Summary
Objective
The aim of this study was to examine how well body mass index (BMI) reflects cardiovascular risk associated with excess adiposity in a Swedish population by examining the association between body fat, BMI and cardiovascular risk factors.
Methods
A total of 3,010 adults participated. Normal weight adiposity was defined as the combination of BMI < 25 kg/m2 and percentage body fat ≥35% for women and ≥25% for men. Associations with blood pressure, blood lipids, apolipoproteins and C‐reactive protein were analysed in age‐adjusted regression models.
Results
The majority of the individuals with overweight and obesity were correctly classified to adiposity, while a wide range of body fat was observed among the normal weight subjects. In total, 9% of the participants were categorised as normal weight with adiposity. Compared with the normal weight leanness group, participants with normal weight adiposity had higher levels of serum triglycerides, low‐density lipoprotein cholesterol, C‐reactive protein, apolipoptotein B and the apolipoprotein B/A‐I ratio. In normal weight men, adiposity was also associated with higher blood pressure and lower high‐density lipoprotein cholesterol.
Conclusions
Higher percentage of body fat was associated with less favourable risk factor profile even in subjects who were normal weight. Thus, it might be relevant to screen for metabolic risk factors in the upper end of the normal weight category.
Keywords: Normal weight obesity, metabolically obese, BMI, body fat, cardiovascular risk
Introduction
Body mass index (BMI) is a commonly used indicator of excess body fat because it is a simple and inexpensive measurement, but it provides only an approximation of body fat and does not reveal fat distribution. The upper cut‐off point for a healthy BMI is also the same for different ages, sexes, ethnicities, etc., to facilitate population comparisons. Moreover, it is possible that the BMI cut‐off for a healthy weight could be set higher than what the previous research has suggested 1.
Thus, individuals with excess body fat might be misclassified as not being at risk, and on the other hand, individuals who have a high body weight in relation to height due to a high muscle mass might be classified as preobese or obese. The fact that individuals classified as normal weight might be ‘metabolically obese’, i.e. display a cluster of obesity‐related abnormalities like reduced insulin sensitivity and atherogenic lipid profile, has been discussed for many years 2, 3, 4, and elevated visceral adipose tissue has been observed even in normal weight subjects 5. Moreover, in American normal weight men and women, higher percentage of body fat has been demonstrated to be associated with increased risk for dyslipidemia, metabolic syndrome and for women, also cardiovascular mortality 6. However, because the relationship between body fat and BMI differs according to ethnicity, studies are needed in various populations 7.
In European populations, the prevalence of ‘normal weight obesity’ has been observed to be lower in men than women 8, 9, 10, which might be one of the reasons why European studies have primarily focused on women. De Lorenzo et al. have demonstrated that percentage of body fat over 30 in a small group of Italian normal‐weight women was associated with obesity‐related inflammation and oxidative stress 11, 12, but elevated levels of serum high‐density lipoprotein (HDL) cholesterol 13. In contrast, Marques‐Vidal showed that normal weight adiposity (NWA) (body fat >38%) in a population of Swiss women was associated with lower HDL cholesterol and with other cardiovascular risk factors but not with inflammatory markers 14. Another study demonstrated less favourable profile regarding serum lipids, insulin sensitivity, blood pressure and C‐reactive protein (CRP) in both women and men in the NWA group when compared with lean subjects 15. Thus, the results are somewhat conflicting regarding associations with specific risk factors and gender.
The results of these studies might not be strictly comparable because they used different cut‐off points to define excess body fat. Some studies have used a single, pre‐set cut‐point, while other studies have compared gender‐specific categories based on the body fat distribution in the specific sample. Thus, more studies are needed on how measures of body fat add complementary information to the BMI classification of overweight on cardiovascular risk and whether these relationships differ with sex. It is also interesting to investigate if such potential additional information could be caught with assessment of specific BMI values instead of merely using the established cut‐of value for healthy BMI.
The aim was to examine how well BMI reflects the cardiovascular risk factor profile associated with excess adiposity in a Swedish population of women and men by examining the association between body fat, BMI and cardiovascular risk factors like blood pressure, inflammation markers, serum lipids and apolipoproteins.
Methods
Population
INTERGENE is a population‐based research programme in western Sweden assessing the INTERplay between GENEtic susceptibility and environmental factors for the risk of chronic diseases. The survey started in April 2001 and continued until December 2004. The study population consists of randomly selected women and men, aged 25–74 years at time of sampling, living in the Västra Götaland County. This is the second largest county in Sweden, consisting of 49 communities of which one is the second largest city in Sweden, Gothenburg. Altogether, the sample consisted of 8,626 eligible subjects. The overall response rate of the invited cohort was 3,614 (1,910 female, 44.5%, and 1,704 men, 39.3%). For the purpose of this study, participants without measurement of bioelectrical impedance (n = 588) were excluded. We also excluded pregnant women (n = 16). Thus, this subsample comprises 3,010 participants. The invitees were asked not to eat during the last 4 h before the physical examination and blood tests.
The INTERGENE research programme study procedures were approved by the regional ethics review board, Forskningsetikkommittén (Ö 237/2000), and have previously been described in detail 16, 17. The study complies with the Declaration of Helsinki. All participants were informed of the aims and procedures of the study and gave their written consent.
