Abstract
Background & Aims
Thrombocytopaenia and hypoalbuminaemia are surrogate markers for portal hypertension and hepatic synthetic dysfunction respectively. Patients infected with hepatitis C virus (HCV) with these surrogates have reduced likelihood of sustained virologic response and increased risk for hepatic decompensation or death when treated with peginterferon/ribavirin plus either telaprevir or boceprevir.
Methods
We conducted a post‐hoc analysis of the TURQUOISE‐II clinical trial in patients with cirrhosis to examine the impact of these surrogates on efficacy and safety of ombitasvir/paritaprevir/ritonavir and dasabuvir with ribavirin.
Results
Of 380 genotype 1‐infected patients in TURQUOISE‐II, 104 had either a platelet count <100 × 109/L or albumin <3.5 g/dl. Sustained virologic response rates were 89 and 97% in patients with thrombocytopaenia, and 84 and 89% in patients with hypoalbuminaemia after 12 and 24 weeks of ombitasvir/paritaprevir/ritonavir and dasabuvir with ribavirin respectively. These rates were similar to those observed in the overall study population (92 and 97% for 12 and 24 weeks). HCV genotype 1a‐infected patients with thrombocytopaenia or hypoalbuminaemia had higher response rates when treated for 24 weeks, whereas only 1 of 35 genotype 1b patients did not achieve a sustained virologic response. Adverse event rates and discontinuations because of adverse events were low.
Conclusions
The findings of these analyses support the use of ombitasvir/paritaprevir/ritonavir and dasabuvir with ribavirin in these subpopulations with cirrhosis. Genotype 1a‐infected patients with indicators of portal hypertension may benefit from a 24‐week treatment duration.
Keywords: direct‐acting antiviral agents, hepatitis C virus, portal hypertension, TURQUOISE‐II
Abbreviations
- 3D
OBV/PTV/r and DSV
- AE
adverse event
- CUPIC
Compassionate Use of Protease Inhibitors in viral C Cirrhosis
- DSV
dasabuvir
- GT
genotype
- HCV
hepatitis C virus
- OBV
ombitasvir
- PTV
paritaprevir
- RBV
ribavirin
- r
ritonavir
- SVR12
sustained virologic response 12 weeks post‐treatment
- SVR
sustained virologic response
Key points box.
Hepatitis C‐infected patients with cirrhosis and thrombocytopaenia or hypoalbuminaemia are at increased risk for serious complications with interferon‐based treatments.
Ombitasvir/paritaprevir/r and dasabuvir with ribavirin for 12 or 24 weeks achieved high sustained virologic response rates in cirrhotic patients with thrombocytopaenia and hypoalbuminaemia that were not statistically different than in patients without these indicators of portal hypertension.
Baseline median platelet counts and albumin did not differ between patients who did or did not achieve a sustained virologic response.
The safety profiles were similar in patients with and without surrogate markers of more advanced liver disease, except for indirect hyperbilirubinaemia.
In patients with cirrhosis, thrombocytopaenia is a surrogate for portal hypertension and hypoalbuminaemia is a surrogate for diminished hepatic synthetic function. Although hepatitis C virus (HCV) triple therapy with first‐generation protease inhibitors, boceprevir or telaprevir, modestly improved sustained virologic response (SVR) rates compared to peginterferon plus ribavirin (RBV), the adverse event (AE) profile was less favourable with an increased risk of rash and potentiation of anaemia 1, 2, 3, 4, 5. In the Compassionate Use of Protease Inhibitors in viral C Cirrhosis (CUPIC) observational study, baseline platelet count ≤100 × 109/L and serum albumin level <3.5 g/dl were associated with an increased risk of serious complications including hepatic decompensation, bacterial infections, and death 6, 7. Treatment efficacy was particularly poor in patients with prior null response to peginterferon/RBV therapy (<20%) suggesting that patients with these characteristics should not be treated with boceprevir or telaprevir triple therapy 6, 7.
