AI and PoA at A 6.00. I: Enlargement of the box in H. Few retrogradely labeled cells within the ventral AI and PoA. J: Schematic drawing of the cells of origin and termination areas within the arcopallium after CTB injections into the contralateral arcopallium. For abbreviations, see list. Scale bars = 1 mm in A; 500 μm in B,H; 50 μm in C,F,G,I; 20 μm in D; 200 μm in E.
A further CTB injection was placed into the left and right medial arcopallium (AM) (case P‐771, P‐656) at A 6.25, with a minute tracer diffusion to the adjacent AI. Contralaterally, a small number of CTB‐labeled neurons were observed in the ventromedial AI (AIvm) close to the border of AM; a few of these retrogradely labeled cells were seen in AM (Fig. 2E). In the anterioposterior direction, the CTB‐labeled neurons extended from A 6.25 to A 7.00. In a more dorsal part of AIvm the retrogradely labeled neurons overlapped with anterogradely labeled fiber endings (Fig. 2E,G).
In two pigeons similar CTB injections were placed at A 6.50 into the caudal portion of the left arcopallium (case P‐04, P‐05). The diffusion area of the tracer in these two cases extended from A 6.25 to A 7.25, within AD and lateral AI. Within the contralateral telencephalon a small number of retrogradely labeled cells were found within the ventral AI (A 6.00 to A 6.50; Fig. 2H,I). These CTB‐labeled neurons extended from AIvm to the ventrolateral edge of AI (Fig. 2H–J). More ventrally and within the lateral edge of PoA, a few further neurons were seen (Fig. 2H,I). In contrast to case P‐771, with tracer injections into the medial AI, the retrogradely labeled cells in case P‐04 and P‐05 extended to the lateral AI. In contrast to the first described case P‐798, the position of the injection site in the last four cases (P‐771, P‐656, P‐04, P‐05) was placed more caudally within the arcopallium. This had an impact on the position of the contralaterally retrogradely CTB‐labeled cells. The injection site in the rostral part of the arcopallium resulted in rostrally located CTB‐labeled cells, while a more caudal position correlated with caudally labeled cells. Small amounts of CTB were also placed into the left PoA (case P‐551) at A 6.25. Diffusion of the tracer was restricted to PoA from A 6.00 to A 6.75 and to the ventral AI. Contralaterally, a few CTB‐labeled neurons were seen within the medial PoA at A 6.50. In case P‐585, small amounts of CTB were placed into the caudal part of right PoA at A 5.00. The tracer diffused to AD and nidopallial areas immediately surrounding PoA. Within the contralateral telencephalon no CTB‐labeled neurons were found.
In sum, injections into the arcopallium and PoA revealed retrogradely labeled cells within the contralateral telencephalon that were restricted to areas within AA, AI, and PoA (Fig. 2J). This cluster of cells shifted from a ventral position caudally to a laterodorsal position rostrally (Fig. 2J).
BDA/CTB anterograde labeling
For a first overview of the projections of the AC, massive injections of BDA or CTB were placed into the rostral portion of either the left or right arcopallium. One of the main projection areas in the contralateral telencephalon was the arcopallium, but the fibers also crossed the borders of the arcopallium and terminated in further pallial and subpallial areas.
Termination areas within contralateral arcopallium
In two birds similar BDA injections were placed at A 7.50 into the left (P‐08) or right (P‐01) arcopallium. Tracer spread was observed in all subareas of the arcopallium (AA, AM, AI, AD) and the posterior pallial amygdala. Additionally, the tracer diffused to the adjacent caudocentral nidopallium (NCC), the ventral part of caudolateral nidopallium (NCL), the lateral striatum and the subpallial amygdala. The injection of BDA into the left (P‐08) or right (P‐01) arcopallium revealed similar termination areas as described in the following. Within the contralateral arcopallium, terminating fibers were visible from A 6.50 to A 7.75. The innervated subareas of the arcopallium in this anterior/posterior extension were mainly AI. A dense network of axon terminals was also observed at A 6.50 within AI, AIvm, and the ventral part of AIdm. This projection pattern was also visible in the already described case P‐771 and P‐656, demonstrating a network of thin and varicose fibers within the dorsomedial AI (A 7.00 to A 7.25), overlapping within AIvm with retrogradely CTB‐labeled neurons (Fig. 2E,G,J). More rostrally, a mixture of thin and thick fibers terminated in a dorsolateral portion of AI (A 7.50). Terminating fibers were also found within the contralateral AA and PoA (Fig. 2J). The ventral parts of AI and PoA were not as densely innervated as the dorsal part, but the innervating fibers were much thicker (Fig. 3A,B). In case P‐798 a precise CTB‐injection could be placed into AA and the rostral parts of AI and PoA. Due to the rostral position of the injection site, a large number of fibers passed contralaterally through AA and rostral AI (Fig. 2B,C). En route, various fibers also terminated within these areas. In a more caudal part of the arcopallium (A 7.00 to A 6.50) a network of thin fibers innervated nearly the whole arcopallium, with a higher concentration of terminating fibers in the dorsal part of AI (Fig. 2B). At A 7.50 these fibers coiled around unlabeled perikarya, building basket‐like arrangements within AI (Fig. 2D). Within the contralateral AD and AM no axon terminals were observed.
Figure 3.
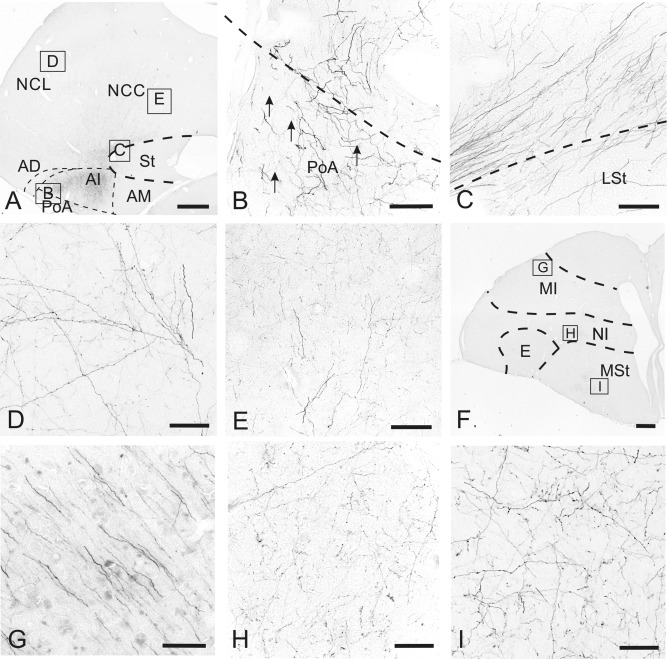
Termination areas within the telencephalon after contralateral arcopallial BDA or CTB injections. A: BDA‐labeled fibers within the contralateral hemisphere at A 6.50 at low magnification. B: Enlargement of the box in A. Thick BDA‐labeled fibers within PoA. C: Enlargement of the box in A. BDA‐labeled fiber bundles run dorsal to the LSt. D: Enlargement of the box in A. BDA‐labeled fibers within the NCL. E: Enlargement of the box in A. Fine network of BDA‐labeled fibers is in the NCC with crossing fibers with a large number of varicosities. F: BDA‐labeled fibers within the contralateral hemisphere at A 11.00 at low magnification. G: Enlargement of the box in F. BDA‐labeled fibers running from MID into the deeper layer of the mesopallium. H: Enlargement of the box in F. Fine network of thin fibers within NI. I: Enlargement of the box in F. Fine network of terminating fibers within the medial MSt. For abbreviations, see list. Scale bars = 1 mm in A,F; 100 μm in B–E,G–I.