Measurements
Body height and weight were measured to the nearest cm and 0.1 kg with the subjects in light clothing and without shoes. Waist circumference was measured at a level midway between the lower rib margin and iliac crest, and hip was measured as the maximum perimeter over the buttocks. Using WHO guidelines 18, overweight was assessed on the basis of BMI (kg/m2), defining overweight as BMI ≥ 25 kg/m2 and obesity as BMI ≥ 30 kg/m2. The category with BMI < 25 kg/m2 will be referred to as normal weight in this article, it includes underweight subjects (n = 17).
Body composition was estimated using bioelectrical impedance analysis. Whole body electrical resistance was measured using BIA series 3–4, 50 kHz (BIACOM Gesundheitsberatung GmbH, Germany), following the instructions given by the manufacturer. The subjects rested in supine position for 10 min before measurement with electrodes on the dorsal surfaces of the right hand, wrist, ankle and foot. The fat‐free mass was derived from prediction equations from a Danish population 19. Cut‐off values for excessive percentage body fat were set to ≥35 for women and ≥25 for men, based on body fat percentage predicted from BMI 25–30 kg/m2 in adult populations 20, 21. This is also the definition of adiposity used by the American Society of Endocrinologists 22.
Physical activity and smoking were assessed by a questionnaire. Blood pressure was measured twice for each person after 5‐min rest, using a validated automatic device (Omron 711 Automatic IS), with the subject in sitting position. The mean value from the two measurements was used. Blood samples were collected into tubes containing 0.1% EDTA for immediate serum lipid (total cholesterol, high‐density cholesterol and triglycerides) and glucose analysis. Serum total cholesterol and triglyceride concentrations were determined using enzymatic assays. Serum HDL cholesterol concentrations were measured after dextran sulphate–magnesium precipitation of apolipoprotein (apo)B‐containing lipoproteins. Low‐density lipoprotein (LDL) cholesterol levels were estimated for all subjects with triglyceride levels below 4.00 mmol/L, using the Friedewald equation. Quantitative determination of apoB and apoA‐I was performed by immunoprecipitation enhanced by polyethylene glycol at 340 nm (Thermo Fisher Scientific, Vantaa, Finland). Plasma glucose was analysed with a hexokinase method. CRP was analysed in serum that had been frozen in −80 °C. It was measured by an ultrasensitive particle‐enhanced immunoturbidimetric method (Orion Diagnostica, Espoo, Finland). All analyses were performed on a Konelab 20 autoanalyser (Thermo Fisher Scientific). Interassay coefficient of variation was for Konelab analyses below 5%. Nine subjects who had not fasted according to instructions were excluded from glucose and triglyceride analyses.
Hypertension was defined as ≥140 mmHg systolic and/or ≥90 mmHg diastolic blood pressure and/or treatment, hyperlipidaemia as LDL cholesterol ≥3 mmol/L and/or treatment, high serum triglycerides as >1.7 mmol/L, low HDL cholesterol as <1.0 mmol/L in men, and <1.2 mmol/L in women 23 . High apoB/apoA‐I ratio was defined as ≥0.9 in men and ≥0.8 in women 24.
The metabolic syndrome was defined by national Cholesterol Education Program, Adult Treatment Panel III, NCEP ATPIII 25, i.e. the presence of three or more of the following five components: waist circumference ≥88 cm in women and ≥102 cm in men; serum triglycerides ≥1.7 mmol/L; HDL cholesterol <1.03 mmol/L in men and <1.29 mmol/L in women; blood pressure ≥130 mmHg systolic and/or ≥85 mmHg diastolic; and fasting plasma glucose ≥6.1 mmol/L.
Statistical analyses
The participants were categorised in four groups according to BMI and percentage of body fat. Normal weight (BMI < 25 kg/m2) leaness (NWL) (body fat <25% for men and <35% for women) was considered as reference. The other groups were NWA, overweight leanness (OWL) and overweight adiposity (OWA).
Differences from reference group in the three other categories, in anthropometrics, blood pressure, serum lipids, apolipoproteins, plasma glucose and CRP, were assessed using age‐adjusted linear regression, stratified by sex, as well as in the whole data set including an interaction term between weight/adiposity status and sex. The distributions of serum lipids, CRP, systolic blood pressure, plasma glucose, weight and body fat were skewed towards large values; therefore, the analyses were performed on the log transformed variables that were normally distributed after transformation. The anti‐log transformation was applied to log‐transformed mean values in order to present the results on the original scale 26.
Differences in prevalence of risk factors in the NWA, OWL and OWA groups compared with the reference group, NWL, was assessed in age‐adjusted logistic regressions. Separate models were used to compare the presence of metabolic syndrome, hypertension, hyperlipidaemia, high serum triglycerides, low HDL cholesterol and high apoB/apoA‐I ratio (yes versus no) by weight–adiposity category. Testing for potential interactions with sex was performed including product terms of sex and weight/adiposity status into each model.
Different cardiovascular risk factors (hypertension, hyperlipidaemia, high serum triglycerides, low HDL cholesterol and high apoB/apoA‐I ratio) were predicted by BMI as continuous variable, by dichotomized BMI (overweight yes versus no), and overweight or central obesity respectively in separate logistic regression models adjusted for sex and age. For each outcome, we compared the area under the receiver operating characteristics (ROC) curve for a model based on BF% as a continuous variable with the corresponding values of the three other logistic models. The statistical analyses were performed using Statistical Analysis Software 9.3 (SAS Institute Inc., NC, USA).
