Abstract
The attributes of specificity and memory enable CD8+ T cells to provide long-lasting protection against a variety of challenges. Although, the importance of CD8+ T cells for protection against intracellular infections and transformation is well-established, the functional type; effector phenotypes (Tc1, Tc2, Tc17 and/or Tcreg) and/or memory (effector or central), of CD8+ T cells most desirable for tumor immunity is not established. To determine the tumor efficacy of various effector types and/or memory CD8 T cells, it is imperative to better understand intrinsic and extrinsic factors that regulate CD8+ T cell differentiation and use this information to generate and test distinct functional cell types in tumor models. The focus of our laboratory investigations is to identify the extrinsic factors such as antigen strength, co-stimulatory molecules, cytokines, and small molecule modifiers that regulate intrinsic programs for various effector and/or memory cell fate in antigen specific CD8 T cells. The use of this information to generate immunity in murine tumor models has facilitated development of new adoptive cell transfer (ACT) as well as immunization strategies for cancer treatment.
Keywords: CD8+ T cell, Cytokines, Transcriptional regulators, Effector and memory cell fate, Adoptive cell transfer and tumor immunity
Introduction
CD8+ T cells are an essential part of the adaptive immune system that control infection by intracellular pathogens and malignant transformation [1, 2]. Their inherent ability to recognize peptides presented by MHC class-I molecules expressed on most nucleated cells, less stringency for requiring co-stimulation, and direct cytolysis of antigen expressing target cells endows them with the unique ability to survey the host for intracellular perturbations and restore homeostasis. Naïve CD8 T cells upon stimulation with cognate antigen/MHC class I molecule, co-stimulatory molecules like B7.1 and/or LFA-1 in the presence of variety of cytokines like IL-12, type 1 interferon and/or gamma chain cytokine; IL-2, IL-21, undergo full activation leading to proliferation and effector functions designed to eradicate the challenge posed [3, 4]. At the peak of the primary response, the clonal expansion undergoes a precipitous contraction phase wherein majority of the induced effector CD8 T cells die due to activation induced cell death (AICD) by apoptosis and a small fraction survive as memory cells [5–7]. Apart from their ability to persist, memory CD8 T cells also possess the ability to rapidly and vigorously respond to a secondary antigen challenge whereby providing deterrence against recurrence of disease [8, 9].
Over the past decade, studies have demonstrated the ability of type I effector T cells (both CD4+ and CD8+ that produce IFN-γ) to be therapeutically beneficial against intracellular infections caused by viruses and bacteria [10–12]. This understanding has been exploited for immunization and/or adoptive cell therapy of cancer with encouraging results [13, 14], but have fallen short of achieving eradication of solid tumors [15]. The inability of adoptively transferred effector CD8+ T cells to persist and promote durable antitumor immunity is thought to be the major reason for their restricted efficacy [16, 17]. Therefore, it is increasingly evident that along with generation of robust effectors cells, it may be essential to generate memory T cells that have the capacity to persist and guard the host against tumor challenge. A few recent reports and our data suggest that memory precursor CD8 T cells are much more effective than robust effector CD8 T cells in mediating long-term tumor immunity [18], (Rao et. al. manuscript under review). However, the mechanisms that determine whether an antigen-stimulated CD8 T cell will undergo robust effector maturation leading to terminal differentiation or will it transition into memory are poorly understood and pose significant hurdles for generating durable immunity against tumors. In our laboratory investigations, we use naïve TCR transgenic CD8 T cells which are reacted with latex beads with defined antigen, co-stimulation and cytokines and the intrinsic signaling pathways, transcriptional factors and gene expression profiles are characterized and evaluated for their ability to determine effector and/or memory cell fate. In this review, we highlight recent insights generated into the mechanisms used by extracellular cues to program effector and/or memory cell fate in naïve CD8 T cells.
