Abstract
Long-acting injectable (LAI) antipsychotics (LAI-APs) have several advantages over oral medications, but deaths reported in Japan during the early post-marketing phase vigilance period have raised safety concerns. We conducted a series of meta-analyses to assess whether LAI-APs affect the mortality of patients with schizophrenia. Three categorical meta-analyses of randomized controlled trials (RCTs) were performed to compare all-cause death (primary outcome) and death due to suicide: individual and pooled LAI-APs vs placebo, individual and pooled LAI-APs vs oral antipsychotics (OAPs), and head-to-head comparisons of LAI-APs. The risk ratios (RRs) and 95% CIs were calculated. We identified 52 RCTs (53 comparisons; total participants = 17 416, LAI-APs = 11 360, OAP = 3910, and placebo = 2146; mean study duration [wk]: LAI-APs vs placebo = 28.9, LAI-APs vs OAPs = 64.5). Neither pooled nor individual LAI-APs (aripiprazole, fluphenazine, olanzapine, paliperidone, and risperidone) differed from the placebo regarding the incidences of all-cause death (pooled LAI-APs: RR = 0.64, P = .37) and death due to suicide (pooled LAI-APs: RR = 0.98, P = .98). However, in a subgroup meta-analysis of only short-duration RCTs (≤13wk), pooled LAI-APs exhibited a trend toward lower incidence of all-cause death than placebo (RR = 0.29, P = .08). Pooled LAI-APs (aripiprazole, fluphenazine, haloperidol, olanzapine, paliperidone, risperidone, and zuclopenthixol) did not differ from pooled OAPs regarding all-cause death (pooled LAI-APs: RR = 0.71, P = .30) and death due to suicide (pooled LAI-APs: RR = 0.94, P = .91). Individual LAI-APs and OAPs were associated with similar risks of death. Data for head-to-head comparisons of individual LAI-APs were insufficient. In conclusion, there was no significant difference between LAI-APs and placebo or OAPs regarding all-cause death and death due to suicide.
Key words: long-acting injectable antipsychotics, mortality, schizophrenia, meta-analysis, systematic review
Introduction
People with schizophrenia have a substantially higher risk of early death in comparison with the general population1,2 (standardized mortality ratio = 3.6–3.73,4). Probable causes for this increased mortality include a greater risk of various comorbid somatic conditions5–7 and higher incidence of suicide.8,9 Second-generation antipsychotics (SGAs) are known to induce severe adverse events such as metabolic syndrome and diabetes, which are important risk factors for cardiovascular and cerebrovascular diseases.10 Moreover, patients with schizophrenia often lead a more sedentary lifestyle and are less motivated to improve their physical condition because of the effects of medication and/or their symptoms, which further increase their risk of developing metabolic syndrome.11 A meta-analysis by Saha et al pertaining to mortality associated with schizophrenia reported that all-cause mortality has increased since the late 1990s when SGAs became widely used. People with schizophrenia are at a high risk of attempting and committing suicide (10%–13%).8 A recent systematic review reported that suicide risk in patients with schizophrenia is particularly associated with affective symptoms, history of suicide attempt, and number of psychiatric hospitalizations for relapse.12 A meta-analysis concluded that antipsychotics are superior to placebo for relapse prevention with large effect size (risk ratio [RR] = 0.40; 95% CI = 0.33−0.49; number needed to treat to benefit = 3).13 In addition, several Finnish nationwide cohort studies reported that current antipsychotic use is associated with a lower mortality risk than non-antipsychotic use.14–16 A recent observational cohort study of schizophrenia in Sweden reported that moderate- (0.5–1.5 defined daily dose/d, ATC/DDD Index, http://www.whocc.no/atc_ddd_index/) and high-dose antipsychotics (≥1.5 defined daily dose/d) are associated with a lower overall mortality in comparison with no antipsychotic exposure (moderate: adjusted hazard ratio [HR] = 0.59, 95% CI = 0.49–0.70; high: adjusted HR = 0.75, 95% CI = 0.63–0.89).17 Furthermore, considering only suicide mortality, exposure to high doses of antipsychotics is associated with a lower risk of death in comparison with no antipsychotic exposure (adjusted HR = 0.43, 95% CI = 0.24–0.78).17 Moreover, the FIN11 study provided evidence that there are significant differences in the risks of mortality associated with individual antipsychotics.14 Considering the current use of perphenazine as the reference, the highest risk for overall mortality has been recorded with the use of quetiapine (adjusted HR = 1.41, 95% CI = 1.09−1.82) and the lowest risk with the use of clozapine (adjusted HR = 0.74, 95% CI = 0.60−0.91). All the studies cited above suggest that antipsychotic dose and type are major factors determining mortality in people with schizophrenia.
