Abstract
Prior cannabis use, compared to nonuse, is reported to be associated with less cognitive impairment in schizophrenia. The age of cannabis use and the persistent influence of cannabis use on cognitive function has not been examined across the psychosis dimension. Ninety-seven volunteers with psychosis (schizophrenia, schizoaffective, or bipolar psychosis) and 64 controls were recruited at the Dallas site of the Bipolar-Schizophrenia Network on Intermediate Phenotypes consortium. Cannabis use history obtained in a semi-structured manner was used to categorize subjects into nonusers, adolescent-onset users, and late-onset users. The a priori hypothesis tested was that individuals with psychosis and a history of adolescent cannabis use (ACU) would have better global neuropsychological performance, as measured by the Brief Assessment of Cognition in Schizophrenia (BACS) battery, compared to those with psychosis and no cannabis use history. BACS Composite scores were significantly higher in individuals with psychosis with ACU compared to individuals with psychosis and no prior cannabis use. In subgroup analyses, ACU influenced global cognition in the schizophrenia/schizoaffective (SCZ) subgroup but not the bipolar psychosis subgroup. Exploratory analyses within the SCZ group, suggest that ACU was associated with better performance in specific domains compared to non-ACU groups. There are distinct associations between age of cannabis use and neuropsychological function across psychotic illnesses. Specifically, ACU is associated with better cognitive function in SCZ but not bipolar psychosis. This age-dependent and diagnosis-specific influence of cannabis may need to be factored into the design of future cognitive studies in SCZ.
Key words: adolescence, neuropsychology, schizophrenia, schizoaffective, bipolar, marijuana
Introduction
The acute use of cannabinoids by healthy individuals can negatively impact cognition1 with evidence of residual cognitive deficits in some cases2,3 but of unclear duration.4–7 Significant adolescent-onset cannabis use (ACU), however, has a more pronounced impact on cognition,7–10 with a recent prospective study finding that adolescent-onset, but not adult-onset, cannabis use is associated with persistent cognitive deficits, most pronounced in executive function and processing speed9 in otherwise healthy controls. In schizophrenia, an illness in which cognitive impairment is a core symptom,11–13 one might expect that ACU would be associated with greater cognitive impairment. On the contrary, there is evidence suggesting that ACU in schizophrenia is associated with better performance on specific cognitive tasks.14–17
In this study, we sought to determine if the association between ACU and cognitive function extends beyond schizophrenia to other psychotic disorders. We made clear distinctions between adolescent-onset and adult-onset cannabis use to determine the potential influence of age of cannabis initiation on cognitive function. We compared neuropsychological performance of individuals with schizophrenia, schizoaffective disorder or bipolar I disorder with psychosis, grouped together as Psychosis, to Control participants. Cognitive function was tested using the BACS (Brief Assessment of Cognition of Schizophrenia) battery, a validated and reliable tool widely used in schizophrenia research.18,19 Psychosis and Control groups were categorized by cannabis use into (1) cannabis nonusers, (2) adolescent-onset cannabis users, and (3) late-onset cannabis users. We tested the hypothesis that Psychosis with ACU group would show better cognitive performance compared to Psychosis nonusers. Next, we tested this hypothesis in schizophrenia/schizoaffective and bipolar I with psychosis subgroups separately to determine if adolescent cannabis use had similar or different effects on cognitive performance between the 2 Psychosis subgroups.
Methods and Materials
Participants
This study uses data from the University of Texas Southwestern site of the Bipolar-Schizophrenia Network on Intermediate Phenotypes study.20 Patient volunteers were eligible if they were between 15 and 65 years old, had a DSM-IV21 diagnosis of schizophrenia, schizoaffective disorder, or bipolar I disorder with psychotic features. Control participants were in the same age range and had no personal history of any psychotic or recurrent mood disorder and no family history of schizophrenia, schizoaffective disorder or bipolar disorder in first- or second-degree relatives (tables 1 and 2). All participants included provided sufficient substance use history and completed cognitive testing. A negative urine drug screen for all illicit substances was required for entrance to study. Exclusion criteria for both groups were (1) organic brain disease, including history of seizures, serious head injury, encephalopathy, and neurological malignancy; (2) intellectual disability; (3) diagnosis of DSM-IV substance abuse within the past month, substance dependence (excluding nicotine) within the past 3 months, or an extensive history of substance dependence; (4) unstable medical illness; and (5) the inability to read and speak English.