Interferon‐free treatment regimens with two or more direct‐acting antiviral agents have recently been approved to treat HCV‐infected patients with and without cirrhosis. One such all‐oral regimen combines three direct‐acting antivirals (3D): ombitasvir (OBV; an NS5A inhibitor); paritaprevir [PTV; an NS3/4A protease inhibitor dosed with ritonavir (r)]; and dasabuvir (DSV; an NS5B RNA polymerase inhibitor). In phase 3 studies among HCV genotype (GT) 1‐infected patients without cirrhosis, the 3D regimen with RBV yielded SVR rates of 96–100% in treatment‐naïve and peginterferon/RBV treatment‐experienced patients with low rates of treatment discontinuation secondary to AEs 8, 9, 10, 11. The efficacy and safety of this regimen among patients with cirrhosis and surrogate markers of portal hypertension and poor synthetic function were explored in this post‐hoc analysis of the TURQUOISE‐II trial.
Patients and methods
The phase 3 TURQUOISE‐II study evaluated the safety and efficacy of the 3D regimen with RBV in 380 HCV GT1‐infected, treatment‐naïve and treatment‐experienced patients with compensated cirrhosis including prior peginterferon/RBV null responders 12. Patients were randomized to receive coformulated OBV/PTV/r (25/150/100 mg QD), DSV (250 mg BID), and weight‐based RBV (1000 mg or 1200 mg total daily dose BID) for 12 or 24 weeks. Baseline demographic and disease characteristics of those enrolled in this study have been described previously 12.
In this post‐hoc analysis, patients with baseline surrogate markers of portal hypertension (platelet count <100 × 109/L) and/or impaired liver function (serum albumin level <3.5 g/dl) were compared to patients without these characteristics. At baseline, 104 patients (27.4%) had either platelet count <100 × 109/L (N = 78, 20.5%) or albumin levels <3.5 g/dl (N = 43, 11.3%). Seventeen (4.5%) patients met both criteria for thrombocytopaenia and hypoalbuminaemia at baseline. Table S1 lists the characteristics for the patients within these subgroups. The majority of patients were white males, infected with HCV GT1a, and most had previously failed peginterferon/RBV treatment. Notably, there were a larger proportion of patients with prior peginterferon/RBV null response among those with baseline thrombocytopaenia (47.4%) compared to those with baseline hypoalbuminaemia (34.9%).
Results
Rates of sustained virologic response at week 12 post‐treatment (SVR12) in the overall population were 91.8 and 96.5% in the 12‐ and 24‐week treatment groups respectively 12. These differences were not statistically different. Patients with GT1a infection and a history of prior null response had higher SVR rates in the 24‐week group than the 12‐week group (93 vs 80%). Among the 78 patients with baseline platelet counts <100 × 109/L, SVR12 rates were 89% and 97% in those receiving 12‐ or 24‐weeks of 3D + RBV respectively (Fig. 1). In the 43 patients with baseline albumin levels <3.5 g/dl, SVR12 rates were 84% and 89% when treated for 12 or 24 weeks respectively. These SVR12 rates were not statistically different than those in patients without thrombocytopaenia or hypoalbuminaemia at baseline. Among patients with both surrogate markers, 8/10 and 7/7 patients receiving 12 or 24 weeks of therapy achieved SVR12 respectively. In patients with either thrombocytopaenia or hypoalbuminaemia, SVR12 was achieved in 88% of patients receiving 12‐week treatment and 93% receiving 24‐week treatment. Although SVR rates were numerically higher among patients with markers of portal hypertension and decreased synthetic function who were treated for 24 weeks than 12 weeks, wide confidence intervals were observed because of small sample sizes. Eleven (14.1%) patients with baseline thrombocytopaenia and 10 (23.3%) patients with baseline hypoalbuminaemia had oesophageal varices or a history of varices at baseline, one of whom did not achieve SVR. Baseline characteristics for patients not achieving SVR12 are presented in Table S2.
Figure 1.
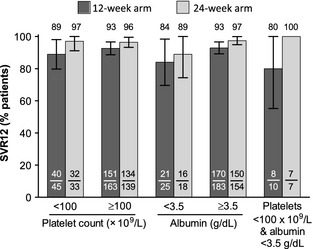
SVR12 rates for patients with baseline platelet count < or ≥100 × 109/L, albumin < or ≥3.5 g/dl, and with platelet count <100 × 109/L and albumin <3.5 g/dl. Whiskers indicate 95% confidence intervals. SVR12, sustained virologic response 12 weeks after the last dose of study drug.