Does the form of antipsychotic drug influence mortality in people with schizophrenia? Long-acting injectable (LAI) antipsychotics (LAI-APs) are considered to have several pharmacokinetic benefits in comparison with oral antipsychotics (OAPs), including (1) an improvement in stability within the blood, (2) more consistent bioavailability, (3) more predictable medication adherence, and (4) an improvement in the pharmacokinetic profile, all of which allow for the use of lower antipsychotic dosages.18 Consequently, the LAI-APs are expected to have greater relapse prevention19 and reduce the incidence of adverse events18 in comparison with OAPs. Therefore, we hypothesized that LAI-APs decrease mortality to a greater extent in comparison with OAPs, at least in part, because of these advantages over OAPs. However, a recent meta-analysis also reported that SGA-LAIs were associated with a higher incidence of extrapyramidal symptoms in comparison with OAPs (RR = 1.45, 95% CI = 1.00–2.10).20 Moreover, from the launch of the SGA-LAI paliperidone palmitate in Japan from November 19, 2013, to May 18, 2014 (the estimated the number of users for this 6-month period was approximately 11 000), 32 patients died during the early post-marketing phase vigilance period.21 Therefore, there is a growing concern among Japanese clinicians and patients that LAI-AP use may increase the risk of death. However, this remains unclear as no meta-analysis of LAI-AP trials regarding mortality in people with schizophrenia has been reported to date.
The overall incidence of death in randomized controlled trials (RCTs) involving pharmacotherapy for the treatment of schizophrenia is very low. Therefore, it is difficult for a single RCT to accurately estimate the risk of such rare adverse events because of low statistical power (ie, insufficient sample size).22 A meta-analysis can increase the statistical power for group comparisons and can overcome the limitation of sample size in underpowered studies.22 Here to determine whether LAI-APs are associated with higher mortality in people with schizophrenia, we performed several categorical meta-analyses of RCTs.
Methods
These meta-analyses were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.23
Search Strategy and Inclusion Criteria of Studies
To identify relevant RCTs, 2 authors (T.K. and S.M.) independently searched MEDLINE, Cochrane Library, and PsycINFO without language restrictions from inception to March 8, 2016 using the following search strategy: schizophrenia AND randomized AND antipsychotic, neuroleptic or an individual LAI-AP name24 (aripiprazole, bromperidol, clopenthixol, flupenthixol, fluphenazine, fluspirilene, haloperidol, iloperidone, olanzapine, oxyprothepin, paliperidone, penfluridol, perphenazine, pipothiazine, risperidone, or zuclopenthixol) AND depot, enanthate, decanoate, long-acting injection, microsphere, once monthly, palmitate, or pamoate. Two authors (T.K. and S.M.) independently assessed the inclusion and exclusion criteria and selected relevant studies. The references of the included articles and review articles (a list of the review articles is presented in supplementary appendix 1) were also searched for citations of further relevant published and unpublished research, such as conference abstracts.
Data Synthesis and Outcome Measures
The outcome measures were all-cause death (primary) and death due to suicide.
Data Extraction
Two authors (T.K. and S.M.) independently extracted data from the included studies. We used intention-to-treat or a modified intention-to-treat for analysis. When the data required for the meta-analysis were missing, we contacted the study investigators and requested the unpublished data. The following 3 categorical meta-analyses of RCTs were performed for evaluating each outcome: (1) individual and pooled LAI-APs vs the placebo; (2) individual and pooled LAI-APs vs OAPs; and (3) head-to-head comparisons of LAI-APs.
