Abstract
Abnormalities in both time processing and dopamine (DA) neurotransmission have been observed in schizophrenia. Time processing seems to be linked to DA neurotransmission. The cognitive dysmetria hypothesis postulates that psychosis might be a manifestation of the loss of coordination of mental processes due to impaired timing. The objective of the present study was to analyze timing abilities and their corresponding functional neuroanatomy in schizophrenia. We performed a functional magnetic resonance imaging (fMRI) study using a predictive motor timing paradigm in 28 schizophrenia patients and 27 matched healthy controls (HC). The schizophrenia patients showed accelerated time processing compared to HC; the amount of the acceleration positively correlated with the degree of positive psychotic symptoms and negatively correlated with antipsychotic dose. This dysfunctional predictive timing was associated with BOLD signal activity alterations in several brain networks, especially those previously described as timing networks (basal ganglia, cerebellum, SMA, and insula) and reward networks (hippocampus, amygdala, and NAcc). BOLD signal activity in the cerebellar vermis was negatively associated with accelerated time processing. Several lines of evidence suggest a direct link between DA transmission and the cerebellar vermis that could explain their relevance for the neurobiology of schizophrenia.
Key words: predictive timing, cognitive dysmetria, schizophrenia, cerebellum, fMRI, dopamine
Introduction
Time is a fundamental dimension of physical reality. Our brains phylogenetically evolved within this reality and developed multiple neural systems to organize events in time. Four known brain systems are currently implicated in time processing. The hippocampal system subserves long-term memory, the chronological storing and recollection of events, and it is capable of dealing with very long time intervals.1 The second system, located in the suprachiasmatic nucleus, is responsible for synchronizing various circadian and ultradian biorhythms to external conditions such as sunlight (the master pacemaker).2 The third system is the cortico-striatal system, which is believed to be responsible for cognitively controlled interval timing (in the seconds-to-minutes range) and functions as a pacemaker-accumulator model (the cortex fires impulses that are accumulated in the striatum; the amount of accumulated impulses measures the amount of elapsed time).3,4 The fourth system is the cerebellar system, which is believed to be implicated in automatic sub-second (millisecond) timing. For more on the neurobiology of time perception, see reviews.5,6
There is convincing evidence that time processing is impaired in schizophrenia. A recent meta-analysis7 reviewed 24 studies from 1956 to 2015 and concluded that “results indicate that schizophrenia individuals are less accurate than healthy controls (HC) in estimating time duration across a wide range of tasks. Subgroup analyses showed that the fundamental timing deficit in schizophrenia is independent from the length of the to-be-timed duration (automatic and cognitively controlled timing) and from methods of stimuli estimation (perceptual and motor timing). Thus, time perception per se is disturbed in schizophrenia (not just task-specific timing processes) and this perturbation is independent from more generalized cognitive impairments”. It has even been hypothesized that time-processing deficits might be one of the core underlying deficits behind schizophrenia.8 Specifically, timing impairments in the millisecond range might lead to discoordination of sensorimotor and mental processes, which could lead to higher-order symptoms of schizophrenia (a concept originally coined by Andreasen in the cognitive dysmetria theory9,10). This loss of fluidity and coordination in mental processes could manifest as psychosis.
Abnormalities in dopamine (DA) neurotransmission have long been considered to be involved in the pathophysiology of schizophrenia11–13 and DA neurotransmission has been linked to changes in time processing. Numerous studies using DA receptor agonists (cocaine and methamphetamine) demonstrated acceleration of the “internal clock,”14–16 while DA receptor antagonists (antipsychotics) demonstrated deceleration.14,16 Experiments probing the effects of various neurotransmitter systems on time processing first demonstrated that there might be 2 time-processing systems in the human brain. One, the interval timing system (supra-second range), is dependent on working memory and is cognitively controlled, influenced by all drugs affecting the working memory; the other, the millisecond timing system, is automatic and influenced only by drugs manipulating the DA system.17,18 Later on, lesions, TMS, and neuroimaging studies supported this notion and localized the interval timing system into the corticostriatal system, while the millisecond timing system was localized to the cerebellum.6,19,20
The present study investigates the neural substrate of time processing in schizophrenia using a predictive motor timing fMRI task.21,22 It is a complex timing task requiring an accurate mental prediction about the future position of a target based on visual input as well as the precisely timed execution of a motor response. The task has been shown to robustly activate the cerebellum and the basal ganglia22 and tests both millisecond timing (50–150ms time window to execute the interception of the target, discrete movement timing) and interval timing (cognition-based mental prediction about a future position, continuous movement of the target). According to previous studies performed by Bareš21,23 who investigated the predictive timing paradigm in patients with spino-cerebellar ataxia (damaged cerebellum) and patients with Parkinson’s disease (damaged basal ganglia), the cerebellar timing system seems to be more important during predictive motor timing than the cortico-striatal timing system. Given these results by Bareš and given the known literature about the involvement of the cerebellum in schizophrenia, we hypothesize that we will find major BOLD signal activity changes in the cerebellar timing system between the schizophrenia patients and controls.
Methods
Subjects
Altogether, 28 patients diagnosed with schizophrenia (32.0±6.9 y, 6F + 22M) and 27 age-matched healthy controls (32.0±6.3 y, 6F + 21M) participated in the study. All of the controls were recruited through advertising within the local community; they underwent an interview with a trained psychiatrist to rule out any psychiatric conditions, were right-handed, were native Czech speakers, had no history of mental illness or drug abuse and no brain disorder, and took no psychiatric medication. All of the patients were recruited from among schizophrenia inpatients of the University Hospital Brno, Czech Republic. The patients were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria using the Mini-International Neuropsychiatric Interview (MINI),24 were right-handed and native Czech speakers. The clinical evaluation of the patients included the Positive and Negative Syndrome Scale (PANSS)25 to rate the symptoms of schizophrenia; the Hamilton Anxiety Rating Scale (HAM-A)26 to rate levels of anxiety; the Wechsler Adult Intelligence Scale (WAIS)27 and Wisconsin Card Sorting Test (WCST)28 to test for distinct aspects of cognitive functions; the International Cooperative Ataxia Rating Scale (ICARS)29 to rate cerebellar symptoms; and the Simpson-Angus Extrapyramidal Side Effects Scale (SAS),30 Abnormal Involuntary Movement Scale (AIMS),31 and Barnes Akathisia Scale (BAS)32 to rate the severity of extrapyramidal symptoms. All patients were treated with second-generation antipsychotics, mean antipsychotic dose was (719±375) mg in CPZ equivalents; mean age (32.0±6.9) years; mean illness duration (6.9±6.8) years; mean number of previous psychotic episodes 2.78; and male/female ratio 22/6. The detailed characteristics of the schizophrenia patients are summarized in supplementary table 1. All participants signed an informed consent form. The study was approved by the local ethics committee at the University Hospital Brno.