Results
BMI in relation to body fat
The average body fat % was equal to 35% in women and 27% in men. In total, 52% of the women had a value ≥35 %, and 69% of the men had a value ≥25%. The impedance assessments indicated that excessive body fat was also present among those with low to normal BMI (Figures 1 and 2). Among these participants with BMI < 25 kg/m2, as many as one fifth were classified to adiposity if the cut‐off for body fat was set to 25% for men and 35% for women. A wide range of body fat was observed among the subjects with normal weight while the majority of the group with overweight had a percentage of body fat corresponding to adiposity.
Figure 1.
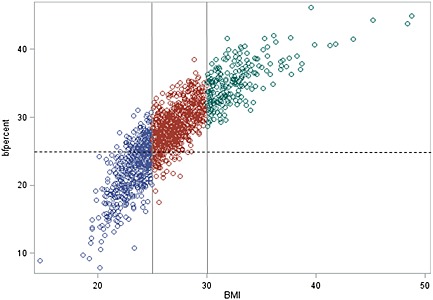
Body mass index (BMI) in relation to percentage of body fat in male. The different colours show BMI categories, and the dotted show line the cut off for excess body fat.
Figure 2.
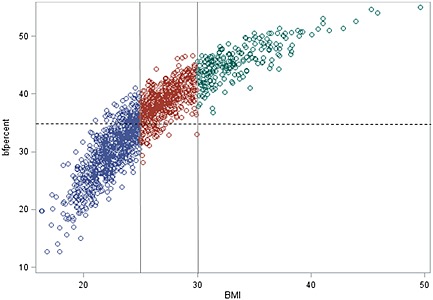
Body mass index (BMI) in relation to percentage of body fat in female. The different colours show BMI categories, and the dotted line shows the cut off for excess body fat.
Characteristics of normal weight and overweight subjects with higher and lower percentage of body fat are shown in the upper panel of Table 1. Slightly more than a third of the participants were in the NWL group. Thus, the majority had overweight and/or adiposity given the sex‐specific cut‐off values for percentage of body fat. In total, 9% of the participants (8% of the women and 10% of the men) were categorised as NWA. When compared with other categories, this group tended to be older (Table 1).
Table 1.
Background characteristics and age adjusted prevalences of risk factors in NWA, OWL and OWA compared with the reference group of NWLa
| Reference group Normal weight leannessb, NWL (n = 1080, 36%) | Normal weight adiposityb, NWA (n = 266, 9%) | Overweight leannessb, OWL (n = 125, 4%) | Overweight adiposityb, OWA (n = 1545, 51%) | |
|---|---|---|---|---|
| Background characteristics | ||||
| Age mean (SD) | 45.4 (12.7) | 57.6 (11.7) | 42.0 (10.3) | 55.0 (12.6) |
| Females, % | 65 | 48 | 48 | 45 |
| Smokers, % | 20 | 13 | 18 | 16 |
| Regular physical activity, %c | 35 | 22 | 46 | 21 |
| Prevalences of risk factorsa | ||||
| Metabolic syndrome, % | 2.3 | 3.9*** | 6.9** | 25.4*** |
| Hypertension, %d | 32.8 | 41.7** | 34.4 | 46.9*** |
| Hyperlipidaemia, %e | 54.3 | 68.0*** | 58.6 | 69.4*** |
| High triglycerides, %f | 9.5 | 18.6*** | 17.3** | 33.2*** |
| Low HDL, %g | 2.0 | 4.7 | 6.7** | 10.1*** |
| High apoB/apoA‐I, %h | 14.3 | 27.5*** | 24.0** | 35.5*** |
NWL, normal weight leanness; NWA, normal weight adiposity; OWL, overweight leanness; OWA, overweight adiposity; SD, standard deviation; HDL, high‐density lipoprotein; BMI, body mass index.
p‐values indicate significant differences compared with NWL, assessed in age adjusted logistic regressions.
Overweight = BMI ≥ 25 kg/m2; adiposity = percentage of body fat ≥25% in men and percentage of body fat ≥35% in women.
Exercise at least 2–3 h a week.
Blood pressure ≥ 140/90 mmHG or treatment.
LDL ≥ 3 mmol/L or treatment.
TG > 1.7 mmol/L.
HDL < 1.2 mmol/L for women and <1.0 mmol/L for men.
apoB/apoA‐I ≥ 0.8 for women and ≥0.9 for men.
p < 0.05;
p < 0.01;
p < 0.001.
On the other hand, the misclassification of obesity due to high muscle mass seems to be minor (Figures 1 and 2). Only 4% were categorised as OWL, merely two overweight men had body fat below 20%, and only two overweight women had body fat below 30%. Among the obese, none had percentage of body fat below cut‐off, only a few men had body fat below 30%, and a few women had body fat below 40%.
Weight/adiposity status and cardiovascular risk factors
The lower panel of Table 1 shows that the prevalence of metabolic syndrome, hypertension, hyperlipidaemia, high triglycerides and a high apoB/apoA‐I ratio was higher in the NWA group compared with the reference group, NWL. There was an interaction between sex and weight/adiposity category (p < 0.05), with a significantly higher prevalence of hypertension in NWA (46.3%) men compared with NWL (32.4%, p < 0.001) but no significant association for women (37.5% vs 32.3%).
