Abstract
Background:
Converging evidence suggests that patients with first-episode psychosis who show a poor treatment response may have a higher degree of neurodevelopmental abnormalities than good Responders. Characterizing the disturbances in the relationship among brain regions (covariance) can provide more information on neurodevelopmental integrity than searching for localized changes in the brain. Graph-based connectomic approach can measure structural covariance thus providing information on the maturational processes. We quantified the structural covariance of cortical folding using graph theory in first-episode psychosis, to investigate if this systems-level approach would improve our understanding of the biological determinants of outcome in psychosis.
Methods:
Magnetic Resonance Imaging data were acquired in 80 first-episode psychosis patients and 46 healthy controls. Response to treatment was assessed after 12 weeks of naturalistic follow-up. Gyrification-based connectomes were constructed to study the maturational organization of cortical folding.
Results:
Nonresponders showed a reduction in the distributed relationship among brain regions (high segregation, poor integration) when compared to Responders and controls, indicating a higher burden of aberrant neurodevelopment. They also showed reduced centrality of key regions (left insula and anterior cingulate cortex) indicating a marked reconfiguration of gyrification. Nonresponders showed a vulnerable pattern of covariance that disintegrated when simulated lesions removed high-degree hubs, indicating an abnormal dependence on highly central hub regions in Nonresponders.
Conclusions:
These findings suggest that a perturbed maturational relationship among brain regions underlies poor treatment response in first-episode psychosis. The information obtained from gyrification-based connectomes can be harnessed for prospectively predicting treatment response and prognosis in psychosis.
Key words: cortical folding, connectome, graph theory, neuroimaging, first-episode psychosis, surface based morphometry
Introduction
Early response to antipsychotic medication is an important indicator of long-term outcome in psychosis1; hence understanding the neurobiological factors contributing to a favorable treatment response may be crucial to understanding the natural course of psychotic disorders. However, the trajectory of response to antipsychotic treatment cannot yet be predicted before patients undergo a treatment trial. Several indirect lines of evidence suggest a relationship between maturational deviations of the developing cortex and poor outcome in psychosis. Presence of obstetric complications,2 early age of onset,3 and neurological soft signs4 are all independently associated with poor response to treatment. These observations have led to a notion that psychotic disorders associated with less favorable outcome are a form of neurodevelopmental disorders.5,6
Cortical folding (gyrification) patterns in adult life reflect the integrity of cortico-cortical and subcortical connectivity during early development,7 suggesting that brain regions that are “wired together, fold together.” A number of early neuroimaging studies have suggested that cortical folding patterns provide important information on the prognosis of schizophrenia.8,9 Indeed, we have recently provided first evidence that lack of response to antipsychotic treatment is associated with widespread cortical folding reduction (hypogyria) at baseline in fronto-temporo-insular regions relevant to the pathophysiology of psychosis.10 Furthermore, we have also shown a reduced integrity of the white matter tracts that connect these regions in patients who do not respond to antipsychotics.11 Our findings have suggested that, across the psychosis spectrum cortical morphology holds clinically relevant prognostic information. However, these univariate approaches do not provide information on the development of the brain at a “system” level. Brain regions do not develop in isolation, and therefore characterizing the disturbances in the relationship among brain regions (covariance) provides more information regarding the developmental integrity than searching for localized changes in the brain.
The use of graph-theory based approaches has provided a means to study abnormalities in the complex network-based organization of the brain that may be especially relevant to disorders like psychosis, where obvious brain anomalies are rare.12–14 In particular, morphological networks (the “connectome”), based on anatomical covariance13,15,16 among brain regions, appear to capture functionally relevant developmental maturation,16 with recent longitudinal imaging data linking anatomical covariance to coordinated brain development.17,18 In primates and other animals, experimental disruption of cortical connections during early stages of brain maturation produces alterations in both proximal and distal cortical folding patterns.7,19 This suggests that brain regions that covary in their degree of cortical folding are likely to be developmentally related, and that connectomic approaches in regional gyrification may provide a window on the developmental pathway of brain connectivity.20
Several neuroimaging studies have utilized graph theoretical approach to study the structural and functional properties of the brain as a connected system in the presence of schizophrenia.21–25 These studies have reported disturbances in crucial organizing principles of a “small-world” biological network formed by the nodal units (brain regions), resulting in a more segregated, less integrated and inefficient system in patients. Several studies also suggest a disturbance in centrality,25–27 affecting the prominence of highly connected brain regions that act as core ‘hubs’ contributing to the efficient organization of the brain networks. However, these studies have only looked at measures of brain volumes or functional activation in relation to healthy controls or family members and none has studied the relationship with clinically meaningful variables such as treatment response.
Here, we construct gyrification-based connectome using structural magnetic resonance imaging (MRI) data and relate it to treatment response in a well-characterized clinical sample.10 More specifically, we advance our previous work by exploring whether the properties of the gyrification-based connectome of this cohort (80 patients) at the time of their first episode was related to their subsequent response to treatment. We hypothesized that Nonresponders would show greater degree of segregated architecture, with poorer integration and resilience across the cortical folding connectome indicating a neurodevelopmental basis for treatment response in psychosis.
Methods
Subjects
Patients with first-episode psychosis (FEP) were recruited from the South London and Maudsley National Health Service Foundation Trust, South East London, England. Details of this sample have been previously described.10 Mainly, all patients with a functional psychotic illness (International Statistical Classification of Diseases, 10th Revision [ICD-10] codes F10-19, excluding coding F1x.0 for acute intoxication; F20-29 and F30-39, psychosis codes) were invited to participate. A sample of healthy controls similar to the patient group in age, sex, ethnicity, educational qualifications, and employment status was recruited from the same geographical area. All participants gave written informed consent and this study received ethical approval from Regional Ethics Committee (London). Further details can be found in supplementary material.
