Abstract
Objectives
Pair-Box 8 (PAX8) is a transcription factor which has been found to be overexpressed in ovarian serous carcinoma (OSC). Silencing PAX8 by using shRNA led to a drop in cell viability in ovarian cancer cell lines, suggesting its use as a targeted therapeutic agent. The prognostic value of PAX8 in OSC is still widely unknown. The aim of this study was to evaluate PAX8 as a prognostic biomarker in patients with advanced stage OSC.
Methods
PAX8 was evaluated using immunohistochemistry on a tissue microarray of 148 OSC and the expression was correlated to the following clinico-pathologic variables; age of diagnosis, tumor stage, optimal debulking, recurrence free survival (RFS) and overall survival (OS).
Results
We found that PAX8 was expressed in 61% of cases. There was no association between PAX8 and tumor stage, optimal debulking and disease recurrence. In addition, PAX8 failed to have a predictive value in disease outcome.
Conclusion
Despite showing that PAX8 protein is not a useful predictive marker in patients with high grade, advanced stage OSC, its overexpression in a large number of these cases makes the inhibition of PAX8 a very attractive targeted therapy.
Keywords: PAX8, Immunoexpression, Ovarian serous cancer, Disease outcome
Introduction
Epithelial ovarian cancer (EOC) is the leading cause of death due to gynecologic malignancies in women in the United States, with 21,990 new cases and 15,460 women estimated to die of ovarian cancer in 2011 [1]. Women with EOC usually present with late stage diseases. The standard treatment for advanced stage disease is staging laparotomy with tumor debulking followed by platinum-taxane based chemotherapy [2]. Approximately 70–80% of patients with EOC will relapse after first-line chemotherapy and the management of relapsed ovarian carcinoma remains a difficult problem open to research [3]. Most patients will eventually die of chemotherapy resistant disease [4,5]. The overall survival rates remain disappointing with little improvement in response rates, disease free interval and median survival rates. Therefore, novel therapies targeting DNA repair genes, angiogenesis, and immune-based therapy are urgently needed to improve patient care.
PAX8 is a member of the pair-box family of transcription factor genes. PAX8 is important in organogenesis of the thyroid, kidney, and mullerian tract [6,7]. Before the recent work of “Project Achilles”, the role of PAX8 in the reproductive system was still shroud in mystery. By testing 100 ovarian cancer cell lines, the team detected PAX8 as the most important gene involved in ovarian cancer cells. Furthermore, in cancer cell lines overexpressing PAX8, silencing PAX8 using shRNA led to cell death, implicating that inhibition of PAX8 could be a novel targeted therapy in ovarian cancer [8]. In another study, PAX8 expression was found to be restricted to secretory cells of the fallopian tube epithelium, which most recently has been suggested as the cell of origin for serous ovarian carcinomas [9]. In the reproductive system, the interest in PAX8 immunoexpression was mainly focused on its use as a marker to distinguish carcinoma of gynecologic origin versus carcinomas from other sites and from mesothelioma [10–12]. The value of PAX8 in women with ovarian cancer is still unknown. Thus, the aims of this study are to determine the expression of PAX8 in high grade, late stage ovarian serous carcinoma and to correlate its expression with clinico-pathologic characteristics.
Methods
Patients and specimens
We searched pathology archives for patients with epithelial ovarian cancer who underwent debulking surgery for their EOC during a 10 year period from 1995 to 2005. All tissue specimens were collected under an approved protocol from the Institutional Review Board (IRB). The medical records of the patients were also retrospectively reviewed under an approved IRB protocol. The review included outpatient and inpatient treatment, including surgery and chemotherapy. Optimal debulking or cytoreduction was defined according to the Gynecologic Oncology Group (GOG) as the largest residual tumor nodule measuring 1 cm or less determined by the operating surgeon [13]. This definition is the most widely accepted among gynecologic oncologists.
Study outcomes included overall survival and time to recurrence, each measured from the time of definitive surgery. The duration of overall survival was the interval between definitive surgery and death. Observation time was the interval between definitive surgery and last contact (death or last follow-up). Data were censored at the last follow-up for patients with no evidence of recurrence, progression or death.
