Abstract
Introduction:
As endophenotypes bridge the gap between genetics and phenotypic disease expression, identifying reliable markers is important for fostering understanding of pathophysiology. The present aim was to conduct current meta-analyses of 3 key auditory event-related potential (ERP) components that have been held as potential endophenotypes for schizophrenia: P50, P300 amplitude and latency, and mismatch negativity (MMN), reflective of sensory gating, attention and classification speed, and perceptual discrimination ability, respectively. In order to assess endophenotype viability, these components were examined in unaffected relatives of patients with schizophrenia and healthy controls.
Methods:
Effect sizes (ES) were examined between relatives and controls for P50 suppression (10 studies, n = 360 relatives, 473 controls), P300 amplitude (20 studies, n = 868 relatives, 961 controls), P300 latency (17 studies, n = 674 relatives, 792 controls), and MMN (11 studies, n = 377 relatives, 552 controls).
Results:
Reliable differences in P50 suppression (ES = 0.86, P < .001), P300 amplitude (ES = −0.52, P < .001), and P300 latency (ES = 0.44, P < .05) were found between unaffected relatives and controls. A trend was found between relatives and controls for MMN (ES = 0.21, P = 0.06), and the use of extraneous channels was found to be a significant moderator (P = 0.01). When MMN was analyzed using frontocentral channel Fz, a significant difference was found (ES = 0.26, P < 0.01).
Discussion:
The results indicate that P50 suppression, P300 amplitude and P300 latency, and MMN may serve as viable endophenotypes for schizophrenia.
Key words: schizophrenia, endophenotype, auditory ERP, relatives
Schizophrenia is a complex disease that involves a combination of numerous genetic and environmental factors.1 Overt illness expression involves the interaction of many of these genotypic and environmental influences, thereby posing severe challenges to genetic dissection of the disease. An endophenotype is defined as an internal phenotype2 that is discoverable using biochemical or microscopic tests.3 Endophenotypes form the causal links between genetic influences and overt phenotypic expression and are therefore key in the understanding of the underlying biological mechanisms of disease risk and expression. Unlike biological markers, which may not be heritable, criteria for an endophenotype include heritability of the marker as well as a higher prevalence of the marker in non-affected family members relative to the general population.4 The current study examines 3 potential neurophysiological endophenotypes of schizophrenia by systematically comparing unaffected relatives of patients with schizophrenia and healthy controls.
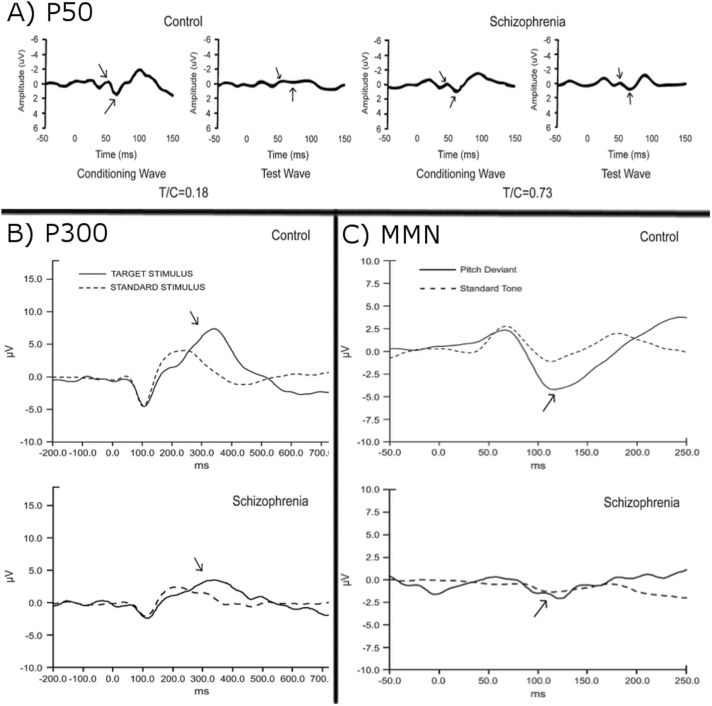
The strategy for validating endophenotypes for schizophrenia first involves identifying deficits in patients, followed by exploring evidence of heritability in unaffected relatives.5 It has been consistently demonstrated that patients with schizophrenia exhibit deficits in sensory and cognitive processing.6–8 Many studies have aimed to further explore these deficits using electroencephalography (EEG) to measure event-related potentials (ERPs). Three specific components, the P50, P300, and mismatch negativity (MMN) have been shown to reliably differ between patients with schizophrenia and healthy controls in response to auditory stimuli (Footnotes can be seen in Supplementary material).i Example waveforms are shown in figure 1.
Fig. 1.
Example component waveforms. (A) Example auditory-evoked response from a healthy control (left) and patient with schizophrenia (right). Arrows mark the location of the P50 wave for the conditioning stimulus and identical test stimulus. T/C indicates the test-to-conditioning ratio for each subject. The P50 response to the second stimulus is attenuated for the healthy control subject, but not for patient with schizophrenia. (B) Grand average waveforms for 38 healthy control subjects (top) and 52 patients with schizophrenia (bottom) in response to an infrequent auditory stimulus to which participants made a button press. As marked by arrows, patients with schizophrenia exhibit smaller P300 amplitudes than healthy controls. (C) Grand average MMN response to an auditory pitch-deviant stimulus for 20 healthy control subjects (top) and 19 patients with schizophrenia (bottom). As marked by arrows, patients with schizophrenia exhibit smaller MMN amplitudes than healthy controls. All example waveforms were adopted and reprinted with permission from Turetsky et al.30 MMN = mismatch negativity.
The P50 ERP component is a positive deflection occurring approximately 50ms after stimulus onset and generally shows a decrease in amplitude to repeated stimuli, termed P50 suppression. Specifically, when two identical stimuli (eg, an auditory click) are presented successively, a decrease in P50 amplitude to the second stimulus is generally found. Many studies have demonstrated robust deficits in P50 suppression, as reflected by larger P50 ratios for patients with schizophrenia relative to healthy controls, which have been confirmed by meta-analytic studies.9,10 There is some debate of the functional significance of P50 suppression deficits. It may reflect increased EEG background noise in overall measurement, or in measuring the response to S2 due to refractory effects,11 but is most commonly posited to reflect a sensory gating mechanism or filtering of redundant stimuli.12,13 Indeed, some studies have shown P50 suppression deficits in patients with schizophrenia to be related to phenomenological dimensions of sensory gating. For example, the P50 ratio is found to be correlated with perceived invasiveness of sounds14 and scores on the Sensory Gating Inventory (SGI) in patients with schizophrenia15 (but see also Jin and coworkers16), suggesting that P50 suppression deficits may indeed be indicative of an inability to inhibit the response to superfluous stimuli.
The P300 component is a positive deflection occurring between 250 and 500ms after stimulus onset and is thought to reflect attentional processes. This component is often recorded using an oddball paradigm, in which the subject is presented with frequent and infrequent stimuli and asked to respond to the infrequent stimuli. An increase in amplitude to the infrequent relative to the frequent stimuli is generally found. As this increase in amplitude to the infrequent target is task dependent (ie, only occurs if the target is meaningful/the participant is asked to respond to the target), this component reflects the engagement of attention17 or context updating.18 It has been demonstrated that P300 amplitude is related to the amount of attentional resources devoted to the task,19,20 whereas P300 latency indexes stimulus classification speed.21,22 Findings of deficient P300 amplitude and latency for patients with schizophrenia relative to healthy controls are robust.9,23 Although the P300 can be studied using visual stimuli, it is the auditory modality that is most commonly studied in schizophrenia, given that this modality has demonstrated the strongest effects,24 has greater genetic influence,25 and reflects a vulnerability trait marker for schizophrenia.26
Also recorded using an oddball paradigm is the MMN component that occurs in response to deviant stimuli. This response is elicited by auditory tones differing in a variety of perceptual features, such as frequency or duration. The MMN generally occurs 150–250ms after the onset of the deviant stimulus, is maximal over frontocentral scalp locations, and is related to the degree of deviance. Unlike the P300, the MMN is elicited even in the absence of attention. The MMN reflects automatic auditory processing, perceptual discrimination ability, and sensory memory.27,28 Deficits in MMN generation are robust in patients with schizophrenia.29
The P50, P300, and MMN Components as Potential Endophenotypes
Examining auditory ERP components in unaffected relatives of patients with schizophrenia offers many benefits beyond studies in patients alone. First, many of these components may be affected by confounds, such as medication usage, which can be problematic when studying patient groups. Additionally, by definition, an endophenotype must be present at a higher rate in unaffected family members relative to the general population.4 Assessing these potential endophenotypes within unaffected relatives is an important and necessary step in evaluating the viability of these markers as endophenotypes and therefore gaining a better understanding of the underlying genetics of schizophrenia.