A total of 80 patients and 46 healthy controls were included in this study. The majority of the patients (n = 63) were taking first-line atypical antipsychotics (35 olanzapine, 18 risperidone, 4 quetiapine fumarate, 1 amisulpride, and 5 aripiprazole), 2 were taking typical antipsychotics (1 each were receiving haloperidol and flupenthixol), and 15 were not on antipsychotics at baseline.
Response was operationalized as a reduction in symptom severity to the levels required by the remission criteria of the Schizophrenia Working Group Consensus.28 This consensus established a set of criteria that provide an absolute threshold in severity of symptoms that should be reached for clinical improvement. We evaluated response to treatment 12 weeks after MRI scan using information obtained from clinical records, patient face-to-face interviews, and reports from informants using the World Health Organization Personal and Psychiatric History Schedule. Using the same method that was employed in our previous work,10 40 patients were classified as Responders and 40 as Nonresponders (Further details provided in the supplementary material).
Gyrification Analysis
Details of the MRI acquisition and processing are provided in the supplementary material. Cortical surfaces were reconstructed using FreeSurfer version 4.5.0, employing standard preprocessing procedures as described by Dale et al.29 Local Gyrification Index was computed using Schaer’s method30 with the aid of Desikan’s atlas31 to obtain regional values for 68 parcellated brain regions as described in the supplementary material.
Constructing Gyrification-Based Networks
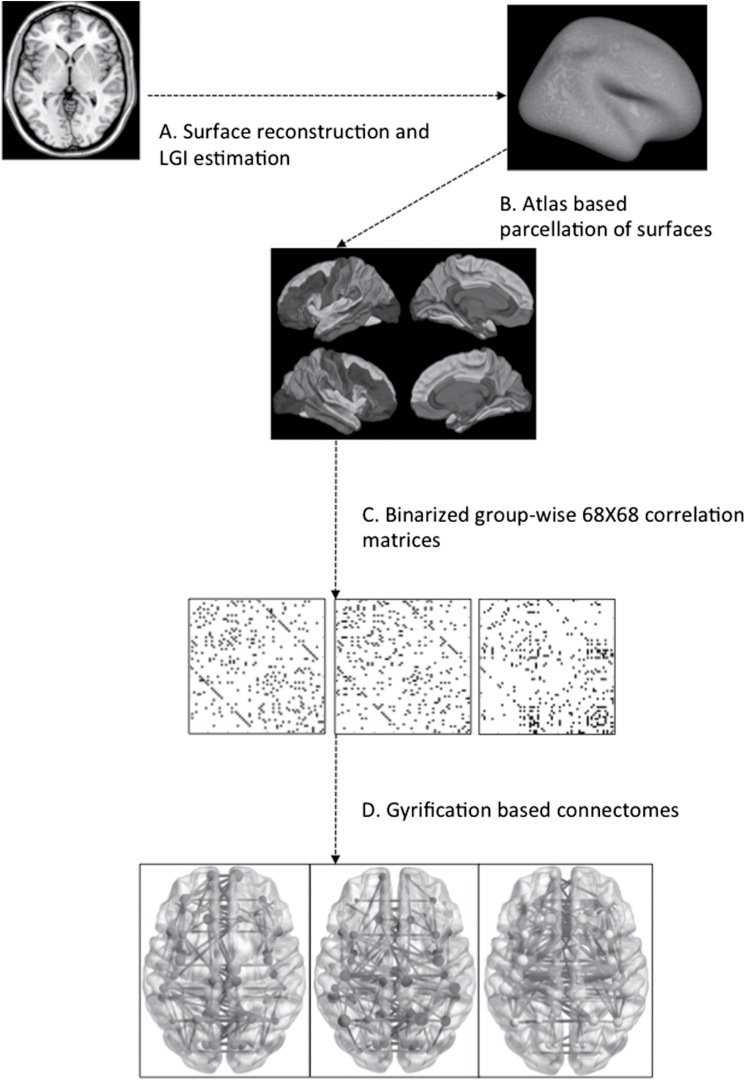
A 68×68 Pearson’s correlation matrix of gyrification indices of each parcellated brain region adjusted for age, gender, affective/nonaffective diagnostic category, intracranial volume and mean overall gyrification index in line with He et al,32 was used to create a binary adjacency matrix for each group, using threshold values for the correlation coefficients. Instead of choosing a single coefficient threshold, we used a range of thresholds determined by connection densities (proportions of connections present in a graph to all possible connections) varying from 0.1 to 0.5 (increments of 0.05) to compare the properties of emerging networks. Across this range in both groups, the resulting graphs were fully connected and not fragmented (minimum density at which fully connected graph was observed = 0.08). The graphs approached random configuration beyond the density of 0.5. The steps involved in obtaining the connectomes are summarized in figure 1.
Fig. 1.
Steps in processing the gyrification-based connectome. (A) Surface reconstruction was carried out using Freesurfer software; Local Gyrification Indices were estimated using Schaer’s procedure; (B) Desikan atlas was used for parcellating the cortical surface to 68 regions (34 on each hemisphere); (C) Association matrices were obtained by calculating the correlations between regional gyrification across subjects within each group separately; (D) Binary adjacency matrices were derived from thresholding at minimum density for fully connected graphs in all groups. The nodes and edges derived from the group-specific matrices are presented using different colors in the online version (red: controls, blue: Responders, yellow: Nonresponders).