The Interception Task
We used an interception task developed and previously published by one of the coauthors of this manuscript.21 During the task, a target moved from left to right on a screen. The test subject was instructed to press a button to shoot a ball from the lower right corner of the screen to intercept the moving target. If the ball hit the target, an animation of an explosion was shown (supplementary figure 1). We varied the properties of the moving target in each trial. The target could move at 3 different speeds (slow, medium, and fast), at 3 different angles (0°, 15°, and 30°) and with 3 types of acceleration (no acceleration, acceleration, and deceleration). This gave 3×3 × 3 = 27 trial types. The whole experiment consisted of 324 trials organized into 6 blocks with 54 trials each. Each trial type was thus presented 12 times. The blocks were separated by a 20-second pause. Within each block, the trials were presented randomly in an event-related design. During each trial, the trial characteristics as well as the subject responses (reaction time, hit, early miss, or late miss) were logged. Each trial lasted an average of about 3.5 seconds, the length of the whole paradigm was about 20 minutes. The paradigm was programmed in E-prime (http://www.pstnet.com/eprime.cfm). The time window to hit the target, ie, the time period during which the button press led to a hit, was 50–175ms, depending on the parameters of the moving target. Measured from the beginning of the trial, the time window between the earliest and the latest moment when a button press led to a hit was 500–2500ms, again depending on the moving target parameters.
Image Acquisition Parameters
The scanning was performed using a 1.5 T Siemens Symphony scanner equipped with Numaris 4 System (MRease). Each functional run acquired 490 volumes (echo time [TE] = 35ms, repetition time (TR) = 2300ms, flip angle (FA) = 90°, 28 axial slices, slice thickness = 4mm, in-plane resolution 220×178.8mm, matrix size 64×52, voxel size 3.4375×3.4375mm). Before each measurement, the paradigm was explained to the subjects, and they each performed one practice run consisting of one block of 54 trials.
Statistical Analysis of Behavioral Data
The behavioral data, consisting of trial characteristics such as the target speed, target acceleration, and target angle, and of subject performance such as reaction times and trial results, were obtained from the log files created during fMRI scanning. These data were analyzed with SPSS (SPSS Inc).
fMRI Data Analysis
Images were preprocessed using Statistical Parametric Mapping 8 (SPM-8) (http://www.fil.ion.ucl.ac.uk/spm). The preprocessing consisted of (1) correction for slice-timing differences, (2) alignment to mean image to correct for head motions, (3) normalization to a common stereotactic space (Montreal Neurological Institute template) using an affine transformation, and (4) smoothing using an isotropic 8-mm Gaussian kernel.
On the single-subject level (first-level analysis), a general linear model (GLM) with 3 regressors was created. The first regressor consisted of all hits (the intercepting ball hit the target), the second regressor of all early misses (the intercepting ball was shot too early), and the third regressor of all late misses (the intercepting ball was shot too late). The 6 motion parameters obtained during realignment and also the 5 time series extracted from 4 white matter and one CSF region of interest (ROI) were entered into the model as nuisance regressors. Four contrasts–HIT, MISS, HIT-MISS, and early MISS-late MISS–were defined using the regressors, and thus 4 contrast maps (maps of parameter estimates β) per subject were obtained.
On a group level (second-level analysis), the first-level contrast maps were evaluated using either 1-sample t tests (to show common activation across all 55 participants) or 2-sample t tests (to show group differences between the healthy controls and schizophrenia patients). The group-level analysis was done using the GLM-Flex Toolbox (http://mrtools.mgh.harvard.edu/index.php/Main_Page).
Results
Behavioral Results
The mean reaction times (time interval from the beginning of a trial to a button press) were 934.9±35.7ms in the schizophrenia group and 944.2±22.1ms in the control group (P < .25).
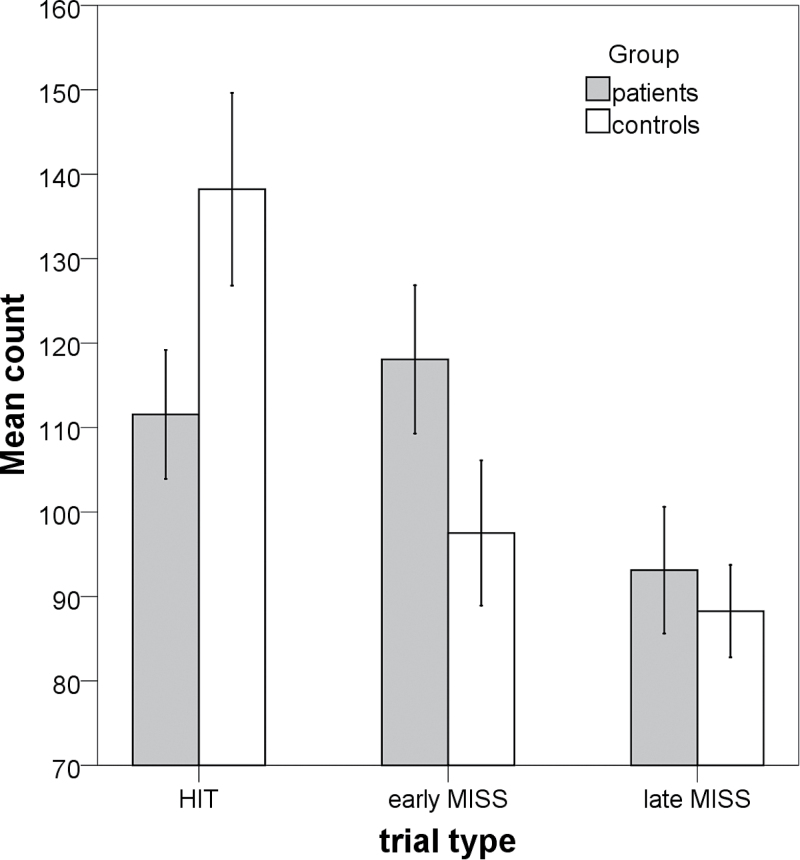
To test the hypothesis that schizophrenia patients have an accelerated time perception, we subdivided the misses into early misses (the intercepting ball passed before the target arrived) and late misses (the intercepting ball passed after the target arrived) and counted the overall number of hits (H), early misses (eM), and late misses (lM) for each subject. There was a significant group difference in the number of hits (P < .001) and early misses (P < .001), but not late misses (P < .29). The results are shown in figure 1.