Instructing CD8 T cell for effector and memory development
The functional fate of CD8 T cells is influenced by the instructions provided during brief period of antigen stimulation [19–21]. The nature and intensity of signals received by a naïve CD8 T cell during antigen stimulation regulates induction of gene programs that determine various effector phenotypes and/or memory [22, 23]. Typically, to achieve functional maturation a naïve CD8 T cell must integrate signals received from the TCR, co-stimulatory molecules and cytokine receptors for activation and proliferation [24–26]. The cytokine milieu in which a naïve CD8 T cell recognizes antigen influences the gene programs induced for distinct functional effector outcomes and/or memory, e.g., IL-12 induced T-bet expression for type I, IL-4 induced GATA-3 for type II, IL-23 induced RORγt for type 17, IL-2 induced Foxp3 for Treg etc. [27]. The ability of inflammatory cytokine; IL-12, to generate T-bet dependent robust type 1 effector maturation as well as enable CD8 T cells for long-term response is well established [28, 29]. However, the mechanisms by which T-bet facilitates transition of type 1 effector CD8 T cells for memory is not well understood. For this review, we will focus on factors regulating type I effector and memory cell fate in CD8 T cells, since type I associated cytokines have been shown to be necessary for antitumor immunity. The following section will specifically focus on how instructions received by a naïve CD8 T cell regulate intrinsic gene programs to regulate fate of a CD8 T cell.
Cell intrinsic regulators of CD8 T cell differentiation
Antigen recognition by a naïve CD8 T cell leads to progressive changes in gene expression and it is now well recognized that the cytokine milieu in which the T cell gets activated has a significant impact on what kind of effector phenotype it will express and whether it will survive to form memory. The differential ability of cytokines to induce various transcriptional networks, commonly known as master regulators of cell fate is important to induce gene programs that impart specific functions to that subset [30–32]. Recent studies have identified a unique pattern of gene expression between the effector and memory subset of CD8 T cells and some of these genes are conserved among effector and memory CD8 T cell subsets [28]. These observations indicate that understanding the regulation of transcriptional networks that program a naïve CD8 T cell for effector and memory differentiation is essential to shed light on mechanism of regulation of these subsets. Moreover, identification of cell intrinsic factors that govern the interplay between transcription factors to impart effector versus memory cell fate in CD8 T cells also remains to be understood.
T-bet and Eomesodermin
T-bet, a member of the T-box family of proteins is the master regulator of type I differentiation in both CD4 as well as CD8 T cells [30]. T-bet expression in CD8 T cells is required for IFN-γ gene expression and also in part for the expression of cytolytic genes (Granzyme-B and Perforin) [33]. It has been previously shown that robust T-bet expression marks CD8 T cells for short-lived effector differentiation, which is associated with the KLRG1hi and CD127lo phenotype [23]. Subsequently, Eomesodermin; a member of the T-box family of transcriptional factors, has also been implicated in CD8 T cell differentiation [34]. Unlike T-bet, the nature and intensity of stimulus that induces Eomesodermin expression in CD8 T cell are not well understood, but there exists an inverse corelation of expression between these two transcription factors; wherein, T-bet expression was observed to be maximal during effector phase whereas Eomes expression increased from the effector to memory phases of a CD8 T cell response [35]. Moreover, IL-12 induces sustained T-bet expression and represses Eomes transcription, consistent with the notion that IL-12 promotes robust, short-lived effector cells whereas Eomes is important for generation of memory CD8 T cells [28]. However, both of these transcription factors play an important role in memory CD8 T cell generation by regulation of CD122 expression; IL-15Rβ which renders CD8 T cells sensitive to IL-15 [35]. The differential expression and function of these transcription factors during effector and memory stages indicate the important role they play in inducing and sustaining gene programs that regulate effector and/or memory CD8 T cell differentiation.