Properties of the Connectome
The patterns of relationship among brain regions within a network can be described using 4 groups of topological properties (integration, segregation, centrality and resilience) quantified using various graph theoretical measures.12,33 In a gyrification network, segregation (or clustered covariance) may suggest modular development or plasticity of related brain regions, indicating a potential for regionally selective functional dependency within the cluster. On the other hand, integration or distributed covariance may result from maturational processes (or constraints) affecting the entire brain. A highly integrated gyrification network can also result from the presence of certain “central” hub regions whose structure covaries with a large number of other brain regions, leading to widely distributed structural coupling. When such hub regions also show a covarying relationship with each other, the entire network will be highly resilient to pathological processes affecting a single hub region, indicating resilience. Integration was measured using Global Efficiency and Characteristic Path Length; segregation was measured using Clustering Coefficient and Local Efficiency; centrality was measured on the basis of Degree and Betweenness; resilience was measured using simulated Random and Targeted Attacks and Assortativity. We also quantified Small-World Index (SWI), a measure of the balance between integration and segregation and Modularity Coefficient that reflects the community structure within a network. The graph metrics used to quantify these properties are explained in detail in the supplementary material.
All topological properties were computed using Graph Analysis Toolbox (GAT)34 (http://brainlens.org/tools.html) that uses computation algorithms from the Brain Connectivity Toolbox (https://sites.google.com/site/bctnet/). The connectomes from the 3 groups were visualized using BrainNet Viewer35 (http://www.nitrc.org/projects/bnv/).
Group Comparison
To test the statistical significance of the difference between the topological parameters, we compared 2 groups at a time (Responders vs Controls, Nonresponders vs Controls and Responders vs Nonresponders) using a nonparametric permutation test with 1000 repetitions. For each iteration, the regional gyrification indices from the 68 parcellated regions of each participant were randomly reassigned to one of 2 new groups with the sample size identical to the original groups that were being compared (eg, controls and Nonresponders). This permutation approach preserves the gyrification index within regions but shuffles across individuals during resampling.
Binary adjacency matrices across a range of network densities (0.1 to 0.5, increments of 0.05) were obtained for each random group. Topological measures were then calculated for the networks and differences between the random groups were computed across the entire range of densities. For the various topological properties, differences in the area under the curves obtained from plotting the values of each random group across the range of densities were obtained for each iteration. This resulted in a null distribution of differences, against which the P values of the actual differences in the curve functions obtained by comparing the 2 contrasted groups (eg, controls vs Nonresponders) were computed. This nonparametric permutation test based on functional data analysis (FDA)36 compares the shape of the curves derived from multiple threshold points, and thus inherently accounts for multiple comparisons.37,38 For regional (n = 68 nodes) properties such as local efficiency, clustering and degree, an additional correction for multiple comparison (False Discovery Rate [FDR]) was used with corrected P < .01 considered as significance threshold. The same permutation approach was also used when comparing the curves obtained from random and targeted attack on each group’s networks. Hubs were defined as the nodes whose FDA-based curve function for regional degree was 2 SDs greater than the mean of corresponding regional degree curve functions obtained from the 1000 random permutations in each group.
Results
Clinical Variables
The clinical and demographic characteristics of the sample are shown in table 1. Responders and Nonresponders did not differ in the distribution of diagnostic categories (proportion with nonaffective psychosis 73% vs 68%, χ2 = 0.24, df = 1, P = .63; table 1). There were no significant differences between the 2 groups in terms of handedness, total intracranial volumes, baseline PANSS negative scores, median DUP, median duration of illness, average dose or duration of antipsychotic treatment or the proportion of antipsychotic-naive subjects at baseline (all P > .05; table 1). Responders had lower baseline total PANSS and PANSS positive symptoms scores than Nonresponders. The patient group as a whole was slightly older than the healthy controls (mean [SD] in patients = 28.0 [8.0], mean [SD] in controls = 24.6 [5.6] y, F = 6.39, P = .013).
Table 1.
Clinical and Demographic Variables
| Nonresponders (n = 40) | Responders (n = 40) | Healthy Controls (n = 46) | F/ χ2 (*P < .05) | |
|---|---|---|---|---|
| Diagnosis (%) | — | χ2 = 3.6 (df = 4) | ||
| Schizophrenia | 64 | 55 | ||
| Schizoaffective disorder | 8 | 8 | ||
| Bipolar disorder | 10 | 18 | ||
| Depressive disorder | 15 | 13 | ||
| Other | 3 | 5 | ||
| Diagnostic groups (Affective/ Nonaffective) | 11/29 | 13/27 | — | χ2 = 0.24 (df = 1) |
| Age in years (SD) | 28.1 (7.9) | 28.0 (8.2) | 24.7 (5.6) | F = 3.17* (2,123) |
| Gender (females/males) | 10/30 | 12/28 | 21/25 | χ2 = 4.5 (df = 2) |
| Education in years (SD) | 13 (3.6) | 13.4 (3.8) | 14.7 (3.2) | F = 2.1 (2,91) |
| IQ (NART, SD) | 93 (11) | 90 (11) | 95 (9) | F = 2.0 (2,94) |
| Handedness (left/right) | 2/38 | 3/37 | 7/39 | χ2 = 2.2 (df = 2) |
| Intracranial volume in cm3 (SD) | 1724 (212.8) | 1753 (213.6) | 1728 (189.4) | F = 0.24 (2,124) |
| Baseline PANSS score (SD) | ||||
| Total | 62.6 (13.4) | 55.6 (12.5) | — | F = 6.38* (1,72) |
| Positive | 16.1 (6.7) | 13.4 (4.6) | — | F = 4.18* (1,72) |
| Negative | 16.6 (6.3) | 14.3 (5.8) | — | F = 2.85 (1,72) |
| No. of days on treatment at the time of scan (SD) | 38.0 (28.1) | 42.6 (32.7) | — | F = 0.43 (1,74) |
| No. of treatment naïve subjects at the time of scan (Affective/nonaffective) | 1/6 | 2/6 | χ2 = 0.08 (df = 1) | |
| Average dose in chlorpromazine equivalents at the time of scan (SD) | 244.9 (167.3) | 223.1 (136.4) | — | F = 0.34 (1,67) |
| Median DUP in weeks (25th; 75th percentiles)a,b | 9 (3;74) | 6 (1;24) | — | Z = −1.52 (df = 61) |
| Median time in weeks between contact with services and the scan (25th; 75th percentiles)a,b | 5 (2;8) | 5 (3;10) | — | Z = −0.41 (df = 61) |
| Median DOI in weeks at the time of scan (25th; 75th percentiles)a,b | 18 (7;81) | 13 (5;26) | — | Z = −1.83 (df = 61) |
Note: NART, National Adult Reading Test; PANSS, Positive and Negative Symptoms Scale; DUP, duration of untreated psychosis; DOI, total duration of illness (both treated + untreated).
