Abstract
For at least a century, a debate has continued as to whether cancer risk is reduced in schizophrenia. Genetic studies have also suggested the 2 conditions may share protein transcriptional pathways. The author predicted that if the pathophysiology of schizophrenia confers protection from cancer, then the immunology of schizophrenia should reflect a state of tumor suppression, ie, the opposite of tumor escape. To examine this possibility, the author performed a literature search for measurements of cytokines in drug-naïve first episode subjects with schizophrenia for comparison with cytokine expression in tumor escape vs tumor suppression. The comparison showed that instead of either tumor suppression or escape, schizophrenia appears to be in a state of tumor equilibrium. Based on this finding, the author hypothesized that the clinical presentation of schizophrenia may involve cell transformation similar to an early stage of cancer initiation or an attenuated tumorigenesis. While this condition could reflect the presence of an actual tumor such as an ovarian teratoma causing anti-NMDA receptor encephalitis, it would only explain a small percentage of cases. To find a more likely tumor model, the author then compared the cytokine profile of schizophrenia to individual cancers and found the best match was melanoma. To demonstrate the viability of the theory, the author compared the hallmarks, emerging hallmarks, and enabling characteristics of melanoma to schizophrenia and found that many findings in schizophrenia are understood if schizophrenia is a condition of attenuated tumorigenesis.
Key words: schizophrenia, cancer, immunology, tumor, melanoma
Introduction
For over 100 years it has been debated whether individuals with schizophrenia have reduced risk of cancer, and many early studies did find schizophrenia seemed to protect from cancer in general.1 Anecdotal observations of heavy cigarette smoking by populations with schizophrenia who appeared free of smoking-related cancers further supported the notion. Recent genetic studies have also supported an inverse correlation between schizophrenia and cancer including a sharing of protein transcriptional pathways between the 2 diseases.2,3 But epidemiological studies have not confirmed any correlation as their results have ranged from decreased,4,5 to no difference6 to increased7 cancer risk in schizophrenia. Some investigators attribute the finding of reduced cancer risk to a protective genetic effect provided by schizophrenia,4 while others have argued the purported change in risk could be explained by medication effects and/or methodological errors.5
The author chose to investigate this uncertain relationship by determining how the immunology of schizophrenia and cancer, specifically the immunological stage of cancer referred to as “tumor escape” are different. Tumor escape is the last of 3 phases referred to as the 3 “Es” of immunoediting,8–10 the first 2 Es being “elimination” and “equilibrium.” During tumor elimination the body’s immune system is able to destroy any cancer cells before any clinical change; in equilibrium which is the most controversial, least understood, longest, and possibly permanent phase,8–10 tumor cell growth is stopped but not eliminated and has been referred to as tumor dormancy10; during tumor escape, the tumor overcomes the immune system and begins to grow, causing clinical symptoms. “Immunoediting” is that process many authors call “sculpting” by the immune system in which tumor cells are selected for their ability to resist the immune system. Most but not all immunoediting occurs within the tumor “microenvironment” which consists of the tumor, immune cells and the surrounding stroma, also referred to as the tumor site or its immediate vicinity.11 These microenvironments are low in oxygen and pH and drain to lymph nodes where tumor-associated microenvironments can be established12 and in later stages can be observed systematically.
Changes in cytokines such as the interleukins are only one aspect of the complex process of immunoediting which is an area of intense research interest due to the recent resurgence of immunological treatments of cancer.13 The numerous mechanisms of tumor or immune escape have been reviewed in detail in several excellent sources8,9,14,15 and can be generally categorized as types of altered antigen presentation, defects in innate immunity such as abnormal NK cells, and accumulation of so-called alternatively activated polarized M2 macrophages; defective T-cell responses including T-cell exhaustion or anergy, and various other mechanisms that include cytokine changes, changes in metabolism that alter apoptosis, and effector immune cell responses. Immunoediting in brain cancer occurs in a similar fashion.16
Interferons and interleukins are cytokines that are proteins like hormones in that they are cell-to-cell messengers but cytokines act in the “microenvironment,” usually target fewer cells than hormones, and are involved in the immune response.17 Interferon-γ, one of several interferons originally named for their regulation of the immune response in response to viral infections, also recruits phagocytic macrophages and promotes the differentiation of CD4+ T cells to the Th1 subset while inhibiting the development of Th2 and Th17 cells.18 Interleukins are cytokines originally believed to originate from and act on leukocytes (thus the name, inter-leuken) although the term has become more generic with the discovery of other sources and actions.17
The author predicted that if schizophrenia reduces the risk of cancer, a significant difference might be observed between the cytokine immunology of tumor escape and that of schizophrenia. More specifically, the author theorized that if schizophrenia reduced cancer risk, then the comparison of cytokine expression would demonstrate schizophrenia was in state of tumor suppression, ie, the opposite of tumor escape. However, as the discussion below shows, this prediction was wrong in predicting “suppression” as it became apparent that the secretion profile of cytokines in medication-naïve first-episode subjects with schizophrenia appears the same as tumor equilibrium as defined by the cytokine expression profiles suggested by Teng et al8 and Lippitz.19 Schizophrenia from the perspective of cytokine expression in the absence of a tumor cannot be differentiated from an initiation phase of cancer, and on comparison to specific tumors, has similarities to the cytokine expression of melanoma.
Methodology
A large review of biomarkers in schizophrenia published in 201020 found that relatively few studies performed measurements in medication-naïve patients. This is important because even though immunological dysfunction was observed in schizophrenia decades before the existence of antipsychotic medications, the immunological side effects of these medications have been known for some time.21 For the current study, the author searched on Pubmed for all studies of cytokines in psychotic subjects published after 2010 (to add to those studies available in the above-mentioned 2010 biomarkers review). To rigorously control for medication effects on cytokine expression only in schizophrenia, studies were then selected that only examined first episode, drug-naïve subjects with only the diagnosis of schizophrenia. This excluded studies of mixed groups of subjects with diverse diagnoses other than schizophrenia like bipolar disorder, schizoaffective disorder or other psychotic disorders; studies that included previously treated subjects who were not taking medications for some period of time, usually weeks, before the measurements or in which subjects had been medicated for very brief periods of time shortly before measurements were obtained; studies in which the medication state or procedures were not clearly described, and reviews and meta-analyses that included medicated patients. This excluded nearly 100 studies published since 2010 of cytokine levels in schizophrenia and left only 10 studies for comparison, 9 of which were published since 2010. The 10 studies described above provided measurements of IFN-γ, TNF-α and 11 interleukins (to include receptor antagonist ILRA) including IL1β, IL1RA, IL2, IL6, IL8, IL10, IL12, IL15, IL17, IL18, and IL23. Information regarding the immunological state of the tumor microenvironment during immune escape and equilibrium to compare to these cytokines was obtained from the extensive monographic literature on the subject of tumor immunology, as well as searches of Pubmed.