Fig. 1.
Comparison of behavioral results of the 2 groups. The Box-and-Whisker bars represent the performance of subjects during the paradigm (the absolute count of hits, early misses, and late misses).
We used partial correlation analysis to investigate the possible mechanisms responsible for this underestimation. The results are summarized in table 1. We found a significant (P < .001) correlation between accelerated time perception and positive symptoms of schizophrenia and a significant anticorrelation (P < .001) between accelerated time perception and antipsychotic medication doses; see table 1. We took care to exclude the effects of possible confounders, controlling the correlations for the effects of age, number of episodes, psychomotor speed (mean reaction times), extrapyramidal symptoms (BAS, AIMS, SAS), and overall level of anxiety (HAMA). We also investigated symptoms of ataxia measured by ICARS subscales (especially the ICARS IV subscale, measuring oculomotor deficits) and cognitive functions measured by selected subscales of the WAIS and WCST tests (working memory and executive function).
Table 1.
Partial Correlation Coefficients (pCC) Showing the Association Between the Overall Number of Hits (H), Early Misses (eM), Late Misses (lM), and PANSS Subscales, Antipsychotic Doses (CPZ), ICARS Subscales, and Subscales of the Wechsler Adult Intelligence Test (WAIS) and Wisconsin Card Sorting Test (WCST)
| Variables Controlled For | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correlated Variables | Age | Nr. EP | mRT | stdRT | CPZ | SAS | AIMS | BAS | HAMA | PANSS P | PANSS N | PANNS G | pCC | Significance | Remark |
| eM vs PP | ● | ● | ● | ● | ● | ● | ● | ● | ● | 0.878 | <.001 | Number of early misses (accelerated time) correlates with positive symptoms (dopamine) and anticorrelates with antipsychotic doses (antidopaminergic) | |||
| eM vs NP | ● | ● | ● | ● | ● | ● | ● | ● | ● | 0.184 | .566 | ||||
| eM vs GP | ● | ● | ● | ● | ● | ● | ● | ● | ● | 0.474 | .120 | ||||
| eM vs CZP | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | −0.879 | .002 | ||
| H vs PP | ● | ● | ● | ● | ● | ● | ● | ● | ● | −0.676 | .022 | Number of hits anticorrelates with all PANSS subscales and positively correlates with antipsychotic doses | |||
| H vs NP | ● | ● | ● | ● | ● | ● | ● | ● | ● | −0.558 | .075 | ||||
| H vs GP | ● | ● | ● | ● | ● | ● | ● | ● | ● | −0.525 | .097 | ||||
| H vs CPZ | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 0.643 | .067 | ||
| lM vs PP | ● | ● | ● | ● | ● | ● | ● | ● | ● | −0.522 | .100 | Number of late misses significantly correlates with antipsychotic doses | |||
| lM vs NP | ● | ● | ● | ● | ● | ● | ● | ● | ● | 0.219 | .518 | ||||
| lM vs GP | ● | ● | ● | ● | ● | ● | ● | ● | ● | −0.181 | .594 | ||||
| lM vs CPZ | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 0.702 | .035 | ||
| ICARS 1 vs eM | ● | ● | ● | ● | ● | ● | ● | ● | ● | 0.671 | .215 | No significant correlations | |||
| ICARS 2 vs eM | ● | ● | ● | ● | ● | ● | ● | ● | ● | 0.714 | .176 | ||||
| ICARS 3 vs eM | ● | ● | ● | ● | ● | ● | ● | ● | ● | 0.338 | .578 | ||||
| ICARS 4 vs eM | ● | ● | ● | ● | ● | ● | ● | ● | ● | 0.246 | .689 | ||||
| eM vs WAIS symbol search | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | −0.556 | .195 | Visual perception/analysis |
| eM vs WAIS similarities | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 0.065 | .889 | Abstract verbal reasoning |
| eM vs WAIS arithmetic | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 0.021 | .965 | Working memory |
| eM vs WAIS picture completion | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | −0.209 | .602 | Ability to quickly perceive visual details |
| eM vs WCST cc | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 0.417 | .410 | Executive function |
Note: The dots represent variables that were controlled for during the partial correlation: mean reaction times (mRT) and standard deviations of reaction times (stdRT), age, number of psychotic episodes (Nr Ep), chlorpromazine equivalents (CPZ), PANSS positive symptoms subscale (PP), PANSS negative symptoms subscale (NP), PANSS general psychopathology subscale (GP), Simpson-Angus Extrapyramidal Side Effects Scale (SAS), Abnormal Involuntary Movement Scale (AIMS), Barnes Akathisia Scale (BAS), and Hamilton Anxiety Rating Scale (HAMA).
fMRI Results
This manuscript uses the following interpretation of hyper/hypoactivation: During the 1-sample t tests (schizophrenia+HC combined), hyperactivation means that the BOLD response in a given region was higher than baseline; hypoactivation means the opposite. During the 2-sample t tests, hyperactivation means that the BOLD signal was stronger in the schizophrenia group than in the control group; hypoactivation means the opposite.