aBased on 63 patients and 29 healthy controls.
bMann-Whitney U tests.
*Group differences significant at P < .05.
Segregation and Integration
Individually, all 3 groups showed small-worldness (mean SWI across densities >1 for all 3 groups). However, Nonresponders had significantly reduced small-worldness when compared to both controls and Responders in the FDA permutation analysis, indicating a global disturbance in the covariance patterns in Nonresponders. Also, Nonresponders showed higher segregation, with mean clustering coefficient higher than those of both controls and Responders. Mean local efficiency, which indicates cliquishness, was also significantly higher in Nonresponders, reaching statistical significance when compared to controls, and a trend-level significance when compared to Responders. Nonresponders also had increased path length and reduced global efficiency when compared to both controls and Responders, indicating a lack of distributed covariance patterns. These results are presented in detail in table 2.
Table 2.
Topological Properties of Gyrification-Based Connectome
| Controls | Responders | Nonresponders | FDA Permutation Test (P values) | |
|---|---|---|---|---|
| Small-world index | 1.86 (0.64) | 1.78 (0.52) | 1.26 (0.19) | Con vs Res |
| *Con > NonRes (.003) | ||||
| *Res > NonRes (.02) | ||||
| Measures of segregation | ||||
| Clustering coefficient | 0.5143 (0.05) | 0.5240 (0.07) | 0.6052 (0.08) | Con vs Res (.73) |
| *Con vs NonRes (.01) | ||||
| *Res vs NonRes (.005) | ||||
| Local efficiency | 0.7234 (0.07) | 0.7278 (0.08) | 0.7629 (0.09) | Con vs Res (.71) |
| *Con < NonRes (.04) | ||||
| Res < NonRes (.06) | ||||
| Measures of integration | ||||
| Characteristic path length | 1.9418 (0.53) | 1.9341 (0.52) | 2.0946 (0.67) | Con vs Res (.59) |
| *Con < NonRes (.04) | ||||
| Res < NonRes (.10) | ||||
| Global efficiency | 0.609 (0.11) | 0.612 (0.11) | 0.592 (0.13) | Con vs Res (.50) |
| Con > NonRes (.09) | ||||
| *Res > NonRes (.04) | ||||
| Measures of resilience | ||||
| Assortativity | 0.185 (0.12) | 0.286 (0.07) | 0.273 (0.13) | *Con < Res (.03) |
| Con vs NonRes (.62) | ||||
| Res vs NonRes (.63) | ||||
| Relative size of large component after targeted attack | 42.2% (34%) | 40.7% (34%) | 30.2% (33%) | Con vs Res (.30) |
| *Con > NonRes (.04) | ||||
| *Res > NonRes (.01) | ||||
| Relative size of large component after random attack | 43.5% (33%) | 43.5% (32%) | 39.7% (30%) | Con vs Res (.50) |
| *Con > NonRes (.04) | ||||
| Res vs NonRes (.15) | ||||
| Modularity coefficient | 0.354 (0.13) | 0.334 (0.10) | 0.253 (0.10) | Con vs Res (.47) |
| *Con > NonRes (.03) | ||||
| *Res vs NonRes (.03) | ||||
| Hubs based on degree centrality | ||||
| Regions with degree 2 SDs greater than group mean | Right posterior cingulate | None | Right rostral middle frontal Right supramarginal | |
Note: FDA, Functional Data Analysis; Con, Controls; Res, Responders; NonRes, Nonresponders. Direction of change shown only for significant results that reach at least a trend level statistical threshold (P = .1). Numbers in brackets refer to SDs across the different densities at which comparison were made.
*P < .05 in FDA permutation analysis.
Resilience of the Connectome
The Nonresponders connectome showed significantly worse resilience to both targeted attack and random attack, when compared to controls. In comparison to controls, random attack produced an 8.7% greater reduction in the size of the largest connected component, while targeted removal of hubs produced a 28.4% greater reduction in Nonresponders, suggesting that the Nonresponder connectome is more vulnerable to targeted removal of hubs (indicating a disturbance in overall binding influences that induce covariance across distributed set of cortical regions). Responders, on the other hand, showed a higher assortativity when compared to controls, suggesting that they have more resilience to hub removal. Responders also showed higher resilience compared to Nonresponders, with a statistically significant difference for targeted attack and a trend-level significance for random attack.