Table 1.
Demographic and Clinical Information for Controls and Psychosis
| Controls | Psychosis | |||||
|---|---|---|---|---|---|---|
| Nonusers | ACU | LCU | Nonusers | ACU | LCU | |
| N | 38 | 16 | 10 | 48 | 33 | 16 |
| Age | 40.8 (11.6) | 37.3 (11.9) | 39.7 (12.2) | 40 (11.7) | 38.2 (10.8) | 40.7 (9) |
| Edu (y)a | 13.8 (1.7) | 14.4 (1.9) | 14.3 (2.1) | 13.6 (2.4) | 12.7 (1.8) | 12.3 (13) |
| WRATa | 99.3 (14.1) | 103.9 (18.6) | 103.6 (12) | 96.2 (13.5) | 96.8 (13.5) | 95.1 (13) |
| SFSa | 145.9 (15) | 139.8 (17.6) | 150.8 (18.9) | 117.4 (27.4) | 116.1 (27.8) | 103.2 (16.1) |
| PANSS positive | 18.4 (4.4) | 18.2 (4.7) | 20.6 (2.8) | |||
| PANSS negative | 14.6 (3.9) | 14.8 (4.3) | 16.4 (3.4) | |||
| PANSS total | 69.3 (14.2) | 67.7 (15.1) | 75.8 (11.8) | |||
| Male (%)b | 37 | 63 | 60 | 38 | 67 | 38 |
| Illicit drugs (%)b | 8 | 69 | 30 | 25 | 94 | 69 |
| AUD (%)b | 0 | 44 | 10 | 15 | 73 | 50 |
| Antipsychotic medications (%) | — | — | — | 92 | 90 | 91 |
Note: Nonusers, cannabis use < 5 times; ACU, adolescent-onset cannabis use; LCU, late-onset cannabis use; Edu, Education; WRAT, Wide Range Achievement Test-4 Reading subtest; SFS, Birchwood Social Functioning Scale; PANSS, Positive and Negative Syndrome Scale; Illicit Drugs, use of illicit drugs other than cannabis; AUD, alcohol use disorder.
aControl different from Psychosis.
bDifferent across all 6 groups.
Table 2.
Demographic and Clinical Information for SCZ and BPP Subgroups
| SCZ | BPP | |||||
|---|---|---|---|---|---|---|
| Nonusers | ACU | LCU | Nonusers | ACU | LCU | |
| N | 33 | 24 | 12 | 15 | 9 | 4 |
| Age | 39.5 (11.9) | 37.9 (11.8) | 40.1 (9.9) | 40.7 (11.7) | 39 (8.03) | 42.5 (6.8) |
| Edu (y)a | 13.1 (2.3) | 12.7 (1.7) | 11.7 (2.4) | 14.7 (2.1) | 12.8 (2.2) | 13.76 (0.5) |
| WRATa | 94.2 (13.2) | 94 (14.7) | 92 (13.5) | 100.6 (13.6) | 100.6 (8.7) | 105 (5.2) |
| SFSa | 110.7 (25.8) | 114.1 (27) | 99.2 (25.8) | 131.3 (26.2) | 121.4 (30.9) | 118 (26.1) |
| PANSS positive | 19.7 (4.2) | 19.5 (4.2) | 21 (2.6) | 15.5 (3.4) | 14.6 (4.1) | 19.5 (3.7) |
| PANSS negative | 15.8 (3.7) | 16.4 (3.6) | 17.1 (2.7) | 12.1 (3.2) | 10.8 (3.3) | 14.5 (4.8) |
| PANSS total | 73.4 (13) | 73.2 (13) | 77.1 (10.3) | 60.3 (12.7) | 53 (9.5) | 72 (17.1) |
| Male (%)b | 42 | 63 | 50 | 27 | 78 | 0 |
| Other drugs (%)b | 18 | 92 | 75 | 40 | 100 | 50 |
| AUD (%)b | 12 | 67 | 50 | 20 | 89 | 50 |
| Antipsychotic medications (%)b | 92 | 90 | 91 | 80 | 78 | 100 |
Note: SCZ, schizophrenia and schizoaffective disorder; BPP, bipolar psychosis. See table 1 for other abbreviations.
aSCZ different from BPP.
bDifferent across all 6 groups.