Examining HCV subgenotypes, patients with GT1a infection and platelets <100 × 109/L had SVR12 rates of 87 and 96% when treated for 12 or 24 weeks respectively (Table S3). The SVR12 rate also increased for GT1a patients with albumin <3.5 g/dl with longer treatment duration, 75% for 12‐week treatment vs 88% for 24 weeks. Among 39 GT1a patients with either thrombocytopaenia or hypoalbuminaemia receiving 24 weeks of 3D + RBV, two patients prematurely discontinued treatment, and 1 (2.6%) relapsed. Of 35 patients infected with GT1b, one patient with low platelets experienced relapse. All GT1b‐infected patients with low albumin achieved SVR12 (Table S3).
For all patients in TURQUOISE‐II, median platelet counts did not differ significantly between patients who did or did not achieve SVR12 (141 × 109/L vs 143 × 109/L respectively; Fig. S1a). Similarly, the median baseline albumin did not significantly differ between patients who did or did not achieve SVR12 (4.0 vs 3.9 g/dl respectively; Fig. S1b).
Fatigue, headache and nausea were the most common treatment‐emergent AEs in the overall population and the majority of AEs were mild or moderate in severity. No treatment‐emergent deaths occurred, nor were marked differences observed in rates of AEs comparing either subgroup to the overall population (Table 1). Among the 21 patients in TURQUOISE‐II who experienced serious AEs, eight (38%) had surrogate markers of either portal hypertension or impaired liver function. Four of these eight had events attributed to 3D and/or RBV including chronic obstructive pulmonary disease, cellulitis, and two events of anaemia. Grade 3 total bilirubin abnormalities were more common in patients with baseline thrombocytopaenia and hypoalbuminaemia compared to the overall population (15.4 and 18.6% vs 9.7% respectively); however, the baseline mean total bilirubin levels were higher in patients with thrombocytopaenia or with hypoalbuminaemia than without (1.08 and 1.13 mg/dl vs 0.82 mg/dl respectively). These laboratory elevations mostly comprised indirect bilirubin and were not associated with aminotransferase elevations. No patient with baseline hypoalbuminaemia and one patient with baseline thrombocytopaenia had a grade 3 alanine aminotransferase elevation.
Table 1.
Common treatment‐emergent adverse events occurring in ≥10% of any treatment group and laboratory abnormalities
| Event, n (%) | All patients (N = 380) | Patients with platelets <100 × 109/L (N = 78) | Patients with albumin <3.5 g/dl (N = 43) |
|---|---|---|---|
| Any AE | 347 (91.3) | 73 (93.6) | 38 (88.4) |
| Any serious AE | 21 (5.5) | 5 (6.4) | 6 (14.0) |
| AE leading to discontinuation | 8 (2.1) | 2 (2.6) | 2 (4.7) |
| Fatigue | 148 (38.9) | 30 (38.5) | 17 (39.5) |
| Headache | 111 (29.2) | 23 (29.5) | 12 (27.9) |
| Nausea | 72 (18.9) | 16 (20.5) | 6 (14.0) |
| Pruritus | 71 (18.7) | 18 (23.1) | 10 (23.3) |
| Insomnia | 63 (16.6) | 14 (17.9) | 8 (18.6) |
| Diarrhoea | 59 (15.5) | 14 (17.9) | 10 (23.3) |
| Asthenia | 51 (13.4) | 6 (7.7) | 2 (4.7) |
| Rash | 48 (12.6) | 9 (11.5) | 5 (11.6) |
| Cough | 43 (11.3) | 9 (11.5) | 4 (9.3) |
| Irritability | 36 (9.5) | 8 (10.3) | 2 (4.7) |
| Anaemia | 34 (8.9) | 10 (12.8) | 4 (9.3) |
| Dyspnoea | 33 (8.7) | 9 (11.5) | 5 (11.6) |
| Decreased appetite | 26 (6.9) | 5 (6.4) | 5 (11.6) |
| Peripheral oedema | 23 (6.1) | 5 (6.4) | 5 (11.6) |
| Laboratory abnormalities | |||
| Haemoglobin G2 | 34 (8.9) | 6 (7.7) | 4 (9.3) |
| Haemoglobin G3 | 4 (1.1) | 3 (3.8)a | 1 (2.3)a |
| Total bilirubin G3 | 37 (9.7) | 12 (15.4) | 8 (18.6) |
| ALT G3 | 6 (1.6) | 1 (2.2) | 0 |
| AST G3 | 1 (0.3) | 0 | 0 |
One patient with a grade 3 decline in haemoglobin had baseline platelet count <100 × 109/L and albumin <3.5 g/dl.