Termination areas within further contralateral pallial areas
After BDA or CTB injections into the arcopallium (cases P‐08, P‐01, P‐798), many fibers passed through the arcopallium within the contralateral telencephalon and continued into neighboring structures (Fig. 3A). Following the trajectory of fibers crossing beyond the LAD and running dorsal to the lateral striatum (LSt) (Fig. 3C), a large number of thin BDA‐labeled fibers constituted a network within NCL (A 6.00 to A 7.75) (Figs. 3D, 4A,B) and NCC (A 6.00 to A 7.00) (Figs. 3E, 4A). A few varicose fibers ran through the NCL and terminated more rostrolaterally within temporo‐parieto‐occipital area (TPO), lateral intermediate nidopallium (NIL), and frontolateral nidopallium (NFL) (Fig. 4C–E). This rostrolaterally oriented trajectory turned dorsomedially at A 10.50 into MID (Fig. 3F,G) and terminated within the frontodorsal mesopallium (MFD) and frontoventral mesopallium (MFV) (A 12.25‐13.50) (Fig. 4E,F). At A 11.50 this network of terminating fibers within NIL expanded rostromedially into the frontal trigeminal nidopallium (NFT) (A 12.50 to A 13.50) (Fig. 4E,F). A distinct network of thin fibers within the intermediate nidopallium (NI) was visible dorsal to the medial striatum and medial to the entopallium at A 11.00 to A 12.50 (Figs. 3H, 4D). A medially oriented fiber stream terminated within the medial striatum and built a network of very thin fibers (A 9.00 to A 11.00) (Figs. 3I, 4D).
Figure 4.
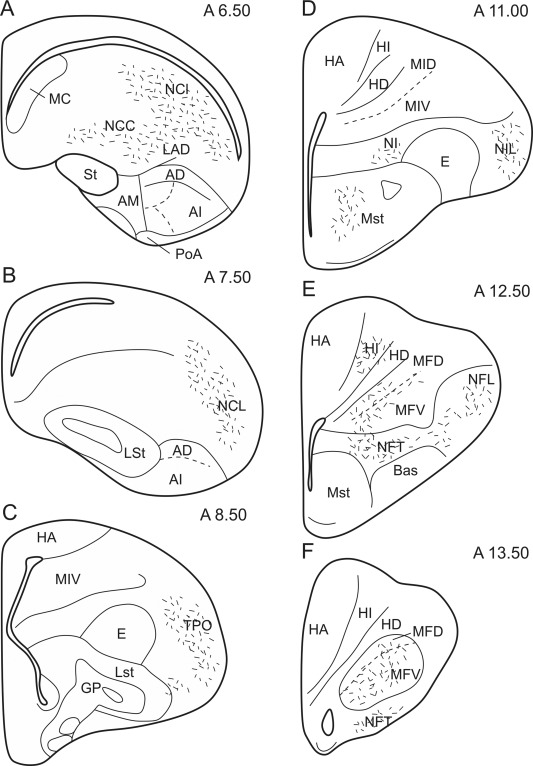
Schematic illustration of the rostrocaudal extent of the termination areas within the telencephalon after arcopallial BDA or CTB injections. Short lines represent anterogradely labeled terminals.
Injections into the nidopallium
CTB retrograde labeling
A CTB injection into the right NCL (case P‐959) was placed at A 6.50, and the tracer diffused to the immediately surrounding NCC (A 7.00 to A 5.75) (Fig. 5A). Contralaterally, a moderate number of CTB‐labeled neurons were seen in the rostral part of AI, extending medially into AM and ventrally into PoA (Fig. 5B,C). A few retrogradely labeled neurons were contralaterally scattered within AA. Beyond the borders of the arcopallium and the posterior pallial amygdala no CTB‐labeled neurons were found in the contralateral telencephalon.
Figure 5.
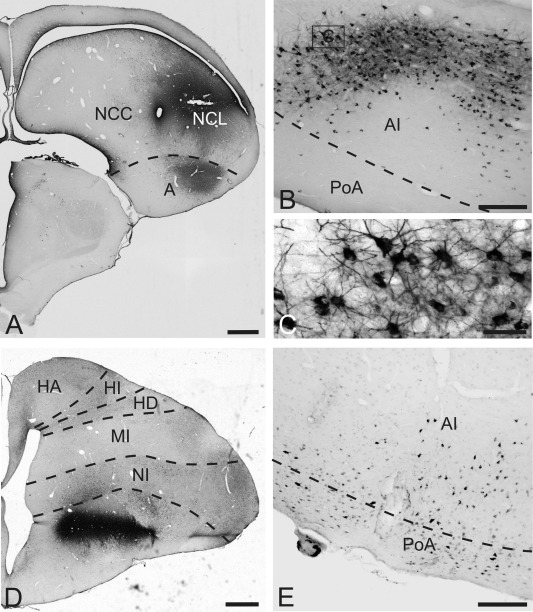
CTB labeling after injections into the contralateral nidopallium, MSt. A: Injection site of CTB into the nidopallium at A 6.50. B: CTB‐labeled neurons within the ventral AI after injections into the contralateral nidopallium. C: Enlargement of the box in B. There are many retrogradely labeled cells within AI. D: Injection site of CTB within MSt at A 11.00. E: Retrogradely labeled cells within the ventral AI and PoA after CTB injections into the contralateral MSt. For abbreviations, see list. Scale bars = 1 mm in A,D; 200 μm in B,E; 50 μm in C.
Injection into the medial striatum
CTB retrograde labeling
The injection of CTB was placed at A 11.00 into the right medial striatum (case P‐669). Diffusion of the tracer was restricted to MSt (A 11.25 to A 10.75) (Fig. 5D). Within the contralateral telencephalon a small number of CTB‐labeled neurons were scattered within the ventrolateral AI and the lateral PoA at A 6.50 (Fig. 5E). Beyond the borders of the arcopallium and the posterior pallial amygdala, no CTB‐labeled neurons were found in the contralateral telencephalon.
Injection into the intercalate hyperpallium
CTB retrograde labeling
Our massive BDA injections into the arcopallium revealed no anterograde fiber staining within the contralateral hyperpallium. However, previous studies had revealed a contribution to the hyperpallium to interhemispheric projections (Zeier and Karten, 1973; Bagnoli and Burkhalter, 1983). We therefore performed CTB injections into subareas of the hyperpallium. Injections of CTB were placed at A 12.00 into the left or right apical hyperpallium (HA) (case P‐62, P‐63). The tracer injection was restricted to HA, with no tracer spread into the adjacent intercalate hyperpallium (HI) (Fig. 6A). Within the contralateral arcopallium no retrogradely labeled neurons were found (Fig. 6B). Further CTB injections were performed into the left or right HI at different anteroposterior positions (case P‐520, P‐449, P‐382, P‐461). In case P‐520 tracer was injected at the dorsal border of HI at A 12.50. Diffusion of the tracer slightly crossed the border to HA but never touched the ventral border to HD or mesopallium (Fig. 6C). Contralaterally, a relatively small number of CTB‐labeled neurons were found in the ventral part of AI and PoA (A 6.50 to A 6.75) (Fig. 6D). Beyond the borders of the arcopallium and the posterior pallial amygdala, no CTB‐labeled neurons were found in the contralateral telencephalon. More caudal tracer injections at A 11.00 to A 10.00 (P‐382, P‐461) revealed no CTB‐labeled cells within the contralateral arcopallium or the remaining contralateral telencephalon.