The viability of neurophysiological endophenotypes of schizophrenia, including P50 suppression, P300, and MMN, were reviewed in 2007 by Turetsky and colleagues30 and have been examined in meta-analyses comparing relatives of patients with healthy controls. Also in 2007, de Wilde and colleagues10 conducted a small meta-analysis of P50 suppression involving six family studies. The results show a moderate-to-large effect size (ES = 0.85), demonstrating evidence for deficits in P50 suppression in relatives of patients with schizophrenia compared with the healthy controls. Additionally, in a meta-analysis involving 11 studies from 1983 to 2003, Bramon and colleagues31 found that P300 amplitude was significantly reduced (ES = 0.61) and latency was significantly longer (ES = 0.50) in relatives compared with the controls. Finally, a recent meta-analysis32 examined MMN impairment across patients with schizophrenia, high-risk individuals, and relatives of patients with schizophrenia. Eight studies of relatives were included and found a nonsignificant trend for relatives to exhibit reduced MMN amplitude compared with the controls (ES = 0.26, P = 0.053).
Current Study
The aim of the current study is to provide a comprehensive, up-to-date review, and meta-analysis of the potential of multiple ERP components, known to reflect different stages of processing of auditory stimuli and serve as endophenotypes for schizophrenia. Importantly, the current analyses include 3 additional studies of P50 suppression, 10 additional studies of the P300 component, and 3 additional studies of the MMN relative to the most recent aforementioned meta-analyses. Furthermore, as it was not their primary analysis, the recent meta-analysis of the MMN did not examine potential moderating variables in relatives. To address this gap and assess these important potential endophenotypes, we have examined three ERP components, the P50, P300, and MMN, and potential moderating variables in unaffected relatives of patients of schizophrenia compared with the healthy controls.
Methods
Eligibility Criteria
Inclusion criteria for the current analyses were as follows: (1) the study included a sample of unaffected relatives of patients with schizophrenia and a healthy control group, (2) EEG was recorded in response to auditory stimuli, (3) at least one of the ERP components of interest (P50, P300, and MMN) was measured, (4) statistics were reported that allowed for calculation of the component of interest for both the relative and the control group, and (5) the article was written in English and published in a peer-reviewed journal. The cutoff date for the literature search was December 1, 2015.
Study Selection
The literature search was conducted using Google Scholar with the following search terms: Schiz* AND P50/P300/Mismatch Negativity/MMN AND Relatives OR Family. The resulting articles were checked for eligibility, and the citations were cross-referenced. For the P50, 10 studies33–42 met eligibility criteria (n = 360 relatives, 473 controls). For P300, 23 studies met eligibility criteria.31,34,35,43–62 In order to prevent biased estimates, 2 studies were excluded from the analysis because subjects participated in another study were used in the current analyses.61,62 In these cases, the most recent study was used for the current analysis. Of the remaining 21 studies, 20 (n = 868 relatives, 961 controls) examined and reported P300 amplitude31,35,39,43–59 and 17 (n = 674 relatives, 792 controls) examined and reported P300 latency.31,35,43–48,50,51,53–58,60 For MMN, 11 studies35,39,55,63–70 met eligibility criteria and were used in the analyses (n = 377 relatives, 552 controls).
Analyses
The variables of interest were P50 suppression, P300 latency, P300 amplitude, and MMN of relatives of patients compared with the healthy controls in response to auditory stimuli. P50 suppression was defined in each study as the ratio of S2:S1 amplitude at the vertex (Cz) within a specified time window ranging from 40ms to 80ms after stimulus onset, where S1 is the first, or conditioning, stimulus and S2 is the second, or testing, stimulus. Therefore, a larger ratio is indicative of less suppression. The P50 methods (eg, 500ms interstimulus interval between paired clicks) and analyses (eg, recorded at channel Cz) were highly consistent between studies. Due to the low number of studies and high consistency between them, no potential moderating variables were analyzed.
The P300 component was defined in each study as a positive deflection generated by the target (infrequent) tones within a specific time window generally ranging from 200ms to 600ms poststimulus onset. For both the P300 amplitude and latency, most studies reported 3 midline sites (Fz, Cz, and Pz). Therefore, when reported separately, amplitude and latency were averaged across channels for each condition. The channels and time windows used in each study are reported in table 1. Differential P300 amplitude has been shown based on gender,71 age,72 and response (eg, button press or silent counting73), making these important moderating variables to consider.
Table 1.
Study Characteristics
| Author(s) | Year | Relative Sample | Relative Psychiatric Disorders (n) | Channel(s) | Time Window |
|---|---|---|---|---|---|
| P50 | |||||
| Clementz et al33 | 1998 | First-degree | SZ (1, moved to clinical group in study), Remitted MDD (5), Current MDD (6) | Cz | 40–80 ms |
| de Wilde et al34 | 2007 | Siblings | No mood disorder, any psychotic symptom, or a substance abuse diagnosis | Cz | 40–80 ms |
| Hall et al35 | 2007 | MZ twins | Remitted MDD (5) | Cz | 40–75 ms |
| Hall et al36 | 2011 | Relatives (degree NR) | No lifetime diagnosis of psychotic disorder, BD, or SZ spectrum PD | Fz, Cz, FC1, FC2 | 40–80 ms |
| Louchart-de la Chapelle et al37 | 2005 | Parents | Schizotypal PD (8) | Cz | 40–80 ms |
| Myles-Worsley38 | 2002 | Parents and siblings | No Axis I disorder | Cz | 40–75 ms |
| Price et al39 | 2006 | First-degree | Diagnosis of SZ were excluded | Cz | 40–70 ms |
| Siegel et al40 | 1984 | Parents and siblings | Diagnosis of SZ were excluded | Cz | NR |
| Turetsky et al.41 | 2012 | First-degree | No axis I psychotic disorder or prodromal symptoms | Cz | 40–75 ms |
| Waldo et al.42 | 1988 | First-degree | No history of psychiatric problems | Cz | 40–70 ms |