mTOR (mammalian target of rapamycin)
The mTOR kinase senses environmental cues, nutrient availability, internal energy stores, and growth factor signaling for regulating cell growth, proliferation and differentiation [36, 37]. Due to its ability to integrate extracellular signals and regulate cell fate, we have been particularly interested in understanding its role in regulating functional cell fate of CD8 T cells. Previously, mTOR inhibition (rapamycin) was shown to induce tolerance in CD4 T cells and some studies have suggested the induction of Foxp3+ Treg phenotype in CD4 T cells [38–41]. A recent report employing genetic approaches demonstrated the critical requirement for mTOR in type I differentiation of CD4 T cells and demonstrated that mTOR deficient CD4+ T cells differentiate into T regulatory cells, at the cost of type I commitment [42]. Moreover, blockade of mTOR by rapamycin administration in vivo has been shown to enhance memory CD8+ T cell generation in a viral infection model [43]. Our studies have demonstrated that instructions received by a naïve CD8+ T cell induces mTOR activity and it plays an essential role in regulating T-bet versus Eomesodermin expression for establishing effector and memory cell fate in CD8+ T cells (Manuscript submitted). In these studies, we have consistently noted that factors that impart type I effector cell fate in CD8+ T cells, enhance and sustain mTOR activity which is required for sustained T-bet expression and type I effector differentiation in CD8+ T cells. Decreased mTOR activity switches the transcriptional program from T-bet to Eomes expression along with phenotype associated with memory-precursor cells; wherein, these cells demonstrate enhanced persistence and antigen recall responses after adoptive transfer. Remarkably, rapamycin treated CD8+ T cells with diminished type I effector functions and enhanced memory-like attributes, demonstrate significantly enhanced ability to control tumor growth. These novel observations implicate mTOR as a central integrator of instructions that regulate cell-fate decisions in CD8 T cells and imply that its use may greatly benefit tumor therapy. Moreover, since mTOR is regulated by the energy state of the cell via AMPK activity, we have initiated studies to better understand the possible impact of host energy imbalance due to states of obesity, aging, and/or starvation on immune surveillance and therapeutic interventions. However, more studies to delineate the mechanisms of its action in vivo are warranted, as they are likely to enable its use for augmenting durable tumor immunity with immunization.
Extrinsic regulators of CD8 T cell differentiation
The regulation of various gene programs that control survival, differentiation, and function is all tightly regulated by the nature and intensity of cues received by a naïve CD8+ T cell [3, 27]. Cognate antigen recognition by the TCR is the primary and essential trigger that itself can determine fate of a naïve CD8+ T cell [22]. How factors other than the antigen recognition/strength influence the functional outcome of a CD8+ T cell is being increasingly studied and understood. In this section, we will discuss the impact of these signals in the effector and/or memory development of CD8+ T cells.
TCR signal strength
Strength of antigen signal induces activation and proliferation of naïve T cells and by regulating survival and cytokine responsiveness play a deterministic role in effector versus memory generation of CD8+ T cells. Previous studies have revealed the critical role played by the strength of signal 1 on the antigen-specific clonal T cell response, i.e., treating the infection with antibiotics (duration) or decreasing the multiplicity of infection (dose), as lower levels of antigen strength promoted memory-precursor formation, as indicated by high levels of CD127 and CD62L expression [22, 44, 45]. In support, a recent study also demonstrated that increasing strength of antigen stimulation by varying infectious insults in vivo promotes high levels of T-bet expression and robust effector maturation, but as the cells were short-lived, they failed to afford durable protection [23]. To better understand how strength of antigen stimulation influences naïve CD8+ T cell response, we have demonstrated that antigen strength regulates Ets-1 dependent IL-12Rβ expression, which sensitizes a naïve CD8+ T cell for IL-12 responsiveness (Li et. al. Manuscript under review). This further explains how increasing strength of antigen favors effector rather than memory development in CD8+ T cells. Collectively, these observations demonstrate that the dose and duration of antigen is a critical factor that regulates the balance between effector and memory development, but it is still unclear as to how a naive CD8+ T cell senses the extent of antigen stimulation to regulate gene programs for effector versus memory development of CD8+ T cells.
