Summary
Objectives
To assess the prevalence, patient–physician communication, treatment and health outcomes associated with urinary incontinence (UI) among the medically complex vulnerable elderly (MCVE) in the United States (US).
Methods
Data from the 2006 to 2012 Medicare Health Outcomes Survey (HOS) were used. MCVE patients were aged 65+ years with a HOS VE score ≥ 3. UI was reported as a small, big or no problem. Descriptive statistics were used to assess patient–physician communication and treatment. Multivariable regression analyses were performed to assess the association of small or big UI problems with various outcomes.
Results
The annual UI prevalence increased among MCVE [from 35.8% (2006) to 38.6% (2012)]. MCVE with big UI problems communicated with their physicians more often than those with small UI problems (77.9% and 49.6%, respectively); however, treatment of UI remained low (48.5% and 29.1%, respectively). Physical component summary (PCS) and mental component summary (MCS) scores were lower among MCVE with small or big UI problems compared with those with no UI problems, respectively. The decrements in PCS and MCS scores associated with big UI problems were greater than the decrements associated with any of the other assessed conditions. MCVE with small or big UI problems, respectively, were more likely to report past falls, depression and activity daily living limitations vs. those without UI. The odds of having experienced these outcomes were greater for those with big UI vs. small UI problems.
Conclusions
Urinary incontinence prevalence in the USA increased among MCVE from 2006 to 2012, although treatment of UI remained low. UI problems, particularly big UI problems, adversely impact health outcomes. Efforts to better identify and manage UI among the MCVE are needed.
What's known
Urinary incontinence (UI) adversely impacts patients' quality of life and is linked to distress, lowered self‐esteem and depression.
Many UI cases are either under‐reported or diagnosed at later stages, leading to inappropriate medical management and treatment.
Previous studies have assessed the prevalence of UI, treatment and patient health outcomes/quality of life associated with UI in the general elderly population; however, such information is lacking for the medically complex vulnerable elderly (MCVE) population.
What's new
The prevalence of UI problems, particularly big UI problems, increased from 2006 to 2012 among the MCVE population. There remains a gap in communication between patients and physicians and treatment for the MCVE with UI problems, especially for those with small UI problems.
UI problems, particularly a big UI problem, adversely impact health outcomes in the MCVE population.
Introduction
Urinary incontinence (UI) is defined as a loss of bladder control with symptoms ranging from mild leaking to uncontrollable wetting 1. UI affects more than 200 million people worldwide, and approximately 25 million adult Americans 2. A recent estimate suggests that UI is more commonly observed among elderly people, especially older women 1. In addition, older individuals reported UI‐related problems more frequently as compared with their younger counterparts 1.
Previous research has demonstrated that UI adversely impacts patients' quality of life and is linked to distress, lowered self‐esteem and depression 3, 4. Among Medicare beneficiaries, recent survey findings indicate that UI is associated with decreased mental component summary (MCS) and physical component summary (PCS) scores as well as chronic conditions, including hypertension, angina, congestive heart failure, history of acute myocardial infarction, stroke, pulmonary diseases, gastrointestinal tract problems, arthritis, sciatica, diabetes and cancer 5.
Studies have shown that many UI cases are either under‐reported or diagnosed at later stages, leading to inappropriate medical management and treatment, facilitating disease progression as well as causing complications, such as urinary tract infections and rashes 6. Physician–patient communication is key to early and successful treatment of UI 7. However, most elderly people do not consider UI as a serious medical problem and discuss UI problems with physicians only when the disease becomes more severe 8. Although a number of pharmacological therapies are available for treating UI, common treatment options, including behavioural treatment, devices and absorbent products, and surgery are given to patients depending on their disease severity 5, 9.
Previous studies have assessed the prevalence of UI and patient health outcomes/quality of life associated with UI in the general elderly population 4, 5; however, such information is lacking for the medically complex vulnerable elderly (MCVE) population. We addressed this knowledge gap by examining the prevalence, patient–physician communication, treatment and health outcomes of UI in the MCVE population using a patient‐reported survey database in the USA. Specific objectives of this study include: (i) to estimate annual prevalence of UI among the MCVE from 2006 to 2012; (ii) to assess patient–physician communication and treatment of UI problems among MCVE from 2006 to 2012; (iii) to compare health and functional status, falls and depression between MCVE with and without UI; (iv) to assess the health impact of UI vis‐à‐vis other chronic conditions among MCVE.