| P300 | |||||
| Black et al43 | 1992 | Relatives (degree NR) | BD excluded | Cz to left mastoid, Cz to right mastoid, Cz to Oz | 44–840 ms |
| Blackwood et al44 | 1991 | Relatives up to 3 generations | None (107), BD (13), unspecified functional psychosis (3), schizoaffective disorder (1), MDD (11), minor depressive disorder (6), GAD (3), panic disorder (2), alcoholism (2), alcoholism with schizotypal features (1), minor depression with schizotypal features (2) | NR | 260–500 ms |
| Bramon et al45 | 2008 | First-degree | Nonpsychotic | Pz (amplitude) Fz, Pz (latency) | NR |
| Bramon et al31 | 2005 | First-degree | Nonpsychotic | Pz, Fz, Cz | 280–500 ms |
| de Wilde et al46 | 2008 | Siblings | No history of mood disorder, any psychotic symptom or substance abuse | Pz, Fz, Cz | 250–450 ms |
| Dutt et al47 | 2012 | First-degree | No illness (59), MDD (13), GAD (1), panic disorder (1) | Pz, Fz, Cz | NR |
| Franguo et al48 | 1997 | First-degree | No illness (47), Remitted Depression (8), Remitted BD (1), Remitted Bulimia Nervosa (1) | Pz, Fz, Cz | 280–500 ms |
| Hall et al35 | 2007 | MZ twins | Remitted MDD (5) | Pz | 280–600 ms |
| Karoumi et al50 | 2000 | Siblings | No Axis I disorder | Pz, Fz, Cz | 280–500 ms |
| Kidogami et al51 | 1991 | Parents and siblings | No history of psychiatric disorders | Pz, Fz, Cz | 260–460 ms |
| Kimble et al52 | 2000 | Children and siblings | No psychotropic medications | Pz, Fz, Cz, Oz | 250–550 ms |
| Lebedeva and Orlova60 | 2001 | Children and siblings | NR | F3, Cz | 280–450 ms |
| Price et al39 | 2006 | First-degree | Diagnosis of SZ were excluded | Pz | 250–550 ms |
| Roxborough et al53 | 1993 | Relatives (degree NR) | None (26), MDD (3), Minor Depressive Disorder (1) | Bipolar between Cz and the left earlobe | 260–500 ms |
| Schreiber et al54 | 1992 | Children | Drug free | Pz | 280–600 ms |
| Şevik et al55 | 2011 | Siblings | No Axis I disorder | Pz | 200–400 ms |
| Simons et al56 | 2011 | Siblings | Non-psychotic | Pz, Fz, Cz | 250–500 ms |
| Turetsky57 | 2000 | Siblings | No Axis I disorder | Pz, Fz, Cz | 280–400 ms |
| Weisbrod58 | 1999 | MZ twins | None (4), remitted BD (2), single depressive episode (2) | Pz | 270–470 ms |
| Winterer et al59 | 2003 | Siblings | No history of psychotic illness. Present but clinically stable depression or PD (14), History but not current nonpsychotic disorder (39) | F3, F4, T5, T6 | 260–420 ms |
| MMN | |||||
| Ahveninen et al63 | 2006 | MZ and DZ twins | No SZ or schizoaffective disorder | F1, Fz, F2, FC1, FCz, FC2 | 100–200 ms |
| Bramon et al64 | 2004 | First-degree | None (28), MDD (5), Panic disorder (1), Schizotypal PD (3) | F3, F4 | 50–200 ms |
| Hall et al35 | 2007 | MZ twins | Remitted MDD (5) | Fz | 50–200 ms |
| Hong et al68 | 2012 | First-degree | No SZ, antipsychotic naive | Fz | 100–250 ms |
| Jessen et al65 | 2001 | First-degree | No history of psychosis | Fz, Cz | 100–250 ms |
| Kim70 | 2014 | First-degree | No prodromal symptoms as measured by SIPS | FPZ, Fz, FCz, Cz, CPz, Pz, POz, Oz | 130–250 ms |
| Lee et al.69 | 2014 | First-degree | No history of psychiatric illness | FP1, FP2, F7, F3, Fz, F4, F8, FC5, FC1, FC2, FC6, T7, C3, Cz, C4, T8, CP5, CP1, CP2, CP6, P7, P3, Pz, P4, P8, O1, O2 | 100–250 ms |
| Magno et al66 | 2008 | First-degree | No psychiatric illness or symptoms | AFz,Fz, FCz, F1, FC1, F2, FC2 | 140–180 ms |
| Michie67 | 2002 | First-degree | No history of psychosis, Remitted depressive episodes (4), social anxiety disorder (1), phobia (1) | F3, F4, Fz, C3, C4, Cz, P3, P4 | 135–205 ms |
| Price et al39 | 2006 | First-degree | Diagnosis of SZ were excluded | Fz | 135–205 ms |
| Şevik et al55 | 2011 | Siblings | No Axis I disorder | Fz, Cz | 100–250 ms |
Note. BD, bipolar disorder; DZ, dizygotic; GAD, generalized anxiety disorder; MDD, major depressive disorder; MZ, monozygotic; NR, not reported; PD, personality disorder; SIPS, Structured Interview for Prodromal Symptoms; SZ, schizophrenia.
MMN was measured in each study by subtracting the averaged amplitude in response to the standard stimuli from the averaged amplitude in response to the deviant stimuli within a specified time window ranging from 50ms to 250ms poststimulus onset, with a larger negative number indicating greater MMN. This is typically measured at frontocentral channels,27 however, some studies have reported MMN as averaged across parietal and/or occipital channels. As patients with schizophrenia typically show the largest MMN deficits at frontal channels,74–76 the inclusion of extraneous (non-frontal) channels for MMN calculation is included as a moderating variable in the current analyses. Other factors that have been shown to impact the MMN are age77 and whether frequency or duration deviants are measured.78 Hence, channel location, relative age, and task (frequency or duration) are all examined as potential moderating variables.
For each study, pooled ES (Hedges’ g) was calculated to define the differences in these ERP components for the relative and control groups.ii Hedges’ g was defined as the difference between group variables divided by pooled within-group SD of both groups. The standardized ES were analyzed using random effects meta-analyses that assumes random variability beyond sampling error between studies.79 Egger’s test and the graphical funnel plot method were used to assess publication bias or the increased probability of statistically significant results to be published. An asymmetrical funnel plot and significant Egger’s regression test of asymmetry suggests publication bias due to negative studies with smaller sample sizes not appearing in the literature.80
Results
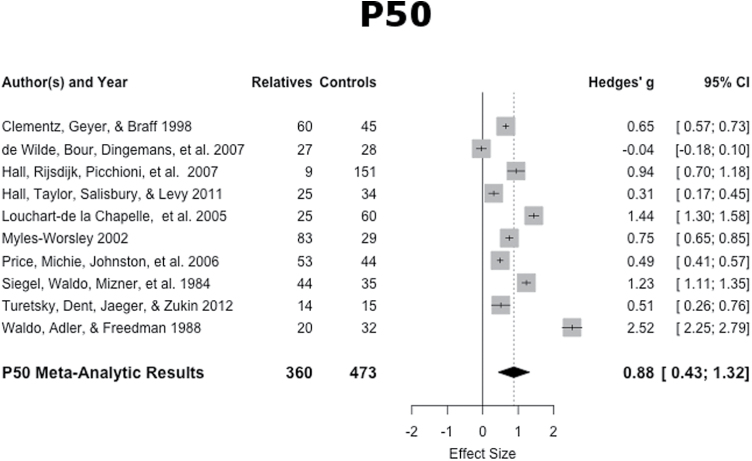
P50: The mean weighted ES of the 10 studies was of large magnitude (ES = 0.86, SE = .21, 95% CI: 0.44, 1.27), with suppression of relatives being smaller than that of healthy controls. This weighted mean ES differed significantly from zero (z = 4.04, P < .001). The forest plot is shown in figure 2. This distribution of the ES indicated heterogeneity (Q9 = 45.54, P < .001), therefore the dispersion of ES is greater than expected from sampling error. The funnel plot was symmetrical and Egger’s regression test of funnel plot asymmetry was not significant (z = 1.36, P = .17).
Fig. 2.
P50 suppression effect sizes and forest plot.