Participants within each group (Psychosis, Controls) were categorized on the basis of cannabis use: (1) Nonuse (cannabis use on less than 5 occasions during their lifetime), (2) Adolescent-onset cannabis use (ACU) before 18 years old, (3) Late-onset cannabis use (LCU) starting at or after 18 years old (table 1). The Psychosis group was also divided by diagnosis into schizophrenia/schizoaffective disorder (SCZ) and bipolar I disorder with psychotic features (BPP) subgroups.
Assessments
Participants completed a clinical interview, as well as structured evaluations, including Structured Clinical Interview for DSM-IV diagnosis (SCID I),22 Positive and Negative Syndrome Scale (PANSS),23 Birchwood Social Functioning Scale24 and Hollingshead Index of Social Position. In addition to the substance abuse module of the SCID, a semi-structured interview carefully detailing substance use history including age of onset, period and frequency of greatest consumption, and most recent use was conducted. Information about cannabis use history determined the participant’s group assignment as above. Participants were considered to have significant non-cannabis substance use (Other subst) if they used illicit drugs other than cannabis on more than 4 occasions or met DSM-IV criteria for an alcohol use disorder. Cognitive testing included the Wide Range Achievement Test-4 (WRAT-4) Reading subtest,25 used as an estimate of premorbid IQ, and the Brief Assessment of Cognition in Schizophrenia (BACS),18 which includes 6 subtests and an overall Composite score. The 6 subtests are Verbal memory (list learning), Digit sequencing, Token motor, Verbal fluency, Symbol coding, and the Tower of London. Written informed consent was obtained from all participants after a detailed explanation of experimental procedures. The study was approved by the Institutional Review Board at UT Southwestern Medical Center.
Statistical Analyses
Demographic and clinical data between groups were compared by ANOVA for continuous or χ2 for categorical variables. All BACS scores were reported as age- and gender-corrected z-scores. A General Linear Model 2-way Analysis of Covariance ANCOVA with 2-by-3 factorial design (group [Psychosis vs Controls] × cannabis use [nonusers, ACU, LCU]) was used for cognitive outcomes, with the primary measure being BACS Composite score. Similar 2-by-3 ANCOVAs were used for subgroup analyses of BACS Composite comparing (SCZ vs Controls) × cannabis use and (BPP vs Controls) × cannabis use. We tested our a priori hypothesis separately. T tests were used for exploratory analyses examining individual BACS domains. We adopted the domain assignment proposed by Keefe et al,19 which decreased the number of outcomes from 6 to 4 by averaging the z-scores for Digit Sequencing, Token Motor, and Verbal Fluency to form a single Processing Speed (PS) score. Verbal memory (VM), Working memory (WM; Digit sequencing task), and Executive function (EF; Tower of London) comprised the remaining 3 domains. The BACS battery is age and gender adjusted. Other demographic variables that differed across groups were evaluated for inclusion as covariates. Those variables that did not significantly affect the primary outcome were removed from the model. Significance level was set at P < .05. All analyses were performed in Statistica (Statsoft, Inc, version 9.1).
Results
Demographics
The demographic and clinical characteristics of Psychosis and Control volunteers are summarized in table 1 (ANOVA and χ2 results in supplementary tables S1 and S2). Controls had higher education, WRAT-4 Reading score, and Birchwood social functioning scale score than the Psychosis group. However, there was no main effect of cannabis use or interaction of group × cannabis use for these variables. Use of other substances (Other subst) was greater in Psychosis compared to Controls. Within the Psychosis subgroups (SCZ, BPP), there was no statistically significant within-group difference in use of antipsychotic medications or of lifetime antipsychotic exposure, measured in chlorpromazine equivalents (F = 0.9, df = 5,56, P = .5).
Primary Analyses of Global Cognitive Performance
For the primary analyses on global cognitive performance, education, WRAT-4 Reading score, Birchwood Social Function Score and Other substance use were evaluated for inclusion as covariates. Of these, only WRAT-4 Reading score significantly impacted the BACS Composite score; the others were thus removed from the final model. All analyses shown below were covaried for WRAT-4 Reading score.
Psychosis vs Controls.