Co-stimulatory molecules
The co-stimulatory signals (signal 2) from receptors such as CD28, CD40, 4-1BB, CD27, and OX40, are required for naive T cell activation that results in proliferation, effector maturation, and survival [46–49]. Activation of T cells without co-stimulation may lead to T cell anergy, T cell deletion, or the development of immune tolerance by ignorance [50]. It remains unclear how those co-stimulatory signals instruct memory development; although a recent study demonstrated that memory CD8 T cells require CD28 co-stimulation to generate maximal secondary responses against pathogens. Moreover, the TNF-members; including OX-40 [51–53], 4-1BB [54, 55], and CD40 [56, 57], can provide an essential signal to antigen stimulated T cells for persistence and memory generation. In our studies, we choose to maintain constant the co-stimulatory signal by providing a fixed amount of B7.1 protein.
CD4 T cell help
The CD4 T cell help for effector and memory development of CD8+ T cell can essentially be argued from two different views. First, for functional effector maturation, which for most part requires the pro-inflammatory cytokine IL-12, can be derived from the CD40–CD40L interaction between CD4+ T cell and dendritic cell wherein the latter is stimulated to produce IL-12 [58]. This could also possibly explain the requirement of early CD4+ T cell help for CD8 T cell priming [59]. Second, the unavailability of CD4+ T cell help leads to generation of a population of CD8+ T cells that largely resembles effector–memory population, responds poorly to re-challenge and are not long-lived [29, 60]. This might probably be attributed to the contribution of IL-2 and/or IL-21 secreted by activated CD4+ T cells which has been shown to be required for durable immune responses [61, 62]. Overall, CD4+ T cells appear to have the capability to modulate effector and memory differentiation of CD8+ T cells, possibly through mechanisms that include CD40:CD40L, cytokines like IL-12, IL-21 and regulation of T-bet expression in CD8+ T cells. The recent demonstration that CD4+/Foxp3+ Treg cells are required for eradication of viral infection by CD8+ T cells [63], suggest new ways by which the CD4+ T cells regulate CD8+ T cell effector and memory cell fate.
Common γ-chain cytokines
IL-2
IL-2 has been recognized as a growth factor for T cells as IL-2 is produced mainly by activated T cells and promotes proliferation of T cells. The role of this cytokine has been difficult to implicate as IL-2-deficient mice develop autoimmune disorders due to a deficiency in CD4+ Foxp3+ Tregs [64]. However, by using an adoptive transfer system with IL-2 or IL-2R-deficient TCR transgenic CD8+ T cells, D’Souza et al. [65, 66] showed that activated CD8+ T cells initially undergo IL-2-independent proliferation, but sustained expansion required IL-2 production. More recently studies by Williams et al. [8] have confirmed that IL-2 signaling though not required during the primary response of CD8+ T cells was required for CD8+ T cell memory generation as in the absence of IL-2 signaling their recall responses were considerably impaired.
IL-7 and IL-15
Although IL-7 and/or IL-15 have probably no instructive role in the generation of short or long-lived memory cells [67, 68], both IL-7 and IL-15 are important for homeostatic self-renewal of memory cells [69–71]. The lack of either of these two cytokines leads to loss of CD8 memory generation [72, 73]. In this regard, it seems like IL-7 is required for the long-term survival of CD8 memory cells, whereas IL-15 supports basal homeostatic proliferation and is required for survival of naïve as well as memory CD8+ T cells [74–76], suggesting that they together support the survival and maintenance of the memory CD8+ T cell pool.
IL-21
IL-21 is produced by activated CD4+ T cells [77]. IL-21 has the ability to sustain long-term CD8+ T cell responses as a result of increased survival and also enhances the capacity of cells to mediate tumor regression upon adoptive transfer [61, 78]. Interestingly, IL-21 promotes proliferation of antigen-specific CD8+ T cells and synergizes with IL-15 in promoting CD8+ T cells expansion in vitro and their anti-tumor effects in vivo [79]. The use of IL-21 as single agent therapeutic for cancer has produced encouraging results and is currently poised for phase three evaluation in melanoma and renal cell carcinoma [80].