Findings
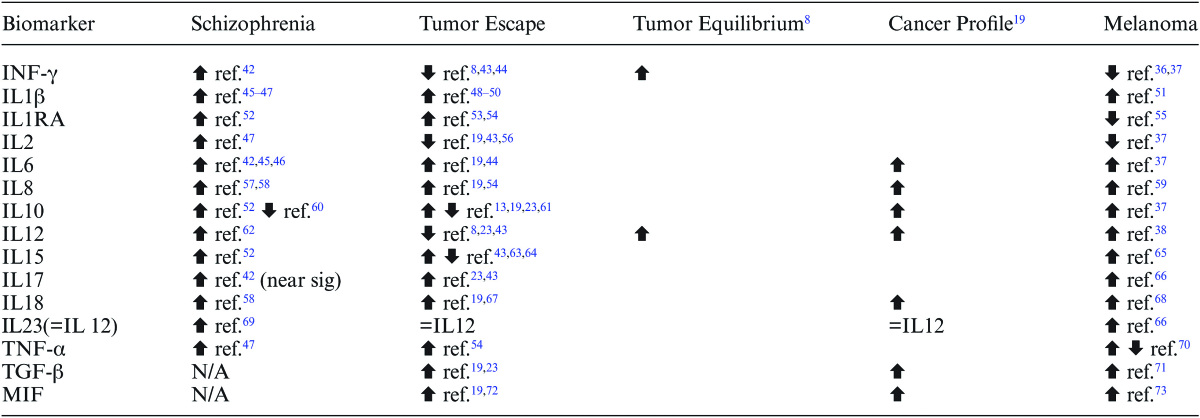
Table 1 illustrates the cytokine changes in schizophrenia including IFN-γ, interleukin and TNF-α compared to their expression in the tumor microenvironment during tumor escape, tumor equilibrium according to Teng et al,8 a cancer “profile” of cytokines suggested by Lippitz as an indicator of simultaneous immunostimulation and immunosuppression,19 and melanoma. Teng et al8 described the essential components of tumor equilibrium as IFN-γ, IL-12 and CD4+ and CD8+ T cells. The current study did not examine CD4+ and CD8+ T cells but a meta-analysis of T-cell subsets found that both subsets are elevated in drug-naïve, first-episode schizophrenia22 which parallels the increases observed in IFN-γ and IL-12 in tumor equilibrium shown on table 1. Table 1 also includes transforming growth factor β (TGF-β) and macrophage migration inhibitory factor (MIF) as part of a proposed cancer profile by Lippitz.19 Although the author found no studies of TGF-β and MIF in schizophrenia that met the stringent criteria described above, both biomarkers have been studied in medicated subjects which will be discussed below.
Table 1.
Cytokine Changes in Drug-Naïve First Episode Schizophrenia Compared to the Tumor Microenvironment
Note: MIF, migration inhibitory factor; IL, Interleukin; TGF-β, transforming growth factor β; TNF-α, Tumor Necrosis Factor-α.
With the exception of TGF-β and MIF which were excluded from the schizophrenia studies on table 1, the table shows an agreement between IFN-γ and interleukin expression in schizophrenia with the tumor equilibrium profile of Teng8 and the balanced immunosuppression and immunostimulation of cancer proposed by Lippitz.19 Combined, these profiles include the levels of IFN-γ, IL6, IL8, IL10, IL12, and IL18. Interleukin 10 has, according to Gopal23 an “enigmatic” function in cancer as this interleukin is both immunosuppressive and immunostimulating, and studies have found both increased and decreased levels in both tumor escape and schizophrenia as shown on table 1. TNFα and the remaining interleukins except for IL2 on table 1 agree with a state of tumor escape, ie, similar expressions in schizophrenia with tumor escape are seen for IL1β, IL1RA, IL15, and IL17. IL23 is considered part of IL12.17
As mentioned above, TGF-β and MIF were excluded from the schizophrenia studies in table 1 although both have been studied in medicated subjects with psychotic illness, and both have roles in tumor immunology. Numerous studies including one meta-analysis have found TGF-β1 elevated in schizophrenia20,24 although other studies have found no difference.25 TGF-β is clearly elevated in cancer19,23 although in the early stages of cancer it can act as a tumor suppressor while becoming a promoter as cancer progresses.23 The activity of TGF-β in medicated subjects with schizophrenia does agree with the cancer profile of Lippitz.19 MIF was identified in a large biomarker survey of schizophrenia as associated with decreased risk of schizophrenia,26 and it does contribute significantly to tumor escape (references in table 1) placing it tentatively in the same category as IL2, ie, having the opposite cytokine expression as observed in tumor escape while not specifically associated with equilibrium. IL2 and MIF, then, are perhaps the only 2 cytokines in table 1 that would correspond to tumor suppression.
This leads to the question whether there are specific cancers with the same cytokine profile as schizophrenia. The most specific marker on table 1 that separates the cancer profile from cancer equilibrium is increased IL-12 although cancers with increased IL-12 might be the minority as IL-12 is frequently tumor-suppressive. To find other cancer(s) with the same profile as schizophrenia, the author assessed IL-12 in cancers that might have either an anatomical or epidemiological association with schizophrenia. For example, schizophrenia could be compared to a glioma brain tumor as schizophrenia is a brain disease, or to a neuroblastoma as schizophrenia involves an abnormality of neurons. However, IL-12 is decreased in both glioma27 and neuroblastoma.28 Several studies have found increased risk of breast cancer in schizophrenia,29 but IL-12 measurements in breast cancer are inconsistent and have been found decreased, unchanged and increased.30–32 Interestingly, one cancer that has consistently decreased risk in schizophrenia5,33–35 and frequent by not consistently increased IL-1236–38 is melanoma.
The melanoma cytokine profile as shown on table 1 does however differ from schizophrenia as melanoma has decreased interferon-γ,36,37 which is consistent with tumor escape rather than equilibrium; but melanoma happens to have increased vascular endothelial growth factor (VEGF)39 compared to reduced VEGF in first episode, drug-naïve schizophrenia.40 VEGF is a potent angiogenic factor closely involved in the angiogenesis hallmark of cancer due to its important role in tumor progression39 and its tumor-promoting actions are suppressed by IFN-γ.41 Under normal conditions, IL-12 increases INF-γ which would sustain tumor equilibrium, but in melanoma IFN-γ is not increased which would allow VEGF to promote tumor angiogenesis. This shows that an important difference between the cytokine profiles of schizophrenia and melanoma is that the tumor-promoting effects of VEGF are suppressed in the former and released in the latter. Other immunological similarities and differences between schizophrenia and melanoma are borne out by comparing other cytokines on table 1 between the 2 conditions. Like schizophrenia, melanoma has elevated IL1β, IL-6, IL-8, IL-10, IL-12, IL-15, IL-17, IL-18, and IL-23; but unlike schizophrenia has decreases of IL-1RA (in uveal melanoma) and IL-2; and increased or decreased TNF-α.
Discussion
The numerous studies of the relationship between schizophrenia and cancer have asked if cancer risk is altered from baseline. While this study set out to address that question, the findings do not equate to any of the epidemiological relations discussed above such as “increased” or “decreased” risk because the immune system in schizophrenia is neither “quiet” as would be expected if cancer risk was unchanged nor is it polarized toward reducing or increasing risk from cancer. Schizophrenia cytokine expression is more like tumor equilibrium than to either tumor suppression or escape. The initial impression is that schizophrenia has some similarity to cancer itself although without a growing tumor, ie, in a state of tumor equilibrium. This is not the first study of schizophrenia to observe that, unlike many studies claim, schizophrenia is not a pure state of inflammation but rather one with both inflammatory and anti-inflammatory characteristics.74
Besides tumor equilibrium, there are 2 other circumstances in which similar findings may appear, namely, chronic infection and the maternal immune tolerance of the semi-allogenic fetus.75,76 Although substantial literature supports maternal infection as a risk factor for schizophrenia, chronic infection of the type necessary to sustain this cytokine profile since birth has not been linked to schizophrenia. While there are many theories that suggest prenatal programming from maternal infection initiates a persistent inflammatory state that results in schizophrenia, the immunology as described above does not support a persistent and purely inflammatory state.
The data instead lead to the hypothesis that the clinical presentation of schizophrenia might involve cell transformation similar to an early stage of cancer initiation in equilibrium.