Methods
Study design and data source
This retrospective database study used data from the Medicare Health Outcome Survey (HOS). The HOS is an annual mail survey with telephone follow‐up sponsored by the Centers for Medicare & Medicaid Services (CMS). The survey collects health and demographic information from a nationally representative sample of Medicare beneficiaries enrolled in Medicare Advantage Organization (MAO) that has a minimum of 500 enrollees.
Study subjects
The study subjects included the HOS respondents aged 65 years or older with complete information on UI status and Veterans Rand 12‐item Health Survey (VR‐12) from 2006 to 2012. The MCVE was determined based on the risk score derived from the Vulnerable Elders Survey of the HOS (VES‐HOS) version 2.0 instrument 10, 11. Adults aged 65+ with a VES‐HOS 2.0 Risk Score greater than or equal to 3 points were defined as the MCVE.
Study variables
Demographic characteristics: age, gender, race, education level, marital status and geographical regions.
Self‐reported UI problems: (i) No UI problem: those who responded ‘not experiencing UI in the past 6 months’ or those describing their recent (i.e. within the past 6 months) UI experience as ‘not a problem’; (ii) Small UI problem: those who responded as having ‘a small problem’ with UI; (iii) Big UI problem: those who responded as having ‘a big problem’ with UI.
Patient–physician communication regarding UI problems and UI treatments: respondents who reported big or small UI problems were asked whether they had talked with a physician or other healthcare providers about the problem. Those who answered in the affirmative were asked if they had received any UI treatments.
Health and functional status: (i) Activities of daily living (ADL) measures: Bathing, dressing, eating, getting in or out of chairs, walking and using the toilet; (ii) VR‐12: the 12 items were summarised to calculate PCS and MCS scale scores 12. The PCS and MCS scores have a range of 0–100 and were normalised to have a mean as 50 and standard deviation as 10 for a representative sample of the US population.
For falls (self‐reported), respondents were asked whether they had any fall in the past 12 months. Those who answered yes were identified as having experienced past falls.
For depression (self‐reported), respondents were asked whether a doctor has ever told them that they had depression. Those who answered yes were identified as having experienced depression.
For comorbid conditions (self‐reported), the HOS respondents were asked whether a doctor has ever told them that they had the following 14 diseases/comorbidities: hypertension or high blood pressure, angina pectoris or coronary artery disease, congestive heart failure, myocardial infarction or heart attack, other heart conditions such as problems with heart valves or the rhythm of heartbeat, stroke, emphysema/asthma/chronic obstructive pulmonary disease (COPD), Crohn's disease/ulcerative colitis/inflammatory bowel diseases, arthritis of hip or knee, arthritis of hand or wrist, osteoporosis, sciatica, diabetes and any cancer (other than skin cancer). Those who answered yes to the comorbidity were identified as having that specific comorbid condition.
Statistical analysis
Annual prevalence of UI by status (small UI problem, big UI problem, and overall UI problem), among MCVE in each survey year between 2006 and 2012, was calculated. Among the MCVE respondents who reported having a small or big UI problem, the number and proportion of patients who had spoken to a physician regarding UI problem and that of patients who had ever received any UI treatment were reported. Results were presented by UI status in each survey year during 2006–2012.
The following analyses were conducted using pooled 2006–2012 data only. Descriptive analyses were conducted to summarize patient demographic characteristics based on UI status among the MCVE. Health outcomes were compared between MCVE with big UI problem and those with no UI problem as well as between MCVE with small UI problem and those with no UI problem. Unadjusted comparisons were conducted using the χ2 test for categorical variables and two‐sample Student's t‐test for continuous variables.
Logistic regression models were conducted to assess the association between binary patient health outcomes (i.e. falls, depression and any ADL limitation) and UI status (i.e. small UI problems vs. no UI problem and big UI problems vs. no UI problem), by adjusting for demographic characteristics and comorbidities.