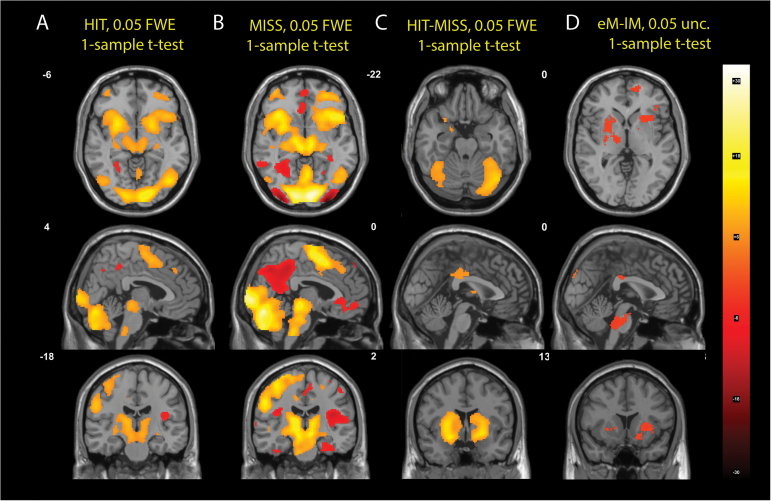
Based on 3 trial types, HIT (H), early MISS (eM), and late MISS (lM), we defined 4 contrasts—HIT (H), MISS (eM+lM), HIT-MISS (2H-eM-lM), and early MISS-late MISS (eM-lM)—and used 1-sample t tests to identify the basic activations of each contrast across all 55 participants; see figure 2. Both the HIT and MISS contrasts “activated” regions in the brainstem (pontine nuclei and olive nucleus), extensive regions within the cerebellum (vermis3, vermis4, vermis5, vermis6, vermis7, vermis8, cerebellum6, cerebellum crus 1, and cerebellum crus 2), thalamus, basal ganglia (striatum), motor and premotor areas of the left side, and the supplementary motor area, and “hypoactivated” regions of the default mode network (DMN). In accordance with previously published results,22 the paradigm thus extensively activated timing networks whose main components are the cerebellar and basal ganglia loops. The cerebellar hemispheres receive afferent projections from the brainstem nuclei (pontine-cerebellar tracts) which in turn receive projections from the cortex (cortico-pontine tracts). The cerebellar hemispheres project onto the deep cerebellar nuclei (DCN) in the vermis. The DCN project efferently onto the thalamus, which in turn projects back onto the cerebral cortex. Thus the closed cerebrum-cerebellum-cerebrum loops are formed.33,34 The HIT and MISS contrasts had similar activations; the MISS activations were stronger (more statistically significant and more surviving thresholding). The HIT-MISS contrast demonstrated that during HITs, the basal ganglia and the cerebellar hemispheres are “hyperactivated,” compared to MISSes. The eM-lM contrast showed “hypoactivations” of the brainstem regions and the basal ganglia during the early MISSes, although the statistical significance was very low (P < .05 uncorr.).
Fig. 2.
Basic activations of the 4 contrasts computed using 1-sample t test across all 55 subjects. The (A) and (B) columns compare the activations for the HIT and MISS contrasts at the same Montreal Neurological Institute (MNI) coordinates. The (C) column shows the HIT-MISS contrast. The (D) column shows the difference in activations between the early MISS and late MISS regressors.
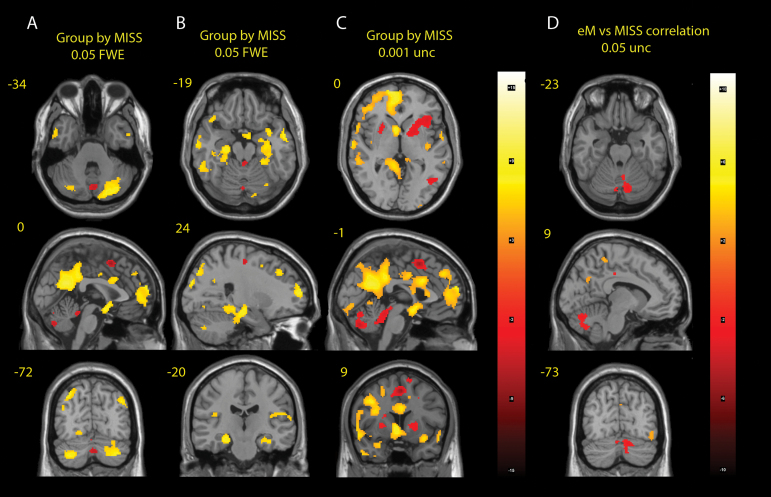
Because the MISS contrast delivered more statistically significant results than the HIT contrast, we used this contrast to investigate the group differences using 2-sample t tests. We found significant differences in brain activation between the schizophrenia group and healthy controls. The schizophrenia group had significant hypoactivations in the cerebellar vermis (vermis3, vermis6, vermis7, and vermis8), the basal ganglia (BG), and the supplementary motor area (SMA), and hyperactivations in the cerebellar hemispheres (lobulus6, crus1, and crus2), the default mode network, the amygdala, the hippocampus, and the nucleus accumbens bilaterally. The results are shown in figures 3A–C and listed in table 2. Figure 3D shows the correlation of the MISS regressor β-coefficients and the total number of early misses. Although the statistical significance is low (.05 unc.), the activation pattern shows relative hypoactivation of the cerebellar vermis in patients with high numbers of early misses, hinting at its role in the pathophysiology of accelerated timing. Supplementary figure 2 shows a group comparison of the parameter estimates (βs) for various ROIs for the MISS regressor.
Fig. 3.
Group differences in the MISS contrast (A), (B), (C) and the correlation of the frequency of early misses (eMs) with the MISS contrast (D).
Table 2.