If so, there are at least 2 possible explanations of how a tumor or tumor-like condition could cause schizophrenia. The first is that some cancers cause psychosis such as ovarian (and other) tumors that cause autoimmune anti-N-methyl-D-aspartate (NMDA)-receptor encephalitis (NMDR-E). NMDR-E, first described in 1997, results from IgG antibodies against the NMDA receptor NR1 subunit. The condition often begins with a viral-like syndrome followed by treatment-resistant psychotic symptoms accompanied by movement disorders, seizures, and catatonia.77
Although only a small percentage of cases of psychosis are anti-NMDA receptor antibody positive78 and there is no altered risk of ovarian cancer in schizophrenia,1 clinicians should consider this diagnosis in treatment-resistant schizophrenia accompanied by neurological abnormalities. Most cases of NMDR-E are associated with germ-cell tumors, especially ovarian teratomas, although non-germ cell tumors can be involved; are more common in women than men, and can result in coma and death if the tumor is not removed.77 When no tumor is found, microscopic, undetectable germ cell tumors are suspected.77 The percentage of new onset psychoses without encephalitis caused by NMDR-E is unknown although such cases are reported.79
Cytokine studies in teratomas are too few to adequately compare to table 1 but there are case reports of increased IL-6 in ovarian and mediastinal teratomas,80,81 increased IL-8 in mediastinal teratoma,81 increased IL-2, 6, and 10 in experimental murine testicular teratoma,82 reduced IL12p40 in embryonic stem cell-extract-treated monocyte-derived dendritic cells83 which may contribute to teratoma formation; increased MIF in bone marrow-derived macrophages after embryonic stem cell transplantation,84 increased TNF-α in embryonic stem cell-exposed macrophages85 and increased TGF-β in a teratocarcinoma cell line.86 This limited information places teratomas more in line with the Cancer Profile than Equilibrium on table 1, mostly due to reduced IL-12.
The comparison of cytokine profiles of specific cancers to schizophrenia described above failed to find an exact match, but did reveal melanoma as a close approximation. This finding is notable as melanocytes and neurons share not only embryological origins in neural crest cells but also many signaling molecules.87 The antipsychotic medication pimozide has even been successfully used to treat melanoma,88 and the inhibitory effect on growth appears related to competitive dopamine (DA) binding89 although this may be related to low affinity binding to melanin.90 Interestingly, melanoma and Parkinson’s Disease (PD) are mutual risk factors,91 PD is a risk factor for schizophrenia,92 and PD is known, like schizophrenia, for a reduced risk of cancer except for breast cancer and melanoma.91 The relationship of DA to melanin are outside the scope of this study but supports melanoma as a good candidate tumor to compare to schizophrenia.
This leads to the question as to how close an approximation is melanoma of schizophrenia when other characteristics of cancer are compared. This can be determined by using Hanahan and Weinberg’s 6 hallmarks of cancer proposed in 200093 and expanded to 8 hallmarks and 2 “enabling characteristics” in 2011.94 The first 6 hallmarks were “sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis” (p. 646).94 The 2 emerging hallmarks were “reprogramming of energy metabolism and evading immune destruction” (p. 646),94 and the 2 “enabling characteristics” were “tumor-promoting inflammation” and “genome instability and mutation.” Having already discussed immune destruction and tumor-promoting inflammation above, the remaining discussion examines how molecular characteristics of schizophrenia are either similar to or are mirror images of the remaining cancer hallmarks and characteristics.
Sustaining Proliferative Signaling
Sustaining proliferative signaling is described as “arguably the most fundamental trait of cancer cells”94 (p. 646). Weinberg’s95 states “…cancer is really a disease of aberrant signal processing.” (p. 176). Aberrant signal processing is also fundamental to schizophrenia as the DA hypothesis of schizophrenia holds, in general, that increased DA signaling in the striatum causes positive symptoms, and decreased DA in cortical regions causes negative and cognitive symptoms.96 This was shown in a recent meta-analysis of the striatal DA hypothesis of schizophrenia that concluded “The locus of the largest dopaminergic abnormality in schizophrenia is presynaptic-affecting DA synthesis capacity, baseline synaptic DA levels and DA release.”97 (p.776).
The most important endogenous proliferative signaling in most cancers including melanoma is the Erk cascade: growth factors—receptor tyrosine kinases—growth factor receptor binder 2 (Grb2)—Son of Sevenless—GTP bound Ras proteins—Raf—mitogen activated protein kinase kinases (ME2K or MEK)—Erk.95 An activating mutation of B-raf protein, eg, is involved in about 50% of melanomas,94 and this activation leads to phosphorylation of at least 60 proteins and loss of phosphorylation in about 30 proteins, all mediated by Erk95 (p. 192). Referring to the DA hypothesis, it is tempting to suggest the dopaminergic state of the striatum in schizophrenia results from increased Erk expression but on the contrary the evidence shows hyperactive striatal DA is actually the proliferative signal.
D2 DA receptor stimulation stimulates mitogenesis so that increased D2 signaling increases Erk,98,99 and if increased Erk is a proliferative signal, then hyperactive DA in schizophrenia should be considered a proliferative signal. In glioblastoma, eg, D2 DA is a mitogenic signal acting through Erk signaling.100 Since a tumor is not present in the striatum in schizophrenia, the D2 signal is either insufficient or blocked from performing similar mitogenic signaling as the Erk pathway appears normal in the hippocampus101 and diminished in the frontal cortex of schizophrenia.102 However, the fact that the Erk pathway is closely involved in the regulation of VEGF and is involved in the control of apoptosis through the regulatory gene BCL-2103 which is also involved in schizophrenia104 is further evidence of the involvement of proliferative signaling in schizophrenia.
There are other paths of proliferative signaling in cancer that could be related to schizophrenia such as the P13 kinase pathway, but one other path will be briefly mentioned because it involves glutamate which forms the basis of an additional schizophrenia theory. The glutamate hypothesis of schizophrenia holds that hypofunction of the glutamatergic NMDA receptor causes striatal DA hyperactivity and cortical DA hypoactivity.105 The metabotropic glutamate receptor 1 encoded by the GRM1 gene was recently found to have oncogenic properties in melanoma,106 and mutations of this gene also appear involved in schizophrenia.107 Similar findings in melanoma have been made for increased expression of mGluR5 and activating mutations in GRM3.106 Both mGluR5 and GRM3 are implicated in schizophrenia,108,109 but overall it appears that melanoma is associated with activated glutamate receptors and schizophrenia with deactivated glutamate receptors. Understanding how and why schizophrenia and melanoma have mirror image defects in these receptor functions will require additional research but is an example of the overlap of molecular pathways in the 2 conditions.
Evading Growth Suppressors
There are 2 major growth suppressors called tumor suppressor genes in cancer biology, one encodes the RB (retinoblastoma-associated) protein (pRb) and the other the TP53 protein.94 Mutations of both have been found in melanoma.110,111 Both pathways are complex, and reviews can be found in Weinberg’s monograph.95 Both have been studied in schizophrenia but only one, pRb, will be discussed in this section. TP53 is discussed in the following section.
In the case of pRb, phosphorylation controls the protein’s ability to block the cell cycle, and hyper-phosphorylation inactivates the protein’s gatekeeping control of the cell cycle and allows cells to proliferate. Hypo-phosphorylation, on the other hand, halts proliferation and facilitates differentiation. Mitogenic signals that hyper-phosphorylate can lead to deregulated proliferation and blocked differentiation which can accompany tumor progression. The cell cycle in schizophrenia does indeed appear altered and is accelerated compared to normal.112 Another study of schizophrenia reported increased phosphorylated pRb in the white matter underling the anterior cingulate gyrus. This was described as diverting the oligodendrocytes that form the white matter from their differentiated state thus possibly causing the demyelination abnormalities in schizophrenia.113 In comparison to melanoma, dedifferentiation is an ominous sign,114 and finding a similar process in oligodendrocytes in schizophrenia is suggestive of a cancer-like state.