Respective multiple regression model, by adjusting for demographic characteristics and comorbidities, was performed to assess difference in PCS/MCS scores between having a condition and not having the condition (e.g. big UI problems vs. no UI problems, small UI problems vs. no UI problems, having a comorbid condition vs. not having the condition).
All analyses were conducted in SAS 9.4 (Cary, NC).
Results
A total of 3,165,135 subjects provided responses to the Medicare HOS from 2006 to 2012. Of these subjects, 1,408,285 aged 65 years or older who had complete information on UI status and VR‐12 were included in the study. Among them, 829,614 were identified as MCVE: 311,524 (37.55%) reported having small or big UI problems, whereas 518,090 (62.45%) reported no UI problems.
Table 1 shows demographic characteristics of MCVE with no, small or big UI problems. Compared with those without UI problems, MCVE with small or big UI problems were slightly older, with a higher proportion of females and whites, a lower proportion of being married and were more likely to live in the Central and Pacific regions.
Table 1.
Demographic characteristics of MCVE with no, small or big UI problem
| No UI problem | Small UI problem | Big UI problem | |
|---|---|---|---|
| N = 518,090 | N = 217,614 | N = 93,910 | |
| Age group (%) | |||
| 65–69 years | 23.6 | 20.0 | 18.6 |
| 70–74 years | 23.8 | 21.7 | 19.5 |
| 75–79 years | 20.7 | 21.3 | 20.1 |
| 80+ years | 31.8 | 37.0 | 41.9 |
| Mean (SD) age | 76.1 (7.4) | 77.0 (7.6) | 78.0 (8.1) |
| Gender (%) | |||
| Male | 45.0 | 31.4 | 25.2 |
| Female | 55.0 | 68.6 | 74.5 |
| Race (%) | |||
| Hispanic | 3.6 | 2.7 | 4.0 |
| White | 78.6 | 84.1 | 82.1 |
| African American | 12.7 | 9.5 | 10.4 |
| Asian | 2.7 | 1.9 | 1.8 |
| Other or missing | 2.4 | 1.8 | 1.8 |
| Education level (%) | |||
| 8th grade or less | 16.0 | 13.4 | 17.9 |
| Some high school, but did not graduate | 16.7 | 15.7 | 16.7 |
| High school graduate or GED | 34.1 | 35.4 | 33.7 |
| Some college or 2 years degree | 18.7 | 20.6 | 19.3 |
| 4 year college graduate | 6.1 | 6.1 | 4.9 |
| More than a 4 year college degree | 5.9 | 6.5 | 4.8 |
| Missing | 2.5 | 2.3 | 2.7 |
| Marital status (%) | |||
| Married | 50.4 | 45.1 | 37.6 |
| Divorced | 11.9 | 12.8 | 13.7 |
| Separated | 1.7 | 1.5 | 1.8 |
| Widowed | 30.2 | 35.4 | 41.4 |
| Never married | 4.0 | 3.4 | 3.7 |
| Missing | 1.8 | 1.9 | 1.9 |
| Geographical region (%) | |||
| Central | 25.1 | 27.6 | 27.3 |
| Eastern | 45.9 | 42.1 | 40.3 |
| Pacific | 14.4 | 16.0 | 16.6 |
| Other | 14.5 | 14.2 | 15.8 |
The mean age of MCVE with a big UI problem (78.0 years, SD = 8.1) was higher than those with a small UI problem (77.0 years, SD = 7.6). In addition, MCVE with a big UI problem had a higher proportion of females (74.9% vs. 68.6%), but a lower proportion of whites (82.1% vs. 84.1%) and lower proportion of being married (37.6% vs. 45.1%), and living in the Eastern region (40.3% vs. 42.1%) vs. those with a small UI problem.
Annual prevalence of UI problems among MCVE
The annual prevalence rate of all UI problems, including small and big UI problems, among the MCVE increased over the 7‐year period, from 35.8% in 2006 to 38.6% in 2012 (Figure 1A). The prevalence of a big UI problem increased from 9.9% in 2006 to 12.1% in 2012 (Figure 1A), whereas the prevalence of a small UI problem remained similar (25.9% in 2006 to 26.5% in 2012, Figure 1A) over the 7‐year period among the MCVE population.
