Abstract
Objective
To assess patterns and predictors of post-partum diabetes screening in a commercially insured, geographically and sociodemographically diverse sample of women with gestational diabetes (GDM).
Methods
Using commercial insurance claims (2000-2012) from all 50 states, we conducted a retrospective cohort study in 447,556 women with at least one delivery and continuous enrollment one year before and after delivery. We identified women with a GDM pregnancy and examined postpartum diabetes screening type and timing, and performed logistic regression to identify screening predictors.
Results
Gestational diabetes mellitus was diagnosed in 32,253 (7.2%) women during the study timeframe. Three fourths received no screening within 1 year postpartum. Rates of recommended 75-g oral glucose tolerance testing within 6–12 weeks were low but increased over time (27 [2%] in 2001 compared with 249 [7%] in 2011, adjusted odds ratio [OR] 3.1, 95% confidence interval [CI] 2.0–47). Among women screened, those in the Northeast (19%) and South (18%) were least likely to receive a 75-g oral glucose tolerance test within 0–12 weeks (adjusted OR 0.4 for each, CI 0.4–0.5) compared with the West (36%). Asian women were most likely to receive any screening (18%; adjusted OR 1.5, CI 1.3–1.6) compared with white women (12%). Black women were most likely to receive hemoglobin A1c (21%; adjusted OR 2.0, CI 1.3–3.2) compared with white women (11%). Antepartum antiglycemic medication (21%; adjusted OR 2.1, CI 2.0–2.3) or visit to a nutritionist–diabetes educator (19%; adjusted OR 1.6, CI 1.4–1.7) or endocrinologist (23%; adjusted OR 1.7, CI 1.6–1.9) predicted screening within 12 weeks postpartum.
Conclusion
Post-partum diabetes screening remains widely underused among commercially insured women with GDM. Differences in screening by geography, race, and antepartum care can inform health system and public health interventions to increase diabetes detection in this high-risk population.
Gestational diabetes mellitus (GDM) is one of the strongest known predictors of type 2 diabetes, conferring a 7-fold higher lifetime risk compared to women without GDM.1 Rates of conversion to diabetes vary by population and range from 2 to 12.5% within one year post-partum,2-5 and from 30% to 60% by10 years.6,7 Black, Hispanic, and Asian women are at greatest risk for development of type 2 diabetes after GDM.8, 9,10
In light of these risks and the clear opportunity for preventive intervention, organizations including the American College of Obstetricians and Gynecologists recommend screening for all women with GDM at 6-12 weeks postpartum with either fasting plasma glucose (FPG) or 75 gram 2-hour oral glucose tolerance test 11,12, Hemoglobin A1C (HbA1C) is not recommended for early post-partum screening. Studies suggest that screening rates remain persistently low despite these recommendations.13-16 . Two systematic reviews found that screening rates range from 25 to 58%, vary by practice patterns, and improve with systems-based interventions.17,18
In the US, the majority of our knowledge about post-partum screening comes from individual practices, particularly specialty referral centers 3,14and an integrated care delivery system on the West Coast.2,4,15 Little is known about trends and determinants of recommended screening across diverse geographic settings, or the presence of variation in care by sociodemographic or clinical characteristics. Understanding these patterns and predictors is central to the development of targeted interventions at the patient, provider, health system, and public health levels. To define these patterns and predictors, we identified women with GDM in a large commercial health plan with enrollees across the entire U.S.
Materials and Methods
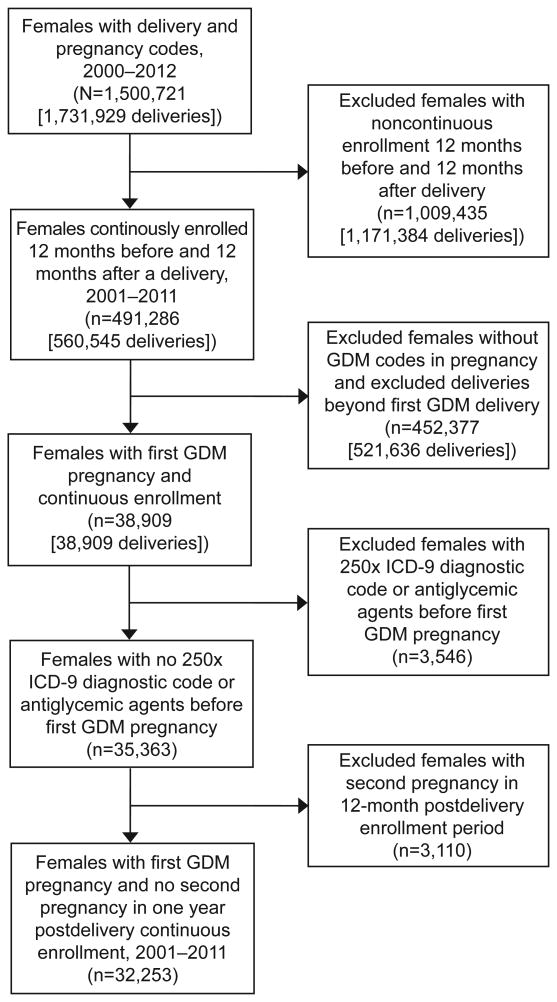
The study was approved by the Harvard Pilgrim Health Care Institute Institutional Review Board. We conducted a retrospective cohort study in a sample of women drawn from the administrative database of a large U.S. health plan that included over 44 million members in all states and Puerto Rico enrolled between 2000 and 2012. From this database we identified 447,556 women with at least one delivery and continuous enrollment 12 months before and after delivery (Figure 1). We then identified women aged 15-44 with pregnancy and delivery in 2001-2011 via International Classification of Diseases, 9th-Revision (ICD-9) and diagnosis related group (DRG) codes. We detected delivery DRG codes during inpatient hospitalizations and required a date of service. We defined the index pregnancy as the first GDM pregnancy meeting enrollment requirements. GDM was defined as one inpatient or two outpatient ICD-9 codes (648.8X). To avoid overlap of glycemic tests with subsequent or prior pregnancies, we included in the analysis only the first GDM pregnancy (the index pregnancy) per woman during the enrollment period.