Regional Topological Properties
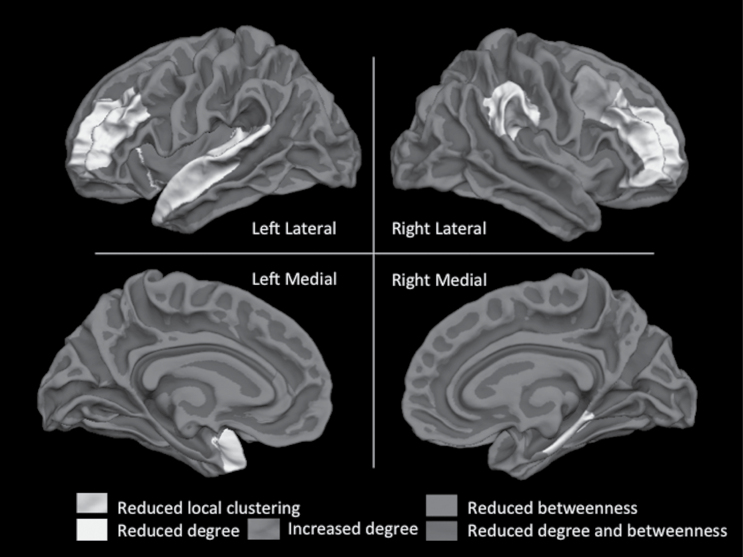
Regional topological characteristics revealed that when compared to Responders, Nonresponders had significant reduction in the node betweenness of the left insula and left rostral anterior cingulate cortex, and reduced clustering and reduced degree of right pars orbitalis, also affecting several fronto-temporal nodes (including the bilateral middle frontal gyrus, left insula, superior temporal and temporal pole regions, and the right parahippocampal region). This suggests that the perturbed relationship between these structures and the rest of the brain is a feature of poor treatment response.
While the posterior cingulate emerged as a significantly central hub in controls, the right rostral middle frontal and supramarginal regions showed the highest degree of centrality (>2 SD of network mean) within the Nonresponder connectome, despite showing reduced centrality when compared to controls and Responders. Further results from the group comparisons of regional topological properties are presented in table 3 and figure 2.
Table 3.
Regional Changes in Topological Properties
| Group Comparisons | Cortical Regions | Permutation-based P Values (FDR-Corrected) |
|---|---|---|
| Nodes with altered local clustering coefficient | ||
| Controls > Nonresponders | Right caudal middle frontal | .001 |
| Left caudal middle frontal | .007 | |
| Left posterior cingulated | .008 | |
| Nonresponders > Controls | Right transverse temporal | .005 |
| Responders > Nonresponders | Right pars orbitalis | .007 |
| Nonresponders > Responders | None | |
| Controls> Responders | None | |
| Responders > Controls | Left inferior temporal | .005 |
| Nodes with altered node betweenness | ||
| Controls > Nonresponders | None | |
| Nonresponders > Controls | None | |
| Responders > Nonresponders | Left insula | .005 |
| Left rostral anterior cingulated | .009 | |
| Nonresponders > Responders | None | |
| Controls > Responders | Left caudal middle frontal | .005 |
| Responders > Controls | None | |
| Nodes with altered degree | ||
| Controls > Nonresponders | Right rostral middle frontal | .001 |
| Left insula | .003 | |
| Left transverse temporal | .009 | |
| Right cuneus | .001 | |
| Nonresponders > Controls | None | |
| Responders > Nonresponders | Right rostral middle frontal | .002 |
| Left insula | .002 | |
| Right supramarginal | .005 | |
| Left transverse temporal | .005 | |
| Left temporal pole | .006 | |
| Right parahippocampal | .007 | |
| Left superior temporal | .008 | |
| Left rostral middle frontal | .009 | |
| Nonresponders > Responders | Right caudal middle frontal | .001 |
| Controls > Responders | Right entorhinal | .005 |
| Left superior temporal | .008 | |
| Responders > Controls | Left lateral occipital | .001 |
Note: FDR, False Discovery Rate.
Fig. 2.
Regional changes in the centrality of the gyrification connectome in Nonresponders compared to Responders. Details of the cortical regions showing centrality changes are shown in table 2. Labels from Desikan atlas are displayed on a Freesurfer-based average reconstructed surface (fsaverage). Online version of this paper has a color figure with regional labels.
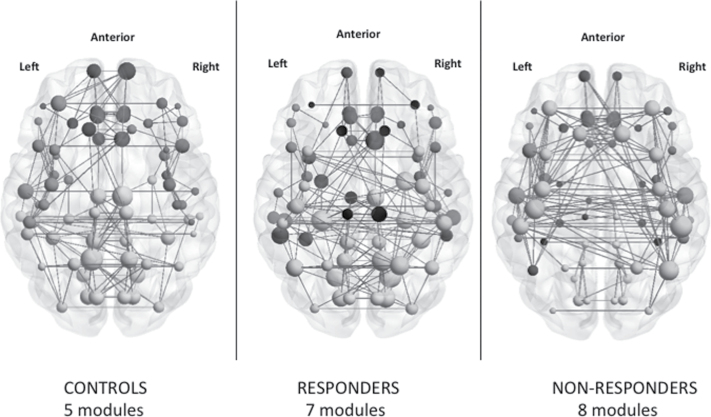
Modularity
The modularity coefficient was significantly lower in Nonresponders when compared to both Responders and controls, suggesting a weaker ability in Nonresponders to partition the gyrification connectome into organized communities. In controls, 5 optimized modules (figure 3) were noted, compared to 7 in Responders and 8 in Nonresponders. The distribution of the module membership in controls revealed 2 perisylvian and 2 posterior lateral (parieto-temporo-occipital) modules on either hemispheres along with a medial module for midline structures and an anterior prefrontal module. In Responders, the 2 perisylvian, the medial (midline structures), and the anterior prefrontal modules were mostly preserved, while a bilateral pericentral and an additional orbitofrontal module were noted. Nonresponders had a more fragmented pattern, with right frontoinsular regions (insula, pars triangularis and pars opercularis) forming a separate restricted module. The modular structure of the connectome is shown in figure 3 with a list of regions indicated in supplementary material.