Figure 6.
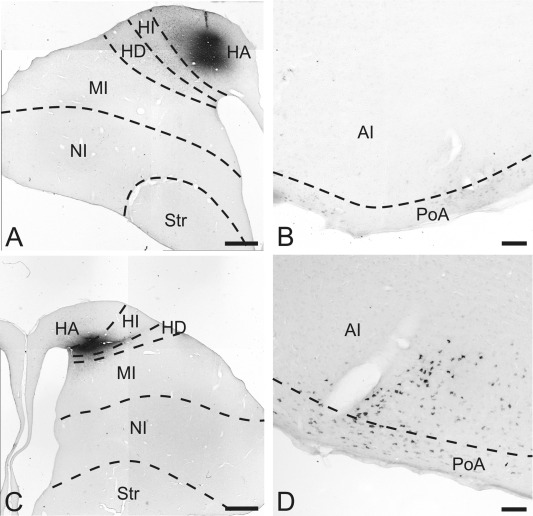
CTB labeling after injections into the contralateral hyperpallium. A: Injection site into HA at A 12.00. B: No CTB labeling within AI and PoA after CTB injections into the contralateral HA. C: Injection site of CTB into HI at A 12.50. D: Retrogradely labeled cells within the ventral AI and PoA after CTB injections into the contralateral HI. For abbreviations, see list. Scale bars = 1 mm in A,C; 100 μm in B,D.
Injections of CTB or BDA after AC commissurectomy
In four of five pigeons the transection of the AC was successful (Fig. 7A). Injections of CTB were placed into right arcopallium (case P‐526) at A 7.50, and the tracer diffused within the ipsilateral arcopallium to AI and AA (Fig. 7B). In another case (P‐477) the CTB injection was placed at A 7.00 into the ventral part of the AI with diffusion observed into PoA. Within the contralateral telencephalon no projections were found after the transection of the AC in any of these cases (Fig. 7C). Further injections of CTB were placed into the nidopallium (case P‐629) at A 6.50 and also showed no labeling within the contralateral telencephalon.
Figure 7.
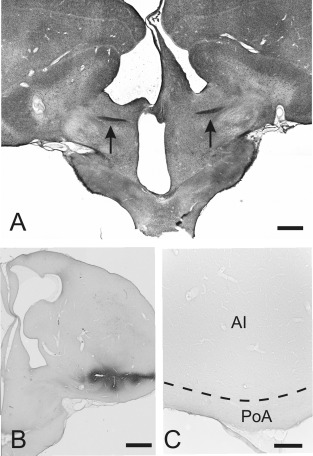
Contralateral projections after AC commissurectomy. A: Transection of the AC at A 7.75. Arrows indicate the remaining fibers of the AC in the left and right hemisphere. B: Injection site of CTB into the arcopallium at A 7.50. C: No CTB labeling within AI and PoA after CTB injections into the contralateral arcopallium of commissurectomized pigeons. For abbreviations, see list. Scale bars = 1 mm in A,B; 200 μm in C.
Cytochemistry of AC projecting cells
GABA‐CTB double labeling
In a first overview at low magnification, different brain areas could easily be distinguished based on the density of GABA‐like fibers and neurons. It was thereby already visible that the large fiber tract of the AC at A 7.75 was unstained (Fig. 8A), and the arcopallium appeared to be labeled less than the remaining telencephalon (Fig. 8A). Within the arcopallium and PoA the GABA‐like staining demonstrated a moderate and regular distribution of perikarya that was similar throughout different arcopallial subareas (AA, AI, AM, AD) and PoA. At a higher magnification, the GABA‐positive neurons could be subdivided into two different cell types (Fig. 8B,C). A very small population had large somata with a diameter around 15 μm (Fig. 8B). The majority of GABA‐positive neurons within the arcopallium was small, with cell body diameters around 10 μm (Fig. 8C). Contrary to the GABA‐positive cells, the commissural CTB‐positive neurons showed a rather uniform perikaryal size with a diameter of around 15 μm (Fig. 8D). With fluorescent double staining, the arcopallial, nidopallial, and medial striatal CTB‐tracing were counterstained with GABA. With confocal microscopy it was shown that the CTB‐labeled commissural cells were not colocalized with GABA (Fig. 8E–G). Thus, commissural neurons of the AC are very likely not GABAergic.
Figure 8.
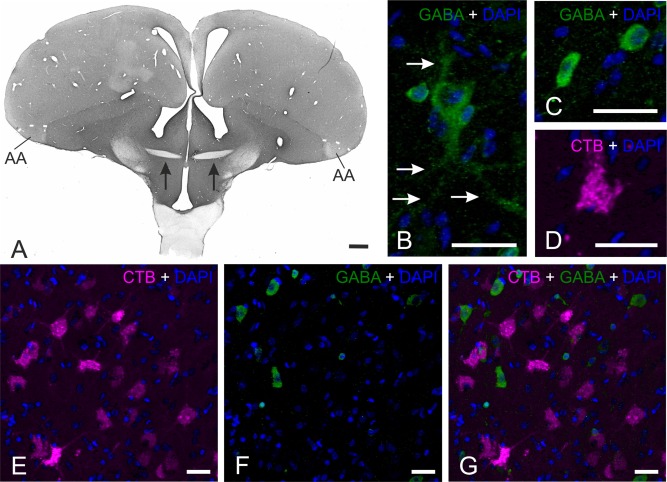
GABA‐CTB double staining. A: Overview of a GABA‐staining (DAB staining) at A 7.75. Arrows indicate the fibers of the AC which are not stained. B: GABA‐positive (green) large neuron within the arcopallium counterstained with DAPI (blue). Arrows indicate the dendrites of the neuron. C: GABA‐positive (green) small cell within the arcopallium counterstained with DAPI (blue). D: CTB‐positive (red) commissural neuron within the arcopallium counterstained with DAPI (blue). E: CTB‐staining (red) within the arcopallium counterstained with DAPI (blue). F: GABA‐staining (green) within the arcopallium counterstained with DAPI (blue). G: Overlay of photo E and F demonstrating no colocalization of commissural CTB and GABA. Scale bars = 1 mm in A; 20 μm in B–G.
TH‐CTB double staining
A fluorescent double staining against TH and CTB in P‐798 (the commissural projection pattern is described above) revealed a very high density of TH‐positive fibers within AD and a high density within AM (for detailed description, see Wynne and Güntürkün, 1995; Durstewitz et al., 1998). Within AI and PoA, the density of TH‐positive fibers was low. However, the double staining with CTB demonstrates a close connection between TH‐positive fibers and commissural projecting CTB‐positive neurons within AI (A 7.50) (Fig. 9A,B). This suggests the possibility that the cells, originating within the arcopallium, receive catecholaminergic and possibly dopaminergic input.
Figure 9.