Figure 1.
Flow diagram of study population by inclusion and exclusion criteria, administrative claims database 2001-2012. First GDM (gestational diabetes mellitus) pregnancy indicates first pregnancy with associated delivery and GDM codes in pregnancy during cohort time frame. ICD-9, International Statistical Classification of Diseases, 9th edition.
We further limited our population to women with continuous enrollment for at least one year before and after the delivery date of the index pregnancy. Requiring at least one year of enrollment before delivery allowed for at least three months (assuming a 280 day gestation) of pre-pregnancy claims to identify and exclude pre-existing diabetes, while requiring at least one year post-partum allowed for assessment of screening within one year. We excluded women with overt type 1 or 2 diabetes, as identified by ICD-9 codes 250.0X prior to date of conception (N=3,037) or one or more pharmacy claims for insulin, sulfonylurea, or other antiglycemic medication (other than metformin) prior to pregnancy (N=105). Because diagnoses for GDM may be miscoded as overt diabetes or vice-versa, we used the ratio of GDM vs. diabetes codes for each woman to refine our determination of likely pregestational diabetes. We excluded 404 women who had more 250.x than 648.8x codes during pregnancy as likely having pre-existing diabetes.
We identified glycemic screening type (75g OGTT, FPG, HbA1c) using Current Procedural Terminology (CPT) codes at < 6, 6-12, and >12-52 weeks post-delivery. Outcome measures of interest were 1) any glycemic screening within 12 weeks or one year post-partum; and 2) among those screened, type (75g OGTT, FPG, or HbA1c) and timing (<6 weeks, recommended 6-12 weeks, >12 weeks up to 1 year) of screening.
We assigned neighborhood sociodemographic characteristics (education, income, and racial/ethnic composition) to subjects using their 2000 census block group of residence (geocoded) and for race/ethnicity, both census and surname analysis. Socioeconomic status was assessed using a geocoded measure of percent of neighborhood residents living at less than the federal poverty level. Education was measured as percent of neighborhood residents with less than high school education. Cut points validated in prior studies19,20were used for both education and poverty measures. We used a combination 2000 US Census neighborhood characteristics and surname analysis to assess race and ethnicity, a validated approach with a high positive predictive value.21,22 We classified members as residing in white, black, or Hispanic neighborhoods based on living in neighborhoods with 75% or more persons of the given race/ethnicity; we assigned census blocks with 75% or more persons of both Hispanic ethnicity and black race to the Hispanic category. We classified members from census block groups that did not fall into one of the three race/ethnicity categories as living in mixed race/ethnicity neighborhoods. We used surname analysis to identify Hispanic and Asian individuals which superseded the neighborhood-based measure, and we classified members as Hispanic if they lived in a predominantly Hispanic neighborhood or had a Hispanic surname.
We identified pre-pregnancy and antepartum co-morbidities (hypertension, depression, polycystic ovarian syndrome [PCOS], hyperlipidemia, fatty liver disease, and preeclampsia) via one inpatient or two outpatient ICD-9 codes. Antepartum visits to nutritionists and diabetes educators were identified via procedure codes for these services, and visits to endocrinologists by endocrinology-specific provider codes. To assess rates of screening by post-partum visit status, we identified outpatient post-partum visits by evaluation and management codes for obstetricians, primary care providers, and endocrinologists. Providers included nurse practitioners, physician's assistants and physicians and timing of visits and timing of screening were categorized within mutually exclusive categories of 12 weeks and 12 weeks to one year.
We used multivariable logistic regression to assess predictors of any screening and, among women screened, type (75g OGTT, FPG, HbA1c) and timing (within 0-12 weeks, within one year) of screening, adjusted for year of index pregnancy. Variables for inclusion in the multivariable models were chosen a priori for relevance to diabetes screening based on prior literature or clinical judgment of study authors and included: age, region of residence, poverty level, race or ethnicity, education, presence of pre-gestational or antenatal co-morbidities, antepartum antiglycemic medication use (insulin, metformin, or glyburide), care by an endocrinologist, or visit to a nutritionist or diabetes educator. Timing of test was analyzed by recommended time frame (6-12 weeks post-partum) for descriptive analyses, but for reasons of sample size and to include women who may have received testing just before 6 weeks, logistic regression time frames 0-6 and 6-12 weeks were combined in a single outcome of within 12 weeks post-partum. Statistical analyses were performed using SAS version 9.3. For the majority of analyses, unadjusted results were similar to adjusted results and we present only adjusted results. Instances in which adjusted and unadjusted results differ are noted in the text.