Fig. 3.
Graphical representation of gyrification connectomes. Connectomes in controls, Responders and Nonresponders are visualized using BrainNet viewer (www.nitrc.org/projects/bnv). The modules are color-coded separately for each network in the online version of this image. The size of the nodes is proportional to the nodal degree (number of edges) within each connectome.
Discussion
To our knowledge, this is the first study that has used a connectomic approach with gyrification data to predict treatment response in psychosis. We had 3 major findings. Firstly, we found that FEP patients with (future) poor response to treatment already show, at illness onset, abnormalities in the structural covariance patterns of the cortical folding, indicating a higher burden of abnormal neurodevelopment in this group. In particular, Nonresponders show a reduction in the distributed relationship among brain regions (high segregation, poor integration) when compared to those who respond to treatment and to healthy individuals. Secondly, reduced centrality of key regions, such as left insula and anterior cingulate cortex is associated with nonresponse, indicating a marked reconfiguration of gyrification in Nonresponders. Thirdly, Nonresponders have a more vulnerable pattern of covariance that disintegrates when simulated lesions remove high degree hubs, indicating that cortical folding of Nonresponders have an abnormal excessive dependence on some highly central hub regions. Such reduced resilience suggests that Nonresponders have a “fragile” covariance pattern prone to disintegration in structural relationships if the cortical folding in certain key brain regions is affected during development. In summary, abnormally segregated, poorly integrated and fragile gyrification covariance may be an important feature that underlies the unfavorable prognosis seen in early Nonresponders with FEP.
We found that Responders only had sparse abnormalities in the gyrification connectome when compared to controls. Apart from a reduction in the betwenness centrality of left caudal middle frontal region and an increased segregation of left inferior temporal region, there were no other notable differences from the controls. This paucity of abnormalities is consistent with our previous report of scant localizable defects in the whole brain gyrification patterns in Responders.10 Using a connectomic approach, we now observe that Responders have a high degree correlation of assortativity, ie, high degree hubs have preferential connections with other high degree hubs, providing increased resilience to localized “lesions” affecting the brain. This property of the Responder connectome may offer a degree of protection from the effects of early developmental deviations and explain a favorable prognosis in psychosis.
Structural covariance connectomes based on morphometric measures represent synchronized developmental changes.39–41 Despite the extensive evidence in favor of widespread gyrification abnormalities in psychosis,42–46 the developmental influence on the gyrification-based connectomes has not yet been demonstrated directly. Evidence from experimentally induced developmental lesions of the white matter, which result in both proximal and distal changes in folding patterns,19 strongly supports the role of maturational covariance, driven by axonal connectivity, in shaping the gyrification connectome. Interestingly, during late childhood and adolescence, the global efficiency (or integration) of covariance networks increases, while local efficiency (segregation) decreases.39–41 This change has been attributed to synaptic fine-tuning and/or pruning processes.15,16 At present, it is not known whether the covariance in gyrification is susceptible to such late maturation influences, as majority of cortical folding is complete in fetal life, therefore suggesting that the connectome abnormalities associated with poor treatment response are already present “in utero.” This also complements our recent report of reduced white matter integrity in distributed tracts (uncinate, cingulum and corpus callosum) in Nonresponders, while Responders were indistinguishable from healthy controls.11
The structural covariance networks are broadly consistent with the patterns of dynamic functional interactions.18,39 In this regard, the reduced global efficiency noted in Nonresponders may also be reflected in dysfunctional information transfer across the brain. Some support for this notion comes from studies showing more extensive sensory processing deficits in poor outcome groups.47 Reduction in symptoms upon antipsychotic treatment may depend on the integrity of functional interactions48 and may require concomitant reconfiguration of connectivity patterns,49 though such acute shifts in connectional patterns may not be sufficient to alter the established structural covariance in gyrification. Interestingly, our own longitudinal data suggest that exposure to antipsychotic medications is in itself associated with an improvement in the integrity of white matter tracts.11 It is tempting to speculate that an efficiently connected brain, where such plastic reconfigurations are more readily permissible, may be an important requirement for the presently available antipsychotics to produce a favorable response.
Compared to Responders, Nonresponders showed reduced centrality of the regions constituting the salience network (insula and anterior cingulate)50 and of several fronto-temporal regions, reduced local clustering of right pars orbitalis (inferior frontal region), and decreased integration. Reduced regional centrality, in the backdrop of an overall increase in segregation, indicate that these regions have reduced integration with other distant, distributed brain regions15 With respect to the salience network, the notion of reduced integration with other networks is consistent with the emerging notions of the primacy of this network in the neural mechanisms relating to the persistence of psychotic symptoms.51,52 Preliminary evidence from poor Responders to antipsychotics switched to second-line treatment with clozapine suggests that clozapine had a specific alleviating effect on the aberrant functional activation of insula, cingulate and thalamus,53 suggesting that further focus on these regions may be warranted when attempting to improve the proportion of treatment Responders.