Figure 1.
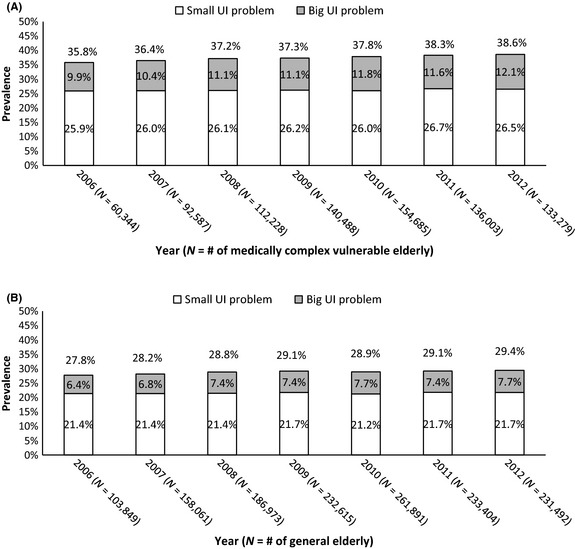
Estimated annual prevalence of UI problems among MCVE by calendar year (A) and estimated annual prevalence of UI problems among general elderly (B). Percentages were based on rounding to the first decimal place
The annual prevalence of UI problems among general elderly was also assessed. The respective prevalence rates for all UI problems, small UI problems and big UI problems among the general elderly (Figure 1B) were all lower than those observed among the MCVE.
Physician–patient communication and treatment among MCVE with UI problems
From 2006 to 2012, about 48–50% of MCVE with a small UI problem and 77–79% of MCVE with a big UI problem communicated with physicians about their UI problems. The percentages were consistent over the years (Figure 2A).
Figure 2.
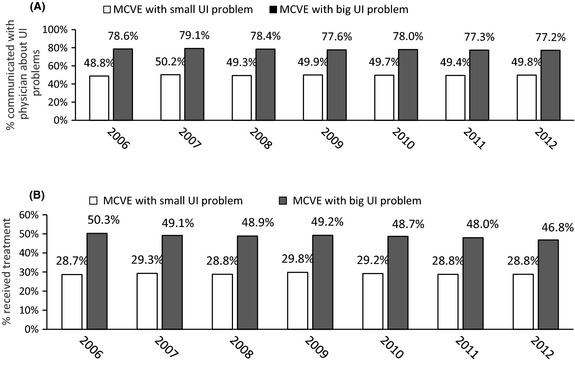
Percentage of MCVE patients who communicated with physicians about UI problems (A) and percentage of MCVE patients receiving treatment (B). Percentages were based on rounding to the first decimal place
Fewer than 30% received any treatment each year from 2006 to 2012 among MCVE with a small UI problem and there was little change over the years. About 50% or fewer received any treatment each year among MCVE with a big UI problem and the percentage decreased from 2006 to 2012 (Figure 2B).
Health and functional outcomes among MCVE with and without UI problems
Medically complex vulnerable elderly with small or big UI problems, when compared with those without a UI problem, were more likely to experience a past fall, depression, ADL limitations and had a lower score on PCS and MCS (all p < 0.0001; Table 2). Those with a big UI problem were most likely to experience a past fall, depression, ADL limitations and had the lowest score on PCS and MCS (Table 2). After adjusting for demographic characteristics, including age, gender, race, education and marital status, and the comorbid conditions, MCVE with a small UI problem were associated with greater odds of having experienced a past fall (OR = 1.59, 95% CI: 1.57–1.60), depression (OR = 1.29, 95% CI: 1.28–1.31) and ADL limitations (OR = 1.52, 95% CI: 1.50–1.53) compared with those without a UI problem. Those with a big UI problem were associated with even greater odds of having experienced a past fall (OR = 2.52, 95% CI: 2.49–2.56), depression (OR = 2.21, 95% CI: 2.18–2.25) and ADL limitations (OR = 3.14, 95% CI: 3.08–3.20) vs. those without UI problem (all p < 0.0001).