Lean body mass was similar in the NWL and NWA groups, while mean body fat mass, BMI and waist to hip ratio were higher among NWA (Tables 2 and 3). The mean fat mass was approximately 5 kg greater in the NWA group than in the NWL group, in both men and women.
Table 2.
Age adjusteda antropometric and metabolic risk characteristics in men with overweight and/or adiposity compared with the reference group of normal weight men with body fat <25%
| Reference group, NWL BMI < 25 kg/m2, BF < 25% | NWA BMI < 25kg/m2, BF ≥ 25% | OWL BMI ≥ 25kg/m2, BF < 25% | OWA BMI ≥ 25kg/m2, BF ≥ 25% | |
|---|---|---|---|---|
| n b/% | 377/26 | 139/10 | 65/5 | 856/59 |
| Body fat, % | 21.0 (20.7–21.4) | 26.6 (26.0–27.1)*** | 24.1 (23.3–24.9)*** | 30.7 (30.5–31.0)*** |
| Body fat, kg | 15.0 (14.7–15.3) | 20.6 (19.9–21.4)*** | 19.6 (18.6–20.6)*** | 27.7 (27.3–28.1)*** |
| LBM, kg | 57.5 (56.9–58.1) | 57.4 (56.5–58.3) | 61.9 (60.5–63.2)*** | 63.2 (62.8–63.5)*** |
| Weight, kg | 72.7 (71.8–73.5) | 77.8 (76.5–79.2)*** | 81.4 (79.2–83.5)*** | 90.9 (90.0–91.6)*** |
| BMI, kg/m2 | 22.8 (22.6–23.1) | 24.1 (23.7–24.6)*** | 25.8 (25.2–26.4)*** | 28.7 (28.6–28.9)*** |
| WHR | 0.88 (0.87–0.89) | 0.91 (0.90–0.92)*** | 0.90 (0.88–0.91)* | 0.95 (0.95–0.96)*** |
| Hip, cm | 96.1 (95.5–96.6) | 98.9 (98.0–99.9)*** | 100.5 (99.1–101.9)*** | 104.8 (104.4–105.1)*** |
| Waist, cm | 84.4 (83.6–85.2) | 89.8 (88.5–91.0)*** | 90.1 (88.2–92.1)*** | 100.0 (99.5–100.6)*** |
| SBP, mmHg | 129 (127–131) | 134 (131–137)** | 132 (128–136) | 135 (134–137)*** |
| DBP, mmHg | 80 (79–81) | 85 (83–86)*** | 80 (78–82) | 84 (84–85)*** |
| Cholesterol, mmol/L | 5.2 (5.1–5.3) | 5.6 (5.4–5.8)*** | 5.2 (5.0–5.5) | 5.5 (5.4–5.6)*** |
| LDL, mmol/L | 3.1 (3.0–3.2) | 3.4 (3.2–3.6)*** | 3.1 (2.9–3.4) | 3.3 (3.2–3.4)*** |
| HDL, mmol/L | 1.6 (1.6–1.6) | 1.5 (1.4–1.6)* | 1.4 (1.3–1.5)*** | 1.3 (1.3–1.4)*** |
| Triglycerides, mmol/L | 1.0 (0.9–1.6) | 1.2 (1.1–1.3)*** | 1.2 (1.0–1.3)* | 1.6 (1.5–1.6)*** |
| ApoB/ApoA‐I | 0.70 (0.67–0.72) | 0.79 (0.76–0.83)*** | 0.76 (0.70–0.81)* | 0.86 (0.85–0.88)*** |
| ApoA‐I, g/L | 1.5 (1.5–1.5) | 1.5 (1.4–1.5) | 1.4 (1.4–1.5) | 1.4 (1.4–1.4)*** |
| ApoB, g/L | 1.0 (1.0–1.0) | 1.2 (1.1–1.2)*** | 1.1 (1.0–1.1) | 1.2 (1.2–1.2)*** |
| Glucose, mmol/L | 5.1 (5.0–5.2) | 5.2 (5.1–5.3) | 5.2 (5.0–5.4) | 5.4 (5.3–5.4)*** |
| CRP. mg/L | 0.8 (0.7–0.9) | 1.4 (1.2–1.7)*** | 1.0 (0.7–1.2) | 1.6 (1.5–1.8)*** |
NWL, normal weight leanness; NWA, normal weight adiposity; OWL, overweight leanness; OWA, overweight adiposity; SD, standard deviation; BMI, body mass index; LBM, lean body mass; WHR, waist to hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL, low‐density lipoprotein; HDL, high‐density lipoprotein; CRP, C‐reactive protein.
Least squares means with 95% confidence intervals adjusted for age in linear regression. p‐values indicate significant differences compared with normal weight lean men.
Total number of participants in the analyses differs between 1,376 (ApoB/ApoA‐I) and 1,437 (weight, height and body composition).
p < 0.05;
p < 0.01;
p < 0.001.
Table 3.