Our study has a number of strengths. We adjusted for the effect of diagnosis when studying the neurobiology of treatment response in FEP, so the group level covariances are influenced by prognosis (response) rather than the diagnosis. We previously noted that the gyrification differences between the treatment response groups was not explained by diagnostic differences, and had very small spatial correspondence with the gyrification differences between the diagnostic groups.10 Graph approaches provide a large number of different theoretical metrics that can be employed to investigate the connectomic properties.33,54 We selected the most intuitive metrics that can be used to meaningfully interpret the structural covariance in a gyrification network.15 Several limitations of this study must be considered. At present, the neurobiological underpinnings of graph metrics are unclear55; so caution must be practiced when making pathophysiological inferences. For example, methodological issues such as the definition of nodes while constructing graph networks remain unsolved55; however, we defined nodes in accordance with meaningful neuroanatomically-defined boundaries of cortical folding. Also, the absolute values of graph metrics are bound to vary if different parcellation schemes are employed.56 Still, anatomically defined parcellations have been shown to have greater convergence with developmental changes in structural covariance networks, possibly due to improvements in signal-to-noise ratio.41 Further, several previous studies have shown that when 2 networks are compared using identical approach for node definition, valid and reliable results can be generated.13
In summary, we provide the first report that poor early response to treatment in FEP is associated with disrupted structural covariance in cortical folding patterns that is likely to be developmentally driven. This would imply that perturbed neurodevelopment is directly relevant to prognostic outcomes in psychosis. Evidence that the coordinated maturation of certain key brain regions is predominantly affected in patients showing poor treatment response raises the possibility of utilizing the information from gyrification-based connectomes for prospectively predicting treatment response in psychosis.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was partly supported by the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, and by a King’s College London Translational Research grant to P.D. P.D.’s research is also supported by the NARSAD and the Psychiatry Research Trust. L.P. was originally supported by a grant from the Wellcome Trust (grant number: 076448/Z/05/Z) and currently by the Department of Psychiatry & Schulich School of Medicine Dean’s Support funds, Western University, Ontario. A.A.T.S.R. was supported by the Netherlands Organization for Scientific Research (www.nwo.nl) (NWO-VENI grant number 451-07-009). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Supplementary Material
Acknowledgments
We acknowledge Ms Jyothika Kumar’s assistance in the preparation of this manuscript. R.M.M. has received honoraria for lectures from Janssen, Astra-Zeneca, Lilly, Novartis, and BMS. A.S.D. has received honoraria for lectures from Janssen and has served on advisory boards for Eli-Lilly and Novartis. L.P. received a Young Investigator travel fellowship sponsored by Eli Lilly (2010) and Travel Support from Magstim Limited (2014) and in the last 1 year, has held shares of Shire Inc. and Glaxo Smith Kline in his/his spousal pension funds. Drs T.R.M., H.T., C.C., F.A., R.W., V.M., S.B., M.D., A.S., C.M.P., and P.D. report no competing interests.
References
- 1. Emsley R, Rabinowitz J, Medori R. Remission in early psychosis: rates, predictors, and clinical and functional outcome correlates. Schizophr Res. 2007;89:129–139. [DOI] [PubMed] [Google Scholar]
- 2. Alvir JM, Woerner MG, Gunduz H, Degreef G, Lieberman JA. Obstetric complications predict treatment response in first-episode schizophrenia. Psychol Med. 1999;29:621–627. [DOI] [PubMed] [Google Scholar]
- 3. Meltzer HY, Rabinowitz J, Lee MA, et al. Age at onset and gender of schizophrenic patients in relation to neuroleptic resistance. Am J Psychiatry. 1997;154:475–482. [DOI] [PubMed] [Google Scholar]
- 4. Das M, Kumari V, Soni W, et al. Neurological soft signs and their relationship to cognitive and clinical efficacy of atypical antipsychotics in schizophrenia. Schizophr Bull. 2004;30:241–253. [DOI] [PubMed] [Google Scholar]
- 5. Murray RM, O’Callaghan E, Castle DJ, Lewis SW. A neurodevelopmental approach to the classification of schizophrenia. Schizophr Bull. 1992;18:319–332. [DOI] [PubMed] [Google Scholar]
- 6. Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. [DOI] [PubMed] [Google Scholar]
- 7. Goldman-Rakic PS. Morphological consequences of prenatal injury to the primate brain. Prog Brain Res. 1980;53:1–19. [PubMed] [Google Scholar]
- 8. Friedman L, Knutson L, Shurell M, Meltzer HY. Prefrontal sulcal prominence is inversely related to response to clozapine in schizophrenia. Biol Psychiatry. 1991;29:865–877. [DOI] [PubMed] [Google Scholar]
- 9. Cachia A, Paillère-Martinot M-L, Galinowski A, et al. Cortical folding abnormalities in schizophrenia patients with resistant auditory hallucinations. Neuroimage. 2008;39:927–935. [DOI] [PubMed] [Google Scholar]
- 10. Palaniyappan L, Marques TR, Taylor H, et al. Cortical folding defects as markers of poor treatment response in first-episode psychosis. JAMA Psychiatry. 2013;70:1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marques TR, Taylor H, Chaddock C, et al. White matter integrity as a predictor of response to treatment in first episode psychosis. Brain. 2013;137:172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. [DOI] [PubMed] [Google Scholar]
- 13. Evans AC. Networks of anatomical covariance. Neuroimage. 2013;80:489–504. [DOI] [PubMed] [Google Scholar]
- 14. Micheloyannis S, Pachou E, Stam CJ, et al. Small-world networks and disturbed functional connectivity in schizophrenia. Schizophr Res. 2006;87:60–66. [DOI] [PubMed] [Google Scholar]
- 15. Griffa A, Baumann PS, Thiran JP, Hagmann P. Structural connectomics in brain diseases. Neuroimage. 2013;80:515–526. [DOI] [PubMed] [Google Scholar]