Meta-analytic Methods
These meta-analyses were conducted using Review Manager software (Version 5.3 for Windows, Cochrane Collaboration, http://tech.cochrane.org/Revman). The random effects model was chosen because of the potential heterogeneity across studies. The RR was estimated along with a 95% CI for each meta-analysis. We also planned to investigate study heterogeneity using a chi-square test of homogeneity (P < .05) together with the I 2 statistic, considering I 2 ≥ 50% indicative of considerable heterogeneity.25
We also performed an individual subgroup meta-analyses of “pooled LAI-APs vs placebo” and “pooled LAI-APs vs OAPs” regarding the primary outcome stratified by antipsychotic group (SGAs vs first-generation antipsychotics [FGAs]), duration of study (short vs long), sponsorship (industry sponsored vs nonindustry sponsored), current disease status (studies including patients with acute symptoms vs studies including only stable patients), and the LAI-AP dose (haloperidol equivalents). We also added the following 2 subgroup meta-analyses of “pooled LAI-APs vs OAPs:” comparator antipsychotic dose (haloperidol equivalents), comparator antipsychotic group (SGAs vs others), and presentation style (published articles vs unpublished conference abstract). The antipsychotic doses were converted into haloperidol equivalents using published guidelines.26–28 For LAI-APs, we used the manufacturers’ recommended equivalent for the depot to oral conversion for the same drug and then converted into oral haloperidol equivalents. The median duration of the “pooled LAI-APs vs placebo” and “pooled LAI-APs vs OAPs” studies were used as cut-offs for distinguishing between short and long-duration studies. The subgroup analyses divided by antipsychotic dose also used a median dose of antipsychotics as cut-offs. Studies including patients with acute symptoms were those accepting patients with acutely symptomatic schizophrenia or an acute exacerbation of schizophrenia. Because the study quality regarding the use of “an unpublished conference abstract” is difficult to assess because of its incompleteness,29 we examined a subgroup analysis regarding presentation style (published articles vs unpublished conference abstracts). For head-to-head comparisons, we conducted the following 3 subgroup meta-analyses: (1) paliperidone palmitate vs other pooled LAI-APs (aripiprazole, haloperidol, and risperidone); (2) zuclopenthixol vs other pooled LAI-APs (flupenthixol, haloperidol, and perphenazine); and (3) SGA-LAIs (paliperidone and risperidone) vs FGA-LAIs (fluphenazine/haloperidol and haloperidol). Both the funnel plot and Egger’s regression test (Comprehensive Meta-Analysis Version 2.0) were used to evaluate publication bias. In addition, we assessed the methodological quality of the trials with the Cochrane risk-of-bias criteria.22
Results
Study Characteristics
Of 6665 hits, we removed 5270 duplicates, 1184 references based on abstract/title review, and 76 articles after a full-text review (56 review articles and 20 identical studies), retaining 135 RCTs for the systematic review (supplementary figure 1). Moreover, we retrieved 16 additional RCTs by searching through the review articles, for a total of 151 RCTs (supplementary figure 1). However, 99 articles did not report data regarding death during the study (supplementary figure 1 and appendix 2). Therefore, only 52 (participant numbers: total n = 17 416; LAI-APs = 11 360; OAPs = 3910; placebo = 2146) were included in the meta-analyses (supplementary figure 1 and appendix 3). The details of each study are described in supplementary table 1. The number of studies for each individual LAI-AP and the corresponding total number of participants are as follows: aripiprazole = 6 (n = 1493), fluphenazine = 9 (n = 376), flupentixol = 1 (n = 30), fluphenazine/haloperidol = 1 (n = 32), haloperidol = 4 (n = 342), olanzapine = 3 (n = 1169), perphenazine = 1 (n = 85), paliperidone = 16 (n = 4092), risperidone = 18 (n = 3562), and zuclopenthixol = 4 (n = 179). The number of studies regarding each individual OAP and the corresponding total number of participants are as follows: aripiprazole = 3 (n = 669), fluphenazine = 3 (n = 139), haloperidol = 2 (n = 275), olanzapine = 4 (n = 903), physicians’ choice among OAPs = 3 (n = 800), physicians’ choice among SGAs = 3 (n = 639), pimozide = 1 (n = 24), risperidone = 4 (n = 441), and zuclopenthixol = 1 (n = 20). The median duration of “LAI-APs vs placebo” studies was 13 weeks and that of “LAI-APs vs OAPs” studies was 52 weeks. Patients in all studies included in the meta-analysis were adults (there were no studies that included specifically adolescent or elderly patients). The median LAI-AP dose (haloperidol equivalent) of the “LAI-APs vs placebo” studies was 4.03mg/d and the “LAI-APs vs OAPs” studies was 3.34mg/d. The median OAPs dose (haloperidol equivalent) of “LAI-APs vs OAPs” studies was 4.07mg/d. One of the 52 RCTs was an unpublished conference abstract.30 Eleven of the 52 RCTs included acute phase patients, such as patients with acutely symptomatic schizophrenia or acute exacerbation of schizophrenia. Of the 52 RCTs, 37 were industry-sponsored, and 18 were not double blinded. The detailed methodological quality of the RCTs based on the Cochrane risk-of-bias criteria are presented in supplementary figures 2 and 3.