Resisting Cell Death
Resistance to cell death in cancer occurs mostly by blocking apoptosis which is accomplished through more than one mechanism, the most important ones being the loss of P53 function and/or inactivation of autophagy.94 Both mechanisms are active in melanoma.115 Apoptosis has been of interest in schizophrenia for some time116 although there has been debate whether it is increased or decreased.117 Some of the variation in findings appears related to the specific brain region being examined and/or the effect of antipsychotic medications which are known to influence apoptosis.117
The p53 gene has indeed been identified as a candidate susceptibility gene for schizophrenia,118 and some of the gene’s role in schizophrenia may occur through pathways involving dysbindin3 and white matter integrity.119 The most important findings for the current study is of altered apoptotic markers in cultured fibroblasts from schizophrenia which indicate that schizophrenia has increased susceptibility to apoptosis, ie, the opposite of cancer.104,120,121 One study found blocked autophagy in the hippocampus of schizophrenia although this was interpreted as indicating increased apoptosis in an alternate pathway.122
How changes of apoptotic or anti-apoptotic signals are interpreted can seem paradoxical. For example, in PD, increased anti-apoptotic signals are interpreted as a compensation for increased neuronal apoptosis.123 In other words, the anti-apoptotic signals result from but do not cause the disease. In contrast, the increased apoptotic signals in schizophrenia have been implicated as a primary cause of schizophrenia by causing a neurodegenerative process similar to aging,121 and this is certainly possible because hyperactive p53 that markedly enhances apoptosis is associated with premature aging and resistance to cancer95 (p.343). But the author suggests that an alternate explanation of increased apoptotic signals in schizophrenia is that the signals are the result not the cause of abnormal neurons, ie, apoptotic mechanisms are attempting to remove abnormal neurons that are altered by neoplastic influences. This process, best described as an “attenuated tumorigenesis” would be a secondary not primary cause of neurodegeneration.
Enabling Replicative Immortality
The widely accepted mechanism that cancer cells use to achieve replicative immortality is blocking telomere shortening.94 Telomeres protect the ends of chromosomes and are progressively shortened in cell propagation to where loss of chromosome protection causes end-to-end fusions that ultimately lead to cell death.94 Proliferation-associated abnormalities such as oncogenic signaling can trigger telomere shortening and result in attenuated tumorigenesis before any tumor growth occurs.94 Most tumors, including melanoma,124 block telomere shortening often through increased telomerase activity, the polymerase that lengthens telomeres by adding telomeric DNA. Shortened telomere lengths have been found in schizophrenia,125 and shortened telomeres are positively associated with severity of symptoms.126 This is the opposite of what is found in cancer, so in the author’s tumor model, decreased telomere length in schizophrenia occurs in response to proliferative signaling before tumor growth occurs which is another form of attenuated tumorigenesis.
Inducing Angiogenesis
This hallmark was briefly discussed above in regard to VEGF which is the primary signaling factor for both normal and pathological blood vessel growth.94 In addition to what was discussed above, abnormal tumor vasculature can develop early in tumor development and is noted for “precocious capillary sprouting, convoluted and excessive vessel branching, distorted and enlarged vessels, erratic blood flow, microhemorrhaging, leakiness, and abnormal levels of endothelial cell proliferation and apoptosis.”94 p. 653. Many of these same abnormalities are reported in several reports of vascular abnormalities in schizophrenia.127–129
Activating Invasion and Metastasis
Schizophrenia is not associated with an invading, metastatic tumor. To the limited extent to which schizophrenia literature can examined for a connection to tumor invasion, the following can be stated. Tumor invasion depends on a complex alteration of the surrounding extracellular matrix.94 Altered adhesion molecules such as N-cadherin are usually upregulated in tumors including melanomas so that cell attachment during invasion can occur.94,130 In schizophrenia, the susceptibility gene disrupted-in-schizophrenia 1 (DISC1) regulates cell-matrix adhesion, and DISC1 overexpression enhances N-cadherin and cell-cell adhesion.131 To date schizophrenia has not been shown to involve an invading tumor in, eg, the striatum but the activity of DISC1 in schizophrenia would appear to allow it.
Genome Instability and Mutation
Genome instability and mutation is an enabling characteristic of cancer,94 and some of the abnormalities discussed above occur as a result of mutations. The number of mutations associated with cancer is quite large and for the purposes of this discussion it is sufficient to quote the authors who reported a decreased risk of melanoma in schizophrenia in Sweden5 who “found that more than 140 schizophrenia-associated genes were mutated in cancer….” p. 532.
Reprogramming of Energy Metabolism
The final emerging hallmark of cancer is the reprogramming of energy metabolism94,132 which might be the most interesting and controversial aspect of cancer biology because the subject involves “aerobic glycolysis” or the so-called Warburg effect95 p.53. Weinberg describes this characteristic of cancer cells as “bizarre”95 p55 since cancer cells inefficiently metabolize glucose to lactate by using aerobic glycolysis when they have sufficient oxygen to use the more efficient mechanism of oxidative phosphorylation. Aerobic glycolysis explains the increased secretion of lactate from tumors.95 There are several reports of impaired glycolysis in schizophrenia including increased levels of lactate in first onset antipsychotic-naïve subjects.133–136 Increased cerebrospinal fluid lactate in schizophrenia has also been attributed to anaerobic glucose metabolism in schizophrenia and bipolar subjects.137
Conclusion
Based on the discussion above, schizophrenia can be understood within the framework of 2 different tumor models although one, the model using NMDR-E, may only explain a small percentage of cases. The more likely tumor model is that schizophrenia is a condition of attenuated tumorigenesis. The author thinks of this as a balance between the emergence of a tumor and suppression of tumorigenic influences. Novel features of this model are: (1) schizophrenia is a suppressed tumor state with similarities to melanoma; (2) DA is the proliferative but blocked signal; (3) schizophrenia would present as a tumor except that certain hallmarks of cancer are oppositely expressed in schizophrenia; and (4) some characteristics of schizophrenia like apoptosis are the results of attenuated tumorigenesis. Finally, if this theory holds up to scrutiny, the root cause of abnormal dopaminergic signaling in schizophrenia should be sought from a tumorigenic standpoint. Consideration should also be given to clinical treatment of schizophrenia based on the principles of oncology to include treatments aimed at normalizing the abnormal immune responses described above.