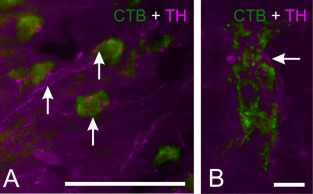
TH‐CTB double staining. A: Fluorescence double‐staining of CTB (green) and TH (red) within the arcopallium at A 7.50. Arrows indicate TH‐positive fibers crossing the CTB‐positive neurons. B: High magnification of a CTB‐positive neuron within the arcopallium. Arrow indicates a TH‐positive fiber close to the CTB‐positive neuron. Scale bars = 50 μm in A; 5 μm in B.
DISCUSSION
The results of the present study show that the AC of pigeons originates in the arcopallium/amygdala‐complex and has reciprocal and homotopic connections to this complex in the contralateral hemisphere. In addition, the arcopallium/amygdala‐complex projects via the AC in a widespread, unidirectional, and heterotopic manner to predominantly secondary sensory, multimodal, and limbic structures of the contralateral telencephalon. Furthermore, the arcopallial cells that give rise to the commissural system are very likely not GABAergic and receive a catecholaminergic input of presumably dopaminergic nature. Overall, different from an often‐repeated statement, the bird cerebrum is not a natural split brain but has prominent interhemispheric connections that reach major portions of the contralateral telencephalon. However, these widespread projections originate from a small cluster of somatomotor and limbic neurons in the caudal telencephalon.
Homotopic projections of the AC in pigeons and other avian species
Amygdaloid projections
The avian amygdala consists of the PoA, the subpallial amygdala, and the nucleus taeniae of the amygdala (Reiner et al., 2004). The present study shows that only the PoA has interhemispheric projections via the AC. The PoA can be subdivided into two parts, a more dorsal part (PoAc) that projects predominantly to the lateral part of the bed nucleus of the stria terminalis, with some fibers reaching the medial hypothalamus, and a more ventral part (PoAb) that extends more rostrally along the base of the telencephalon and projects heavily onto the hypothalamus (Atoji et al., 2006). According to the present study, it is only the PoAb that exclusively participates in interhemispheric projections and forms homotopic connections with the contralateral PoAb.
Arcopallial projections
The arcopallium consists of four subareas: the anterior (AA), dorsal (AD), intermediate (AI), and medial (AM) arcopallium. While the connections of the first three components clearly show a somatomotor instead of an amygdaloid character, the somatic or limbic identity of AM is less clear (Reiner et al., 2004). AA and AI are somatomotor structures that innervate subpallial areas down to medullary levels (Zeier and Karten, 1971; Dubbeldam, 2014). At the same time, AA and AI are associative structures that receive input from the caudolateral nidopallium (NCL), auditory (Wild et al., 1993), trigeminal (Wild et al., 1985), somatosensory (Kröner and Güntürkün 1999), and visual structures (Bagnoli and Burkhalter, 1983; Husband and Shimizu, 1999). According to Shanahan et al. (2013), the AI represents one of the most central hubs of the pigeons' connectome. The present study demonstrates that the reciprocal and homotopic interhemispheric projections via the AC are primarily mediated by the somatomotor structures AA and ventral AI. The AIvm is the auditory component of AI (Wild et al., 1993) and also projects to the contralateral arcopallium. The present findings accord with some previous studies in pigeons (Zeier and Karten, 1973), chicken (Davies et al., 1997), and mallards (Dubbeldam et al., 1997). All of these studies demonstrated commissural afferents to AA and projections from AA towards the contralateral arcopallium. Projections towards the contralateral AI were only reported for pigeons (Zeier and Karten, 1973) and mallards (Dubbeldam et al., 1997).
Heterotopic projections of the AC in pigeons and other avian species
Projections to higher sensory areas
Trigeminal system
Somatosensory input from the oral region in birds is conveyed to the nucleus sensorius principalis nervi trigemini in the brainstem and is then sent via the tractus quintofrontalis without thalamic relay to the nucleus basorostralis pallii (Bas) in the forebrain (Wild et al., 1985; Schall et al., 1986). Bas gives rise to efferents towards the frontal trigeminal nidopallium (NFT), which then sends connections to AI and the trigeminal component of the NCL (Wild et al., 1985; Kröner and Güntürkün, 1999). The present study shows that the arcopallium projects via the AC to the contralateral NFT. This is similar to mallards which, in addition, also have connections to the contralateral dorsal (MD) and ventral (MV) mesopallium (Dubbeldam et al., 1997). In pigeons, there is a second trigeminal pathway that runs through the frontoventral mesopallium (MFV) that is reciprocally connected to Bas and NFT (Atoji and Wild, 2012). The present study shows that both NFT and MFV are interhemispherically connected via arcopallial projections. Thus, interhemispheric trigeminal information is integrated both at primary (Bas) and at secondary (NFT, MFV) levels.
Visual system
Visual information is conveyed from the retina to the telencephalon via two main visual pathways, the tectofugal and the thalamofugal pathway. Within the tectofugal pathway visual information is transferred from the thalamic nucleus rotundus to the entopallial core, which then projects to a surrounding shell, the perientopallium. The perientopallium receives a relatively sparse direct projection from nucleus rotundus and is itself a major source of projections to wider regions of the hemisphere, including NFL, lateral intermediate nidopallium (NIL), NCL, the temporo‐parieto‐occipital area (TPO) and arcopallium (Husband and Shimizu, 1999; Krützfeldt and Wild, 2005). The present study reveals that the arcopallium projects via the AC to the contralateral NFL, NIL, and TPO. This result is in line with previous studies in pigeons (Zeier and Karten, 1973) and mallards (Dubbeldam et al., 1997). Furthermore, these results indicate that the arcopallium modulates visual tectofugal information in a widespread telencephalic network through the AC.
In the thalamofugal pathway of pigeons, visual information is transferred from the thalamic lateral geniculate nucleus, pars dorsalis to the interstitial part of the hyperpallium apicale / hyperpallium densocellulare, pars lateralis of the visual Wulst (Güntürkün and Karten, 1991). The visual information is then primarily sent to the supragranular layer in the visual Wulst (HA), which, in turn, projects to NFL, NCL, intermediate nidopallium (NI), AI, lateral hyperpallium (HL), and LSt/MSt (Shimizu et al., 1995; Kröner and Güntürkün, 1999). NFL receives a projection from the perientopallium and constitutes reciprocal connections with HA. Thus, NFL is an area of integration between the thalamofugal and the tectofugal pathway (Shimizu et al., 1995). In contrast to pigeons, Davies et al. (1997) did not report interhemispheric commissural projections from the arcopallium to NFL or NIL in chicken. Instead, the chicken AA seems to have strong interhemispheric connections with all layers of the Wulst, including HA. Thus, both in chicken and in pigeons the visual system is interhemispherically connected via the AC. But while the AC of chicken only interconnects the thalamofugal system, the AC of pigeons integrates information from tertiary visual areas of both visual pathways. Additionally, the present study revealed an interhemispheric connection between AI and PoA and the contralateral HI. This projection was already described by Zeier and Karten (1973) and Bagnoli and Burkhalter (1983) in pigeons. A similar commissural projection was also discovered by Dubbeldam et al. (1997) in the mallard. Additionally, the present study demonstrated that primarily the anterior part of HI receives contralateral input from the arcopallium/amygdala‐complex. This part of HI receives afferents from the thalamic nucleus dorsolateralis and nucleus superficialis, parvocellularis, which are both thought to belong to the somatosensory, spinothalamic tract (Karten and Revzin, 1966). Accordingly, the anterior part of HI primary processes somatosensory rather than visual information, suggesting that somatosensory information is interhemispheric, controlled by the contralateral arcopallium/amygdala complex on a primary processing level.