LAI-APs vs Placebo
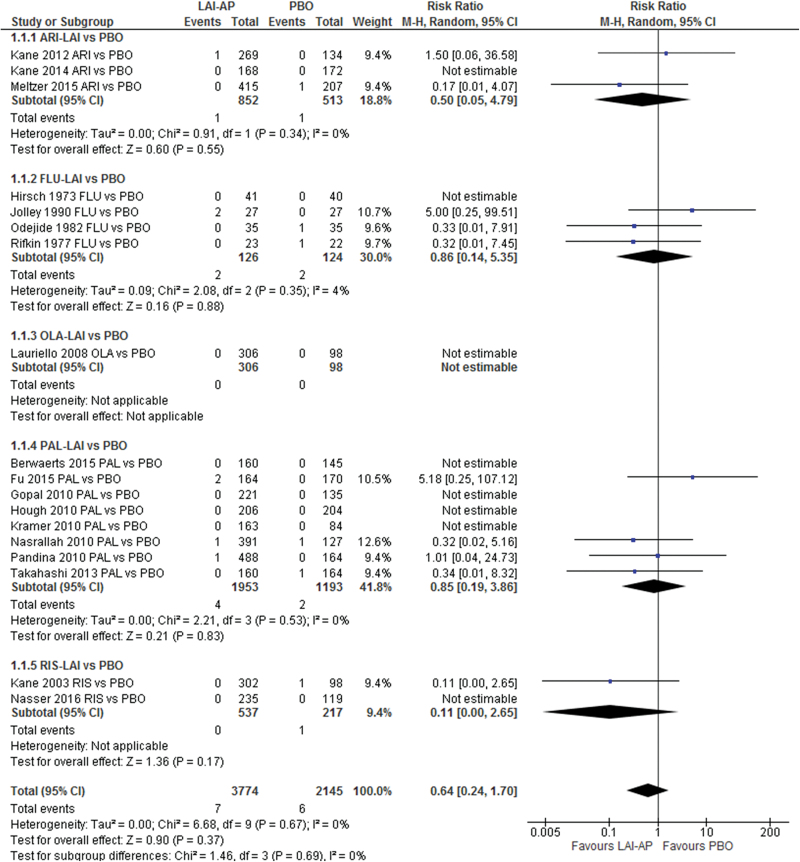
Neither the pooled LAI-APs nor any single individual LAI-AP (aripiprazole, fluphenazine, olanzapine, paliperidone, and risperidone) outperformed the placebo regarding the incidence of all-cause death (figure 1) and death due to suicide (supplementary figure 4). We did not find significant heterogeneity with respect to the primary outcome in the meta-analysis. In addition, we also did not detect publication bias (Egger’s regression test: P = .548, funnel plot: supplementary figure 5). However, in a subgroup meta-analysis using only short-duration RCTs (≤13wk), pooled LAI-APs exhibited a trend towards a lower incidence of all-cause death in comparison with the placebo (RR = 0.29; 95% CI = 0.07 − 1.16; P = .08; I 2 = 0%; N = 10; n = 4217; table 1). We did not find significant differences in any of the other subgroup analyses between LAIs and placebo (table 1).
Fig. 1.
All-cause death: long-acting injectable antipsychotics vs placebo. AP: antipsychotic; ARI: aripiprazole; FLU: fluphenazine; LAI: long-acting injectable; OLA: olanzapine; PAL: paliperidone; PBO: placebo; RIS: risperidone.
Table 1.