G2 haemoglobin is <10‐8 g/dl, G3 haemoglobin is <8 g/dl, G3 ALT and AST are > 5 × ULN.
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; G2, grade 2; G3, grade 3; ULN, upper limit of normal.
Thirty‐four (9.0%) patients in TURQUOISE‐II reduced RBV dose because of anaemia‐related AEs including 9 (11.5%) with thrombocytopaenia and 4 (9.3%) with hypoalbuminaemia at baseline; all achieved SVR. One patient with thrombocytopaenia, and one patient with both thrombocytopaenia and hypoalbuminaemia required a transfusion; no patient received erythropoietin.
Discussion
TURQUOISE‐II included a sufficient number of HCV GT1‐infected patients with cirrhosis to support this post‐hoc analysis focusing on patients with moderate thrombocytopaenia and/or hypoalbuminaemia at study entry. Sustained virologic response rates in those with markers of portal hypertension ranged from 84 to 97%. Our analysis suggests that patients with cirrhosis and moderate thrombocytopaenia, hypoalbuminaemia, or the combination of both does not significantly impact the efficacy or safety of 3D + RBV. Importantly, these results were achieved despite a high representation of HCV GT1a infection and prior null response to peginterferon/RBV. Nevertheless, there was a trend towards higher SVR12 rates in GT1a‐infected patients treated for 24 weeks, suggesting that this duration may be considered for those with low platelets or albumin.
Efficacy and safety data are limited in patients with cirrhosis, particularly in peginterferon/RBV prior null responders and in those with thrombocytopaenia or hypoalbuminaemia. In the CUPIC observational trial, predictors of lower efficacy included prior null response, GT1a infection, and a baseline platelet count <100 × 109/L 6. Efficacy rates among treatment‐experienced patients with compensated cirrhosis were 54–74% among prior relapsers, and 0–19% among prior null responders 7. In TURQUOISE‐II, there were no significant differences in SVR rates by treatment history in the subgroups of patients with thrombocytopaenia or hypoalbuminaemia at baseline (Table S3).
Published data with interferon‐free regimens in patients with cirrhosis are limited, particularly in prior null responders and in those with surrogate markers of portal hypertension or decreased synthetic function. In a study of previously treated patients with cirrhosis, the combination of ledipasvir and sofosbuvir yielded SVR rates of 82–86% in the 12‐week arm and 100% in the 24‐week arm 13. The addition of ribavirin to sofosbuvir and ledipasvir for 12 weeks increased SVR rates in treatment‐experienced patients with compensated cirrhosis; however, SVR rates are unknown for prior null responders and/or small sample sizes limit conclusions about patients with baseline thrombocytopaenia or baseline hypoalbuminaemia 13, 14, 15, 16. In a phase 2 trial of grazoprevir plus elbasvir, SVR rates of 92% were achieved in prior null responders with cirrhosis, and SVR rates were 72% among 25 patients with baseline platelet counts <90 × 109/L 17. A response rate of 71% was observed in GT1b‐infected patients with baseline platelet count <90 × 109/L receiving the combination of daclatasvir and asunaprevir 18. By comparison, 94% of GT1b‐infected patients with baseline platelet count <90 × 109/L achieved SVR12 in TURQUOISE‐II 12. The safety profiles of these interferon‐free regimens in patients with cirrhosis have generally been favourable with low rates of treatment discontinuations related to AEs 12, 13, 14, 15, 16, 17, 18.
The safety profile of the 3D + RBV regimen was similar in patients with cirrhosis with and without the surrogate markers of more advanced liver disease, although higher rates of hyperbilirubinaemia were observed mainly comprising indirect bilirubin and not coinciding with aminotransferase elevations. Within these subgroups, RBV dose adjustment for anaemia‐related AEs occurred at similar rates to those observed in the entire study population. In contrast, patients in the CUPIC trial with platelet counts <100 × 109/L or albumin levels <3.5 g/dl at baseline were at increased risk for serious bacterial infections, hepatic decompensation, death and the frequency of Grade 3 anaemia was high (4–13%) with frequent need for transfusions (6–18%) and erythropoietin (46–57%) 6, 7.