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Leucht S, Burkard T, Henderson JH, Maj M, Sartorius N. Physical Illness and Schizophrenia: A Review of the Evidence. Cambridge, UK: Cambridge University Press; 2007:208. [DOI] [PubMed] [Google Scholar]
- 2. Ibáñez K, Boullosa C, Tabarés-Seisdedos R, Baudot A, Valencia A. Molecular evidence for the inverse comorbidity between central nervous system disorders and cancers detected by transcriptomic meta-analyses. PLoS Genet. 2014;10:e1004173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ma X, Fei E, Fu C, Ren H, Wang G. Dysbindin-1, a schizophrenia-related protein, facilitates neurite outgrowth by promoting the transcriptional activity of p53. Mol Psychiatry. 2011;16:1105–1116. [Google Scholar]
- 4. Catts VS, Catts SV, O’Toole BI, Frost AD. Cancer incidence in patients with schizophrenia and their first-degree relatives - a meta-analysis. Acta Psychiatr Scand. 2008;117:323–336. [DOI] [PubMed] [Google Scholar]
- 5. Ji J, Sundquist K, Ning Y, Kendler KS, Sundquist J, Chen X. Incidence of cancer in patients with schizophrenia and their first-degree relatives: a population-based study in Sweden. Schizophr Bull. 2013;39:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Osborn DP, Limburg H, Walters K, et al. Relative incidence of common cancers in people with severe mental illness. Cohort study in the United Kingdom THIN primary care database. Schizophr Res. 2013;143:44–49. [DOI] [PubMed] [Google Scholar]
- 7. McGinty EE, Zhang Y, Guallar E, et al. Cancer incidence in a sample of Maryland residents with serious mental illness. Psychiatr Serv. 2012;63:714–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teng MWL, Kershaw MH, Smyth MJ. Chapter 7. In: Prendergast GC, Jaffee EM, eds. Cancer Immunotherapy: Immune Suppression and Tumor Growth. Amsterdam, the Netherlands: Elsevier; 2013:85–99. [Google Scholar]
- 9. Bhatia A, Kumar Y.Chapter 12. In: Rezaei N, ed. Cancer Immunology: A Translational Medicine Context. Berlin, Germany: Springer-Verlag; 2015:195–208. [Google Scholar]
- 10. Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. [DOI] [PubMed] [Google Scholar]
- 11. Yefenof E.Foreword. In: Yefenof E, ed. Innate and Adaptive Immunity in the Tumor Microenvironment. New York, NY: Springer; 2008:v–viii. [Google Scholar]
- 12. Kim CH. Chapter 6. In: Rezaei N, ed. Cancer Immunology: A Translational Medicine Context. Berlin, Germany: Springer-Verlag; 2015:77–91. [Google Scholar]
- 13. Rezaei N, Aalaei-Andabili SH, Kaufman HL. Chapter 1. In: Rezaei N, ed. Cancer Immunology: A Translational Medicine Context. Berlin, Germany: Springer-Verlag; 2015:1–8. [Google Scholar]
- 14. Demaria S.Chapter 11. In: Prendergast GC, Jaffee EM, eds. Cancer Immunotherapy: Immune Suppression and Tumor Growth. Amsterdam, the Netherlands: Elsevier; 2013:149–164. [Google Scholar]
- 15. Aptsiauri N, Garcia-Lora AM, Cabrera T. MHC class I Antigens in Malignant Cells: Immune Escape and Response to Immunotherapy. New York, NY: Springer; 2013:51. [Google Scholar]
- 16. Sonabend AM, Showers CR, Anderson RCE. Chapter 18. In: Rezaei N, ed. Cancer Immunology: Cancer Immunotherapy for Organ-Specific Tumors. Heidelberg, Germany: Springer; 2015:333–362. [Google Scholar]
- 17. Dembic Z. The Cytokines of the Immune System: The Role of Cytokines in Disease Related to Immune Response. Amsterdam, the Netherlands: Elsevier; 2015. [Google Scholar]
- 18. Abbas AK, Lichtman AH, Pillai S. Cellular and Molecular Immunology. 8th ed. Philadelphia, PA: Elsevier; 2015. [Google Scholar]
- 19. Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14:e218–e228. [DOI] [PubMed] [Google Scholar]
- 20. Chan MK, Guest PC, Levin Y, et al. In: Guest PC, Bahn S, eds. Biomarkers of Neurological and Psychiatric Disease. Amsterdam, the Netherlands: Elsevier; 2011:95–144. [Google Scholar]
- 21. DeLisi LE. Chapter 25. In: Kurstak E, Lipowski ZJ, Morozov PV, eds. Viruses, Immunity, and Mental Disorders. New York, NY: Plenum; 1987:271–283. [Google Scholar]
- 22. Miller BJ, Gassama B, Sebastian D, Buckley P, Mellor A. Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2013;73:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gopal M.Chapter 7. In: Rezaei N, ed. Cancer Immunology: A Translational Medicine Context. Heidelberg, Germany: Springer; 2015:93–119. [Google Scholar]
- 24. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin CC, Chang CM, Chang PY, Huang TL. Increased interleukin-6 level in Taiwanese schizophrenic patients. Chang Gung Med J. 2011;34:375–381. [PubMed] [Google Scholar]
- 26. Li Y, Yolken R, Cowan DN, et al. Biomarker identification and effect estimation on schizophrenia - a high dimensional data analysis. Front Public Health. 2015;3:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumar R, Kamdar D, Madden L, et al. Th1/Th2 cytokine imbalance in meningioma, anaplastic astrocytoma and glioblastoma multiforme patients. Oncol Rep. 2006;15:1513–1516. [DOI] [PubMed] [Google Scholar]
- 28. Walker SR, Redlinger RE, Jr, Barksdale EM., Jr Neuroblastoma-induced inhibition of dendritic cell IL-12 production via abrogation of CD40 expression. J Pediatr Surg. 2005;40:244–249; discussion 249. [DOI] [PubMed] [Google Scholar]
- 29. Bushe CJ, Bradley AJ, Wildgust HJ, Hodgson RE. Schizophrenia and breast cancer incidence: a systematic review of clinical studies. Schizophr Res. 2009;114:6–16. [DOI] [PubMed] [Google Scholar]
- 30. Jafarzadeh A, Minaee K, Farsinejad AR, et al. Evaluation of the circulating levels of IL-12 and IL-33 in patients with breast cancer: influences of the tumor stages and cytokine gene polymorphisms. Iran J Basic Med Sci. 2015;18:1189–1198. [PMC free article] [PubMed] [Google Scholar]
- 31. Derin D, Soydinc HO, Guney N, et al. Serum IL-8 and IL-12 levels in breast cancer. Med Oncol. 2007;24:163–168. [DOI] [PubMed] [Google Scholar]
- 32. Hussein MZ, Al Fikky A, Abdel Bar I, Attia O. Serum IL-6 and IL-12 levels in breast cancer patients. Egypt J Immunol. 2004;11:165–170. [PubMed] [Google Scholar]
- 33. Mortensen PB. The occurrence of cancer in first admitted schizophrenic patients. Schizophr Res. 1994;12:185–194. [DOI] [PubMed] [Google Scholar]
- 34. Catalá-López F, Suárez-Pinilla M, Suárez-Pinilla P, et al. Inverse and direct cancer comorbidity in people with central nervous system disorders: a meta-analysis of cancer incidence in 577,013 participants of 50 observational studies. Psychother Psychosom. 2014;83:89–105. [DOI] [PubMed] [Google Scholar]
- 35. Goldacre MJ, Kurina LM, Wotton CJ, Yeates D, Seagroat V. Schizophrenia and cancer: an epidemiological study. Br J Psychiatry. 2005;187:334–338. [DOI] [PubMed] [Google Scholar]
- 36. Moretti S, Chiarugi A, Semplici F, et al. Serum imbalance of cytokines in melanoma patients. Melanoma Res. 2001;11:395–399. [DOI] [PubMed] [Google Scholar]
- 37. Lauerova L, Dusek L, Simickova M, et al. Malignant melanoma associates with Th1/Th2 imbalance that coincides with disease progression and immunotherapy response. Neoplasma. 2002;49:159–166. [PubMed] [Google Scholar]