Results
Over the 12 year period, 447,556 women had at least one delivery and met the continuous enrollment criteria. Of these, 32,253 (7.2%) had a pregnancy complicated by GDM (Table 1). The majority were white (59%),in their thirties (65%) from the South (43%) or Midwest (29%), and lived in an area with <10% of residents living below the federal poverty level (64%) One-quarter of women with GDM were treated with an antiglycemic medication during pregnancy. Insulin (54%) and glyburide (37%) were the most common agents used in women on antiglycemic medications.
Table 1. Sociodemographic and clinical characteristics of women with pregnancy complicated by gestational diabetes, 2001-2011, by screening status within one year post-partum.
| Sociodemographic and clinical characteristics | All N = 32,253 N (%) |
Unscreened N = 24,531 (%) |
Screened* N = 7722 (%) |
Unadjusted Odds Ratio** (95% CI) |
|---|---|---|---|---|
| Age during years of index delivery (years) | ||||
| 15-29 | 8342 (26) | 6,638 (27) | 1,704 (22) | 1.0 (ref) |
| 30-39 | 21,159 (65) | 15, 891 (65) | 5,268 (68) | 1.2 (1.1-1.2) |
| 40-44 | 2752 (9) | 2,002 (8) | 750 (10) | 1.2 (1.1-1.3) |
| Region | ||||
| West | 5118 (16) | 3547 (14) | 1571 (20) | 1.0 (ref) |
| South | 13,772 (43) | 10693 (44) | 3079 (40) | 0.9 (0.8-0.9) |
| Midwest | 9389 (29) | 7245 (30) | 2143 (28) | 0.9 (0.9-1.0) |
| Northeast | 3944 (12) | 3020 (12) | 924 (12) | 1.0 (0.9-1.0) |
| % neighborhood with <high school education | ||||
| <5 | 19,864 (62) | 14,880 (61) | 4, 984 (65) | 1.0 (ref) |
| 5-<25 | 6766 (21) | 5,254 (21) | 1,512 (20) | 0.9 (0.8-1.0) |
| 25-<40 | 4006 (12) | 3,140 (13) | 866 (11) | 0.9 (0.8-1.0) |
| >40 | 1558 (5) | 1,215 (5) | 343 (4) | 0.9 (0.8-1.0) |
| Missing | 59 (0) | 42 (0) | 17 (0) | |
| % neighborhood below poverty level | ||||
| <5 | 15,349 (48) | 11,573 (47) | 3,776 (49) | 1.0 (ref) |
| 5-<10 | 8322 (26) | 6,294 (26) | 2,028 (26) | 1.0 (1.0-1.1) |
| 10-<20 | 5846 (18) | 4,547 (19) | 1,299 (17) | 0.9 (0.8-1.0) |
| ≥ 20 | 2677 (8) | 2,075 (8) | 602 (8) | 0.9 (0.8-1.0) |
| Missing | 59 (0) | 42 (0) | 17 (0) | |
| Race/ethnicity& | ||||
| White | 19,161 (59) | 14,909 (61) | 4,252 (55) | 1.0 (ref) |
| Black | 597 (2) | 471 (2) | 126 (2) | 0.9 (0.9-1.0) |
| Asian | 3743 (12) | 2,524 (10) | 1,219 (16) | 1.6 (1.5-1.8) |
| Hispanic | 4086 (13) | 3,047(13) | 1,039 (13) | 1.1 (1.0-1.2) |
| Mixed | 4588 (14) | 3,527(14) | 1,061 (14) | 1.0 (0.9-1.0) |
| Missing | 78 (0) | 53 (0) | 25(0) | |
| Comorbidity in or prior to pregnancy | 10,173 (32) | 7,438 (30) | 2,735 (35) | 1.2 (1.2-1.3)§ |
| Seen by nutritionist or diabetes educator during pregnancy | 5472 (17) | 3,660 (15) | 1,812 (23) | 1.8 (1.6-1.9)§ |
| Seen by endocrinologist during pregnancy | 3881 (12) | 2,374 (10) | 1,507 (20) | 2.3 (2.1-2.4)§ |
| Any antiglycemic agent during pregnancy | 8082 (25) | 4,985 (20) | 3,097 (40) | 2.6 (2.5-28)§ |
Screened with any test (75g OGTT, HbA1C, or FPG) within one year post-partum.
Unadjusted ORs compare screening vs. no screening by sociodemographic and clinical characteristics.
Race/ethnicity is derived from surname analysis and neighborhood-level census data. Mixed race/ethnicity reflects neighborhood with residents of undefined race/ethnicity by surname analysis and census-level data.
As compared to “No” (ref).