Other cytokines
IL-12
IL-12 is a well studied inflammatory cytokine produced by dendritic cells (DCs) and macrophages in response to Toll-like receptor signaling. The role of this cytokine in programming robust type I CD8+ T cell effector differentiation has been well documented [81]. Although, IL-12 provides an essential third signal for acquisition of full effector functions [82], its role in generation of CD8 memory pool remains unclear. We and others have shown that IL-12 signaling during the priming phase promotes long-term CD8+ T cell responses [81]. In another report, however, Pearce et al. [83] shown that greater numbers of memory CD8+ T cells formed in the IL-12−/− mice and they underwent more extensive homeostatic proliferation than in wild type animals. Nevertheless, recent studies addressed the mechanisms by which IL-12 regulates transition and turnover of memory CD8+ T cells. In these studies, IL-12 has emerged as a key factor of regulation, which determines the levels of T-bet and Eomes expression and thereby controls effector versus memory cell formation. They demonstrated that IL-12 inversely regulates the expression of T-bet and Eomes during a CD8+ T-cell response, with IL-12 promoting the expression of T-bet while suppressing the expression of Eomes [28]. Moreover, Joshi et al. [23] provided further support for the role of IL-12 and T-bet in CD8+ T cell effector differentiation. Based on these studies, a model of CD8+ T cell effector/memory differentiation has been proposed, wherein antigen activated CD8+ T cells proliferate and differentiate into effector cells. The amount of IL-12 determines the balance between T-bet and Eomes expression and correspondingly makes cell fate decisions [84]. In our lab, we found that IL-12 enhanced T-bet expression programs CD8+ T cells for not only robust effector response but enables them for IL-15 mediated transition to memory (Li et. al. manuscript under review). The ability of IL-12 conditioned T-bet expression to “tune” effector CD8+ T cells for sensitivity for IL-15 by increasing their IL-15Rα and CD122 expression, promotes IL-15 mediated survival and homeostatic renewal.
IFN-α/β
Type I IFNs (IFN-α/β) is a common cytokine produced in response to infection. Early studies shown that IFN-α/β signaling is critical for enhancing the activation, proliferation, expansion, and memory generation of CD8+ T cells [85–87]. Studies have also suggested that IFN-α/β signaling could also provide the necessary third signal to support CD8 clonal expansion and development of effector function via a STAT4-dependent pathways to stimulate survival, development of cytolytic function, and production of IFN-γ [88]. Both IL-12 and IFN-α/β as the third signal initiate many in common as well as a unique complex differentiation program for CD8+ T cell memory generation [3].
Concluding remarks
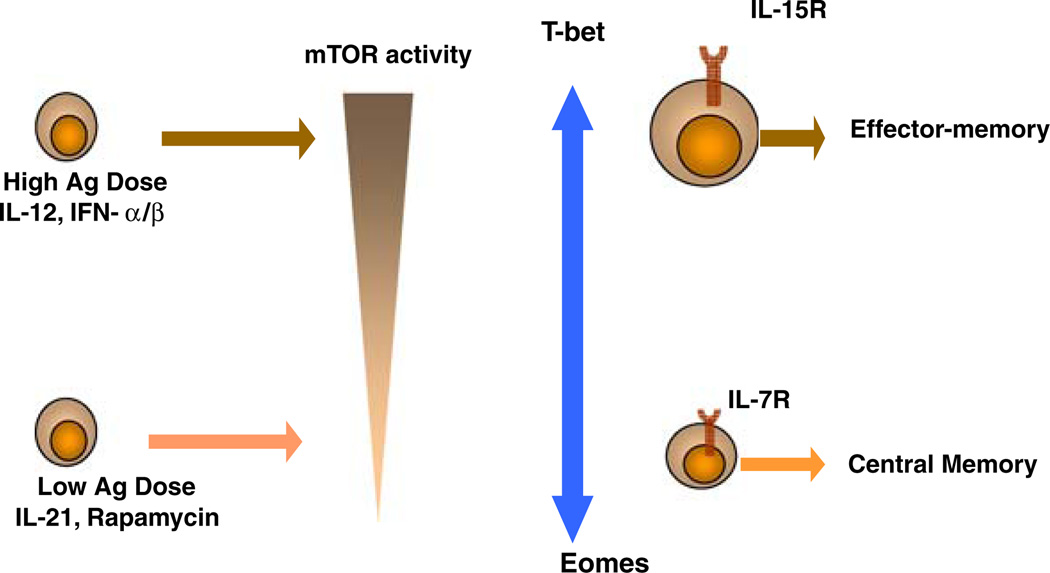
A CD8+ T cell response is programmed very early during the priming phase of an immune response. Therefore, it is essential to have an understanding of various extrinsic and intrinsic factors that regulate effector and memory differentiation of CD8+ T cells. With the recent identification of fate deterministic cytokines and transcription factors it is becoming increasingly necessary to study the mechanism by which extrinsic factors integrate into a cell and induce gene programs that specify cell fate of CD8+ T cells. The role of mTOR in integrating extracellular instructions and more importantly sensing the extent of inflammation to direct effector versus memory cell fate is becoming evident and detailed mechanistic studies are required to delineate how mTOR regulates transcriptional programs depending upon the status of its phosphorylation (Fig. 1).