Table 2.
Descriptive statistics of the health and functional outcomes among MCVE with and without UI problems
| No UI problem | Small UI problem | Big UI problem | |
|---|---|---|---|
| N = 518,090 | N = 217,614 | N = 93,910 | |
| Past falls (%) | 24.9 | 37.1 | 51.1 |
| Depression (%) | 20.7 | 25.7 | 39.5 |
| Any ADL limitations (%) | |||
| Yes | 54.7 | 67.7 | 83.2 |
| No | 45.3 | 32.3 | 16.8 |
| PCS, mean (SD) | 33.7 (10.6) | 31.3 (10.1) | 26.7 (9.8) |
| MCS mean (SD) | 49.1 (11.7) | 47.0 (11.9) | 42.1 (13.3) |
PCS, Physical Component Summary; MCS, Mental Component Summary; UI, urinary incontinence; ADL, activity of daily living.
Difference in PCS and MCS between having and not having a condition among MCVE
Medically complex vulnerable elderly with a small UI problem had lower PCS (Coeff = −1.17, SE = 0.03, p < 0.0001) and MCS scores (Coeff = −1.89, SE = 0.03, p < 0.0001) compared with those without UI problems, after adjusting for demographic characteristics and the comorbid conditions. MCVE with a big UI problem had even lower PCS (Coeff = −4.47, SE = 0.04, p < 0.0001) and MCS scores (Coeff = −5.95, SE = 0.04, p < 0.0001) than those without UI problems after adjusting for demographic characteristics and the comorbid conditions. For most of the comorbid conditions assessed in this study, MCVE with the condition also had lower PCS and MCS scores compared with those without the condition, after adjusting for demographic characteristics and the conditions other than the one being evaluated (Table 3). But the decrements in the PCS and MCS scores associated with big UI problems were bigger than the decrements associated with any of the other conditions being assessed (Table 3).
Table 3.
Difference in PCS and MCS scores between having and not having a condition among MCVE patients (N = 829,614)
| Mean difference in PCS (95% confidence interval) | Mean difference in MCS (95% confidence interval) | |
|---|---|---|
| UI problem (Reference: no UI problem) | ||
| Small UI problem | −1.17 (−1.22, −1.12)* | −1.89 (−1.95, −1.83)* |
| Big UI problem | −4.47 (−4.53, −4.40)* | −5.95 (−6.03, −5.87)* |
| Comorbid conditions (Reference: no condition) | ||
| Hypertension | −1.01 (−1.06, −0.96)* | 0.26 (0.20, 0.32)* |
| Angina pectoris or coronary artery disease | −0.92 (−0.98, −0.86)* | −0.74 (−0.81, −0.66)* |
| Congestive heart failure | −2.48 (−2.55, −2.41)* | −1.66 (−1.74, −1.57)* |
| Myocardial infarction | −0.83 (−0.90, −0.76)* | 0.02 (−0.06, 0.10) |
| Other heart conditions | −1.00 (−1.05, −0.95)* | −0.25 (−0.31, −0.19)* |
| Stroke | −2.47 (−2.53, −2.41)* | −2.20 (−2.27, −2.12)* |
| Emphysema/asthma/COPD | −3.15 (−3.20, −3.10)* | −0.97 (−1.03, −0.91)* |
| Inflammatory bowel disease | −0.24 (−0.32, −0.15)* | −2.99 (−3.09, −2.89)* |
| Arthritis of hip or knee | −3.23 (−3.27, −3.18)* | 0.19 (0.14, 0.25)* |
| Arthritis of hand or wrist | −1.14 (−1.19, −1.09)* | −1.17 (−1.22, −1.11)* |
| Osteoporosis | −1.42 (−1.48, −1.37)* | −1.47 (−1.53, −1.41)* |
| Sciatica | −1.93 (−1.98, −1.88)* | −1.85 (−1.91, −1.79)* |
| Diabetes | −1.60 (−1.64, −1.55)* | −0.68 (−0.73, −0.62)* |
| Cancer (except skin cancer) | −1.08 (−1.14, −1.03)* | −0.06 (−0.13, 0.00) |
PCS, Physical Component Summary; MCS, Mental Component Summary; UI, Urinary Incontinence; COPD, chronic obstructive pulmonary disease. The difference in PCS and MCS was estimated by respective multiple regression models controlling for age, gender, race, education and marital status, and the conditions included in this table other than the one being evaluated. *Statistically significant (two‐sided p < 0.0001).