P300 Amplitude
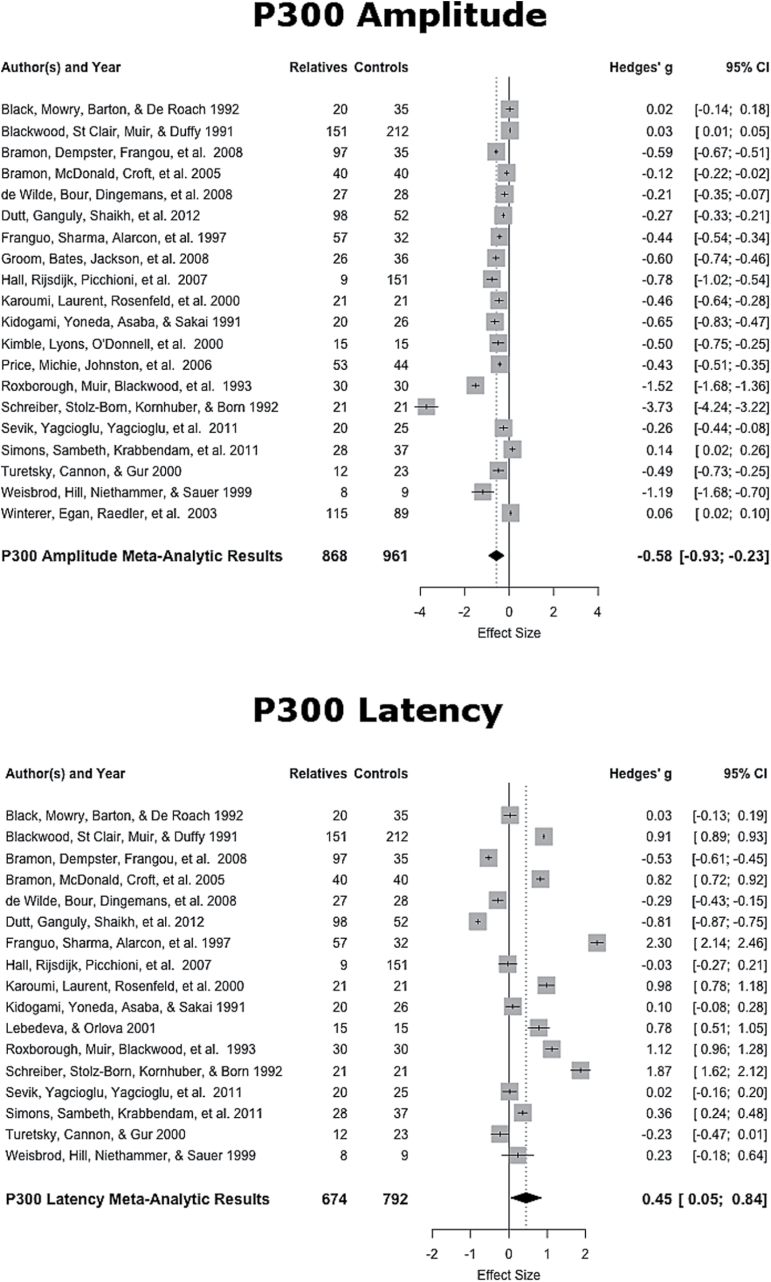
The mean weighted ES of the 20 studies was of medium magnitude (ES = −0.52, SE = .15, 95% CI: −0.82, −0.23), with amplitude of relatives being smaller than that of healthy controls (see figure 3). This weighted mean ES differed significantly from zero (z = −3.52, P < .001). This distribution of the ES indicated heterogeneity (Q19 = 91.70, P < .001), therefore the dispersion of ES is greater than expected from sampling error. The funnel plot was asymmetrical and Egger’s regression test of funnel plot asymmetry was significant (z = −4.32, P < .001). The following moderating variables were tested using a mixed effects model: (1) the ratio of males to females in the relative sample, (2) the average age of relatives, and (3) the response required of participants. Results indicate that ratio (P = .43), relative age (P = .15), and no response (P = .38) were significant moderators. These moderators did not account for significant heterogeneity in ES, (QE16 = 90.12, P < .001).
Fig. 3.
P300 effect sizes and forest plot.
P300 Latency
The mean weighted ES of the 17 studiesiii was of small-to-medium magnitude (ES = 0.44, SE = .20, 95% CI: 0.04, 0.84), with latency of relatives being longer than that of healthy controls (see figure 3). This weighted mean ES differed significantly from zero (z = 2.17, P < .05). This distribution of the ES indicated heterogeneity (Q16 = 178.96, P < .001), therefore the dispersion of ES is greater than expected from sampling error. The funnel plot was asymmetrical and Egger’s regression test of funnel plot asymmetry was not significant (z = 0.62, P = .54). Moderating variables were again tested using a mixed effects model, which showed that the male to female ratio (P = .61), relative age (P = .51), and no response (P = .39) were significant moderators and that these moderators did not account for significant heterogeneity in ES (QE13 = 135.39, P < .001).
Mismatch Negativity
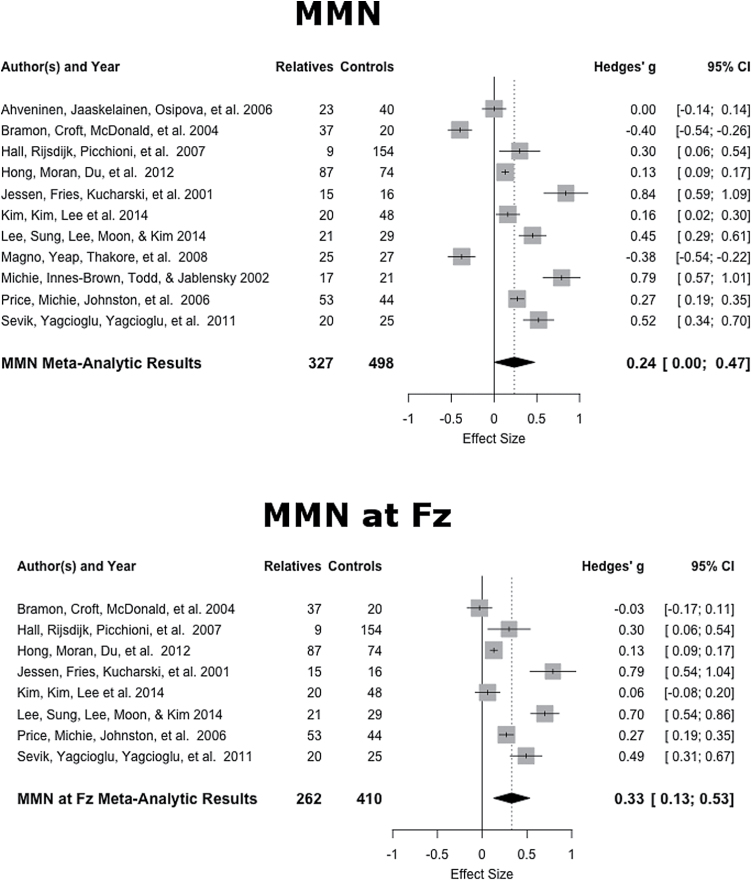
The mean weighted ES of the 11 studiesiv was of small magnitude (ES = 0.21, SE = .11, 95% CI: −0.01, 0.42). Although there was a trend, this weighted mean ES did not differ significantly from zero (z = 1.90, P = .06). This distribution of the ES indicated heterogeneity (Q10 = 18.57, P = .05), therefore the dispersion of ES is greater than expected from sampling error. The funnel plot was symmetrical and Egger’s regression test of funnel plot asymmetry was not significant (z = 1.16, P = .25).v The following moderating variables were tested using a mixed effects model: (1) the average age of relatives, (2) whether the reported amplitudes included additional channels (eg, Oz) outside of the frontal channels, and (3) whether MMN was calculated based on a duration or frequency deviation task. The results showed that neither age (P = .63) nor task (P = .36) were significant moderators. There was, however, a significant effect of reported channels (z = −2.47, P = .01). The moderating variables accounted for the previously found significant heterogeneity in ES (QE7 = 9.10, P = .25).
In order to further explore whether MMN deficits are found in relatives of patients compared with the controls, an additional exploratory meta-analysis was conducted looking only at MMN amplitude in the Fz channel. Of the 11 studies35,39,55,64,65,68–70, 8 provided sufficient data for ES calculation in Fz. The mean weighted ES of the 8 studies was again of small magnitude (ES = 0.26, SE = .08, 95% CI: 0.09, 0.42), however, this weighted mean ES differed significantly from zero (z = 3.02, P < .01). This distribution of the ES did not indicate heterogeneity (Q7 = 7.80, P = .35), therefore the dispersion of ES is not greater than expected from sampling error. The MMN forest plot is shown in in figure 4.
Fig. 4.
MMN effect sizes and forest plot. MMN = mismatch negativity.