Fig. 1.
Model for the role of mTOR in sensing extracellular signals and regulating gene programs for effector and memory differentiation of CD8+ T cells. Increasing dose of antigen along with inflammatory cytokines promotes heightened mTOR activity leads to sustained T-bet expression. Low dose antigen and/or IL-21, rapamycin induce low levels of mTOR activity leading to decreased mTOR phosphorylation and increased Eomes expression. CD8+ T cells expressing high levels of T-bet are dependent on IL-15 for their survival and become effector memory like cells, whereas cells expressing Eomes are intrinsically programmed for central memory-like cells which is IL-7 dependent.
Despite all the progress made in recent years, we still lack a clear understanding of how extrinsic factors regulate intrinsic molecular programs for CD8+ T cell differentiation into effector and/or memory functional fates. By revealing the molecular basis by which extrinsic factors govern optimal effector and memory cell fates in CD8 T cells, we expect to develop new treatment modalities for acute and chronic challenges such as infectious diseases and cancer.
References
- 1.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moskophidis D, Kioussis D. Contribution of virus-specific CD8+ cytotoxic T cells to virus clearance or pathologic manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J Exp Med. 1998;188:223–232. doi: 10.1084/jem.188.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 4.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, et al. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- 5.Williams MA, Holmes BJ, Sun JC, Bevan MJ. Developing and maintaining protective CD8+ memory T cells. Immunol Rev. 2006;211:146–153. doi: 10.1111/j.0105-2896.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 6.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 8.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sprent J, Surh CD. T cell memory. Annu Rev Immunol. 2002;20:551–579. doi: 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- 10.Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 11.Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, et al. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 13.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 20.Bevan MJ, Fink PJ. The CD8 response on autopilot. Nat Immunol. 2001;2:381–382. doi: 10.1038/87676. [DOI] [PubMed] [Google Scholar]
- 21.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 23.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation and maintenance. Curr Opin Immunol. 2007;19:315–319. doi: 10.1016/j.coi.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 27.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 28.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 29.Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, et al. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 31.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 33.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 34.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 35.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 36.Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 37.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, et al. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 39.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162:2775–2784. [PubMed] [Google Scholar]
- 40.Kang J, Huddleston SJ, Fraser JM, Khoruts A. De novo induction of antigen-specific CD4+ CD25+ Foxp3+ regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. J Leukoc Biol. 2008;83:1230–1239. doi: 10.1189/jlb.1207851. [DOI] [PubMed] [Google Scholar]