Multisensory systems
NCL is reciprocally connected with the secondary sensory areas of all modalities (Kröner and Güntürkün, 1999). Due to this multimodal input and the broad overlap of terminations, the NCL is a true associative forebrain structure and the largest hub in the pigeons' forebrain connectome (Shanahan et al., 2013). The present study revealed that AI sends a massive projection to the contralateral NCL that mostly terminate in the representational areas of the trigeminal, tectofugal visual, and somatosensory systems (Kröner and Güntürkün, 1999). This observation is in line with the results from Zeier and Karten (1973) in the pigeon.
Limbic system
The frontomedial nidopallium (NFM) is reciprocally connected to the PoA (Atoji et al., 2006) and also projects to the medial MSt (Veenman et al., 1995; Kröner and Güntürkün, 1999). Another limbic pathway is processed via the frontodorsal mesopallium (MFD), which connects reciprocally with NFM, receives thalamic input from the subrotundus, and projects to AD, AI, and medial MSt (Atoji and Wild, 2012). In addition, MFD has descending fibers to the hypothalamus and connects reciprocally with limbic ventral arcopallium, PoA, and the area corticoidea dorsolateralis (Zeier and Karten, 1971; Atoji et al., 2006). The present study demonstrates a contralateral connection from the arcopallium to entire MFD and MID. The origin of this projection within the arcopallium seems to be the AI (Atoji and Wild, 2012). MID is connected to the limbic caudocentral nidopallium (NCC) and appears to be associated with the limbic system (Atoji and Wild, 2009). As shown in the present study, especially the limbic NCC receives strong input from the contralateral arcopallial AI (Atoji and Wild, 2009).
Motoric system
The striatum of birds are constituted by a lateral and a medial component. The present study reveals an exclusive projection from limbic PoA and ventral AI to the contralateral MSt via the AC. The MSt can be further subdivided into a lateral and a medial part. The more lateral MSt receives pallial input from somatic regions, such as those involved in somatosensory, visual, auditory, and motor functions (Karten and Dubbeldam, 1973; Nottebohm et al., 1976; Brauth et al., 1978; Wild, 1987; Wild et al., 1993; Veenman et al., 1995). By contrast, medial MSt appears to be more viscerolimbic, since its pallial input arises from such regions as hippocampus, piriform cortex, and the limbic subareas of NCL (Veenman et al., 1995, 1997; Kröner and Güntürkün, 1999; for review, see Kuenzel et al., 2011). In the present study, the anterogradely labeled fibers in the contralateral MSt were primarily found in the lateral part of MSt, suggesting an interhemispheric influence onto striatal information within the more somatic lateral MSt.
Comparative aspects of the AC in other vertebrates
AC in fish, amphibians, and reptiles
Both the hippocampal (pallial) and the AC also exist in fish, amphibians, and reptiles. Unfortunately, in none of these vertebrate species were we able to find a publication that exclusively analyzes the pattern of the AC. Tracing studies in fish primary focus on the connectivity of the olfactory bulb, and in this context also some anterior commissural connections were described. In agnathans, cartilaginous fish and teleosts the AC interconnects the olfactory bulbs (agnatha: Nieuwenhuys and Nicholson, 1998; cartilaginous fish: Yáñez et al., 2011; teleost: Folgueira et al., 2004; Northcutt, 2006). In birds, the olfactory bulb is not interconnected via the anterior but via the habenular commissure (Zeier and Karten, 1973; Davies et al., 1997; Patzke et al., 2011). Interestingly, secondary olfactory axons of cartilaginous and bony fish decussate not only through the AC, but also through the habenular and the postoptic commissure (Northcutt, 2011; Yáñez et al., 2011; for review, see Suárez et al., 2014). Studies of commissural systems in amphibians and reptiles report interhemispheric projections that could be transmitted via either the hippocampal and/or the AC (amphibians: Kokoros and Northcutt, 1977; Northcutt and Ronan, 1992; reptiles: Bruce and Butler, 1984; Hoogland, 1977; for review, see Northcutt, 1981). Overall, these studies show that most of the extent of the pallium of amphibians and reptiles is interhemispherically connected through commissural systems without clearly separating which commissural system contributes to which pathway.
AC in eutherian and metatherian mammals
Projections of the paleocortex
In all mammals, the paleocortex, including the olfactory bulbs (OB), their associated nuclei, and the amygdala (human: Di Virgilio et al., 1999; higher eutherians: Jouandet et al., 1979, 1982, 1984; Pandya et al., 1973; lower eutherians: Jouandet and Hartenstein, 1983) of both hemispheres, are interconnected through the AC (Fig. 10A,B). In contrast, the present study could not reveal olfactory interconnections through the AC in pigeons (Fig. 10C). In pigeons, olfaction plays an important role in navigation. Olfactory information is bilaterally transferred from the OB to several telencephalic areas, including the olfactory tuberculum, prepiriform cortex, nucleus taeniae of the amygdala, OB, and piriform cortex (Patzke et al., 2011; Atoji and Wild, 2014). Rieke and Wenzel (1978) described that the contralateral efferents of the OB run through the AC. Also, Zeier and Karten (1973) described a number of projections through the AC to olfactory nuclei like olfactory tuberculum and piriform cortex in pigeons. Davies et al. (1997) described efferents from AA to the contralateral OB, piriform cortex, and olfactory tuberculum through the AC in chickens. In contrast, Reiner and Karten (1985) showed that a bundle of fibers from the OB enters the diencephalon via the stria medullaris, crosses in the habenular commissure, and ascends to the contralateral telencephalon. The projection to the contralateral hemisphere via the habenular commissure has also been reported in reptiles and amphibians but not in mammals (Scalia et al., 1968; Northcutt, 1970, 1981; Reiner and Karten, 1985). Thus, there is strong evidence that interhemispheric olfactory information in birds is not transferred via the AC, but the habenular commissure. This is in accordance with the present results that could not evince an olfactory projection via the AC in pigeons (Fig. 10C). In sum, while the transfer of interhemispheric olfactory information in mammals is achieved via the AC, this is not the case for birds, reptiles, and amphibians. Besides olfactory areas, the AC in mammals also interconnects amygdaloid areas. This is also true for pigeons, as demonstrated in the present study (Fig. 10C).
Figure 10.
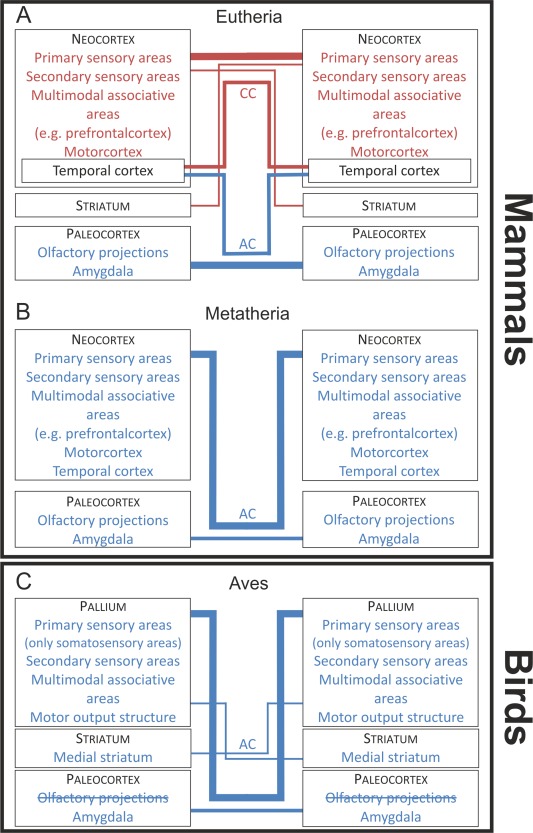
Schematic diagram showing the main interhemispheric connections through the CC and the AC in A: eutherian mammals B: metatherian mammals C: Aves. Line widths between the blocks are about proportional to the magnitude of the connections.