Age adjusteda anthropometric and metabolic risk characteristics in women with overweight and/or adiposity compared with the reference group of normal weight women with body fat <35%
| Reference group, NWL BMI < 25kg/m2, BF < 35% | NWA BMI < 25kg/m2, BF ≥ 35% | OWL BMI ≥ 25kg/m2, BF < 35% | OWA BMI ≥ 25kg/m2, BF ≥ 35% | |
|---|---|---|---|---|
| n b/% | 703/44 | 127/8 | 60/4 | 689/44 |
| Body fat, % | 29.4 (29.2–29.7) | 35.4 (34.8–36.1)*** | 34.3 (33.4–35.2)*** | 40.9 (40.6–41.2)*** |
| Body fat, kg | 17.6 (17.3–17.9) | 23.3 (22.4–24.1)*** | 23.9 (22.6–25.2)*** | 32.0 (31.5–32.5)*** |
| LBM, kg | 42.8 (42.5–43.1) | 42.9 (42.2–43.6) | 45.9 (44.9–46.9)*** | 46.7 (46.4–47.0)*** |
| Weight, kg | 60.6 (60.0–61.2) | 66.0 (64.7–67.4)*** | 69.6 (67.6–71.7)*** | 78.6 (78.2–79.6)*** |
| BMI, kg/m2 | 22.1 (21.9–22.3) | 24.1 (23.6–24.6)*** | 25.8 (25.1–26.5)*** | 29.3 (29.1–29.5)*** |
| WHR | 0.79 (0.78–0.79) | 0.81 (0.80–0.82)*** | 0.81 (0.80–0.83)** | 0.85 (0.84–0.85)*** |
| Hip, cm | 94.8 (94.2–95.3) | 98.9 (97.7–100.0)*** | 100.5 (98.7–102.3)*** | 107.7 (107.2–108.3)*** |
| Waist, cm | 74.5 (73.9–75.1) | 79.9 (78.5–81.3)*** | 81.6 (79.6–83.7)*** | 91.2 (90.6–91.8)*** |
| SBP, mmHg | 125 (124–126) | 125 (122–128) | 125 (122–128) | 129 (128–130)*** |
| DBP, mmHG | 80 (79–81) | 80 (79–82) | 79 (76–81) | 83 (82–83)*** |
| Cholesterol, mmol/L | 5.3 (5.2–5.4) | 5.6 (5.4–5.7)** | 5.4 (5.1–5.6) | 5.5 (5.4–5.5)** |
| LDL, mmol/L | 2.9 (2.8–3.0) | 3.1 (3.0–3.3)* | 3.0 (2.8–3.2) | 3.2 (3.1–3.2)*** |
| HDL, mmol/L | 1.8 (1.8–1.9) | 1.8 (1.7–1.9) | 1.7 (1.6–1.8)* | 1.6 (1.6–1.6)*** |
| Triglycerides, mmol/L | 0.9 (0.9–1.0) | 1.1 (1.0–1.2)*** | 1.1 (1.0–1.2)** | 1.3 (1.2–1.3)*** |
| ApoB/apoA‐I | 0.60 (0.59–0.62) | 0.66 (0.62–0.70)** | 0.66 (0.61–0.71)* | 0.73 (0.71–0.75)*** |
| ApoA‐I, g/L | 1.66 (1.63–1.68) | 1.67 (1.62–1.72) | 1.61 (1.54–1.68) | 1.57 (1.55–1.59)*** |
| ApoB, g/L | 0.98 (0.96–1.00) | 1.07 (1.02–1.12)*** | 1.03 (0.97–1.10) | 1.12 (1.10–1.14)*** |
| Glucose, mmol/L | 4.9 (4.8–4.9) | 4.9 (4.8–5.0) | 4.9 (4.8–5.1) | 5.1 (5.0–5.1)*** |
| CRP. mg/L | 0.8 (0.7–0.9) | 1.2 (0.9–1,4)** | 1.1 (0.1–1.5)* | 2.4 (2.2–2.6)*** |
NWL, normal weight leanness; NWA, normal weight adiposity; OWL, overweight leanness; OWA, overweight adiposity; SD, standard deviation; BMI, body mass index; LBM, lean body mass; WHR, waist to hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL, low‐density lipoprotein; HDL, high‐density lipoprotein; CRP, C‐reactive protein.
Least‐squares means with 95% confidence intervals adjusted for age in linear regression. p‐values indicate significant differences compared to normal weight lean women.
Total number of participants in the analyses differs between 1484 (ApoB/ApoA‐I) and 1573 (weight, height and body composition).
p < 0.05;
p < 0.01;
p < 0.001.
In Tables 2 and 3, mean values of those with adiposity and/or overweight are compared with the reference group NWL. The analyses are stratified by sex because interactions between sex and weight/adiposity status were significant for several outcome variables. The OWA group had less favourable values for all measured risk factors than the reference group in both men and women. Also, among those with BMI below 25, higher body fat was associated with more unfavourable risk factor profile than lower body fat. Compared with NWL, NWA subjects of both sexes had higher levels of CRP, serum triglycerides, LDL cholesterol, apoB and apoB/apoA‐I ratio. In men, NWA was also associated with higher blood pressure and lower HDL cholesterol.
In sensitivity analyses, we further adjusted for BMI and waist circumference, respectively, to account for the higher BMI and waist circumference observed in NWA compared with NWL subjects (Table 2 and 3). When adjusted for BMI, the difference between NWA and NWL was still observed for systolic and diastolic blood pressure, total and LDL serum cholesterol, serum triglycerides, apoB/apoA‐I, apoB and CRP in men, and for serum cholesterol, serum triglycerides and apoB in women. When adjusting for waist circumference, the difference between NWA and NWL was still observed for systolic and diastolic blood pressure, total and LDL serum cholesterol, apoB/apoA‐I, apoB and CRP in men, and for serum cholesterol and apoB in women. Thus, the association with serum triglycerides became non‐significant in both women and men when adjusting for waist circumference.