In summary, HCV GT1‐infected patients with cirrhosis with surrogate markers of portal hypertension or impaired liver function at baseline achieved high SVR rates with 3D + RBV, and treatment was well tolerated. Patients with GT1a infection and indicators of portal hypertension may benefit from extending treatment to 24 weeks, whereas treatment duration did not impact SVR rates in GT1b‐infected patients. The 3D + RBV regimen is a promising treatment approach in these particularly vulnerable patient populations.
Supporting information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1111/liv.12931/suppinfo
Acknowledgements
Financial support: AbbVie sponsored this study (NCT01704755), contributed to its design, participated in the collection, analysis and interpretation of the data, and in the writing, reviewing and approval of the manuscript. The authors thank Douglas E. Dylla, PhD, of AbbVie for medical writing support.
Conflict of interest: X Forns: Grant support: Janssen; Advisor: Janssen, MSD, Gilead, AbbVie. F Poordad: Grant/Research support: AbbVie, Achillion Pharmaceuticals, Anadys Pharmaceuticals, Biolex Therapeutics, Boehringer Ingelheim, Bristol‐Myers Squibb, Genentech, Gilead Sciences, GlaxoSmithKline, GlobeImmune, Idenix Pharmaceuticals, Idera Pharmaceuticals, Intercept Pharmaceuticals, Janssen, Medarex, Medtronic, Merck, Novartis, Santaris Pharmaceuticals, Scynexis Pharmaceuticals, Vertex Pharmaceuticals, ZymoGenetics; Speaker: Gilead, Kadmon, Merck, Onyx/Bayer, Genentech, GlaxoSmithKline, Salix, Vertex; Consultant/Advisor: AbbVie, Achillion Pharmaceuticals, Anadys Pharmaceuticals, Biolex Therapeutics, Boehringer Ingelheim, Bristol‐Myers Squibb, Gilead Sciences, GlaxoSmithKline, GlobeImmune, Idenix, Merck, Novartis, Tibotec/Janssen, Theravance, Vertex. M Berenguer: Advisory board: Bristol‐Myers Squibb, Janssen, Roche, MSD, Novartis, AbbVie. H Wedemeyer: Honoraria for consulting/speaking: Abbott, AbbVie, Achillion, Bristol‐Myers Squibb, Boehringer Ingelheim, Gilead, GlaxoSmithKline, ITS, Janssen, Merck, Novartis, Roche, Roche Diagnostics, Siemens, Transgene; Grant support: Abbott, Bristol‐Myers Squibb, MSD, Novartis, Roche. P Ferenci: Advisory committees/Speaker: Roche, Rottapharm‐Madaus; Consultant: Boehringer Ingelheim, Janssen, Bristol‐Myers Squibb Austria, Idenix, Achillion, GlaxoSmithKline, Gilead Sciences, MSD; Research grants: Roche Austria. ML Shiffman: Advisor: AbbVie, Achillion, Bristol‐Myers Squibb, Boehringer Ingelheim, Gen‐Probe, Gilead, GlaxoSmithKline, Janssen, Merck, Roche/Genentech; grant/research support: AbbVie, Achillion, Beckman‐Colter, Bristol‐Myers Squibb, Boehringer Ingelheim, Gen‐Probe, Gilead, Globeimmune, Idenix, Intercept, Lumena, Novartis, Roche/Genentech; Speaker: Bayer, Gilead, GlaxoSmithKline, Janssen, Merck. MW Fried: Research grant: AbbVie, Bristol‐Myers Squibb, Genentech, Gilead, Janssen, Merck, Vertex; Consultant: AbbVie, Bristol‐Myers Squibb, Gilead, Janssen, Merck, Vertex. G Everson: Grant/Research support: AbbVie, Vertex, Bristol‐Myers Squibb, Merck, Roche/Genentech, Gilead/Pharmasset, Glaxo‐SmithKline, Novartis, Tibotec, Janssen; Consultant/Advisor: AbbVie, Vertex, Bristol‐Myers Squibb, Merck, Roche/Genentech, Gilead, GlaxoSmithKline, Novartis, Esai, Biotest, Boehringer Ingelheim, Janssen; Equity interest/Manager: HepQuant LLC. M Pedrosa, S Lovell, R Trinh: Employees of AbbVie and may hold AbbVie stock or options. JC Lopez‐Talavera: Former employee of AbbVie and may hold AbbVie stock or options.