Discussion
To our knowledge, this is the first study to assess the prevalence, patient–physician communication, treatment and health and functional outcomes associated with UI problems in the MCVE population. Our study shows that the prevalence of UI problems among the MCVE increased from 35.78% in 2006 to 38.60% in 2012, suggesting a continued increasing trend in the overall burden of UI.
The increasing prevalence of UI observed in this study is consistent with the published literature. The study by Irwin et al. 13 reported an increasing projected prevalence of UI in North America and worldwide from 2008 to 2018. Irwin and co‐authors attributed the trend of increasing prevalence to the ageing of the population 13, which might partly explain the finding here. The increasing prevalence rate in this study is primarily driven by an increase in big UI problems. A prior study consistently showed that patients tended to report UI‐related problems when symptoms became more severe 8. The prevalence estimate in this study is similar to that reported by Gnanadesigan et al. 14 (36.0%) and Mardon et al. 5 (37.3%). However, it is higher than that reported by Ko et al. 4 (24.7%), which might be because of a different base population (MCVE population in current study vs. general elderly population in the previous study) and different study time period (2006–2012 HOS data in the current study vs. earlier HOS data in the previous study).
Consistent with published literature 5, 15, our study also indicates a potential gap in patient–physician communication and treatment among MCVE patients with UI problems. We found that among MCVE with small UI problems, about 50% or fewer had communicated their UI problems with their physicians, less than 30% had received any UI treatments and there was no improvement of communication or treatment rate from 2006 to 2012. MCVE with big UI problems were more likely to discuss their UI problems with physicians than those with small UI problems. However, there were still about 20% of MCVE with big UI problems who did not discuss those UI problems with physicians. Although more likely to receive treatments than those with small UI problems, more than half of the MCVE with big UI problems never received any UI treatments and the treatment rate decreased from 2006 to 2012. It is not completely clear why some MCVE patients with UI problems, including those with big UI problems, did not receive any treatments. Inadequate quality of care for UI provided to the vulnerable elderly 14 and patients' perceptions may partly explain these findings. Even though UI can be managed by a number of treatment options 16, previous literature has demonstrated that older adults with UI considered it not as a medical condition but as a normal part of ageing 17. Because of this perception, they did not raise this health subject with their physicians and believed they could cope with UI 8.
Without proper management, small UI problems may develop into big UI problems that may lead to complications, such as falls/fractures and depression. This study showed that both small and big UI problems were significantly associated with past falls, depression and limitations in ADL among MCVE patients. The potential impacts of these outcomes were more prominent for those with big UI problems. Our findings of UI on depression were consistent with the study by Ko et al. 4, which showed that elderly people with UI problems were about twice as likely to report feeling depressed as those without UI problems.
The mean PCS and MCS scores were significantly lower in MCVE patients with small or big UI problems vs. those without UI problems. Our finding is consistent with previous published studies 4, 5, although the mean PCS and MCS scores were slightly lower in the current study. The lower PCS and MCS scores could possibly be explained by the different base study populations (current study: MCVE vs. previous studies: general elderly 4, 5). The lower PCS score among those with UI problems may be explained by the negative impact of UI problems on the physical functioning domain, such as difficulty in performing moderate activities, climbing stairs and having physical health problems. The lower MCS score among those with UI problems suggests that UI may hinder the patients' emotional well‐being, their activities at work and their social interaction. The literature indicates that there is a strong association between UI and psychosocial problems/social isolation 17, 18.