- 41.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+ Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bullock TN, Colella TA, Engelhard VH. The density of peptides displayed by dendritic cells affects immune responses to human tyrosinase and gp100 in HLA-A2 transgenic mice. J Immunol. 2000;164:2354–2361. doi: 10.4049/jimmunol.164.5.2354. [DOI] [PubMed] [Google Scholar]
- 45.Wherry EJ, Puorro KA, Porgador A, Eisenlohr LC. The induction of virus-specific CTL as a function of increasing epitope expression: responses rise steadily until excessively high levels of epitope are attained. J Immunol. 1999;163:3735–3745. [PubMed] [Google Scholar]
- 46.Pardigon N, Bercovici N, Calbo S, Santos-Lima EC, Liblau R, Kourilsky P, et al. Role of co-stimulation in CD8+ T cell activation. Int Immunol. 1998;10:619–630. doi: 10.1093/intimm/10.5.619. [DOI] [PubMed] [Google Scholar]
- 47.Borowski AB, Boesteanu AC, Mueller YM, Carafides C, Topham DJ, Altman JD, et al. Memory CD8+ T cells require CD28 costimulation. J Immunol. 2007;179:6494–6503. doi: 10.4049/jimmunol.179.10.6494. [DOI] [PubMed] [Google Scholar]
- 48.Laderach D, Movassagh M, Johnson A, Mittler RS, Galy A. 4–1BB co-stimulation enhances human CD8(+) T cell priming by augmenting the proliferation and survival of effector CD8(+) T cells. Int Immunol. 2002;14:1155–1167. doi: 10.1093/intimm/dxf080. [DOI] [PubMed] [Google Scholar]
- 49.Grewal IS, Foellmer HG, Grewal KD, Xu J, Hardardottir F, Baron JL, et al. Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science. 1996;273:1864–1867. doi: 10.1126/science.273.5283.1864. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 51.Ruby CE, Redmond WL, Haley D, Weinberg AD. Anti-OX40 stimulation in vivo enhances CD8+ memory T cell survival and significantly increases recall responses. Eur J Immunol. 2007;37:157–166. doi: 10.1002/eji.200636428. [DOI] [PubMed] [Google Scholar]
- 52.Salek-Ardakani S, Croft M. Regulation of CD4 T cell memory by OX40 (CD134) Vaccine. 2006;24:872–883. doi: 10.1016/j.vaccine.2005.07.108. [DOI] [PubMed] [Google Scholar]
- 53.Maxwell JR, Weinberg A, Prell RA, Vella AT. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J Immunol. 2000;164:107–112. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi C, Mittler RS, Vella AT. Cutting edge: 4–1BB is a bona fide CD8 T cell survival signal. J Immunol. 1999;162:5037–5040. [PubMed] [Google Scholar]
- 55.Lee HW, Park SJ, Choi BK, Kim HH, Nam KO, Kwon BS. 4–1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol. 2002;169:4882–4888. doi: 10.4049/jimmunol.169.9.4882. [DOI] [PubMed] [Google Scholar]
- 56.Maxwell JR, Campbell JD, Kim CH, Vella AT. CD40 activation boosts T cell immunity in vivo by enhancing T cell clonal expansion and delaying peripheral T cell deletion. J Immunol. 1999;162:2024–2034. [PubMed] [Google Scholar]
- 57.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 58.Stuber E, Strober W, Neurath M. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996;183:693–698. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang JC, Livingstone AM. Cutting edge: CD4+ T cell help can be essential for primary CD8+ T cell responses in vivo. J Immunol. 2003;171:6339–6343. doi: 10.4049/jimmunol.171.12.6339. [DOI] [PubMed] [Google Scholar]
- 60.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 61.Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J Immunol. 2004;173:900–909. doi: 10.4049/jimmunol.173.2.900. [DOI] [PubMed] [Google Scholar]
- 62.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 65.D’Souza WN, Lefrancois L. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J Immunol. 2003;171:5727–5735. doi: 10.4049/jimmunol.171.11.5727. [DOI] [PubMed] [Google Scholar]
- 66.D’Souza WN, Schluns KS, Masopust D, Lefrancois L. Essential role for IL-2 in the regulation of antiviral extralymphoid CD8 T cell responses. J Immunol. 2002;168:5566–5572. doi: 10.4049/jimmunol.168.11.5566. [DOI] [PubMed] [Google Scholar]