Hematoxylin and eosin slides were available for histologic review. The tumor subtypes and grade were re-reviewed for confirmation by a single experienced pathologist (PMF). Histologic subtypes were based on World Health organization (WHO) guidelines [14]. For our study, we used the two-tier grading system for ovarian carcinoma grading [15]. Only serous types, high grade and late stage tumors were included in the study. Out of total 250 cases, 148 cases fit our inclusion criteria and they were considered for further evaluation.
Immunohistochemistry
Paraffin-embedded tissues from 148 patient samples were included in this study. A tissue microarray was constructed as described previously by Kononen et al. [16]. Briefly, after carefully choosing the morphologically representative region from the hematoxylin–eosin (HE) section, a 0.6 mm core was punched from the individual paraffin-embedded block (donor block), and transferred to the receiver paraffin-embedded block (receiver block). To overcome tumor heterogeneity, 3 core biopsies were performed from three different areas of each tumor. One section was stained with H&E to confirm the presence of the tumor by light microscopy.
Four µm sections from the formalin-fixed, paraffin-embedded tissue were processed for immunohistochemistry (IHC). IHC for PAX8 was done as described previously [17]. Endogenous peroxidase was blocked with 0.3% hydrogen peroxidase for 5 min. Antigen retrieval was carried out in high citrate buffer for 3 min in a steam-cooker. Then, sections were incubated overnight with Pax8 antibody (monoclonal antibody, 1:400 dilutions; Biocare lab, San Francisco, CA). A subsequent reaction was performed with the biotin-free HRP enzyme labeled polymer of the Envision plus detection system (Dakocytomation, Carpinteria, CA-USA). Diaminobenzidine complex was used as chromogen. Normal kidney and thyroid tissues were used as positive control. In negative controls, a normal goat serum was used instead of the primary antibody resulting in a lack of detectable staining. Two pathologists (PMF and DS) reviewed the IHC slides on a double-headed microscope, and the staining intensity was interpreted as negative, weak, moderate and strong. Only positive nuclear staining was considered positive. Only the staining intensity was taken into consideration for primary statistical analysis and it was divided into the following 2 groups; negative was considered as negative (group I) and weak/moderate/strong was considered as positive (group II).
Statistical analysis
Statistical analyses were performed by R (http://www.r-project.org/). The clinical parameters used for modeling are age, histologic subtype, tumor grade, recurrence, status, optimal debulking, recurrence time and survival time. To test the association between the biomarker and the clinical parameters, Fisher's exact test was performed for categorical parameters and the logistic regression model was used for the continuous variable age. For survival analysis, the Kaplan–Meier method with log-rank test was used to calculate the cumulative survival time, and check both the overall survival (OS) and recurrence free survival (RFS) difference between patients with different PAX8 statuses. All reported p values were two sided and p values<0.05 were considered statistically significant.
Results
The characteristics of the study population are presented in Table 1. The mean age of the study population was 62 years (range 33–89 years), and the median duration of follow-up was 39 months (ranging 0.6–161 months). One hundred twenty-five out of 148 patients (84%) were stage III and 23/148 (16%) were stage IV. As for disease outcome, 41% of patients had recurrences, 52% had persistent disease, and 6% had no recurrences. The median survival was 39 months (0.6–161 months). 66% of patients were considered to have optimal debulking and 33% had suboptimal tumor debulking. As for disease status at follow-up, 84% were dead of disease (DOD), 9% alive with evidence of disease (AWED), and only 7% were alive with no evidence of disease (ANED).
Table 1.
Clinical and pathologic features of 148 patients with ovarian serous carcinoma (OSC).