Overview of MISS Contrast Brain Activation Group Differences
| Extent in Voxels | t Value | MNI Coordinates | Side | Region Name | Remark | |||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Timing networks | 71 | −6.60 | 2 | −74 | −34 | Cerebellum Vermis VII | Structures known to be involved during timing paradigms in healthy controls. The cerebellar vermis contains the fastigial and dentate nuclei and is known as the “limbic cerebellum” with bilateral connections to dopaminergic regions (VTA, hippocampus, NAcc). Reduced vermis volume is the most common cerebellar structural deficit in schizophrenia | |
| 36 | −5.71 | 0 | −38 | −18 | Cerebellum Vermis I/II | |||
| 17 | −5.32 | −2 | −74 | −18 | Cerebellum Vermis VI | |||
| 1193 | 8.45 | 18 | −82 | −26 | R | Cerebellum Crus I | ||
| 1193 | 8.23 | 34 | −77 | −39 | R | Cerebellum Crus II | ||
| 1193 | 5.51 | 30 | −70 | −22 | R | Cerebellum Crus VI | ||
| 226 | 7.95 | −32 | −74 | −39 | L | Cerebellum Crus II | ||
| 131 | −8.51 | 2 | 10 | 52 | R | Supplementary Motor Area | ||
| 37 | −5.27 | 12 | 8 | 68 | R | Supplementary Motor Area | ||
| 15 | −5.29 | −15 | −6 | 57 | L | Supplementary Motor Area | ||
| 168 | −4.82 | 22 | 12 | 2 | R | Putamen | ||
| 41 | −5.60 | 18 | 6 | 1 | R | Putamen/Pallidum | ||
| Limbic structures | 4609 | 6.36 | −42 | −26 | −14 | L | hippocampus | All limbic and reward processing structures hyperactivate in schizophrenia compared to controls; at the same time, all these structures are part of the dopaminergic mesolimbic pathway |
| 4609 | 8.15 | −25 | −19 | −17 | L | Hippocampus | ||
| 632 | 6.53 | 29 | −14 | −18 | R | Hippocampus | ||
| 4609 | 6.64 | −26 | −32 | −12 | L | Parahippocampal Gyrus | ||
| 632 | 6.51 | 24 | −6 | −18 | R | Amygdala | ||
| 481 | 7.39 | 0 | 2 | −13 | Nucleus Accumbens/ olfactory tubercle | |||
| 208 | 6.96 | 2 | 14 | 30 | R | Anterior Cingulate Cortex | ||
| Default Mode Network | 4609 | 9.66 | −2 | −56 | 24 | L | Precuneus | DMN is known to fail to deactivate in schizophrenia, hence the relative hyperactivation in schizophrenia patients compared to controls |
| 4609 | 7.81 | 12 | −53 | 31 | R | Precuneus | ||
| 4609 | 9.62 | 8 | −38 | 32 | R | Posterior Cingulate Cortex | ||
| 4609 | 8.31 | −5 | −41 | 43 | L | Middle Cingulate Cortex | ||
| 343 | 8.85 | −64 | −10 | −12 | R | Middle Temporal Gyrus | ||
| 159 | 7.64 | 56 | 0 | −18 | L | Middle Temporal Gyrus | ||
| 1680 | 9.73 | −8 | 52 | 6 | L | Superior Frontal Gyrus— Medial Part | ||
| 135 | 6.3 | 4 | 44 | 38 | R | Superior Frontal Gyrus— Medial Part | ||
| 1099 | 7.8 | −36 | −71 | 46 | L | Angular Gyrus | ||
| 288 | 6.8 | 42 | −72 | 36 | R | Angular Gyrus | ||
| Motor regions | 854 | 6.21 | −53 | 1 | 22 | L | Precentral Gyrus | motor cortices, button pressing |
| 854 | 6.02 | −50 | −11 | 35 | L | Postcentral Gyrus | ||
| 602 | 7.37 | 58 | −6 | 16 | R | Postcentral Gyrus | ||
| 132 | 7.28 | 27 | −27 | 53 | R | Precentral Gyrus | ||
| Insula | 103 | 6.04 | −40 | −4 | −10 | L | Insula | interoceptive awareness, known to be involved in hallucinations in schizophrenia |
| 632 | 8.02 | 36 | 4 | −14 | R | Insula | ||
| 168 | −6.64 | 26 | 20 | 6 | R | Insula | ||
| 168 | −4.81 | 38 | 16 | 2 | R | Insula | ||
| 602 | 7.24 | 43 | −11 | 24 | R | Rolandic Operculum | ||
| 854 | 7.64 | −46 | −10 | 22 | L | Rolandic Operculum | ||
| Fusiform | 4609 | 6.34 | −23 | −41 | −11 | L | Fusiform Gyrus | face recognition |
| 632 | 7.01 | 26 | −36 | −22 | R | Fusiform Gyrus | ||
| 118 | 7.38 | 38 | −48 | −24 | R | Fusiform Gyrus | ||
| Frontal | 1680 | 9.01 | −26 | 58 | 12 | L | Superior Frontal Gyrus | |
| 91 | 7.76 | −22 | 32 | 38 | L | Superior Frontal Gyrus | ||
| 37 | −5.82 | 12 | 0 | 72 | R | Superior Frontal Gyrus | ||
| 854 | 7.46 | −40 | 10 | 38 | L | Middle Frontal Gyrus | ||
| 29 | −5.79 | 40 | 38 | 30 | R | Middle Frontal Gyrus | ||
| 7 | −5.06 | 27 | −2 | 56 | R | Middle Frontal Gyrus | ||
| 588 | 6.93 | −50 | 34 | 8 | L | Inferior Frontal Gyrus—pars triangulars | ||
| 588 | 6.34 | −43 | 30 | −12 | L | Inferior Frontal Gyrus—pars orbitalis | ||
| 168 | −5.98 | 40 | 24 | −2 | R | Inferior Frontal Gyrus—pars triangulars | ||
| 1680 | 10.12 | −12 | 62 | −2 | L | Medial Surface of the Frontal Lobe— Orbital Part | ||
| Occipital | 1099 | 8.85 | −32 | −86 | 24 | L | Middle Occipital Gyrus | |
| 1099 | 7.43 | −43 | −65 | 25 | L | Middle Occipital Gyrus | ||
| 100 | 7.53 | 24 | −90 | 34 | R | Superior Occipital Gyrus | ||
| 68 | 7.85 | 12 | −102 | 10 | R | Superior Occipital Gyrus | ||
| 18 | −5.91 | 40 | −64 | 4 | R | Middle Occipital Gyrus | ||
| 7 | −5.19 | 36 | −72 | 22 | R | Middle Occipital Gyrus | ||
| Pariet | 6 | −5.29 | −30 | −56 | 52 | L | Inferior Parietal Lobule | |
| 23 | −5.43 | 53 | −37 | 34 | R | SupraMarginal Gyrus | ||
| Temp | 588 | 6.28 | −57 | 12 | −11 | L | Superior Temporal Pole | |
| 22 | 6.35 | 36 | 24 | −24 | R | Superior Temporal Pole | ||
Note: MNI, Montreal Neurological Institute. All regions surviving the whole-brain 0.05 FWE multiple comparisons correcture are shown (P < 1.67×10−6). Bold shows hypoactivations and unbold shows hyperactivations in schizophrenia subjects compared to controls.