Projections of the neocortex
In most mammals, the AC also contains fibers originating from the neocortex. In eutherians, it is primarily the temporal pole that is interconnected through the AC (Di Virgilio et al., 1999), while the remaining neocortical structures are interconnected by the corpus callosum (Fig. 10A) (man: Clarke and Miklossy, 1990; rhesus monkey: Jacobson and Trojanowski, 1974; LaMantia and Rakic, 1990; cat: Ebner and Myers, 1965; Jacobson and Trojanowski, 1974; rat: Jacobson and Trojanowski, 1974). Metatherian mammals like wallaby and opossum do not possess a corpus callosum. In these animals, the whole neocortical mantle projects through the AC (Fig. 10B) (Martin, 1967; Granger et al., 1985). So, the corpus callosum in eutherians overtook most of the interhemispheric connectivity that is subserved by the AC in metatherians (Martin, 1967; Granger et al., 1985). Although, the pigeon's AC resembles the commissural systems of eutherian and metatherian mammals in some aspects, there are some important differences: One of them is that the AC of pigeons predominantly does not interconnect primary sensory forebrain areas (Fig. 10C). This is different from eutherians with respect to the corpus callosum (Jacobson and Trojanowski, 1974; LaMantia and Rakic, 1990; Clarke and Miklossy, 1990) and to metatherians with respect to the AC (Fig. 10A,B) (Martin, 1967; Granger et al., 1985). In contrast, secondary sensory areas are connected via the AC in pigeons and metatherians (Ebner and Myers, 1965; Granger et al., 1985) and the corpus callosum in eutherians (Clarke and Miklossy, 1990). Multimodal and “prefrontal” areas are interconnected via the AC in pigeons (present study) and via the corpus callosum in eutherians (Fig. 10A,C) (LaMantia and Rakic, 1990).
Differences in anatomical organization between birds and mammals
In contrast to the AC of birds, the connections of the mammalian corpus callosum are largely homotopic and reciprocal (Fig. 11A) (Innocenti, 1986). Also, the mammalian AC has a large amount of homotopic and reciprocal connections, but in comparison to the corpus callosum the proportion of homotopic reciprocal to heterotopic unidirectional connections is different from the corpus callosum (Fig. 11B) (Lent and Schmidt, 1992). In eutherians, an example for a nonreciprocal AC connection is the heterotopic connection between the olfactory bulb and the piriform cortex (Fig. 11B) (Haberly and Price, 1978). The AC of metatherians is much larger than that of eutherians and has both strong homotopic and heterotopic projections (Fig. 11B). The present study in the pigeon shows that a homotopic reciprocal fiber organization is only true for arcopallial and amygdaloid projections. These constitute only a small fraction of the connectivity that runs through the AC. The majority of AC connections in birds are heterotopic and nonreciprocal (Fig. 11C). In addition, the avian AC is constituted by a rather small population of arcopallial and amygdaloid fibers. Although these neurons reach a vast contralateral territory with heterotopic projections, their constituent cell population is restricted to a small part of the bird pallium.
Figure 11.
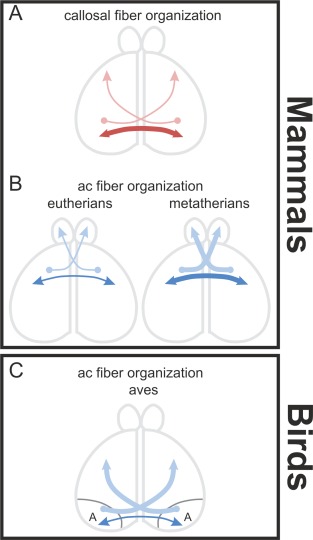
Some principles of the structural organization of interhemispheric connections. A: Callosal connections are largely homotopic‐reciprocal but heterotopic‐unidirectional projections also exist as shown in the dorsal view of the hemispheres. B: AC connections in mammals show nearly the same amount of fibers for homotopic‐reciprocal as well as heterotopic‐unidirectional connections. C: AC connections in Aves are largely heterotopic‐unidirectional. Only a small amount of homotopic, reciprocal projecting fibers exist.
CONCLUSION
In summary, the present study demonstrates that the AC of pigeons interconnects a wide network of forebrain structures. The telencephalic commissures in mammals and birds overlap in some aspects of organization but also evince several important differences. In contrast to the mammalian AC, the avian AC interconnects no olfactory areas. Furthermore in birds, interhemispheric information is predominantly integrated at a later processing stage due to the absence of interconnections between primary sensory areas (Fig. 12). The only exception is somatosensory information, which is already interhemispherically integrated at a primary processing level.
Figure 12.
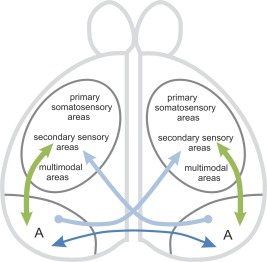
Schematic drawing of an interhemispheric circuit. The arcopallium is the source of interhemispheric homotopic‐reciprocal and heterotopic‐unidirectional projections and also for ipsilateral‐reciprocal connections of all modalities.
But the main differences in the interhemispheric connectivity between birds and mammals are found at two levels of structural organization. First, the AC in birds differs from the corpus callosum and the AC of mammals in its proportion of homotopic reciprocal to heterotopic unidirectional projections. In contrast to the situation in mammals, in birds only a small amount of cells interconnect the two hemispheres in a homotopic and reciprocal fashion. Instead, most of the cells project heterotopically and in unidirectional manner. Second, in birds the absolute majority of pallial areas do not participate by themselves in interhemispheric exchange. Instead, a rather small arcopallial and amygdaloid cluster is key for commissural interactions. According to Ehrlich and Mills (1985), only 89,000 crossing fibers constitute the AC in chicken. Thus, the colloquial statement that birds are “natural split‐brains” is wrong, when the pallial areas are considered that interhemispherically interact via the AC. It is true, however, when taking into account how small the proportion of pallial neurons is that constitute interhemispheric exchange.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
ROLE OF THE AUTHORS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: S.L., O.G. Acquisition of data: S.L., A.S. Analysis and interpretation of data: S.L., O.G. Drafting of the article: S.L. Critical revision of the article for important intellectual content: S.L., O.G.
ACKNOWLEDGMENTS
We thank Carsten Theiss for help with confocal microscopy and Emre Ünver for help with commissurectomy.