- 16. Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 2013;14:322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee NR, Raznahan A, Wallace GL, et al. Anatomical coupling among distributed cortical regions in youth varies as a function of individual differences in vocabulary abilities Hum Brain Mapp. 2014;35:1885–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raznahan A, Lerch JP, Lee N, et al. Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron. 2011;72:873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goldman-Rakic P, Rakic P. Experimental modification of gyral patterns. In: Geshwind N, Galaburda AM. (eds). Cerebral Dominance: The Biological Foundations. Cambridge, MA: Harvard University Press; 1984. [Google Scholar]
- 20. White T, Hilgetag CC. Gyrification and neural connectivity in schizophrenia. Dev Psychopathol. 2011;23:339–352. [DOI] [PubMed] [Google Scholar]
- 21. Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. NeuroImage. 2012;62:2296–2314. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Y, Lin L, Lin C-P, et al. Abnormal topological organization of structural brain networks in schizophrenia. Schizophr Res. 2012;141:109–118. [DOI] [PubMed] [Google Scholar]
- 23. Lynall M-E, Bassett DS, Kerwin R, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Q, Su T-P, Zhou Y, et al. Anatomical insights into disrupted small-world networks in schizophrenia. Neuroimage. 2012;59:1085–1093. [DOI] [PubMed] [Google Scholar]
- 25. Van den Heuvel MP, Sporns O, Collin G, et al. Abnormal Rich Club Organization and Functional Brain Dynamics in Schizophrenia. JAMA Psychiatry. 2013;70:783–792. [DOI] [PubMed] [Google Scholar]
- 26. Palaniyappan L, Liddle PF. Diagnostic discontinuity in psychosis: a combined study of cortical gyrification and functional connectivity. Schizophr Bull. 2014;40:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van den Heuvel MP, Mandl RC, Stam CJ, Kahn RS, Hulshoff Pol HE. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J Neurosci. 2010;30:15915–15926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andreasen NC, Carpenter WT Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162:441–449. [DOI] [PubMed] [Google Scholar]
- 29. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. [DOI] [PubMed] [Google Scholar]
- 30. Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran JP. A surface-based approach to quantify local cortical gyrification. IEEE Trans Med Imaging. 2008;27:161–170. [DOI] [PubMed] [Google Scholar]
- 31. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. [DOI] [PubMed] [Google Scholar]
- 32. He Y, Chen ZJ, Evans AC. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex. 2007;17:2407–2419. [DOI] [PubMed] [Google Scholar]
- 33. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. [DOI] [PubMed] [Google Scholar]
- 34. Hosseini SM, Hoeft F, Kesler SR. GAT: a graph-theoretical analysis toolbox for analyzing between-group differences in large-scale structural and functional brain networks. PLoS One. 2012;7:e40709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ramsay JO, Dalzell CJ. Some tools for functional data analysis. J Royal Stat Soc Series B Method. 1991;53:539–572. [Google Scholar]
- 37. Bassett DS, Nelson BG, Mueller BA, Camchong J, Lim KO. Altered resting state complexity in schizophrenia. Neuroimage. 2012;59:2196–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Singh MK, Kesler SR, Hadi Hosseini SM, et al. Anomalous gray matter structural networks in major depressive disorder. Biol Psychiatry. 2013;75:777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zielinski BA, Gennatas ED, Zhou J, Seeley WW. Network-level structural covariance in the developing brain. Proc Natl Acad Sci U S A. 2010;107:18191–18196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khundrakpam BS, Reid A, Brauer J, et al. Developmental changes in organization of structural brain networks. Cereb Cortex. 2013;23:2072–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alexander-Bloch A, Raznahan A, Bullmore E, Giedd J. The convergence of maturational change and structural covariance in human cortical networks. J Neurosci. 2013;33:2889–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Palaniyappan L, Liddle PF. Aberrant cortical gyrification in schizophrenia: a surface-based morphometry study. J Psychiatry Neurosci. 2012;37:110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nanda P, Tandon N, Mathew IT, et al. Local gyrification index in probands with psychotic disorders and their first-degree relatives. Biol Psychiatry. 2014;76:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. White T, Gottesman I. Brain connectivity and gyrification as endophenotypes for schizophrenia: weight of the evidence. Curr Top Med Chem. 2012;12:2393–2403. [DOI] [PubMed] [Google Scholar]
- 45. Gay O, Plaze M, Oppenheim C, et al. Cortex morphology in first-episode psychosis patients with neurological soft signs. Schizophr Bull. 2013;39:820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harris JM, Moorhead TWJ, Miller P, et al. Increased prefrontal gyrification in a large high-risk cohort characterizes those who develop schizophrenia and reflects abnormal prefrontal development. Biol Psychiatry. 2007;62:722–729. [DOI] [PubMed] [Google Scholar]
- 47. Mitelman SA, Buchsbaum MS. Very poor outcome schizophrenia: clinical and neuroimaging aspects. Int Rev Psychiatry. 2007;19:345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sarpal DK, Argyelan M, Robinson DG, et al. Baseline striatal functional connectivity as a predictor of response to antipsychotic drug treatment. Am J Psychiatry. 2015;173:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bolding MS, White DM, Hadley JA, Weiler M, Holcomb HH, Lahti AC. Antipsychotic drugs alter functional connectivity between the medial frontal cortex, hippocampus, and nucleus accumbens as measured by H215O PET. Front Psychiatry. 2012;3:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF. Neural primacy of the salience processing system in schizophrenia. Neuron. 2013;79:814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci. 2012;37:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Molina V, Tamayo P, Montes C, et al. Clozapine may partially compensate for task-related brain perfusion abnormalities in risperidone-resistant schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32: 948–954. [DOI] [PubMed] [Google Scholar]
- 54. Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28:9239–9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fornito A, Zalesky A, Breakspear M. Graph analysis of the human connectome: promise, progress, and pitfalls. Neuroimage. 2013;80:426–444. [DOI] [PubMed] [Google Scholar]
- 56. Zalesky A, Fornito A, Harding IH, et al. Whole-brain anatomical networks: does the choice of nodes matter? Neuroimage. 2010;50:970–983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.