Discussion
An analysis of both behavioral and neuroimaging data obtained during a predictive motor timing paradigm in schizophrenia patients and healthy controls resulted in 3 principal findings:
The schizophrenia patients demonstrated disturbed timing during a predictive motor timing paradigm. They had more early misses. The count of early misses was positively associated with the degree of positive psychotic symptoms as measured by PANSS and negatively associated with antipsychotic doses (CPZ equivalents). This result can reflect an accelerated time perception/timing in schizophrenia/psychosis.
This dysfunctional predictive timing was associated with alterations in several brain networks, especially those previously described as timing networks (basal ganglia, cerebellum, SMA, and insula), reward networks (hippocampus, amygdala, and NAcc), the DMN, and regions of the frontal, temporal and parietal lobes.
The count of early misses negatively correlated with the activation of the vermis within the schizophrenia group, ie, the more early misses a given schizophrenic patient had, the weaker the BOLD activity in the vermis. The vermis was the only region which anticorrelated with the BOLD signal activity.
Although our data show a distributed neuroanatomical pattern of regions involved in predictive motor timing in schizophrenia, the link between the performance in the task, the severity of psychosis, and the antipsychotic dose, and the relationship among all of that and the cerebellar vermis BOLD signal, suggest a prominent role of the cerebellar vermis in subinterval timing deficits in schizophrenia.
There is a wealth of literature describing the involvement of the cerebellar vermis in psychosis. A study conducted on a large sample of 1700 subjects demonstrated that 50% of the subjects who had functional psychosis had cerebellar vermal atrophy.35 Reduced volume of the vermis is the most common cerebellar structural deficit reported in schizophrenia.36 Post-mortem studies showed a reduced gyrification index in the cerebellar vermis in schizophrenia,37 decreased neuronal integrity in the vermis in schizophrenia,38 a reduced volume of the cerebellar vermis in neuroleptic-naive schizophrenia,39 and a smaller cerebellar vermis, but not smaller hemisphere volumes, in patients with chronic schizophrenia.40 These findings indicate that reduced/atrophic vermis/vermis hypoactivations are associated with psychosis, which is in agreement with our finding that the BOLD signal activity was reduced (hypoactive) in probands with schizophrenia. Additionally, we showed that the vermal BOLD activity negatively correlated with the number of early misses, ie, with timing. The number of early misses positively correlated with positive psychotic symptoms (psychosis) and negatively correlated with antipsychotic dose. These results may offer indirect support for the cognitive dysmetria hypothesis.
Our data suggest a link between time processing (as measured by the number of early misses) and DA neurotransmission. The evidence is provided by the opposing effects of antipsychotic medication (DA receptor antagonists) and positive psychotic symptoms (that may reflect DA hyperactivity) on the number of early misses.
Several lines of evidence suggest a link between the DA system, the cerebellar vermis, and the millisecond timing that may explain our pattern of findings. A seminal study investigating dopaminergic innervation of the cerebellum conducted on primates using immunohistochemistry methods41 found that “axons immunoreactive for the DA membrane transporter, a specific marker of DA axons, were present in high density, but only in certain lobules of the cerebellar vermis”. A later study confirmed the result.42 Indirect but striking evidence comes from a vast body of literature linking the cerebellar vermis to several psychiatric disorders with the involvement of DA neurotransmission and treated with dopaminergic drugs. Several morphometric studies demonstrated cerebellar vermis reductions in bipolar disorder patients43–45 and subsequent reductions with every bipolar episode. Several studies have shown a reduced vermis in ADHD, a disorder linked to DA abnormalities and treated with psychostimulant dopaminergic drugs.46–48 Children treated with methylphenidate had larger vermi than drug-naive children.47,48 There is evidence linking the cerebellar vermis to stimulant addiction.49 However, it seems unclear in which direction the causality between the vermis and disturbed DA neurotransmission goes. Neuroanatomical studies of vermal connectivity have shown that connections with the dopaminergic areas in the brainstem—the ventral tegmental area (VTA)—go both ways. Fluorescent retrograde double-labeling in rats has shown that VTA projects to the cerebellum.50 Other histologic staining studies have demonstrated that vermal Purkynje cells project directly and indirectly to the VTA and the substantia nigra.51,52 Snider demonstrated that artificial lesions on the vermis in rats led to altered DA metabolism in the forebrain,53 which would suggest a primary role of the vermis. Other experiments demonstrated that dopaminergic drugs can influence millisecond timing.14,15,17
The link between aberrant DA neurotransmission in schizophrenia and the cerebellar vermis functioning might be an alternative explanation of the cognitive dysmetria hypothesis—or it may complement the possibility of abnormal wiring within the cortico-thalamo-cerebello-cortical loops.9 The link is provided by the following chain of arguments: aberrant DA neurotransmission in schizophrenia influences the cerebellar vermis. The vermis is implicated in millisecond timing across many domains of motor, perceptual, and cognitive tasks. Disturbed millisecond timing leads to discoordination and a loss of fluency in mental processes, which manifests as psychosis. The cognitive dysmetria hypothesis is based on an analogy with motor dysmetria. Several experiments with cerebellar patients demonstrated that disturbed timing in sequential muscle contractions of agonists and antagonists leads to discoordination and thus dysmetria, which leads to a loss of tracking accuracy and creates disjointed responses.54 The best examples are provided by ball-throwing experiments,55,56 by paced finger-tapping tasks,57 and by dysdiadochokinesis in cerebellar patients. Mauk looked at the relationship between timing, coordination, and learning.58 Specifically relating to vermis, one study59 found that transcranial magnetic stimulation of the cerebellar vermis caused timing dysfunctions. Accumulating evidence has demonstrated that the cerebellum is involved not only in motor control and coordination,60 but also in perception34 and cognition.61,62 This is further corroborated by the anatomical connections of the cerebellum, which is connected to motor areas as well as to limbic regions and the frontal cortices.33,63,64 Interestingly, many motor deficits commonly observed in cerebellar patients are observed in schizophrenia.36
To keep this manuscript focused and short, we moved our discussion of other brain activations to supplementary material. Of interest are the altered activations in the reward circuitry (hippocampus, amygdala, and ncl. accumbens) of the schizophrenia patients, because all these regions are projection sites of the dopaminergic neurons in the VTA.
Limitations
The study was performed on older fMRI hardware with a resolution of 1.5T. The resolution was insufficient for differentiating the individual structures within the vermis, ie, differentiating the individual cerebellar nuclei.