Association of anthropometric measures with prevalence of cardiovascular risk factors
As the range of BMI and percentage of body fat are rather broad within the normal weight category, narrower BMI categories may better reflect risk related to percentage of body fat. Therefore, we compared by contrasting ROC curves how well BMI as continuous variable and the dichotomous variable overweight, respectively, predicted hypertension and dyslipidemia in comparison with percentage of body fat. As shown in Table 4, the area under the ROC curve was smaller for the dichotomous BMI variable (overweight or not) than for percentage of body fat as continuous variable for all analysed risk factors. The predictive ability was still inferior to percentage of body fat when also considering waist circumference. When instead analysing the predictive ability of BMI as continuous variable in comparison with percentage of body fat, the accuracy only differed significantly for hyperlipidemia (p < 0.05).
Table 4.
Comparison of prediction of individual cardiovascular risk factors by four different anthropometric predictors in terms of the area under the ROC curvea
| Anthropometric variables (predictor) | ||||
|---|---|---|---|---|
| Risk factor (outcome) | Reference BF% | BMI ≥ 25 | BMI ≥ 25 or WC > 88/102b | BMI |
| Hypertension | 0.81 | 0.80** | 0.80** | 0.81 |
| Hyperlipidemia | 0.73 | 0.72** | 0.72** | 0.72* |
| High triglycerides | 0.74 | 0.71*** | 0.71*** | 0.74 |
| Low HDL | 0.71 | 0.67** | 0.68* | 0.72 |
| High apoB/apoAI | 0.72 | 0.68*** | 0.68*** | 0.71 |
BF%, percentage of body fat; BMI, body mass index; WC, waist circumference; ROC, receiver operating characteristics; HDL, high‐density lipoprotein.
For each outcome variable, the area under the ROC curve of a model with percentage of body fat, BF%, as a continuous predictor (reference) is compared with the corresponding value for the other three models (all models adjusted for age and sex).
Waist circumference >88 cm for women and >102 cm for men.
p‐value for comparison with the reference.
p < 0.05;
p < 0.01;
p < 0.001.
Thus, the specific BMI values (continuous) predicted cardiovascular risk factor profile associated with excess adiposity better than the cut‐off point of BMI ≥ 25 (dichotomous). However, percentage of body fat had only slightly better predictive ability than BMI for hyperlipidaemia.
Discussion
The results indicate that NWA exists in a Swedish population, and that the prevalence is considerable, as almost one out of ten was classified as NWA. The average body fat mass was higher in this group compared with the NWL, while the lean body mass was the same. Thus, within the group of participants who were classified as normal or underweight based on their weight and height, 20% had excessive adiposity. It is of relevance for health because adiposity among individuals with normal weight, NWA, was associated with less favourable cardiovascular risk factor profile. That higher percentage of body fat at normal body weight is associated with metabolic disorders has also been demonstrated in other populations 6, 11, 12, 13, 27, 28, 29, 30, 31. The predictive ability of BMI as continuous variable for cardiovascular risk factors seems to be more accurate than merely categorising as overweight or not. Thus, relying on the dichotomous BMI classification of healthy weight might be misleading in the clinic as well as in research. Consequently, the present study supports the suggestion by St‐Onge et al. that screening for metabolic abnormalities in individuals with normal weight is important in the prevention of diabetes and cardiovascular disease 32.
However, BMI as an indicator of adiposity as well as simple measures of the distribution of fat to the abdomen, like waist circumference or waist‐to‐height ratio, might be used as early indicators of cardiovascular risk 33, 34, 35. Even if the present result indicates that BMI cut‐off values underestimate adiposity, it has high specificity to detect excessive body fat when BMI is ≥30 kg/m2. This is in line with the conclusion of a meta‐analysis 36 of studies in different populations, on sensitivity and specificity of BMI to identify excessive body adiposity. In fact, in the present population, only 7% of the participants with overweight had a body percentage of fat below cut‐off. Moreover, in contrast to previous analyses by Romero‐Corral 37, BMI correlated much stronger with body fat than lean body mass (data not shown). Average body fat, BMI, hip and waist were also significantly higher in the OWL group compared with the NWL, and the mean HDL cholesterol was lower, and the apoB/apoA‐I ratio and serum triglycerides were higher compared with the NWL group (Tables 2 and 3). The situation might, however, be otherwise for a population with high fitness status. Furthermore, there are individual differences, and subjects are more or less susceptible to development of metabolic disturbance because of obesity 38. Research has even suggested that a metabolically healthy obese phenotype, which is protected from increased cardiometabolic risk, may exist 39, 40. A high BMI alone might even be protective in patients with coronary artery disease, while central obesity is associated with risk of mortality 41. Thus, knowledge for phenotyping of obesity is needed 4.
Even if the prevalence of NWA and the patterns of associations with cardiovascular risk factors were rather similar for men and women in the present study, gender differences existed. NWA was associated with higher blood pressure and lower HDL cholesterol in men but not women, and the relations between NWA and cardiovascular risk factors were in general somewhat stronger for men than women. In women, all the associations became weaker or disappeared when adjusting for BMI or waist circumference, while most associations remained significant in men. Another striking gender aspect in this Swedish population was that only one‐quarter of the men were in the NWL group, while among women, 44% were categorised as NWL.