- 38. Fang S, Wang Y, Chun YS, et al. The relationship between blood IL-12p40 level and melanoma progression. Int J Cancer. 2015;136:1874–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nevala WK, Vachon CM, Leontovich AA, Scott CG, Thompson MA, Markovic SN; Melanoma Study Group of the Mayo Clinic Cancer Center Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin Cancer Res. 2009;15:1931–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee BH, Hong JP, Hwang JA, et al. Alterations in plasma vascular endothelial growth factor levels in patients with schizophrenia before and after treatment. Psychiatry Res. 2015;228:95–99. [DOI] [PubMed] [Google Scholar]
- 41. Sun T, Yang Y, Luo X, et al. Inhibition of tumor angiogenesis by interferon-γ by suppression of tumor-associated macrophage differentiation. Oncol Res. 2014;21:227–235. [DOI] [PubMed] [Google Scholar]
- 42. Ding M, Song X, Zhao J, et al. Activation of Th17 cells in drug naïve, first episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:78–82. [DOI] [PubMed] [Google Scholar]
- 43. May KFJ, Jinushi M, Dranoff G. Chapter 8. In: Prendergast GC, Jaffee EM, eds. Cancer Immunotherapy: Immune Suppression and Tumor Growth. Amsterdam, the Netherlands: Elsevier; 2013:101–113. [Google Scholar]
- 44. Serafini P, Bronte V.In: Gabrilovich DI, Hurwitz AA, eds. Tumor-Induced Immune Suppression. New York, NY: Springer; 2008:157–195. [Google Scholar]
- 45. Song X, Fan X, Song X, et al. Elevated levels of adiponectin and other cytokines in drug naïve, first episode schizophrenia patients with normal weight. Schizophr Res. 2013;150:269–273. [DOI] [PubMed] [Google Scholar]
- 46. Song X, Fan X, Zhang J, et al. Prolactin serum levels correlate with inflammatory status in drug-naïve first-episode schizophrenia. World J Biol Psychiatry. 2014;15:546–552. [DOI] [PubMed] [Google Scholar]
- 47. Pesce M, Ferrone A, Rizzuto A, et al. The SHP-1 expression is associated with cytokines and psychopathological status in unmedicated first episode schizophrenia patients. Brain Behav Immun. 2014;41:251–260. [DOI] [PubMed] [Google Scholar]
- 48. Kholmanskikh O, van Baren N, Brasseur F, et al. Interleukins 1alpha and 1beta secreted by some melanoma cell lines strongly reduce expression of MITF-M and melanocyte differentiation antigens. Int J Cancer. 2010;127:1625–1636. [DOI] [PubMed] [Google Scholar]
- 49. Elkabets M, Ribeiro VS, Dinarello CA, et al. IL-1β regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur J Immunol. 2010;40:3347–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kwong C, Gilman-Sachs A, Beaman K. Tumor-associated a2 vacuolar ATPase acts as a key mediator of cancer-related inflammation by inducing pro-tumorigenic properties in monocytes. J Immunol. 2011;186:1781–1789. [DOI] [PubMed] [Google Scholar]
- 51. Okamoto M, Liu W, Luo Y, et al. Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of interleukin-1beta. J Biol Chem. 2010;285:6477–6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. de Witte L, Tomasik J, Schwarz E, et al. Cytokine alterations in first-episode schizophrenia patients before and after antipsychotic treatment. Schizophr Res. 2014;154:23–29. [DOI] [PubMed] [Google Scholar]
- 53. Di Mitri D, Toso A, Chen JJ, et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature. 2014;515:134–137. [DOI] [PubMed] [Google Scholar]
- 54. Hirbod-Mobarakeh A, Amirzargar AA, Nikbin B, Nicknam MH, Kutikhin A, Rezaei N. Chapter 17. In: Rezaei N, ed. Cancer Immunology: A Translational Medicine Context. Berlin, Germany: Springer-Verlag; 2015:295–341. [Google Scholar]
- 55. Nagarkatti-Gude N, Bronkhorst IH, van Duinen SG, Luyten GP, Jager MJ. Cytokines and chemokines in the vitreous fluid of eyes with uveal melanoma. Invest Ophthalmol Vis Sci. 2012;53:6748–6755. [DOI] [PubMed] [Google Scholar]
- 56. Dutta A, Banerjee A, Saikia N, Phookan J, Baruah MN, Baruah S. Negative regulation of natural killer cell in tumor tissue and peripheral blood of oral squamous cell carcinoma. Cytokine. 2015;76:123–130. [DOI] [PubMed] [Google Scholar]
- 57. Hayes LN, Severance EG, Leek JT, et al. Inflammatory molecular signature associated with infectious agents in psychosis. Schizophr Bull. 2014;40:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reale M, Patruno A, De Lutiis MA, et al. Dysregulation of chemo-cytokine production in schizophrenic patients versus healthy controls. BMC Neurosci. 2011;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ene CD, Anghel AE, Neagu M, Nicolae I. 25-OH Vitamin D and Interleukin-8: Emerging Biomarkers in Cutaneous Melanoma Development and Progression. Mediators Inflamm. 2015;2015:904876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xiu MH, Yang GG, Tan YL, et al. Decreased interleukin-10 serum levels in first-episode drug-naïve schizophrenia: relationship to psychopathology. Schizophr Res. 2014;156:9–14. [DOI] [PubMed] [Google Scholar]
- 61. Jinushi M, Baghdadi M.Chapter 3. In: Rezaei N, ed. Cancer Immunology: A Translational Medicine Context. Berlin, Germany: Springer-Verlag; 2015:29–46. [Google Scholar]
- 62. Crespo-Facorro B, Carrasco-Marín E, Pérez-Iglesias R, et al. Interleukin-12 plasma levels in drug-naïve patients with a first episode of psychosis: effects of antipsychotic drugs. Psychiatry Res. 2008;158:206–216. [DOI] [PubMed] [Google Scholar]
- 63. Liu D, Song L, Wei J, et al. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. J Clin Invest. 2012;122:2221–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Epardaud M, Elpek KG, Rubinstein MP, et al. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 2008;68:2972–2983. [DOI] [PubMed] [Google Scholar]
- 65. Wagner SN, Schultewolter T, Wagner C, et al. Immune response against human primary malignant melanoma: a distinct cytokine mRNA profile associated with spontaneous regression. Lab Invest. 1998;78:541–550. [PubMed] [Google Scholar]
- 66. Ganzetti G, Rubini C, Campanati A, et al. IL-17, IL-23, and p73 expression in cutaneous melanoma: a pilot study. Melanoma Res. 2015;25:232–238. [DOI] [PubMed] [Google Scholar]
- 67. Ostrand-Rosenberg S, Sinha P, Beury DW, Chornoguz O, Parker KH.Chapter 28. In: Prendergast GC, Jaffee EM, eds. Cancer Immunotherapy: Immune Suppression and Tumor Growth. Amsterdam, the Netherlands: Elsevier; 2013:473–496. [Google Scholar]
- 68. Park H, Byun D, Kim TS, et al. Enhanced IL-18 expression in common skin tumors. Immunol Lett. 2001;79:215–219. [DOI] [PubMed] [Google Scholar]
- 69. Borovcanin M, Jovanovic I, Dejanovic SD, Radosavljevic G, Arsenijevic N, Lukic ML. Increase systemic levels of IL-23 as a possible constitutive marker in schizophrenia. Psychoneuroendocrinology. 2015;56:143–147. [DOI] [PubMed] [Google Scholar]
- 70. Bertrand F, Rochotte J, Colacios C, et al. Blocking Tumor Necrosis Factor α Enhances CD8 T-cell-Dependent Immunity in Experimental Melanoma. Cancer Res. 2015;75:2619–2628. [DOI] [PubMed] [Google Scholar]
- 71. Tang MR, Wang YX, Guo S, Han SY, Li HH, Jin SF. Prognostic significance of in situ and plasma levels of transforming growth factor β1, -2 and -3 in cutaneous melanoma. Mol Med Rep. 2015;11:4508–4512. [DOI] [PubMed] [Google Scholar]