LITERATURE CITED
- Arendt D, Denes AS. Jékely G, Tessmar‐Raible K. 2008. The evolution of nervous system centralization. Philos Trans R Soc Lond B Biol Sci 1496:1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atoji Y, Wild JM. 2009. Afferent and efferent projections of the central caudal nidopallium in the pigeon (Columba livia) . J Comp Neurol 517:350–370. [DOI] [PubMed] [Google Scholar]
- Atoji Y, Wild JM. 2012. Afferent and efferent projections of the mesopallium in the pigeon (Columba livia) . J Comp Neurol 520:717–741. [DOI] [PubMed] [Google Scholar]
- Atoji Y, Wild JM. 2014. Efferent and afferent connections of the olfactory bulb and prepiriform cortex in the pigeon (Columba livia) . J Comp Neurol 522:1728–1752. [DOI] [PubMed] [Google Scholar]
- Atoji Y, Saito S, Wild JM. 2006. Fiber connections of the compact division of the posterior pallial amygdala and lateral part of the bed nucleus of the stria terminalis in the pigeon (Columba livia) . J Comp Neurol 499:161–182. [DOI] [PubMed] [Google Scholar]
- Bagnoli P, Burkhalter A. 1983. Organization of the afferent projections to the wulst in the pigeon. J Comp Neurol 214:103–113. [DOI] [PubMed] [Google Scholar]
- Brauth S, Ferguson JL, Kitt CA. 1978. Prosencephalic pathways related to the paleostriatum of the pigeon (Columba livia) . Brain Res 147:205–221. [DOI] [PubMed] [Google Scholar]
- Bruce LL, Butler AB. 1984. Telencephalic connections in lizards. I. Projections to cortex. J Comp Neurol 229:585–601. [DOI] [PubMed] [Google Scholar]
- Clarke S, Miklossy J. 1990. Occipital cortex in man: organization of callosal connections, related myelo‐ and cytoarchitecture and putative boundaries of functional visual areas. J Comp Neurol 298:188–214. [DOI] [PubMed] [Google Scholar]
- Davies DC, Csillag A, Szekely AD, Kabai P. 1997. Efferent connections of the domestic chick archistriatum: a phaseolus lectin anterograde tracing study. J Comp Neurol 389:679–693. [DOI] [PubMed] [Google Scholar]
- Di Virgilio G, Clarke S, Pizzolato G, Schaffner T. 1999. Cortical regions contributing to the anterior commissure in man. Exp Brain Res 124:1–7. [DOI] [PubMed] [Google Scholar]
- Dubbeldam JL, Den Boer‐Visser AM, Bout RG. 1997. Organization and efferent connections of the archistriatum of the mallard, Anas platyrhynchos L.: an anterograde and retrograde tracing study. J Comp Neurol 388:632–657. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Kröner S, Hemmings HC, Güntürkün O. 1998. The dopaminergic innervation of the pigeon telencephalon: distribution of DARP‐32 and co‐occurrence with glutamate decarboxylase and tyrosine hydroxylase. Neuroscience 83:763–779. [DOI] [PubMed] [Google Scholar]
- Ebner FF, Myers RE. 1965. Distribution of corpus callosum and anterior commissure in cat and raccoon. J Comp Neurol 124:353–366. [DOI] [PubMed] [Google Scholar]
- Ehrlich D, Mills D. 1985. Myelogenesis and estimation of the number of axons in the anterior commissure of the chick (Gallus gallus) . Cell Tissue Res 239:661–666. [DOI] [PubMed] [Google Scholar]
- Folgueira M, Anadón R, Yáñez J. 2004. An experimental study of the connections of the telencephalon in the rainbow trout (Oncorhynchus mykiss). I: olfactory bulb and ventral area. J Comp Neurol 480:180–203. [DOI] [PubMed] [Google Scholar]
- Granger EM, Masterton RB, Glendenning KK. 1985. Origin of interhemispheric fibers in acallosal opossum (with a comparison to callosal origins in rat). J Comp Neurol 241:82–98. [DOI] [PubMed] [Google Scholar]
- Graves JA, Goodale MA. 1977. Failure of interocular transfer in the pigeon (Columba livia) . Physiol Behav 3: 425–428. [DOI] [PubMed] [Google Scholar]
- Güntürkün O. 2005. The avian ‘prefrontal cortex’ and cognition. Curr Opin Neurobiol 15:686–693. [DOI] [PubMed] [Google Scholar]
- Güntürkün O, Böhringer PG. 1987. Lateralization reversal after intertectal commissurotomy in the pigeon. Brain Res 408:1–5. [DOI] [PubMed] [Google Scholar]
- Güntürkün O, Karten HJ. 1991. An immunocytochemical analysis of the lateral geniculate complex in the pigeon (Columba livia) . J Comp Neurol 314:721–749. [DOI] [PubMed] [Google Scholar]
- Haberly LB, Price JL. 1978. Association and commissural fiber systems of the olfactory cortex of the rat. J Comp Neurol 181:781–808. [DOI] [PubMed] [Google Scholar]
- Hellmann B, Güntürkün O. 1999. Visual‐field‐specific heterogeneity within the tecto‐rotundal projection of the pigeon. Eur J Neurosci 11:2635–2650. [DOI] [PubMed] [Google Scholar]
- Hoogland PV. 1977. Efferent connections of the striatum in Tupinambis nigropunctatus . J Morphol 152:229–246. [DOI] [PubMed] [Google Scholar]
- Husband SA, Shimizu T. 1999. Efferent projections of the ectostriatum in the pigeon (Columba livia) . J Comp Neurol 406:329–345. [PubMed] [Google Scholar]
- Innocenti GM. 1986. General organization of callosal connections in the cerebral cortex In: Jones EG, Peters A, editors. Cerebral cortex, vol. 5 New York: Plenum Press; p 291–353. [Google Scholar]
- Jacobson S, Trojanowski JQ. 1974. The cells of origin of the corpus callosum in rat, cat and rhesus monkey. Brain Res 74:149–155. [DOI] [PubMed] [Google Scholar]
- Jouandet ML. 1982. Neocortical and basal telencephalic origins of the anterior commissure of the cat. Neuroscience 7:1731–1752. [DOI] [PubMed] [Google Scholar]
- Jouandet ML, Gazzaniga MS. 1979. Cortical field of origin of the anterior commissure of the rhesus monkey. Exp Neurol 66:381–397. [DOI] [PubMed] [Google Scholar]
- Jouandet ML, Hartenstein V. 1983. Basal telencephalic origins of the anterior commissure of the rat. Exp Brain Res 50:183–192. [DOI] [PubMed] [Google Scholar]
- Jouandet ML, Garey LJ, Lipp HP. 1984. Distribution of the cells of origin of the corpus callosum and anterior commissure in the marmoset monkey. Anat Embryol 169:45–59. [DOI] [PubMed] [Google Scholar]
- Karten HJ, Dubbeldam JL. 1973. The organization and projections of the paleostriatal complex in the pigeon (Columba livia) . J Comp Neurol 148:61–90. [DOI] [PubMed] [Google Scholar]
- Karten HJ, Hodos WJH. 1967. A stereotaxic atlas of the brain of the pigeon (Columba livia). Baltimore, MD: Johns Hopkins Press. [Google Scholar]
- Karten HJ, Revzin AM. 1966. The afferent connections of the nucleus rotundus in the pigeon. Brain Res 2:368–377. [DOI] [PubMed] [Google Scholar]
- Kokoros JJ, Northcutt RG. 1977. Telencephalic efferents of the tiger Salamander Ambystoma tigrinurn tigrinum (Green). J Comp Neurol 173:613–628. [DOI] [PubMed] [Google Scholar]
- Kröner S, Güntürkün O. 1999. Afferent and efferent connections of the caudolateral neostriatum in the pigeon (Columba livia): a retro‐ and anterograde pathway tracing study. J Comp Neurol 407:228–260. [DOI] [PubMed] [Google Scholar]
- Krützfeldt NOE, Wild JM. 2005. Definition and novel connections of the entopallium in the pigeon (Columba livia) . J Comp Neurol 490:40–56. [DOI] [PubMed] [Google Scholar]