The decrements in PCS or MCS associated with big UI problems vs. no UI problems are not only statistical significant but also clinically meaningful based on the published literature 19, 20. Mean PCS score for MCVE with big UI problems was 4.47‐point lower than those without UI problems, even after controlling for age, gender, race, education, marital status and the presence of other comorbid conditions. When comparing the magnitude of difference in PCS score associated with having a condition vs. not having the condition among the MCVE patients, the decrement in PCS score associated with big UI problems was among the largest of any condition examined. The potential impact of big UI problems on the MCS score among MCVE patients was even more prominent, with mean MCS score among those with big UI problems being 5.95‐point lower than those without UI problems. The decrement in the MCS score associated with big UI problems was again the largest of all conditions assessed.
Several limitations from this study need to be recognised when interpreting the results. Typically in surveys, self‐reported data are subject to recall bias. HOS respondents were sampled nationally from the MAO that has a minimum of 500 enrollees. Even though it is a nationally representative sample, the results are only representative of Medicare advantage health plan participants without end‐stage renal disease and cannot be generalised to the overall Medicare population. This study only includes HOS respondents with complete information on UI status and VR‐12. The exclusion of HOS respondents with missing information on any of these items may lead to selection bias. Because of the nature of the cross‐sectional analysis, causal relationship cannot be inferred from study results. Although multiple regression analyses were performed to adjust for potential confounders when assessing the association of UI with study outcomes, unobservable confounders (e.g. prior healthcare resource utilisation, such as prior inpatient stay or emergency room visit, and/or other comorbidities not assessed in this study, such as dementia and renal disease) might lead to biased estimates.
Conclusions
The prevalence of UI problems, particularly big UI problems, increased from 2006 to 2012 among the MCVE population. However, there remains a gap in communication between the patients and physicians and treatment for UI problems, especially for those with small UI problems. MCVE with both small and big UI problems were more likely to have experienced negative health outcomes, such as past falls, depression, any ADL limitations and worse PCS and MCS scores, than those without UI problems. The negative impact of UI problems on health outcomes and quality of life were most remarkable among MCVE with big UI problems. Strategies should be implemented to raise the awareness of UI problems and facilitate patient communication with physicians about the problems among MCVE. Increased efforts to appropriately manage and treat UI, especially big UI problems, among the MCVE population are warranted.
Author contributions
Xuemei Luo conceived the study, participated in its design and led the drafting of the manuscript. Chien‐Chia Chuang participated in the design of the study, developed the study methodology and helped to draft the manuscript. Erru Yang helped to develop the methodology, performed the data analysis and helped to draft the manuscript. Kelly H. Zou participated in the design of the study, developed the methodology and helped to draft the manuscript. Anna Araiza participated in the design of the study and helped to draft the manuscript. Tarun Bhagnani assisted in the statistical analysis of the database and helped to draft the manuscript.
Acknowledgments
This observational study was sponsored by Pfizer Inc. K.H. Zou and X. Luo are employees and shareholders of Pfizer. A. Araiza was a short‐term contractor at Pfizer. C. C. Chuang was a former employee and E. Yang and T. Bhagnani are current employees of Evidera who were paid consultants by Pfizer in connection with the development of this manuscript. Journal formatting support for this manuscript was provided by Complete Healthcare Communications, Inc. and funded by Pfizer Inc.
Disclosures
This observational study was sponsored by Pfizer Inc. K.H. Zou and X. Luo are employees and shareholders of Pfizer. A. Araiza was a short‐term contractor at Pfizer. C. C. Chuang was a former employee and E. Yang and T. Bhagnani are current employees of Evidera who were paid consultants by Pfizer in connection with the development of this manuscript. Data analysis was conducted by Evidera, which is also responsible for data storage.