- 72. Mittelbronn M, Platten M, Zeiner P, et al. Macrophage migration inhibitory factor (MIF) expression in human malignant gliomas contributes to immune escape and tumour progression. Acta Neuropathol. 2011;122:353–365. [DOI] [PubMed] [Google Scholar]
- 73. Yaddanapudi K, Rendon BE, Lamont G, et al. MIF Is necessary for late-stage melanoma patient MDSC immune suppression and differentiation. Cancer Immunol Res. 2016;4:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Drexhage RC, Hoogenboezem TA, Cohen D, et al. An activated set point of T-cell and monocyte inflammatory networks in recent-onset schizophrenia patients involves both pro- and anti-inflammatory forces. Int J Neuropsychopharmacol. 2011;14:746–755. [DOI] [PubMed] [Google Scholar]
- 75. Bubanovic I. Origin of Anti-Tumor Immunity Failure in Mammals. New York, NY: Kluwer Academic/Plenum Publishers; 2004. [Google Scholar]
- 76. Gajewski TF.Chapter 4. In: Yefenof E, ed. Innate and Adaptive Immunity in the Tumor Microenvironment. New York, NY: Springer; 2008:77–89. [Google Scholar]
- 77. Braverman JA, Marcus C, Garg R. Anti-NMDA-receptor encephalitis: a neuropsychiatric syndrome associated with ovarian teratoma. Gynecol Oncol Rep. 2015;14:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pollak TA, McCormack R, Peakman M, Nicholson TR, David AS. Prevalence of anti-N-methyl-D-aspartate (NMDA) receptor [corrected] antibodies in patients with schizophrenia and related psychoses: a systematic review and meta-analysis. Psychol Med. 2014;44:2475–2487. [DOI] [PubMed] [Google Scholar]
- 79. Arboleya S, Clemente A, Deng S, et al. Anti-NMDAR antibodies in new-onset psychosis. Positive results in an HIV-infected patient [published online ahead of print May 4, 2016]. Brain Behav Immun. doi:10.1016/j.bbi.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 80. Sánchez Andrés A, Valdés Diéguez E, Marco Macián A, Carrasco Moreno JI. [Immature ovarian tumour and dilated myocardiopathy]. An Pediatr (Barc). 2010;73:347–351. [DOI] [PubMed] [Google Scholar]
- 81. Maeyama R, Uchiyama A, Tominaga R, Ichimiya H, Kuroiwa K, Tanaka M. Benign mediastinal teratoma complicated by cardiac tamponade: report of a case. Surg Today. 1999;29:1206–1208. [DOI] [PubMed] [Google Scholar]
- 82. Sundström J, Veräjnkorva E, Salminen E, Pelliniemi LJ, Pöllänen P. Experimental testicular teratoma promotes formation of humoral immune responses in the host testis. J Reprod Immunol. 1999;42:107–126. [DOI] [PubMed] [Google Scholar]
- 83. Mohib K, Allan D, Wang L. Human embryonic stem cell-extracts inhibit the differentiation and function of monocyte-derived dendritic cells. Stem Cell Rev. 2010;6:611–621. [DOI] [PubMed] [Google Scholar]
- 84. Wang X, Chen T, Leng L, et al. MIF produced by bone marrow-derived macrophages contributes to teratoma progression after embryonic stem cell transplantation. Cancer Res. 2012;72:2867–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chen T, Wang X, Guo L, et al. Embryonic stem cells promoting macrophage survival and function are crucial for teratoma development. Front Immunol. 2014;5:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Weima SM, van Rooijen MA, Mummery CL, et al. Differentially regulated production of platelet-derived growth factor and of transforming growth factor beta by a human teratocarcinoma cell line. Differentiation. 1988;38:203–210. [DOI] [PubMed] [Google Scholar]
- 87. Yaar M, Park HY. Melanocytes: a window into the nervous system. J Invest Dermatol. 2012;132:835–845. [DOI] [PubMed] [Google Scholar]
- 88. Lorenzo CR, Koo J. Pimozide in dermatologic practice: a comprehensive review. Am J Clin Dermatol. 2004;5:339–349. [DOI] [PubMed] [Google Scholar]
- 89. Krummel TM, Neifeld JP, Taub RN. Effects of dopamine agonists and antagonists on murine melanoma: correlation with dopamine binding activity. Cancer. 1982;49:1178–1184. [DOI] [PubMed] [Google Scholar]
- 90. Böni R, Steinert H, Böni RA, et al. Lack of expression of dopamine D2 receptors in malignant melanoma: evidence for interaction of iodobenzofurans with melanin. Dermatology. 1996;193:198–202. [DOI] [PubMed] [Google Scholar]
- 91. Disse M, Reich H, Lee PK, Schram SS. A review of the association between parkinson disease and malignant melanoma. Dermatol Surg. 2016;42:141–146. [DOI] [PubMed] [Google Scholar]
- 92. Lin HL, Lin HC, Chen YH. Psychiatric diseases predated the occurrence of Parkinson disease: a retrospective cohort study. Ann Epidemiol. 2014;24:206–213. [DOI] [PubMed] [Google Scholar]
- 93. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. [DOI] [PubMed] [Google Scholar]
- 94. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 95. Weinberg RA. The Biology of Cancer. 2nd ed. New York, NY: Garland Science; 2014:876. [Google Scholar]
- 96. Kambeitz J, Abi-Dargham A, Kapur S, Howes OD. Alterations in cortical and extrastriatal subcortical dopamine function in schizophrenia: systematic review and meta-analysis of imaging studies. Br J Psychiatry. 2014;204:420–429. [DOI] [PubMed] [Google Scholar]
- 97. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Luo Y, Kokkonen GC, Wang X, Neve KA, Roth GS. D2 dopamine receptors stimulate mitogenesis through pertussis toxin-sensitive G proteins and Ras-involved ERK and SAP/JNK pathways in rat C6-D2L glioma cells. J Neurochem. 1998;71:980–990. [DOI] [PubMed] [Google Scholar]
- 99. Oak JN, Lavine N, Van Tol HH. Dopamine D(4) and D(2L) Receptor Stimulation of the Mitogen-Activated Protein Kinase Pathway Is Dependent on trans-Activation of the Platelet-Derived Growth Factor Receptor. Mol Pharmacol. 2001;60:92–103. [DOI] [PubMed] [Google Scholar]
- 100. Li J, Zhu S, Kozono D, et al. Genome-wide shRNA screen revealed integrated mitogenic signaling between dopamine receptor D2 (DRD2) and epidermal growth factor receptor (EGFR) in glioblastoma. Oncotarget. 2014;5:882–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Szamosi A, Kelemen O, Kéri S. Hippocampal volume and the AKT signaling system in first-episode schizophrenia. J Psychiatr Res. 2012;46:279–284. [DOI] [PubMed] [Google Scholar]
- 102. Yuan P, Zhou R, Wang Y, et al. Altered levels of extracellular signal-regulated kinase signaling proteins in postmortem frontal cortex of individuals with mood disorders and schizophrenia. J Affect Disord. 2010;124:164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Trisciuoglio D, Iervolino A, Zupi G, Del Bufalo D. Involvement of PI3K and MAPK signaling in bcl-2-induced vascular endothelial growth factor expression in melanoma cells. Mol Biol Cell. 2005;16:4153–4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gassó P, Mas S, Molina O, Lafuente A, Bernardo M, Parellada E. Increased susceptibility to apoptosis in cultured fibroblasts from antipsychotic-naïve first-episode schizophrenia patients. J Psychiatr Res. 2014;48:94–101. [DOI] [PubMed] [Google Scholar]
- 105. Ginovart N, Kapur S.Chapter 16. In: Neve KA, ed. The Dopamine Receptors. New York, NY: Humana Press; 2010:431–477. [Google Scholar]