- Kuenzel WJ, Medina L, Csillagc A, Perkel DJ, Reiner A. 2011. The avian subpallium: new insights into structural and functional subdivisions occupying the lateral subpallial wall and their embryological origins. Brain Res 1424:67–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMantia AS, Rakic P. 1990. Cytological and quantitative characteristics of four cerebral commissures in the rhesus monkey. J Comp Neurol 291:520–537. [DOI] [PubMed] [Google Scholar]
- Lent R, Schmidt SL. 1992. The ontogenesis of the forebrain commissures and the determination of brain asymmetries. Prog Neurobiol 40:249–276. [DOI] [PubMed] [Google Scholar]
- Letzner S, Patzke N, Verhaal J, Manns M. 2014. Shaping a lateralized brain: asymmetrical light experience modulates access to visual interhemispheric information in pigeons. Sci Rep 4:4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns M, Römling J. 2012. The impact of asymmetrical light input on cerebral hemispheric specialization and interhemispheric. Nat Commun 3:696. [DOI] [PubMed] [Google Scholar]
- Martin GF. 1967. Interneocortical connections in the opossum, Didelphis virginiana . Anat Rec 157:607–616. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Nicholson C. 1998. Lampreys, Petromyzontoidea In: Nieuwenhuys R, ten Donkelaar HJ, Nicholson C, editors. The central nervous system of vertebrates, vol. 1. Berlin: Springer; p 397–495. [Google Scholar]
- Northcutt RG. 1970. The telencephalon of the western painted turtle, Chrysemys picta belli. III. Biol Monogr, No 43. Urbana, IL: University of Illinois Press. [Google Scholar]
- Northcutt RG. 1981. Evolution of the telencephalon in nonmammals. Annu Rev Neurosci 4:301–350. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. 2006. Connections of the lateral and medial divisions of the goldfish telencephalic pallium. J Comp Neurol 494:903–943. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. 2011. Olfactory projections in the white sturgeon, Acipenser transmontanus: an experimental study. J Comp Neurol 519:1999–2022. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Ronan M. 1992. Afferent and efferent connections of the bullfrog medial pallium. Brain Behav Evol 1:1–16. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. 1976. Central control of song in the canary, Serinus canaries . J Comp Neurol 165:457–486. [DOI] [PubMed] [Google Scholar]
- Nottelmann F, Wohlschlager A, Güntürkün O. 2002. Unihemispheric memory in pigeons‐knowledge, the left hemisphere is reluctant to share. Behav Brain Res 133:309–315. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Karol EA, Lele PP. 1973. The distribution of the anterior commissure in the squirrel monkey. Brain Res 49:177–180. [DOI] [PubMed] [Google Scholar]
- Patzke N, Manns M, Güntürkün O. 2011. Telencephalic organization of the olfactory system in homing pigeons (Columba livia) . Neuroscience 194:53–61. [DOI] [PubMed] [Google Scholar]
- Reiner A, Karten HJ. 1985. Comparison of olfactory bulb projections in pigeons and turtles. Brain Behav Evol 27:11–27. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce L, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Güntürkün O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas‐Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. 2004. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol 473:377–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke GK, Wenzel BM. 1978. Forebrain projections of the pigeon olfactory bulb. J Morphol 158:41–56. [DOI] [PubMed] [Google Scholar]
- Scalia F, Halpern M, Knapp H, Riss W. 1968. The efferent connexions of the olfactory bulb in the frog: a study of degenerating unmyelinated fibers. J Anat 103(Pt 2):245–262. [PMC free article] [PubMed] [Google Scholar]
- Schall U, Güntürkün O, Delius JD. 1986. Sensory projections to the nucleus basalis prosencephali of the pigeon. Cell Tissue Res 245:539–546. [DOI] [PubMed] [Google Scholar]
- Semmler H, Chiodin M, Bailly X, Martinez P. 2010. Steps towards a centralized nervous system in basal bilaterians: insights from neurogenesis of the acoel. Dev Growth Differ 52:701–713. [DOI] [PubMed] [Google Scholar]
- Shanahan M, Bingman VP, Shimizu T, Wild M, Güntürkün O. 2013. Large‐scale network organization in the avian forebrain: a connectivity matrix and theoretical analysis. Front Comp Neurosci 7:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Cox K, Karten HJ. 1995. Intratelencephalic projections of the visual wulst in pigeons (Columba livia) . J Comp Neurol 359:551–572. [DOI] [PubMed] [Google Scholar]
- Skiba M, Diekamp B, Prior H, Güntürkün O. 2000. Lateralized interhemispheric transfer of color cues: evidence for dynamic coding principles of visual lateralization in pigeons. Brain Lang 73:254–273. [DOI] [PubMed] [Google Scholar]
- Suárez R, Gobius I, Richards LJ. 2014. Evolution and development of interhemispheric connections in the vertebrate forebrain. Front Hum Neurosci 8:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenman CL. 1997. Pigeon basal ganglia: insights into the neuroanatomy underlying telencephalic sensorimotor processes in birds. Eur J Morphol 35:220–233. [DOI] [PubMed] [Google Scholar]
- Veenman CL, Wild JM, Reiner A. 1995. Organization of the avian “corticostriatal” projection system: a retrograde and anterograde pathway tracing study in pigeons. J Comp Neurol 354:87–126. [DOI] [PubMed] [Google Scholar]
- Watanabe S. 1980. Conditional discrimination training and interocular transfer in pigeons. Behav Brain Res 1:125–137. [DOI] [PubMed] [Google Scholar]
- Wild JM. 1987. The avian somatosensory system: connections of regions of body representation in the forebrain of the pigeon. Brain Res 412:205–223. [DOI] [PubMed] [Google Scholar]
- Wild JM, Arends JJA, Zeigler HP. 1985. Telencephalic connections of the trigeminal system in the pigeon (Columba livia): a trigeminal sensorimotor circuit. J Comp Neurol 234:441–464. [DOI] [PubMed] [Google Scholar]
- Wild JM, Karten HJ, Frost BJ. 1993. Connections of the auditory forebrain in the pigeon (Columba livia). J Comp Neurol 337:32–62. [DOI] [PubMed] [Google Scholar]
- Wynne B, Güntürkün O. 1995. Dopaminergic innervation of the telencephalon of the pigeon (Columba livia): a study with antibodies against tyrosine hydroxylase and dopamine. J Comp Neurol 357:446–464. [DOI] [PubMed] [Google Scholar]
- Yáñez J, Folgueira M, Köhler E, Martínez C, Anadón R. 2011. Connections of the terminal nerve and the olfactory system in two galeomorph sharks: an experimental study using a carbocyanine dye. J Comp Neurol 519:3202–3217. [DOI] [PubMed] [Google Scholar]
- Zeier H, Karten HJ. 1971. The archistriatum of the pigeon: organization of afferent and efferent connections. Brain Res 2:313–326. [DOI] [PubMed] [Google Scholar]
- Zeier H, Karten HJ. 1973. Connections of the anterior commissure in the pigeon (Columba livia) . J Comp Neurol 150:201–216. [DOI] [PubMed] [Google Scholar]