| # of cases | % | |
|---|---|---|
| No. of evaluable patients | 148 | 100 |
| Age | ||
| Median | 62 | |
| Range | 33–89 | |
| Recurrence | ||
| No | 9 | 6 |
| PD | 77 | 52 |
| Yes | 61 | 41 |
| Missing | 1 | 1 |
| PAX8 | ||
| Negative (0) | 57 | 39 |
| Positive (1,2,3) | 91 | 61 |
| Stage | ||
| III | 125 | 84 |
| IV | 23 | 16 |
| Status | ||
| ANED | 11 | 7 |
| AWED | 13 | 9 |
| DOD | 124 | 84 |
| Optimal debulking | ||
| No | 49 | 33 |
| Yes | 98 | 66 |
| Unknown | 1 | 1 |
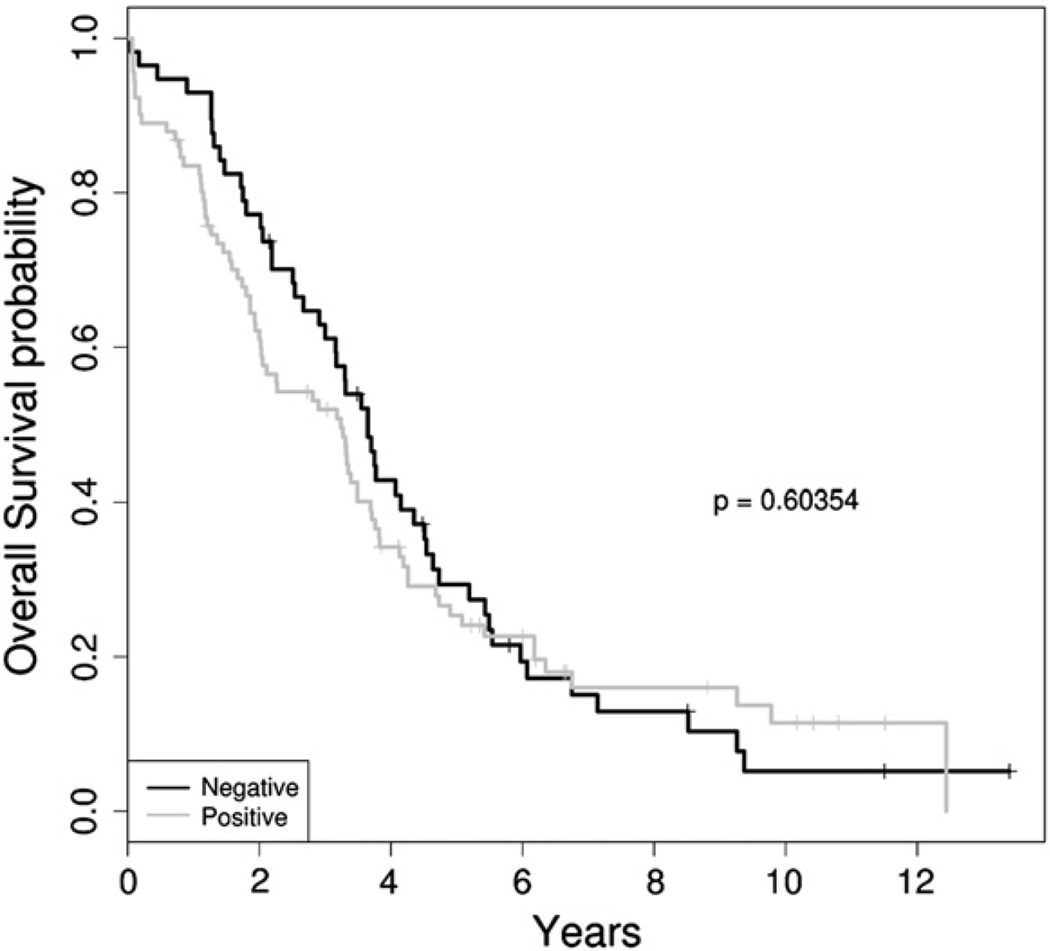
PAX8 was immunoexpressed in 91/148 tumors (61%), and negative in 57/148 (39%) of tumors (Figs. 1a–c). There was no association between PAX8 expression and tumor debulking (p=0.28, OR=1.46 [CI: 0.69–3.12]), tumor stage (p=0.82, OR=1.21 [CI: 0.44–3.55]), and tumor recurrence (p=0.73, OR=0.72 [CI: 0.11–3.78]). Lastly, there was no association between PAX8 and DOD (p=0.36, OR=0.61 [CI: 0.2–1.69]) (Table 2 and Figs. 2, 3).
Fig. 1.
An example of strong PAX8 expression (a), weak expression of PAX8 (b), and negative PAX8 expression (c).
Table 2.