The Subgroup Meta-analysis of Long-acting Injectable Antipsychotics vs Placebo
| Number of Studies | Number of Patients | Risk Ratio | 95% CI | P | I 2 | ||
|---|---|---|---|---|---|---|---|
| Antipsychotic group | Second-generation antipsychotics-long acting injectable | 14 | 5669 | 0.56 | 0.17–1.80 | .33 | 0% |
| First-generation antipsychotics-long acting injectable | 4 | 250 | 0.86 | 0.14–5.35 | .88 | 4% | |
| Duration of study | ≤13 wk | 10 | 4217 | 0.29 | 0.07–1.16 | .08 | 0% |
| >13 wk | 8 | 1702 | 1.40 | 0.35–5.60 | .64 | 0% | |
| Sponsorship | Industry | 14 | 5669 | 0.56 | 0.17–1.80 | .33 | 0% |
| Non industry | 3 | 180 | 1.32 | 0.09–19.66 | .84 | 35% | |
| Unknown | 1 | 70 | 0.33 | 0.01–7.91 | .50 | not applicable | |
| Current disease status | Acute phase | 9 | 3827 | 0.72 | 0.16–3.28 | .67 | 0% |
| Other phase | 9 | 2092 | 0.58 | 0.16–2.10 | .41 | 0% | |
| Long acting injectable- antipsychotic dose | Haloperidol equivalent > 4.03mg/d | 8 | 3032 | 1.28 | 0.21–7.82 | .79 | 0% |
| Haloperidol equivalent ≤ 4.03mg/d | 8 | 2788 | 0.31 | 0.08–1.24 | .10 | 0% | |
| Unknown | 2 | 99 | 1.32 | 0.09–19.66 | .84 | 35% |
LAI-APs vs OAPs
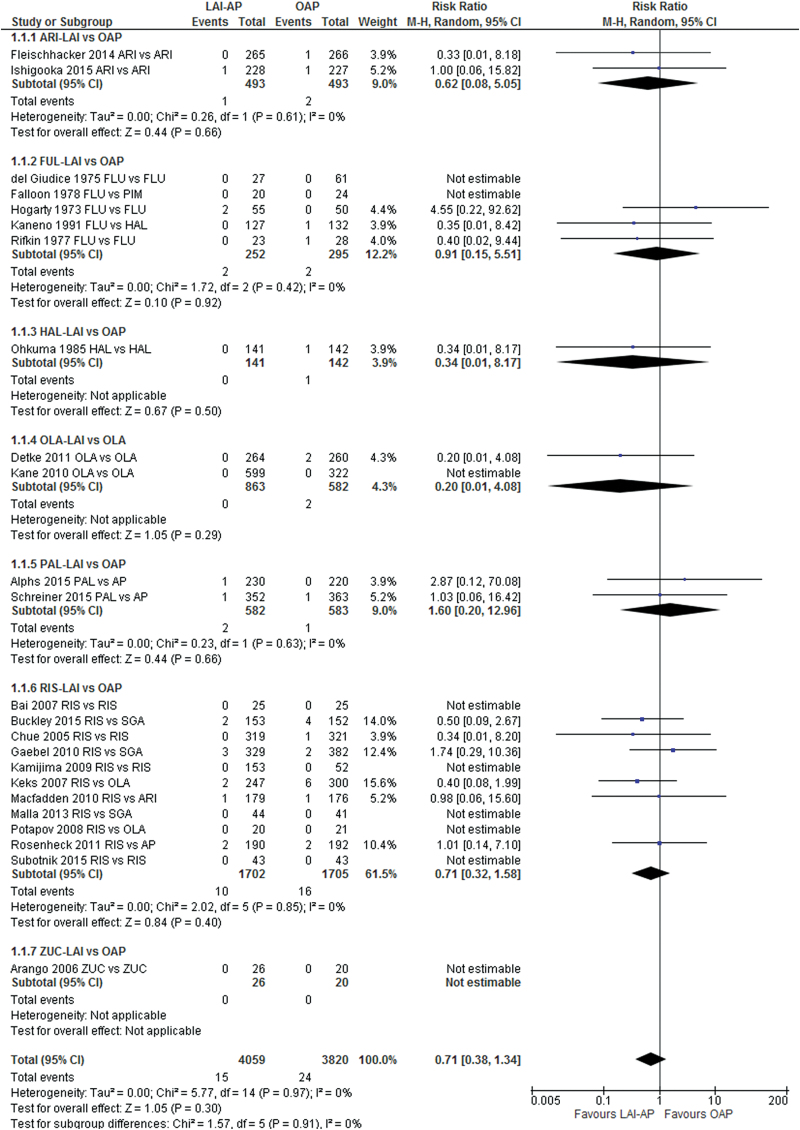
Neither the pooled LAI-APs nor any single individual LAI-AP (aripiprazole, fluphenazine, haloperidol, olanzapine, paliperidone, risperidone, and zuclopenthixol) differed from OAPs regarding the incidence of all-cause death (figure 2) and death due to suicide (supplementary figure 6). We did not find significant heterogeneity with respect to the primary outcome in the meta-analysis. We did not detect publication bias (Egger’s regression test: P = .949; funnel plot: supplementary figure 7) or significant differences in any of the subgroup analyses between the pooled LAI-APs and OAPs (supplementary table 2).
Fig. 2.