Discussion
An ideal neurophysiological endophenotype is one that exhibits a robust deficit in both patients with schizophrenia and unaffected family members, is easily measured with limited subject demands, and demonstrates high reliability.30 We have assessed whether 3 ERP components may fit these criteria by specifically examining whether these components, which are easily measured, demonstrate high reliability, show robust and stable deficits in the patient population (for a review, see Turetsky et al30), and are also reliably deficient in unaffected relatives. The present study comprehensively reviewed all current literature examining 3 key ERP components assessing sensory and attentional processing of auditory stimuli: P50 suppression, P300, and MMN. The results suggest that deficits in these components may all be viable candidates for schizophrenia endophenotypes.
Twin and family studies have shown heritability in each of the components examined in the current review. For example, a twin study calculated heritability estimates of 68% for P50 suppression, 63% for MMN peak amplitude, and 69% for P300 amplitude.81 In an intriguing study, these 3 components were studied concomitantly within a sample of monozygotic and dizygotic twins in order to assess the genetic overlap between them. Interestingly, there was little evidence of a genetic association between these separate components, suggesting that they each may represent different cognitive processes that are influenced by independent sets of genes.82 Therefore, individuals who exhibit abnormalities in more than one of those components may carry a higher genetic loading. Studying all 3 of these components within high-risk populations would therefore offer greater power in determining susceptibility of phenotypic disease expression.
Patients with schizophrenia have been shown to have deficits in P50 suppression relative to healthy controls, thereby being potentially susceptible to sensory overload.9,10 The current analysis demonstrated that relatives of patients also exhibit this deficit relative to healthy controls, which is consistent with the heritability of P50 suppression that has been shown in genetic studies.81,83 The magnitude of the ES found in the current analysis (ES = 0.86) is large and replicates the previous smaller meta-analysis of this population (ES = 0.8510). Although smaller, this ES is comparable to the large ES magnitude for patients with schizophrenia relative to healthy controls (eg, ES = 1.2810). Thus, P50 suppression meets heritability criteria needed to serve as an endophenotype. Furthermore, P50 suppression is measured without the need of an explicit response from the participant. Although there are potential disadvantages, such as excessive boredom and potential for subjects to fall asleep, the lack of subject demands may make the P50 an ideal neurophysiological endophenotype for schizophrenia.
The P300 component is an exogenous component that is involved in the engagement of attention, processing of novelty, and context updating to changes in the environment.84 Patients with schizophrenia have been shown to exhibit deficits in both P300 amplitude and latency.9,23 The current study has demonstrated reliable differences in P300 amplitude and latency between unaffected relatives of patients and healthy controls, suggesting deficits in attention and novelty processing (amplitude) as well as perceptual processing speed (latency) in unaffected relatives. The ES values in the current analysis (amplitude ES = 0.52; latency ES = 0.44) were marginally smaller than those previously shown in a meta-analytic review of the P300 in schizophrenia (amplitude ES = 0.85; latency ES = 0.579) and previously reported for relatives (amplitude ES = 0.61; latency ES = 0.5031). Furthermore, we did not find male-to-female ratio, relative age, or response required by participants to have a significant influence on the ES or to explain the heterogeneity found. The reliable deficits found in the current study suggest that P300 deviances are a premorbid marker of risk, irrespective of subject demands and are not dependent on the phenotypic expression of the disease.
In the primary analysis, it was shown that although there was a trend, no reliable difference between MMN was found between relatives and healthy controls. This is in stark contrast to the large magnitude of the ES for differences in MMN between patients with schizophrenia and healthy controls (ES = 0.9929) and is consistent with the results of a recent meta-analysis of relatives (ES = 0.2632). However, the use of extraneous channels was shown to be a significant moderating variable and accounted for a great deal of heterogeneity. Interestingly, differences in scalp topography of the MMN in patients with schizophrenia relative to healthy controls have been shown with impairment in frontal but not temporal regions in patients.74–76 Indeed, there is evidence that two distinct neural generators underlie the MMN, superior temporal generators and frontal generators,86,87 as evidenced by current source density maps88,89 and equivalent current dipole modeling.90 Although still an area of debate, it has been posited that upon detection of a stimulus change in the temporal circuits, the frontal generator is used for involuntary attention switching.91 When only the results reported for channel Fz were examined, a significant deficit in MMN amplitude was found for relatives. This may be a result of increased noise when irrelevant posterior channels are included or may be indicative of deficits in the frontal MMN subcomponent. It has been suggested that MMN deficits are related to disease progression or imminent conversion to psychosis rather than genetic vulnerability to the disease.29,32 Indeed, MMN reduction has also been shown to predict conversion to psychosis in clinical high-risk individuals,92–95 and a previous meta-analysis of the MMN in schizophrenia found a systematic increase in ES as a function of illness duration.29 However, a more recent meta-regression between patients and healthy controls failed to find significant linear relationship between illness duration and ES, suggesting that progressive impairment is not a linear process.32 Additional research on the MMN in unaffected relatives of patients with schizophrenia is warranted.
The current analysis may have implications for both the genetic associations underlying the phenotypical expression of schizophrenia and the cognitive functioning of those at risk of the disease. The concept of a multilayered information processing system has been posited,82 and it has been suggested that although separate, these layers may overlap to some extent.96 P50 suppression and MMN are thought to be pre-attentional processes involved in sensory “gating out” of irrelevant sensory input and “gating in” of important information, respectively.97 Successful stimulus encoding involves both these processes, and the processed stimuli are then evaluated,21 and environmental changes are updated,98 as indexed by P300 latency and amplitude. The lack of inter-component heritability of these measures,82 as well as varying deficits of these measures in unaffected relatives, suggests distinct underlying genetic influences of these cognitive processes. Our results suggest both attentional deficits and deficient inhibition of irrelevant auditory input in unaffected relatives. It remains possible that only some relatives exhibit one or more of these deficits. Indeed, bimodal distributions have been found in independent studies of unaffected relatives for the P30044,48 and for P50 suppression.40 The phenotypic expression of schizophrenia may involve a combination of these deficits. Studies of individual differences in these components in both patients and relatives would inform the genetic underpinnings of schizophrenia and may guide the development of specific drugs and therapies for treatment.5 For example, P50 suppression deficits have been associated with the alpha-7 nicotinic receptor gene.99 Therefore, alpha-7 agonists may be feasible treatments for auditory sensory gating impairments in schizophrenia.100
Conclusion
The systematic examination of potential endophenotypes is a critical step in understanding underlying genetic influences of the disease. Here, we have identified 3 auditory ERP components that meet the criteria for endophenotypes of schizophrenia. The results of these meta-analyses suggest that relatives of patients with schizophrenia reliably demonstrate deficits in sensory gating, attentional processing, stimulus classification, and perceptual discrimination ability, as indexed by P50 suppression, P300 amplitude, P300 latency, and MMN, respectively.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
H.A.E. and T.C. were supported by National Institutes of Health grant R01MH096698 and National Science Foundation grant #SMA-1041755 to the Temporal Dynamics of Learning Center. V.M. was supported by National Institutes of Health grants R01MH094650 and R21/R33MH103231.
Supplementary Material
Acknowledgment
All authors declare no biomedical financial interests or potential conflicts of interest.