Association of Pax8 protein immunoexpression and the clinical factors.
| Pax8 neg. | Pax8 pos. | p Value | Odds ratio |
CI for odds ratio |
|
|---|---|---|---|---|---|
| Age | 59.7a | 63.8 | 0.043 | 1.03 | 1.00–1.06 |
| Stage | |||||
| III | 49 (85.96)b | 76 (83.52) | 0.817 | 1.21 | 0.44–3.55 |
| IV | 8 (14.04) | 15 (16.48) | |||
| Optimal debulking | |||||
| No | 22 (38.6) | 27 (30) | 0.2883 | 1.46 | 0.69–3.12 |
| Yes | 35 (61.4) | 63 (70) | |||
| Recurrence | |||||
| No | 3 (10.71) | 6 (14.29) | 0.7322 | 0.72 | 0.11–3.78 |
| Yes | 25 (89.29) | 36 (85.71) | |||
| PD | |||||
| No | 28 (49.12) | 42 (46.67) | 0.8667 | 1.08 | 0.53–2.2 |
| Yes | 29 (50.88) | 48 (53.33) | |||
| DOD | |||||
| No | 7 (12.28) | 17 (18.68) | 0.3644 | 0.61 | 0.2–1.69 |
| Yes | 50 (87.72) | 74 (81.32) | |||
| ANED | |||||
| No | 53 (92.98) | 84 (92.31) | 1 | 1.1 | 0.27–5.39 |
| Yes | 4 (7.02) | 7 (7.69) |
Continuous variables are tested by logistic regression and categorical variables are tested by the fisher exact test.
Mean.
Count (%).
Fig. 2.
Kaplan–Meier survival analysis revealed the association of PAX8 with overall survival (p=0.603). The black curve is for PAX8 negative patients, while the gray curve is for PAX8 positive patients.
Fig. 3.
Kaplan–Meier survival analysis revealed the association of PAX8 with recurrence free survival (p=0.414). The black curve is for PAX8 negative patients, while the gray curve is for PAX8 positive patients.
Discussion
Pair-Box 8 (PAX8) plays an essential role in the normal development of the thyroid gland and the female genital tract [6,7]. In the reproductive tract, PAX8 is immunoexpressed in 89% of mullerian-type neoplasms including those from the ovary, uterus, peritoneum and fallopian tubes [10–12]. Multiple studies showed that PAX8 deficient mice are infertile due to the absence of a uterus and vaginal openings, indicating that PAX8 is important for the development of the mullerian duct [6]. One study showed that PAX8 expression was restricted to secretory cells of healthy fallopian tube mucosal lining and it is expressed in ovarian cancer but absent in the normal ovarian surface epithelium, suggesting that PAX8 may play a critical role in the development of high grade serous ovarian cancer [9]. PAX8 gene was found to be a lineage-specific survival gene that is essential for proliferation of ovarian cancer cells, overexpressed in ovarian cancer lines and amplified in 16% of primary ovarian tumors [8]. In addition, silencing PAX8 using sh-RNA led to a 50% decrease in cell viability in ovarian cancer cell lines overexpressing PAX8. Despite the fact that PAX8 plays an important role in ovarian cancer pathogenesis, its prognostic value in human ovarian samples is still widely unknown. Our work is an attempt to explore the prognostic value of PAX8 protein in ovarian carcinoma. Because the majority of ovarian cancers are of the serous histological subtype (90%) and 70% of patients with ovarian carcinomas present with advanced stage disease, and they are of high grade, we chose to limit our study to this category of patients.
In our series of 148 patients with late stage, high grade serous carcinoma, we found PAX8 to be expressed in a large number of cases (61%) which confirms previous studies. However, when we evaluated PAX8 for its prognostic significance, PAX8 failed to show any association with residual tumor size after cytoreduction, which is considered one of the most important prognostic factors after tumor grade and stage [4]. In addition, our study showed that PAX8 failed to predict patient outcome including disease recurrence, death of disease or overall survival. We clearly showed that even though PAX8 is overexpressed in OSC, it does not have an independent value in predicting disease outcome in patients with high grade, advanced stage OSC. These findings are in concordance with Kobel et al. where the authors failed to show PAX8 immunoexpression as a significant prognostic factor in high grade ovarian serous carcinoma [18]. Despite this, the high number of human OSC cases expressing PAX8 protein makes silencing PAX8 by targeted antibody an attractive therapeutic approach. Targeted therapy for OSC is especially important given the limited therapeutic options for such a lethal disease.