A weakness is our reliance on bioelectrical impedance to estimate percentage body fat. Furthermore, the cut‐off points for percentage of body fat are arbitrarily chosen. The different proposed upper limits have varied between 30% and 37% for women, and 20% and 25% for men 22. While the definitions of healthy weight and BMI categories of obesity are based on empirical data, there is not enough evidence for defining corresponding cut‐offs for healthy percentage of body fat and degree of adiposity. Therefore, it is a strength that we also compared body fat percentage as continuous variable with overweight, high waist circumference and BMI as predictors of cardiovascular risk factors.
In conclusion, excess body fat was associated with less favourable cardiovascular risk factor profile even in those without overweight. Thus, the established definition of a healthy weight might result in underestimation of risk in persons with normal weight. This is of importance because the prevalence of NWA was 9% in the present population. However, even if specific BMI values or percentage of body fat have better ability to predict cardiometabolic risk factors, it is not possible to determine the cardiovascular risk. Thus, the results suggest that screening for metabolic risk factors is also relevant in the upper end of the normal weight BMI category.
Conflict of interest statement
No Conflict of Interest Statement.
Acknowledgements
This study was supported by grants from the Västra Götaland County Council, the Swedish Council for Working life and Social Research, the Swedish Research Council, the Swedish Heart and Lung Foundation and the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning.
Berg, C. , Strandhagen, E. , Mehlig, K. , Subramoney, S. , Lissner, L. , and Björck, L. (2015) Normal weight adiposity in a Swedish population: how well is cardiovascular risk associated with excess body fat captured by BMI?. Obesity Science & Practice, 1: 50–58. doi: 10.1002/osp4.4.
References
- 1. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all‐cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta‐analysis. JAMA 2013; 309: 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruderman NB, Schneider SH, Berchtold P. The “metabolically‐obese,” normal‐weight individual. Am J Clin Nutr 1981; 34: 1617–21. [DOI] [PubMed] [Google Scholar]
- 3. Karelis AD, St‐Pierre DH, Conus F, Rabasa‐Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab 2004; 89: 2569–75. [DOI] [PubMed] [Google Scholar]
- 4. Ahima RS, Lazar MA. Physiology. The health risk of obesity—better metrics imperative. Science 2013; 341: 856–8. [DOI] [PubMed] [Google Scholar]
- 5. Thomas EL, Frost G, Taylor‐Robinson SD, Bell JD. Excess body fat in obese and normal‐weight subjects. Nutr Res Rev 2012; 25: 150–61. [DOI] [PubMed] [Google Scholar]
- 6. Romero‐Corral A, Somers VK, Sierra‐Johnson J, et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J 2009; 31: 737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord 1998; 22: 1164–71. [DOI] [PubMed] [Google Scholar]
- 8. Marques‐Vidal P, Pecoud A, Hayoz D, et al. Prevalence of normal weight obesity in Switzerland: effect of various definitions. Eur J Nutr 2008; 47: 251–7. [DOI] [PubMed] [Google Scholar]
- 9. Marques‐Vidal P, Chiolero A, Paccaud F. Large differences in the prevalence of normal weight obesity using various cut‐offs for excess body fat. Eur E‐J Clin Nut Metabolism 2008; 3: e159–e62. [Google Scholar]
- 10. Mannisto S, Harald K, Kontto J, et al. Dietary and lifestyle characteristics associated with normal‐weight obesity: the National FINRISK 2007. Study. Br J Nutr 2013: 1–8. [DOI] [PubMed] [Google Scholar]
- 11. De Lorenzo A, Del Gobbo V, Premrov MG, Bigioni M, Galvano F, Di Renzo L. Normal‐weight obese syndrome: early inflammation? Am J Clin Nutr 2007; 85: 40–5. [DOI] [PubMed] [Google Scholar]
- 12. Di Renzo L, Galvano F, Orlandi C, et al. Oxidative stress in normal‐weight obese syndrome. Obesity (Silver Spring) 2010; 18: 2125–30. [DOI] [PubMed] [Google Scholar]
- 13. De Lorenzo A, Martinoli R, Vaia F, Di Renzo L. Normal weight obese (NWO) women: an evaluation of a candidate new syndrome. Nutr Metab Cardiovasc Dis 2006; 16: 513–23. [DOI] [PubMed] [Google Scholar]
- 14. Marques‐Vidal P, Pecoud A, Hayoz D, et al. Normal weight obesity: relationship with lipids, glycaemic status, liver enzymes and inflammation. Nutr Metab Cardiovasc Dis 2010; 20: 669–75. [DOI] [PubMed] [Google Scholar]
- 15. Gomez‐Ambrosi J, Silva C, Galofre JC, et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes (Lond) 2012; 36: 286–94. [DOI] [PubMed] [Google Scholar]
- 16. Berg CM, Lissner L, Aires N, et al. Trends in blood lipid levels, blood pressure, alcohol and smoking habits from 1985 to 2002: results from INTERGENE and GOT‐MONICA. Eur J Cardiovasc Prev Rehabil 2005; 12: 115–25. [DOI] [PubMed] [Google Scholar]