References
- 1. Mittal VA, Ellman LM, Cannon TD. Gene-environment interaction and covariation in schizophrenia: the role of obstetric complications. Schizophr Bull. 2008;34:1083–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. John B, Lewis KR. Chromosome variability and geographical distribution in insects: chromosome rather than gene variation provide the key to differences among populations. Science. 1966;151:711–721. [DOI] [PubMed] [Google Scholar]
- 3. Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122:15–30. [DOI] [PubMed] [Google Scholar]
- 4. Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. [DOI] [PubMed] [Google Scholar]
- 5. Braff DL, Freedman R, Schork NJ, Gottesman II. Deconstructing schizophrenia: an overview of the use of endophenotypes in order to understand a complex disorder. Schizophr Bull. 2007;33:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. [DOI] [PubMed] [Google Scholar]
- 7. Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5:249–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silverstein SM, Keane BP. Perceptual organization impairment in schizophrenia and associated brain mechanisms: review of research from 2005 to 2010. Schizophr Bull. 2011;37:690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res. 2004;70:315–329. [DOI] [PubMed] [Google Scholar]
- 10. de Wilde OM, Bour LJ, Dingemans PM, Koelman JH, Linszen DH. A meta-analysis of P50 studies in patients with schizophrenia and relatives: differences in methodology between research groups. Schizophr Res. 2007;97:137–151. [DOI] [PubMed] [Google Scholar]
- 11. Hillyard SA, Kutas M. Electrophysiology of cognitive processing. Annu Rev Psychol. 1983;34:33–61. [DOI] [PubMed] [Google Scholar]
- 12. Freedman R, Adler LE, Baker N, Waldo M, Mizner G. Candidate for inherited neurobiological dysfunction in schizophrenia. Somat Cell Mol Genet. 1987;13:479–484. [DOI] [PubMed] [Google Scholar]
- 13. Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. [DOI] [PubMed] [Google Scholar]
- 14. Micoulaud-Franchi J, Aramaki M, Merer A, et al. Toward an exploration of feeling of strangeness in schizophrenia: Perspectives on acousmatic and everyday listening. J Abnorm Psychol. 2012;121:628. [DOI] [PubMed] [Google Scholar]
- 15. Micoulaud-Franchi J, Hetrick WP, Aramaki M, et al. Do schizophrenia patients with low P50-suppression report more perceptual anomalies with the sensory gating inventory? Schizophr Res. 2014;157:157–162. [DOI] [PubMed] [Google Scholar]
- 16. Jin Y, Bunney WE, Sandman CA, et al. Is P50 suppression a measure of sensory gating in schizophrenia? Biol Psychiatry. 1998;43:873–878. [DOI] [PubMed] [Google Scholar]
- 17. Posner MI. Psychobiology of attention. In: Gazzaniga M, Blakemore C, eds. Handbook of Psychobiology. New York, NY: Academic Press; 1975:441–480. [Google Scholar]
- 18. Donchin E, Coles MG. Is the P300 component a manifestation of context updating? Behav Brain Sci. 1988;11:357–374. [Google Scholar]
- 19. Kramer AF, Strayer DL. Assessing the development of automatic processing: an application of dual-task and event-related brain potential methodologies. Biol Psychol. 1988;26:231–267. [DOI] [PubMed] [Google Scholar]
- 20. Kramer AF, Wickens CD, Donchin E. An analysis of the processing requirements of a complex perceptual-motor task. Hum Factors. 1983;25:597–621. [DOI] [PubMed] [Google Scholar]
- 21. Kutas M, McCarthy G, Donchin E. Augmenting mental chronometry: the P300 as a measure of stimulus evaluation time. Science. 1977;197:792–795. [DOI] [PubMed] [Google Scholar]
- 22. Polich J. P300 development from auditory stimuli. Psychophysiology. 1986;23:590–597. [DOI] [PubMed] [Google Scholar]
- 23. Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. [DOI] [PubMed] [Google Scholar]
- 24. Pfefferbaum A, Ford JM, White PM, Roth WT. P3 in schizophrenia is affected by stimulus modality, response requirements, medication status, and negative symptoms. Arch Gen Psychiatry. 1989;46:1035–1044. [DOI] [PubMed] [Google Scholar]
- 25. Bestelmeyer PE, Phillips LH, Crombie C, Benson P, St Clair D. The P300 as a possible endophenotype for schizophrenia and bipolar disorder: evidence from twin and patient studies. Psychiatry Res. 2009;169:212–219. [DOI] [PubMed] [Google Scholar]
- 26. Duncan CC. Event-related brain potentials: a window on information processing in schizophrenia. Schizophr Bull. 1988;14:199–203. [DOI] [PubMed] [Google Scholar]
- 27. Näätänen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin Neurophysiol. 2007;118:2544–2590. [DOI] [PubMed] [Google Scholar]
- 28. Garrido MI, Kilner JM, Stephan KE, Friston KJ. The mismatch negativity: a review of underlying mechanisms. Clin Neurophysiol. 2009;120:453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. [DOI] [PubMed] [Google Scholar]
- 30. Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bramon E, McDonald C, Croft RJ, et al. Is the P300 wave an endophenotype for schizophrenia? A meta-analysis and a family study. Neuroimage. 2005;27:960–968. [DOI] [PubMed] [Google Scholar]
- 32. Erickson MA, Ruffle A, Gold JM. A meta-analysis of mismatch negativity in schizophrenia: from clinical risk to disease specificity and progression [published online ahead of print August 25, 2015]. Biol Psychiatry. doi:10.1016/j.biopsych.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clementz BA, Geyer MA, Braff DL. Multiple site evaluation of P50 suppression among schizophrenia and normal comparison subjects. Schizophr Res. 1998;30:71–80. [DOI] [PubMed] [Google Scholar]
- 34.de Wilde O, Bour L, Dingemans P, Koelman J, Linszen D. Failure to find P50 suppression deficits in young first-episode patients with schizophrenia and clinically unaffected siblings. Schizophr Bull. 2007;33:1319–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hall M, Rijsdijk F, Picchioni M, et al. Substantial shared genetic influences on schizophrenia and event-related potentials. Am J Psychiatry. 2007;164:804–812. [DOI] [PubMed] [Google Scholar]
- 36. Hall MH, Taylor G, Salisbury DF, Levy DL. Sensory gating event-related potentials and oscillations in schizophrenia patients and their unaffected relatives. Schizophr Bull. 2011;37:1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Louchart-de la Chapelle S, Nkam I, Houy E, et al. A concordance study of three electrophysiological measures in schizophrenia. Am J Psychiatry. 2005;162:466–474. [DOI] [PubMed] [Google Scholar]
- 38. Myles-Worsley M. P50 sensory gating in multiplex schizophrenia families from a Pacific island isolate. Am J Psychiatry. 2002;159:2007–2012. [DOI] [PubMed] [Google Scholar]
- 39. Price GW, Michie PT, Johnston J, et al. A multivariate electrophysiological endophenotype, from a unitary cohort, shows greater research utility than any single feature in the Western Australian family study of schizophrenia. Biol Psychiatry. 2006;60:1–10. [DOI] [PubMed] [Google Scholar]
- 40. Siegel C, Waldo M, Mizner G, Adler LE, Freedman R. Deficits in sensory gating in schizophrenic patients and their relatives. Evidence obtained with auditory evoked responses. Arch Gen Psychiatry. 1984;41:607–612. [DOI] [PubMed] [Google Scholar]
- 41. Turetsky BI, Dent G, Jaeger J, Zukin SR. P50 amplitude reduction: a nicotinic receptor-mediated deficit in first-degree relatives of schizophrenia patients. Psychopharmacology (Berl). 2012;221:39–52. [DOI] [PubMed] [Google Scholar]
- 42. Waldo MC, Adler LE, Freedman R. Defects in auditory sensory gating and their apparent compensation in relatives of schizophrenics. Schizophr Res. 1988;1:19–24. [DOI] [PubMed] [Google Scholar]