- 67.Haring JS, Jing X, Bollenbacher-Reilley J, Xue HH, Leonard WJ, Harty JT. Constitutive expression of IL-7 receptor alpha does not support increased expansion or prevent contraction of antigen-specific CD4 or CD8 T cells following Listeria monocytogenes infection. J Immunol. 2008;180:2855–2862. doi: 10.4049/jimmunol.180.5.2855. [DOI] [PubMed] [Google Scholar]
- 68.Klonowski KD, Williams KJ, Marzo AL, Lefrancois L. Cutting edge: IL-7-independent regulation of IL-7 receptor alpha expression and memory CD8 T cell development. J Immunol. 2006;177:4247–4251. doi: 10.4049/jimmunol.177.7.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yajima T, Yoshihara K, Nakazato K, Kumabe S, Koyasu S, Sad S, et al. IL-15 regulates CD8+ T cell contraction during primary infection. J Immunol. 2006;176:507–515. doi: 10.4049/jimmunol.176.1.507. [DOI] [PubMed] [Google Scholar]
- 70.Bradley LM, Haynes L, Swain SL. IL-7: maintaining T-cell memory and achieving homeostasis. Trends Immunol. 2005;26:172–176. doi: 10.1016/j.it.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 71.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 72.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, et al. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells. J Exp Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 75.Osborne LC, Dhanji S, Snow JW, Priatel JJ, Ma MC, Miners MJ, et al. Impaired CD8 T cell memory and CD4 T cell primary responses in IL-7R alpha mutant mice. J Exp Med. 2007;204:619–631. doi: 10.1084/jem.20061871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carrio R, Rolle CE, Malek TR. Non-redundant role for IL-7R signaling for the survival of CD8+ memory T cells. Eur J Immunol. 2007;37:3078–3088. doi: 10.1002/eji.200737585. [DOI] [PubMed] [Google Scholar]
- 77.Suto A, Kashiwakuma D, Kagami S, Hirose K, Watanabe N, Yokote K, et al. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008;205:1369–1379. doi: 10.1084/jem.20072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu S, Lizee G, Lou Y, Liu C, Overwijk WW, Wang G, et al. IL-21 synergizes with IL-7 to augment expansion and anti-tumor function of cytotoxic T cells. Int Immunol. 2007;19:1213–1221. doi: 10.1093/intimm/dxm093. [DOI] [PubMed] [Google Scholar]
- 79.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thompson JA, Curti BD, Redman BG, Bhatia S, Weber JS, Agarwala SS, et al. Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J Clin Oncol. 2008;26:2034–2039. doi: 10.1200/JCO.2007.14.5193. [DOI] [PubMed] [Google Scholar]
- 81.Li Q, Eppolito C, Odunsi K, Shrikant PA. IL-12-programmed long-term CD8+ T cell responses require STAT4. J Immunol. 2006;177:7618–7625. doi: 10.4049/jimmunol.177.11.7618. [DOI] [PubMed] [Google Scholar]
- 82.Valenzuela J, Schmidt C, Mescher M. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J Immunol. 2002;169:6842–6849. doi: 10.4049/jimmunol.169.12.6842. [DOI] [PubMed] [Google Scholar]
- 83.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 84.Hamilton SE, Jameson SC. CD8(+) T cell differentiation: choosing a path through T-bet. Immunity. 2007;27:180–182. doi: 10.1016/j.immuni.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 85.Ogasawara K, Hida S, Weng Y, Saiura A, Sato K, Takayanagi H, et al. Requirement of the IFN-alpha/beta-induced CXCR3 chemokine signalling for CD8+ T cell activation. Genes Cells. 2002;7:309–320. doi: 10.1046/j.1365-2443.2002.00515.x. [DOI] [PubMed] [Google Scholar]
- 86.Hida S, Ogasawara K, Sato K, Abe M, Takayanagi H, Yokochi T, et al. CD8(+) T cell-mediated skin disease in mice lacking IRF-2, the transcriptional attenuator of interferon-alpha/beta signaling. Immunity. 2000;13:643–655. doi: 10.1016/s1074-7613(00)00064-9. [DOI] [PubMed] [Google Scholar]
- 87.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]