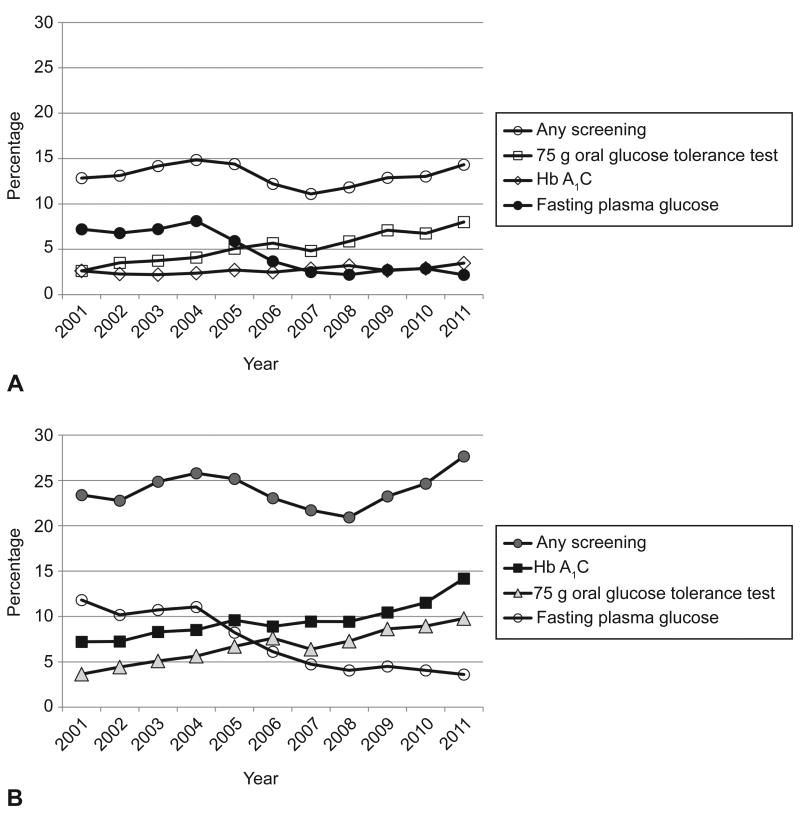
For the entire study period including all 32, 253 women with gestational diabetes, only 23.9% received any testing for overt diabetes in the first year. During the first 0-12 weeks post-partum, 13.1% received any testing, and another 13.6% of women were tested between 12 and 52 weeks post partum. Of the 4230 women screened in the first 12 weeks, 41.9%received the recommended 75g OGTT. OGTT was the most frequently used test at in the first 12 weeks (Table 2). Twenty one percent of tests within 12 weeks were HbA1c alone. Contrary to guidelines, 17.1% of women were tested prior to 6 weeks post partum. Screening occurred between 12 and 52 weeks for 56.8% of the total screened population of women. After 12 weeks, HbA1c was the most common test used (69.3%). Figure 2 demonstrates rates of post-partum screening by testing type over the study period.
Table 2. Type and timing of postpartum glycemic screening among women with GDM with any screening in first year post-delivery (N= in 7722 women).
| Screening type | All N=7722 |
< 6 weeks N=1320 (17%) |
6- <12 weeks N=3034 (39) |
12 weeks-1year N= 4393 (57) |
|---|---|---|---|---|
| 75g OGTT | 2281 (30) | 230 (17) | 1548 (51) | 520 (12) |
| HbA1c only | 2721(35) | 256 (19) | 645 (21) | 2503 (57) |
| FPG only | 1844 (24) | 764 (58) | 647 (21) | 825 (19) |
| HbA1c and FPG | 876 (11) | 70 (5) | 194 (6) | 545 (12) |
Note: Percents are column percents. Column percents and row totals do not equal 100% as 374 (4%) are repeat of same test within the year post-partum. 75g OGTT= any 75g OGTT alone or in combination with A1C or FPG.
Figure 2.
Any screening and type of screening among 32,256 females with pregnancy complicated by gestational diabetes mellitus. Screening within 12 weeks of delivery (A); screening within one year of delivery (B).
Among all women with GDM, rates of recommended screening with the 75-g OGTT at 6–12 weeks increased from 2% (27) to 7% (249) over the study period (adjusted odds ratio [OR] for 2011 compared with 2001 2.4, 2.0–4.7; data not shown). Use of the 75-g OGTT within 12 weeks increased from 3% (33) in 2001 to 8.0% (286) in 2011 (adjusted OR 3.2, confidence interval [CI] 2.2–4.7; Table 3). Within 1 year postpartum, rates of the 75-g OGTT increased from 4% (46) to 10% (349) (adjusted OR 2.9, CI 2.0–4.1; Appendix Table 1); FPG alone decreased from 7% (91) to 2% (78; adjusted OR 0.2, CI 0.1–0.3) and from 12% (149) to 4% (129; OR 0.2, CI 0.1–0.2), respectively. Use of A1C alone within 1 year increased from 25% (74) to 45% (985; adjusted OR 2.3, CI 1.7–3.1; Appendix Table 1).
Table 3.