- 43. Black JL, Mowry BJ, Barton DA, De Roach JN. Auditory P300 studies in schizophrenic subjects and their first degree relatives. Australas Phys Eng Sci Med. 1992;15:65–73. [PubMed] [Google Scholar]
- 44. Blackwood DH, St Clair DM, Muir WJ, Duffy JC. Auditory P300 and eye tracking dysfunction in schizophrenic pedigrees. Arch Gen Psychiatry. 1991;48:899–909. [DOI] [PubMed] [Google Scholar]
- 45. Bramon E, Dempster E, Frangou S, et al. Neuregulin-1 and the P300 waveform—a preliminary association study using a psychosis endophenotype. Schizophr Res. 2008;103:178–185. [DOI] [PubMed] [Google Scholar]
- 46. de Wilde OM, Bour LJ, Dingemans PM, Koelman JH, Boerée T, Linszen DH. P300 deficits are present in young first-episode patients with schizophrenia and not in their healthy young siblings. Clin Neurophysiol. 2008;119:2721–2726. [DOI] [PubMed] [Google Scholar]
- 47. Dutt A, Ganguly T, Shaikh M, et al. Association between hippocampal volume and P300 event related potential in psychosis: support for the Kraepelinian divide. Neuroimage. 2012;59:997–1003. [DOI] [PubMed] [Google Scholar]
- 48. Frangou S, Sharma T, Alarcon G, et al. The Maudsley Family Study, II: Endogenous event-related potentials in familial schizophrenia. Schizophr Res. 1997;23:45–53. [DOI] [PubMed] [Google Scholar]
- 49. Groom MJ, Bates AT, Jackson GM, Calton TG, Liddle PF, Hollis C. Event-related potentials in adolescents with schizophrenia and their siblings: a comparison with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:784–792. [DOI] [PubMed] [Google Scholar]
- 50. Karoumi B, Laurent A, Rosenfeld F, et al. Alteration of event related potentials in siblings discordant for schizophrenia. Schizophr Res. 2000;41:325–334. [DOI] [PubMed] [Google Scholar]
- 51. Kidogami Y, Yoneda H, Asaba H, Sakai T. P300 in first degree relatives of schizophrenics. Schizophr Res. 1991;6:9–13. [DOI] [PubMed] [Google Scholar]
- 52. Kimble M, Lyons M, O’Donnell B, Nestor P, Niznikiewicz M, Toomey R. The effect of family status and schizotypy on electrophysiologic measures of attention and semantic processing. Biol Psychiatry. 2000;47:402–412. [DOI] [PubMed] [Google Scholar]
- 53. Roxborough H, Muir WJ, Blackwood DH, Walker MT, Blackburn IM. Neuropsychological and P300 abnormalities in schizophrenics and their relatives. Psychol Med. 1993;23:305–314. [DOI] [PubMed] [Google Scholar]
- 54. Schreiber H, Stolz-Born G, Kornhuber HH, Born J. Event-related potential correlates of impaired selective attention in children at high risk for schizophrenia. Biol Psychiatry. 1992;32:634–651. [DOI] [PubMed] [Google Scholar]
- 55. Şevik AE, Anıl Yağcıoğlu AE, Yağcıoğlu S, Karahan S, Gürses N, Yıldız M. Neuropsychological performance and auditory event related potentials in schizophrenia patients and their siblings: a family study. Schizophr Res. 2011;130:195–202. [DOI] [PubMed] [Google Scholar]
- 56. Simons CJ, Sambeth A, Krabbendam L, Pfeifer S, van Os J, Riedel WJ. Auditory P300 and N100 components as intermediate phenotypes for psychotic disorder: familial liability and reliability. Clin Neurophysiol. 2011;122:1984–1990. [DOI] [PubMed] [Google Scholar]
- 57. Turetsky BI, Cannon TD, Gur RE. P300 subcomponent abnormalities in schizophrenia: III. Deficits In unaffected siblings of schizophrenic probands. Biol Psychiatry. 2000;47:380–390. [DOI] [PubMed] [Google Scholar]
- 58. Weisbrod M, Hill H, Niethammer R, Sauer H. Genetic influence on auditory information processing in schizophrenia: P300 in monozygotic twins. Biol Psychiatry. 1999;46:721–725. [DOI] [PubMed] [Google Scholar]
- 59. Winterer G, Egan MF, Raedler T, et al. P300 and genetic risk for schizophrenia. Arch Gen Psychiatry. 2003;60:1158–1167. [DOI] [PubMed] [Google Scholar]
- 60. Lebedeva I, Orlova V. Features of auditory P300 in relatives of schizophrenic patients. Hum Physiol. 2001;27:289–293. [Google Scholar]
- 61. Schreiber H, Stolz G, Rothmeier J, Kornhuber H, Borne J. Prolonged latencies of the N2 and P3 of the auditory event-related potential in children at risk for schizophrenia. Eur Arch Psychiatry Neurol Sci. 1989;238:185–188. [DOI] [PubMed] [Google Scholar]
- 62. Schreiber H, Stolz-Born G, Rothmeier J, Kornhuber A, Kornhuber HH, Born J. Endogenous event-related brain potentials and psychometric performance in children at risk for schizophrenia. Biol Psychiatry. 1991;30:177–189. [DOI] [PubMed] [Google Scholar]
- 63. Ahveninen J, Jääskeläinen IP, Osipova D, et al. Inherited auditory-cortical dysfunction in twin pairs discordant for schizophrenia. Biol Psychiatry. 2006;60:612–620. [DOI] [PubMed] [Google Scholar]
- 64. Bramon E, Croft RJ, McDonald C, et al. Mismatch negativity in schizophrenia: a family study. Schizophr Res. 2004;67:1–10. [DOI] [PubMed] [Google Scholar]
- 65. Jessen F, Fries T, Kucharski C, et al. Amplitude reduction of the mismatch negativity in first-degree relatives of patients with schizophrenia. Neurosci Lett. 2001;309:185–188. [DOI] [PubMed] [Google Scholar]
- 66. Magno E, Yeap S, Thakore JH, Garavan H, De Sanctis P, Foxe JJ. Are auditory-evoked frequency and duration mismatch negativity deficits endophenotypic for schizophrenia? High-density electrical mapping in clinically unaffected first-degree relatives and first-episode and chronic schizophrenia. Biol Psychiatry. 2008;64:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Michie PT, Innes-Brown H, Todd J, Jablensky AV. Duration mismatch negativity in biological relatives of patients with schizophrenia spectrum disorders. Biol Psychiatry. 2002;52:749–758. [DOI] [PubMed] [Google Scholar]
- 68. Hong LE, Moran LV, Du X, O’Donnell P, Summerfelt A. Mismatch negativity and low frequency oscillations in schizophrenia families. Clin Neurophysiol. 2012;123:1980–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee SH, Sung K, Lee KS, Moon E, Kim CG. Mismatch negativity is a stronger indicator of functional outcomes than neurocognition or theory of mind in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:213–219. [DOI] [PubMed] [Google Scholar]
- 70. Kim M, Kim SN, Lee S, et al. Impaired mismatch negativity is associated with current functional status rather than genetic vulnerability to schizophrenia. Psychiat Res Neuroimag. 2014;222:100–106. [DOI] [PubMed] [Google Scholar]
- 71. Hoffman LD, Polich J. P300, handedness, and corpus callosal size: gender, modality, and task. International Journal of Psychophysiology. 1999;31:163–174. [DOI] [PubMed] [Google Scholar]
- 72. Dujardin K, Derambure P, Bourriez J, Jacquesson J, Guieu J. P300 component of the event-related potentials (ERP) during an attention task: effects of age, stimulus modality and event probability. International Journal of Psychophysiology. 1993;14:255–267. [DOI] [PubMed] [Google Scholar]
- 73. Salisbury DF, Rutherford B, Shenton ME, McCarley RW. Button-pressing affects P300 amplitude and scalp topography. Clinical Neurophysiology. 2001;112:1676–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Alain C, Hargrave R, Woods DL. Processing of auditory stimuli during visual attention in patients with schizophrenia. Biol Psychiatry. 1998;44:1151–1159. [DOI] [PubMed] [Google Scholar]
- 75. Baldeweg T, Klugman A, Gruzelier JH, Hirsch SR. Impairment in frontal but not temporal components of mismatch negativity in schizophrenia. International Journal of Psychophysiology. 2002;43:111–122. [DOI] [PubMed] [Google Scholar]
- 76. Sato Y, Yabe H, Todd J, et al. Impairment in activation of a frontal attention-switch mechanism in schizophrenic patients. Biol Psychol. 2003;62:49–63. [DOI] [PubMed] [Google Scholar]
- 77. Näätänen R, Kujala T, Escera C, et al. The mismatch negativity (MMN)—a unique window to disturbed central auditory processing in ageing and different clinical conditions. Clin Neurophysiol. 2012;123:424–158. [DOI] [PubMed] [Google Scholar]