All of the patients were on long-term antipsychotic medication, which may have influenced their test performances. Because the effect of medication can be manifested by extra-pyramidal symptoms and a potential sedative effect, we would expect a higher count of late misses and slower reaction times. Quite contrary to that, we saw the opposite effect—antipsychotic medication tended to normalize the timing performance. Furthermore, we controlled for the medication effects in our analyses by measuring the EPS (BAS, AIMS, and SAS scales) and the total daily antipsychotic doses (CPZ equivalents), and we included those as covariates wherever possible. We did not observe any effect of the EPS on the task performance, neither did we observe any prolongation of reaction times; we observed the opposite: early misses were more frequent and reaction times were shorter in the schizophrenia group.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Ministry of Health of the Czech Republic (15-31063A).
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35:86–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gillette MU, Tischkau SA. Suprachiasmatic nucleus: the brain’s circadian clock. Recent Prog Horm Res. 1999;54:33–58; discussion 58–39. [PubMed] [Google Scholar]
- 3. Jin DZ, Fujii N, Graybiel AM. Neural representation of time in cortico-basal ganglia circuits. Proc Natl Acad Sci U S A. 2009;106:19156–19161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jech R, Dusek P, Wackermann J, Vymazal J. Cumulative blood oxygenation-level-dependent signal changes support the ‘time accumulator’ hypothesis. Neuroreport. 2005;16:1467–1471. [DOI] [PubMed] [Google Scholar]
- 5. Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. [DOI] [PubMed] [Google Scholar]
- 6. Ivry RB, Spencer RM. The neural representation of time. Curr Opin Neurobiol. 2004;14:225–232. [DOI] [PubMed] [Google Scholar]
- 7. Ciullo V, Spalletta G, Caltagirone C, Jorge RE, Piras F. Explicit time deficit in schizophrenia: systematic review and meta-analysis indicate it is primary and not domain specific. Schizophr Bull. 2016;42:505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gómez J, Jesús Marín-Méndez J, Molero P, Atakan Z, Ortuño F. Time perception networks and cognition in schizophrenia: a review and a proposal. Psychiatry Res. 2014;220:737–744. [DOI] [PubMed] [Google Scholar]
- 9. Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry. 1999;46:908–920. [DOI] [PubMed] [Google Scholar]
- 10. Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–378. [DOI] [PubMed] [Google Scholar]
- 11. Carlsson A, Carlsson ML. A dopaminergic deficit hypothesis of schizophrenia: the path to discovery. Dialogues Clin Neurosci. 2006;8:137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guillin O, Abi-Dargham A, Laruelle M. Neurobiology of dopamine in schizophrenia. Int Rev Neurobiol. 2007;78:1–39. [DOI] [PubMed] [Google Scholar]
- 14. Buhusi CV, Meck WH. Differential effects of methamphetamine and haloperidol on the control of an internal clock. Behav Neurosci. 2002;116:291–297. [DOI] [PubMed] [Google Scholar]
- 15. Meck WH, Cheng RK, MacDonald CJ, Gainetdinov RR, Caron MG, Cevik MÖ. Gene-dose dependent effects of methamphetamine on interval timing in dopamine-transporter knockout mice. Neuropharmacology. 2012;62:1221–1229. [DOI] [PubMed] [Google Scholar]
- 16. Rammsayer T. Temporal discrimination in schizophrenic and affective disorders: evidence for a dopamine-dependent internal clock. Int J Neurosci. 1990;53:111–120. [DOI] [PubMed] [Google Scholar]
- 17. Rammsayer TH. Neuropharmacological evidence for different timing mechanisms in humans. Q J Exp Psychol B. 1999;52:273–286. [DOI] [PubMed] [Google Scholar]
- 18. Meck WH, Benson AM. Dissecting the brain’s internal clock: how frontal-striatal circuitry keeps time and shifts attention. Brain Cogn. 2002;48:195–211. [DOI] [PubMed] [Google Scholar]
- 19. Koch G, Oliveri M, Caltagirone C. Neural networks engaged in milliseconds and seconds time processing: evidence from transcranial magnetic stimulation and patients with cortical or subcortical dysfunction. Philos Trans R Soc Lond B Biol Sci. 2009;364:1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: evidence from neuroimaging. Curr Opin Neurobiol. 2003;13:250–255. [DOI] [PubMed] [Google Scholar]
- 21. Bares M, Lungu OV, Husárová I, Gescheidt T. Predictive motor timing performance dissociates between early diseases of the cerebellum and Parkinson’s disease. Cerebellum. 2010;9:124–135. [DOI] [PubMed] [Google Scholar]
- 22. Husárová I, Lungu OV, Mareček R, et al. Functional imaging of the cerebellum and basal ganglia during predictive motor timing in early Parkinson’s disease. J Neuroimaging. 2014;24:45–53. [DOI] [PubMed] [Google Scholar]
- 23. Bares M, Lungu O, Liu T, Waechter T, Gomez CM, Ashe J. Impaired predictive motor timing in patients with cerebellar disorders. Exp Brain Res. 2007;180:355–365. [DOI] [PubMed] [Google Scholar]
- 24. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33;quiz 34–57. [PubMed] [Google Scholar]
- 25. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 26. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. [DOI] [PubMed] [Google Scholar]
- 27. Wechsler D. The Measurement and Appraisal of Adult Intelligence. Fourth Edition. [With a Bibliography.]. London, UK: Tindall & Cox; 1958. [Google Scholar]
- 28. Nyhus E, Barceló F. The Wisconsin Card Sorting Test and the cognitive assessment of prefrontal executive functions: a critical update. Brain Cogn. 2009;71:437–451. [DOI] [PubMed] [Google Scholar]
- 29. Trouillas P, Takayanagi T, Hallett M, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. 1997;145:205–211. [DOI] [PubMed] [Google Scholar]
- 30. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. [DOI] [PubMed] [Google Scholar]
- 31. Guy W. ECDEU Assessment Manual for Psychopharmacology: Revised (DHEW Publication Number ADM 76-338). Rockville, MD: US Department of Health, Education and Welfare, Public Health Service, Alcohol, Drug Abuse and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976:534–537. [Google Scholar]