In summary, PAX8 protein was overexpressed in a large number of high grade late stage OSC and PAX8 seems to have no value in predicting outcome in these patients. However, inhibition of PAX8 could be a promising novel therapeutic agent for these patients.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.The National Cancer Institute's Surveillance, Epidemiology and End Results Program < http://seer.cancer.gov/>. The Center for Disease Control and Prevention's National Program of Cancer Registries Web site < http://www.cdc.gov/cancer/npcr/uscs/index.htm>. 2012
- 2.Colombo N, Van Gorp T, Parma G, Amant F, Gatta G, Sessa C, et al. Ovarian cancer. Crit Rev Oncol Hematol. 2006;60:159–179. doi: 10.1016/j.critrevonc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Winter WE, III, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2007;25:3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 4.Skirnisdòtir I, Sorbe B. Prognostic factors for surgical outcome and survival in 447 women treated for advanced (FIG0-stages III–IV) epithelial ovarian carcinoma. Int J Oncol. 2007;30:727–734. [PubMed] [Google Scholar]
- 5.Wimbeger P, Lehmann N, Kimmig R, Burges A, Meier W, Du Bois A. Prognostic factors for complete debulking in advanced ovarian cancer and its impact on survival. An explatory analysis of a prospectively randomized phase III study of the Arbeitsgemeinschaft Gynaekologische Onkologie ovarian cancer study group (AGO-AVAR) Gynecol Oncol. 2007;106:69–74. doi: 10.1016/j.ygyno.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Mittag J, Winterhager E, Bauer K, Grummer R. Congenital hypothyroid female pax8-deficient mice are infertile despite thyroid hormone replacment therapy. Endocrinology. 2007;148:719–725. doi: 10.1210/en.2006-1054. [DOI] [PubMed] [Google Scholar]
- 7.Bouchard M. Transcriptional control of kidney development. Differentiation. 2004;72:295–306. doi: 10.1111/j.1432-0436.2004.07207001.x. [DOI] [PubMed] [Google Scholar]
- 8.Cheung HW, Cowley GS, Weir BA, Boehm JS, Rusin S, Scott JA, et al. Systematic investigation of genetic vulnerabilities across cancer cell lines reveals lineage-specific dependencies in ovarian cancer. Proc Natl Acad Sci U S A. 2011;108:12372–12377. doi: 10.1073/pnas.1109363108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowen NJ, Logani S, Dickerson EB, Kapa LB, Akhtar M, Benigno BB, et al. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol Oncol. 2007;104:331–337. doi: 10.1016/j.ygyno.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 10.Laury AR, Hornick JL, Perets R, Krane JF, Corson J, Drapkin R, et al. PAX8 reliably distinguishes ovarian serous tumors from malignant mesothelioma. Am J Surg Pathol. 2010;34:627–635. doi: 10.1097/PAS.0b013e3181da7687. [DOI] [PubMed] [Google Scholar]
- 11.Laury AR, Peters R, Piao H, Krane JF, Barletta JA, French C, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol. 2011;35:816–826. doi: 10.1097/PAS.0b013e318216c112. [DOI] [PubMed] [Google Scholar]
- 12.Nonaka D, Chiriboga L, Soslow RA. Expression of Pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am J Surg Pathol. 2008;32:1566–1571. doi: 10.1097/PAS.0b013e31816d71ad. [DOI] [PubMed] [Google Scholar]
- 13.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 14.Creasman WT. Announcements: FIGO stages: 1988 revision. Gynecol Oncol. 1989;35:125–127. [Google Scholar]
- 15.Malpica A, Deavers MT, Lu K, Bodurka DC, Atkinson EN, Gershenson DM, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28:496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 17.Mhawech-Fauceglia P, Wang D, Menesses T, Chandavarkar U, Ough F, Lin Y, et al. PAX8 is a reliable marker in making the diagnosis in advanced stage epithelial ovarian carcinoma and primary peritoneal carcinoma for neoadjuvant chemotherapy on cell block and biopsy specimens. Histopathology. 2012;60:1019–1020. doi: 10.1111/j.1365-2559.2011.04172.x. [DOI] [PubMed] [Google Scholar]
- 18.Kobel M, Kalloger SE, Boyd N, McKinney S, Mehl E, Palmer C, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. Plos one. 2008;12:e232. doi: 10.1371/journal.pmed.0050232. [DOI] [PMC free article] [PubMed] [Google Scholar]