- 106. Teh JL, Chen S. Glutamatergic signaling in cellular transformation. Pigment Cell Melanoma Res. 2012;25:331–342. [DOI] [PubMed] [Google Scholar]
- 107. Ayoub MA, Angelicheva D, Vile D, et al. Deleterious GRM1 mutations in schizophrenia. PLoS One. 2012;7:e32849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Matosin N, Fernandez-Enright F, Lum JS, Newell KA. Shifting towards a model of mGluR5 dysregulation in schizophrenia: consequences for future schizophrenia treatment [published online ahead of print May 4, 2016]. Neuropharmacology. doi:10.1016/j.neuropharm.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 109. Mounce J, Luo L, Caprihan A, Liu J, Perrone-Bizzozero NI, Calhoun VD. Association of GRM3 polymorphism with white matter integrity in schizophrenia. Schizophr Res. 2014;155:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Halaban R. Rb/E2F: a two-edged sword in the melanocytic system. Cancer Metastasis Rev. 2005;24:339–356. [DOI] [PubMed] [Google Scholar]
- 111. Shain AH, Yeh I, Kovalyshyn I, et al. The genetic evolution of melanoma from precursor lesions. N Engl J Med. 2015;373:1926–1936. [DOI] [PubMed] [Google Scholar]
- 112. Fan Y, Abrahamsen G, McGrath JJ, Mackay-Sim A. Altered cell cycle dynamics in schizophrenia. Biol Psychiatry. 2012;71:129–135. [DOI] [PubMed] [Google Scholar]
- 113. Katsel P, Davis KL, Li C, et al. Abnormal indices of cell cycle activity in schizophrenia and their potential association with oligodendrocytes. Neuropsychopharmacology. 2008;33:2993–3009. [DOI] [PubMed] [Google Scholar]
- 114. Knappe N, Novak D, Weina K, et al. Directed dedifferentiation using partial reprogramming induces invasive phenotype in melanoma cells. Stem Cells. 2016;34:832–846. [DOI] [PubMed] [Google Scholar]
- 115. Bhattacharya A, Schmitz U, Raatz Y, et al. miR-638 promotes melanoma metastasis and protects melanoma cells from apoptosis and autophagy. Oncotarget. 2015;6:2966–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Catts VS, Catts SV. Apoptosis and schizophrenia: is the tumour suppressor gene, p53, a candidate susceptibility gene? Schizophr Res. 2000;41:405–415. [DOI] [PubMed] [Google Scholar]
- 117. Jarskog LF. Apoptosis in schizophrenia: pathophysiologic and therapeutic considerations. Curr Opin Psychiatry. 2006;19:307–312. [DOI] [PubMed] [Google Scholar]
- 118. Ni X, Trakalo J, Valente J, et al. Human p53 tumor suppressor gene (TP53) and schizophrenia: case-control and family studies. Neurosci Lett. 2005;388:173–178. [DOI] [PubMed] [Google Scholar]
- 119. Molina V, Papiol S, Sanz J, et al. Convergent evidence of the contribution of TP53 genetic variation (Pro72Arg) to metabolic activity and white matter volume in the frontal lobe in schizophrenia patients. Neuroimage. 2011;56:45–51. [DOI] [PubMed] [Google Scholar]
- 120. Catts VS, Catts SV, McGrath JJ, et al. Apoptosis and schizophrenia: a pilot study based on dermal fibroblast cell lines. Schizophr Res. 2006;84:20–28. [DOI] [PubMed] [Google Scholar]
- 121. Batalla A, Bargalló N, Gassó P, et al. Apoptotic markers in cultured fibroblasts correlate with brain metabolites and regional brain volume in antipsychotic-naive first-episode schizophrenia and healthy controls. Transl Psychiatry. 2015;5:e626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Merenlender-Wagner A, Malishkevich A, Shemer Z, et al. Autophagy has a key role in the pathophysiology of schizophrenia. Mol Psychiatry. 2015;20:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Yalçınkaya N, Haytural H, Bilgiç B, et al. Expression changes of genes associated with apoptosis and survival processes in Parkinson’s disease. Neurosci Lett. 2016;615:72–77. [DOI] [PubMed] [Google Scholar]
- 124. Parris CN, Jezzard S, Silver A, MacKie R, McGregor JM, Newbold RF. Telomerase activity in melanoma and non-melanoma skin cancer. Br J Cancer. 1999;79:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Rao S, Ye N, Hu H, Shen Y, Xu Q. Variants in TERT influencing telomere length are associated with paranoid schizophrenia risk. Am J Med Genet B Neuropsychiatr Genet. 2016;171:317–324. [DOI] [PubMed] [Google Scholar]
- 126. Pawelczyk T, Szymanska B, Grancow-Grabka M, Kotlicka-Antczak M, Pawelczyk A. Telomere length in blood cells is related to the chronicity, severity, and recurrence rate of schizophrenia. Neuropsychiatr Dis Treat. 2015;11:1493–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Meier MH, Shalev I, Moffitt TE, et al. Microvascular abnormality in schizophrenia as shown by retinal imaging. Am J Psychiatry. 2013;170:1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Uranova NA, Zimina IS, Vikhreva OV, Krukov NO, Rachmanova VI, Orlovskaya DD. Ultrastructural damage of capillaries in the neocortex in schizophrenia. World J Biol Psychiatry. 2010;11:567–578. [DOI] [PubMed] [Google Scholar]
- 129. Sinka L, Kovari E, Santos M, et al. Microvascular changes in late-life schizophrenia and mood disorders: stereological assessment of capillary diameters in anterior cingulate cortex. Neuropathol Appl Neurobiol. 2012;38:696–709. [DOI] [PubMed] [Google Scholar]
- 130. Siret C, Terciolo C, Dobric A, et al. Interplay between cadherins and α2β1 integrin differentially regulates melanoma cell invasion. Br J Cancer. 2015;113:1445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hattori T, Shimizu S, Koyama Y, et al. DISC1 regulates cell-cell adhesion, cell-matrix adhesion and neurite outgrowth. Mol Psychiatry. 2010;15:778, 798–778, 809. [DOI] [PubMed] [Google Scholar]
- 132. Jayachandran A, Lo PH, Chueh AC, et al. Transketolase-like 1 ectopic expression is associated with DNA hypomethylation and induces the Warburg effect in melanoma cells. BMC Cancer. 2016;16:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Herberth M, Koethe D, Cheng TM, et al. Impaired glycolytic response in peripheral blood mononuclear cells of first-onset antipsychotic-naive schizophrenia patients. Mol Psychiatry. 2011;16:848–859. [DOI] [PubMed] [Google Scholar]
- 134. Du F, Cooper AJ, Thida T, et al. In vivo evidence for cerebral bioenergetic abnormalities in schizophrenia measured using 31P magnetization transfer spectroscopy. JAMA Psychiatry. 2014;71:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Stone WS, Faraone SV, Su J, Tarbox SI, Van Eerdewegh P, Tsuang MT. Evidence for linkage between regulatory enzymes in glycolysis and schizophrenia in a multiplex sample. Am J Med Genet B Neuropsychiatr Genet. 2004;127B:5–10. [DOI] [PubMed] [Google Scholar]
- 136. Martins-de-Souza D, Maccarrone G, Wobrock T, et al. Proteome analysis of the thalamus and cerebrospinal fluid reveals glycolysis dysfunction and potential biomarkers candidates for schizophrenia. J Psychiatr Res. 2010;44:1176–1189. [DOI] [PubMed] [Google Scholar]
- 137. Halim ND, Lipska BK, Hyde TM, et al. Increased lactate levels and reduced pH in postmortem brains of schizophrenics: medication confounds. J Neurosci Methods. 2008;169:208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]