All-cause death: long-acting injectable antipsychotics vs oral antipsychotics. AP: antipsychotic; ARI: aripiprazole; FLU: fluphenazine; HAL: haloperidol; LAI: long-acting injectable; OAP: oral antipsychotics; OLA: olanzapine; PAL: paliperidone; PIM: pimozide; RIS: risperidone; SGA: second-generation antipsychotic; ZUC: zuclopenthixol.
LAI-AP vs LAI-AP
The meta-analyses of the head-to-head LAI-AP comparisons did not exhibit any significant differences in all-cause death and suicide (supplementary figures 8 and 9). There were also no differences in the incidence of all-cause death in any of the subgroup meta-analyses (supplementary table 3).
Discussion
This is the first meta-analysis of RCTs (52 studies, 53 comparisons) examining the differences in mortality among people with schizophrenia treated with LAI-APs, placebo, or OAPs (total participants = 17 416; LAI-APs = 11 360, OAP = 3910, and placebo = 2146). Several clinicians and patients in Japan have concerns regarding LAI-AP safety considering the deaths reported during the early post-marketing phase vigilance period.21 However, the results of the current study suggest that there are no significant differences in the incidence of death between LAI-APs and OAPs or the placebo treatment groups. Moreover, we identified a trend towards a lower mortality rate in the pooled LAI-APs group in comparison with the placebo within 13 weeks of treatment onset (RR = 0.29; 95% CI = 0.07−1.16; P = .08). Furthermore, it is possible that this result was a type II error because of the small sample size. According to the early post-marketing phase vigilance period regarding paliperidone palmitate in Japan, 32 Japanese patients died after paliperidone palmitate injection (over a 6-month period).21 Of the 32 patients, the percent of males (1 patient was unknown) was 67.7% and the mean age was 51.2 years. Moreover, 37.5% patients (n = 12) were diagnosed as sudden death based on the International Classification of Diseases 10th revision. Other causes of death were suspected sudden death (12.5%, n = 4), suicide (21.9%, n = 7), suspected neuroleptic malignant syndrome (12.5%, n = 4), and physical illness such as pancreatic cancer (15.6 %, n = 5). The mean and median duration from the first paliperidone palmitate injection to death were 7.61 and 5.86 weeks, respectively. When analyzing the publicly available information regarding the deceased Japanese patients (n = 27), 33.3% of patients (n = 9) died within 4 weeks and 77.8% (n = 21) of patients died within 13 weeks after the first paliperidone palmitate injection. However, our subgroup meta-analysis (≤13wk of treatment onset) revealed that the pooled LAI-APs trended toward a lower mortality rate in comparison with the placebo. This evidence may be representative of conflicting findings. Therefore, our results must be discussed in light of the following limitations regarding the current study. First, all studies included in these current meta-analyses were RCTs, and most (71.2%) were industry-sponsored clinical trials. RCTs are considered to be effective for detecting large effects in homogeneous populations. Importantly, in RCTs, patients who are medically compromised have psychiatric comorbidities, including substance dependence, those who are suicidal, and who receive different co-medications, are systematically excluded. Moreover, the use of concomitant medications is limited during the study. On the other hand, when analyzing publicly available information regarding the deceased Japanese patients (n = 27), those who used antipsychotics, benzodiazepines, or mood stabilizers as concomitant medications were 85.2%, 59.3%, and 44.4%, respectively. However, detailed information regarding these drugs was unknown (ie, when the patients took these drugs, the dose of these drugs taken by the patients, and/or how long the patients took these drugs).
Moreover, the traditional RCTs of LAI-APs confirmed that the participants responded to oral same antipsychotics, and that the participants were well tolerated to the same OAPs before the LAI-APs were given. Therefore, homogeneous patient cohorts and strictly controlled clinical environments associated with RCTs may not reflect real-world practice and outcomes.31 In fact, 1 recent meta-analysis of RCTs of LAI-APs vs OAPs regarding relapse prevention in people with schizophrenia found no evidence for the superiority of LAI-APs over OAPs.32 However, another meta-analysis that included mirror image studies (which have been suggested to better reflect the real-world impact of LAI-APs than RCTs) concluded that LAI-APs were superior to OAPs for reducing relapse.19 Thus, the traditional RCT may not be the most appropriate trial design to compare the effectiveness of LAI-APs vs OAPs because participants are strictly selected and the treatments varied.31 Therefore, we could not deny the possibility that our results included a risk of selection bias. Moreover, because the medications may be differently dosed in clinical care settings, patients at a higher risk of death were treated in usual care settings, which could have affected the mortality rates. Further study is needed to examine that the potential effect of these factors on the risk of death. Given the above discussion, we emphasize that LAI-APs use did not increase the risk of death in comparison with OAPs or placebo when used as directed by the drug package insert. This is because the drug package insert is composed based on the industry-sponsored RCTs.