Sociodemographic and pregnancy characteristics associated with the adjusted odds of any postpartum glycemic screening (N=32,152), and type of screening (N= 7,693) among those screened within 12 weeks post-partum in . women with a pregnancy complicated by gestational diabetes mellitus, 2001-2011
| Sociodemographic and Pregnancy Characteristics | Any Postpartum Screening OR (95% CI) |
75g OGTT OR (95% CI) |
HbA1c OR (95% CI) |
FPG OR (95% CI) |
|---|---|---|---|---|
| Age at delivery (yrs) | ||||
| 15-29 (ref) | 1.0 | 1.0 | 1.0 | 1.0 |
| 30-39 | 1.1 (1.0-1.2) | 1.0 (0.8-1.1) | 1.2 (1-1.4) | 0.9 (0.8-1.0) |
| 40-44 | 1.2 (1.1-1.4) | 0.7 (0.6- 0.9) | 1.2 (0.9-1.6) | 1.1 (0.9-1.4) |
| Region | ||||
| West (ref) | 1.0 | 1.0 | 1.0 | 1.0 |
| South | 0.7 (0.7-0.8) | 0.4 (0.4-0.5) | 1.5 (1.2-1.9) | 2.0 (1.7-2.5) |
| Midwest | 0.7 (0.6-0.8) | 0.5 (0.4-0.6) | 1.3 (1.0-1.6) | 1.8 (1.5-2.2) |
| Northeast | 0.6 (0.5-0.6) | 0.4 (0.4-0.5) | 1.7 (1.3-2.3) | 0.7 (0.5-0.9) |
| Neighborhood % < HS education | ||||
| <5 (ref) | 1.0 | 1.0 | 1.0 | 1.0 |
| 5-<25 | 0.9 (0.8-1.0) | 1.0 (0.9-1.2) | 0.8 (0.7-1) | 1.1 (1.0-1.4) |
| 25-40 | 0.9 (0.8-1.1) | 1.0 (0.8-1.2) | 1.1 (0.9-1.5) | 1.1 (0.9-1.4) |
| >40 | 1.0 (0.8-1.2) | 1.0 (0.7-1.4) | 0.7 (0.5-1.2) | 1.4 (1.0-2.0) |
| Neighborhood % below poverty level | ||||
| <5 (ref) | 1.0 | 1.0 | 1.0 | 1.0 |
| 5-<10 | 1.0 (0.9-1.1) | 1.0 (0.8-1.1) | 0.9 (0.8-1.1) | 0.9 (0.8-1.1) |
| 10-<20 | 1.0 (0.9-1.1) | 1.0 (0.8-1.2) | 1.0 (0.8-1.3) | 1.0 (0.8-1.2) |
| ≥ 20 | 1.0 (0.9-1.2) | 0.8 (0.6-1.1) | 1.2 (0.9-1.7) | 1.2 (0.9-1.5) |
| Race/ethnicity | ||||
| White (ref) | 1.0 | 1.0 | 1.0 | 1.0 |
| Black | 1.1 (0.8-1.4) | 0.6 (0.4-1.1) | 2.0 (1.3-3.2) | 0.5 (0.3-0.9) |
| Asian | 1.5 (1.3-1.6) | 1.2 (1.0-1.4) | 0.9 (0.7-1.2) | 0.9 (0.7-1.0) |
| Hispanic | 1.2 (1.1-1.3) | 1.0 (0.8-1.1) | 1.1 (0.8-1.3) | 1.0 (0.8-1.2) |
| Mixed* | 1.1 (1.0-1.2) | 1.1 (0.9-1.3) | 1.0 (0.8-1.2) | 1.0 (0.8-1.2) |
| Co-morbidities | ||||
| No (ref) | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 1.0 (0.9-1.1) | 0.7 (0.6-0.8) | 1.3 (1.1-1.6) | 0.7 (0.6-0.9) |
| Seen by nutritionist or diabetes educator | ||||
| No (ref) | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 1.6 (1.4-1.7) | 1.4 (1.2-1.6) | 0.7 (0.6-0.9) | 1.0 (0.9-1.2) |
| Antiglycemic agent | ||||
| No (ref) | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 2.1 (2.0-2.3) | 1.0 (0.9-1.1) | 1.2 (1.1-1.4) | 0.9 (0.7-1.0) |
| Seen by Endocrinologist | ||||
| No (ref) | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 1.7 (1.6-1.9) | 0.7 (0.6-0.9) | 2.1 (1.8-2.4) | 0.8 (0.7-1.0) |
| Year of index delivery | ||||
| 2001 (ref) | 1.0 | 1.0 | 1.0 | 1.0 |
| 2002 | 1.0 (0.8-1.2) | 1.3 (0.9-2.1) | 0.9 (0.5-1.4) | 1 (0.8-1.4) |
| 2003 | 1.1 (0.9-1.3) | 1.3 (0.8-1.9) | 0.8 (0.5-1.2) | 1 (0.7- 1.4) |
| 2004 | 1.1 (0.9-1.4) | 1.4 (0.9-2.1) | 0.7 (0.5-1.1) | 1.1 (0.8-1.5) |
| 2005 | 1 (0.9-1.3) | 1.9 (1.3-2.9) | 0.9 (0.6- 1.4) | 0.7 (0.5-1) |
| 2006 | 0.9 (0.7-1.1) | 2.5 (1.7- 3.8) | 0.9 (0.6-1.4) | 0.5 (0.3- 0.6) |
| 2007 | 0.8 (0.6- 1) | 2.3 (1.5-3.4) | 1.1 (0.7-1.7) | 0.3 (0.2-0.5) |
| 2008 | 0.8 (0.7-1) | 3.1 (2-4.6) | 1.4 (0.9-2.1) | 0.3 (0.2-0.4) |
| 2009 | 0.9 (0.7- 1.1) | 3.3 (2.2-4.9) | 1 (0.6-1.5) | 0.3 (0.2-0.5) |
| 2010 | 0.9 (0.8- 1.1) | 3 (2-4.5) | 1 (0.6-1.5) | 0.3 (0.2-0.5) |
| 2011 | 1.1 (0.9- 1.3) | 3.2 (2.2-4.7) | 1.1 (0.7-1.7) | 0.2 (0.1-0.3) |
Data are presented as odds ratios (ORs) with 95% confidence interval. Subjects with any missing values were excluded from the analysis. Education is % neighborhood with < HS education. Poverty is % neighborhood living below poverty level. Race/ethnicity is derived from surname analysis and neighborhood-level census data. Mixed race/ethnicity reflects neighborhood with residents of undefined race/ethnicity by surname analysis and census-level data. 75g OGTT is any 75g OGTT, whereas FPG and HbA1c represent FPG or HbA1c only.