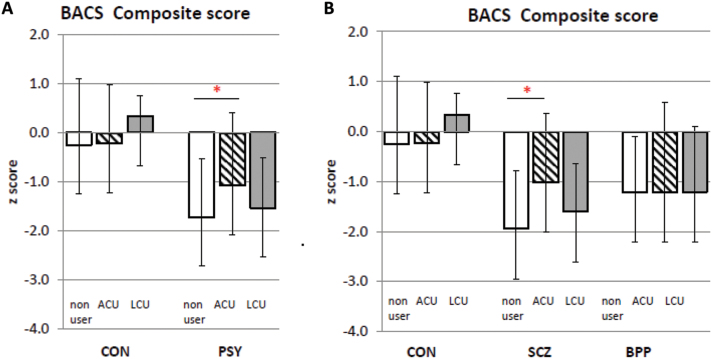
BACS Composite scores were different between the Psychosis and Control groups (F = 28.9, df = 1,155, P < .001) but not affected by cannabis use (F = 1.56, df = 2,154, P = .31) and did not show a group × cannabis use interaction (F = 2.17, df = 2,154, P = .11; figure 1A). Testing our a priori hypothesis, we found that the Psychosis with ACU group performed significantly better on the BACS Composite score than Psychosis nonusers (F = 5.54, df = 1,76, P = .02).
Fig. 1.
Brief Assessment of Cognition in Schizophrenia (BACS) Composite score: interaction of group and cannabis use. Panel A: Performance on BACS composite scores between Control (CON) and psychosis (PSY) diagnostic groups, each divided into nonusers (white bars), adolescent cannabis users (ACU; striped bars) and late onset cannabis users (LCU; hatched bars). Panel B shows schizophrenia/schizoaffective (SCZ) and Bipolar with psychosis (BPP) groups (that together comprise the PSY group shown in Panel A) compared to CON. Significant differences within groups are shown by an asterix (P < .05). Data shown are mean ± SD.
SCZ vs Controls.
BACS Composite scores differed significantly between SCZ and Controls (F = 26.3, df = 1,127, P < .001), with no effect of cannabis use (F = 2.2, df = 2,126, P = .11) and a significant diagnosis × cannabis use interaction (F = 3.25, df = 2,126, P = .042; figure 1). Post hoc analysis revealed that SCZ with ACU performed significantly better on the BACS Composite compared to SCZ nonusers (F = 5.44, df = 1, 54, P = .022). In contrast, SCZ with ACU group performance on BACS Composite was not significantly different from Controls with ACU group (F = 0.89, df = 1,37, P = .35).
BPP vs Controls.
ANCOVA of the BACS Composite score found differences between BPP and Controls (F = 14.7, df = 1,86, P < .001) but no differences among cannabis use groups (F = 0.07, df = 2,85, P = .93) nor any interaction between diagnosis and cannabis use (F = 0.47, df = 2,85, P = .62; figure 1).
Exploratory Analyses of Individual Domains
Given the absence of an effect of cannabis use on BACS composite score for the BPP group, we report only the exploratory analyses between the Control and SCZ groups for the BACS domain scores.
Differences Among Control Groups.
We did not observe any significant differences between the Control nonusers and Control with ACU groups (all F < 1.7, all P > .2). Similarly, there were no differences between the Control (CON) nonusers and Control with LCU (all F < 2.6, all P > .1) or between Control with ACU and Control with LCU groups (all F< 2.45, all P > .13).
Differences Among SCZ Groups.