Liver Int. 2015; 35: 2358–2362
Handling Editor: Alessio Aghemo
Trial registration number: ClinicalTrials.gov: NCT01704755
References
- 1. Reiberger T, Rutter K, Ferlitsch A, et al Portal pressure predicts outcome and safety of antiviral therapy in cirrhotic patients with hepatitis C virus infection. Clin Gastroenterol Hepatol 2011; 9: 602–8 e1. [DOI] [PubMed] [Google Scholar]
- 2. Jacobson IM, McHutchison JG, Dusheiko G, et al Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011; 364: 2405–16. [DOI] [PubMed] [Google Scholar]
- 3. Zeuzem S, Andreone P, Pol S, et al Telaprevir for retreatment of HCV infection. N Engl J Med 2011; 364: 2417–28. [DOI] [PubMed] [Google Scholar]
- 4. Bacon BR, Gordon SC, Lawitz E, et al Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med 2011; 364: 1207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Colombo M, Fernandez I, Abdurakhmanov D, et al Safety and on‐treatment efficacy of telaprevir: the early access programme for patients with advanced hepatitis C. Gut 2014; 63: 1150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hézode C, Fontaine H, Dorival C, et al Triple therapy in treatment‐experienced patients with HCV‐cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20‐CUPIC) – NCT01514890. J Hepatol 2013; 59: 434–41. [DOI] [PubMed] [Google Scholar]
- 7. Hézode C, Fontaine H, Dorival C, et al Effectiveness of telaprevir or boceprevir in treatment‐experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology 2014; 147: 132–42.e4. [DOI] [PubMed] [Google Scholar]
- 8. Andreone P, Colombo MG, Enejosa JV, et al ABT‐450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment‐experienced patients with HCV genotype 1b infection. Gastroenterology 2014; 147: 359–65. [DOI] [PubMed] [Google Scholar]
- 9. Feld JJ, Kowdley KV, Coakley E, et al Treatment of HCV with ABT‐450/r–ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014; 370: 1594–603. [DOI] [PubMed] [Google Scholar]
- 10. Ferenci P, Bernstein D, Lalezari J, et al ABT‐450/r–ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med 2014; 370: 1983–92. [DOI] [PubMed] [Google Scholar]
- 11. Zeuzem S, Jacobson IM, Baykal T, et al Retreatment of HCV with ABT‐450/r–ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014; 370: 1604–14. [DOI] [PubMed] [Google Scholar]
- 12. Poordad F, Hezode C, Trinh R, et al ABT‐450/r‐ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 2014; 370: 1973–82. [DOI] [PubMed] [Google Scholar]
- 13. Afdhal N, Reddy KR, Nelson DR, et al Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014; 370: 1483–93. [DOI] [PubMed] [Google Scholar]
- 14. Bourliere M, Bronowicki JP, de Ledinghen V, et al Ledipasvir‐sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non‐responsive to previous protease‐inhibitor therapy: a randomised, double‐blind, phase 2 trial (SIRIUS). Lancet Infect Dis 2015; 15: 394–404. [DOI] [PubMed] [Google Scholar]
- 15. Flamm SL, Everson GT, Charlton MR, et al Ledispasvir/sofosbuvir with ribavirin for the treatment of HCV in patients with decompensated cirrhosis: preliminary results of a prospective, multicenter study. Presented at American Association for the Study of Liver Disease, Boston, MA, November 11, 2014.
- 16. Reddy KR, Everson GT, Flamm SL, et al Ledipasvir/sofosbuvir with ribavirin for the treatment of HCV in patients with post transplant recurrence: preliminary results of a prospective, multicenter study. Presented at American Association for the Study of Liver Diseases, Boston, MA, November 9, 2014.
- 17. Lawitz E, Gane E, Pearlman B, et al Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK‐5172) and elbasvir (MK‐8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C‐WORTHY): a randomised, open‐label phase 2 trial. Lancet 2015; 385: 1075–86. [DOI] [PubMed] [Google Scholar]
- 18. Manns M, Pol S, Jacobson IM, et al All‐oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet 2014; 384: 1597–605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1111/liv.12931/suppinfo