- 17. Strandhagen E, Berg C, Lissner L, et al. Selection bias in a population survey with registry linkage: potential effect on socioeconomic gradient in cardiovascular risk. Eur J Epidemiol 2010; 25: 163–72. [DOI] [PubMed] [Google Scholar]
- 18. WHO . Obesity, Preventing and Managing the Global Epidemic. World Health Organisation: Geneva, 2000. [PubMed] [Google Scholar]
- 19. Heitmann BL. Prediction of body water and fat in adult Danes from measurement of electrical impedance. A validation study. Int J Obes 1990; 14: 789–802. [PubMed] [Google Scholar]
- 20. Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr 2000; 72: 694–701. [DOI] [PubMed] [Google Scholar]
- 21. Zhu S, Wang Z, Shen W, Heymsfield SB, Heshka S. Percentage body fat ranges associated with metabolic syndrome risk: results based on the third National Health and Nutrition Examination Survey (1988–1994). Am J Clin Nutr 2003; 78: 228–35. [DOI] [PubMed] [Google Scholar]
- 22. Oliveros E, Somers VK, Sochor O, Goel K, Lopez‐Jimenez F. The concept of normal weight obesity. Prog Cardiovasc Dis 2014; 56: 426–33. [DOI] [PubMed] [Google Scholar]
- 23. Perk J, De Backer G, Gohlke H, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Atherosclerosis 2012; 223: 1–68. [DOI] [PubMed] [Google Scholar]
- 24. Walldius G, Jungner I. The apoB/apoA‐I ratio: a strong, new risk factor for cardiovascular disease and a target for lipid‐lowering therapy—a review of the evidence. J Intern Med 2006; 259: 493–519. [DOI] [PubMed] [Google Scholar]
- 25. National Institutes of health . Third report of National Cholesterol Education Programme Expert Panel on Detection, Evaluation and Treatment of High blood Cholesterol in Adults (Adult treatment Panel III). National Institute of Health: Bethesda, 2001. [Google Scholar]
- 26. Bland JM, Altman DG. Transformations, means, and confidence intervals. BMJ 1996; 312: 1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim MK, Han K, Kwon HS, et al. Normal weight obesity in Korean adults. Clin Endocrinol (Oxf) 2014; 80: 214–20. [DOI] [PubMed] [Google Scholar]
- 28. Batsis JA, Sahakyan KR, Rodriguez‐Escudero JP, Bartels SJ, Somers VK, Lopez‐Jimenez F. Normal weight obesity and mortality in United States subjects >/=60 years of age (from the Third National Health and Nutrition Examination Survey). Am J Cardiol 2013; 10: 1592–8. [DOI] [PubMed] [Google Scholar]
- 29. Shea JL, King MT, Yi Y, Gulliver W, Sun G. Body fat percentage is associated with cardiometabolic dysregulation in BMI‐defined normal weight subjects. Nutr Metab Cardiovasc Dis 2012; 22: 741–7. [DOI] [PubMed] [Google Scholar]
- 30. Tanaka S, Togashi K, Rankinen T, et al. Is adiposity at normal body weight relevant for cardiovascular disease risk? Int J Obes Relat Metab Disord 2002; 26: 176–83. [DOI] [PubMed] [Google Scholar]
- 31. Madeira FB, Silva AA, Veloso HF, et al. Normal weight obesity is associated with metabolic syndrome and insulin resistance in young adults from a middle‐income country. PLoS One 2013; 8(3): e60673. doi:10.1371/journal.pone.0060673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. St‐Onge MP, Janssen I, Heymsfield SB. Metabolic syndrome in normal‐weight Americans: new definition of the metabolically obese, normal‐weight individual. Diabetes Care 2004; 27: 2222–8. [DOI] [PubMed] [Google Scholar]
- 33. Abbasi F, Blasey C, Reaven GM. Cardiometabolic risk factors and obesity: does it matter whether BMI or waist circumference is the index of obesity? Am J Clin Nutr 2013; 98: 637–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ashwell M, Gunn P, Gibson S. Waist‐to‐height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta‐analysis. Obes Rev 2012; 13: 275–86. [DOI] [PubMed] [Google Scholar]
- 35. Huxley RR, Jacobs DR, Jr. Size still matters…but not in the way we once thought. Lancet 2011; 377: 1051–2. [DOI] [PubMed] [Google Scholar]
- 36. Okorodudu DO, Jumean MF, Montori VM, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta‐analysis. Int J Obes (Lond) 2010; 34: 791–9. [DOI] [PubMed] [Google Scholar]
- 37. Romero‐Corral A, Somers VK, Sierra‐Johnson J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 2008; 32: 959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bastien M, Poirier P, Lemieux I, Despres JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis 2014; 56: 369–81. [DOI] [PubMed] [Google Scholar]
- 39. Phillips CM. Metabolically healthy obesity: definitions, determinants and clinical implications. Rev Endocr Metab Disord 2013; 14: 219–27. [DOI] [PubMed] [Google Scholar]
- 40. Plourde G, Karelis AD. Current issues in the identification and treatment of metabolically healthy but obese individuals; Nutr Metab Cardiovasc Dis: 2014. [DOI] [PubMed] [Google Scholar]
- 41. Coutinho T, Goel K, Correa de Sa D, et al. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: role of “normal weight central obesity”. J Am Coll Cardiol 2013; 61: 553–60. [DOI] [PubMed] [Google Scholar]