Both the likelihood of receiving any screening and the type of screening obtained were associated with geography, race or ethnicity, and clinical factors. Geographic location was a strong predictor of postpartum screening practices. Women in the West (Table 3) were the most likely to receive any screening at 0–12 weeks (18%) and at 1 year (31%) (Appendix Table 1). Among women screened, they were most likely to receive the recommended 75-g OGTT at 0–12 weeks (36%) and least likely to receive Hb A1C only (8%). Women in the Northeast were least likely to receive any screening (11%; adjusted OR 0.6) compared with the West (CI 0.5–0.6) and, along with women in the South, least likely to get recommended 75-g OGTT (19% and 18%, respectively, adjusted OR 0.4 compared with the West, CI 0.4–0.5). Women in the Northeast were most likely to receive Hb A1C only (14%; adjusted OR 1.7 compared with the West, CI 1.3–2.3), and women in the South were most likely to receive FPG only (20%; adjusted OR 2.0 compared with the West, CI 1.7–2.5).
There are differences in screening by race or ethnicity. Twelve percent of white women received any screening within 0–12 weeks postpartum. Asian (18%; adjusted OR 1.5, CI 1.3–1.6 compared with white) and Hispanic (14%; adjusted OR 1.2, CI 1.1–1.3 compared with white) women were more likely to receive any screening than were black women, who received any screening at rates similar to those of white women (11%; adjusted OR 1.1, CI 0.8–1.4). On unadjusted analyses, Asian women were also most likely to get the 75-g OGTT (31%; OR 1.6 compared with white [21%], CI 1.4–1.9) and black women least likely (11%; OR 0.4 compared with white, CI 0.3–0.8); however, these relationships were attenuated in the adjusted model. Black women were more likely to receive Hb A1C than any other group at either 12 weeks (21%; adjusted OR 2.0 compared with white [11%], CI 1.3–3.2) or 1 year (52%; adjusted OR 1.8 compared with white 1,471 [35%], CI 1.2–2.6; Appendix Table 1).
In unadjusted analyses, lower education and poverty were both associated with modestly higher odds of receiving FPG only and decreased odds of receiving the 75-g OGTT; however, neither relationship held with adjustment for other factors. Measures of disease severity and antepartum care influenced likelihood and type of screening at 0–12 weeks postpartum. Women on antiglycemic medication in pregnancy (21%) were twice as likely to receive any screening than those who were not (10%; adjusted OR 2.1 compared with no antiglycemic agent, CI 2.0–2.3). Only 17% of women saw a nutritionist or diabetes educator during pregnancy. However, these women were more likely to receive any screening (19%; adjusted OR 1.6 compared with no visit to nutrition or diabetes education, CI 1.4–1.7) and, among those screened, more likely to get 75-g OGTT and less likely to receive Hb A1C only. Women seen by an endocrinologist (12%) were also more likely (23%) to get any screening than those who were not (12%; adjusted OR 1.7, CI 1.6–1.9), but when screened were less likely to get the 75-g OGTT (20%; adjusted OR 0.7, CI 0.6–0.9) and twice as likely to get Hb A1C (18%; adjusted OR 2.1, CI 1.8–2.4). Adjusted ORs for screening within 1 year postpartum were similar to those at 0–12 weeks and are shown in Appendix Table 1.
Forty percent of women had a visit with an obstetrics, primary care, or endocrinology provider within 12 weeks postpartum, and 81% within one year. The large majority of visits at both time points were with obstetrics or primary care (93% <12 weeks and 96% at 12 weeks to one year).
Among the 5,467 women seen by obstetricians for a postpartum visit within 12 weeks 16% had screening within 12 weeks and an additional 11% within one year. Among the 7,341 women who had a post-partum obstetrician visit at 12 weeks to one year (and no visit with obstetrics, primary care, or endocrinology prior to 12 weeks) 10% received screening <12 weeks and a further 15% received screening at 12 weeks to one year.
Discussion
In this large sample of commercially insured women in the U.S., rates of recommended post-partum diabetes screening among women with a history of GDM were very low but marginally improving, and varied by geographic location, race–ethnicity, and clinical characteristics.
Screening rates observed are at the lower end, but similar to baseline rates, of those previously reported. Ferrara et al. studied screening within one year post-partum in 14,448 women with GDM in Kaiser Permanente Northern California from 1995 to 2006. Rates of screening with either FPG or 75g OGTT rose from 20.7% to 53.8% following implementation of a nurse-managed intervention, and rates of 75g OGTT within one year rose from 5% to 71%.4
A recent study in over one million pregnant women using a national laboratory found that only 19% of women with GDM had post-partum glucose screening of any type within six months of delivery.16
The strongest predictor of screening was antepartum antiglycemic medication, a finding similar to several2,23 but not all prior studies. Studies have also found that Asian and Hispanic race or ethnicity predict screening.2,18 As compared to whites, Hispanic and Asian women have higher rates of GDM and, particularly among Asian women of South Indian origin, higher rates of conversion to type 2 diabetes.8-10 Black women are not consistently observed to have higher rates of GDM, but have the highest rates of conversion to type 2 diabetes of any group8,9 and the highest rate of adverse pregnancy outcomes with GDM.24,25 In the current study, black women were least likely to receive 75g OGTT, and most likely to receive HbA1c alone. Use of HbA1c may indicate disparities in care or practical barriers to obtaining 75g OGTT. It could also reflect seeing a provider after 12 weeks post-partum, when there may be less clarity among providers as to the type of recommended testing. However as claims data allow assessment of completed tests rather than ordered tests, we are unable to ascertain if observed patterns are due to provider orders or patient completion of ordered tests.