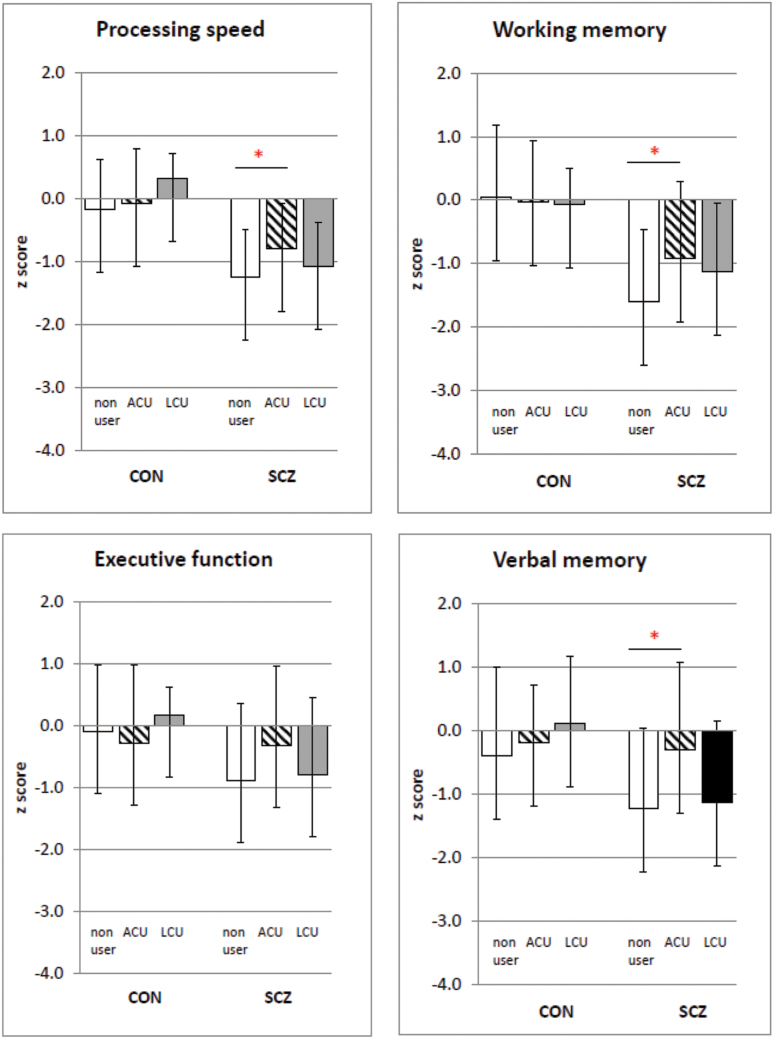
There were significant differences between SCZ nonusers and SCZ with ACU on most domains tested with SCZ with ACU performing better on most domains including PS (F = 6.2, df 1,54, P = .02), WM (F = 5.0, df 1,54, P = .03), and VM (F = 7.6, df 1,54, P = .01) but not EF (F = 3.1, df 1,54, P = .08). There were no differences in any of the subtests between SCZ nonusers and SCZ with LCU (all F < 1.5, all P > .22). There were also no differences between SCZ with ACU and SCZ with LCU groups (all F < 2.8, all P > .1). In the SCZ with ACU group, the mean age of cannabis initiation was 14.3±2.05 years of age while mean age of onset of psychosis was 22.9±9.1 years of age. There were, however, 6 volunteers in this group who reported onset of psychosis before that of cannabis use. We conducted analyses without these 6 cases and find that the significant differences between SCZ with ACU and SCZ nonusers on BACS total scores are not affected (F = 26.6, df = 49, P = .013) with the ACU group demonstrating better cognitive function.
Differences Between SCZ and Control Groups.
Comp arisons between SCZ with ACU vs Controls with ACU did not reveal differences in most domains including WM (F = 2.9, df 38, P = .1), EF (F = 1.31, df 1,38, P = .3) or VM (F = 0.02, df 1,38, P = .9; figure 2) except for PS (F = 4.4, df 1,38, P < .04). On the other hand, the comparisons between Control nonusers vs SCZ nonusers showed significant differences in all the domains tested (all F > 4.5, all P < .03), as would be expected. Similarly, Control with LCU vs SCZ with LCU comparisons showed differences in all domains (all F > 4.2, all P < .05), except for EF (F = 6.3, df 1,19, P = .02).
Fig. 2.
Brief Assessment of Cognition in Schizophrenia (BACS) domain scores for Control (CON) and schizophrenia/schizoaffective (SCZ) groups, divided into nonusers, adolescent-onset cannabis users (ACU) and late-onset cannabis users (LCU). Significant differences within each diagnostic group are shown by an asterisk (P < .05). Data shown are mean ± SD. Significant differences within each diagnostic group are shown by an asterisk (P < .05). Data shown are mean ± SD.
PANSS Scores
This analysis was conducted to determine if cannabis use in SCZ or BPP is associated with symptoms captured in the PANSS scores. A 2×2 ANCOVA (diagnosis [SCZ vs BPP] × cannabis use [nonusers, ACU]) for PANSS positive, negative, and total scores showed an effect of diagnosis (all F 17.5 and 23.3, df = 1, all P < .001), but no effect of cannabis use (all F between 0.2 and 1.46, df = 1, all P > .23) or diagnosis × cannabis use interaction (all F between 0.16 and 1.3, df = 1, all P = .25; supplementary figure S1).