- 78. Tervaniemi M, Lehtokoski A, Sinkkonen J, Virtanen J, Ilmoniemi R, Näätänen R. Test–retest reliability of mismatch negativity for duration, frequency and intensity changes. Clin Neurophysiol. 1999;110:1388–1393. [DOI] [PubMed] [Google Scholar]
- 79. Lipsey MW, Wilson DB. Practical Meta-analysis. Thousand Oaks, CA: Sage Publications; 2001. [Google Scholar]
- 80. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hall MH, Schulze K, Rijsdijk F, et al. Heritability and reliability of P300, P50 and duration mismatch negativity. Behav Genet. 2006;36:845–857. [DOI] [PubMed] [Google Scholar]
- 82. Hall MH, Schulze K, Bramon E, Murray RM, Sham P, Rijsdijk F. Genetic overlap between P300, P50, and duration mismatch negativity. Am J Med Genet B: Neuropsychiatr Genet. 2006;141:336–343. [DOI] [PubMed] [Google Scholar]
- 83. Anokhin AP, Vedeniapin AB, Heath AC, Korzyukov O, Boutros NN. Genetic and environmental influences on sensory gating of mid-latency auditory evoked responses: a twin study. Schizophr Res. 2007;89:312–319. [DOI] [PubMed] [Google Scholar]
- 84. Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Näätänen R, Michie PT. Early selective-attention effects on the evoked potential: a critical review and reinterpretation. Biol Psychol. 1979;8:81–136. [DOI] [PubMed] [Google Scholar]
- 86. Rinne T, Alho K, Ilmoniemi R, Virtanen J, Näätänen R. Separate time behaviors of the temporal and frontal mismatch negativity sources. Neuroimage. 2000;12:14–19. [DOI] [PubMed] [Google Scholar]
- 87. Giard M, Perrin F, Pernier J, Bouchet P. Brain generators implicated in the processing of auditory stimulus deviance: a topographic event-related potential study. Psychophysiology. 1990;27:627–640. [DOI] [PubMed] [Google Scholar]
- 88. Shalgi S, Deouell LY. Direct evidence for differential roles of temporal and frontal components of auditory change detection. Neuropsychologia. 2007;45:1878–1888. [DOI] [PubMed] [Google Scholar]
- 89. Restuccia D, Della Marca G, Marra C, Rubino M, Valeriani M. Attentional load of the primary task influences the frontal but not the temporal generators of mismatch negativity. Cognitive Brain Research. 2005;25:891–899. [DOI] [PubMed] [Google Scholar]
- 90. Schönwiesner M, Krumbholz K, Rübsamen R, Fink GR, von Cramon DY. Hemispheric asymmetry for auditory processing in the human auditory brain stem, thalamus, and cortex. Cereb Cortex. 2007;17:492–499. [DOI] [PubMed] [Google Scholar]
- 91. Näätänen R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behav Brain Sci. 1990;13:201–233. [Google Scholar]
- 92. Bodatsch M, Ruhrmann S, Wagner M, et al. Prediction of psychosis by mismatch negativity. Biol Psychiatry. 2011;69:959–966. [DOI] [PubMed] [Google Scholar]
- 93. Shaikh M, Valmaggia L, Broome MR, et al. Reduced mismatch negativity predates the onset of psychosis. Schizophr Res. 2012;134:42–48. [DOI] [PubMed] [Google Scholar]
- 94. Higuchi Y, Sumiyoshi T, Seo T, Miyanishi T, Kawasaki Y, Suzuki M. Mismatch negativity and cognitive performance for the prediction of psychosis in subjects with at-risk mental state. PLoS One. 2013;8:e54080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Perez VB, Woods SW, Roach BJ, et al. Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biol Psychiatry. 2014;75:459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Näätänen R. The mismatch negativity. In: Näätänen R, ed. Attention and Brain Function. Mahwah, NJ: Laurence Erbaum Associates; 1992:136–200. [Google Scholar]
- 97. Näätänen R, Gaillard AW, Mäntysalo S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychol (Amst). 1978;42:313–329. [DOI] [PubMed] [Google Scholar]
- 98. Donchin E. Surprise!… surprise? Psychophysiology. 1981;18:493–513. [DOI] [PubMed] [Google Scholar]
- 99. Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl). 2004;174:54–64. [DOI] [PubMed] [Google Scholar]
- 100. Olincy A, Harris JG, Johnson LL, et al. Proof-of-concept trial of an α7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63:630–638. [DOI] [PubMed] [Google Scholar]
- 101. Spreng M. Influence of impulsive and fluctuating noise upon physiological excitations and short-time readaptation. Scand Audiol Suppl. 1980;(Suppl. 12):299–306. [PubMed] [Google Scholar]
- 102. Keidel WD, Spreng M. Neurophysiological evidence for the Stevens power function in man. J Acoust Soc Am. 1965;38:191–195. [DOI] [PubMed] [Google Scholar]
- 103. Butler RA. Effect of changes in stimulus frequency and intensity on habituation of the human vertex potential. J Acoust Soc Am. 1968;44:945–950. [DOI] [PubMed] [Google Scholar]
- 104. Davis H, Mast T, Yoshie N, Zerlin S. The slow response of the human cortex to auditory stimuli: recovery process. Electroencephalogr Clin Neurophysiol. 1966;21:105–113. [DOI] [PubMed] [Google Scholar]
- 105. Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182:177–180. [DOI] [PubMed] [Google Scholar]
- 106. Ogura C, Nageishi Y, Matsubayashi M, Omura F, Kishimoto A, Shimokochi M. Abnormalities in event-related potentials, N100, P200, P300 and slow wave in schizophrenia. Psychiatry Clin Neurosci. 1991;45:57–65. [DOI] [PubMed] [Google Scholar]
- 107. Ford JM, Mathalon DH, Kalba S, Marsh L, Pfefferbaum A. N1 and P300 abnormalities in patients with schizophrenia, epilepsy, and epilepsy with schizophrenialike features. Biol Psychiatry. 2001;49:848–860. [DOI] [PubMed] [Google Scholar]
- 108. O’Donnell B, Vohs J, Hetrick W, Carroll C, Shekhar A. Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. International Journal of Psychophysiology. 2004;53:45–55. [DOI] [PubMed] [Google Scholar]
- 109. Salisbury DF, Collins KC, McCarley RW. Reductions in the N1 and P2 auditory event-related potentials in first-hospitalized and chronic schizophrenia. Schizophr Bull. 2010;36:991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Clementz BA, Blumenfeld LD. Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Exp Brain Res. 2001;139:377–390. [DOI] [PubMed] [Google Scholar]
- 111. Javitt DC, Sweet RA. Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nat Rev Neurosci. 2015;16:535–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Brockhaus-Dumke A, Schultze-Lutter F, Mueller R, et al. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biol Psychiatry. 2008;64:376–384. [DOI] [PubMed] [Google Scholar]
- 113. Boutros NN, Belger A, Campbell D, D’Souza C, Krystal J. Comparison of four components of sensory gating in schizophrenia and normal subjects: a preliminary report. Psychiatry Res. 1999;88:119–130. [DOI] [PubMed] [Google Scholar]
- 114. Foxe JJ, Yeap S, Snyder AC, Kelly SP, Thakore JH, Molholm S. The N1 auditory evoked potential component as an endophenotype for schizophrenia: high-density electrical mapping in clinically unaffected first-degree relatives, first-episode, and chronic schizophrenia patients. Eur Arch Psychiatry Clin Neurosci. 2011;261:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sumich A, Kumari V, Dodd P, et al. N100 and P300 amplitude to Go and No–Go variants of the auditory oddball in siblings discordant for schizophrenia. Schizophr Res. 2008;98:265–277. [DOI] [PubMed] [Google Scholar]
- 116. Force RB, Venables NC, Sponheim SR. An auditory processing abnormality specific to liability for schizophrenia. Schizophr Res. 2008;103:298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Turetsky BI, Greenwood TA, Olincy A, Radant AD, Braff DL, Cadenhead KS, et al. Abnormal auditory N100 amplitude: a heritable endophenotype in first-degree relatives of schizophrenia probands. Biol Psychiatry. 2008;64:1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.