- 32. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. [DOI] [PubMed] [Google Scholar]
- 33. Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. [DOI] [PubMed] [Google Scholar]
- 34. Baumann O, Borra RJ, Bower JM, et al. Consensus paper: the role of the cerebellum in perceptual processes. Cerebellum. 2015;14:197–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heath RG, Franklin DE, Walker CF, Keating JW., Jr Cerebellar vermal atrophy in psychiatric patients. Biol Psychiatry. 1982;17:569–583. [PubMed] [Google Scholar]
- 36. Picard H, Amado I, Mouchet-Mages S, Olié JP, Krebs MO. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr Bull. 2008;34:155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmitt A, Schulenberg W, Bernstein HG, et al. Reduction of gyrification index in the cerebellar vermis in schizophrenia: a post-mortem study. World J Biol Psychiatry. 2011;12(suppl 1):99–103. [DOI] [PubMed] [Google Scholar]
- 38. Deicken RF, Feiwell R, Schuff N, Soher B. Evidence for altered cerebellar vermis neuronal integrity in schizophrenia. Psychiatry Res. 2001;107:125–134. [DOI] [PubMed] [Google Scholar]
- 39. Ichimiya T, Okubo Y, Suhara T, Sudo Y. Reduced volume of the cerebellar vermis in neuroleptic-naive schizophrenia. Biol Psychiatry. 2001;49:20–27. [DOI] [PubMed] [Google Scholar]
- 40. Okugawa G, Sedvall GC, Agartz I. Smaller cerebellar vermis but not hemisphere volumes in patients with chronic schizophrenia. Am J Psychiatry. 2003;160:1614–1617. [DOI] [PubMed] [Google Scholar]
- 41. Melchitzky DS, Lewis DA. Tyrosine hydroxylase- and dopamine transporter-immunoreactive axons in the primate cerebellum. Evidence for a lobular- and laminar-specific dopamine innervation. Neuropsychopharmacology. 2000;22:466–472. [DOI] [PubMed] [Google Scholar]
- 42. Hurley MJ, Mash DC, Jenner P. Markers for dopaminergic neurotransmission in the cerebellum in normal individuals and patients with Parkinson’s disease examined by RT-PCR. Eur J Neurosci. 2003;18:2668–2672. [DOI] [PubMed] [Google Scholar]
- 43. DelBello MP, Strakowski SM, Zimmerman ME, Hawkins JM, Sax KW. MRI analysis of the cerebellum in bipolar disorder: a pilot study. Neuropsychopharmacology. 1999;21:63–68. [DOI] [PubMed] [Google Scholar]
- 44. Kim D, Cho HB, Dager SR, et al. Posterior cerebellar vermal deficits in bipolar disorder. J Affect Disord. 2013;150:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mills NP, Delbello MP, Adler CM, Strakowski SM. MRI analysis of cerebellar vermal abnormalities in bipolar disorder. Am J Psychiatry. 2005;162:1530–1532. [DOI] [PubMed] [Google Scholar]
- 46. Bledsoe J, Semrud-Clikeman M, Pliszka SR. A magnetic resonance imaging study of the cerebellar vermis in chronically treated and treatment-naïve children with attention-deficit/hyperactivity disorder combined type. Biol Psychiatry. 2009;65:620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mackie S, Shaw P, Lenroot R, et al. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am J Psychiatry. 2007;164:647–655. [DOI] [PubMed] [Google Scholar]
- 48. Anderson CM, Polcari A, Lowen SB, Renshaw PF, Teicher MH. Effects of methylphenidate on functional magnetic resonance relaxometry of the cerebellar vermis in boys with ADHD. Am J Psychiatry. 2002;159:1322–1328. [DOI] [PubMed] [Google Scholar]
- 49. Anderson CM, Maas LC, Frederick Bd, et al. Cerebellar vermis involvement in cocaine-related behaviors. Neuropsychopharmacology. 2006;31:1318–1326. [DOI] [PubMed] [Google Scholar]
- 50. Ikai Y, Takada M, Shinonaga Y, Mizuno N. Dopaminergic and non-dopaminergic neurons in the ventral tegmental area of the rat project, respectively, to the cerebellar cortex and deep cerebellar nuclei. Neuroscience. 1992;51:719–728. [DOI] [PubMed] [Google Scholar]
- 51. Snider RS, Maiti A, Snider SR. Cerebellar pathways to ventral midbrain and nigra. Exp Neurol. 1976;53:714–728. [DOI] [PubMed] [Google Scholar]
- 52. Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–580. [DOI] [PubMed] [Google Scholar]
- 53. Snider SR, Snider RS. Alterations in forebrain catecholamine metabolism produced by cerebellar lesions in the rat. J Neural Transm. 1977;40:115–128. [DOI] [PubMed] [Google Scholar]
- 54. Ivry R. Cerebellar timing systems. Int Rev Neurobiol. 1997;41:555–573. [PubMed] [Google Scholar]
- 55. Hore J, Timmann D, Watts S. Disorders in timing and force of finger opening in overarm throws made by cerebellar subjects. Ann N Y Acad Sci. 2002;978:1–15. [DOI] [PubMed] [Google Scholar]
- 56. Kornhuber HH. Motor functions of cerebellum and basal ganglia: the cerebellocortical saccadic (ballistic) clock, the cerebellonuclear hold regulator, and the basal ganglia ramp (voluntary speed smooth movement) generator. Kybernetik. 1971;8:157–162. [DOI] [PubMed] [Google Scholar]
- 57. Ivry RB, Keele SW. Timing functions of the cerebellum. J Cogn Neurosci. 1989;1:136–152. [DOI] [PubMed] [Google Scholar]
- 58. Mauk MD, Medina JF, Nores WL, Ohyama T. Cerebellar function: coordination, learning or timing? Curr Biol. 2000;10:R522–R525. [DOI] [PubMed] [Google Scholar]
- 59. Théoret H, Haque J, Pascual-Leone A. Increased variability of paced finger tapping accuracy following repetitive magnetic stimulation of the cerebellum in humans. Neurosci Lett. 2001;306:29–32. [DOI] [PubMed] [Google Scholar]
- 60. Manto M, Bower JM, Conforto AB, et al. Consensus paper: roles of the cerebellum in motor control–the diversity of ideas on cerebellar involvement in movement. Cerebellum. 2012;11:457–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129:290–292. [DOI] [PubMed] [Google Scholar]
- 62. Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage. 2012;59:1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. [DOI] [PubMed] [Google Scholar]
- 64. Voogd J. The human cerebellum. J Chem Neuroanat. 2003;26:243–252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.