Discussion
This study is, to the best of our knowledge, the first to compare the influence of a history of adolescent cannabis use on cognitive function in individuals within the psychosis dimension, followed by comparison of the respective contributions of either schizophrenia/schizoaffective or bipolar I with psychosis subgroups to this cognitive performance.
The main finding of this study is that adolescent-onset cannabis use has a moderating effect on cognitive function in individuals with psychotic illnesses. When individuals with psychosis are divided into SCZ and BPP groups, we find that cannabis use in the BPP group is not associated with cognitive function. In the SCZ group, however, a history of cannabis use is associated with cognitive function, particularly when cannabis use starts during the adolescent years. SCZ with ACU individuals have better global cognitive function than all other SCZ individuals (ie, nonusers and those who start using cannabis at or after age 18) and better performance on most of the cognitive domains tested. In fact, global neuropsychological performance in the SCZ and ACU group was not significantly different from Controls with ACU. Our exploratory analyses comparing Control with ACU and SCZ with ACU suggest that specific BACS domains, namely executive function, working memory and verbal memory, are not significantly different between groups. These data suggest that the SCZ with ACU group may have discrete deficits in contrast to other SCZ cases where deficits are more global in nature.
The reasons for this paradoxical effect on cognitive function in SCZ with ACU are not known. There are several possibilities to consider. One is that schizophrenia is a heterogeneous illness and ACU could define a schizophrenia subgroup. There is a substantial body of literature implicating cannabis as a risk factor for psychosis,26–32 with greater risk with use at a younger age.33–35 It is possible that individuals who will inevitably develop a psychotic illness may have greater biological loading than those who have a genetic predisposition but require an additional environmental insult, such as cannabis use during brain maturation, to trigger conversion to psychosis. The former may have more generalized brain dysfunction, whereas the path to psychosis induced by ACU may have more selective effects on psychosis and a subset of cognitive domains. This could explain our findings of similar psychosis symptom scale scores but less impairment in global cognition in SCZ with ACU.
A second possibility could be that the process of obtaining cannabis self-selects for individuals with better cognition. While the association between premorbid function and cannabis use is mixed,14,36–39 the assumption is that obtaining cannabis requires a high level of social functioning. In this study, we did not find any differences between SCZ groups in psychosocial function as measured by the Birchwood Social Functioning Scale and Hollingshead index scores.
A third theory suggests that cannabis is neuroprotective, based upon effects of cannabinoids in animal models40 and increased serum levels of brain derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in medication-naïve schizophrenia volunteers with a history of cannabis abuse.41,42 Imaging studies, however, demonstrate greater loss of brain volume in first episode schizophrenia participants who use cannabis43,44 which is not consistent with a neuroprotective effect. Further, there is no evidence that healthy controls with ACU have improved cognitive function, rather reports suggest greater impairment.2,10
Our findings, based on a priori hypotheses and using a validated and reliable battery to measure cognition in schizophrenia, suggest that ACU is associated with less severe deficits in cognition in SCZ but not in bipolar psychosis. The former finding is consistent with 2 prior subgroup analyses of ACU.14,16 However, neither of these studies compared ACU to a group with negligible cannabis exposure (in our study less than 5 occasions). One study defines nonuse as less than weekly use for at least 12 months14 while the other compares early- to late-onset cannabis use rather than nonuse.16 They both examined heavy use; thus, our study suggests a greater susceptibility to the effects of cannabis than was previously supported. We also show that cannabis use does not impact cognitive function in bipolar psychosis, which to our knowledge has not been previously described. Two previous studies in bipolar disorder, irrespective of the age of cannabis use,45,46 report better cognitive performance in some domains for cannabis users as compared to nonusers. We do not find an effect of adolescent cannabis use in the control population which is contrary to previous studies that have reported deficits in cognitive function following varying periods of abstinence.9,47 One reason for this discrepancy could be our stringent definition of cannabis use as use on 5 or more occasions, a relatively low threshold compared to the studies that find persistent cognitive deficits with cannabis use.
There are limitations to consider in this study. First, the cannabis use data is based on retrospective self-report, restricting the reliable estimation of cannabis use in terms of amount and frequency. Similarly, other substance use is also based on self-report. Substance dependence for the prior 3 months or substance abuse for the prior 1 month was an exclusion criterion for entry to this study. We evaluated alcohol use disorders and other substance use (besides cannabis) as a potential covariate in our analysis and find that this did not influence our outcome measures, similar to findings in previous reports. Another potential factor to take into account is sample size in the LCU groups. Dividing participants into groups of cannabis users resulted in these cells having 10–12 subjects that could lead to type I or type II errors. The major comparisons between nonusers and ACU groups, however, were still adequately represented. Finally, while the BACS was designed specifically to be sensitive to the generalized cognitive deficits seen most commonly in schizophrenia, it might not be attuned to other deficits that could be related to cannabis use.