We also found variation screening by region, with women in the west more likely to get recommended screening. This may be influenced in part by California, where the California Diabetes and Pregnancy Program Sweet Success program provides standardized training and resources to providers across the state (www.cdph.ca.gov). Lastly, antepartum visit to nutrition or diabetes education was predictive of recommend screening, suggesting benefit of these visits beyond nutrition guidance and GDM education.
This study includes women from all 50 states and represents a large segment of the commercially insured population in the U.S. The wide range of clinical settings can provide a more broadly representative assessment of screening rates across the country. However the study has several limitations. Insurance claims are used in pregnancy-related research26 and are particularly useful as the date of delivery (not routinely available in outpatient EHR) is known from billing codes. However, laboratory values are not routinely available and identification of GDM via ICD-9 codes alone can overestimate GDM prevalence if, the diagnosis is carried over from a prior pregnancy. Requiring at least one inpatient or two outpatient GDM diagnoses, as we did, reduces this possibility.27
Other clinical parameters that may influence screening, such as family history and body mass index, are also not routinely available in claims data. The population in our sample may differ from populations covered by other commercial insurers or by Medicaid and may not be generalizable to these populations.
The definition of race or ethnicity used is a combination of surname analysis and neighborhood level geocoded data. This approach has been shown to have high positive predictive value 21,22, but is a proxy measure that does not have the same accuracy as individual self-report.
Our finding that the majority of women had a post-partum visit with obstetrician or primary care within a year post-partum, but of these only a minority had a screening test completed, strongly suggests a widespread missed opportunity for intervention in the post-partum time frame. In addition, the use of HgbA1c testing is not recommended in the early post-partum period as vascular changes in pregnancy and influence of glycemic control towards the end of pregnancy make HgbA1c less reliable12 . Studies suggest correlations between HbA1C and other measures of glucose are low in the first year post-partum and improving thereafter28. It can reasonably be concluded that there is a pressing need for continued innovations for implementation of timely, appropriate post partum screening for diabetes.
Interventions will require local adaptation to overcome care fragmentation between obstetrical teams and primary care clinicians, provide vehicles for integration and increase access to diabetes education, nutrition counseling, and endocrinology support. Particular attention is needed to target outreach to groups at particularly high risk of conversion, with tailoring by culture or other patient centered factors to educate women and encourage diabetes screening. Whether at the level of health system or population, quality improvement efforts must identify effective means of post-partum screening that are feasible for both women and providers, and based on risk factors rather than geography or disparities in care.
Supplementary Material
Acknowledgments
Funding: Drs. Oken and Ross-Degnan are supported in part by the Health Delivery Systems Center for Diabetes Translational Research (HDS-CDTR) [NIDDK grant 1P30-DK092924]. Dr. Oken received funding from NIH K24 HD069408. Drs. Eggleston, Wharam, Ross-Degnan, and Zhang are supported by the Natural Experiments in Diabetes Translation (NEXT-D) Program (CDC grant 5U58DP002719).
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Contributor Information
Emma Morton Eggleston, Department of Population Medicine, Harvard Medical School, Harvard Pilgrim Health Care Institute, Boston, MA; Brigham and Women's Hospital, Division of Endocrinology, Diabetes and Hypertension, Boston, MA.
Robert Franklin LeCates, Department of Population Medicine, Harvard Medical School, Harvard Pilgrim Health Care Institute, Boston, MA.
Fang Zhang, Department of Population Medicine, Harvard Medical School/Harvard Pilgrim Health Care Institute, Boston, MA.
James Franklin Wharam, Department of Population Medicine, Harvard Medical School, Harvard Pilgrim Health Care Institute, Boston, MA.
Dennis Ross-Degnan, Department of Population Medicine, Harvard Medical School, Harvard Pilgrim Health Care Institute, Boston, MA.
Emily Oken, Department of Population Medicine, Harvard Medical School, Harvard Pilgrim Health Care Institute, Boston, MA.