A second limitation was that the duration of studies included in the meta-analysis of LAI-APs vs placebo was relatively short (median = 13wk, mean = 28.9wk). The median and the mean duration of all studies included in the meta-analysis of LAI-APs vs OAPs were 52 weeks and 64.5 weeks, respectively. Therefore, because of this short duration, the evidence from our meta-analyses does not substantiate a similar death risk between LAI-APs and OAPs or placebo over several years. Third, the studies included in the current meta-analyses differed regarding patient characteristics (eg, race and ethnicity, the state of disease, antipsychotic dose, and study duration), which could generate heterogeneity when combining data. However, we did not detect significant heterogeneity regarding the primary outcome in any of the categorical meta-analyses, so we performed subgroup meta-analyses for the primary outcomes. The pooled LAI-APs group exhibited a trend towards a reduced mortality rate within 13 weeks in comparison with the placebo, while no significant differences were found in the subgroup analyzes between LAI-APs and the placebo or OAPs. Fourth, although we detected 151 RCTs of LAI-APs, we included only 52 RCTs in the meta-analysis because the articles of 99 RCTs (93 of 99 RCTs were published before the year 2000; (supplementary appendix 2) did not include any information regarding death during the study. We contacted the authors requesting raw data, but we have, in most cases, received no reply. In addition, although we searched the data using the review articles (supplementary appendix 1), we could not locate all necessary data. However, if we included the data from the 99 RCTs in the meta-analysis, the results may be different from the current results (reporting bias). Fifth, because LAI-APs may be more harmful to elderly patients than younger patients,33 there were no RCT that included only elderly patients. Sixth, because we did not use Embase as a database for our literature search because of the limited access to our university hospital, this was a possible weakness of our literature study. Therefore, it was possible that some appropriate articles could have been missed.
To conclude, our results suggest that there is no significant difference in all-cause death and death due to suicide between LAI-APs and placebo or OAPs. On the other hand, there was a modest trend for reduced mortality in the pooled LAI-APs group in comparison with the placebo within 13 weeks. Data were insufficient for meaningful head-to-head comparisons of individual LAI-APs. Data for individual LAI-APs vs individual OAPs were also insufficient.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
The authors have no support or funding to report.
Supplementary Material
Acknowledgments
We thank Dr Mosolov (Department of Mental Disorders Therapy, Moscow Research Institute of Psychiatry, Moscow, Russia), Dr Koshikawa (Department of Neuropsychiatry, Kansai Medical University, Osaka, Japan) and Dr Takekita (Department of Neuropsychiatry, Kansai Medical University, Osaka, Japan and Department of Biomedical and Neuromotor Sciences, University of Bologna, Bologna, Italy) for providing additional, unpublished data on his study relevant for this meta-analysis. The authors have declared that there are no conflicts of interest in relation to the subject of this study. No grants or other funding sources were used for this study. T.K. has received speaker’s honoraria from Abbvie, Astellas, Daiichi Sankyo, Dainippon Sumitomo, Eisai, Eli Lilly, GlaxoSmithKline, Janssen, Yoshitomi, Otsuka, Meiji, Mochida, Shionogi, Tanabe-Mitsubishi, Tsumura, Novartis, and Pfizer and has a Fujita Health University School of Medicine research grant and Grant-in-Aid for Young Scientists (B). S.M. has received speaker’s honoraria from Eisai, Janssen, Novartis, Daiichi Sankyo, Ono, Eli Lilly, Takeda, and Otsuka and had a Fujita Health University School of Medicine research grant and Grant-in-Aid for Young Scientists (B). N.I. has received speaker’s honoraria from Astellas, Dainippon Sumitomo, Eli Lilly, GlaxoSmithKline, Janssen, Yoshitomi, Otsuka, Meiji, Shionogi, Novartis, and Pfizer and had research grants from Dainippon Sumitomo, GlaxoSmithKline, Tanabe-Mitsubishi and Otsuka. T.K. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept, design, and statistical analysis were performed by T.K. Interpretation of data and acquisition of data were performed by T.K. and S.M. The manuscript was written by T.K., S.M., and N.I. N.I. supervised the review.