In summary, we found superior performance on a measure of global cognition, as well as certain cognitive domains, in Psychosis with adolescent-onset cannabis use as compared to Psychosis nonusers. This difference was attributable to the Schizophrenia/schizoaffective subgroup and not the Bipolar psychosis subgroup.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was supported by the National Institutes of Mental Health grants MH077851 (to C.A.T.), MH 078113 (to M.K.), MH 077945 (to G.P.), MH 077852 (to Gunvant K. Thaker), MH 077862 (to J.A.S.) and MH100228 (to S.G.).
Supplementary Material
Acknowledgments
Gunvant K. Thaker, MD, the former Director of the Schizophrenia Research Program at the Maryland Psychiatric Research Center, University of Maryland School of Medicine was closely involved with the conceptual and methodological aspects of the B-SNIP consortium. We tremendously appreciate his contribution. The authors also thank the families at each site who took part in this study. None of the authors report potential conflicts of interest related to this publication.
References
- 1. D’Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. [DOI] [PubMed] [Google Scholar]
- 2. Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. J Int Neuropsychol Soc. 2003;9:679–689. [DOI] [PubMed] [Google Scholar]
- 3. Solowij N, Battisti R. The chronic effects of cannabis on memory in humans: a review. Curr Drug Abuse Rev. 2008;1:81–98. [DOI] [PubMed] [Google Scholar]
- 4. Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. [DOI] [PubMed] [Google Scholar]
- 5. Fried P, Watkinson B, James D, Gray R. Current and former marijuana use: preliminary findings of a longitudinal study of effects on IQ in young adults. CMAJ. 2002;166:887–891. [PMC free article] [PubMed] [Google Scholar]
- 6. Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58:909–915. [DOI] [PubMed] [Google Scholar]
- 7. Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. [DOI] [PubMed] [Google Scholar]
- 8. Solowij N, Jones KA, Rozman ME, et al. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology. 2011;216:131–144. [DOI] [PubMed] [Google Scholar]
- 9. Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109:E2657–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Curr Drug Abuse Rev. 2008;1:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palmer BW, Dawes SE, Heaton RK. What do we know about neuropsychological aspects of schizophrenia? Neuropsychol Rev. 2009;19:365–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. [DOI] [PubMed] [Google Scholar]
- 13. Keefe RS, Eesley CE, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57:688–691. [DOI] [PubMed] [Google Scholar]
- 14. Yucel M, Bora E, Lubman DI, et al. The impact of cannabis use on cognitive functioning in patients with schizophrenia: a meta-analysis of existing findings and new data in a first-episode sample. Schizophr Bull. 2012;38:316–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de la Serna E, Mayoral M, Baeza I, et al. Cognitive functioning in children and adolescents in their first episode of psychosis: differences between previous cannabis users and nonusers. J Nerv Ment Dis. 2010;198:159–162. [DOI] [PubMed] [Google Scholar]
- 16. Jockers-Scherubl MC, Wolf T, Radzei N, et al. Cannabis induces different cognitive changes in schizophrenic patients and in healthy controls. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1054–1063. [DOI] [PubMed] [Google Scholar]
- 17. Kumra S, Thaden E, DeThomas C, Kranzler H. Correlates of substance abuse in adolescents with treatment-refractory schizophrenia and schizoaffective disorder. Schizophr Res. 2005;73:369–371. [DOI] [PubMed] [Google Scholar]
- 18. Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–297. [DOI] [PubMed] [Google Scholar]
- 19. Keefe RS, Harvey PD, Goldberg TE, et al. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophr Res. 2008;102:108–115. [DOI] [PubMed] [Google Scholar]
- 20. Tamminga CA, Ivleva EI, Keshavan MS, et al. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170:1263–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed., text rev. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 22.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 23. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 24. Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br J Psychiatry. 1990;157:853–859. [DOI] [PubMed] [Google Scholar]