References
- 1.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet. 2009;373(9677):1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence JM, Black MH, Hsu JW, Chen W, Sacks DA. Prevalence and timing of postpartum glucose testing and sustained glucose dysregulation after gestational diabetes mellitus. Diabetes Care. 2010;33(3):569–576. doi: 10.2337/dc09-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunt KJ, Conway DL. Who returns for postpartum glucose screening following gestational diabetes mellitus? Am J Obstet Gynecol. 2008;198(4):404 e401–406. doi: 10.1016/j.ajog.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrara A, Peng T, Kim C. Trends in postpartum diabetes screening and subsequent diabetes and impaired fasting glucose among women with histories of gestational diabetes mellitus: A report from the Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care. 2009;32(2):269–274. doi: 10.2337/dc08-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwak SH, Choi SH, Jung HS, Cho YM, Lim S, Cho NH, et al. Clinical and genetic risk factors for type 2 diabetes at early or late post partum after gestational diabetes mellitus. J Clin Endocrinol Metab. 2013;98(4):E744–752. doi: 10.1210/jc.2012-3324. [DOI] [PubMed] [Google Scholar]
- 6.Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM screening program. Diabetes Care. 2005;28:579–584. doi: 10.2337/diacare.28.3.579. [DOI] [PubMed] [Google Scholar]
- 7.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care. 2002;25(10):1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto W, Samoa R, Wotring A. Gestational Diabetes in High-Risk Populations. Clinical Diabetes. 2013;31:90–94. [Google Scholar]
- 9.Xiang AH, Li BH, Black MH, Sacks DA, Buchanan TA, Jacobsen SJ, et al. Racial and Ethnic Disparities after Gestational Diabetes Mellitus Diabetologia. 2011;54:3016–321. doi: 10.1007/s00125-011-2330-2. [DOI] [PubMed] [Google Scholar]
- 10.Lee AJ, Hiscock RJ, Wein P, Walker SP, Permezel M. Gestational diabetes mellitus: clinical predictors and long-term risk of developing type 2 diabetes: A retrospective cohort study using survival analysis. Diabetes Care. 2007;30(4):878–883. doi: 10.2337/dc06-1816. [DOI] [PubMed] [Google Scholar]
- 11.ACOG Committee Opinion No. 435: Postpartum screening for abnormal glucose tolerance in women who had gestational diabetes mellitus. Obstet Gynecol. 2009;113(6):1419–1421. doi: 10.1097/AOG.0b013e3181ac06b6. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association. Standards of medical care in diabetes 2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 13.Almario CV, Ecker T, Moroz LA, Bucovetsky L, Berghella V, Baxter JK. Obstetricians seldom provide postpartum diabetes screening for women with gestational diabetes. Am J Obstet Gynecol. 2008;198(5):528 e521–525. doi: 10.1016/j.ajog.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Stasenko M, Cheng YW, McLean T, Jelin A, Rand L, Caughey AB. Postpartum follow-up for women with gestational diabetes mellitus. Am J Perinatol. 2010;27(09):737–742. doi: 10.1055/s-0030-1253557. [DOI] [PubMed] [Google Scholar]
- 15.Dietz PM, Vesco KK, Callaghan WM, Bachman DJ, Bruce FC, Berg CJ, et al. Postpartum screening for diabetes after a gestational diabetes mellitus-affected pregnancy. Obstet Gynecol. 2008;112(4):868–874. doi: 10.1097/AOG.0b013e318184db63. [DOI] [PubMed] [Google Scholar]
- 16.Blatt AJ, Nakamoto JM, Kaufman HW. Gaps in diabetes screening during pregnancy and postpartum. Obstet Gynecol. 2011;117:61–68. doi: 10.1097/AOG.0b013e3181fe424b. [DOI] [PubMed] [Google Scholar]
- 17.Tovar A, Chasan-Taber, Eggleston E, Oken E. Postpartum screening for diabetes among women with a history of gestational diabetes mellitus. Prev Chronic Dis. 2011;8(6):A124. [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt KJ, Logan SL, Conway DL, Corte JE. Postpartum screening following GDM: How well are we doing? Curr Diab Rep. 2010;10(3):235–241. doi: 10.1007/s11892-010-0110-x. [DOI] [PubMed] [Google Scholar]
- 19.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: A comparison of area-based socioeconomic measures—the public health disparities geocoding project. Am J Public Health. 2003;93(10):1655–1671. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: The Public Health Disparities Geocoding Project. Am J Public Health. 2005;95(2):312–323. doi: 10.2105/AJPH.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. [2014];Ethnic Technologies. Available at: http://www.ethnictechnologies.com/
- 22.Fiscella K, Fremont AM. Use of geocoding and surname analysis to estimate race and ethnicity. Health Serv Res. 2006;41:1482–1500. doi: 10.1111/j.1475-6773.2006.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Retnakaran R, Qi Y, Connelly PW, Sermer M, Hanley AJ, Zinman B. Risk of early progression to pre-diabetes or diabetes in women with recent gestational dysglycemia but normal glucose tolerance at 3 months postpartum. Clin Endocrinol. 2010;73:776–483. doi: 10.1111/j.1365-2265.2010.03834.x. [DOI] [PubMed] [Google Scholar]
- 24.Esakoff TF, Caughey AB, Block-Kurbish I, Inturrisi M, Cheng YW. Perinatal outcomes in patients with gestational diabetes mellitus by race/ethnicity. J Matern Fetal Neonatal Med. 2011;24(3):422–426. doi: 10.3109/14767058.2010.504287. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen BT, Cheng YW, Snowden JM, Esakoff TF, Frias AE, Caughey AB. The effect of race/ethnicity on adverse perinatal outcomes among patients with gestational diabetes mellitus. Am J Obstet Gynecol. 2012;207:322–e1. doi: 10.1016/j.ajog.2012.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozhimannil KB, Pereira MA, Harlow BL. Association between diabetes and perinatal depression among low-income mothers. JAMA. 2009;301:842–847. doi: 10.1001/jama.2009.201. [DOI] [PubMed] [Google Scholar]
- 27.Shah BR, Lipscombe LL, Feig DS, Lowe JM. Missed opportunities for type 2 diabetes testing following gestational diabetes: a population-based cohort study. BJOG: Int J Obstet Gynaecol. 2011;118:1484–1490. doi: 10.1111/j.1471-0528.2011.03083.x. [DOI] [PubMed] [Google Scholar]
- 28.Kim C, Herman WH, Cheung NW, Gunderson EP, Richardson C. Comparison of hemoglobin A1c with fasting plasma glucose and 2-h post-challenge glucose for risk stratification among women with recent gestational diabetes mellitus. Diabetes Care. 2011;34(9):1949–1951. doi: 10.2337/dc11-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.