References
- 1. Nielsen RE, Uggerby AS, Jensen SO, McGrath JJ. Increasing mortality gap for patients diagnosed with schizophrenia over the last three decades–a Danish nationwide study from 1980 to 2010. Schizophr Res. 2013;146:22–27. [DOI] [PubMed] [Google Scholar]
- 2. Hoang U, Stewart R, Goldacre MJ. Mortality after hospital discharge for people with schizophrenia or bipolar disorder: retrospective study of linked English hospital episode statistics, 1999-2006. BMJ. 2011;343:d5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72:1172–1181. [DOI] [PubMed] [Google Scholar]
- 4. Reininghaus U, Dutta R, Dazzan P, et al. Mortality in schizophrenia and other psychoses: a 10-year follow-up of the ӔSOP first-episode cohort. Schizophr Bull. 2015;41:664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DE Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10:52–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Osby U, Westman J, Hallgren J, Gissler M. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987–2010 [published online ahead of print January 8, 2016]. Eur J Public Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64:1123–1131. [DOI] [PubMed] [Google Scholar]
- 9. Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in schizophrenia. Annu Rev Clin Psychol. 2014;10:425–448. [DOI] [PubMed] [Google Scholar]
- 10. De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2012;8:114–126. [DOI] [PubMed] [Google Scholar]
- 11. Henderson DC, Vincenzi B, Andrea NV, Ulloa M, Copeland PM. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry. 2015;2:452–464. [DOI] [PubMed] [Google Scholar]
- 12. Popovic D, Benabarre A, Crespo JM, et al. Risk factors for suicide in schizophrenia: systematic review and clinical recommendations. Acta Psychiatr Scand. 2014;130:418–426. [DOI] [PubMed] [Google Scholar]
- 13. Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Davis JM. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. 2012;5:CD008016. [DOI] [PubMed] [Google Scholar]
- 14. Tiihonen J, Lonnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374:620–627. [DOI] [PubMed] [Google Scholar]
- 15. Tiihonen J, Wahlbeck K, Lonnqvist J, et al. Effectiveness of antipsychotic treatments in a nationwide cohort of patients in community care after first hospitalisation due to schizophrenia and schizoaffective disorder: observational follow-up study. BMJ. 2006;333:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX, Korhonen P. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168:603–609. [DOI] [PubMed] [Google Scholar]
- 17. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, Alexanderson K, Tanskanen A. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study [published online ahead of print December 7, 2015]. Am J Psychiatry. [DOI] [PubMed] [Google Scholar]
- 18. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9:310–317. [DOI] [PubMed] [Google Scholar]
- 19. Kishimoto T, Nitta M, Borenstein M, Kane JM, Correll CU. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74:957–965. [DOI] [PubMed] [Google Scholar]
- 20. Fusar-Poli P, Kempton MJ, Rosenheck RA. Efficacy and safety of second-generation long-acting injections in schizophrenia: a meta-analysis of randomized-controlled trials. Int Clin Psychopharmacol. 2013;28:57–66. [DOI] [PubMed] [Google Scholar]
- 21. Fujii Y. [What Lessons Should We Learn from the Death of Patients on Xeplion?]. Seishin Shinkeigaku Zasshi. 2015;117:132–145. [PubMed] [Google Scholar]
- 22. Button KS, Ioannidis JP, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–376. [DOI] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Risio A, Lang AP. History and therapeutic rationale of long acting antipsychotics. Curr Clin Pharmacol. 2014;9:39–52. [DOI] [PubMed] [Google Scholar]
- 25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. [DOI] [PubMed] [Google Scholar]
- 28. Expert Consensus Panel for Optimizing Pharmacologic Treatment of Psychotic D. The expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. J Clin Psychiatry. 2003;64:2–97, quiz 98–100. [PubMed] [Google Scholar]
- 29. McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet. 2000;356:1228–1231. [DOI] [PubMed] [Google Scholar]
- 30. Potapov A, Eduard T, Sergey M. Response, remission and relapse during the long-term treatment of schizophrenia patients with long-acting injectable risperidone versus olanzapine. Int J Neuropsychop. 2008;11:158–158. [Google Scholar]
- 31. Haddad PM, Kishimoto T, Correll CU, Kane JM. Ambiguous findings concerning potential advantages of depot antipsychotics: in search of clinical relevance. Curr Opin Psychiatry. 2015;28:216–221. [DOI] [PubMed] [Google Scholar]
- 32. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40:192–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Masand PS, Gupta S. Long-acting injectable antipsychotics in the elderly: guidelines for effective use. Drugs Aging. 2003;20:1099–1110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.