- 25. Wilkinson GS, Robertson GJ. Wide Range Achievement Test—Fourth Edition. Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- 26. Andreasson S, Allebeck P, Engstrom A, Rydberg U. Cannabis and schizophrenia. a longitudinal study of Swedish conscripts. Lancet. 1987;2:1483–1486. [DOI] [PubMed] [Google Scholar]
- 27. Arendt M, Rosenberg R, Foldager L, Perto G, Munk-Jørgensen P. Cannabis-induced psychosis and subsequent schizophrenia-spectrum disorders: follow-up study of 535 incident cases. Br J Psychiatry. 2005;187:510–515. [DOI] [PubMed] [Google Scholar]
- 28. Henquet C, Krabbendam L, Spauwen J, et al. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. BMJ. 2005;330:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kristensen K, Cadenhead KS. Cannabis abuse and risk for psychosis in a prodromal sample. Psychiatry Res. 2007;151:151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33:15–21. [DOI] [PubMed] [Google Scholar]
- 31. van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol. 2002;156:319–327. [DOI] [PubMed] [Google Scholar]
- 32. Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ. 2002;325:1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Caspi A, Moffitt TE, Cannon M, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–1127. [DOI] [PubMed] [Google Scholar]
- 34. Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Konings M, Henquet C, Maharajh HD, Hutchinson G, Van Os J. Early exposure to cannabis and risk for psychosis in young adolescents in Trinidad. Acta Psychiatr Scand. 2008;118:209–213. [DOI] [PubMed] [Google Scholar]
- 36. Leeson VC, Harrison I, Ron MA, Barnes TR, Joyce EM. The effect of cannabis use and cognitive reserve on age at onset and psychosis outcomes in first-episode schizophrenia. Schizophr Bull. 2012;38:873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ringen PA, Vaskinn A, Sundet K, et al. Opposite relationships between cannabis use and neurocognitive functioning in bipolar disorder and schizophrenia. Psychol Med. 2010;40:1337–1347. [DOI] [PubMed] [Google Scholar]
- 38. Stirling J, Lewis S, Hopkins R, White C. Cannabis use prior to first onset psychosis predicts spared neurocognition at 10-year follow-up. Schizophr Res. 2005;75:135–137. [DOI] [PubMed] [Google Scholar]
- 39. Rodriguez-Sanchez JM, Ayesa-Arriola R, Mata I, et al. Cannabis use and cognitive functioning in first-episode schizophrenia patients. Schizophr Res. 2010;124:142–151. [DOI] [PubMed] [Google Scholar]
- 40. Pope C, Mechoulam R, Parsons L. Endocannabinoid signaling in neurotoxicity and neuroprotection. Neurotoxicology. 2010;31:562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jockers-Scherubl MC, Danker-Hopfe H, Mahlberg R, et al. Brain-derived neurotrophic factor serum concentrations are increased in drug-naive schizophrenic patients with chronic cannabis abuse and multiple substance abuse. Neurosci Lett. 2004;371:79–83. [DOI] [PubMed] [Google Scholar]
- 42. Jockers-Scherubl MC, Matthies U, Danker-Hopfe H, Lang UE, Mahlberg R, Hellweg R. Chronic cannabis abuse raises nerve growth factor serum concentrations in drug-naive schizophrenic patients. J Psychopharmacol. 2003;17:439–445. [DOI] [PubMed] [Google Scholar]
- 43. Bangalore SS, Prasad KM, Montrose DM, Goradia DD, Diwadkar VA, Keshavan MS. Cannabis use and brain structural alterations in first episode schizophrenia--a region of interest, voxel based morphometric study. Schizophr Res. 2008;99:1–6. [DOI] [PubMed] [Google Scholar]
- 44. Rais M, Cahn W, Van Haren N, et al. Excessive brain volume loss over time in cannabis-using first-episode schizophrenia patients. Am J Psychiatry. 2008;165:490–496. [DOI] [PubMed] [Google Scholar]
- 45. Ringen PA, Melle I, Berg AO, et al. Cannabis use and premorbid functioning as predictors of poorer neurocognition in schizophrenia spectrum disorder. Schizophr Res. 2013;143:84–89. [DOI] [PubMed] [Google Scholar]
- 46. Braga RJ, Burdick KE, Derosse P, Malhotra AK. Cognitive and clinical outcomes associated with cannabis use in patients with bipolar I disorder. Psychiatry Res. 2012;200:242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front Psychiatry. 2013;4:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.