References
- 1. Appell RA, Abrams P, Drutz HP et al. Treatment of overactive bladder: long‐term tolerability and efficacy of tolterodine. World J Urol 2001; 19: 141–7. [DOI] [PubMed] [Google Scholar]
- 2. Bang L, Easthope S, Perry C. Transdermal oxybutynin: for overactive bladder. Drugs Aging 2003; 20: 857–64. [DOI] [PubMed] [Google Scholar]
- 3. Broome BA. The impact of urinary incontinence on self‐efficacy and quality of life. Health Qual Life Outcomes 2003; 1: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ko Y, Lin SJ, Salmon JW, Bron MS. The impact of urinary incontinence on quality of life of the elderly. Am J Manag Care 2005; 11: S103–11. [PubMed] [Google Scholar]
- 5. Mardon RE, Halim S, Pawlson L, Haffer SC. Management of urinary incontinence in medicare managed care beneficiaries: Results from the 2004 medicare health outcomes survey. Arch Intern Med 2006; 166: 1128–33. [DOI] [PubMed] [Google Scholar]
- 6. Irwin DE, Milsom I, Kopp Z et al. Symptom bother and health care‐seeking behavior among individuals with overactive bladder. Eur Urol 2008; 53: 1029–37. [DOI] [PubMed] [Google Scholar]
- 7. Smith AL, Nissim HA, Le TX et al. Misconceptions and miscommunication among aging women with overactive bladder symptoms. Urology 2011; 77: 55–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dugan E, Roberts CP, Cohen SJ et al. Why older community‐dwelling adults do not discuss urinary incontinence with their primary care physicians. J Am Geriatr Soc 2001; 49: 462–5. [DOI] [PubMed] [Google Scholar]
- 9. Dmochowski RR, Sand PK, Zinner NR et al. Comparative efficacy and safety of transdermal oxybutynin and oral tolterodine versus placebo in previously treated patients with urge and mixed urinary incontinence. Urology 2003; 62: 237–42. [DOI] [PubMed] [Google Scholar]
- 10. Final Report on Identifying Elderly HOS Beneficiaries at Risk for Mortality Using the Updated 2009 VES‐HOS Risk Scoring: Health Services Advisory Group, 2013.
- 11. Saliba D, Elliott M, Rubenstein LZ et al. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc 2001; 49: 1691–9. [DOI] [PubMed] [Google Scholar]
- 12. Iqbal SU, Rogers W, Selim A et al. The Veterans RAND 12 Item Health Survey (VR‐12): What it is and How it is used. http://www.hosonline.org/surveys/hos/download/Veterans_RAND_12_Item_Health_Survey_VR-12_2007.pdf (accessed November 4, 2014).
- 13. Irwin DE, Kopp ZS, Agatep B et al. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int 2011; 108: 1132–8. [DOI] [PubMed] [Google Scholar]
- 14. Gnanadesigan N, Saliba D, Roth CP et al. The quality of care provided to vulnerable older community‐based patients with urinary incontinence. J Am Med Dir Assoc 2004; 5: 141–6. [DOI] [PubMed] [Google Scholar]
- 15. Shaw C. A review of the psychosocial predictors of help‐seeking behaviour and impact on quality of life in people with urinary incontinence. J Clin Nurs 2001; 10: 15–24. [DOI] [PubMed] [Google Scholar]
- 16. Gormley EA, Lightner DJ, Burgio KL et al. Diagnosis and treatment of overactive bladder (non‐neurogenic) in adults: AUA/SUFU guideline. J Urol 2012; 188(6 Suppl.): 2455–63. [DOI] [PubMed] [Google Scholar]
- 17. Brown JS, Subak LL, Gras J et al. Urge incontinence: the patient's perspective. J Womens Health 1998; 7: 1263–9. [DOI] [PubMed] [Google Scholar]
- 18. Gardner J, Fonda D. Urinary incontinence in the elderly. Disabil Rehabil 1994; 16: 140–8. [DOI] [PubMed] [Google Scholar]
- 19. Ware JEJ, Kosinski M, Bjorner JB, Turner‐Bowker DM, Gandek B, Maruish ME. User's Manual for the SF‐36v2TM Health Survey, 2nd edn Lincoln, RI: QualityMetric Incorporated, 2007. [Google Scholar]
- 20. Blum SI, Tourkodimitris S, Ruth A. Evaluation of functional health and well‐being in patients receiving levomilnacipran ER for the treatment of major depressive disorder. J Affect Disord 2014; 170c: 230–6. [DOI] [PubMed] [Google Scholar]


